Abstract
With high rates of morbidity and mortality, cancer continues to pose a serious threat to public health on a global scale. Considering the discrepancies in metabolism between cancer and normal cells, metabolism-based anti-cancer biopharmaceuticals are gaining importance. Normal cells can synthesize arginine, but they can also take up extracellular arginine, making it a semi-essential amino acid. Arginine auxotrophy occurs when a cancer cell has abnormalities in the enzymes involved in arginine metabolism and relies primarily on extracellular arginine to support its biological functions. Taking advantage of arginine auxotrophy in cancer cells, arginine deprivation, which can be induced by introducing recombinant human arginase I (rhArg I), is being developed as a broad-spectrum anti-cancer therapy. This has led to the development of various rhArg I variants, which have shown remarkable anti-cancer activity. This article discusses the importance of arginine auxotrophy in cancer and different arginine-hydrolyzing enzymes that are in various stages of clinical development and reviews the need for a novel rhArg I that mitigates the limitations of the existing therapies. Further, we have also analyzed the necessity as well as the significance of using rhArg I to treat various arginine-auxotrophic cancers while considering the importance of their genetic profiles, particularly urea cycle enzymes.
Keywords: Arginase I, Auxotrophy, Cancer, PEGylation, Fusion technology
Introduction
In spite of recent advances in the therapeutic field, cancer remains a severe hazard that challenges the progress and development made thus far. According to the World Health Organization (WHO), cancer is the second leading cause of death globally, with an estimated 10 million deaths reported (Cancer-WHO 2021). In India, around 2.25 million people are living with different types of cancer, and every year approximately 1.2 million new cases are being registered (Cancer Statistics-India Against Cancer 2020). Cancer incidence and fatalities are estimated to grow to 29.5 and 16.3 million, respectively, by 2040 (Kulothungan et al. 2022). Cancer refers to a vast range of disorders that can affect any region of the body, defined by uncontrolled proliferation, invasion, and metastasis as a result of a multistep process that results in the buildup of multiple genetic abnormalities (Gupta and Massagué 2006). There are several methods and medications available to treat cancer, with many more under investigation. Some therapies are “local,” such as radiation therapy and surgery, and are used to treat a specific tumour or body part. Because drug therapies such as chemotherapy, targeted therapy, and immunotherapy may influence the entire body, they are commonly referred to as “systemic” treatments. For certain cancers, a single form of treatment may be appropriate, whereas for others optimum combination therapy may be required (Hellman and Vokes 1996; Society AC 2022). These treatments, while useful, have their own set of drawbacks. Chemotherapy, for example, has little selectivity between normal and cancer cells and causes severe toxicity (Padma 2015). Targeted treatment using a monoclonal antibody or a small molecule inhibitor to target one or more receptors in cancer cells is also less effective in cancers that are weakly vascularized or located in inaccessible areas (Zhou et al. 2014). Thus, these problems have driven the quest for a safe and broad-spectrum agent(s) to effectively combat the global cancer threat. Arginine depletion by administering arginine-hydrolyzing enzymes has been shown to inhibit the growth and progression of various arginine-auxotrophic cancers with lower expression of urea cycle enzymes.
Thus, this article discusses the importance of arginine auxotrophy in cancer and different arginine-hydrolyzing enzymes that are in various stages of their clinical development and reviews the need for a novel rhArg I that mitigates the limitations of the existing therapies. Further, we have also analyzed the necessity as well as the significance of using rhArg I to treat various arginine-auxotrophic cancers, while considering the importance of their genetic profiles, particularly urea cycle enzymes.
Auxotrophy in cancer
In addition to being involved in the synthesis of peptides and proteins, amino acids are known to regulate crucial cellular processes in both normal and cancerous cells (Andersen et al. 2014). To ensure that cellular activities are not disrupted, the physiological level of amino acids must be maintained in the body (Patil et al. 2016). Cancer cells require additional nutrients than healthy cells due to their rapid growth and multiplication. Furthermore, some cancer cells become incapable of synthesizing one or more amino acids (Fernandes et al. 2017). To continue their growth and metabolism, cancer cells, therefore, become dependent on exogenous amino acid sources, or ‘auxotrophic’ for such amino acids. Since such auxotrophic cancer cells are destroyed when the availability of amino acids is limited, studies are now being conducted to develop amino acid deprivation therapy (Wang et al. 2021). Also, normal cells are unaffected in such conditions since they are less demanding and can synthesize these amino acids (Fernandes et al. 2017). For example, children with acute lymphoblastic leukaemia (ALL) have been effectively treated with the l-asparaginase enzyme, which serves as the rationale for amino acid depletion for cancer therapy (Pieters et al. 2011; Phillips et al. 2013; Lomelino et al. 2017). Several amino acids are now being targeted for the treatment of various cancers and many of the enzymes that are employed to deplete these amino acids are in the advanced phases of their development (Table 1) (Jeon et al. 2016; Wang et al. 2021).
Table 1.
Amino acid targets in amino acid deprivation therapy
| Targeted amino acid | Enzyme used for amino acid depletion | References |
|---|---|---|
| Arginine | Arginase 1, arginine deiminase | Dillon et al. (2004), Bowles et al. (2008) |
| Glutamine | Glutaminase | Jiang et al. (2019) |
| Methionine | Methioninase | Hu and Cheung (2009) |
| Threonine | Threonine deaminase | Greenfield and Wellner (1977) |
| Asparagine | Asparaginase | Egler et al. (2016) |
| Lysine | Lysine-α-oxidase | Lukasheva et al. (2021) |
Arginine auxotrophy
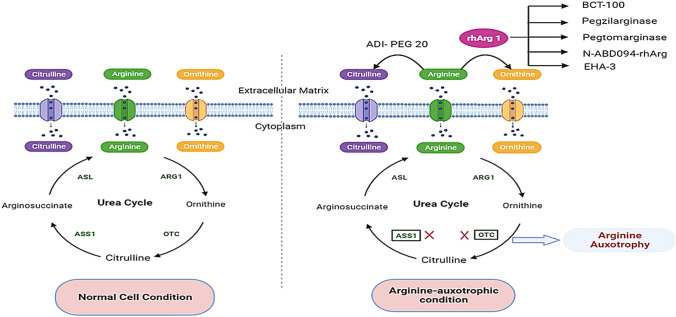
Arginine is a cationic, semi-essential amino acid that is required for the synthesis of amino acids such as proline and glutamate, as well as polyamines, urea, and agmatine (Wu and Morris 1998; Anakha et al. 2022). It has immunomodulatory properties like T cell receptor expression, formation of immunological memory, required for the production of nitric oxide (NO) through inducible nitric oxide synthase (NOS), which results in vasodilation, promotes the production of growth hormones, ammonia detoxification, and aids in wound healing (Alba-Roth et al. 1988; Bronte and Zanovello 2005; Stechmiller et al. 2005; Luiking et al. 2012). The enzymes argininosuccinate synthetase (ASS1) and argininosuccinate lyase (ASL) are responsible for converting citrulline to arginine. ASS1 converts citrulline and aspartic acid to argininosuccinate, which is then converted to arginine and fumaric acid by ASL. Arginase I then hydrolyses arginine into ornithine and urea and ornithine transcarbamylase (OTC) acts on ornithine and converts it to citrulline (Fig. 1) (Feun et al. 2008). ASS1 and/or ASL and/or OTC gene deficiencies or low levels of expression result in arginine auxotrophy, which makes such cancer cells entirely reliant on extracellular arginine for survival and maintenance (Tsai et al. 2009; Delage et al. 2010). The transcriptional silencing (hypermethylation) of OTC and ASS1 genes are thought to be the cause of their downregulation in cancer cells (Delage et al. 2012). Normal cells enter the G0/G1 phase of the cell cycle, become quiescent and survive for several weeks when arginine levels fall, whereas cancer cells continue to go through the cell cycle despite the lack of arginine, resulting in a significant imbalance and cell death (Fung and Chan 2017; Chen et al. 2021). Thus, arginine auxotrophy renders these cancers amenable to arginine-degrading enzyme therapy (Fig. 1).
Fig. 1.
The expression of ASS1 and OTC in normal cells ensures a steady supply of arginine through the catalysis of citrulline and ornithine, respectively. However, the loss of ASS1 and OTC expression causes arginine metabolism to be dysfunctional in arginine-auxotrophic cancer. ADI-PEG 20 and rhArg I deplete extracellular arginine to citrulline and ornithine, respectively, which cause the apoptosis and autophagy of the cancer cells (the figure was created using free medical images available from Servier Medical Art at: smart.servier.com)
Enzymes such as human arginase I (hArg I), NOS, arginine deiminase (ADI) and arginine decarboxylase were explored for arginine-auxotrophic cancer therapy and hArg I and ADI are found to be potential drug candidates (Fultang et al. 2016; Patil et al. 2016). For a variety of reasons, other enzymes were found to be not suitable. Arginine decarboxylase, for example, can efficiently metabolize arginine, but is hazardous to human cells as it generates agmatine and CO2 (Stasyk et al. 2015). ADI metabolizes arginine to ammonia, and citrulline and is a promising anti-cancer agent (Kozai et al. 2009) (Fig. 1). ADI-PEG 20 (pegargiminase), is a US FDA-approved engineered arginine deiminase molecule, developed by Polaris pharmaceuticals, USA, with reduced immunogenicity and enhanced plasma stability (compared to the native enzyme) and is being used for the treatment of hepatocellular carcinoma (HCC) as well as malignant melanomas (Tsai et al. 2009; Dhankhar et al. 2018; Drugbank online-Accession Number-DB06592). ADI-PEG 20 is currently being developed for a range of arginine-auxotrophic cancers including pancreatic cancer, AML, non-small cell lung cancer, mesothelioma, melanoma, etc., and to date, certain phase I and phase II studies have had promising results! However, there are several major limits to using ADI-PEG 20, which includes the generation of a high level of ammonia that has been linked to hyperammonaemia, which causes neurological damage as well as neutropenia in many patients on ADI-PEG 20 treatment (Yoon et al. 2013). Strong neutralizing antibody responses resulting in anaphylactic events in recipients have also been observed due to the microbial origin of the ADI enzyme (Fung and Chan 2017). In light of these limitations of ADI-PEG 20, recombinant hArg I (rhArg I) has emerged as a potential candidate for the development of a broad-spectrum anti-cancer drug for arginine-auxotrophic cancers.
Human arginase I (hArg I)
hArg I (EC 3.5.3.1) is a homotrimeric, manganese (Mn2+) metalloenzyme (Mol. wt. ~105 kDa) that catalyzes the hydrolysis of arginine to form urea and ornithine (Stone et al. 2010). Mn2+, which acts as a cofactor, and contributes to catalysis by generating a metal-bound hydroxide ion (OH−) from a water molecule that serves as a nucleophile attacking the guanidium carbon of arginine (Romero et al. 2012). Humans have two isoforms of the arginase enzyme: arginase I (Arg I), which is mostly found in hepatocytes, and arginase II (Arg II) present in non-hepatic organs (Srivastava and Ratha 2013). Both isoforms have ~60% amino acid sequence similarity, although they differ in gene location and functionality. The Arg I gene is found on chromosome 6 (6q.23) in humans, and its main physiological role is in the urea cycle. The Arg II gene, on the other hand, is found on chromosome 14 (14q.24.1) and is involved in cellular development and proliferation through proline and polyamine synthesis (Ash 2004; Romero et al. 2012). It has been demonstrated that Arg I knock-out animals exhibit lethal phenotypes, whereas Arg II knock-out animals show minimal physiological repercussions (Fultang et al. 2016). Furthermore, hyperargininaemia is a rare arginase I deficiency disorder in humans, caused by the formation of shortened protein due to a point mutation (R291X) in its gene, which leads to stunted development and hyperammonaemia (Lavulo et al. 2001). As discussed above, arginine auxotrophy is identified as a major characteristic of human malignancies, which has been discovered in a wide range of advanced solid tumours, including prostate carcinoma, HCC, malignant melanoma, pancreatic carcinoma, breast cancer, and many more (Dillon et al. 2004; Bowles et al. 2008; Kim et al. 2009a, b). This metabolic anomaly is also seen in acute myeloid leukaemia (AML), as well as lymphomas including Hodgkin’s lymphoma and non-Hodgkin’s lymphoma (Delage et al. 2012; Miraki-Moud et al. 2015). Arginine depletion by administering hArg I enzyme has been shown to inhibit the growth and progression of such arginine-auxotrophic cancers with lower expression of OTC and/or ASS1 enzymes (Cheng et al. 2007; Lam et al. 2010). However, at physiological pH, the native form of hArg I is less efficient since it has an optimum pH of 9.6. Moreover, it has a Km of 10.5 mM for arginine, indicating that a large quantity of the enzyme is required to obtain the desired outcomes. Also, the circulatory half-life of hArg1 is ~30 min, which contributes to its poor pharmacokinetic and pharmacodynamic characteristics. This limits the usefulness of this enzyme in its native form, necessitating the quick development of modifications to overcome these constraints (Patil et al. 2016; Anakha et al. 2022). To address this issue, PEGylated- and fusion-rhArg I molecules are being developed by pharmaceutical firms and are under various stages of their clinical development.
BCT-100, developed by Bio-cancer Treatment International Limited, Hong Kong, is a PEGylated (PEG5000) recombinant human arginase I (rhArg I) with improved pharmacokinetic properties (in vivo half-life of ~3 days) and enhanced arginine-hydrolyzing activity (Table 2) (Cheng et al. 2007). Weekly administration of BCT-100 (1600 U/kg) depletes arginine and has a favourable toxicity profile, according to a clinical study (Yau et al. 2015). BCT-100 is now being tested in phase II/III clinical studies for AML as well as liver cancer (Bio Cancer 2022). Nevertheless, there have been reports showing that BCT-100 has reduced catalytic activity and stability in serum at physiological pH (Georgiou and Stone 2013, 2014).
Table 2.
Recombinant human arginase I (rhArg I) under clinical development
| Therapeutic | Developed by | Modifying modality | Development stage | Condition or disease | References |
|---|---|---|---|---|---|
| BCT-100 | Bio-cancer Treatment International Limited, Hong Kong | PEGylation (multiple and random) | Phase III | Hepatocellular carcinoma, leukaemia, lymphoma, melanoma, prostate cancer, paediatric AML | ClinicalTrials.gov Identifier: NCT00988195, NCT01551628, NCT02089633, NCT02285101, NCT02089763, NCT03455140 |
| Pegzilarginase (AEB1102) | Aeglea BioTherapeutics, USA | PEGylation (multiple and random) | Phase II | Arginase I deficiency or hyperargininaemia, small-cell lung cancer | ClinicalTrials.gov Identifier: NCT03378531, NCT03921541, NCT05676853, NCT03371979 |
| Pegtomarginase (PT01) | Athenex, Inc. USA | PEGylation (single and specific) | Phase I | Advanced solid malignancies | ClinicalTrials.gov Identifier: NCT04136834 |
| N-ABD094-rhArg | Hong Kong Polytechnic University | Fusion protein (ABD as HLEP) | Pre-clinical | Obese-related metabolic disorders | Leung and Shum (2020) |
| LBURGINAZE (EHA-3) | National Institute of Pharmaceutical Education and Research (NIPER), Mohali, India | Fusion protein (HSA fragment as HLEP) | Pre-clinical | Liver### cancer | Pande et al. (2022) |
ABD albumin-binding domain; AML acute myeloid leukaemia; HLEP half-life extension partner; HSA human serum albumin; PEG polyethylene glycol; EHA-3 engineered human arginase-3
Pegzilarginase (AEB1102) is another PEGylated rhArg I developed by Aeglea BioTherapeutics, USA, with improved plasma stability and arginine-hydrolyzing activity due to the replacement of Mn2+ present in the active site of the native form with Co2+ (Table 2) (Glazer et al.2011). This molecule, which is also known as HuArgI (Co)-PEG5000, has proved to be beneficial in decreasing plasma arginine levels in mouse models of arginase deficiency and shows considerable inhibition of HCC and pancreatic cancer xenografts in animal studies (Mauldin et al. 2012; Burrage et al. 2015). It was approved by the US FDA in 2019 and granted the designation of breakthrough therapy for the treatment of arginase I deficiency or hyperarginaemia. Aeglea BioTherapeutics and Immedica recently signed a marketing partnership for pegzilarginase for hyperarginaemia in Europe and the Middle East (Aeglea 2022).
Pegtomarginase (PT01), developed by Athenex, Inc. USA, is a linear PEGylated form of rhArg I with two cysteines changed to serines at positions 168 and 303, leaving a single cysteine at position 45 and allowing site-specific PEGylation (PEG 20000) via maleimide–thiol conjugation (Table 2) (Yu et al. 2020). In mice xenograft models of prostate cancer and pancreatic cancer, preclinical studies with pegtomarginase revealed predictable pharmacodynamic behaviour (Athenex 2022). It is now being tested in phase I clinical study for the treatment of advanced solid tumours (ClinicalTrials.gov Identifier: NCT04136834).
Despite the fact that PEGylated protein therapies have shown to be effective and have been in clinical use for a long time, it is now recognized that they too have a number of drawbacks. As PEG is a non-biodegradable polymer, it causes vacuolization in the hepatic, renal as well splenic regions of the body (Gaberc-Porekar et al. 2008). Another severe concern of PEGylated protein therapeutics are its unexpected characteristics, such as lower activity, heterogeneity and increased aggregation in some cases. It is also reported that the efficacy of PEGylated therapeutics has been reduced due to the formation of antibodies against them, which causes them to be removed quickly (Langenheim and Chen 2009; Fee and Alstine 2011; Li et al. 2013; Haeckel et al. 2016). Because of these drawbacks, there is a dire need in the field to produce rhArg I variants that are both safe and have a longer circulatory half-life.
In this regard, the development of fusion proteins, in which a therapeutic protein is genetically linked to the domain of another protein, has emerged as a potent method for improving therapeutic protein pharmacokinetic features (Kontermann 2011). Protein clearance processes like receptor-mediated endocytosis, proteolysis and hepatic and renal clearance are being used to develop fusion proteins (Kintzing et al. 2016). The development of half-life extension technology, in which therapeutic proteins are fused with half-life extension partners (HLEPs), has been shown to significantly improve the circulatory half-lives of these proteins and hence their overall pharmacokinetic properties (Zaman et al.2019). Human transferrin (Strohl 2015), human IgG Fc domain (Sockolosky et al. 2012) and human serum albumin (HSA)-full length or domain (Dennis et al. 2002) are some of the effective HLEPs, which can be used to enhance the half-life of rhArg I by fusing it with any of these HLEPs using appropriate linkers (Chen et al. 2013). N-ABD094-rhArg is a rhArg I fusion protein with an albumin-binding domain developed by Hong Kong Polytechnic University for the treatment of obese-related disorders, which has shown to be effective in xenograft models with increased circulatory life (~7 days) and pharmacokinetic properties (Table 2) (Leung and Shum 2020). We have also developed hArg I variants using the fusion technique to treat arginine-auxotrophic cancers, in which the hArg I enzyme is linked via a peptide linker to a variety of HLEPs (Pande et al. 2022). The ‘lead’ engineered human arginase I (EHA-3 or LBURGINAZE), which has the third domain of HSA serves as the HLEP, in conjunction with 5-fluorouracil shows enhanced in vivo circulatory half-life and has considerable anti-cancer effectiveness in HCC xenograft model, indicating its potential utility as an anti-cancer agent (Table 2) (Pande et al. 2022).
ASS1 and/or OTC expression in arginine-auxotrophic cancer
Arginine depletion by enzymatic approaches is now being used/explored to treat a variety of malignancies. However, drug resistance induction was found to be a significant barrier to employing arginine deprivation-inducing therapeutics (Haines et al. 2011). As discussed above, cells meet their arginine requirements in physiological conditions by taking it directly from the circulation or through its biosynthesis (catalyzed by urea cycle enzymes). Arg I enzyme catalyze the conversion of arginine to urea and ornithine, which is then converted to citrulline by OTC. The two-step conversion of citrulline to arginosuccinate and then to arginine is mediated by ASS1 and ASL (Feun et al. 2008) (Fig. 1). As a result, the efficiency of arginine deprivation therapy using any arginine-depleting enzyme is reliant on these recycling enzymes. It is reported that ASS1 acts as a tumour suppressor gene in addition to its enzymatic activity. ASS1 deficiency was found in approximately half of all nasopharyngeal cancer patients; it was found to be highly associated with advanced tumour status and is a potential biomarker of poor disease-free survival (Lan et al. 2014; Huang et al. 2021). Patients with bladder cancer as well as myxofibrosarcoma also show similar features. ASS1 epigenetic silencing has also been shown to increase tumour cell proliferation and migration in bladder cancer, demonstrating that ASS1 can act as a tumour suppressor gene (Huang et al. 2013; Allen et al. 2014). Similarly, reduced or deficient expression of OTC enzyme in HCC, prostate cancer and melanoma have been shown to increase their sensitivity toward rhArg I-induced growth inhibition (Cheng et al. 2007; Lam et al. 2010; Hsueh et al. 2012). These findings suggest that any arginine-degrading enzyme can be used in clinical settings depending on the patient’s genetic profiles, particularly, ASS1 and/or OTC expression. In this context, many in vitro studies have been conducted and it has been observed that cancerous cells with ASL and/or ASS1 deficits are more susceptible to ADI-PEG20 treatment, whereas those with ASS1 and/or OTC impairments are more responsive to rhArg I therapeutics (Hsueh et al. 2012; Lam et al. 2010; Allen et al. 2014; Qiu et al. 2015). Interestingly, more than 25 arginine-auxotrophic tumours have been reported so far; however, around 9 of them only had more than 60% ASS1 and/or OTC deficiency in immunohistochemical (IHC) studies (Table 3).
Table 3.
ASS1 and/or OTC deficiency in different cancer using immunohistochemical studies
| Arginine-auxotrophic cancer | Ratio of patients with minimal/deficit expression of ASS1 and/or OTC enzymes with total enrolled patients | Percentage of ASS1 and/or OTC deficiency | References |
|---|---|---|---|
| Hepatocellular carcinoma |
51/51-ASS1 30/42-OTC |
100%-ASS1 71%-OTC |
Dillon et al. (2004) He et al. (2019) |
| Prostate cancer | 13/13-ASS1 | 100% | Dillon et al. (2004) |
| Hodgkin’s lymphoma | 173/179; 50/50-ASS1 | 98% | Delage et al. (2012) |
| Non-Hodgkin’s lymphoma | 288/303-ASS1 | 95% | Delage et al. (2012) |
| Malignant melanoma | 119/119; 75/102-ASS1 | 88% | Dillon et al. (2004); Khadeir et al. (2015) |
| Pancreatic cancer | 41/47; 7/14-ASS1 | 69% | Bowles et al. (2008); Liu et al. (2014) |
| Breast cancer | 95/149-ASS1 | 63.8% | Qiu et al. 2014 |
| Malignant pleural mesothelioma | 52/82-ASS1 | 63% | Szlosarek et al. (2006) |
| Osteosarcoma | 39/62-ASS1 | 63% | Kobayashi et al. (2010) |
| Nasopharyngeal carcinoma | 64/124-ASS1 | 51.6% | Lan et al. (2014) |
| Myxofibrosarcoma | 44/90-ASS1 | 48.8% | Huang et al. (2013) |
| Small cell lung carcinoma | 7/16-ASS1 | 43.7% | Kelly et al. (2011) |
| Ovarian cancer | 23/54-ASS1 | 42.5% | Nicholson et al. (2009) |
| Bladder cancer | 190/478-ASS1 | 39.7% | Allen et al. (2014) |
| Glioblastoma | 8/22-ASS1 | 36% | Syed et al. (2013) |
| Renal cell carcinoma | 6/21-ASS1 | 28.5% | Yoon et al. (2007) |
| Esophageal carcinoma | 6/32-ASS1 | 18.7% | Lagarde et al. (2008) |
| Seminoma | 2/12-ASS1 | 16.6% | Dillon et al. (2004) |
The nine arginine-auxotrophic cancers with more than 60% ASS1 and/or OTC impairments in IHC studies are discussed here, along with the potential therapeutic role of arginine-hydrolyzing enzymes in their treatment.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC), also called malignant hepatoma, is a major type of primary liver cancer that accounts for around 75% of all liver cancer cases (Petrick and McGlynn 2019; Dasgupta et al. 2020). With an estimated 905,677 new cases and 830,180 deaths in 2020, HCC is the sixth most often diagnosed cancer and the second major cause of cancer mortality globally (Globocan World 2020). In India, HCC is the fourth leading cause of cancer deaths with approximately 66,279 deaths in 2020 (Fig. 2) (Globocan India 2020). The optimal therapy for HCC is a multidisciplinary strategy that takes into account the tumour stage, patient performance status, and liver function reserve. The short-term survival of HCC has improved in recent years due to major breakthroughs in locoregional as well as surgical treatments, but the recurring disease remains a serious concern, and this has fuelled the search for novel target agents (Fig. 3) (Raza and Sood 2014). Growth inhibition of the human hepatoma cell line (HLE) by the administration of 5 ng/ml mycoplasma-derived ADI revealed the possibility of arginine-deprivation therapy for HCC. It is also reported that the urea cycle is suppressed in most HCC cell lines, and free arginine levels are elevated 5- to 20-fold in animals with HCC (Moyer and Pitot 1974; Miyazaki et al. 1990). In addition, an IHC study of HCC biopsy samples obtained from 51 patients to determine ASS1 expression exhibited 100% ASS1 deficiency (Table 3) (Dillon et al. 2004). Interestingly, Feun et al. conducted a study on various HCC cell lines and found that the arginine auxotrophy of these cell lines were caused by a deficiency of ASS1 enzyme, which made them sensitive to mycoplasma-derived ADI (Feun and Savaraj 2006). As a result, ADI-PEG 20, or pegargiminase was developed and got certified as an ‘orphan drug’ by US-FDA and the European agency for HCC treatment (Polaris pharmaceuticals 2022). It is currently being studied in phase III clinical trials for advanced HCC (ClinicalTrials.gov Identifier: NCT01287585). Recently, IHC studies show a minimal or absent expression of OTC in 71% (30 out of 42) of HCC cell lines collected from patients suffering from advanced HCC, which indicates its functional role in HCC progression (Table 3) (He et al. 2019).
Fig. 2.
Arginine-auxotrophic cancer statistics, 2020 (the figure was created using Prism—GraphPad software)
Fig. 3.
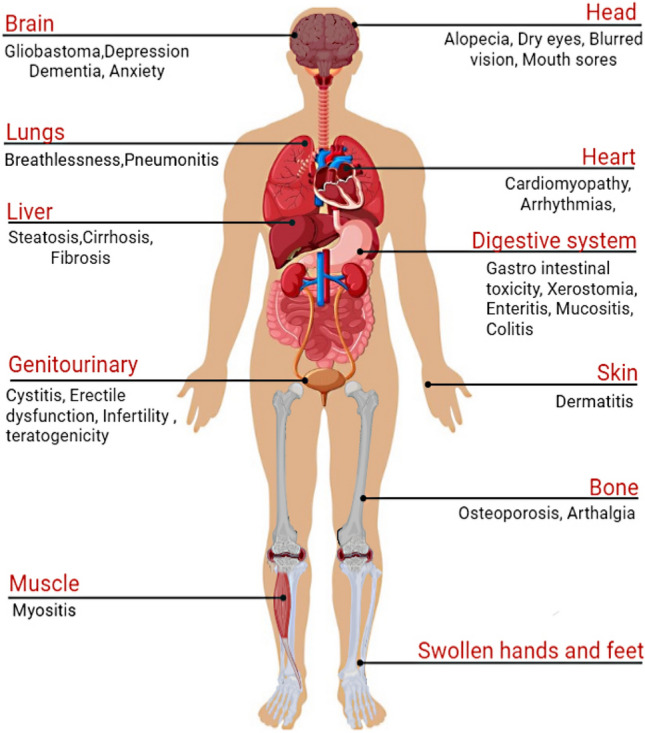
Adverse effects of different treatments of cancer (the figure was created using free medical images available from Servier Medical Art at: smart.servier.com)
Based on these findings, arginine-hydrolyzing enzymes are also being studied alone as well as with combinations, for the treatment of HCC. BCT-100 completed phase II clinical trials and shows effective results in patients with advanced HCC, when it is administered alone (1600 U/kg/week in 16 patients) as well as in dose-escalated PACOX administration, which is a combination regimen of BCT-100 with capecitabine and oxaliplatin drugs (ClinicalTrials.gov Identifier: NCT01092091, NCT02089633). Furthermore, in vivo study using EHA-3 in combination with 5-fluorouracil in a mouse xenograft model of HCC (10 mg/kg/week) showed a significant reduction in the growth rate of the tumour, suggesting its tumour-suppressing effect (Pande et al. 2022). Hence, these findings imply that arginine depletion utilizing rhArg I variants could be a feasible strategy for the management of HCC.
Malignant melanoma
Malignant melanoma, a neoplasm of melanocytes, occurs due to genetic abnormalities in melanocytes present in the skin, genitals, mouth, ear, anal area, and so on (Tolleson 2005). It is considered the deadliest form of skin cancer, which makes up less than 5% of all skin cancer cases (Lee et al. 2012). Although it was long thought to be rare, the annual incidence has risen considerably in recent decades with an estimated 57,043 deaths and 324,635 new cases in 2020 (Globocan World 2020). When compared to the global statistics, India had a low incidence of melanoma, with 2296 fatalities and 3916 new cases, a 0.7% rise over the previous Indian statistic report (Fig. 2) (Globocan India 2018, 2020). The US-FDA has authorized many drugs for its treatment such as dacarbazine, interleukin-2, vemurafenib, etc. (Domingues et al. 2018). Apart from these, surgical excision, targeted therapy, radiation treatment, immunotherapy and/or photodynamic therapy may be used depending on the characteristics of the tumour (Batus et al. 2013; Miller et al. 2016; Van Zeijl et al. 2017). Despite the fact that these surgical techniques and treatments have made remarkable progress, there is still a pressing need to reduce the rising burden caused by their limitations such as lack of selectivity, reduced efficacy, and gastrointestinal and cutaneous toxicity (Fig. 3) (Widakowich et al. 2007; Li et al. 2010; Austin et al. 2017). Real-time quantitative PCR (qPCR) and IHC studies conducted in melanoma cell lines show ASS1 deficiency, and hence its susceptibility toward arginine-degrading enzymes (Table 3) (Dillon et al. 2004; Khadeir et al. 2015; Sugimura et al. 1992). Furthermore, a dose-escalation (40, 80, or 160 IU/m2) clinical trial with ADI-PEG 20 reveals 100% plasma arginine depletion in 30 out of 31 advanced melanoma patients in 8 days (ClinicalTrials.gov Identifier: NCT00520299). In this context, a phase I study employing ADI-PEG20 (36 mg/m2) in combination with ipilimumab (1 mg/kg) and nivolumab (240 mg/kg) is now being conducted in nine advanced uveal melanoma patients (ClinicalTrials.gov Identifier: NCT03922880). Apart from this, BCT-100 (2.7 mg/kg) is also in its phase I clinical trial using 22 patients with advanced melanoma (ClinicalTrials.gov Identifier: NCT02285101). Thus, the development of a safe and long-acting variant of rhArg I will be a milestone in the treatment of melanoma.
Prostate cancer
Prostate cancer, the most common cancer in males after the age of 50 years, is a condition in which nonlocalized tumours are potentially very dangerous and difficult to treat (Kim et al. 2009a, b). It is the second most prevalent cancer in males and the third most common cancer globally with an estimated 1,414,259 new cases and 375,304 fatalities (Globocan World 2020). Despite the fact that prostate cancer rates in India are lower than in Western nations, it is the fifth most common cancer in men, with an estimated 16,783 deaths and 34,540 new cases (Fig. 2) (Globocan India 2020; Mathur et al. 2020). Prostatectomy, chemotherapy, biphosphonate therapy, targeted therapy, external beam radiation therapy, brachytherapy, and immunotherapy are the major treatments available for prostate cancer (Prostate Cancer Treatment (PDQ®)–Patient Version 2022). However, these therapies have a number of drawbacks, including bladder incontinence, rectal ulcers and bleeding, erectile dysfunction, proctitis, and many more (Fig. 3) (Pearlstein et al. 2019). IHC analysis of 13 prostate tumour biopsy specimens revealed total ASS1 impairment, and paved the possibility for therapy with an arginine-degrading enzyme (Table 3) (Dillon et al. 2004). Moreover, the Polaris group completed a phase 1 clinical trial and concluded that weekly administration of ADI-PEG 20 to castration-resistant prostate cancer patients results in increased tumour cell apoptosis with manageable toxicity (ClinicalTrials.gov Identifier: NCT01497925). Later studies revealed that ADI therapy, or any arginine deprivation therapy, effectively kills ASS1-deficient prostate tumour cells by inducing the formation of mitochondrial reactive oxygen species (mitoROS), morphological changes in mitochondria and decreased synthesis of mitochondrial metabolites, all of which led to mitochondrial dysfunction (Changou et al. 2014; Cheng et al. 2018). Henceforth, these results suggested that arginine depletion employing an enzyme (rhArg I) that hydrolyses arginine is a promising treatment strategy for prostate cancer.
Hodgkin’s lymphoma and non-Hodgkin’s lymphoma
Lymphoma is a malignant haematologic cancer that originates in the lymph system, comes in almost 70 distinct varieties and is broadly classified into Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL) (Ng et al. 2011). HL most commonly affects young adults (20s–30s) having a high rate of cure using available treatments such as radiotherapy, steroid therapy, chemotherapy, and combined modality therapy (Shanbhag and Ambinder 2018). However, people who had these treatments for HL have a higher chance of getting other diseases or ailments later in life, according to the reports. The development of a second form of cancer such as breast cancer, AML, NHL, and lung cancer later in life is an unusual, but a highly significant adverse effect of HL therapy (Rathore and Kadin 2010; Shanbhag and Ambinder 2018). Infertility has also been reported in teens and adults treated with the ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) and BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone) chemotherapies, which are often used to treat HL (Amin et al. 2021). In addition to this, the prevalence of HL has been rising in recent years, with an estimated 23,376 annual deaths and representing around 10% of newly diagnosed lymphoma instances globally (Fig. 2) (Globocan World 2020).
NHL, on the other hand, is the ninth most common cancer in India and the twelfth most prevalent cancer globally in terms of new incidence and mortality (Fig. 2) (Globocan India 2020; Globocan World 2020). These lymphoproliferative tumours are found to be less predictable than HL, with a higher proclivity for spreading to extranodal sites. Approximately, a quarter of NHL cases occur in non-nodal sites, with the majority of these including both nodal and extranodal regions. NHL accounts for around 5% of head and neck cancers and has a wide variety of symptoms that are similar to HL (Armitage et al. 2017; Singh et al. 2020). Despite the availability of several therapies for NHL, such as chemotherapy, radiation and bone marrow transplant, it has been noted that similar to HL treatments, there is a greater chance of acquiring late effects (Armitage 1993). This includes left ventricular dysfunction in NHL patients treated with doxorubicin (dosage >200 mg/m2) (Haddy et al. 1998; Moser et al. 2006), high risk of acquiring secondary malignancies such as HCC, bladder cancer, glioblastoma, AML, melanoma, etc. (Travis et al. 1993; Hemminki et al. 2008), a higher chance of being permanently sterile in patients who had cyclophosphamide and radiation treatment in the pelvic area (Mudie et al. 2006) and the risk of developing osteoporosis (Fig. 3) (Westin et al. 2013).
Burkitt lymphoma, a type of NHL that requires arginine for proliferation, paved the way for arginine-degrading enzyme treatment for lymphomas (Osunkoya et al. 1970). The majority of lymphoma cell lines lack ASS1 and are vulnerable to mycoplasma-derived ADI (Delage et al. 2012). Also, in vitro rhArg I-treatment studies (0.02–2 IU/ml) in NHL cell lines revealed considerable cell growth inhibition due to G1 phase arrest, as well as ASS1 and OTC deficiency as determined by western blot analysis (Zeng et al. 2013). The Polaris Group has completed a phase II clinical study with ADI-PEG 20 in 18 NHL patients who had previously failed systemic therapy (ClinicalTrials.gov Identifier: NCT01910025). Thus, these data suggest that arginine depletion using rhArg I could inhibit lymphoma proliferation.
Pancreatic cancer
The prevalence of pancreatic cancer (PC) has grown in recent years and is one of the leading causes of cancer-related death with a poor prognosis. With 1,414,259 new cases and 375,304 deaths worldwide, it is now the third most common cancer (Fig. 2) (Globocan World 2020). The majority of patients have no symptoms while their illness develops into advanced pancreatic metastasis, which explains the low 5-year survival rate of patients (2–9%) (Gillen et al. 2010; McGuigan et al. 2018). Available treatments include pancreatectomy, radiation therapy such as proton beam therapy and SBRT (Stereotactic body radiation therapy), immunotherapy and chemotherapy, with most regimens comprising capecitabine, nab-paclitaxel, oxaliplatin, 5-fluorouracil, leucovorin and others (McGuigan et al. 2018; Robatel and Schenk 2022). Most of these therapies are less effective and have a number of drawbacks, including peripheral neuropathy, a high risk of diabetes, hand–foot syndrome, and other complications (Fig. 3) (Kubota 2011; Robatel and Schenk 2022). As a result, novel therapies targeting the metabolic activity of tumour are becoming more appealing and are expected to aid pancreatic cancer patients in the future. IHC studies conducted on pancreatic biopsy specimens show an ASS1 deficiency rate of 69% (41/47), indicating the feasibility of arginine-depletion treatment employing enzymes (Table 3) (Bowles et al. 2008). Additionally, in a clinical trial conducted with 18 advanced pancreatic adenocarcinoma, patients were given ADI-PEG 20 (36 mg/m2) weekly for 3 weeks in combination with nab-paclitaxel (125 mg/m2) and gemcitabine (1000 mg/m2). When compared to the toxicity of the FDA-approved first-line therapy for stage 3 pancreatic adenocarcinoma, gemcitabine and nab-paclitaxel regimen, this phase 1/1B clinical trial shows moderate toxicity, providing the chance for a more effective pancreatic cancer treatment (ClinicalTrials.gov Identifier: NCT02101580; Lowery et al. 2017) (Fig. 3). Thus, the successful development of rhArg I variants will provide a potentially ground-breaking treatment regimen for pancreatic cancer, which will help the suffering patients.
Breast cancer
Breast cancer is the most frequent cancer worldwide and in India ranks first in terms of incidence and mortality rate (Fig. 2) (Globocan World 2020; Globocan India 2020). Based on the presence or absence of molecular markers human epidermal growth factor receptor 2 (HER2), progesterone receptors and oestrogen receptors, breast cancer is divided into three groups. Around 15% of all breast cancer patients have triple negative cancer, which lacks these three molecular markers, whereas ~70% of patients have HER2-negative or hormone receptor-positive cancer and the remaining ~15% have HER2-positive cancer (Waks and Winer 2018; Bhattacharyya et al. 2020). Mastectomy or lumpectomy, radiation therapy such as brachytherapy, intra-operative or external beam radiation, hormone therapy and chemotherapy regimen (doxorubicin, gemcitabine, cisplatin, capecitabine, cyclophosphamide, etc.) are some of the therapies available (Richie and Swanson 2003; Sharma et al. 2010). Despite the fact that 70–80% of people with breast cancer may be treated in the early stages with currently available medicines, advanced cancer with metastasis is considered incurable (Harbeck et al. 2019). Apart from that, a major drawback of most of these treatments is the substantial threat of recurrence (Bhattacharyya et al. 2020). Interestingly, ASS1 deficiency in cytoplasmic ASS1 staining was seen in roughly 64% of samples from breast cancer patients (Table 3) (Qiu et al. 2014). Moreover, ADI-PEG 20 (36 mg/m2) in combination with doxorubicin (240 mg/m2) was given weekly for around 4 months to nine patients with HER2-negative metastatic breast cancer and this phase I clinical trial revealed a satisfactory safety profile, as well as lower arginine levels in the blood (ClinicalTrials.gov Identifier: NCT01948843; Yao et al. 2022). Therefore, utilizing rhArg I to manage breast cancer will be a promising therapeutic approach.
Malignant pleural mesothelioma
Malignant pleural mesothelioma (MPM) is an incurable, deadly form of mesothelioma that is primarily caused by frequent exposure to asbestos. In spite of extensive asbestos manufacturing and supply constraints, MPM prevalence keeps rising, with 30,870 new cases globally (Fig. 2) (Globocan World 2020; Asciak et al. 2021). According to statistics, MPM patients have a short median survival time of 9–13 months, with fewer biomarkers and no treatment options. Because it has such a broad range of symptoms, diagnosing it can be difficult, making treatments such as oncoviral therapies, angiogenesis inhibitors, radiation therapy, and microRNA replacement ineffective (Nicolini et al. 2020). IHC studies demonstrated a 63% minimal or loss of ASS1 expression in biopsies collected from 82 MPM patients (Table 3) (Szlosarek et al. 2006). Furthermore, ~94% disease management rate was achieved in patients with non-epithelioid ASS1-deficient MPM subtypes by the administration of ADI-PEG 20 (18, 27 and 36 mg/m2) in combination with cisplatin (75 mg/m2) and pemetrexed (500 mg/m2) every 3 weeks (ClinicalTrials.gov Identifier: NCT02029690). ADI-PEG20 or placebo (36 mg/m2) coupled with the conventional dosage of cisplatin and pemetrexed for a period of 18 weeks is now being tested on 386 MPM patients in phase II/III study (ClinicalTrials.gov Identifier: NCT02709512; Szlosarek et al. 2017). These findings indicate that the treatment of MPM via arginine depletion using an enzyme is very effective.
Osteosarcoma
Osteosarcoma (OS) is defined by the formation of a premature osteoid matrix by mesenchymal spindle cells (Campanacci 2013). In adolescents and young adults, it is the most prevalent primary bone cancer with a global incidence rate of 3.25 new cases per million every year, despite its rarity (Misaghi et al. 2018; Osteosarcoma-American Cancer Society 2022). Generally, adjuvant and neoadjuvant chemotherapy, as well as amputation or limb salvage are used to treat OS. Cisplatin, ifosfamide, doxorubicin and methotrexate combined with the antidote leucovorin are the common treatment regimens used in chemotherapy (O’Kane et al. 2015; Misaghi et al. 2018). Despite the fact that these therapies have been proven to be beneficial, it has been observed that considerable OS patients may acquire fatal secondary malignancies or severe treatment-related difficulties, suggesting the necessity of novel therapies (Longhi et al. 2006; Durfee et al. 2016; Schwartz et al. 2016). IHC examination of samples from 62 osteosarcoma patients revealed a 63% ASS1 deficit, suggesting that arginine-degrading enzyme therapy could be used to treat OS (Table 3) (Kobayashi et al. 2010). A phase II clinical trial is presently underway employing ADI-PEG 20 (36 mg/m2) in conjunction with docetaxel (60 mg/m2) and gemcitabine (600 mg/m2), which are the standard chemotherapy regimens for soft tissue sarcoma treatments (ClinicalTrials.gov Identifier: NCT03449901). These results imply that arginine deprivation using arginine-hydrolyzing enzymes (rhArg I) could prevent OS proliferation.
Conclusion
Cancer, a common and enduring disease that is neither an epidemic nor a pandemic, took more than 10 million lives globally in 2020. Depending on the type and stage of cancer, numerous types of therapy options are available, which include chemotherapy, radiation therapy, surgery, targeted therapy, photodynamic therapy, and many more. The quest for a safe and broad-spectrum agent that can be effective against a wide range of cancers has been spurred by the severity of side effects linked to some of these treatments and/or their specificity towards a particular cancer (Society AC 2022). Therefore, it has been shown that focusing on the metabolism of cancer cells may be a more effective strategy with fewer side effects (Vettore et al. 2020; Lieu et al. 2020). In this context, numerous malignancies have been identified that are arginine auxotrophic and, thus, treatments based on the enzymes that break down arginine appear to be promising (Patil et al. 2016). As a result, arginine deprivation, which can be induced by rhArg I, has been explored as a novel approach to treating cancer, since it makes use of the characteristic arginine auxotrophy in cancers (Burrage et al. 2015). However, the native enzyme has poor pharmacokinetic properties, which constrains its development for clinical use. PEGylated-rhArg I molecules, such as BCT-100, pegzilarginase (AEB1102) and pegtomarginase (PT01), are being developed by the pharmaceutical companies to address this issue, which are at different phases of their clinical development. However, because of the limitations associated with the clinical use of PEGylated proteins, the development of recombinant variants employing fusion technology, in which the therapeutic protein is genetically fused to another protein/domain, has emerged as an effective approach to enhance its pharmacokinetic features. Aberrant expression of ASS1 and/or OTC is essential for the effectiveness of arginine deprivation therapy and only nine out of the more than 25 arginine-auxotrophic cancers have been reported thus far exhibited 60% or more ASS1 and/or OTC deficiency. Therefore, taking into account the significance of genetic profiles, particularly these urea cycle enzymes, the successful development of fusion variants of rhArg I with increased circulatory half-life will be a step toward generating a safer and longer-acting Arg I molecule for the treatment of the nine arginine-auxotrophic cancers discussed in this literature.
Abbreviations
- ADI
Arginine deiminase
- ALL
Acute lymphoblastic leukaemia
- AML
Acute myeloid leukaemia
- ASL
Argininosuccinate
- ASS1
Argininosuccinate synthetase
- BCT
Bio-cancer treatment
- CO2
Carbon dioxide
- Co
Cobalt
- EHA
Engineered human arginase
- HCC
Hepatocellular carcinoma
- HER
Human epidermal growth factor
- HL
Hodgkin’s lymphoma
- HLEPs
Half-life extension partners
- HSA
Human serum albumin
- IHC
Immunohistochemistry
- mitoROS
Mitochondrial reactive oxygen species
- MPM
Malignant pleural mesothelioma
- Mn
Manganese
- NO
Nitric oxide
- NHL
Non-Hodgkin’s lymphoma
- OTC
Ornithine transcarbamylase
- OS
Osteosarcoma
- PC
Pancreatic cancer
- PEG
Polyethylene glycol
- rhArg I
Recombinant human arginase I
- SBRT
Stereotactic body radiation therapy
- WHO
World Health Organization
Author contributions
AJ and YRP contributed equally. AJ, YRP and NS were involved in conceptualization, data curation and writing the manuscript. AHP was involved in conceptualization, visualization, investigation, supervision, reviewing and editing. All authors read and approved the final manuscript.
Funding
The authors would like to thank the Department of Biotechnology (New Delhi, Government of India; grant # BT/PR23283/MED/30/1953/2018) and the National Institute of Pharmaceutical Education and Research, S.A.S. Nagar (NPLC-AHP), for providing financial support.
Data availability
Not Applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
J. Anakha, Email: anakhasopanam@gmail.com
Yenisetti Rajendra Prasad, Email: yrprajendra129@gmail.com.
Nisha Sharma, Email: nishasharma.nics8@gmail.com.
Abhay H. Pande, Email: apande@niper.ac.in
References
- A Phase I open-label study for subjects with advanced malignancies (PT01). ClinicalTrials.gov Identifier: NCT04136834. https://clinicaltrials.gov/
- ADI-PEG 20 in combination with gemcitabine and docetaxel for the treatment of soft tissue sarcoma, osteosarcoma, Ewing’s sarcoma, and small cell lung cancer. ClinicalTrials.gov Identifier: NCT03449901. https://clinicaltrials.gov/
- Aeglea-enzymes for rare metabolic diseases-Pegzilarginase. https://www.aeglea.com/pegzilarginase/. Accessed 2 Sep 2022
- Alba-Roth J, Müller OA, Schopohl J, Von Werder K. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. J Clin Endocrinol Metab. 1988;67:1186–1189. doi: 10.1210/jcem-67-6-1186. [DOI] [PubMed] [Google Scholar]
- Allen MD, Luong P, Hudson C, Leyton J, Delage B, Ghazaly E, Cutts R, Yuan M, Syed N, Lo Nigro C, Lattanzio L, Chmielewska-Kassassir M, Tomlinson I, Roylance R, Whitaker HC, Warren AY, Neal D, Frezza C, Beltran L, Jones LJ, Chelala C, Wu BW, Bomalaski JS, Jackson RC, Lu YJ, Crook T, Lemoine NR, Mather S, Foster J, Sosabowski J, Avril N, Li CF, Szlosarek PW. Prognostic and therapeutic impact of argininosuccinate synthetase 1 control in bladder cancer as monitored longitudinally by PET imaging. Cancer Res. 2014;74(3):896–907. doi: 10.1158/0008-5472.can-13-1702. [DOI] [PubMed] [Google Scholar]
- American Cancer Society . Cancer treatment & survivorship facts & figure 2022–2024. Atlanta: American Cancer Society; 2022. [Google Scholar]
- Amin MS, Brunckhorst O, Scott C, Wrench D, Gleeson M, Kazmi M, Ahmed K. ABVD and BEACOPP regimens’ effects on fertility in young males with Hodgkin lymphoma. Clin Transl Oncol. 2021;23(6):1067–1077. doi: 10.1007/s12094-020-02483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anakha J, Kawathe PS, Datta S, Jawalekar SS, Banerjee UC, Pande AH. Human arginase 1, a Jack of all trades? 3 Biotech. 2022;12:264. doi: 10.1007/s13205-022-03326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JT, Dalhus B, Viuff D, Ravn BT, Gunnarsen KS, Plumridge A, Bunting K, Antunes F, Williamson R, Athwal S, Allan E, Evans L, Bjørås M, Kjærulff S, Sleep D, Sandlie I, Cameron J. Extending serum half-life of albumin by engineering neonatal Fc receptor (FcRn) binding. J Biol Chem. 2014;289(19):13492–13502. doi: 10.1074/jbc.m114.549832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage JO. Treatment of non-Hodgkin’s lymphoma. New Engl J Med. 1993;328(14):1023–1030. doi: 10.1056/nejm199304083281409. [DOI] [PubMed] [Google Scholar]
- Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet. 2017;390(10091):298–310. doi: 10.1016/s0140-6736(16)32407-2. [DOI] [PubMed] [Google Scholar]
- Asciak R, George V, Rahman NM. Update on biology and management of mesothelioma. Eur Respir Rev. 2021;30(159):200226. doi: 10.1183/16000617.0226-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash DE. Structure and function of arginases. J Nutr. 2004;134(10):2760S–2764. doi: 10.1093/jn/134.10.2760s. [DOI] [PubMed] [Google Scholar]
- Athenex—improving the life of cancer patients everywhere. https://www.athenex.com/. Accessed 10 Sep 2022
- Austin E, Mamalis A, Ho D, Jagdeo J. Laser and light-based therapy for cutaneous and soft-tissue metastases of malignant melanoma: a systematic review. Arch Dermatol Res. 2017;390(4):229–242. doi: 10.1007/s00403-017-1720-9. [DOI] [PubMed] [Google Scholar]
- Batus M, Waheed S, Ruby C, Petersen L, Bines SD, Kaufman HL. Optimal management of metastatic melanoma: current strategies and future directions. Am J Clin Dermatol. 2013;14(3):179–194. doi: 10.1007/s40257-013-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya GS, Doval DC, Desai CJ, Chaturvedi H, Sharma S, Somashekhar SP. Overview of breast cancer and implications of overtreatment of early-stage breast cancer: an Indian perspective. JCO Glob Oncol. 2020;6:789–798. doi: 10.1200/go.20.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bio Cancer—treatment of human malignancies and immune disorders. http://www.bio-cancer.com/. Accessed 1 Sep 2022
- Bowles TL, Kim R, Galante J, Parsons CM, Virudachalam S, Kung HJ, Bold RJ. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer. 2008;123(8):1950–1955. doi: 10.1002/ijc.23723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- Burrage LC, Sun Q, Elsea SH, Jiang MM, Nagamani SC, Frankel AE, Stone E, Alters SE, Johnson DE, Rowlinson SW, Georgiou G, Members of Urea Cycle Disorders Consortium. Lee BH. Human recombinant arginase enzyme reduces plasma arginine in mouse models of arginase deficiency. Hum Mol Genet. 2015;24(22):6417–6427. doi: 10.1093/hmg/ddv352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanacci M. Bone and soft tissue tumors: clinical features, imaging, pathology and treatment. Berlin: Springer; 2013. [Google Scholar]
- Cancer Statistics (2020) India against cancer, 2020. http://cancerindia.org.in/cancer-statistics/. Accessed 15 Sep 2022
- Cancer-WHO (2021). https://www.who.int/news-room/fact-sheets/detail/cancer Accessed 8 Sep 2022
- Changou CA, Chen YR, Xing L, Yen Y, Chuang FY, Cheng RH, Bold RJ, Ann DK, Kung HJ. Arginine starvation-associated atypical cellular death involves mitochondrial dysfunction, nuclear DNA leakage, and chromatin autophagy. Proc Natl Acad Sci. 2014;111(39):14147–14152. doi: 10.1073/pnas.1404171111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zaro JL, Shen WC. Fusion protein linkers: property, design and functionality. Adv Drug Deliv Rev. 2013;65(10):1357–1369. doi: 10.1016/j.addr.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Hsu SC, Ann DK, Yen Y, Kung HJ. Arginine signaling and cancer metabolism. Cancers. 2021;13(14):3541. doi: 10.3390/cancers13143541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PN, Lam TL, Lam WM, Tsui SM, Cheng AW, Lo WH, Leung YC. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 2007;67(1):309–317. doi: 10.1158/0008-5472.can-06-1945. [DOI] [PubMed] [Google Scholar]
- Cheng CT, Qi Y, Wang YC, Chi KK, Chung Y, Ouyang C, Chen YR, Oh ME, Sheng X, Tang Y, Liu YR. Arginine starvation kills tumor cells through aspartate exhaustion and mitochondrial dysfunction. Commun Biol. 2018;1(1):1–5. doi: 10.1038/s42003-018-0178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta P, Henshaw C, Youlden DR, Clark PJ, Aitken JF, Baade PD. Global trends in incidence rates of primary adult liver cancers: a systematic review and meta-analysis. Front Oncol. 2020;28(10):171. doi: 10.3389/fonc.2020.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T, Szlosarek PW. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2010;126(12):2762–2772. doi: 10.1002/ijc.25202. [DOI] [PubMed] [Google Scholar]
- Delage B, Luong P, Maharaj L, O’Riain C, Syed N, Crook T, Hatzimichael E, Papoudou-Bai A, Mitchell TJ, Whittaker SJ, Cerio R, Gribben J, Lemoine N, Bomalaski J, Li CF, Joel S, Fitzgibbon J, Chen LT, Szlosarek PW. Promoter methylation of argininosuccinate synthetase-1 sensitises lymphomas to arginine deiminase treatment, autophagy and caspase-dependent apoptosis. Cell Death Dis. 2012;3(7):e342. doi: 10.1038/cddis.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MS, Zhang M, Meng YG, Kadkhodayan M, Kirchhofer D, Combs D, Damico LA. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J Biol Chem. 2002;277(38):35035–35043. doi: 10.1074/jbc.m205854200. [DOI] [PubMed] [Google Scholar]
- Dhankhar R, Gulati P, Kumar S, Kapoor RK. Arginine-lowering enzymes against cancer: a technocommercial analysis through patent landscape. Expert Opin Ther Pat. 2018;28(8):603–614. doi: 10.1080/13543776.2018.1508452. [DOI] [PubMed] [Google Scholar]
- Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: a method for identifying cancers sensitive to arginine deprivation. Cancer. 2004;100(4):826–833. doi: 10.1002/cncr.20057. [DOI] [PubMed] [Google Scholar]
- Domingues B, Lopes JM, Soares P, Pópulo H. Melanoma treatment in review. Immunotargets Ther. 2018;7:35–49. doi: 10.2147/itt.s134842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugbank online. Database for drug and target info, Pegargiminase. DrugBank Accession Number-DB06592. https://go.drugbank.com/drugs/DB06592
- Durfee RA, Mohammed M, Luu HH. Review of osteosarcoma and current management. Rheumatol Ther. 2016;3(2):221–243. doi: 10.1007/s40744-016-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egler RA, Ahuja SP, Matloub Y. L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J Pharmacol Pharmacother. 2016;7(2):62–71. doi: 10.4103/0976-500x.184769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee CJ, Van Alstine JM. Purification of pegylated proteins. Methods Biochem Anal. 2011;54:339–362. doi: 10.1002/9780470939932.ch14. [DOI] [PubMed] [Google Scholar]
- Fernandes HS, Silva Teixeira CS, Fernandes PA, Ramos MJ, Cerqueira NM. Amino acid deprivation using enzymes as a targeted therapy for cancer and viral infections. Expert Opin Therap Pat. 2017;27(3):283–297. doi: 10.1080/13543776.2017.1254194. [DOI] [PubMed] [Google Scholar]
- Feun L, Savaraj N. Pegylated arginine deiminase: a novel anticancer enzyme agent. Expert Opin Investig Drugs. 2006;15(7):815–822. doi: 10.1517/13543784.15.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr M, Spector S, Savaraj N. Arginine deprivation as a targeted therapy for cancer. Curr Pharm Des. 2008;14:1049–1057. doi: 10.2174/138161208784246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultang L, Vardon A, De Santo C, Mussai F. Molecular basis and current strategies of therapeutic arginine depletion for cancer. Int J Cancer. 2016;139(3):501–509. doi: 10.1002/ijc.30051. [DOI] [PubMed] [Google Scholar]
- Fung MKL, Chan GC. Drug-induced amino acid deprivation as strategy for cancer therapy. J Hematol Oncol. 2017;10(1):144. doi: 10.1186/s13045-017-0509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaberc-Porekar V, Zore I, Podobnik B, Menart V. Obstacles and pitfalls in the PEGylation of therapeutic proteins. Curr Opin Drug Discov Devel. 2008;11(2):242–250. [PubMed] [Google Scholar]
- Georgiou G, Stone E (2013) Compositions of engineered human arginases and methods for treating cancer. Google Patents. US20100111925A1. https://patents.google.com/
- Georgiou G, Stone E (2014) Methods for purifying pegylated arginase. Google patents. US8679479B2. https://patents.google.com/
- Gillen S, Schuster T, Meyer zumBüschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer ES, Stone EM, Zhu C, Massey KL, Hamir AN, Curley SA. Bioengineered human arginase I with enhanced activity and stability controls hepatocellular and pancreatic carcinoma xenografts. Transl Oncol. 2011;4(3):138–146. doi: 10.1593/tlo.10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield RS, Wellner D. Effects of threonine deaminase on growth and viability of mammalian cells in tissue culture and its selective cytotoxicity toward leukemia cells. Cancer Res. 1977;37(8 Part 1):2523–2529. [PubMed] [Google Scholar]
- Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Haddy TB, Adde MA, McCalla J, Domanski MJ, Datiles M, 3rd, Meehan SC, Pikus A, Shad AT, Valdez I, Lopez Vivino L, Magrath IT. Late effects in long-term survivors of high-grade non-Hodgkin’s lymphomas. J Clin Oncol. 1998;16(6):2070–2079. doi: 10.1200/jco.1998.16.6.2070. [DOI] [PubMed] [Google Scholar]
- Haeckel A, Appler F, Ariza de Schellenberger A, Schellenberger E. XTEN as biological alternative to PEGylation allows complete expression of a protease-activatable Killin-based cytostatic. PLoS One. 2016;11(6):e0157193. doi: 10.1371/journal.pone.0157193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines RJ, Pendleton LC, Eichler DC. Argininosuccinate synthase: at the center of arginine metabolism. Int J Biochem Mol Biol. 2011;2(1):8–23. [PMC free article] [PubMed] [Google Scholar]
- Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer. Nat Rev Dis Primers. 2019;5(1):1–31. doi: 10.1038/s41572-019-0122-z. [DOI] [PubMed] [Google Scholar]
- He L, Cai X, Cheng S, Zhou H, Zhang Z, Ren J, Ren F, Yang Q, Tao N, Chen J. Ornithine transcarbamylase downregulation is associated with poor prognosis in hepatocellular carcinoma. Oncol Lett. 2019;17(6):5030–5038. doi: 10.3892/ol.2019.10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman S, Vokes EE. Advancing current treatments for cancer. Sci Am. 1996;275(3):118–123. doi: 10.1038/scientificamerican0996-118. [DOI] [PubMed] [Google Scholar]
- Hemminki K, Lenner P, Sundquist J, Bermejo JL. Risk of subsequent solid tumors after non-Hodgkin’s lymphoma: effect of diagnostic age and time since diagnosis. J Clin Oncol. 2008;26(11):1850–1857. doi: 10.1200/jco.2007.14.6068. [DOI] [PubMed] [Google Scholar]
- Hsueh EC, Knebel SM, Lo WH, Leung YC, Cheng PN, Hsueh CT. Deprivation of arginine by recombinant human arginase in prostate cancer cells. J Hematol Oncol. 2012;30(5):17. doi: 10.1186/1756-8722-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Cheung NK. Methionine depletion with recombinant methioninase: in vitro and in vivo efficacy against neuroblastoma and its synergism with chemotherapeutic drugs. Int J Cancer. 2009;124(7):1700–1706. doi: 10.1002/ijc.24104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Wu WR, Wang YH, Wang JW, Fang FM, Tsai JW, Li SH, Hung HC, Yu SC, Lan J, Shiue YL, Hsing CH, Chen LT, Li CF. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res. 2013;9(11):2861–2872. doi: 10.1158/1078-0432.ccr-12-2641. [DOI] [PubMed] [Google Scholar]
- Huang H, Li S, Tang Q, Zhu G. Metabolic reprogramming and immune evasion in nasopharyngeal carcinoma. Front Immunol. 2021;12:680955. doi: 10.3389/fimmu.2021.680955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- India; source: Globocan 2020—International agency for research on cancer, WHO. The Global Cancer Observatory 2021. https://gco.iarc.fr/
- Jeon H, Kim JH, Lee E, Jang YJ, Son JE, Kwon JY, Lim TG, Kim S, Park JH, Kim JE, Lee KW. Methionine deprivation suppresses triple-negative breast cancer metastasis in vitro and in vivo. Oncotarget. 2016;7(41):67223–67234. doi: 10.18632/oncotarget.11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Srivastava S, Zhang J. Starve cancer cells of glutamine: break the spell or make a hungry monster? Cancers (Basel) 2019;11(6):804. doi: 10.3390/cancers11060804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Jungbluth AA, Wu BW, Bomalaski J, Old LJ, Ritter G. Arginine deiminase PEG20 inhibits growth of small cell lung cancers lacking expression of argininosuccinate synthetase. Br J Cancer. 2011;106(2):324–332. doi: 10.1038/bjc.2011.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadeir RS, Phillips MM, Sgoo MS, Arora A, Cohen V, Thaung C, Szlosarek PW (2015) Abstract 1156: widespread deficiency of ASS1 in uveal melanoma and sensitivity to pegylated arginine deiminase. In: Conference: molecular and cellular biology, p 1156. 10.1158/1538-7445.am2015-1156
- Kim RH, Bold RJ, Kung HJ. ADI, autophagy and apoptosis: metabolic stress as a therapeutic option for prostate cancer. Autophagy. 2009;5(4):567–568. doi: 10.4161/auto.5.4.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Coates JM, Bowles TL, McNerney GP, Sutcliffe J, Jung JU, Gandour-Edwards R, Chuang FY, Bold RJ, Kung HJ. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res. 2009;69(2):700–708. doi: 10.1158/0008-5472.can-08-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintzing JR, FilsingerInterrante MV, Cochran JR. Emerging strategies for developing next-generation protein therapeutics for cancer treatment. Trends Pharmacol Sci. 2016;37(12):993–1008. doi: 10.1016/j.tips.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Masuda M, Nakayama R, Ichikawa H, Satow R, Shitashige M, Honda K, Yamaguchi U, Shoji A, Tochigi N, Morioka H. Reduced argininosuccinate synthetase is a predictive biomarker for the development of pulmonary metastasis in patients with osteosarcoma. Mol Cancer Ther. 2010;9(3):535–544. doi: 10.1158/1535-7163.mct-09-0774. [DOI] [PubMed] [Google Scholar]
- Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol. 2011;22(6):868–876. doi: 10.1016/j.copbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Kozai M, Sasamori E, Fujihara M, Yamashita T, Taira H, Harasawa R. Growth inhibition of human melanoma cells by a recombinant arginine deiminase expressed in Escherichia coli. J Vet Med Sci. 2009;71(10):1343–1347. doi: 10.1292/jvms.001343. [DOI] [PubMed] [Google Scholar]
- Kubota K. Recent advances and limitations of surgical treatment for pancreatic cancer. World J Clin Oncol. 2011;2(5):225–228. doi: 10.5306/wjco.v2.i5.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulothungan V, Sathishkumar K, Leburu S, Ramamoorthy T, Stephen S, Basavarajappa D, Tomy N, Mohan R, Geetha RM, Prashant M. Burden of cancers in India—estimates of cancer crude incidence, YLLs, YLDs and DALYs for 2021 and 2025 based on National Cancer Registry Program. BMC Cancer. 2022;22:527. doi: 10.1186/s12885-022-09578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde SM, Ver Loren PE, van Themaat MPD, Gilhuijs-Pederson TK, Reitsma PH. Analysis of gene expression identifies differentially expressed genes and pathways associated with lymphatic dissemination in patients with adenocarcinoma of the esophagus. Ann Surg Oncol. 2008;15:3459–3470. doi: 10.1245/s10434-008-0165-y. [DOI] [PubMed] [Google Scholar]
- Lam TL, Wong GK, Chow HY, Chong HC, Chow TL, Kwok SY, Cheng PN, Wheatley DN, Lo WH, Leung YC. Recombinant human arginase inhibits the in vitro and in vivo proliferation of human melanoma by inducing cell cycle arrest and apoptosis. Pigment Cell Melanoma Res. 2010;24(2):366–376. doi: 10.1111/j.1755-148x.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- Lan J, Tai HC, Lee SW, Chen TJ, Huang HY, Li CF. Deficiency in expression and epigenetic DNA Methylation of ASS1 gene in nasopharyngeal carcinoma: negative prognostic impact and therapeutic relevance. Tumor Biol. 2014;35(1):161–169. doi: 10.1007/s13277-013-1020-8. [DOI] [PubMed] [Google Scholar]
- Langenheim JF, Chen WY. Improving the pharmacokinetics/pharmacodynamics of prolactin, GH, and their antagonists by fusion to a synthetic albumin-binding peptide. J Endocrinol. 2009;203(3):375–387. doi: 10.1677/joe-09-0211. [DOI] [PubMed] [Google Scholar]
- Lavulo LT, Sossong Jr TM, Brigham-Burke MR, Doyle ML, Cox JD, Christianson DW, Ash DE. Subunit–subunit interactions in trimeric arginase. Generation of active monomers by mutation of a single amino acid. J Biol Chem. 2001;276(17):14242–14248. doi: 10.1074/jbc.m010575200. [DOI] [PubMed] [Google Scholar]
- Lee B, Mukhi N, Liu D. Current management and novel agents for malignant melanoma. J Hematol Oncol. 2012;5(1):1–7. doi: 10.1186/1756-8722-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung YC, Shum AS (2020) Composition and application of arginine- depleting agents for cancer, obesity, metabolic disorders, and related complications and comorbidities. Google patents. 20200181597A1. https://patents.google.com/
- Li X, Naylor MF, Le H, Nordquist RE, Teague TK, Howard CA, Murray C, Chen WR. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10(11):1081–1087. doi: 10.4161/cbt.10.11.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang Y, Chen J, Cheng B, Hu J, Zhou Y, Gao X, Gao L, Mei X, Sun M, Zhang Z, Song H. An engineered arginase FC protein inhibits tumor growth in vitro and in vivo. Evid Based Complement Alternat Med. 2013;2013:423129. doi: 10.1155/2013/423129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52(1):15–30. doi: 10.1038/s12276-020-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ma J, Wu Z, Li W, Zhang D, Han L, Wang F, Reindl KM, Wu E, Ma Q. Arginine deiminase augments the chemosensitivity of argininosuccinate synthetase-deficient pancreatic cancer cells to gemcitabine via inhibition of NF-κB signaling. BMC Cancer. 2014;14:686. doi: 10.1186/1471-2407-14-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomelino CL, Andring JT, McKenna R, Kilberg MS. Asparagine synthetase: function, structure, and role in disease. J Biol Chem. 2017;292(49):19952–19958. doi: 10.1074/jbc.R117.819060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32(6):423–436. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Lowery MA, Yu KH, Kelsen DP, Harding JJ, Bomalaski JS, Glassman DC, Covington CM, Brenner R, Hollywood E, Barba A, Johnston A, Liu KC, Feng X, Capanu M, Abou-Alfa GK, O’Reilly EM. A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma. Cancer. 2017;123(23):4556–4565. doi: 10.1002/cncr.30897. [DOI] [PubMed] [Google Scholar]
- Luiking YC, Ten Have GAM, Wolfe RR, Deutz NEP. Arginine de novo and nitric oxide production in disease states. AJP Endocrinol Metab. 2012;303:E1177–E1189. doi: 10.1152/ajpendo.00284.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasheva EV, Babayeva G, Karshieva SS, Zhdanov DD, Pokrovsky VS. L-Lysine α-oxidase: enzyme with anticancer properties. Pharmaceuticals (Basel) 2021;14(11):1070. doi: 10.3390/ph14111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Sathishkumar K, Chaturvedi M, et al. Cancer statistics, 2020: report from national cancer registry programme, India. JCO Glob Oncol. 2020;6:1063–1075. doi: 10.1200/go.20.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauldin JP, Zeinali I, Kleypas K, Woo JH, Blackwood RS, Jo CH, Stone EM, Georgiou G, Frankel AE. Recombinant human arginase toxicity in mice is reduced by citrulline supplementation. Transl Oncol. 2012;5(1):26–31. doi: 10.1593/tlo.11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- Miraki-Moud F, Ghazaly E, Ariza-McNaughton L, Hodby KA, Clear A, Anjos-Afonso F, Liapis K, Grantham M, Sohrabi F, Cavenagh J, Bomalaski JS, Gribben JG, Szlosarek PW, Bonnet D, Taussig DC. Arginine deprivation using pegylated arginine deiminase has activity against primary acute myeloid leukemia cells in vivo. Blood. 2015;125:4060–4068. doi: 10.1182/blood-2014-10-608133. [DOI] [PubMed] [Google Scholar]
- Misaghi A, Goldin A, Awad M, Kulidjian AA. Osteosarcoma: a comprehensive review. SICOT J. 2018;4:12. doi: 10.1051/sicotj/2017028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K, Takaku H, Umeda M, Fujita T, Huang WD, Kimura T, Yamashita J, Horio T. Potent growth inhibition of human tumor cells in culture by arginine deiminase purified from a culture medium of a Mycoplasma-infected cell line. Cancer Res. 1990;50(15):4522–4527. [PubMed] [Google Scholar]
- Moser EC, Noordijk EM, van Leeuwen FE, le Cessie S, Baars JW, Thomas J, Carde P, Meerwaldt JH, van Glabbeke M, Kluin-Nelemans HC. Long-term risk of cardiovascular disease after treatment for aggressive non-Hodgkin lymphoma. Blood. 2006;107(7):2912–2919. doi: 10.1182/blood-2005-08-3392. [DOI] [PubMed] [Google Scholar]
- Moyer GH, Pitot HC. Static and dynamic aspects of amino acid pools in rat liver and Morris hepatomas 9618A and 7800. Cancer Res. 1974;334:2647–2653. [PubMed] [Google Scholar]
- Mudie NY, Swerdlow AJ, Higgins CD, Smith P, Qiao Z, Hancock BW, Hoskin PJ, Linch DC. Risk of second malignancy after non-Hodgkin’s lymphoma: a British Cohort Study. J Clin Oncol. 2006;24(10):1568–1574. doi: 10.1200/jco.2005.04.2200. [DOI] [PubMed] [Google Scholar]
- Ng AK, LaCasce A, Travis LB. Long-term complications of lymphoma and its treatment. J Clin Oncol. 2011;29(14):1885–1892. doi: 10.1200/jco.2010.32.8427. [DOI] [PubMed] [Google Scholar]
- Nicholson LJ, Smith PR, Hiller L, et al. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. Int J Cancer. 2009;125(6):1454–1463. doi: 10.1002/ijc.24546. [DOI] [PubMed] [Google Scholar]
- Nicolini F, Bocchini M, Bronte G, Delmonte A, Guidoboni M, Crinò L, Mazza M. Malignant pleural mesothelioma: state-of-the-art on current therapies and promises for the future. Front Oncol. 2020 doi: 10.3389/fonc.2019.01519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kane GM, Cadoo KA, Walsh EM, Emerson R, Dervan P, O’keane C, Hurson B, O’toole G, Dudeney S, Kavanagh E, Eustace S. Perioperative chemotherapy in the treatment of osteosarcoma: a 26-year single institution review. Clin Sarcoma Res. 2015;5(1):1–8. doi: 10.1186/s13569-015-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteosarcoma-American Cancer Soceity 2022. https://www.cancer.org/cancer/osteosarcoma.html Accessed 12 Aug 2022
- Osunkoya BO, Adler WH, Smith RT. Effect of arginine deficiency on synthesis of DNA and immunoglobulin receptor of Burkitt lymphoma cells. Nature. 1970;227:398–399. doi: 10.1038/227398a0. [DOI] [PubMed] [Google Scholar]
- Padma VV. An overview of targeted cancer therapy. Biomedicine. 2015 doi: 10.7603/s40681-015-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande AH, Jawalekar SS, Kawathe PS, Sharma N, Tikoo K (2022) Engineered arginase, method of generation and uses thereof. Indian Patent application # 202211013970
- Patil M, Bhaumik J, Babykutty S, Banerjee U, Fukumura D. Arginine dependence of tumor cells: targeting a chink in cancer’s armor. Oncogene. 2016;35(38):4957. doi: 10.1038/onc.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein KA, Basak R, Chen RC. Comparative effectiveness of prostate cancer treatment options: limitations of retrospective analysis of cancer registry data. Int J Radiat Oncol Biol Phys. 2019;103(5):1053–1057. doi: 10.1016/j.ijrobp.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Pegylated recombinant human arginase 1 in combination with oxaliplatin and capecitabine for the treatment of HCC (PACOX). ClinicalTrials.gov Identifier: NCT02089633. https://clinicaltrials.gov/
- Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep. 2019;6(2):104–111. doi: 10.1007/s40471-019-00188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ph 1 ADI-PEG 20 plus doxorubicin; patients with HER2 Negative metastatic breast cancer Yao. ClinicalTrials.gov Identifier: NCT01948843. https://clinicaltrials.gov/
- Ph 1 study in subjects with tumors requiring arginine to assess ADI-PEG 20 with pemetrexed and cisplatin (TRAP). ClinicalTrials.gov Identifier: NCT02029690. https://clinicaltrials.gov/
- Ph 1 trial of ADI-PEG 20 plus docetaxel in solid tumors with emphasis on prostate cancer and non-small cell lung cancer. ClinicalTrials.gov Identifier: NCT01497925. https://clinicaltrials.gov/
- Ph 1B trial with ADI-PEG 20 plus nab-paclitaxel and gemcitabine in subjects with pancreatic cancer. ClinicalTrials.gov Identifier: NCT02101580. https://clinicaltrials.gov/
- PH 2 ADI-PEG 20 study in non-hodgkin’s lymphoma subjects who have failed prior systemic therapy Gillen. ClinicalTrials.gov Identifier: NCT01910025. https://clinicaltrials.gov/
- Ph 2/3 study in subjects with MPM to assess ADI-PEG 20 with pemetrexed and cisplatin (ATOMIC). ClinicalTrials.gov Identifier: NCT02709512. https://clinicaltrials.gov/
- Ph 3 ADI-PEG 20 versus placebo in subjects with advanced hepatocellular carcinoma who have failed prior systemic therapy (ADI-PEG 20). ClinicalTrials.gov Identifier: NCT01287585. https://clinicaltrials.gov/
- Phillips MM, Sheaff MT, Szlosarek PW. Targeting arginine-dependent cancers with arginine-degrading enzymes: opportunities and challenges. Cancer Res Treat Off J Korean Cancer Assoc. 2013;45(4):251. doi: 10.4143/crt.2013.45.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, Goekbuget N, Schrappe M, Pui CH. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117(2):238–249. doi: 10.1002/cncr.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polaris Pharmaceuticals 2022. https://polarispharma.com Accessed 10 Aug 2022
- Prostate Cancer Treatment (PDQ®)–Patient Version (2022) NIH-National Cancer Institute. https://www.cancer.gov/types/prostate/patient/prostate-treatment-pdq Accessed 15 Aug 2022
- Qiu F, Chen YR, Liu X, Chu CY, Shen LJ, Xu J, Gaur S, Forman HJ, Zhang H, Zheng S, Yen Y, Huang J, Kung HJ, Ann DK. Arginine starvation impairs mitochondrial respiratory function in ASS1-deficient breast cancer cells. Sci Signal. 2014;7(319):ra31. doi: 10.1126/scisignal.2004761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Huang J, Sui M. Targeting arginine metabolism pathway to treat arginine-dependent cancers. Cancer Lett. 2015;364(1):1–7. doi: 10.1016/j.canlet.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Rathore B, Kadin ME. Hodgkin’s lymphoma therapy: past, present, and future. Expert Opin Pharmacother. 2010;11(17):2891–2906. doi: 10.1517/14656566.2010.515979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Sood GK. Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20(15):4115–4127. doi: 10.3748/wjg.v20.i15.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recombinant human arginase 1 (rhArg1) in patients with advanced arginine auxotrophic solid tumors. ClinicalTrials.gov Identifier: NCT02285101. https://clinicaltrials.gov/
- Richie RC, Swanson JO. Breast cancer: a review of the literature. J Insur Med N Y Denver. 2003;35(2):85–101. [PubMed] [Google Scholar]
- Robatel S, Schenk M. Current limitations and novel perspectives in pancreatic cancer treatment. Cancers (Basel) 2022;14(4):985. doi: 10.3390/cancers14040985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero PA, Stone E, Lamb C, Chantranupong L, Krause A, Miklos AE, Hughes RA, Fechtel B, Ellington AD, Arnold FH, Georgiou G. SCHEMA-designed variants of human Arginase I and II reveal sequence elements important to stability and catalysis. ACS Synth Biol. 2012;1(6):221–228. doi: 10.1021/sb300014t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CL, Wexler LH, Krailo MD, Teot LA, Devidas M, Steinherz LJ, Goorin AM, Gebhardt MC, Healey JH, Sato JK, Meyers PA, Grier HE, Bernstein ML, Lipshultz SE. Intensified chemotherapy with dexrazoxane cardioprotection in newly diagnosed nonmetastatic osteosarcoma: a report from the children’s oncology group. Pediatr Blood Cancer. 2016;63(1):54–61. doi: 10.1002/pbc.25753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag S, Ambinder RF. Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–132. doi: 10.3322/caac.21438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma GN, Dave R, Sanadya J, Sharma P, Sharma K. Various types and management of breast cancer: an overview. J Adv Pharm Technol Res. 2010;1(2):109. [PMC free article] [PubMed] [Google Scholar]
- Singh R, Shaik S, Negi BS, Rajguru JP, Patil PB, Parihar AS, Sharma U. Non-Hodgkin’s lymphoma: a review. J Fam Med Prim Care. 2020;9(4):1834–1840. doi: 10.4103/jfmpc.jfmpc_1037_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockolosky JT, Tiffany MR, Szoka FC. Engineering neonatal Fc receptor-mediated recycling and transcytosis in recombinant proteins by short terminal peptide extensions. Proc Natl Acad Sci. 2012;109(40):16095–16100. doi: 10.1073/pnas.1208857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Ratha BK. Unique hepatic cytosolic arginase evolved independently in ureogenic freshwater air-breathing teleost, Heteropneustes fossilis. PLoS One. 2013;8(6):e66057. doi: 10.1371/journal.pone.0066057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasyk OV, Boretsky YR, Gonchar MV, Sibirny AA. Recombinant arginine-degrading enzymes in metabolic anticancer therapy and bioanalytics. Cell Biol Int. 2015;39(3):246–252. doi: 10.1002/cbin.10383. [DOI] [PubMed] [Google Scholar]
- Stechmiller JK, Childress B, Cowan L. Arginine supplementation and wound healing. Nutr Clin Pract. 2005;20:52–61. doi: 10.1177/011542650502000152. [DOI] [PubMed] [Google Scholar]
- Stone EM, Glazer ES, Chantranupong L, Cherukuri P, Breece RM, Tierney DL, Curley SA, Iverson BL, Georgiou G. Replacing Mn(2+) with Co(2+) in human arginase 1 enhances cytotoxicity toward l-arginine auxotrophic cancer cell lines. ACS Chem Biol. 2010;5(3):333–342. doi: 10.1021/cb900267j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl WR. Fusion proteins for half-life extension of biologics as a strategy to make biobetters. BioDrugs. 2015;29(4):215–239. doi: 10.1007/s40259-015-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Study of ADI-PEG 20 in patients with advanced melanoma. ClinicalTrials.gov Identifier: NCT00520299. https://clinicaltrials.gov/
- Study of immunotherapy plus ADI-PEG 20 for the treatment of advanced uveal melanoma. ClinicalTrials.gov Identifier: NCT03922880. https://clinicaltrials.gov/
- Study of pegylated human recombinant arginase for liver cancer (BCT-100-002) (BCT-100-002). ClinicalTrials.gov Identifier: NCT01092091. https://clinicaltrials.gov/
- Sugimura K, Ohno T, Kusuyama T, Azuma I. High sensitivity of human melanoma cell lines to the growth inhibitory activity of mycoplasmal arginine deiminase in vitro. Melanoma Res. 1992;2(3):191–196. doi: 10.1097/00008390-199209000-00007. [DOI] [PubMed] [Google Scholar]
- Syed N, Langer J, Janczar K, Singh P, Lo Nigro C, Lattanzio L, Coley HM, Hatzimichael E, Bomalaski J, Szlosarek P, Awad M, O’Neil K, Roncaroli F, Crook T. Epigenetic status of argininosuccinate synthetase and argininosuccinate lyase modulates autophagy and cell death in glioblastoma. Cell Death Dis. 2013;4(1):e458. doi: 10.1038/cddis.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szlosarek PW, Klabatsa A, Pallaska A, Sheaff M, Smith P, Crook T, Grimshaw MJ, Steele JP, Rudd RM, Balkwill FR, Fennell DA. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res. 2006;12(23):7126–7131. doi: 10.1158/1078-0432.ccr-06-1101. [DOI] [PubMed] [Google Scholar]
- Szlosarek PW, Baas P, Ceresoli GL, Fennell DA, Gilligan D, Johnston A, Lee P, Mansfield AS, Nolan L, Nowak AK, Steele JP. ATOMIC-Meso: a randomized phase 2/3 trial of ADI-PEG20 or placebo with pemetrexed and cisplatin in patients with argininosuccinate synthetase 1-deficient non-epithelioid mesothelioma. J Clin Oncol. 2017 doi: 10.1200/JCO.2017.35.15_suppl.TPS8582. [DOI] [Google Scholar]
- Tolleson WH. Human melanocyte biology, toxicology, and pathology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2005;23(2):105–161. doi: 10.1080/10590500500234970. [DOI] [PubMed] [Google Scholar]
- Travis LB, Curtis RE, Glimelius B, Holowaty E, Van Leeuwen FE, Lynch CF, Adami J, Gospodarowicz M, Wacholder S, Inskip P, et al. Second cancers among long-term survivors of non-Hodgkin’s lymphoma. J Natl Cancer Inst. 1993;85(23):1932–1937. doi: 10.1093/jnci/85.23.1932. [DOI] [PubMed] [Google Scholar]
- Tsai WB, Aiba I, Lee SY, Feun L, Savaraj N, Kuo MT. Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1alpha/Sp4. Mol Cancer Ther. 2009;8(12):3223–3233. doi: 10.1158/1535-7163.mct-09-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zeijl M, van den Eertwegh A, Haanen J, Wouters M. (Neo) adjuvant systemic therapy for melanoma. Eur J Surg Oncol. 2017;43(3):534–543. doi: 10.1016/j.ejso.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Vettore L, Westbrook RL, Tennant DA. New aspects of amino acid metabolism in cancer. Br J Cancer. 2020;122(2):150–156. doi: 10.1038/s41416-019-0620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2018;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xie Q, Zhou H, Zhang M, Shen J, Ju D. Amino acid degrading enzymes and autophagy in cancer therapy. Front Pharmacol. 2021 doi: 10.3389/fphar.2020.582587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin JR, Thompson MA, Cataldo VD, Fayad LE, Fowler N, Fanale MA, Neelapu S, Samaniego F, Romaguera J, Shah J, McLaughlin P, Pro B, Kwak LW, Sanjorjo P, Murphy WA, Jimenez C, Toth B, Dong W, Hagemeister FB. Zoledronic acid for prevention of bone loss in patients receiving primary therapy for lymphomas: a prospective, randomized controlled phase III trial. Clin Lymphoma Myeloma Leuk. 2013;13(2):99–105. doi: 10.1016/j.clml.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widakowich C, de Castro G, De Azambuja E, Dinh P, Awada A. Side effects of approved molecular targeted therapies in solid cancers. Oncologist. 2007;12(12):1443–1455. doi: 10.1634/theoncologist.12-12-1443. [DOI] [PubMed] [Google Scholar]
- World; source: Globocan 2020-International agency for research on cancer, WHO. The Global Cancer Observatory 2021. https://gco.iarc.fr/
- Wu G, Morris SM. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Janku F, Koenig K, Tsimberidou AM, Piha-Paul SA, Shi N, Stewart J, Johnston A, Bomalaski J, Meric-Bernstam F, Fu S. Phase 1 trial of ADI-PEG 20 and liposomal doxorubicin in patients with metastatic solid tumors. Cancer Med. 2022;11(2):340–347. doi: 10.1002/cam4.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau T, Cheng PN, Chan P, Chen L, Yuen J, Pang R, Fan ST, Wheatley DN, Poon RT. Preliminary efficacy, safety, pharmacokinetics, pharmacodynamics and quality of life study of pegylated recombinant human arginase 1 in patients with advanced hepatocellular carcinoma. Invest New Drugs. 2015;33(2):496–504. doi: 10.1007/s10637-014-0200-8. [DOI] [PubMed] [Google Scholar]
- Yoon CY, Shim YJ, Kim EH, Lee JH, Won NH, Kim JH, Park IS, Yoon DK, Min BH. Renal cell carcinoma does not express argininosuccinate synthetase and is highly sensitive to arginine deprivation via arginine deiminase. Int J Cancer. 2007;120(4):897–905. doi: 10.1002/ijc.22322. [DOI] [PubMed] [Google Scholar]
- Yoon JK, Frankel AE, Feun LG, Ekmekcioglu S, Kim KB. Arginine deprivation therapy for malignant melanoma. Clin Pharmacol. 2013;5:11–19. doi: 10.2147/cpaa.s37350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KM, Pang TP, Cutler M, Tian M, Huang L, Lau JY, Chung SF, Lo TW, Leung TY. Rational design, engineer, and characterization of a novel pegylated single isomer human arginase for arginine depriving anti-cancer treatment. Life Sci. 2020;264:118674. doi: 10.1016/j.lfs.2020.118674. [DOI] [PubMed] [Google Scholar]
- Zaman R, Islam RA, Ibnat N, Othman I, Zaini A, Lee CY, Chowdhury EH. Current strategies in extending half-lives of therapeutic proteins. J Control Rel. 2019;301:176–189. doi: 10.1016/j.jconrel.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Zeng X, Li Y, Fan J, Zhao H, Xian Z, Sun Y, Wang Z, Wang S, Zhang G, Ju D. Recombinant human arginase induced caspase-dependent apoptosis and autophagy in non-Hodgkin’s lymphoma cells. Cell Death Dis. 2013;4(10):e840. doi: 10.1038/cddis.2013.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Xu N, Sun Y, Liu XM. Targeted biopharmaceuticals for cancer treatment. Cancer Lett. 2014;352(2):145–151. doi: 10.1016/j.canlet.2014.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.