Abstract
Background
Postoperative atrial fibrillation (POAF) increases postoperative morbidity, mortality, and length of hospital stay. Propofol is reported to modulate atrial electrophysiology and the cardiac autonomic nervous system. Therefore, we retrospectively examined whether propofol suppresses POAF in patients undergoing video-assisted thoracoscopic surgery (VATS) compared to desflurane.
Methods
We retrospectively recruited adult patients who underwent VATS during the period from January 2011 to May 2018 in an academic university hospital. Between continuous propofol and desflurane administration during anesthetic maintenance, we investigated the incidence of new-onset POAF (within 48 hours after surgery) before and after propensity score matching.
Results
Of the 482 patients, 344 received propofol, and 138 received desflurane during anesthetic maintenance. The incidence of POAF in the propofol group was less than that in the desflurane group (4 [1.2%] vs. 8 patients [5.8%], odds ratio [OR]; 0.161, 95% confidence interval (CI), 0.040–0.653, p = 0.011) in the present study population. After adjustment for propensity score matching (n = 254, n = 127 each group), the incidence of POAF was still less in propofol group than desflurane group (1 [0.8%] vs. 8 patients [6.3%], OR; 0.068, 95% CI: 0.007–0.626, p = 0.018).
Conclusions
These retrospective data suggest propofol anesthesia significantly inhibits POAF compared to desflurane anesthesia in patients undergoing VATS. Further prospective studies are needed to elucidate the mechanism of propofol on the inhibition of POAF.
Introduction
Atrial fibrillation (AF) is the most common type of arrhythmia after surgery and is associated with increased morbidity, mortality, length of hospital stay, and medical costs [1–5]. Lung surgery is a high-risk surgery for postoperative atrial fibrillation (POAF) in the range of 3% to 20% [1, 2, 4, 6, 7], compared to general surgery (less than 10%) [8, 9]. As risk factors for POAF in lung surgery, increasing age, male sex, and increasing extent of pulmonary resection have been reported [2, 4, 10, 11]. High-risk electrocardiographic findings for POAF, which reflect atrial structural and/or electrical remodeling [12, 13], were also studied in lung and cardiac surgery.
From the anesthetic standpoint, either an intravenous or a volatile anesthetic agent is administered for anesthetic maintenance. The selection of anesthetic agents is generally based on the anesthesiologists’ discretion and/or hospital practices. In lung surgery, clinical advantages for postoperative pulmonary complications between anesthetic agents are comparable [14–16]. Regarding perioperative arrhythmias, several studies showed propofol suppressed supraventricular tachycardia, and propofol modulated atrial electrophysiology, atrioventricular conduction, and cardiac autonomic nervous system [17–19]. This evidence suggests that propofol can potentially contribute to preventing POAF. On the other hand, volatile anesthetic agents have not shown more antiarrhythmic effects than propofol [20–23].
Therefore, we retrospectively examined the effects of propofol on POAF compared to the volatile anesthetic agent with desflurane in video-assisted thoracoscopic surgery (VATS), along with possible underlying mechanisms by some literature reviews.
Materials and methods
Ethical approval
The Jikei University Review Board approved this retrospective clinical study, which provided a waiver of patient consent (reception number 30–369 [9390]).
Patients
From January 2011 to May 2018, we analyzed data for patients with lung disease who underwent VATS at our institution. We excluded patients < 20 years of age, with insufficient preoperative electrocardiography (ECG), or with a history of chronic or paroxysmal AF or congenital heart disease. We also excluded if they underwent converted open thoracotomy or pneumonectomy, received a mixture of propofol and desflurane during anesthesia maintenance, or received general anesthesia alone without epidural anesthesia. The data analyzed were incidence of POAF, age, sex, height and body weight, comorbidities, American Society of Anesthesiologists Physical Status (ASA PS), and preoperative ECG. Comorbidities included hypertension (HT), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), and current smoker status (had smoked within the past year). We also assessed electrolyte status on postoperative day (POD) 1.
Data collection and definitions
We reviewed medical records, anesthesia records, and the intensive care unit (ICU) database to obtain patient background, anesthetic management, surgical procedure, and POAF outcome data. The primary outcome was POAF incidence, defined as new onset within 48 hours after surgery. In addition, we use the Clavien-Dindo classification of surgical classifications (grade I-V) to categorize POAF; grade Ⅰ did not need intervention, grade Ⅱ required pharmacological treatment, grade Ⅲ required surgical interventions, grade Ⅳ was a life-threatening complication, and grade Ⅴ was death [16].
Preoperative electrocardiography
Preoperative ECG indicates atrial structural and/or electrical remodeling and predicts POAF after lung and cardiac surgery [12, 13]. Therefore, we analyzed preoperative ECG for all patients performed by medical technologists within 6 weeks before surgery to evaluate basic structural substrate. Standard 12-lead ECG was documented with an automatic electrocardiographic recorder (model FX-7432; Fukuda Denshi, Tokyo, Japan) at a paper speed of 25 mm/s. The following parameters were measured: P-wave duration, P-wave amplitude, and QRS duration. The P-wave duration was measured between P-wave onset and offset. The P-wave dispersion was defined as the difference between the longest and the shortest P-wave durations across 12 leads. The longest PR interval was defined as the longest interval in any of the 12 leads measured between the beginning of the P-wave and Q-wave onset. The QRS duration was measured between the Q-wave onset and the J-point, and the longest QRS duration was examined for all leads. In addition, left atrial enlargement (LAE) was defined as a P-wave duration >120 ms at lead II or a terminal negative P-wave in V1 >40 ms wide and >1 mm deep (P-wave terminal force > 40 ms × mm) [24]. LVH was present if the sum of the S-wave in V1 and the R-wave in V5 or V6 >35 mm (Sokolow-Lyon index) [25].
Perioperative management
Routine monitoring, including ECG, pulse oximetry, noninvasive blood pressure monitoring with continuous arterial pressure via a radial artery, and end-tidal carbon dioxide pressure, was performed from induction to the end of anesthesia. Before anesthetic induction, we routinely inserted an epidural catheter without contraindications for better perioperative analgesia. For anesthetic induction in the propofol group, we administered propofol using a target-controlled infusion device (Terufusion syringe pump system; Terumo, Tokyo, Japan). Fentanyl (1–2 μg/kg) (fentanyl citrate; Sankyo, Tokyo, Japan) and/or remifentanil (0.1–0.25 μg/kg/min) (Ultiva; Janssen Pharmaceutical, Tokyo, Japan) and rocuronium (0.6–1.0 mg/kg) (Eslax; MSD, Tokyo, Japan) were also administered.
In the desflurane group, we administered propofol (1–2 mg/kg) just for induction, and opioids and neuromuscular blockade were administered as described above, followed by inhaled desflurane (0.6–1.0 minimal alveolar concentration (MAC)) (Suprane; Baxter, Deerfield, IL, US) for maintenance.
Depth of anesthesia was maintained at 40 to 60 by bispectral index (BIS-XP system; Medotronic, Minneapolis, MN, USA) throughout the operation in both groups. Dexamethasone (0.1–0.15 mg/kg, max 6.6 mg) was administered intraoperatively to prevent postoperative nausea and vomiting [26]. The selection of an anesthetic agent and the use of dexamethasone were at the anesthesiologist’s discretion. The type of operation was segmentectomy or lobectomy with lymphoidectomy by VATS.
After surgery, we administered 1–4 mg/kg of sugammadex as needed to antagonize residual neuromuscular paralysis by monitoring with train-of-4 monitoring. After extubation, we transferred all patients to the ICU for overnight observation. Postoperative management, including laboratory data in the ICU, was performed by ICU specialists. Electrocardiographic monitoring was continued 48 hours after surgery, even if the patient returned to the ward the following day.
Statistical analysis
Quantitative data are presented as median (interquartile range) and qualitative data as number (%). For quantitative data, an unpaired t test or Mann-Whitney U test was performed per statistical data normality, and qualitative data were analyzed by chi-square test (if the chi-square test was not eligible for the condition, the Fisher exact probability method was used). After univariate analysis, multivariate logistic regression analysis was examined to investigate the association with POAF.
Given the inherent differences between the two groups, propensity score matching was performed to compare the incidence of POAF. The participants were 1:1 matched with nearest neighbor method without replacement, within caliper <0.1: the propofol group and the desflurane group. The propensity score was calculated by a multivariate logistic regression model involving the following covariates. Covariates included anesthetic agents, age, gender, height, body weight, past history with HT, DM, COPD, current smoker, ASA PS, procedure type, anesthesia time, operation time, one lung ventilation time, bleeding, in-out fluid balance [1, 2, 4, 6, 10, 27], serum potassium [28], and ECG findings of the P-wave dispersion [29], the maximum QRS duration, left atrial enlargement [12], and left ventricular hypertrophy [30]. A p value <0.05 was considered statistically significant, and confidence intervals (CIs) were 95%. For all statistical analyses, data were analyzed by EZR version 1.55 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.7–1, Vienna, Austria) [31].
Results
Patients and perioperative management
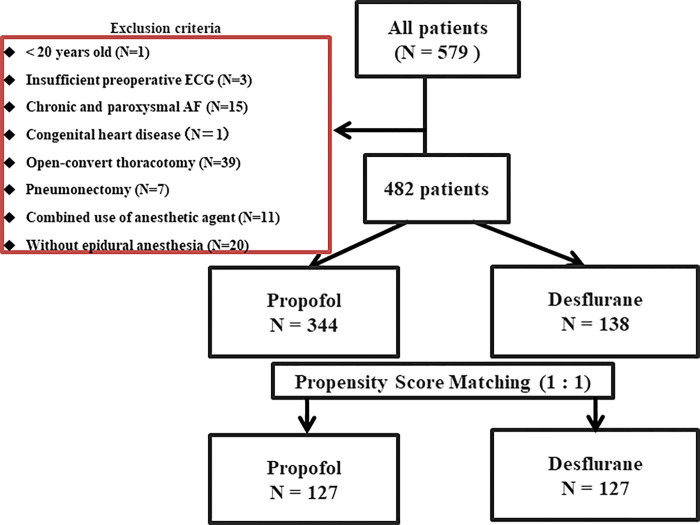
A total of 579 patients were identified, and 97 were excluded (Fig 1). Among the remaining 482 patients, 344 received continuous propofol, and 138 were maintained with desflurane for general anesthesia. In the current study, factors that affected the incidence of POAF were age, beta-blocker, and anesthetic agent (Table 1). Patient characteristics, anesthetic management, electrolyte findings, and procedure type between propofol and desflurane groups were summarized in Table 2. Patient characteristics were comparable between groups. However, during the intraoperative period, the propofol group showed significantly longer anesthesia, longer operation time, longer one-lung ventilation time, and more bleeding than the desflurane group. In addition, electrolyte serum potassium on POD 1 was significantly lower in the propofol group.
Fig 1. Flow chart of the study population.
AF: atrial fibrillation, ECG: electrocardiogram.
Table 1. Patient characteristics, anesthetic management, and surgical procedure based on POAF incidence.
| POAF (n = 12) | Non-POAF (n = 470) | p value | ||
|---|---|---|---|---|
| Patient characteristics | Age (yr) | 75 (68–76) | 67 (61–74) | 0.013 |
| Gender male, N (%) | 10 (83.3) | 288 (61.3) | 0.852 | |
| Height (cm) | 168 (162–172) | 163 (156–169) | 0.956 | |
| Body weight (kg) | 69 (63–75) | 59 (52–67) | 0.169 | |
| HT, N (%) | 6 (50.0) | 203 (43.2) | 0.244 | |
| DM, N (%) | 2 (16.7) | 73 (15.5) | 0.393 | |
| COPD, N (%) | 2 (16.7) | 57 (12.1) | 0.686 | |
| Current smoker, N (%) | 2 (16.7) | 35 (7.4) | 0.147 | |
| ASA PS Ⅰ, N (%) | 1 (8.3) | 78 (16.6) | 0.627 | |
| Ⅱ, N (%) | 11 (91.7) | 372 (79.1) | ||
| Ⅲ, N (%) | 0 (0) | 20 (4.3) | ||
| Beta-blocker, N (%) | 3 (25.0) | 20 (4.3) | 0.012 | |
| Type of surgery | Lobectomy, N (%) | 11 (91.7) | 414 (88.1) | 0.897 |
| Segmentectomy, N (%) | 1 (8.3) | 56 (11.9) | ||
| Anesthetic management | Propofol, N (%) | 4 (30.8) | 340 (72.3) | 0.044 |
| Desflurane, N (%) | 8 (69.2) | 130 (27.7) | ||
| Anesthesia time (min) | 351 (280–417) | 349 (301–409) | 0.559 | |
| Operation time (min) | 260 (212–331) | 264 (213–320) | 0.496 | |
| OLV time (min) | 229 (201–323) | 249 (201–304) | 0.835 | |
| Bleeding (ml) | 85 (50–138) | 50 (0–100) | 0.296 | |
| In-out fluid balance (ml/kg/hr) | 2.45 (1.89–3.16) | 3.58 (2.41–5.05) | 0.072 | |
| Electrolyte on POD1 | Serum potassium (mEq/L) | 4.20 (4.00–4.43) | 4.00 (3.80–4.20) | 0.091 |
| ECG markers | The P-wave dispersion (ms) | 54 (32–75) | 62 (30–74) | 0.436 |
| The longest PR duration (ms) | 176 (159–209) | 176 (160–190) | 0.953 | |
| The longest QRS duration (ms) | 111 (105–115) | 108 (102–116) | 0.997 | |
| Left atrial enlargement (%) | 5 (41.7) | 143 (30.4) | 0.943 | |
| Left ventricular hypertrophy (%) | 0 (0) | 72 (15.3) | 0.993 | |
Data are presented as median (interquartile range) or number (percent).
POAF: postoperative atrial fibrillation, HT: hypertension, DM: diabetes mellitus, COPD: chronic obstructive pulmonary disease, ASA PS: American society of anesthesiologist physical status, OLV: one-lung ventilation, ECG: electrocardiogram POD: postoperative day, PSM: propensity score matching, SMD: standardized mean difference
Table 2. Patient characteristics, anesthetic management, and surgical procedure (Before and after propensity score matching).
| Before PSM | After PSM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Propofol (n = 344) | Desflurane (n = 138) | p value | SMD | Propofol (n = 127) | Desflurane (n = 127) | p value | SMD | ||
| Patient characteristics | Age (yr) | 67 (61–73) | 69 (63–75) | 0.064 | 0.175 | 71 (64–76) | 68 (62–75) | 0.213 | 0.161 |
| Gender male, N (%) | 217 (63.1) | 81 (58.7) | 0.407 | 0.09 | 75 (59.1) | 75 (59.1) | 1.000 | <0.001 | |
| Height (cm) | 163 (157–169) | 163 (156–169) | 0.531 | 0.064 | 161 (155–167) | 162 (156–169) | 0.322 | 0.121 | |
| Body weight (kg) | 60 (52–68) | 59 (52–66) | 0.457 | 0.077 | 58 (51–66) | 59 (52–66) | 0.94 | 0.003 | |
| HT, N (%) | 144 (41.9) | 65 (47.1) | 0.310 | 0.106 | 59 (46.5) | 55 (43.3) | 0.705 | 0.063 | |
| DM, N (%) | 46 (13.4) | 29 (21.0) | 0.051 | 0.204 | 29 (22.8) | 21 (16.5) | 0.269 | 0.159 | |
| COPD, N (%) | 41 (11.9) | 18 (13.0) | 0.759 | 0.034 | 16 (12.6) | 15 (11.8) | 1.000 | 0.024 | |
| Current smoker, N (%) | 23 (6.7) | 14 (10.1) | 0.255 | 0.125 | 14 (11.0) | 12 (9.4) | 0.836 | 0.052 | |
| ASA PS Ⅰ, N (%) | 61 (17.7) | 18 (13.0) | 0.059 | 0.228 | 12 (9.4) | 18 (14.2) | 0.465 | 0.155 | |
| Ⅱ, N (%) | 273 (79.4) | 110 (79.7) | 109 (85.8) | 102 (80.3) | |||||
| Ⅲ, N (%) | 10 (2.9) | 10 (7.2) | 6 (4.7) | 7 (5.5) | |||||
| Beta-blocker, N (%) | 18 (5.2) | 5 (3.6) | 0.637 | 0.078 | 9 (7.1) | 4 (3.1) | 0.254 | 0.179 | |
| Type of surgery | Lobectomy, N (%) | 301 (87.5) | 124 (89.9) | 0.535 | 0.074 | 112 (88.2) | 113 (89.0) | 1.000 | 0.025 |
| Segmentectomy, N (%) | 43 (12.5) | 14 (10.1) | 15 (11.8) | 14 (11.0) | |||||
| Anesthetic management | Anesthesia time (min) | 356 (308–423) | 331 (280–367) | <0.001 | 0.411 | 330 (290–387) | 331 (280–370) | 0.490 | 0.056 |
| Operation time (min) | 271 (223–331) | 242 (197–292) | <0.001 | 0.374 | 246 (202–297) | 242 (200–293) | 0.525 | 0.035 | |
| OLV time (min) | 253 (206–315) | 227 (191–273) | <0.001 | 0.337 | 236 (193–276) | 228 (198–277) | 0.593 | 0.059 | |
| Bleeding (ml) | 50 (0–120) | 45 (0–100) | 0.001 | 0.304 | 50 (0–100) | 40 (0–100) | 0.438 | 0.114 | |
| In-out fluid balance(ml/kg/hr) | 3.65 (2.53–5.08) | 3.23 (2.15–4.76) | 0.093 | 0.153 | 3.54 (2.35–4.94) | 3.45 (2.17–4.79) | 0.718 | 0.019 | |
| Electrolyte on POD1 | Serum potassium (mEq/L) | 4.00 (3.80–4.10) | 4.10 (3.90–4.30) | <0.001 | 0.355 | 4.10 (3.85–4.20) | 4.10 (3.90–4.20) | 0.588 | 0.016 |
| ECG markers | The P-wave dispersion (ms) | 62 (28–75) | 63 (34–74) | 0.553 | 0.076 | 66 (31–78) | 62 (34–72) | 0.254 | 0.115 |
| The longest PR duration (ms) | 176 (158–190) | 178 (160–192) | 0.312 | 0.11 | 178 (162–192) | 176 (160–192) | 0.608 | 0.050 | |
| The longest QRS duration (ms) | 108 (102–116) | 108 (102–116) | 0.818 | 0.028 | 108 (103–116) | 108 (102–117) | 0.950 | 0.073 | |
| Left atrial enlargement (%) | 101 (29.4) | 47 (34.1) | 0.327 | 0.101 | 41 (32.3) | 41 (32.3) | 1.000 | <0.001 | |
| Left ventricular hypertrophy (%) | 58 (16.9) | 14 (10.1) | 0.067 | 0.197 | 14 (11.0) | 14 (11.0) | 1.000 | <0.001 | |
Data are presented as median (interquartile range) or number (percent).
HT: hypertension, DM: diabetes mellitus, COPD: chronic obstructive pulmonary disease, ASA PS: American society of anesthesiologist physical status, OLV: one-lung ventilation, POD: postoperative day, ECG: electrocardiogram, PSM: propensity score matching
SMD: standardized mean difference.
After adjustment for propensity score matching, 127 pairs were matched. There was no significant difference between propofol and desflurane groups, and standardized mean differences were less than 0.2 (Table 2 and S1 Data). The intraoperative maximal effect-site target concentration (Ce) of propofol was 2.7 (2.5–3.0) μg/mL, the minimal Ce was 2.5 (2.0–2.6) μg/mL.
Incidence of POAF
The overall incidence of POAF before propensity score matching (new onset, within 48 hours after VATS) was 12 of 482 patients (2.5%) in this study; 4 (1.2%) in the propofol group, and 8 (5.8%) in the desflurane group. There was less POAF incidence in the propofol group than in the desflurane group (odds ratio [OR]; 0.161, 95% confidence interval (CI), 0.040–0.653, p = 0.011) in the present study population. The POAF incidence after propensity score matching was 9 of 254 patients (3.5%) in this study; 1 (0.8%) in the propofol group and 8 (6.3%) in the desflurane group. There was also less POAF incidence in the propofol group than in the desflurane group (OR; 0.068, 95% CI: 0.007–0.626, p = 0.018) (Table 3).
Table 3. The incidence of postoperative atrial fibrillation.
| Before PSM | All | Propofol | Desflurane | p value | SMD |
| POAF incidence, N (%) | 12/482 (2.5%) | 4/344 (1.2%) | 8/138 (5.8%) | 0.006 | 0.255 |
| Odds ratio (95% Confidence Interval); 0.161 (0.040–0.653), p = 0.011 | |||||
| After PSM | All | Propofol | Desflurane | p value | SMD |
| POAF incidence, N (%) | 9/254 (3.5%) | 1/127 (0.8%) | 8/127 (6.3%) | 0.036 | 0.302 |
| Odds ratio (95% Confidence Interval); 0.068 (0.007–0.626), p = 0.018 | |||||
POAF: postoperative atrial fibrillation, PSM: propensity score matching
SMD: standardized mean difference
Clinical course, classification of surgical complications, and management of POAF
After adjustment for propensity score matching, a total of 6 patients with POAF (67%) recovered to regular sinus rhythm without pharmacologic therapy (grade І) (Table 4). We performed pharmacologic therapy for rate or rhythm control in 3 patients (grade Ⅱ). In the desflurane group, a 78-year-old POAF patient developed transient cardiac arrest due to pulmonary thromboembolism in the general ward. However, the POAF was not supposed to be the direct cause of the incident. Except for the case, patients with POAF returned to the general ward on POD1 with ECG monitoring and experienced no adverse events.
Table 4. Clinical data of POAF patients and classification of surgical complications.
| Anesthetic agent | Age (yr) | ASA PS | Procedure | Onset | Classification of complication | Treatment | Included after PSM |
|---|---|---|---|---|---|---|---|
| Propofol | 62 | 2 | Lobectomy | POD2 | Ⅰ | None | No |
| 83 | 2 | Lobectomy | POD1 | Ⅰ | None | No | |
| 67 | 1 | Lobectomy | POD1 | Ⅱ | Rate and rhythm control | No | |
| 75 | 2 | Lobectomy | POD1 | Ⅱ | Rate and rhythm control | Yes | |
| Desflurane | 67 | 2 | Lobectomy | POD1 | Ⅰ | None | Yes |
| 68 | 2 | Lobectomy | POD1 | Ⅰ | None | Yes | |
| 75 | 2 | Lobectomy | POD1 | Ⅰ | None | Yes | |
| 75 | 2 | Lobectomy | POD1 | Ⅰ | None | Yes | |
| 75 | 2 | Segmentectomy | POD1 | Ⅰ | None | Yes | |
| 78 | 2 | Lobectomy | POD1 | Ⅰ | None | Yes | |
| 69 | 2 | Lobectomy | POD1 | Ⅱ | Rate control, Bolus crystalloid | Yes | |
| 83 | 2 | Lobectomy | POD1 | Ⅱ | Rate control, Magnesium | Yes |
ASA PS: American society of anesthesiologist physical status, PSM: propensity score matching, POD: postoperative day
Discussion
There has been little clinical research on POAF incidence and anesthetic agents in lung surgery. Therefore, we investigated the novel comparison between standard anesthetic agents (propofol vs. desflurane) on the incidence of POAF after VATS. Our results showed that the incidence of new-onset POAF after VATS was significantly less for propofol anesthesia than for desflurane anesthesia.
Non-cardiac thoracic surgery is a high-risk procedure for POAF, with a reported incidence of 3% to 40% [2, 4–6, 32]. For lung surgery, the incidence of POAF is reported to be 10% to 20% for open thoracotomy, whereas it is 3% to 20% for VATS [32–35]. The lower incidence of POAF in VATS is thought to be due to that being a less-invasive procedure [27, 33]. In the present study, before matching, the incidence of POAF in the propofol group (1.2%) was less than the overall incidence (2.5%), and less than previously reported [32–35]. In subgroup analysis for the Perioperative Ischemic Evaluation (POISE) trial, although an anesthetic agent was not described, the incidence of POAF was predicted by 5.3% from the risk model, which includes age and type of surgery [5]. It is consistent with the desflurane group by 5.8% in our study before and similar even after matching (6.3%). Therefore, we speculate that this study’s difference in POAF incidence was due to propofol’s beneficial effect, not desflurane’s adverse effect.
In lung surgery, as standard practice, an intravenous or volatile anesthetic agent is administered for anesthetic maintenance. The outcome of major postoperative complications, including pulmonary complications, has been comparable between anesthetic agents [14–16]. Among volatile anesthetics, desflurane is mainly used in our hospital because of its short-acting work and is tolerated to be administered in a clinical setting [36, 37]. In addition, some studies have claimed that a high concentration of desflurane has been linked to tachycardia in healthy volunteers due to increased sympathetic stimulation [38, 39]. Nonetheless, as pointed out in the review [40], the difference between volatile anesthetics was believed to be limited during balanced maintenance of anesthesia with less than 1MAC of desflurane in our study. No difference was also observed between sevoflurane and desflurane anesthesia regarding postoperative pulmonary complications in lung surgery [28].
Two steps to POAF
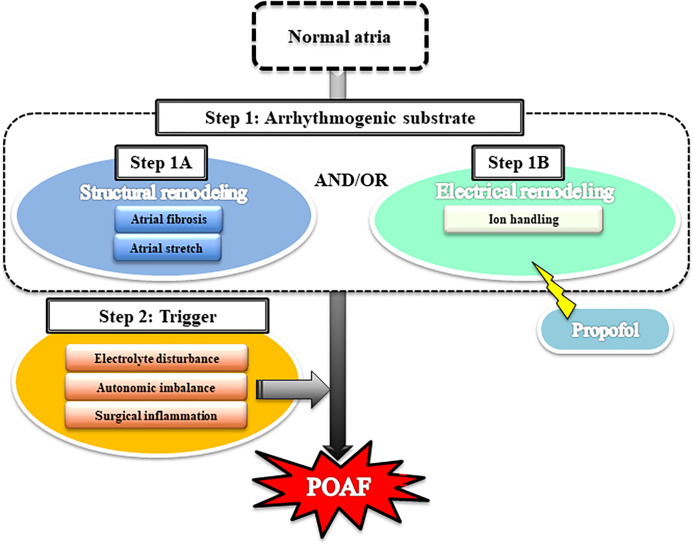
As shown in Fig 2, AF is thought to develop via the following 2 steps: the creation of an arrhythmogenic substrate (step 1), followed by a trigger event (step 2) [11, 41]. With respect to step 1, structural remodeling (step 1A) is caused by atrial fibrosis or atrial stretch, and electrical remodeling (step 1B) is caused by the modulation of cardiac ion currents. After step 1, POAF might be triggered by multiple factors such as electrolyte disturbance, surgical inflammation, and/or cardiac autonomic imbalance (step 2). We discuss the 2 steps for POAF development below. AF is thought to develop via 2 steps: an arrhythmogenic substrate (step 1) that involves structural remodeling and/or electrical remodeling, followed by a trigger event such as electrolyte disturbance, autonomic imbalance, and/or surgical inflammation (step 2).
Fig 2. Schematic of the mechanism underlying the development of postoperative atrial fibrillation (POAF).
Factors related to structural and electrical remodeling
In our study, factors related to structural and electrical remodeling, such as age, male sex, intraoperative fluid balance, and preoperative high-risk ECG findings, were matched even before propensity score matching. In the context of thoracic surgery, increasing age and male sex are reported preoperative risk factors for POAF [2, 4, 10]. Furthermore, increasing age is associated with atrial fibrosis [11], and the age-related prevalence of AF is less in women than in men, given that estrogen has been shown to prolong atrial action potential duration (APD) [42, 43].
Regarding perioperative management, atrial stretch due to volume overload may be associated with POAF, suggesting that intraoperative in-out fluid balance may contribute to the development of POAF [44]. In the present study, ICU specialists performed postoperative fluid management after surgery. Moreover, none of the patients developed sepsis or shock, which are also risk factors for POAF [44, 45]. The patients in the present study were comparable even before matching from the point of preoperative high-risk ECG findings, speculated to reflect atrial remodeling. Especially, increased P-wave dispersion, prolonged QRS duration, and LAE are reported to be the risk factors for POAF after lung surgery [12, 46]. For example, LAE causes interatrial electromechanical delay (electrical remodeling) related to decreased calcium flow [47].
Factors related to the trigger of POAF
Electrolyte disturbance is a factor related to the trigger of POAF [41]. However, little research on electrolyte derangement has been performed in the context of lung surgery, in contrast to cardiac surgery [48]. Gong et al. reported an association between POAF and higher potassium concentration in lung surgery [28]. The present study matched the serum potassium level on POD1 to minimize the influence.
The autonomic nervous system plays an important role in initiating and maintaining POAF [11, 18, 49, 50]. Amar et al. [51] concluded that one of the pathogenesis of POAF in lung surgery is increased parasympathetic nervous activity with sympathetic predominance. We did not examine heart rate variability in the present study. However, pain control, which strongly correlates with sympathetic nerve activity, was performed uniformly by the acute pain service team in the propofol and desflurane groups.
Some reports demonstrated that mediastinal lymph node dissection was associated with POAF [33, 52], although there was no association found between them in this study (p = 0.765 in nodal dissection 0/1 vs. 2/3). In addition, surgical inflammation around the vagal nerve and pericardium with mediastinal lymph node dissection may be supposed to trigger POAF [53], but the previous studies included open approaches and pneumonectomy in contrast to our study.
Potential mechanism of POAF inhibition by propofol: Attenuation of electrical remodeling and prevention of trigger event
Among cardiac ion currents, potassium plays an important role in human atrial myocytes by modulating repolarization (electrical remodeling). Ultrarapid-delayed-rectifier potassium current (IKur) and transient-outward potassium current (Ito) are major outward currents existing in the human atrium [54] but not the ventricle [55]. Thus, IKur and Ito are a target for developing anti-AF therapy [56, 57]. Commonly distributed in plants, Acacetin prolongs APD by suppressing IKur and Ito in human atrial myocytes and preventing AF in anesthetized dogs [58]. Similarly, propofol is demonstrated to prolong human atrial APD by inhibiting IKur and Ito in a dose-dependent manner [59, 60]; therefore, propofol might act as an anti-arrhythmogenic agent in the clinic, like Acacetin. In addition, the solvent for propofol contains n-3 α-linolenic acid, which blocks the human voltage-gated Kv1.5 channel, leading to IKur modulation [61]. Desflurane has not been reported to have modulating effects on atrial APD.
In the trigger event of AF, propofol has decreased vagal nerve activity [62] and P-wave dispersion compared to volatile anesthetic agents [36]. It suggests propofol might modulate cardiac autonomic imbalances and improve atrial electrophysiologic heterogeneity compared to volatile anesthetic agents. The present study did not evaluate the postoperative neural and electrical changes; however, ICU specialists should have performed postoperative care uniformly in the propofol and desflurane groups.
Surgical procedure and clinical outcome
Whether the type of surgical procedure affects the incidence of POAF remains controversial [6, 7, 10, 63, 64]. In the present study, surgical procedures, such as left or right resection, did not affect POAF incidence (p = 0.389 in POAF vs. Non-POAF, and p = 0.171 in propofol vs. desflurane). Neither the magnitude of resection also affected POAF incidence (p = 0.763 in POAF vs. Non-POAF, and p = 0.769 in propofol vs. desflurane) (S2 Data). For the relation between surgical location and incidence of embolism, 2 cohort studies have identified left-upper lobectomy as a risk factor for stroke [63, 65]. None of the patients with POAF in the present study developed atherothrombotic brain infarction during their hospital stay.
From a pathological standpoint, we included patients with small cell lung cancer (n = 10), not only non-small cell lung cancer and so on. (n = 472). Although the general condition tends to be severe in small cell lung cancer by the rapid progression of its pathophysiology, they were classified as ASA-PS 1 or 2 and did not cause POAF in the current study.
Strengths and limitations
Strengths of the present study include the fact that we evaluated a common postoperative arrhythmia after lung surgery in a homogeneous population. In addition, our results provide anesthesiologists with important information for selecting an anesthetic agent in lung surgery, considering the various perioperative complications of POAF [1–4, 27].
Whereas this study presents novel findings on the incidence of POAF between propofol and desflurane, there are a few limitations. First, anesthetic management was performed by each anesthesiologist at their discretion. Nonetheless, patients’ backgrounds were comparable between the two groups, and anesthetic management was not highly variable. Second, we might not have detected POAF after POD2 because POD2 terminated ECG monitoring. Some studies have suggested that the peak onset of postoperative dysrhythmias is a few days after surgery [1, 10]. Therefore, we focused on the effect of an anesthetic agent on immediate POAF; any potential slow-onset POAF was not assessed. Third, we included patients with and without preoperative beta-blocker medication, which was the risk factor associated with POAF by our multivariate logistic regression analysis. However, we matched the factor with the propensity score and confirmed that patients taking beta-blockers before surgery continued them in the postoperative period to avoid beta-blockade withdrawal [1], keeping hemodynamic stability [66].
Conclusion
Our retrospective data suggest that propofol anesthesia exerts significant inhibition of POAF compared to desflurane in patients with lung disease who underwent VATS. Although we did not identify the mechanism of propofol on inhibition of POAF incidence, our results correspond with the literature. Prospective studies are needed to elucidate this effect and the mechanism of propofol inhibition on POAF.
Supporting information
(XLSX)
The standardized mean difference distribution of many covariances was lower than 0.2 after propensity score matching. ASA PS: American Society of Anesthesiologists Physical Status.
(DOCX)
Data are presented as number (percent). POAF: postoperative atrial fibrillation.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Frendl G, Sodickson AC, Chung MK, Waldo AL, Gersh BJ, Tisdale JE, et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J Thorac Cardiovasc Surg. 2014;148(3):e153–93. Epub 2014/08/19. doi: 10.1016/j.jtcvs.2014.06.036 ; PubMed Central PMCID: PMC4454633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onaitis M, D’Amico T, Zhao Y, O’Brien S, Harpole D. Risk factors for atrial fibrillation after lung cancer surgery: analysis of the Society of Thoracic Surgeons general thoracic surgery database. Ann Thorac Surg. 2010;90(2):368–74. Epub 2010/07/30. doi: 10.1016/j.athoracsur.2010.03.100 . [DOI] [PubMed] [Google Scholar]
- 3.Riddersholm S, Tayal B, Kragholm K, Andreasen JJ, Rasmussen BS, Sogaard P, et al. Incidence of Stroke After Pneumonectomy and Lobectomy. Stroke. 2019;50(5):1052–9. Epub 2019/04/23. doi: 10.1161/STROKEAHA.118.024496 . [DOI] [PubMed] [Google Scholar]
- 4.Vaporciyan AA, Correa AM, Rice DC, Roth JA, Smythe WR, Swisher SG, et al. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: analysis of 2588 patients. J Thorac Cardiovasc Surg. 2004;127(3):779–86. Epub 2004/03/06. doi: 10.1016/j.jtcvs.2003.07.011 . [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Coello P, Cook D, Xu SC, Sigamani A, Berwanger O, Sivakumaran S, et al. Predictors, Prognosis, and Management of New Clinically Important Atrial Fibrillation After Noncardiac Surgery: A Prospective Cohort Study. Anesthesia and analgesia. 2017;125(1):162–9. doi: 10.1213/ANE.0000000000002111 . [DOI] [PubMed] [Google Scholar]
- 6.De Decker K, Jorens PG, Van Schil P. Cardiac complications after noncardiac thoracic surgery: an evidence-based current review. The Annals of Thoracic Surgery. 2003;75(4):1340–8. doi: 10.1016/s0003-4975(02)04824-5 [DOI] [PubMed] [Google Scholar]
- 7.Riber LP, Larsen TB, Christensen TD. Postoperative atrial fibrillation prophylaxis after lung surgery: systematic review and meta-analysis. Ann Thorac Surg. 2014;98(6):1989–97. Epub 2014/10/07. doi: 10.1016/j.athoracsur.2014.06.069 . [DOI] [PubMed] [Google Scholar]
- 8.Bhave PD, Goldman LE, Vittinghoff E, Maselli J, Auerbach A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am Heart J. 2012;164(6):918–24. doi: 10.1016/j.ahj.2012.09.004 ; PubMed Central PMCID: PMC4096141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chebbout R, Heywood EG, Drake TM, Wild JRL, Lee J, Wilson M, et al. A systematic review of the incidence of and risk factors for postoperative atrial fibrillation following general surgery. Anaesthesia. 2018;73(4):490–8. doi: 10.1111/anae.14118 . [DOI] [PubMed] [Google Scholar]
- 10.Fernando HC, Jaklitsch MT, Walsh GL, Tisdale JE, Bridges CD, Mitchell JD, et al. The Society of Thoracic Surgeons practice guideline on the prophylaxis and management of atrial fibrillation associated with general thoracic surgery: executive summary. Ann Thorac Surg. 2011;92(3):1144–52. Epub 2011/08/30. doi: 10.1016/j.athoracsur.2011.06.104 . [DOI] [PubMed] [Google Scholar]
- 11.Aldhoon B, Melenovsky V, Peichl P, Kautzner J. New insights into mechanisms of atrial fibrillation. Physiological research. 2010;59(1):1–12. doi: 10.33549/physiolres.931651 . [DOI] [PubMed] [Google Scholar]
- 12.Jiang J, He M, Xu Y. Preoperative Electrocardiogram and Perioperative Methods for Predicting New-Onset Atrial Fibrillation During Lung Surgery. J Cardiothorac Vasc Anesth. 2021;35(5):1424–30. doi: 10.1053/j.jvca.2020.09.107 . [DOI] [PubMed] [Google Scholar]
- 13.Gu J, Andreasen JJ, Melgaard J, Lundbye-Christensen S, Hansen J, Schmidt EB, et al. Preoperative Electrocardiogram Score for Predicting New-Onset Postoperative Atrial Fibrillation in Patients Undergoing Cardiac Surgery. J Cardiothorac Vasc Anesth. 2017;31(1):69–76. doi: 10.1053/j.jvca.2016.05.036 . [DOI] [PubMed] [Google Scholar]
- 14.Senoner T, Velik-Salchner C, Luckner G, Tauber H. Anesthesia-Induced Oxidative Stress: Are There Differences between Intravenous and Inhaled Anesthetics? Oxid Med Cell Longev. 2021;2021:8782387. Epub 2021/12/08. doi: 10.1155/2021/8782387 ; PubMed Central PMCID: PMC8643269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck-Schimmer B, Bonvini JM, Braun J, Seeberger M, Neff TA, Risch TJ, et al. Which Anesthesia Regimen Is Best to Reduce Morbidity and Mortality in Lung Surgery?: A Multicenter Randomized Controlled Trial. Anesthesiology. 2016;125(2):313–21. doi: 10.1097/ALN.0000000000001164 . [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien P-A. Classification of Surgical Complications. Annals of Surgery. 2004;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Kong AL, Chen R, Qian C, Liu SW, Sun BG, et al. Propofol and arrhythmias: two sides of the coin. Acta Pharmacol Sin. 2011;32(6):817–23. Epub 2011/06/07. doi: 10.1038/aps.2011.42 ; PubMed Central PMCID: PMC3505762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen PS, Tan AY. Autonomic nerve activity and atrial fibrillation. Heart Rhythm. 2007;4(3 Suppl):S61–4. Epub 2007/03/06. doi: 10.1016/j.hrthm.2006.12.006 ; PubMed Central PMCID: PMC1852524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cervigon R, Moreno J, Perez-Villacastin J, Castells F. Profound sedation with propofol modifies atrial fibrillation dynamics. Pacing Clin Electrophysiol. 2013;36(9):1176–88. Epub 2013/04/27. doi: 10.1111/pace.12137 . [DOI] [PubMed] [Google Scholar]
- 20.Jakobsen CJ, Berg H, Hindsholm KB, Faddy N, Sloth E. The influence of propofol versus sevoflurane anesthesia on outcome in 10,535 cardiac surgical procedures. J Cardiothorac Vasc Anesth. 2007;21(5):664–71. doi: 10.1053/j.jvca.2007.03.002 . [DOI] [PubMed] [Google Scholar]
- 21.Min JJ, Kim G, Lee JH, Hong KY, Kim WS, Lee YT. Does the Type of Anesthetic Technique Affect In-Hospital and One-Year Outcomes after Off-Pump Coronary Arterial Bypass Surgery? PLoS One. 2016;11(4):e0152060. Epub 2016/04/08. doi: 10.1371/journal.pone.0152060 ; PubMed Central PMCID: PMC4824512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Hert SG, Van der Linden PJ, Cromheecke S, Meeus R, Nelis A, Van Reeth V, et al. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004;101(2):299–310. doi: 10.1097/00000542-200408000-00009 . [DOI] [PubMed] [Google Scholar]
- 23.Li F, Yuan Y. Meta-analysis of the cardioprotective effect of sevoflurane versus propofol during cardiac surgery. BMC Anesthesiol. 2015;15:128. Epub 2015/09/26. doi: 10.1186/s12871-015-0107-8 ; PubMed Central PMCID: PMC4583176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazen MS, Marwick TH, Underwood DA. Diagnostic accuracy of the resting electrocardiogram in detection and estimation of left atrial enlargement: an echocardiographic correlation in 551 patients. Am Heart J. 1991;122(3 Pt 1):823–8. Epub 1991/09/01. doi: 10.1016/0002-8703(91)90531-l . [DOI] [PubMed] [Google Scholar]
- 25.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. American heart journal. 1949;37(2):161–86. doi: 10.1016/0002-8703(49)90562-1 [DOI] [PubMed] [Google Scholar]
- 26.Weibel S, Schaefer MS, Raj D, Rucker G, Pace NL, Schlesinger T, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: an abridged Cochrane network meta-analysis. Anaesthesia. 2021;76(7):962–73. Epub 2020/11/11. doi: 10.1111/anae.15295 . [DOI] [PubMed] [Google Scholar]
- 27.Ivanovic J, Maziak DE, Ramzan S, McGuire AL, Villeneuve PJ, Gilbert S, et al. Incidence, severity and perioperative risk factors for atrial fibrillation following pulmonary resection. Interact Cardiovasc Thorac Surg. 2014;18(3):340–6. Epub 2013/12/18. doi: 10.1093/icvts/ivt520 ; PubMed Central PMCID: PMC3930225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong J, Wang X, Liu Z, Yao S, Xiao Z, Zhang M, et al. Risk factors and survival analysis of arrhythmia following lung cancer surgery: a retrospective study. J Thorac Dis. 2021;13(2):847–60. Epub 2021/03/16. doi: 10.21037/jtd-20-2740 ; PubMed Central PMCID: PMC7947489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshizawa T, Niwano S, Niwano H, Igarashi T, Fujiishi T, Ishizue N, et al. Prediction of new onset atrial fibrillation through P wave analysis in 12 lead ECG. International Heart Journal. 2014;55(5):422–7. doi: 10.1536/ihj.14-052 [DOI] [PubMed] [Google Scholar]
- 30.Aizawa Y, Watanabe H, Okumura K. Electrocardiogram (ECG) for the prediction of incident atrial fibrillation: an overview. Journal of atrial fibrillation. 2017;10(4). doi: 10.4022/jafib.1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanda Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. Epub 2012/12/05. doi: 10.1038/bmt.2012.244 ; PubMed Central PMCID: PMC3590441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenna RJ, Houck W, Fuller CB. Video-Assisted Thoracic Surgery Lobectomy: Experience With 1,100 Cases. The Annals of Thoracic Surgery. 2006;81(2):421–6. doi: 10.1016/j.athoracsur.2005.07.078 [DOI] [PubMed] [Google Scholar]
- 33.Muranishi Y, Sonobe M, Menju T, Aoyama A, Chen-Yoshikawa TF, Sato T, et al. Atrial fibrillation after lung cancer surgery: incidence, severity, and risk factors. Surg Today. 2017;47(2):252–8. doi: 10.1007/s00595-016-1380-y . [DOI] [PubMed] [Google Scholar]
- 34.Garner M, Routledge T, King JE, Pilling JE, Veres L, Harrison-Phipps K, et al. New-onset atrial fibrillation after anatomic lung resection: predictive factors, treatment and follow-up in a UK thoracic centre. Interact Cardiovasc Thorac Surg. 2017;24(2):260–4. Epub 2016/11/03. doi: 10.1093/icvts/ivw348 . [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Xia Y, Yang Y, Ni ZZ, He WX, Wang HF, et al. Risk factors for major adverse events of video-assisted thoracic surgery lobectomy for lung cancer. Int J Med Sci. 2014;11(9):863–9. Epub 2014/07/12. doi: 10.7150/ijms.8912 ; PubMed Central PMCID: PMC4081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owczuk R, Wujtewicz MA, Sawicka W, Polak-Krzeminska A, Suszynska-Mosiewicz A, Raczynska K, et al. Effect of Anaesthetic Agents on P-Wave Dispersion on the Electrocardiogram: Comparison of Propofol and Desflurane. Clinical and Experimental Pharmacology and Physiology. 2008;35(9):1071–6. doi: 10.1111/j.1440-1681.2008.04963.x [DOI] [PubMed] [Google Scholar]
- 37.Kawanishi R, Kakuta N, Sakai Y, Hari Y, Sasaki H, Sekiguchi R, et al. Desflurane improves lung collapse more than propofol during one-lung ventilation and reduces operation time in lobectomy by video-assisted thoracic surgery: a randomized controlled trial. BMC Anesthesiol. 2022;22(1):125. Epub 2022/04/30. doi: 10.1186/s12871-022-01669-7 ; PubMed Central PMCID: PMC9052625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muzi M, Ebert TJ, Hope WG, Robinson BJ, Bell LB. Site (s) mediating sympathetic activation with desflurane. The Journal of the American Society of Anesthesiologists. 1996;85(4):737–47. doi: 10.1097/00000542-199610000-00008 [DOI] [PubMed] [Google Scholar]
- 39.Ebert TJ, Muzi M. Sympathetic hyperactivity during desflurane anesthesia in healthy volunteers: a comparison with isoflurane. The Journal of the American Society of Anesthesiologists. 1993;79(3):444–53. [DOI] [PubMed] [Google Scholar]
- 40.Eger EI 2nd. Characteristics of anesthetic agents used for induction and maintenance of general anesthesia. Am J Health Syst Pharm. 2004;61 Suppl 4:S3–10. Epub 2004/11/10. doi: 10.1093/ajhp/61.suppl_4.S3 . [DOI] [PubMed] [Google Scholar]
- 41.Bosch NA, Cimini J, Walkey AJ. Atrial Fibrillation in the ICU. Chest. 2018;154(6):1424–34. doi: 10.1016/j.chest.2018.03.040 ; PubMed Central PMCID: PMC6335260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odening KE, Deiss S, Dilling-Boer D, Didenko M, Eriksson U, Nedios S, et al. Mechanisms of sex differences in atrial fibrillation: role of hormones and differences in electrophysiology, structure, function, and remodelling. Europace. 2019;21(3):366–76. Epub 2018/10/24. doi: 10.1093/europace/euy215 . [DOI] [PubMed] [Google Scholar]
- 43.Andrade JG, Deyell MW, Lee AYK, Macle L. Sex Differences in Atrial Fibrillation. Can J Cardiol. 2018;34(4):429–36. Epub 2018/02/20. doi: 10.1016/j.cjca.2017.11.022 . [DOI] [PubMed] [Google Scholar]
- 44.Shaver CM, Chen W, Janz DR, May AK, Darbar D, Bernard GR, et al. Atrial Fibrillation Is an Independent Predictor of Mortality in Critically Ill Patients. Critical care medicine. 2015;43(10):2104–11. doi: 10.1097/CCM.0000000000001166 ; PubMed Central PMCID: PMC4725582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seguin P, Signouret T, Laviolle B, Branger B, Malledant Y. Incidence and risk factors of atrial fibrillation in a surgical intensive care unit. Critical care medicine. 2004;32(3):722–6. doi: 10.1097/01.ccm.0000114579.56430.e0 . [DOI] [PubMed] [Google Scholar]
- 46.Materazzo C, Piotti P, Mantovani C, Miceli R, Villani F. Atrial fibrillation after non-cardiac surgery: P-wave characteristics and Holter monitoring in risk assessment. Eur J Cardiothorac Surg. 2007;31(5):812–6. Epub 2007/03/06. doi: 10.1016/j.ejcts.2007.02.007 . [DOI] [PubMed] [Google Scholar]
- 47.Gunes H, Sokmen A, Kaya H, Gungor O, Kerkutluoglu M, Guzel FB, et al. Evaluation of Atrial Electromechanical Delay to Predict Atrial Fibrillation in Hemodialysis Patients. Medicina (Kaunas). 2018;54(4). Epub 2018/10/23. doi: 10.3390/medicina54040058 ; PubMed Central PMCID: PMC6174336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lancaster TS, Schill MR, Greenberg JW, Moon MR, Schuessler RB, Damiano RJ Jr., et al. Potassium and Magnesium Supplementation Do Not Protect Against Atrial Fibrillation After Cardiac Operation: A Time-Matched Analysis. Ann Thorac Surg. 2016;102(4):1181–8. Epub 2016/09/07. doi: 10.1016/j.athoracsur.2016.06.066 ; PubMed Central PMCID: PMC5030158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zafeiropoulos S, Doundoulakis I, Farmakis IT, Miyara S, Giannis D, Giannakoulas G, et al. Autonomic Neuromodulation for Atrial Fibrillation Following Cardiac Surgery: JACC Review Topic of the Week. J Am Coll Cardiol. 2022;79(7):682–94. Epub 2022/02/19. doi: 10.1016/j.jacc.2021.12.010 . [DOI] [PubMed] [Google Scholar]
- 50.He G, Yao T, Zhao L, Geng H, Ji Q, Zuo K, et al. Atrial fibrillation and alteration of heart rate variability after video-assisted pulmonary lobectomy versus thoracotomy pulmonary lobectomy. J Cardiothorac Surg. 2020;15(1):220. Epub 2020/08/17. doi: 10.1186/s13019-020-01260-6 ; PubMed Central PMCID: PMC7427877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precedethe onset of postoperative atrial fibrillation. Journal of the American College of Cardiology. 2003;42(7):1262–8. doi: 10.1016/s0735-1097(03)00955-0 [DOI] [PubMed] [Google Scholar]
- 52.Wu DH, Xu MY, Mao T, Cao H, Wu DJ, Shen YF. Risk factors for intraoperative atrial fibrillation: a retrospective analysis of 10,563 lung operations in a single center. Ann Thorac Surg. 2012;94(1):193–7. Epub 2012/05/23. doi: 10.1016/j.athoracsur.2012.03.057 . [DOI] [PubMed] [Google Scholar]
- 53.Amar D. Postthoracotomy atrial fibrillation. Curr Opin Anaesthesiol. 2007;20(1):43–7. Epub 2007/01/11. doi: 10.1097/ACO.0b013e32801158bb . [DOI] [PubMed] [Google Scholar]
- 54.Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovascular research. 1999;42(2):477–89. Epub 1999/10/26. doi: 10.1016/s0008-6363(99)00034-6 . [DOI] [PubMed] [Google Scholar]
- 55.Li GR, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circulation research. 1996;78(4):689–96. doi: 10.1161/01.res.78.4.689 . [DOI] [PubMed] [Google Scholar]
- 56.Chiamvimonvat N, Chen-Izu Y, Clancy CE, Deschenes I, Dobrev D, Heijman J, et al. Potassium currents in the heart: functional roles in repolarization, arrhythmia and therapeutics. J Physiol. 2017;595(7):2229–52. Epub 2016/11/04. doi: 10.1113/JP272883 ; PubMed Central PMCID: PMC5374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nattel S, Khairy P, Roy D, Thibault B, Guerra P, Talajic M, et al. New approaches to atrial fibrillation management: a critical review of a rapidly evolving field. Drugs. 2002;62(16):2377–97. doi: 10.2165/00003495-200262160-00005 . [DOI] [PubMed] [Google Scholar]
- 58.Li GR, Wang HB, Qin GW, Jin MW, Tang Q, Sun HY, et al. Acacetin, a natural flavone, selectively inhibits human atrial repolarization potassium currents and prevents atrial fibrillation in dogs. Circulation. 2008;117(19):2449–57. doi: 10.1161/CIRCULATIONAHA.108.769554 . [DOI] [PubMed] [Google Scholar]
- 59.Wu MH, Su MJ, Sun SS. Comparative direct electrophysiological effects of propofol on the conduction system and ionic channels of rabbit hearts. British journal of pharmacology. 1997;121(4):617–24. doi: 10.1038/sj.bjp.0701155 ; PubMed Central PMCID: PMC1564725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang L, Liu H, Sun HY, Li GR. Intravenous anesthetic propofol inhibits multiple human cardiac potassium channels. Anesthesiology. 2015;122(3):571–84. doi: 10.1097/ALN.0000000000000495 . [DOI] [PubMed] [Google Scholar]
- 61.Guizy M, David M, Arias C, Zhang L, Cofan M, Ruiz-Gutierrez V, et al. Modulation of the atrial specific Kv1.5 channel by the n-3 polyunsaturated fatty acid, alpha-linolenic acid. Journal of molecular and cellular cardiology. 2008;44(2):323–35. doi: 10.1016/j.yjmcc.2007.11.004 . [DOI] [PubMed] [Google Scholar]
- 62.Kanaya N, Hirata N, Kurosawa S, Nakayama M, Namiki A. Differential effects of propofol and sevoflurane on heart rate variability. Anesthesiology. 2003;98(1):34–40. Epub 2002/12/28. doi: 10.1097/00000542-200301000-00009 . [DOI] [PubMed] [Google Scholar]
- 63.Ohtaka K, Hida Y, Kaga K, Kato T, Muto J, Nakada-Kubota R, et al. Thrombosis in the pulmonary vein stump after left upper lobectomy as a possible cause of cerebral infarction. Ann Thorac Surg. 2013;95(6):1924–8. Epub 2013/04/30. doi: 10.1016/j.athoracsur.2013.03.005 . [DOI] [PubMed] [Google Scholar]
- 64.Xin Y, Hida Y, Kaga K, Iimura Y, Shiina N, Ohtaka K, et al. Left lobectomy might be a risk factor for atrial fibrillation following pulmonary lobectomy. Eur J Cardiothorac Surg. 2014;45(2):247–50. Epub 2013/08/08. doi: 10.1093/ejcts/ezt383 . [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto T, Suzuki H, Nagato K, Nakajima T, Iwata T, Yoshida S, et al. Is left upper lobectomy for lung cancer a risk factor for cerebral infarction? Surg Today. 2016;46(7):780–4. Epub 2015/08/15. doi: 10.1007/s00595-015-1233-0 . [DOI] [PubMed] [Google Scholar]
- 66.Jakobsen CJ, Bille S, Ahlburg P, Rybro L, Hjortholm K, Andresen EB. Perioperative metoprolol reduces the frequency of atrial fibrillation after thoracotomy for lung resection. J Cardiothorac Vasc Anesth. 1997;11(6):746–51. Epub 1997/11/05. doi: 10.1016/s1053-0770(97)90169-5 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
The standardized mean difference distribution of many covariances was lower than 0.2 after propensity score matching. ASA PS: American Society of Anesthesiologists Physical Status.
(DOCX)
Data are presented as number (percent). POAF: postoperative atrial fibrillation.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.