Abstract
Bacterial proteases have extensive applications in various fields of industrial microbiology. In this study, protease-producing organisms were screened on skimmed milk agar media using serial dilution. Through microbial biomass production, biochemical tests, protease-specific activity, and 16 s RNA gene sequencing, the isolates were identified as Bacillus subtilis and submitted to NCBI. The strain accession numbers were designated as A1 (MT903972), A2 (MT903996), A4 (MT904091), and A5 (MT904796). The strain A4 Bacillus subtilis showed highest protease-specific activity as 76,153.84 U/mg. A4 Bacillus subtilis was unaffected by Ca2+, Cu2+, Fe2+, Hg2+, Mg2+, Na, Fe2+, and Zn2+ but was inhibited by 80% by Mn2+ (5 mM). The protease activity was inhibited by up to 30% by iodoacetamide (5 mM). These findings confirm the enzyme to be a cysteine protease which was further confirmed by MALDI-TOF. The identified protease showed 71% sequence similarity with Bacillus subtilis cysteine protease. The crude cysteine protease significantly aided in fabric stain removal when added to a generic detergent. It also aided in the recovery of silver from used X-ray films and de-hairing of goat skin hides and showed decent application in meat tenderization. Thus, the isolated cysteine protease has high potential for industrial applications.
Keywords: Cysteine protease, Skimmed milk agar, Metal ions, Protease inhibitors, MALDI-TOF, Protease industrial applications
Introduction
Proteases are a universal entity of existing life on earth which are found in microbes, plants and animals. Microbial proteases are widely studied and the most important hydrolytic enzymes, which gained the most attention in the past few decades due to their potential applications in various industrial sectors like dairy, pharmacy, textile, detergent, leather, and meat industry [1]. These proteases also play a crucial role in the physiological and metabolic processes of all living organisms including cell growth, differentiation, digestion, high blood pressure regulation, signal transduction, angiogenesis, neurogenesis, fertilization, necrosis, programmed cell death, and many others [2–5]. The most prominent industrial enzymes are proteases that account for about 60% of the entire global enzyme market [6, 7]. Bacterial proteases have more applications compared with animal and fungal proteases. Bacillus proteases have been most widely characterized, and among them bacterial, alkaline and fungal acidic proteases are the major contributors [8].
Among the microbial proteases, Bacillus species are the most widely exploited as industrial enzymes [9, 10] and have been observed to have numerous industrial applications. For example, toxicity and quantity of wastewater effluents from the leather industry can be reduced by keratinolytic protease treatment and for de-hairing also proteases were used [11–13]. In the leather industry, a conventional process is employed, where sodium sulfide, limes, and amides are used to remove goat skin hair, but this process causes a high amount of environmental pollution. Therefore, proteases produced from microbes are good alternatives to these toxic chemicals. For example, a keratinolytic protease from Pseudomonas aeruginosa YK17 is also used as a de-hairing agent and also good alternative to toxic chemicals, which reduces environmental pollution [14].
Silver recovery by traditional methods involves burning used X-ray films which generates a foul smell and complicates the recovery by a lengthy process. Environmental pollution-free processes such as microbiological methods are preferable in this scenario. Recovering silver from used X-rays is a lucrative business around the world, accounting for 20% of the world’s silver needs [15]. Some studies previously explored the potential of microorganisms for silver recovery. For instance, Bacillus subtilis and Aspergillus versicolor protease were used to recover metallic silver from used X-ray films showed appreciable efficiency of recovery [16, 17]. However, the exact mechanism of recovery, efficiency, and cost dynamics is yet to be extensively studied for a wider application to circumvent the practice of chemical recovery.
Proteases have the ability to remove fabric stains in combination with detergents efficiently and thus can be used as additives for detergents [18, 19]. Proteases regulate several metabolic processes such as fibrinolysis, blood coagulation, complement activation, phagocytosis, and blood pressure [20–22]. Industrially, they are employed for applications such as contact lens cleaning [9, 23], biofilm removal [24], isolation of nucleic acids [25, 26], pest control [27], and degumming of silk [28, 29].
As some of the most widely used enzymes, proteases have a higher commercialization rate and research potential. Since bacterial proteases play a major role in diverse industrial sectors, we endeavored to isolate protease-producing bacteria from soil, which is a rich source of such bacterial species. With increasing global demand, proteases have a huge scope for application. Microbial proteases are preferred over plant and animal sources due to their biochemical diversity, ease of production and purification, and the convenience with which the enzymes can be genetically engineered for optimizing productivity. Several Bacillus proteases have been previously studied for their high proteolytic activity and stability. Bacillus species offer the advantage of shorter fermentation durations and low cost of downstream purification.
There are no specific studies on proteases produced by soil bacteria from the local Hyderabad region where this study was carried out. Osmania University has rich microbial diversity due to its urban forest on campus which includes diverse flora and fauna. The goal of the present study therefore is to isolate a protease producing bacterial strain. This is the first report describing protease-producing bacteria isolated from Osmania University. The cysteine protease enzyme screened from simple soil source, showed various industrial eco-friendly and environment friendly applications, and hence can have potential applications in food processing, detergent industry and agriculture.
Materials and methods
Materials
Skimmed milk powder, tryptone, dextrose, yeast extract, agar agar, nutrient agar, L-tyrosine, sodium carbonate, potassium phosphate, trichloroacetic acid (TCA), glacial acetic acid, Tris–HCl, bromophenol blue, commacie brilliant blue, bovine serum albumin (BSA), Cacl2, CuSO4, MnCl2, FeSO4, HgCl2, MgSO4, NaCl, FeCl2, cobalt, and ZnSO4 (Hi-media). Folin & Ciocalteus Phenol (FCP) reagent (SRL), sodium dodecyl sulfate (SDS), acrylamide, bis-acrylamide, ido-acetamide and phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich, USA), ethylene diaminetetraacetic acid (EDTA), dithiothreitol (DTT), and β-mercaptoethanol (β-ME). All other chemicals were analytical grade.
Isolation and selection
The soil sample was taken from the garden soil in Osmania University campus, Hyderabad. The sample was collected from a 15 cm depth of the soil surface, kept in a sterile container, and stored at 4 °C.
Screening of protease-producing bacteria from the soil
To reduce the microbial load, the serial dilution technique was employed. Briefly, 1 g of collected soil was added to the first tube, mixed well, and diluted up to 10−9 dilutions. The diluted sample was cultured on skimmed milk agar plates (skimmed milk powder, 28 g; tryptone, 5 g; yeast extract, 2.5 g; dextrose, 1 g; and agar agar, 15 g dissolved in 1 L distilled water) and incubated at 37 °C for 48 h and observed for microbial growth. Bacterial colonies showing clear zones around the colonies indicating casein hydrolysis were isolated and characterized further. Similar forms of colonies were considered to be the same bacteria, whereas different colony morphologies are exhibited as different microorganisms. The nutrient agar plates were prepared and then streaked with each distinct colony by quadrant streaking. The plates were incubated at 37 °C for 24 h and observed for single isolated pure colonies of protease-producing bacteria obtained from the soil which were further isolated for obtaining pure cultures [30].
Gram staining and biochemical tests: The colonies obtained from soil samples were sub-cultured in nutrient agar broth and streaked on nutrient agar plates to obtain pure culture. The colonies were Gram stained and IMViC tests were performed using HiIMViC™ Biochemical Test Kit according to the manufacturer’s protocol (KB001, HiMedia, India). For catalase test, pure cultures isolated from protease producing colonies were grown on nutrient agar plate for 24 h at 37 °C. After incubation, a loopful of culture was taken on a glass slide in aseptic conditions and mixed with a drop of 3% hydrogen peroxide, and the mixture was observed for effervescence.
Molecular identification of the isolates
16S rRNA gene sequencing and phylogenetic construction
The pure isolates were inoculated on skimmed milk broth (skimmed milk powder, 28 g; tryptone, 5 g; yeast extract, 2.5 g; dextrose, 1 g per 1 L) and incubated at 37 °C for 24 h for isolation of genomic DNA. By using genomic DNA of isolates and 16 s RNA universal primers, PCR amplification was carried out. The primers used were as follows: Universal 16S rRNA bacterial primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1392R (5′-GGTTACCTTGTTACGACTT-3′) were used for amplification. For amplification by PCR, 50 ng of template DNA was used. The PCR was performed in final volume 25 μL, containing 2.5 μL 10 × PCR buffer, 2 μL dNTP mix (2.5 mmol/L), 1.0 μL forward primer (10 μmol/L), 1.0 μL reverse primer (10 μmol/L), 0.25 μL Taq DNA polymerase (5 U/μL), 2 μL extracted DNA (25 ng/μL), and ddH2O to make up the volume, and the products were detected on 1% agarose gel and submitted to Sandor for DNA sequencing. The partial 16 s rRNA sequences of isolates were searched within the Nucleotide BLAST tool, and a phylogenetic tree was constructed by using MEGA X with 1000 replicates of bootstrap values 10. The four isolates were subsequently identified by above method, and their 16 s RNA sequences were submitted in the NCBI database.
Production of protease from isolates
For the production of protease enzyme, 24 h fresh culture of isolated Bacillus species (A1, A2, A3, A4, and A5) were inoculated into a 50 mL medium containing (skimmed milk powder, 28 g; tryptone, 5 g; yeast extract, 2.5 g; dextrose, 1 g; NaCl, 0.5 g per L) and incubated at 37 °C for 48 h. After incubation, the culture supernatant was used as the protease source.
Estimation of protease activity
The protease activity of the isolates was estimated by universal protease activity assay using casein as substrate [31]. Briefly, 5 mL of 0.65% casein w/v as the substrate was added to 1 mL of cell-free culture filtrate and incubated at 37 °C for 10 min. To stop the enzyme reaction, 5 mL of 110 mM trichloroacetic acid (TCA) was added to the reaction mixture, and the samples were incubated at 37 °C for 30 min. After incubation, tyrosine standard dilutions were brought to the volume of 2 mL. To each test sample, 5 mL of 500 mM of Na2CO3 and 1 mL of 0.5 M Folin phenol reagents were added, and the reaction mixture was incubated at 37 °C for 30 min. After 30 min, the protease activity was measured at 660 nm. The sample without any enzyme was used as a control. One unit of protease activity was defined as the amount of enzyme required to liberate 1 µM tyrosine/mL / min. s
Protease activity was determined by the following equation:
Effect of inhibitors and metal ions on protease activity
To identify, the nature of protease belonging to, A4 Bacillus subtilis strain was studied by inhibitory assay. To determine the effect of various protease inhibitors such as PMSF (inhibitor of serine protease) and EDTA (inhibitor of metalloprotease), β-mercaptoethanol, DTT and iodoacetamide, IAA (cysteine protease inhibitor), and also metal ions such as cobalt, HgCl2, NaCl, FeCl2, FeSO4, CuSO4, CaCl2, MgSO4, MnCl2, and ZnCl2 were added to 1 mL of protease enzyme added and incubated at 37 °C 30 min. The final concentration of metal ions and protease inhibitors was 5 mM. After 30 min of incubation, 5 mL of 0.65% casein w/v as the substrate was added to all the tubes, and the reactions were started by placing them at 37 °C, and consequently the protease assay was done as described above. The percent residual enzyme activity was calculated with reference to the activity of the enzyme without these supplements.
Effect of pH and temperature on the activity of the cysteine protease
The effect of pH and temperature on protease activity was studied in the pH range 5 to 12 and temperature range of 30 to 70 °C, with increments of 10 °C. For effect of pH, the cultures were incubated in pH range as mentioned above at 37 °C, and for effect of temperature, the cultures were incubated at pH 7 in the temperature range mentioned as above for a period of 48 h. At the end of incubation, the culture supernatant was taken, and specific activity of the protease was calculated as previously described.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/TOF MS)
The crude protease enzyme was mixed with HCCA (α-cyano-4-hydroxycinnamic acid) matrix (5 mg/mL α-cyano-4-hydroxycinnamic acid in 1:2 ratio of 0.1% TFA and 100% ACN) in 1:1 ratio, and the resulting 2 μL was spotted onto the MALDI plate [MTP 384 ground steel (Bruker Daltonics, Germany)]. After air drying, the sample was analyzed on the MALDI TOF/TOF ULTRAFLEX III instrument (Bruker Daltonics, Germany). External calibration was done with standard peptide (PEPMIX Mixture) supplied by Bruker, with masses ranging from 1046 to 3147 Da. Further analysis was done with FLEX ANALYSIS SOFTWARE (version 3.3) in reflection ion mode with an average of 500 laser shots at mass detection range between 500 and 5000 m/z for obtaining the MS/MS. The masses obtained in the MS/MS were submitted for Mascot search in “Bacillus subtilis” database for identification of the protease and peptide mass figure printing (PMF).
Cysteine protease on blood stain removal
Here, crude protease enzymes were used to remove the cloth’s blood stain. Three sets of clothes were selected for testing. Clean cotton fabric pieces of sized 4 × 4 cm were stained with blood and were allowed to dry. After 60 min of incubation, one set of fabric cloth was kept as a control without any treatment, the next piece was washed with distilled water to remove the blood stain, and then crude protease was used to remove the blood stain.
Washing performance of protease from Bacillus species in combination with a commercial detergent
The enzyme compatibility of crude protease obtained from Bacillus subtilis was investigated experimentally with solid detergents like Tide, Ariel, and Rin-Shakti. The detergents were diluted in tap water to 10 mg/mL concentration and incubated at 65 °C for 1 h to inactivate the endogenous enzymes present in these detergents, before the addition of crude protease enzyme. The small white cloth pieces (4 × 4 cm) were stained with Red label Tea (Brooke Bond, India) and air dried for 2 days. The stained clothes were incubated in 100 mL distilled water (control), and later 1 mL of 10 mg/mL commercial detergent (Tide) was added to a beaker containing 100 mL of distilled water with our without 1 mL protease (76,000 U/mL). The flasks were incubated at 50 °C for 1 h to determine the residual activity of crude protease and to study the enzyme compatibility.
Recovery of silver from used X-ray films
X-ray films (made into 33 cm in size) were first washed with distilled water followed by cotton immersed with ethanol and then dried at 37 °C for 30 min. After incubation, X-ray films were treated with 3 mL of crude protease enzyme and kept them at 37 °C under continuous shaking until the silver is removed from the X-ray films which can be observed in the form of turbidity of the solution.
Enzymatic action of cysteine protease on goat skin hide
The sample of goat skin was brought from the nearest slaughterhouse and used for the de-hairing of hides using the cysteine protease. Goat skin was immersed with 10 mL of protease enzyme and incubated at 37 °C for 48 h. After incubation, goat skin was gently washed under tap water and observed for the efficiency of hair-removal from the skin.
Meat tenderization: Warner–Bratzler shear force
Fresh Buffalo meat cut from longissimus thoracis et lumborum (near the lumbar vertebrae) from a nearby slaughterhouse in Hyderabad was collected. This meat is suitable for tenderization because of its uniform muscle. The meat sample was treated with cysteine protease, raw papaya extract, and distilled water for 24 h. After treatment, the samples were washed with distilled water to remove excess enzyme and cooked at 100 °C for 30 min. The cooked samples were stored at 4 °C overnight and measured for tenderness after warmed to room temperature through the Warner–Bratzler shear force (WBSF). Samples were measured in sizes with fibers perpendicular to the direction of the blade (Model no.81031307, GR Elect. Mfg. Co., USA). The shear force required for the samples was recorded in N/Cm2 [32].
Results
Identification and screening of microorganisms
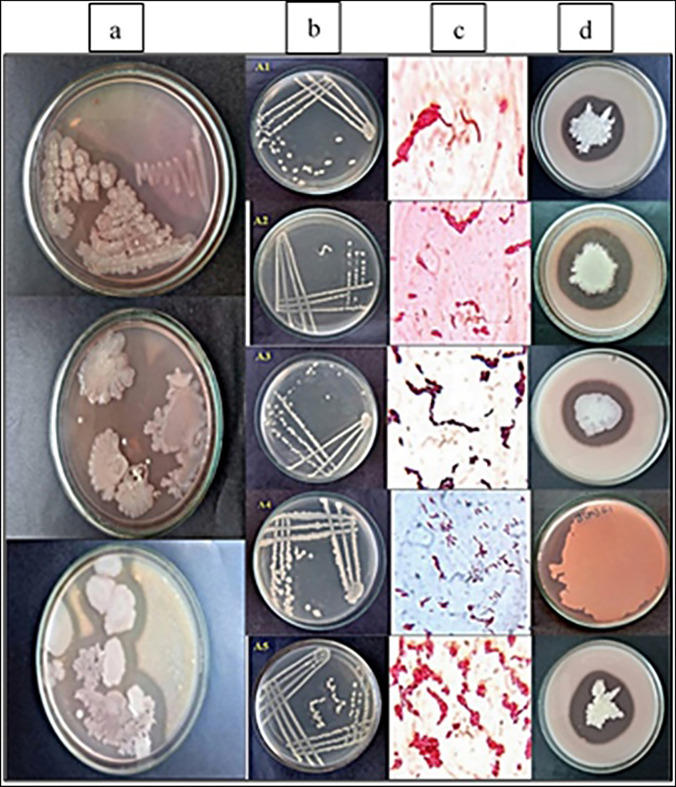
A local soil sample in Hyderabad was used to screen the protease-producing organisms. Five isolated colonies were selected according to the clear zone of hydrolysis of casein in skimmed milk plates (Fig. 1). From the morphological observation, physiological characteristics, the arrangement, color of colonies in nutrient agar, and based on Gram staining and biochemical tests (IMViC tests), the microorganisms were identified as Gram-positive bacilli, which were catalase positive. These five isolates were named as A1, A2, A3, A4, and A5 and were further used for protease enzyme production through the submerged fermentation process.
Fig. 1 .
Screening and identification of protease-producing bacterial strains (A1–A5) from soil. a Primary screening of protease-producing microorganisms on skimmed milk agar media. b Secondary screening of protease-producing organisms on nutrient agar plates by streak plate method to obtain isolated colonies. c Gram stained images of isolates. d Zone of hydrolysis of protease-producing bacterial isolates on skimmed milk agar plates showing zone of proteolysis
16S rRNA sequencing and phylogenetic studies
The phylogenetic tree of 16S rRNA sequences was constructed by using the ten valid representative species of the genus Bacillus to know the relationship of the strains A1, A2, A4, and A5. The BLAST result showed that they were close match in the neighbor-joining tree (Fig. 2). The four isolates were identified as Bacillus subtilis and submitted to NCBI, designated as Bacillus subtilis A1 (MT903972), Bacillus subtilis A2 (MT903996), Bacillus subtilis A4 (MT904091), and Bacillus subtilis A5 (MT904796) which have the closest sequence similarity of 99%.
Fig. 2.
16S rRNA gene sequencing and phylogenetic analysis. Phylogenetic tree constructed from 16 s rRNA gene sequencing of Bacillus subtilis using a maximum likelihood method
Enzyme production and activity
The growth pattern and enzyme production of the isolated Bacillus strains were observed by cultivation in skimmed milk broth at 37 °C (pH 8) after 48 h. The protease-specific activity of all isolates A1–A5 is shown in Table 1. The supernatant containing extracellular protease showed specific activity of A1, A2, A3, A4, and A5 strains as 32,694.44 U/mg, 36,666.66 U/mg, 13,605.26U/mg, 76,153.84 U/mg, and 44,687.50 U/mg protein, respectively. Bacillus subtilis A4 (MT904091) isolate showed the highest specific activity, and therefore, it was used for further characterization of the secreted protease.
Table 1.
Specific activity of cysteine protease produced by bacterial isolates A1, A2,A3,A4 and A5
| Isolates (Crude Enzyme) |
Total Activity (U/ml) |
Protein concentration (mg/ml) | Specific Activity (U/mg) |
|---|---|---|---|
| A1 | 5885 | 0.18 | 32,694.44 |
| A2 | 6600 | 0.18 | 36,666.66 |
| A3 | 2585 | 0.19 | 13,605.26 |
| A4 | 9900 | 0.13 | 76,153.84 |
| A5 | 7150 | 0.16 | 44,687.50 |
*data represented is the mean of three replicates
Effect of metal ions and protease inhibitors
The protease secreted by Bacillus subtilis A4 strain was studied for its activity in presence of metals ions, and it was observed to be unaffected by Ca2+, Cu2+, Fe2+, Hg2+, Mg2+, Na+, and Zn2+ but was inhibited by almost 80% by Mn2+ (5 mM). PMSF and EDTA did not affect the activity of the protease, whereas its activity was enhanced by 40% and 73% in the presence of β-ME and DTT (5 mM). The protease was inhibited by up to 30% by iodoacetamide (5 mM) (Table 2). These observations confirm the enzyme to be a cysteine protease.
Table 2.
Effect of Metal Ions and Inhibitors on the Cysteine Protease secreted by Bacillus subtilis A4 strain
| S.nO | Name | Relative activity (%) |
|---|---|---|
| 1 | CaCl2 | 100 |
| 2 | CuSO4 | 105.63 |
| 3 | FeSO4 | 104.22 |
| 4 | HgCl2 | 90 |
| 5 | MnCl2 | 18.30 |
| 6 | MgSO4 | 111.26 |
| 7 | Cobalt | 100 |
| 8 | Nacl | 105 |
| 9 | FeCl2 | 102.81 |
| 10 | ZnSO4 | 97 |
| 11 | A4 Protease (Control) | 100% |
| 12 | PMSF | 100% |
| 13 | EDTA | 100% |
| 14 | DTT | 172.53% |
| 15 | Beta-mercaptoethanol | 139.43% |
| 16 | Iodoacetamide | 69.62% |
*data represented is the mean of three replicates
Effect of pH and temperature on cysteine protease enzyme
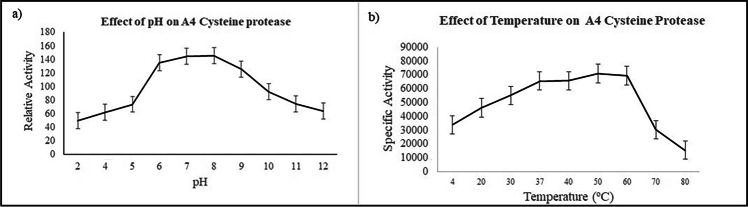
After incubation of B. subtilis culture at various temperatures, the culture supernatant was utilized to measure the specific activity of the secreted cysteine protease. The specific activity of the enzyme did not deviate significantly between 30 and 60 °C after which the activity plunged drastically, indicating resilience of the enzyme in the above range and its thermo-tolerant nature (Fig. 3). The cysteine protease also showed high specific activity in a broad pH range of 6–9, with an optimum pH value at 7.5. The enzyme hence has good pH stability as it shows tolerance to changes in pH and temperature. This is an advantageous property during fermentation wherein side products and growth conditions lead to changes in pH and temperature of the medium. Hence, the enzyme can have potential application on an industrial scale.
Fig. 3.
Effect of pH and temperature on A4 cysteine protease enzyme. a Effect of protease activity at different pH ranging from pH 2 to pH 12. b Effect of protease activity at different temperatures ranging from 4 to 80 °C
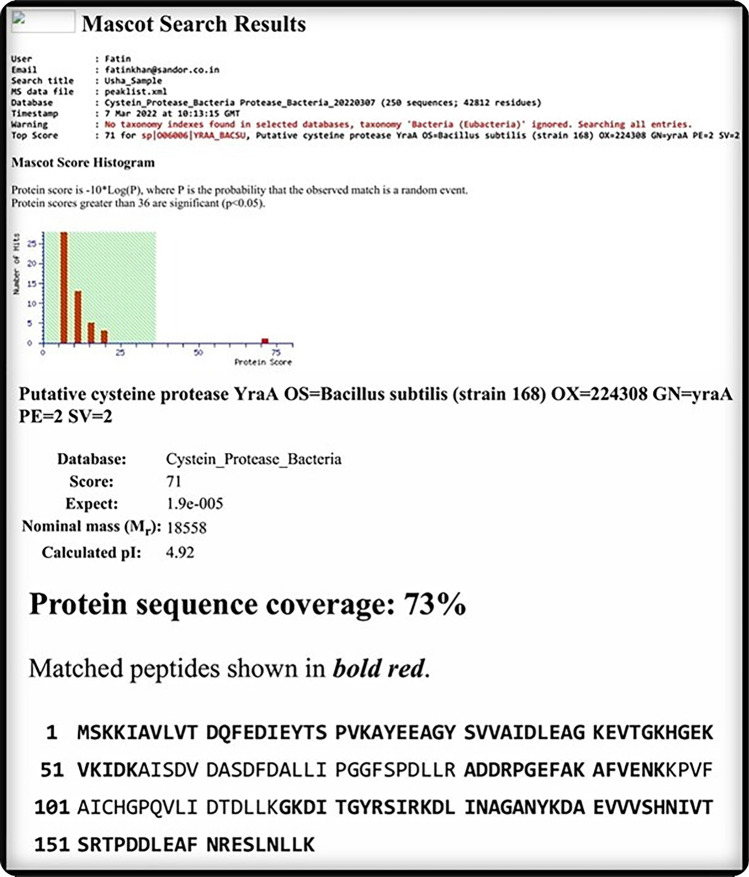
Protease identification by MALDI-TOF and PMF
MALDI-TOF-based identification and analysis by Mascot search for the protease from Bacillus subtilis A4 strain revealed the protease to have ~ 73% protein sequence coverage with cysteine protease (Fig. 4).
Fig. 4.
MALDI-TOF and peptide mass fingerprinting (PMF) identification of cysteine pProtease isolated from Bacillus subtilis. MASCOT search followed by PMF analysis showed the matched amino acid residues (in bold) of the peptide fragments with the putative cysteine protease of Bacillus subtilis
Application of cysteine proteases
Application of cysteine protease in fabric stain removal
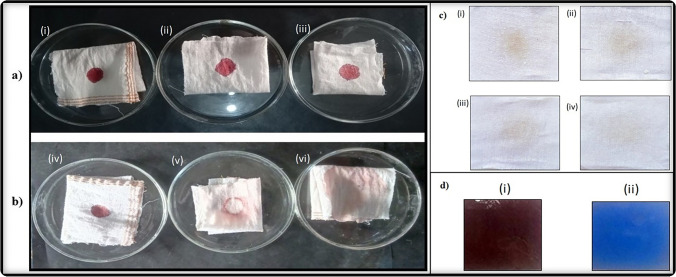
The spent bacterial culture medium containing cysteine protease was investigated for its application in stain removal. A 3 × 3 cm white cotton cloth sample was stained in the center with sheep blood obtained from a local abattoir and allowed to dry for 30 min. After drying, distilled water and the crude protease enzyme was used for removing the blood stain from the cloth. After treatment with protease enzyme, the cloth samples were washed and dried and observed that the blood stain was removed within a few minutes (Fig. 5a). The results revealed that blood stains were completely removed with the crude enzyme extract within 3 min, and no stains were removed when distilled water or plain culture medium were used.
Fig. 5.
Blood stain removal, detergent activity, and silver recovery from used X-ray films with B. subtilis cysteine protease. a Blood stain removal: sterile cotton cloth was stained with blood and dried. First blood stain – untreated (i, iv), second stain was washed with distilled water (ii, v), and the third blood stain was treated with 200–500 µl of Bacillus subtilis cysteine protease for 30 s (iii, vi). The first row of images represents before treatment (i, ii, iii), and the second row represents images post treatment with distilled water or protease. b Washing performance of protease from Bacillus subtilis in combination with commercial detergent. c Decomposition of gelatin layer of X-ray film by protease
In another set, the cotton fabrics were stained with Red Label Tea (Brooke bond, India) and air dried for two days. The stained clothes after de-staining with a mixture of detergent and protease revealed that the protease plus detergent combination clears the stain much more effectively compared with detergent or protease alone, indicating that the cysteine protease has significant stain removing abilities and hence can be effectively used in the detergent industry for enhanced stain removal.
Recovery of silver halides by degradation of gelatin from X-ray films
Upon further investigation, the cysteine protease from spent skimmed milk broth culture of B. subtilis A4 was utilized for the regeneration of silver halide from used or waste X-ray films. The cysteine protease was observed to completely decompose the gelatinous coating on the X-ray film at 37 °C within 20 min of incubation (Fig. 5d-ii). This indicates that the crude cysteine protease from the B. subtilis (A4) has a high capability of recovering of silver from the used X-ray films.
The enzymatic action of cysteine protease on de-haring of goat skin hide
The sample of goat skin was brought from the nearest slaughterhouse and used for de-hairing of hides using B. subtilis cysteine protease. After 48 h of treatment with cysteine protease, it was noticed that goat skin hair was very easily removed without much damage to the skin (Fig. 6c). This indicates that this cysteine protease can be a good source for removing skin hides and can be used in leather industries.
Fig. 6.
De-hairing of the goat skin and meat tenderization by B. subtilis cysteine protease. Enzymatic de-hairing of goat skin: a skin sample treated with skimmed milk broth (control), b skin sample treated with distilled water, c skin sample treated with protease enzyme. Meat tenderization: scanning electron micrographs (SEM) of cooked intra-muscular tissue of buffalo meat. d Untreated, e protease-treated sample, and f raw papaya extract treated sample. Scale bars are adjusted to 10 µm per unit
Meat tenderization using cysteine protease
To check for the meat tenderization properties of the cysteine protease isolated, analysis of the shear force values (N/cm2) in buffalo meat samples was calculated after treatment with the protease. The shear force values were much lower compared with untreated (control, 27), samples treated with the raw papaya extract (24.03), and samples treated with protease (17.05), respectively, indicating that the sample treated with protease were significantly more tender. The breakdown of proteins was clearly visible in protease treated samples indicating prominent proteolysis of muscle proteins due to the degradation of myosin which can be observed in the scanning electron micrographs (Fig. 6d, e, and f). Higher shear force values and scanning electron micrographs clearly indicate that the isolated bacterial cysteine protease can be used as a good meat tenderizing agent instead of papain.
Discussion
The soil ecosystem is highly diverse and is capable of producing various nutrients and resources for the development of living microorganisms [33]. The genus Bacillus is one of the most predominant soil bacteria [34] which are the most significant group of protease producers. Bacillus produces a wide variety of extra-cellular proteases. An abundant amount of proteases can be produced from B. sterothermophilus, B. mojavensis, B. megaterium, B. cereus, and B. subtilis and are used for various industrial applications [35]. B. subtilis is the most extensively used organism for the production of various proteases such as serine, cysteine, aspartic, and metalloproteases [36]. Bacterial proteases are essential hydrolytic enzymes with a wide range of industrial and medical applications [8, 37]. Cysteine proteases are less prevalent in bacteria, although their presence in prokaryotes is as common as in eukaryotes [38]. Cysteine proteases are present in a wide range of organisms, from bacteria to mammals. They play a crucial role in diverse biological processes such as apoptosis, inflammation, and pathogenesis. Additionally, they are becoming increasingly valuable in various industrial applications due to their unique properties, high proteolytic activity along with temperature and pH stability, and broad substrate specificity.
In the present study, we isolated and identified a protease-secreting Bacillus subtilis strain with very high protease activity (76,153.84 U/mg) (Table 1), when compared with previous strains of Bacillus subtilis proteases (Table 3). After identification of its protease activity using various metal ions and salts (Table 2) and MALDI-TOF analysis (Fig. 3), the protease was identified as cysteine protease. Cysteine proteases show a similar function to that of pepsin in gastric juices and are most widely studied proteolytic enzymes [39]. They possess enormous biological value and can be found in all prokaryotes, eukaryotes, and also viruses. The crude cysteine protease showed high specific activity in a pH range from pH 6–9 which is similar to several previous studies [40, 41].
Table 3.
An overview of Cysteine Proteases isolated by previous groups when compared with Bacillus subtilis A4 Cysteine Protease
| S.No | Organism Name | Molecular weight | Protease type | Specific activity | Reference |
|---|---|---|---|---|---|
| 1 | B. subtilis I-2 | 42, 48 and 60 kDa | fibrinolytic protease | (797.28 U/mL) | Bijender Kumar Bajaj et al., Braz. Arch. Biol. Technol. v.57 n.5: pp. 653–662, Sept/Oct 2014 |
| 2 | Bacillus subtilis P13 |
∼66, 43 and 20 kDa… ∼31 kDa protease |
Keratinolytic serine protease |
Pillai, Priya, et al., Process Biochemistry 46 (2011) 1110–1117 |
|
| 3 | B. subtilis Y-108 | 44 kDa | neutral protease | 20.2 U/ml | Jen-Kuo Yang et al., Enzyme and Microbial Technology 26 (2000) 406–413 |
| 4 | Bacillus subtilis KIBGE HAS | 56 kDa | α-amylase | 13,011 U/mg | Saeeda Bano et al., American Association of Pharmaceutical Scientists, Vol. 12, No. 1, March 2011 |
| 5 | Bacillus sp. IMD 434 | 69.2 kDa | α-amylase | Lynn M. Hamilton et al., Biotechnology Letters 21: 111–115, 1999 | |
| 6 | Bacillus subtilis KS-1 | 25.4 kDa | keratinolytic enzyme | 538.2 units/mg | Hyung Joo Suh et al., Journal of Protein Chemistry, Vol. 20, No. 2, 2001 |
| 7 | Bacillus sp. SSR1 | 29 and 35 kDa | serine alkaline protease | 2865 U/mg | J. Singh, N et al., Process Biochemistry 36 (2001) 781–785 |
| 8 | Bacillus subtilis RD7 | 43 kDa | serine alkaline protease | 6.24 U/mg | Yewande Suberu et al., Biocatalysis and Agricultural Biotechnology 20 (2019) 101,264 |
| 9 | Bacillus subtilis QK02 | 42 kDa and 28 k Da | Two fibrinolytic enzymes (QK-1 and QK-2) | Ju Ho Ko et al., Comparative Biochemistry and Physiology Part C 137 (2004) 65–74 | |
| 10 | Bacillus subtilis YY88 | 28,500 and 39,500 | Membrane-bound proteases | 359 U/mg | PEKKA MANTSALA et al., JOURNAL OF BACTERIOLOGY, Feb. 1980, p. 493–501 |
| 11 | Bacillus subtilis A4 (MT904091) | 20 kDa | Cysteine Protease | 76,153.84 U/mg | Present study |
In our study, the spent media containing secreted cysteine protease was studied for various industrial applications. The protease could effectively remove fabric blood stains within 30 s, which is much faster compared with previous studies, such as the one by S.K. Marathe et al., who demonstrated the de-staining activity of their protease in removing the blood stain from the cotton fabric [42]. Microbial enzymes occupy nearly one-third of the commercial enzymes used as key ingredients in detergent making. Amylases and proteases have extensive applications in detergent manufacturing process for their efficiency in removal of hard to remove protein and fat stains. Proteases form amphipathic structures when combined with detergents and help in breaking down protein and fat stains in fabrics. Previously, several microbial proteases such as serine alkaline protease from Micromonospora chaiyaphumensis which is extremely thermotolerant and an intracellular serine protease from Bacillus megaterium were shown to have efficient stain removal properties [43]. Properties of thermotolerance and pH stability make proteases such as the cysteine protease isolated in this study into an ideal fit for applications in detergent manufacturing. The cysteine protease isolated in the present study showed much higher specific activity compared with these microbial proteases and also showed faster rates of stain removal from fabrics (Table 3), indicating that the protease can significantly increase the efficiency of a detergent in fabric stain removal and therefore can be used as a detergent additive.
With recent technological advancements, filmless X-ray image development has overtaken traditional method of developing the films in the dark room. However, the traditionally developed films occupy a very large amount of space in film libraries and databanks. Several medical practices also still use the traditional X-ray films as the conversion to filmless system is expensive and in many cases not justifiable. Previously, X-ray films would be incinerated to recover the silver from the ash which would require several stages for further purification. This is an environmentally harmful and inefficient method. Hence, biological methods of silver recovery have attractive industrial applications as it is eco-friendly and cost-effective. In this context, we checked for the application of the B. subtilis A4 cysteine protease in silver recovery from waste X-ray films. Some previous reports have revealed that complete recovery of silver from X-ray films can take 30 min or mor, whereas in our study, we were able to recover it within 15 min [17] (Fig. 5d-ii). Proteases act by degrading the gelatin layer of the x-ray film which helps in recovery of the silver halides, which can be further purified in simple steps. It is possible that since the activity of the cysteine protease was quite high, it can concurrently hydrolyze the gelatin rapidly to help with the recovery of silver halides.
In addition to these applications, cysteine proteases also showed good de-hairing properties when tested on goat skin, as protease-treated goat skin hides could be easily de-haired, suggesting potential application for the enzyme in the leather industry. With growing consumption of meat with increasing population around the world, the opportunity for leather industry to capitalize on the availability of animal skin has significant economic implications. Eco-friendly de-hairing using microbial proteases has the advantage of elimination of chemical pollutants such as sodium sulfide which is an important effluent released during conventional methods of de-hairing [43]. Previously, several fungal proteases have been isolated from the soil of leather and hair dumping areas. These enzymes were found to be useful in depilation of goat skin. Proteases from actinomycetes such as Streptomyces nogalator and Streptomyces sp. DP2 showed high activity with resilient characters of stability at pH 8 and high temperatures of 90 °C [43, 44]. These enzymes show good depilation properties, although the cysteine protease isolated in the study shows much higher activity with resilience comparable with these proteases and hence has several advantages of high specific activity, thermotolerance, and pH stability.
Tenderness is an important aspect of meat which makes it more palatable and appealing for consumption. A cysteine protease calpain 1 (calcium dependant) is an important enzyme utilized in softening of meat. Papain has been utilized since centuries in softening the meat and recent findings show that Rmpro-A from Rhizomucor miehei exhibits tenderization much better than papain at even lower concentrations [45]. Another cysteine protease Chinese kiwifruit and a newly studied proline iminopeptidase from lactic acid bacteria have been demonstrated to effectively tenderize cattle meat, pork, etc. Recently, plant-derived proteases from Cucumis trigonus and Zingiber officinale have been shown to have better tenderizing capacity for buffalo meat than papain [43]. Interestingly, the cysteine protease isolated from B. subtilis A4 shown in this study could also tenderize buffalo meat without affecting its consistency. Further investigation could lead to its application for consumption. Since B. subtilis is a non-pathogenic species and the cysteine protease like any protein or enzyme can be denatured by boiling or cooking, it could be safe for application in food industry, if the texture the treated meat shows good palatability. From our observations, the protease treated and cooked meat showed clear muscle protein breakdown, higher than papain treated meat samples.
Considering the diverse applications of the cysteine protease in the present study, it can be stated that the presented work on the given cysteine protease has several industrial applications, especially due to its high activity and various applications that suggest its economic and eco-friendly importance.
Conclusion
In conclusion, cysteine proteases are versatile enzymes with wide range of industrial applications. Their stability and specificity make them an attractive alternative to other enzymes. We have isolated a thermo-tolerant and pH tolerant cysteine protease from Bacillus subtilis A4 with high activity and applicability. The protease has multiple applications such as good stain removal properties and hence has the potential to be added to generic detergents and can recover silver from discarded X-ray films. The cysteine protease was effective in aiding the de-hairing of goat skin hides and the shear force values of Buffalo meat reveal that it can be used as a meat tenderizing agent. These findings suggest that this cysteine protease of A4 Bacillus subtilis has potential applications in both industrial and environmental sectors and can function as an eco-friendly microbial cysteine protease.
Funding
This work has been partially funded by OU-DST-PURSE intramural grant and RUSA 2.0.
Data availability
The data that support the findings of this study are available on request from the corresponding author, SB.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Responsible Editor: Gisele Monteiro
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paula S, Mona A, Carolina C, et al. A biotechnology perspective of fungal proteases. Braz J Microbiol. 2015;46(2):337–346. doi: 10.1590/2FS1517-838246220140359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett AJ, Rawlings ND, O’Brien EA. The MEROPS database as a protease information system. J struct boil. 2001;134(2–3):95–102. doi: 10.1006/jsbi.2000.4332. [DOI] [PubMed] [Google Scholar]
- 3.Asha B, Palaniswamy M. Optimization of alkaline protease production by Bacillus cereus FT 1 isolated from soil. J Appl Pharm Sci. 2018;8(02):119–127. doi: 10.7324/JAPS.2018.8219. [DOI] [Google Scholar]
- 4.Theron W, Divol B. Microbial aspartic proteases: current and potential applications in industry. Appl Microbiol Biotechnol. 2014;98:8853–8868. doi: 10.1007/s00253-014-6035-6. [DOI] [PubMed] [Google Scholar]
- 5.Satbir S, Bijender K. Potential application spectrum of microbial proteases for clean and green industrial production. Energ. Ecol. Environ. 2017;2(6):370–386. doi: 10.1007/s40974-017-0076-5#citeas. [DOI] [Google Scholar]
- 6.Yogesh P, Akshaya G, Shilpa G. Production, partial purification, characterization and detergent compatibility of alkaline protease from soil isolate Bacillus cereus AG1. Int J Curr Microbiol App Sci. 2018;7(8):587–600. doi: 10.20546/ijcmas.2018.708.064. [DOI] [Google Scholar]
- 7.Vijitra L, Yotchaisarn M, Saengha W, et al. Protease-producing bacteria from soil in Nasinuan community forest, Mahasarakham Province Thailand. Biomed Pharmacol. 2019;12(2):587–595. doi: 10.13005/bpj/1678. [DOI] [Google Scholar]
- 8.Preeti S, Chayanika P, Anil K, et al. Microbial proteases: ubiquitous enzymes with innumerable uses. 3 Biotech. 2021;11:428. doi: 10.1007/s13205-021-02928-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iuliia D, Margarita S. The practical potential of Bacilli and their enzymes for industrial production. Front Microbiol. 2020;11:1782. doi: 10.3389/fmicb.2020.01782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabiano J, de Melo Ricardo R, Sato Helia H. An overview of Bacillus proteases: from production to application. Crit Rev Biotechnol. 2017;38(3):1549–780. doi: 10.1080/07388551.2017.1354354. [DOI] [PubMed] [Google Scholar]
- 11.Zhen F, Yang-C JZ, et al. Keratinolytic protease: a green biocatalyst for leather industry. Appl Microbiol Biotechnol. 2017;101:7771–7779. doi: 10.1007/s00253-017-8484-1. [DOI] [PubMed] [Google Scholar]
- 12.Yuhong H, Mateusz Ł, Alexander H, et al. Novel keratinolytic enzymes, discovered from a talented and efficient bacterial keratin degrader. Sci Rep. 2020;10:10033. doi: 10.1038/s41598-020-66792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renata B, Violeta V, Virgilijus V. Enzymes for leather processing: effect on pickling and chroming. Materials. 2021;14(6):1480. doi: 10.3390/2Fma14061480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeasmin A, Javed F, Aa H, et al. Keratinolytic protease from Pseudomonas aeruginosa for leather skin processing. JGEB. 2021;19:53. doi: 10.1186/s43141-021-00149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayant P, Pallavi S, Il Patil, et al. Extraction of silver from waste x-ray films using protease enzyme. IJABR. 2015;6(2):220–226. [Google Scholar]
- 16.Vaishali C. Recovery of silver from used x-ray films by Aspergillus versicolor protease. JAIR. 2013;2(1):2278–5213. [Google Scholar]
- 17.Amira H, Eida M. Recovery of silver from used x-ray film using alkaline protease from Bacillus subtilis sub sp. Subtilis Afr J Biotechnol. 2016;15(26):1413–1416. doi: 10.5897/AJB2016.15340. [DOI] [Google Scholar]
- 18.Ibrahim A, Elbadawi Y, Mohamed Al, et al. Alkaline serine protease from the new halotolerant alkaliphilic Salipaludibacillus agaradhaerens strain AK-R: purifcation and properties. Biotech. 2019;9(11):391. doi: 10.1007/2Fs13205-019-1928-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamilvendan M, Arulmani M, Rl S, et al. Identification of a novel thermostable alkaline protease from Bacillus megaterium-TK1 for the detergent and leather industry. Biology. 2020;9:472. doi: 10.3390/biology9120472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuanliang H, Dan Y, Wang Z, et al. Purification and characterization of a novel, highly potent fbrinolytic enzyme from Bacillus subtilis DC27 screened from Douchi, a traditional Chinese fermented soybean food. Nature- Sci Rep. 2019;9:9235. doi: 10.1038/s41598-019-45686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farwa A, Shourong W, Kasim V. Role of fibrinolytic enzymes in anti-thrombosis therapy. Front Mol Biosci. 2021;8:680397. doi: 10.3389/fmolb.2021.680397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fathma S, Elisabeth K, Pl N, et al. Fibrinolytic bacteria of Indonesian fermented soybean: preliminary study on enzyme activity and protein profile, Food Sci. Technol, Campinas. 2020;40(2):458–465. doi: 10.1590/fst.23919. [DOI] [Google Scholar]
- 23.Rasika P, Vasudeo Z, Siddhivinayak B, et al. Application of protease isolated from Bacillus sp 158 in enzymatic cleansing of contact lenses. Biotechnol J. 2009;8(2):276–280. doi: 10.3923/biotech.2009.276.280. [DOI] [Google Scholar]
- 24.Olga M, Ayslu M, Vladimir E et all (2017) Effects of Bacillus serine proteases on the bacterial biofilms. BioMed Res Int 8525912. 10.1155/2017/8525912 [DOI] [PMC free article] [PubMed]
- 25.Janos A, Ferenc T, Jozsef T. Research applications of proteolytic enzymes in molecular biology. Biomolecules. 2013;3:923–942. doi: 10.3390/biom3040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasir A, Rita D, Alexandre D et all (2017) Current Nucleic Acid Extraction Methods and Their Implications to Point-of-Care Diagnostics. Biomed Res Int 2017:9306564. 10.1155/2017/9306564 [DOI] [PMC free article] [PubMed]
- 27.Swati J, Satyanarayana T. Characteristics and applications of a recombinant alkaline serine protease from a novel bacterium Bacillus lehensis. Bioresour Technol. 2013;131:76–85. doi: 10.1016/j.biortech.2012.12.124. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Kwon MiYeon, Kim S. Biological degumming of silk fabrics with proteolytic enzymes. J Nat Fibers. 2016;13(6):629–639. doi: 10.1080/15440478.2015.1093578. [DOI] [Google Scholar]
- 29.Lei Z, Junxiong LP, et al. Recent advances in environmentally friendly and green degumming processes of silk for textile and non-textile applications. Polymers. 2022;14:659. doi: 10.3390/polym14040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sneha U, Poornima R, Sridhar S. Optimization and decolorization of malachite green using Pseudomonas putida. J Chem Pharm Res. 2014;6(12):50–57. [Google Scholar]
- 31.Anson ML. The estimation of pepsin, trypsin, papain, and cathepsin with haemoglobin. J Gen Physiol. 1938;22(1):79–89. doi: 10.1085/jgp.22.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandran M, Getachew G, Mesfin T. Isolation, screening, characterization, and identification of alkaline protease producing bacteria from leather industry effluent. Ann Microbiology. 2021;71:24. doi: 10.1186/s13213-021-01631-x. [DOI] [Google Scholar]
- 33.Naveena BM, Mendiratta SK, Anjaneyulu ASR. Tenderization of buffalo meat using plant proteases from Cucumis trigonus Roxb (Kachri) and Zingiber officinale roscoe (Ginger rhizome) Meat Sci. 2004;68(3):363–369. doi: 10.1016/j.meatsci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Furtak K, Gajda AM (2018) Activity and Variety of Soil Microorganisms Depending on the Diversity of the Soil Tillage System [Internet]. Sustainability of Agroecosystems. InTech 2018. Available from: 10.5772/intechopen.72966
- 35.Saxena A, Kumar M, Chakdar H, et al. Bacillus species in soil as a natural resource for plant health and nutrition. J Appl Microbiol. 2019;128(6):1583–1594. doi: 10.1111/jam.14506. [DOI] [PubMed] [Google Scholar]
- 36.Prashant S, Shilpa A, Kiransinh N et al (2020) Thermostable alkaline proteases from bacteria: A review. NCIBS 978(93):5407–322-9. 10.2139/ssrn.3562387
- 37.Harwood CR, Yoshimi K. The ins and outs of Bacillus proteases: activities, functions and commercial significance. FEMS Microbiol Rev. 2022;46(1):1–20. doi: 10.1093/femsre/fuab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayuri S, Yogesh G, Shalini A, et al. A review on microbial alkaline protease: an essential tool for various industrial approaches. Ind Biotechnol. 2019;15(2):69–78. doi: 10.1089/ind.2018.0032. [DOI] [Google Scholar]
- 39.Rayan S, Elham M, Saeed A. Cohnella 1759 cysteine protease shows significant long term half-life and impressive increased activity in presence of some chemical reagents. Sci Rep. 2021;11:4573. doi: 10.1038/2Fs41598-021-84267-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juan L, Anupama S, Marie J, et al. Papain-like cysteine proteases in Carica papaya: lineage-specific gene duplication and expansion. BMC Genomics. 2018;19:26. doi: 10.1186/s12864-017-4394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leila K, Mohammad Y, Mehdi I. The effects of pH and temperature on cysteine protease (cathepsin B) activity in miracidia and eggs of Fasciola hepatica. Iran J Parasitol. 2020;15(2):233–239. [PMC free article] [PubMed] [Google Scholar]
- 42.Sarika M, Manisha A, Aishwarya P. Isolation, partial purification, biochemical characterization and detergent compatibility of alkaline protease produced by Bacillus subtilis, Alcaligenes faecalis and Pseudomonas aeruginosa obtained from sea water samples. JGEB. 2018;16(1):39–46. doi: 10.1016/j.jgeb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurumallesh P, Alagu K, et al. A systematic reconsideration on proteases. Int J of Biol Macromol. 2019;128:254–267. doi: 10.1016/j.ijbiomac.2019.01.081. [DOI] [PubMed] [Google Scholar]
- 44.Bajaj BK, Sharma P. An alkali-thermotolerant extracellular protease from a newly isolated Streptomyces sp. DP2. New Biotech. 2011;28(6):725–732. doi: 10.1016/j.nbt.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 45.James David Morton, Zuhaib Fayaz Bhat, Alaa El-Din Ahmed Bekhit (2019) Proteases and meat tenderization. Encyof Food Chem 2:309–313. 10.1016/B978-0-08-100596-5.21663-6.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, SB.