Abstract
Background:
Delayed cold-storage of room temperature platelets may extend shelflife from 5 to 14 days. We hypothesized the use of delayed cold-stored platelets in cardiac surgery would be associated with decreased postoperative platelet count increments but similar transfusion and clinical outcomes compared to room temperatuve stored platelets.
Methods:
This is an observational cohort study of adults transfused with platelets intraoperatively during elective cardiac surgery between April 2020 and May 2021. Intraoperative platelets were either room temperature or delayed cold-stored based on blood bank availability rather than clinical features or provider preference. Differences in transfusion and clinical outcomes, including a primary outcome of allogenic transfusion exposure in the first 24 hours postoperatively, were compared between groups.
Results:
713 patient encounters were included: 529 (74%) room temperature, 184 (26%) delayed cold-stored platelets. Median (interquartile range) intraoperative platelet volumes were 1 (1, 2) units in both groups. Patients receiving delayed cold-stored platelets had higher odds of allogeneic transfusion in the first 24 hours postoperatively (81/184 (44%) vs. 169/529 (32%); adjusted odds ratio [aOR], 95% confidence interval [CI], 1.65 (1.13, 2.39); p=0.009), including both RBCs (65/184 (35%) vs. 135/529 (26%); aOR 1.54 (1.03, 2.29); p=0.035) and platelets (48/184 (26%) vs. 79/529 (15%); aOR 1.91 (1.22, 2.99); p=0.005). There was no difference in the number of units administered postoperatively amongst those transfused. Platelet counts were modestly lower in the delayed cold-stored platelet group (−9 × 109/L [95% CI: −16, −3]) through the first 3 days postoperatively. There were no significant differences in reoperation for bleeding, postoperative chest tube output, or clinical outcomes.
Conclusions:
In adults undergoing cardiac surgery, delayed cold-stored platelets were associated with higher postoperative transfusion utilization and lower platelet counts compared to room temperature stored platelets without differences in clinical outcomes. The use of delayed cold-stored platelets in this setting may offer a viable alternative when facing critical platelet inventories but is not recommended as a primary transfusion approach.
Keywords: COVID-19, hemostasis, platelets, surgery, transfusion
Summary Statement:
The use of delayed cold-stored platelets during cardiac surgery is associated with modestly higher postoperative blood product utilization and lower platelet counts than room temperature platelets.
Introduction
The COVID-19 pandemic has strained medical resources around the world and has exposed vulnerability in blood inventories a. Platelets are the blood component most vulnerable to shortages. While other products may be stored long-term by freezing or have pharmacological alternatives such as coagulation factor concentrates, platelets have neither2. Typically, platelets are stored at 20–24 degrees C with gentle agitation for up to 5–7 days2. This short outdate has been selected to balance the increased risk of bacterial contamination seen with non-refrigerated storage and the need to maintain an adequate inventory2. The introduction of pathogen reduction technology (PRT) in platelet manufacturing has added an element of safety with minimizing bacterial and viral contamination, however this has not yet prolonged the shelf life beyond 5 days2. Altogether, this makes platelet inventories dependent on reliable donations and predictable and judicious utilization.
It has long been recognized that refrigeration, or cold storage, may diminish the risk of bacterial contamination, however it comes at a cost. In the 1960s, investigators demonstrated that cold-stored platelets (CSPs) had dramatically shorter lifespans (i.e. 1–2 days) following transfusion compared to their room temperature (RTPs) counterparts (i.e., 7–9 days)3,4. As most platelet transfusions in this time were given to those with hypoproliferative thrombocytopenia in need of prolonged platelet survival, this work essentially excluded CSPs from clinical practice. However, subsequent work has shown that CSPs may have a more favorable hemostatic profile and improved metabolic characteristics than RTPs, which may potentially be beneficial for the management of acute bleeding5–7. Indeed, early clinical work has suggested that CSPs may at least be equally efficacious in bleeding patients8–10. A recent pilot trial in cardiac surgery lends support to the non-inferiority of CSPs compared to RTPs, and a large multicenter randomized trial is currently enrolling participants11.
In early 2020 in anticipation of blood shortages and unpredictable platelet usage, the Mayo Clinic in Rochester, Minnesota notified the United States Food and Drug Administration (FDA) that it would convert about-to-expire (i.e., day 5 of room temperature storage) pathogen-reduced RTPs to cold-storage. These delayed cold-stored platelets (dCSPs) would be stored at 1–6 degrees C without agitation for up to an additional 9 days (i.e., 14 days total storage)12. Previous work, though limited in nature, has suggested preserved metabolic and activation profiles of dCSPs compared to CSPs, which are immediately refrigerated following collection rather than having a period of room-temperature storage13. Assuming that dCSPs would display similar features to CSPs (i.e., shorter circulation duration and potential favorable hemostatic response compared to RTPs), dCSPs were earmarked for use in bleeding patients (i.e., surgical, trauma, massive hemorrhage) when RTPs inventories were low or unavailable12. Early clinical review of this practice, including transfusion of 61 units of dCSPs (58% in cardiac surgery), showed signs of hemostatic efficacy without signal for patient harm.
Given our dual inventory of dCSPs and RTPs in which bleeding cardiac surgery patients may receive either product intraoperatively based upon blood bank availability rather than provider preference, there is an excellent opportunity to compare the clinical efficacy of dCSPs against traditional RTPs. We hypothesized that dCSPs would be associated with decreased postoperative platelet increments compared to RTPs but comparable transfusion and clinical outcomes.
Methods
This is a retrospective observational cohort study conducted under appropriate institutional review board approval at the Mayo Clinic in Rochester, Minnesota with a waived requirement for written informed consent. The Strengthening the Reporting of Observational Studies in Epidemiology guidelines were employed in study design and conduct.14
All adults (≥18 years) undergoing elective cardiac surgery between April 20, 2020 and May 21, 2021 with administration of at least 1 unit of platelets intraoperatively were eligible for inclusion. Patient records were excluded for the following reasons: emergency surgery, primary extracorporeal membrane oxygenation (ECMO) cannulation, wash-out, or weaning, or irrigation and debridement procedures. Additionally, patient records could only be included once, such that the first qualifying cardiac surgery and associated hospital admission was included for each patient. This is a primary analysis of these data, although 28 patients in this investigation were included in an earlier publication regarding dCSP use at our institution12.
dCSP and RTP availability and blood bank processes
The Mayo Clinic in Minnesota maintains a local blood donor program, with approximately 80% of blood products derived from local donors and an additional 20% received from regional suppliers. All platelets undergo pathogen reduction. As previously described, the in April 2020 we began transitioning pathogen-reduced RTPs on day 5 of their shelf-life to cold-storage for up to an additional 9 days (i.e., 14 days total storage), thereby creating a parallel dCSP inventory. The dCSP inventory was earmarked for use in patients with active bleeding (i.e., surgical, trauma, massive transfusion) when RTP inventories were low (i.e. <10% of optimal levels) or unavailable. RTPs remained available for bleeding patients when supplies were adequate and were maintained for patients with non-bleeding thrombocytopenia. Clinicians were made aware of the dual platelet inventories via various internal communications. No changes were made in blood ordering processes, and clinicians could not request dCSPs or RTPs. When an order for a platelet transfusion was placed from the operating room for a cardiac surgery patient, the Transfusion Medicine team would distribute either a RTP or dCSP unit based on blood bank availability rather than patient features or provider request.
Transfusion utilization in cardiac surgery
Intraoperative platelet transfusions in cardiac surgery at the study institution are administered to patients with clinical bleeding assessed by the attending anesthesiologist and surgeon in accordance with a previously published protocol15. Briefly, this includes platelet administration for thrombocytopenia of less than 102 ×10^9 or a maximal amplitude of less than 48 on kaolin thromboelastography (Haemonetics Corporation, Boston, MA, USA). Thromboelastography use is at the discretion of the anesthesiologist and is not standardized. Supplemental coagulation factor concentrates may be used based on the clinical judgement of the attending anesthesiologist. Similar criteria for platelet transfusion are used postoperatively. During the study time period, RBCs were administered for hemoglobin concentrations less than 8 g/dL, during rapid hemorrhage, or when there was evidence of depressed oxygen delivery on cardiopulmonary bypass. All postoperative transfusion orders for platelets and RBCs must be accompanied by an indication for transfusion entered into the electronic medical record, which may include the presence of bleeding and/or a platelet count or hemoglobin concentration falling below a given threshold.
Exposure
The primary exposure of interest was intraoperative administration of dCSP versus RTPs, with all transfusion episodes extracted from our institutional Transfusion DataMart. Patients in the dCSP group must have received at least 1 unit of intraoperative dCSPs but could also have received additional units of either platelet type (dCSP or RTP) intraoperatively. Patients in the RTP group only received RTPs intraoperatively.
Outcomes
Both transfusion/bleeding and clinical outcomes were evaluated. The primary outcome was allogeneic transfusion in the first 24 hours postoperatively, evaluated as present or absent and by the total number of units transfused. Secondary transfusion/bleeding outcomes included: allogenic transfusions of red blood cells (RBCs) and platelets in the first 24 and 72 hours after surgery, total allogeneic transfusions in first the 72 hours postoperatively, unanticipated return to the operating room for bleeding in the first 24 hours after surgery, and total chest tube output in the first 24 hours after surgery. Maximum daily postoperative platelet counts were evaluated through postoperative day 7. Secondary clinical outcomes of interest included: venous thromboembolism, stroke or myocardial infarction, infection, transfusion reactions, and hospital mortality. Clinical outcomes were assessed through the first 7 postoperative days, apart from mortality which was evaluated through the duration of hospitalization. Venous thromboembolism was defined by radiographic evidence of acute deep venous thrombosis or pulmonary embolism during hospitalization following surgery. Stroke was defined by radiographic evidence of acute or subacute infarction during hospitalization following surgery and/or clinical diagnosis by Neurology consultation. Myocardial infarction was defined according to the fourth universal definition of myocardial infarction.16 Infection included those diagnosed clinically during postoperative hospital admission and treated with systemic antimicrobial therapy (e.g., oral or intravenous antibiotics) with or without positive microbial culture. This could include infections of the bloodstream, urinary tract, lungs, skin or soft tissues, or surgical site, amongst others. Transfusion reactions were those documented in clinical notes and/or reported to the Transfusion Medicine service through our internal reporting system.
Statistical Analysis
A statistical analysis plan was developed and filed with the IRB prior to accessing study data, with any additional analyses labeled as post-hoc. The statistical analysis plan is provided as supplemental digital content. Baseline demographic, clinical, and surgical characteristics were described with median (interquartile range) and number (percent) for continuous and categorial data elements, respectively. Unadjusted outcomes were compared between groups with Mann-Whitney U tests and Pearson’s Chi-square tests for continuous and categorial data elements, respectively. In cases where expected cell counts were less than 5, categorical endpoints were compared with Fisher’s exact tests. Surgeries were classified as complex or not, with the complex group including, combination procedures (i.e. coronary artery bypass grafting + valve surgery and/or other procedure; valve surgery + other procedures), adult congenital surgery, ventricular assist device and mechanical circulatory support surgery, and heart transplantation. To account for the large proportion of zero values, multivariable hurdle models were created to assess the association between treatment group and postoperative transfusion outcomes without pre-specification of minimum clinically meaningful effect sizes. The results of the zero and the count portions of the hurdle models were reported as: 1) an adjusted odds ratio (aOR) representing the estimated multiplicative increase in odds for receiving any transfusion after surgery for dCSP compared to RTP for the zero portion, and 2) an adjusted rate ratio representing the estimated multiplicative increase in total number of units administered after surgery for dCSP compared to RTP in those who receive postoperative transfusion for the count portion. Both the zero and count portions of the hurdle models were adjusted for the same covariates. While there was no selection of platelet type by clinicians (i.e., units distributed solely by blood bank inventory), thereby minimizing potential between-group differences in important prognostic and confounding variables, several adjustment variables were selected a priori during study design. These variables included age, sex, preoperative hemoglobin concentration, preoperative platelet count, complex surgery, and total cardiopulmonary bypass time, with 3 additional variables added at the request of peer reviewers including preoperative use of antiplatelet therapy (i.e., aspirin, clopidogrel, or ticagrelor within 7 days of surgery), redo sternotomy, and total number of transfused platelet units intraoperatively. Multivariable linear regression models with the same adjustment variables were employed for the outcomes of postoperative platelet counts and total chest tube output in the first 24 hours postoperatively. For the outcome of return to the operating room for bleeding, a multivariable logistic regression model was utilized and adjusted only for preoperative platelet count and total cardiopulmonary bypass time. No adjustment was performed for clinical outcomes given limited events. Recognizing that some patients receiving more than 1 intraoperative platelet unit in the dCSP group may also receive RTPs, a predefined sensitivity analysis was performed limited to those receiving only dCSPs in the dCSP group. As a post-hoc analysis, runs test was employed to evaluate potential clustering of treatment assignment by calendar time. Subsequently, potential clustering of 24-hour transfusion outcomes over time was evaluated with runs tests (i.e., transfusion yes or no) and Durbin-Watson (DW) tests (i.e., units transfused). Additionally, amongst patients receiving a single unit of intraoperative dCSPs, we evaluated the relationships between the age of the unit in days and transfusion outcomes using multivariable models as described previously. Finally, we used the coefficients from the final models to estimate the total number of additional platelet and RBC units administered in the first 72 hours postoperatively under the hypothetical scenarios where either all patients in the sample were treated with dCSPs or all patients were treated with RTPs. No power analysis was performed for this hypothesis-generating study, which employed all available clinical data at the time of data extraction. All continuously scaled covariates were included as linear effects in the multivariable models. When not all data were available, complete case analyses were utilized under the assumption that data were missing completely at random. All analyses were performed with R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria), and two-tailed p-values <0.05 were considered significant.
Results
713 patient encounters with intraoperative platelet transfusion during cardiac surgery were included (Supplemental Figure 1). RTPs were administered in 529 cases (74%) and dCSPs in 184 cases (26%), with 154 patients (84%) in the dCSP group receiving only dCSPs intraoperatively and 30 patients (16%) also receiving intraoperative RTPs. The median age of dCSP units was 7 (6, 8) days. The RTP and dCSP groups were similar in all baseline demographic, clinical, and surgical characteristics (Table 1). Overall, the cohort had a median age of 65 (51, 73) years with a male predominance (64%) and a median Charlson Score of 4 (2, 6). Half of the cohort underwent complex cardiac surgery, and 34% of operations were redo sternotomies. The most common procedures were: 179 valvular plus other procedures (25%), 140 isolated valvular procedures (20%), and 110 isolated CABG procedures (15%). The median CPB time was 138 (99, 196) minutes, and the median cross-clamp time was 102 (67, 149) minutes.
Table 1.
Baseline demographic, clinical, and surgical features
| Platelet Transfusion Type | |||
|---|---|---|---|
| Characteristic | Overall N = 7131 |
RTPs N = 5291 |
dCSPs N = 1841 |
| Age (yrs) | 65 (51, 73) | 64 (51, 73) | 66 (56, 74) |
| Sex | |||
| Female | 255 (36%) | 191 (36%) | 64 (35%) |
| Male | 458 (64%) | 338 (64%) | 120 (65%) |
| Charlson Score | 4 (2, 6) | 4 (2, 6) | 4 (3, 7) |
| Preop Platelet count (×10 9 /L; n=685) | 188 (154, 233) | 188 (153, 232) | 184 (154, 237) |
| Preop Antiplatelet Use | 357 (50%) | 257 (49%) | 100 (54%) |
| Aspirin | 346 (49%) | 248 (47%) | 98 (53%) |
| Clopidogrel/Ticagrelor | 82 (12%) | 57 (11%) | 25 (14%) |
| Procedure Type | |||
| CABG Onlya | 110 (15%) | 72 (14%) | 38 (21%) |
| CABG + Other | 12 (2%) | 10 (2%) | 2 (1%) |
| CABG + Valve | 50 (7%) | 42 (8%) | 8 (4%) |
| CABG + Valve + Other | 13 (2%) | 9 (2%) | 4 (2%) |
| Congenital | 67 (9%) | 50 (9%) | 17 (9%) |
| HCM/Myectomy | 40 (6%) | 32 (6%) | 8 (4%) |
| Otherb | 58 (8%) | 43 (8%) | 15 (8%) |
| Pericardiectomy | 10 (1%) | 8 (2%) | 2 (1%) |
| Transplant | 24 (3%) | 17 (3%) | 7 (4%) |
| VAD and MCS | 10 (1%) | 4 (1%) | 6 (3%) |
| Valve | 140 (20%) | 107 (20%) | 33 (18%) |
| Valve + Otherc | 179 (25%) | 135 (26%) | 44 (24%) |
| Redo Sternotomy | 240 (34%) | 183 (35%) | 57 (31%) |
| Complex surgery | 355 (50%) | 267 (50%) | 88 (48%) |
| CPB time (min; n=709) | 138 (99, 196) | 141 (100, 199) | 129 (96, 185) |
| Cross-clamp time (min; n=646) | 102 (67, 149) | 104 (66, 152) | 99 (70, 138) |
Median (IQR); n (%)
Two procedures performed off-pump, both in the room-temperature group
Including aneurysm repair, myxoma resections, pulmonary thromboendarterectomy
Including Bentall procedure.
Abbreviations: CABG; coronary artery bypass graft, HCM; hypertrophic cardiomyopathy, VAD; ventricular assist device, MCS; mechanical circulatory support
Intraoperative transfusions were generally similar between groups (Table 2). Patients in both groups received a median of 1 (1, 2) unit of platelets, representing 777 units of intraoperative RTPs and 292 units of dCSPs. Fifty-three percent of those in the RTP group received an RBC transfusion compared to 50% in the dCSP group, and patients in the dCSP group received a median of 3 (1, 4) units of RBCs as compared to 2 (1, 4) units. Cryoprecipitate was given in 49% of cases in the RTP group and 37% of cases in the dCSP group. Cell salvage volumes were modestly higher in the RTP group (605 [406, 872] mL vs. 527 [347, 812] mL). Autologous fresh whole blood from acute normovolemic hemodilution was transfused in 12% and 14% of cases for RTPs and dCSPs, respectively, with modestly higher volumes in the RTP group.
Table 2.
Intraoperative transfusion features
| Platelet Transfusion Type | |||
|---|---|---|---|
| Transfusion Type | Overall, N = 7131 | RTPs, N = 5291 | dCSPs, N = 1841 |
| Any Platelets | 713 (100%) | 529 (100%) | 184 (100%) |
| Platelets (units; n=713) | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) |
| Any RBC | 371 (52%) | 279 (53%) | 92 (50%) |
| RBC (units; n=371) | 2 (1, 4) | 2 (1, 4) | 3 (1, 4) |
| Any Plasma | 314 (44%) | 233 (44%) | 81 (44%) |
| Plasma (units; n=314) | 2 (1, 2) | 2 (1, 2) | 2 (2, 3) |
| Any Cryoprecipitate | 329 (46%) | 261 (49%) | 68 (37%) |
| Cryoprecipitate (units; n=329) | 2 (1, 2) | 2 (1, 2) | 2 (1, 2) |
| Any Cell Salvage | 675 (95%) | 498 (94%) | 177 (96%) |
| Cell salvage (mL; n=675) | 578 (388, 846) | 605 (406, 872) | 527 (347, 812) |
| Any Fresh whole blood | 92 (13%) | 66 (12%) | 26 (14%) |
| Fresh whole blood (mL; n=92) | 742 (451, 902) | 775 (454, 902) | 642 (449, 826) |
n (%); Median (IQR)
Abbreviations: dCSPs; delayed cold-stored platelets, RBC; red blood cell, RTPs; room-temperature platelets
Unadjusted analyses of postoperative transfusions and other transfusion outcomes revealed significant between-group differences (Supplemental Table 1), including a lower incidence of transfusion of platelets (79/529 [15%] vs. 48/184 [26%], p<0.001) and RBCs (135/529 [26%] vs. 65/184 [35%], p=0.011) in the first 24 hours after surgery in the RTP group. Amongst those receiving platelets in the first 24 hours after surgery, the proportions of patients receiving a platelet transfusion for active bleeding were 90% (71/79) and 96% (46/48) for RTP and dCSP groups, respectively (p=0.317). Amongst those receiving RBCs in the first 24 hours after surgery, the proportions transfused for active bleeding were 60% (81/135) and 71% (46/65) for RTP and dCSP groups, respectively (p=0.160). Differences in maximum platelet count on each postoperative day are displayed graphically (Figure), showing modestly lower platelet counts on each day in the dCSP group. There were no significant differences in chest tube output in the first 24 hours after surgery (762 [510, 1089] ml for RTPs vs. 766 [554, 1191] ml for dCSPs, p=0.215), return to the operating room for bleeding (24/529 [5%] for RTPs vs. 11/184 [6%] for dCSPs, p=0.436), or clinical outcomes between groups (Table 3).
Figure 1.
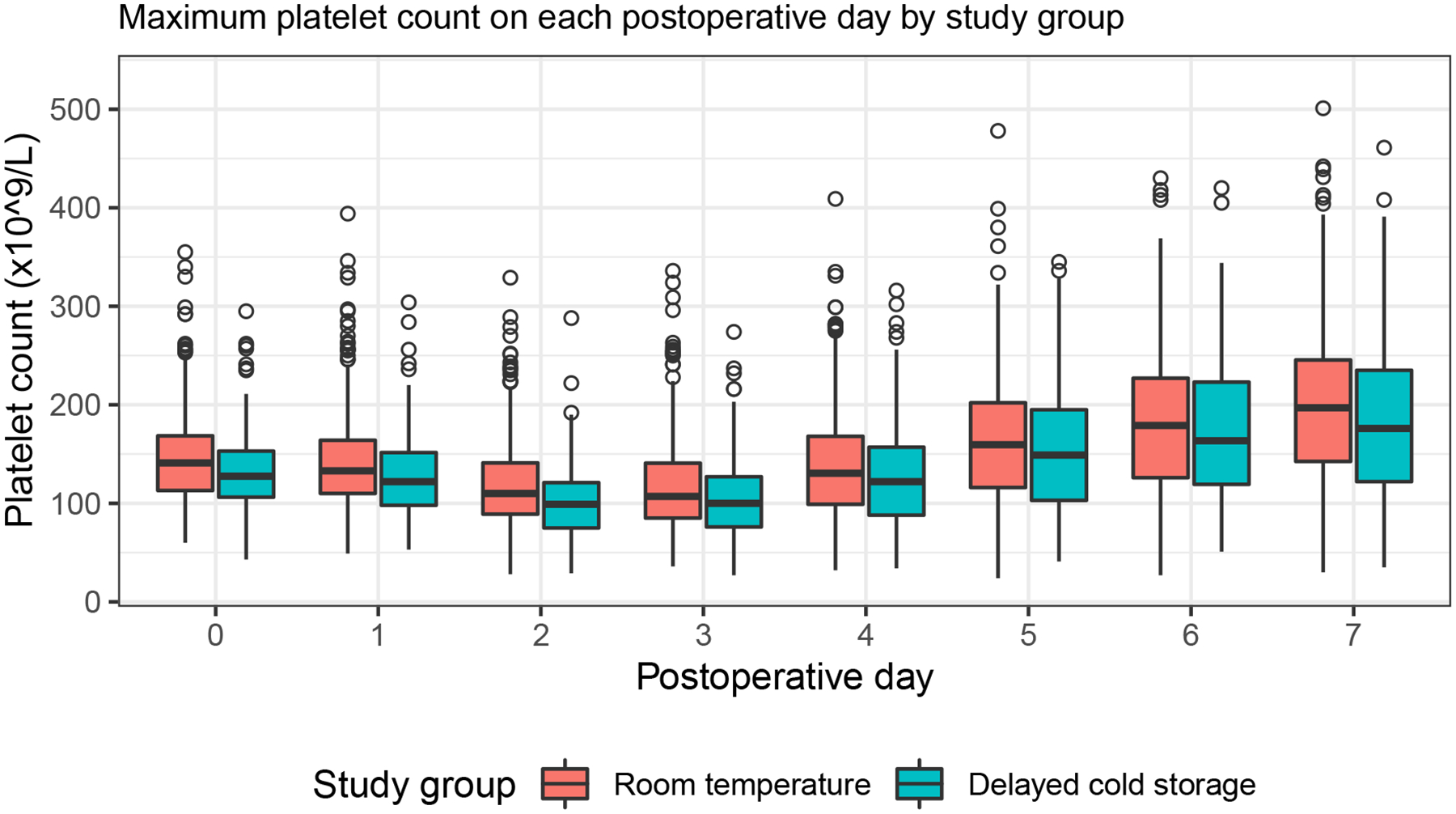
Maximum platelet counts on each postoperative day.
Distribution of maximum platelet count presented as box-and-whisker plots according to study group and postoperative day. For each box-and-whisker plot, the lower, middle, and upper horizontal lines represent the 25th, 50th, and 75th percentiles. The difference between the 75th and 25th percentiles is defined as the interquartile range (IQR). Vertical lines are drawn from the upper and lower quartiles to the maximum and minimum values within 1.5 IQR respectively. Values outside of those ranges are plotted as circles. Ninety-five percent of patients had available platelet counts through postoperative day 4, with 89%, 74%, and 58% having available platelet count on days 5, 6, and 7 respectively.
Table 3.
Unadjusted seven-day clinical outcomes
| Platelet Transfusion Type | ||||
|---|---|---|---|---|
| Outcome | Overall N = 7131 |
RTPs N = 5291 |
dCSPs N = 1841 |
P-value2 |
| Venous thromboembolism | 9 (1%) | 5 (1%) | 4 (2%) | 0.247 |
| Stoke or myocardial infarction | 10 (1%) | 8 (2%) | 2 (1%) | 0.999 |
| Infection | 52 (7%) | 37 (7%) | 15 (8%) | 0.603 |
| Hospital mortality | 16 (2%) | 12 (2%) | 4 (2%) | 0.999 |
| Transfusion Reaction (n=712) | 6 (1%) | 4 (1%) | 2 (1%) | 0.650 |
n (%)
All p-values were from Fisher’s exact tests except the p-value comparing infection between groups which was from Pearson’s Chi square test
Abbreviations: dCSPs; delayed cold-stored platelets, RBC; red blood cell RTPs; room-temperature platelets
In adjusted analyses for the primary outcome, intraoperative dCSPs were associated with higher odds receiving any allogeneic transfusion (aOR, 95% CI, 1.65 (1.13, 2.39); p=0.009), any RBCs (aOR 1.54 (1.03, 2.29); p=0.035), and any platelets (aOR 1.91 (1.22, 2.99); p=0.005) in the first 24 hours after surgery (Table 4). Only an increased odds for platelet transfusion remained statistically significant at 72 hours postoperatively. Amongst those transfused, there were no significant differences in the number of units administered (Table 4). In adjusted analyses, patients receiving dCSPs had an estimated −12 (95% CI −19, −5) × 109/L and −9 (95% CI −16, −3) × 109/L difference in maximum platelet count on postoperative days 0 (p<0.001) and 3 (p=0.004), respectively, compared to those receiving RTPs (Table 5). The difference in platelet counts on postoperative day 7 was similar but not statistically significant. There was no significant difference in odds of returning to the operating room for bleeding with dCSPs (aOR 0.85 (0.40, 1.98), P=0.688), nor was there any significant difference in chest tube output in the first 24 hours after surgery (−31 (95% CI −168, 106) ml; p=0.660). Study results were consistent in a predefined sensitivity analysis limited to those only receiving dCSPs in the dCSP group (n=154; Supplemental Tables 2–5).
Table 4.
Adjusted postoperative transfusion outcomes for patients receiving dCSPs vs RTPs
| Outcome* | aOR# (95% CI) | P-value | Rate Ratio^ (95% CI) | P-value |
|---|---|---|---|---|
| 24 hours | ||||
| Any allogeneic transfusion | 1.65 (1.13, 2.39) | 0.009 | 0.90 (0.55, 1.46) | 0.665 |
| RBC transfusion | 1.54 (1.03, 2.29) | 0.035 | 0.93 (0.56, 1.55) | 0.792 |
| Platelet transfusion | 1.91 (1.22, 2.99) | 0.005 | 0.69 (0.33, 1.45) | 0.327 |
| 72 hours | ||||
| Any allogeneic transfusion | 1.26 (0.87, 1.84) | 0.217 | 1.10 (0.73, 1.66) | 0.651 |
| RBC transfusion | 1.30 (0.90, 1.89) | 0.167 | 1.05 (0.73, 1.53) | 0.776 |
| Platelet transfusion | 1.86 (1.20, 2.89) | 0.005 | 0.76 (0.361, 1.62) | 0.482 |
Models adjusted for age, sex, preoperative hemoglobin concentration, preoperative platelet count, preoperative use of antiplatelet agents, complex surgery, redo sternotomy, total number of transfused intraoperative platelet units, and total cardiopulmonary bypass time. Analysis limited to observations with complete covariate information (n=681/713, 96%).
aOR represent estimated multiplicative increase in odds of receiving 1 or more of the given transfusion associated with receipt of cold-stored platelets intraoperatively compared to room temperature platelets.
Rate ratios represent the estimated multiplicative increase in the number of units administered postoperatively (limited to those who received a given postoperative transfusion type) associated with receipt of cold platelets intraoperatively compared to room temperature platelets.
Abbreviations: dCSPs; delayed cold-stored platelets, RBC; red blood cell, RTPs; room-temperature platelets
Table 5.
Adjusted platelet recovery and bleeding outcomes for patients receiving dCSPs vs RTPs
| Outcome* | Estimate# | 95% CI | P-value |
|---|---|---|---|
| Platelet count on postoperative day 0, × 109/L; n=669 | −12 | −19, −5 | <0.001 |
| Platelet count on postoperative day 3, × 109/L; n=667 | −9 | −16, −3 | 0.004 |
| Platelet count on postoperative day 7, × 109/L; n=396 | −11 | −29, 6 | 0.199 |
| Return to operating room for bleeding; n=681 | 0.85 | 0.40, 1.98 | 0.688 |
| Chest tube output in 1st 24 hours after surgery, ml; n=678 | −31 | −168, 106 | 0.660 |
Models adjusted for age, sex, preoperative hemoglobin concentration, preoperative platelet count, preoperative use of antiplatelet agents, complex surgery, redo sternotomy, total number of transfused intraoperative platelet units, and total cardiopulmonary bypass time, except for return to the operating room for bleeding which is adjusted for preoperative platelet count and total cardiopulmonary bypass time. Number of observations with complete outcome and covariate information and included in each analysis is summarized.
Estimates represent the estimated difference in outcome associated with receipt of cold platelets intraoperatively compared to room temperature platelets. For example, receipt of cold platelets was associated with an estimated 12 (95% CI: 5, 19) × 109/L lower immediate postoperative platelet count compared to room temperature platelets.
Abbreviations: dCSPs; delayed cold-stored platelets, RBC; red blood cell, RTPs; room-temperature platelets
In post-hoc analyses, there was evidence of clustering of treatment assignment over time (Supplemental Figure 2, runs test p<0.001), but no statistically significant outcome clustering over time was detected for any transfusion (runs test p=0.097), any RBC transfusion (runs test p=0.155), any platelet transfusion (runs test p=0.974), total transfusion units (DW p=0.677), total RBC units (DW p=0.580), or total platelet units (DW p=0.606) in the first 24 hours after surgery. Amongst patients receiving intraoperative dCSPs, the age of the unit was not associated with the presence or absence of postoperative transfusion. However, in those receiving a postoperative transfusion of platelets in the first 24 hours after surgery, each 1-day increase in age of the intraoperative dCSP unit was associated with a 3.7 times greater rate of platelet transfusions (Supplemental Table 6). Similarly, each 1-day increase in intraoperative dCSP age was associated with a reduction of −5.7 (−9.0, −2.3) in the maximum platelet count on postoperative day 0 (p<0.001). There were no significant associations with intraoperative dCSP unit age and postoperative platelet counts on days 3 and 7. The total model-estimated number of RBC and platelet units administered postoperatively through the first 72 hours under the hypothetical dCSP-only scenario compared to the RTP-only scenario was 1060 vs. 927 (14% increase with dCSPs) and 411 vs. 341 (20% increase with dCSPs), respectively.
Discussion
In this observational study of intraoperative dCSPs versus RTPs for adult cardiac surgery patients, more than 180 patients (25% of included cases) received dCSPs over the 13-month study period, thereby receiving platelet units that would otherwise have been discarded. Those receiving dCSPs had higher allogeneic transfusion requirements through the first 3 days after surgery. Post-transfusion platelet counts were modestly lower in the dCSP group (approximately 10 × 10^9/L less through the first week postoperatively), though this was not accompanied by an increased rate of reoperation for bleeding or higher chest tube output. There was no signal for adverse clinical outcomes such as transfusion reactions or thrombotic events in those receiving dCSPs.
Following the discovery in the 1960s that CSPs exhibited a significantly reduced circulation time after transfusion, CSPs were nearly abandoned clinically for decades3. Collectively, this makes CSPs a relatively rare product in modern clinical practice outside of the military and clinical trial situations12,17,18. Despite this rarity, accumulating evidence has suggested although they remain in circulation for less time than RTPs, CSPs have improved metabolism and reduced oxidative stress, demonstrate ‘prohemostatic’ characteristics due to improved aggregation and clot strength, and release fewer proinflammatry markers during storage5–7,19. In addition, CSPs appear to be more resistant to bacterial growth than RTPs up to 21 days of cold storage2,20,21. These properties have led to a growing interest in apheresis derived CSP concentrates in bleeding patients (mostly trauma) since the introduction of an FDA variance in 2015 and a renewed interest in studying their clinical efficacy and safety in actively bleeding patients in other clinical areas12,17,18.
Although few studies have evaluated CSPs clinically, those that have generally demonstrate comparable early post transfusion efficacy in actively bleeding patients with no significant safety concerns and consistently demonstrate a decreased post-transfusion circulation time4,8,10,22. In a recent study by Cohn et al., 10 volunteers underwent a dual arm crossover and were evaluated for the ability of RTP or CSP autotransfusions to correct aspirin and clopidogrel-influenced bleeding times23. While neither group was effective in reversing antiplatelet effects, the two groups were generally comparable23. In a recent report of platelet functional recovery, Stolla et al. transfused 21 patients with radiolabeled CSPs stored for 5, 10, 15, or 20 days9. They found a continuous decline in post-transfusion platelet recovery with increasing storage duration with the low nadir platelet recovery occurring after 10 days of storage, however in vitro assessments showed preserved metabolic profiles, integrin activation and mitochondrial membrane integrity up to 20 days of cold storage9. Although recovery may be worse in vivo, in vitro testing shows platelet reactivity is prolonged up to 20 days in CSPs. A single randomized controlled pilot trial in Norway has directly compared CSPs to RTPs in complex cardiac surgery11. There were no differences in the median chest tube output between the groups, and no differences in total blood product usage, adverse events, or ICU length of stay11. The results of this trial were used to inform a large multicenter trial of RTPs vs. CSPs in complex cardiac surgery (CHIPs), which is currently recruiting and will likely take several years before results are available (NCT04834414).
Few studies have specifically focused on delayed CSPs, where there is at least some period of “typical” storage at room temperature before cooling to 1–6 degrees C. Two recent studies have demonstrated preserved metabolic profiles and hemostatic properties of dCSPs for up to 21 days13,24. However, Braathen et al. demonstrated that although both CSPs and dCSPs showed reasonable in vitro hemostatic profiles and remained functional throughout their storage life, dCSPs showed lower maximal aggregation responses with multiple functional studies compared to CSPs24. Our previously published observational data of 61 dCSP transfusions for bleeding patients demonstrated adequate hemostasis and no evidence of patient harm, though there was no RTP comparator12.
In this study of dCSPs in adult cardiac surgery, we found that while dCSPs demonstrated clinical efficacy without signal for patient harm, they appear less efficacious than RTPs within their first 5-day storage window for the prevention of postoperative transfusions or maintenance of postoperative platelet counts. Rather than having greater hemostatic efficacy as has been previously postulated for CSPs, dCSPs may instead be functionally similar to day 5 RTPs but with shorter circulatory life span. Hence, apart from times of shortages in RTPs, it is unlikely that dCSPs should play a major role in the management of thrombocytopenia-related acute bleeding during cardiac surgery. That being said, in times of severe inventory shortages, dCSPs may represent a viable option with an encouraging safety and hemostatic profile. However, it is important to consider the implications of using a blood inventory management strategy that may reduce wastage of platelet units prior to surgery but may come at a cost of increased platelet and RBC utilization after surgery. In this study, the use of dCSPs intraoperatively was associated with model-predicted absolute increases in postoperative RBC and platelet units through the first 72 hours of 133 and 70 units, respectively. These utilization numbers are substantially lower than the 292 dCSP units administered intraoperatively that otherwise would have been discarded, suggesting positive net benefit on inventory. Regarding financial implications, employing estimated acquisition costs of $585 and activity-based costs of $1360 per unit of platelets,25 saving 222 units in net platelet utilization would translate to approximately $129,870 to $301,920 in cost savings over the 13-month study period based on acquisition and activity-based costs, respectively. Considering the additional 133 units of RBCs administered postoperatively in the dCSP group with estimated acquisition and activity-based costs of $210 and $761,26,27 these savings would be offset by approximately $27,930 to $101,213, yielding total transfusion-related cost savings of $101,940 (acquisition costs only) to $200,707 (activity-based costs). Importantly, these estimates do not include potential costs related to the management of a dual-platelet inventory.
Our report has several limitations worth highlighting. First, these findings are representative of a single tertiary referral center performing cardiac surgery with a large in-house blood donor and product manufacturing capability. As such, the approach presented therein (i.e., maintenance of dual platelet inventory) may be impractical for many centers. Secondly, our study is observational which may introduce relevant biases related to residual confounding or incomplete data. Third, our institution utilizes transfusion algorithms based on standard blood counts. Without correcting for the expected differences in post transfusion platelet recovery, there may be a bias towards increased transfusion of platelets in the dCSP group despite no difference in hemostatic efficacy. Our practice also allows the individualized use of coagulation factor concentrates that were not captured herein. Fourth, some patients (16%) in the dCSP group also received RTPs intraoperatively, which may bias outcome estimates. We performed a predefined sensitivity analysis excluding patients receiving both dCSPs and RTPs with similar results. Fifth, as an observational design, adverse events are identified and evaluated by bedside clinicians rather than prospective surveillance, which may predispose to under-reporting. As such, similar clinical outcomes between groups does not necessarily imply comparable safety of dCSPs and RTPs. Sixth, although dCSP versus RTP use was based on blood bank availability and not directly related to patient acuity, important measured or unmeasured confounders might exist and could contribute to a missing covariable bias, although we attempted to limit this with thoughtful adjustment for clinically relevant confounders. Further, as a post hoc analysis we evaluated for clustering of treatment over time based on inventory levels, which could introduce bias in treatment assignment. Despite evidence of clustering of treatment assignment over time, there was no evidence of outcome clustering. However, the study was not designed to assess outcome clustering, therefore the nonsignificant findings should not be considered definitive. Seventh, a small proportion of observations were excluded due to missing covariate information, and bias may be introduced by using a complete case approach to missing data. Eighth, it is known that PRT platelets used for cold storage have slightly altered clotting dynamics compared to those cold stored without PRT treatment, and the results of this study may not be generalizable to non-PRT platelets28. Finally, the data presented herein pertain specifically to cardiac surgical patients and therefore cannot be extrapolated to other bleeding clinical situations.
In summary, our data demonstrate the utility of dCSPs in bleeding cardiac surgery patients. Despite reasonable clinical efficacy as highlighted by no differences in chest tube output or reoperation for bleeding, dCSPs were associated with increased odds of allogenic transfusion through the first 3 postoperative days. Since dCSPs undergo a period of typical room temperature storage before refrigeration, determining the clinical efficacy and safety of CSPs, which undergo immediate refrigeration, will require well designed, randomized controlled trials, of which one is currently underway. Finally, these results show that nearly 300 units of platelets that would have otherwise been discarded were able to be utilized in clinical practice, which far outweighs any increase in downstream platelet utilization related to intraoperative dCSP use. In the face of ongoing blood shortages and financial concerns related to the maintenance of adequate platelet inventories due to their short outdate and high wastage rates, further discussion is needed regarding the potential utility of dCSP use in bleeding patients, especially when the alternative may be to forego platelet transfusion due to lack of inventory.
Supplementary Material
Supplemental Figure 1. Consort diagram
Supplemental Figure 2. Treatment allocation over time.
Supplemental Table 1. Unadjusted postoperative transfusion and bleeding outcomes
Supplemental Table 2. Baseline demographic, clinical, and surgical features limited to those with only a single type of platelet transfusion intraoperatively
Supplemental Table 3. Intraoperative transfusion features limited to those with only a single type of platelet transfusion intraoperatively
Supplemental Table 4. Unadjusted clinical outcomes limited to those with only a single type of platelet transfusion intraoperatively
Supplemental Table 5. Adjusted postoperative transfusion outcomes for patients receiving dCSPs vs RTPs, limited to those with only a single type of platelet transfusion intraoperatively
Supplemental Table 6. Adjusted postoperative transfusion outcomes in patients receiving intraoperative dCSPs for each 1-day increase in age of the platelet unit
Data Analysis Plan
Acknowledgements:
The authors would like to thank Scott A. Hammell for his support in producing this manuscript.
The authors would like to acknowledge the Anesthesia Clinical Research Unit (ACRU) Data Specialist Kellie Robbins for her help with data extraction.
Funding:
This work is supported by the National Heart Lung and Blood Institute (NHLBI) of the United States National Institutes of Health (NIH) grant K23HL153310 (MAW). Study design and conduct are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Additional funding for statistical support was provided through the Mayo Clinic Department of Anesthesiology & Perioperative Medicine Small Grant Program.
Conflicts of Interest:
Allan Klompas: Serves as the site principal investigator for the CHIPS randomized controlled trial (NCT04834414)
Simon Zec: No conflicts of interest to disclose
Andrew Hanson: No conflicts of interest to disclose
Tim Weister: No conflicts of interest to disclose
James Stubbs: Serves as a co-investigator for the CHIPS randomized controlled trial (NCT04834414)
Daryl Kor: Has received research support from the NIH and consulting fees from NIH, UpToDate, and Dynocardia.
Matthew Warner: Receives research support from the National Institutes of Health. Serves on the board of directors for the Society for the Advancement of Patient Blood Management.
References:
- 1.Shander A, Goobie SM, Warner MA, Aapro M, Bisbe E, Perez-Calatayud AA, Callum J, Cushing MM, Dyer WB, Erhard J, Faraoni D, Farmer S, Fedorova T, Frank SM, Froessler B, Gombotz H, Gross I, Guinn NR, Haas T, Hamdorf J, Isbister JP, Javidroozi M, Ji H, Kim YW, Kor DJ, Kurz J, Lasocki S, Leahy MF, Lee CK, Lee JJ, Louw V, Meier J, Mezzacasa A, Munoz M, Ozawa S, Pavesi M, Shander N, Spahn DR, Spiess BD, Thomson J, Trentino K, Zenger C, Hofmann A, International Foundation of Patient Blood M, Society for the Advancement of Blood Management Work G: Essential Role of Patient Blood Management in a Pandemic: A Call for Action. Anesth Analg 2020; 131: 74–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stubbs JR, Homer MJ, Silverman T, Cap AP: The current state of the platelet supply in the US and proposed options to decrease the risk of critical shortages. Transfusion 2021; 61: 303–312 [DOI] [PubMed] [Google Scholar]
- 3.Cohen P, Gardner FH: Platelet preservation. IV. Preservation of human platelet concentrates by controlled slow freezing in a glycerol medium. N Engl J Med 1966; 274: 1400–7 [DOI] [PubMed] [Google Scholar]
- 4.Slichter SJ, Harker LA: Preparation and storage of platelet concentrates. Transfusion 1976; 16: 8–12 [DOI] [PubMed] [Google Scholar]
- 5.Bynum JA, Meledeo MA, Getz TM, Rodriguez AC, Aden JK, Cap AP, Pidcoke HF: Bioenergetic profiling of platelet mitochondria during storage: 4 degrees C storage extends platelet mitochondrial function and viability. Transfusion 2016; 56 Suppl 1: S76–84 [DOI] [PubMed] [Google Scholar]
- 6.Nair PM, Pandya SG, Dallo SF, Reddoch KM, Montgomery RK, Pidcoke HF, Cap AP, Ramasubramanian AK: Platelets stored at 4 degrees C contribute to superior clot properties compared to current standard-of-care through fibrin-crosslinking. Br J Haematol 2017; 178: 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk CG, Aden JK, Ramasubramanian AK, Cap AP: Hemostatic function of apheresis platelets stored at 4 degrees C and 22 degrees C. Shock 2014; 41 Suppl 1: 54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker GA, Tuccelli M, Kunicki T, Chalos MK, Aster RH: Studies of platelet concentrates stored at 22 C nad 4 C. Transfusion 1973; 13: 61–8 [DOI] [PubMed] [Google Scholar]
- 9.Stolla M, Bailey SL, Fang L, Fitzpatrick L, Gettinger I, Pellham E, Christoffel T: Effects of storage time prolongation on in vivo and in vitro characteristics of 4 degrees C-stored platelets. Transfusion 2020; 60: 613–621 [DOI] [PubMed] [Google Scholar]
- 10.Valeri CR: Circulation and hemostatic effectiveness of platelets stored at 4 C or 22 C: studies in aspirin-treated normal volunteers. Transfusion 1976; 16: 20–3 [DOI] [PubMed] [Google Scholar]
- 11.Strandenes G, Sivertsen J, Bjerkvig CK, Fosse TK, Cap AP, Del Junco DJ, Kristoffersen EK, Haaverstad R, Kvalheim V, Braathen H, Lunde THF, Hervig T, Hufthammer KO, Spinella PC, Apelseth TO: A Pilot Trial of Platelets Stored Cold versus at Room Temperature for Complex Cardiothoracic Surgery. Anesthesiology 2020; 133: 1173–1183 [DOI] [PubMed] [Google Scholar]
- 12.Warner MA, Kurian EB, Hammel SA, van Buskirk CM, Kor DJ, Stubbs JR: Transition from room temperature to cold-stored platelets for the preservation of blood inventories during the COVID-19 pandemic. Transfusion 2021; 61: 72–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood B, Johnson L, Hyland RA, Marks DC: Maximising platelet availability by delaying cold storage. Vox Sang 2018 [DOI] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S: The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–9 [DOI] [PubMed] [Google Scholar]
- 15.Nuttall GA, Oliver WC, Santrach PJ, Bryant S, Dearani JA, Schaff HV, Ereth MH: Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology 2001; 94: 773–81; discussion 5A-6A [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial I: Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018; 72: 2231–2264 [DOI] [PubMed] [Google Scholar]
- 17.Jimenez-Marco T, Castrillo A, Hierro-Riu F, Vicente V, Rivera J: Frozen and cold-stored platelets: reconsidered platelet products. Platelets 2022; 33: 27–34 [DOI] [PubMed] [Google Scholar]
- 18.Stubbs JR, Tran SA, Emery RL, Hammel SA, Haugen AL, Zielinski MD, Zietlow SP, Jenkins D: Cold platelets for trauma-associated bleeding: regulatory approval, accreditation approval, and practice implementation-just the “tip of the iceberg”. Transfusion 2017; 57: 2836–2844 [DOI] [PubMed] [Google Scholar]
- 19.Nair PM, Meledeo MA, Wells AR, Wu X, Bynum JA, Leung KP, Liu B, Cheeniyil A, Ramasubramanian AK, Weisel JW, Cap AP: Cold-stored platelets have better preserved contractile function in comparison with room temperature-stored platelets over 21 days. Transfusion 2021; 61 Suppl 1: S68–S79 [DOI] [PubMed] [Google Scholar]
- 20.Brown BL, Wagner SJ, Hapip CA, Fischer E, Getz TM, Thompson-Montgomery D, Turgeon A: Time from apheresis platelet donation to cold storage: Evaluation of platelet quality and bacterial growth. Transfusion 2022; 62: 439–447 [DOI] [PubMed] [Google Scholar]
- 21.Ketter PM, Kamucheka R, Arulanandam B, Akers K, Cap AP: Platelet enhancement of bacterial growth during room temperature storage: mitigation through refrigeration. Transfusion 2019; 59: 1479–1489 [DOI] [PubMed] [Google Scholar]
- 22.Filip DJ, Aster RH: Relative hemostatic effectiveness of human platelets stored at 4 degrees and 22 degrees C. J Lab Clin Med 1978; 91: 618–24 [PubMed] [Google Scholar]
- 23.Cohn SM, Jimenez JC, Khoury L, Perez JM, Panzo M: Inability to Reverse Aspirin and Clopidogrel-induced Platelet Dysfunction with Platelet Infusion. Cureus 2019; 11: e3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braathen H, Sivertsen J, Lunde THF, Kristoffersen EK, Assmus J, Hervig TA, Strandenes G, Apelseth TO: In vitro quality and platelet function of cold and delayed cold storage of apheresis platelet concentrates in platelet additive solution for 21 days. Transfusion 2019; 59: 2652–2661 [DOI] [PubMed] [Google Scholar]
- 25.Hofmann A, Ozawa S, Shander A: Activity-based cost of platelet transfusions in medical and surgical inpatients at a US hospital. Vox Sang 2021; 116: 998–1004 [DOI] [PubMed] [Google Scholar]
- 26.Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR: Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010; 50: 753–65 [DOI] [PubMed] [Google Scholar]
- 27.Toner RW, Pizzi L, Leas B, Ballas SK, Quigley A, Goldfarb NI: Costs to hospitals of acquiring and processing blood in the US: a survey of hospital-based blood banks and transfusion services. Appl Health Econ Health Policy 2011; 9: 29–37 [DOI] [PubMed] [Google Scholar]
- 28.Agey A, Reddoch-Cardenas K, McIntosh C, Sharma U, Cantu C, Cap A, Bynum J: Effects of Intercept pathogen reduction treatment on extended cold storage of apheresis platelets. Transfusion 2021; 61: 167–177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Consort diagram
Supplemental Figure 2. Treatment allocation over time.
Supplemental Table 1. Unadjusted postoperative transfusion and bleeding outcomes
Supplemental Table 2. Baseline demographic, clinical, and surgical features limited to those with only a single type of platelet transfusion intraoperatively
Supplemental Table 3. Intraoperative transfusion features limited to those with only a single type of platelet transfusion intraoperatively
Supplemental Table 4. Unadjusted clinical outcomes limited to those with only a single type of platelet transfusion intraoperatively
Supplemental Table 5. Adjusted postoperative transfusion outcomes for patients receiving dCSPs vs RTPs, limited to those with only a single type of platelet transfusion intraoperatively
Supplemental Table 6. Adjusted postoperative transfusion outcomes in patients receiving intraoperative dCSPs for each 1-day increase in age of the platelet unit
Data Analysis Plan


