Abstract
目的
丹参酮ⅡA具有广泛的心肌保护作用。AK003290是1种在心肌组织中高表达的长链非编码RNA(long noncoding RNA,lncRNA),在心肌发生损伤时表达下调。本研究旨在探讨丹参酮ⅡA减轻氧糖剥夺(oxygen glucose deprivation,OGD)诱导心肌细胞损伤的机制。
方法
以H9C2大鼠心肌细胞为研究对象,构建OGD损伤模型。采用转染siRNA的方法敲低AK003290的表达。将H9C2细胞分为6组:对照(control)组、丹参酮ⅡA(TAN)组、OGD组、丹参酮ⅡA+OGD(TAN+OGD)组、scrambled siRNA转染+丹参酮ⅡA+OGD(scrambled siRNA+TAN+OGD)组、AK003290 siRNA转染+丹参酮ⅡA+OGD(AK003290 siRNA+TAN+OGD)组。TAN组只用40 μmol/L的丹参酮ⅡA预处理细胞12 h。OGD组只进行OGD处理12 h。TAN+OGD组先用40 μmol/L的丹参酮ⅡA预处理12 h,再行OGD处理12 h。Scrambled siRNA+TAN+OGD组和AK003290 siRNA+TAN+OGD组在转染相应siRNA 24 h后进行后续处理。采用实时反转录PCR(real-time reverse transcription PCR,real-time RT-PCR)检测AK003290的表达,分光光度法检测细胞培养液中乳酸脱氢酶(lactate dehydrogenase,LDH)的水平以反映LDH漏出率,酶联免疫吸附测定(enzyme-linked immunosorbent assay,ELISA)检测细胞培养液中白细胞介素-1β(interleukin-1β,IL-1β)和白细胞介素-18(interleukin-18,IL-18)的含量,蛋白质印迹法检测磷酸化的核因子κB(phospho-nuclear factor-κB,p-NF-κB)的蛋白质表达水平。
结果
与control组比较,OGD组LDH漏出率及细胞培养液中IL-1β和IL-18含量增加,p-NF-κB的蛋白质表达水平上调,差异均有统计学意义(P<0.01或P<0.001);与OGD组相比,TAN+OGD组LDH漏出率及细胞培养液中IL-1β和IL-18含量减少,p-NF-κB的蛋白质表达水平下调,差异均有统计学意义(P<0.05或P<0.01)。与control组比较,TAN组细胞中AK003290的表达水平明显上调(P<0.01),OGD组细胞中AK003290的表达水平明显下调(P<0.05);与OGD组相比,TAN+OGD组细胞中AK003290的表达水平明显上调(P<0.05)。与scrambled siRNA+TAN+OGD组相比,AK003290 siRNA+TAN+OGD组LDH漏出率及细胞培养液中IL-1β和IL-18含量增加,p-NF-κB的蛋白质表达水平上调,差异均有统计学意义(P<0.05或P<0.01)。
结论
丹参酮ⅡA通过上调AK003290抑制NF-κB活性,从而减轻OGD导致的心肌细胞炎症损伤。
Keywords: 丹参酮ⅡA, 心肌细胞损伤, 长链非编码RNA, AK003290, 核因子κB, 炎症, 氧糖剥夺
Abstract
Objective
Tanshinone IIA has a wide range of myocardial protective effects. AK003290 is a long noncoding RNA (lncRNA) that is highly expressed in myocardial tissue, and its expression is down-regulated when myocardial injury occurs. This study aims to explore the mechanism for tanshinone IIA in alleviating myocardial cell damage induced by oxygen glucose deprivation (OGD).
Methods
OGD model was established in rat H9C2 cardiomyocytes. siRNA was transfected to reduce AK003290 expression. H9C2 cells were divided into 6 groups: A control group, a tanshinone IIA (TAN) group, an OGD group, a tanshinone IIA+OGD (TAN+OGD) group, a scrambled siRNA transfection+tanshinone IIA+OGD (scrambled siRNA+TAN+OGD) group, and a AK003290 siRNA transfection+tanshinone IIA+OGD (AK003290 siRNA+TAN+OGD) group. H9C2 cells in the TAN group were treated with 40 μmol/L tanshinone IIA for 12 h. The TAN+OGD group was treated with 40 μmol/L tanshinone IIA for 12 h, followed by OGD treatment for 12 h. The scrambled siRNA+TAN+OGD group and AK003290 siRNA+TAN+OGD group were transfected with the scrambled siRNA or AK003290 siRNA. Twenty-four hours later, the cells were treated with tanshinone IIA and OGD. Real-time RT-PCR was used to detect the expression of AK003290. Spectrophotometry was used to detect the content of lactate dehydrogenase (LDH) in cell culture medium to reflect LDH leakage rate, and enzyme-linked immunosorbent assay (ELISA) was used to detect the content of interleukin-1β (IL-1β) and interleukin-18 (IL-18). Western blotting was used to detect the protein expression of phospho-nuclear factor- κB (p-NF-κB).
Results
Compared with the control group, the leakage rate of LDH, the content of IL-1β and IL-18 in culture medium, and the protein expression level of p-NF-κB were increased in the OGD group (P<0.01 or P<0.001). Compared with the OGD group, the leakage rate of LDH, the content of IL-1β and IL-18 in culture medium, and the protein expression level of p-NF-κB were decreased in the TAN+OGD group (P<0.05 or P<0.01). Compared with the control group, the AK003290 expression was increased in the TAN group (P<0.01) and it was decreased in the OGD group (P<0.05). Compared with the OGD group, the AK003290 expression was increased in the TAN+OGD group (P<0.05). Compared with the scrambled siRNA+TAN+OGD group, the leakage rate of LDH, the content of IL-1β and IL-18 in culture medium, and the protein expression level of p-NF-κB were increased in the AK003290 siRNA+TAN+OGD group (P<0.05 or P<0.01).
Conclusion
Tanshinone IIA inhibits NF-κB activity and attenuates OGD-induced inflammatory injury of cardiomyocytes through up-regulating AK003290.
Keywords: tanshinone IIA, cardiomyocyte injury, long noncoding RNA, AK003290, nuclear factor- κB, inflammatory, oxygen glucose deprivation
心肌缺血作为临床心血管疾病最常见的症状之一,冠心病是导致心肌缺血的最主要的原因[1]。随着生活水平的提高,心肌缺血的患病率逐年上升,且呈现年轻化趋势[2]。缺血时心肌细胞出现坏死和凋亡,大量炎症细胞浸润后进一步加剧心肌损伤,加速心功能恶化,炎症反应是心肌缺血后损伤的重要因素[3]。丹参酮ⅡA作为丹参最主要的脂溶性成分,具有广泛的心肌保护作用,研究[4]报道其也具有抗炎作用。此外,丹参酮ⅡA通过调控多种长链非编码RNA(long noncoding RNA,lncRNA)发挥其药理学作用[5]。作者团队前期研究[6]发现丹参酮ⅡA能促进AK003290表达。AK003290是1种在心脏和肌组织高表达的lncRNA,在心肌损伤时表达降低[7]。但丹参酮ⅡA调控AK003290后如何影响心肌细胞炎症损伤尚不明确。考虑到核因子κB(nuclear factor-κB,NF-κB)作为关键转录因子,在炎症因子瀑布级联反应中起重要的调节作用[8]。本研究通过构建H9C2心肌细胞氧糖剥夺(oxygen glucose deprivation,OGD)模型,以lncRNA和炎症损伤作为切入点,探讨丹参酮ⅡA通过AK003290调控NF-κB影响心肌细胞炎症反应的作用机制,为丹参酮ⅡA在心肌缺血性疾病中的应用提供实验依据和理论基础。
1. 材料与方法
1.1. 材料
大鼠心肌细胞H9C2购自中国科学院上海细胞库。丹参酮ⅡA(20 mg/支,T109794)购自上海阿拉丁生化科技股份有限公司。高糖/低糖Dulbecco改良的Eagle培养基(Dulbecco’s modified Eagle’s medium,DMEM)、胎牛血清均购自美国Gibco公司。Lipofectamine 2000为美国Invitrogen公司产品。Scrambled siRNA和AK003290 siRNA均购自上海吉玛生物公司。乳酸脱氢酶(lactate dehydrogenase,LDH)检测试剂盒购自上海碧云天生物技术有限公司。RNAiso Plus、PrimeScriptTM RT Master Mix试剂盒、TB Green® Premix Ex TaqTM II试剂盒均购自宝生物工程(大连)有限公司。小鼠中白细胞介素-1β(interleukin-1β,IL-1β)和白细胞介素-18(interleukin-18,IL-18)酶联免疫吸附测定(enzyme-linked immunosorbent assay,ELISA)试剂盒均购自武汉博士德生物工程有限公司。兔抗NF-κB/磷酸化的NF-κB(phospho-NF-κB,p-NF-κB)抗体购自美国CST公司。
三气培养箱为美国Thermo Fisher Scientific公司产品。StepOne Plus荧光定量PCR仪为美国ABI公司产品。蛋白质电泳分离、转膜和成像系统为美国Bio-Rad公司产品。Lx800酶标仪为美国Bio Tek公司产品。
1.2. 方法
1.2.1. 细胞分组和处理
用含10%胎牛血清的DMEM,在37 ℃含5% CO2的细胞培养箱中培养H9C2细胞,细胞融合度约85%时进行传代,取对数生长期状态良好的细胞进行实验研究。将H9C2细胞分为以下6组:对照(control)组、丹参酮ⅡA(TAN)组、氧糖剥夺(OGD)组、丹参酮ⅡA+氧糖剥夺(TAN+OGD)组、scrambled siRNA转染+丹参酮ⅡA+氧糖剥夺(scrambled siRNA+TAN+OGD)组、AK003290 siRNA转染+丹参酮ⅡA+氧糖剥夺(AK003290 siRNA+TAN+OGD)组。TAN组只用 40 μmol/L的丹参酮ⅡA预处理H9C2细胞12 h。OGD组只进行OGD处理12 h。TAN+OGD组用40 μmol/L的丹参酮ⅡA预处理H9C2细胞12 h,再行OGD处理12 h。Scrambled siRNA+TAN+OGD组和AK003290 siRNA+TAN+OGD组在转染相应siRNA 24 h后进行后续处理。对照组仅常规培养。
1.2.2. 细胞OGD模型构建
参考文献[9]构建细胞OGD模型。H9C2细胞吸去培养基,用PBS清洗2次,加入低糖DMEM,并放置于37 ℃、5% CO2、95% N2的环境中培养12 h。
1.2.3. 细胞转染
取对数生长期的H9C2细胞接种于6孔板,待细胞融合度达30%~50%时进行转染,按Lipofectamine 2000说明书步骤转染相应的AK003290 siRNA或scrambled siRNA,转染后24 h检测AK003290的表达情况以确定转染是否成功,并进行后续分组处理。
1.2.4. Real-time RT-PCR
采用实时反转录PCR(real-time reverse transcription PCR,real-time RT-PCR)检测各组细胞中AK003290的表达情况。收集各组细胞,用RNAiso Plus提取总RNA,PrimeScriptTM RT Master Mix试剂盒将RNA反转录为cDNA,TB Green® Premix Ex TaqTM II试剂盒行荧光定量PCR扩增AK003290及内参GAPDH,引物序列见表1。实时PCR总体系为20 µL,包含TB Green Premix Ex Taq II 10 μL,正反向引物各0.8 μL,cDNA 2 μL,ROX 0.4 μL和双蒸水6 μL。反应条件为:先95 ℃预变性30 s,然后95 ℃ 5 s和60 ℃ 30 s,循环40次。用2-ΔΔCt法计算目的基因的相对表达量。
表1.
实时反转录PCR引物序列
Table 1 Sequences of real-time RT-PCR primers
| 引物名称 | 序列 | |
|---|---|---|
| AK003290 | 正向 | 5'-CAAGCTCTTCCAGATATGGTG-3' |
| 反向 | 5'-ACCTTTGGTATGCCTTTCCAC-3' | |
| GAPDH | 正向 | 5'-TGTGTCCGTCGTGGATCTG-3' |
| 反向 | 5'-CCTGCTTCACCACCTTCTTG-3' | |
1.2.5. 细胞培养液中LDH水平的检测
采用分光光度法检测细胞培养液中LDH的水平以反映细胞中LDH的漏出率。收集各组细胞的培养液,按照LDH检测试剂盒说明书,使用酶标仪测定450 nm波长处的吸光度值,以control组或TAN+OGD组LDH为100%,计算其他各组的LDH漏出率。
1.2.6. 细胞培养液中IL-1β和IL-18含量的检测
采用酶联免疫吸附测定(enzyme-linked immunosorbent assay,ELISA)检测细胞培养液中IL-1β和IL-18的含量。收集各组细胞的培养液,按照试剂盒说明书,使用酶标仪测定450 nm波长处的吸光度值,通过标准曲线分别计算各组IL-1β和IL-18的含量。
1.2.7. 蛋白质印迹法
收集各组细胞,用RIPA裂解液提取细胞总蛋白质,行BCA法蛋白质定量后煮沸变性。取30 μg总蛋白质经10%的SDS聚丙烯酰胺凝胶电泳(SDS polyacrylamide gel electrophoresis,SDS-PAGE)分离后转移至聚偏二氟乙烯(polyvinylidenefluoride,PVDF)膜,将膜在室温下用5%脱脂奶粉封闭1 h后,加入兔抗NF-κB、p-NF-κB抗体,并在4 ℃下孵育过夜。加入二抗(1꞉3 000),并在室温下孵育1 h。用增强型化学发光(enhanced chemiluminescence,ECL)成像,以NF-κB作为参照,采用Image Lab分析p-NF-κB相对表达水平。
1.3. 统计学处理
采用GraphPad Prism 8软件进行数据的统计分析和图形绘制。数据用均数±标准差( ±s)表示,多组间比较采用单因素方差分析,两两比较采用t检验,P<0.05表示差异有统计学意义。
2. 结 果
2.1. 丹参酮IIA抑制OGD处理导致的细胞中LDH漏出及细胞炎症因子释放的增加
Control组与TAN组在LDH漏出率、细胞培养液中IL-1β和IL-18含量方面的差异均无统计学意义(均P>0.05);与control组比较,OGD组细胞LDH漏出率及细胞培养液中IL-1β和IL-18含量增加,差异均有统计学意义(P<0.01或P<0.001);与OGD组相比,TAN+OGD组细胞LDH漏出率及细胞培养液中IL-1β和IL-18含量减少,差异均有统计学意义(P<0.05或 P<0.01,图1)。
图1.
丹参酮IIA和/或OGD处理对LDH漏出率(A)及细胞培养液中IL-1β(B)和IL-18(C)含量的影响
Figure 1 Effect of treatment with tanshinone IIA and/or OGD on the LDH leakage rate (A), and content of IL-1β (B) and IL-18 (C) in cell culture medium
Pretreatment of H9C2 cells with 40 μmol/L tanshinone IIA for 12 h inhibited the increased leakage of LDH and release of inflammatory cytokines in H9C2 cells induced by OGD treatment. Data are represented as the means±standard deviation, n=6. **P<0.01, ***P<0.001 vs the control group; †P<0.05, ††P<0.01 vs the OGD group. LDH: Lactate dehydrogenase; IL-1β: Interleukin-1β; IL-18: Interleukin-18; OGD: Oxygen glucose deprivation.
2.2. 丹参酮IIA抑制OGD处理导致的细胞中NF-κB活性的增加
蛋白质印迹法结果显示:Control组和TAN组之间p-NF-κB水平的差异无统计学意义(P>0.05);与control组比较,OGD组细胞中p-NF-κB水平明显上调(P<0.01);与OGD组相比,TAN+OGD组细胞中p-NF-κB水平明显下调(P<0.05,图2)。
图2.
丹参酮IIA和/或OGD处理对p-NF-κB蛋白质表达水平的影响
Figure 2 Effect of treatment with tanshinone IIA and/or OGD on the protein expression level of p-NF-κB
The protein expression level of p-NF-κB in H9C2 cells was detected by Western blotting. Pretreatment of H9C2 cells with 40 μmol/L tanshinone IIA for 12 hours inhibited the increased protein expression level of p-NF-κB in H9C2 cells induced by OGD treatment. Data are represented as the means±standard deviation, n=3. **P<0.01 vs the control group; †P<0.05 vs the OGD group. p-NF-κB: Phospho-nuclear factor-κB; OGD: Oxygen glucose deprivation.
2.3. 丹参酮IIA抑制OGD处理导致的细胞中 AK003290 表达的下调
Real-time RT-PCR结果显示:与control组比较,TAN组细胞中AK003290的表达水平明显上调(P<0.01),OGD组细胞中AK003290的表达水平明显下调(P<0.05);与OGD组相比,TAN+OGD组细胞中AK003290的表达水平明显上调(P<0.05,图3)。
图3.
丹参酮IIA和/或OGD处理对 AK003290 表达水平的影响
Figure 3 Effect of treatment with tanshinone IIA and/or OGD on the expression level of AK003290
The expression level of AK003290 in H9C2 cells was detected by real-time RT-PCR. Pretreatment of H9C2 cells with 40 μmol/L tanshinone IIA for 12 h inhibited the decreased expression of AK003290 in H9C2 cells induced by OGD treatment. Data are represented as the means±standard deviation, n=6. *P<0.05, **P<0.01 vs the control group; †P<0.05 vs the OGD group. OGD: Oxygen glucose deprivation.
2.4. 敲低 AK003290 基因表达可增加OGD处理细胞LDH漏出率及炎症因子IL-1β和IL-18释放
Real-time RT-PCR结果显示:转染AK003290 siRNA后,AK003290的表达水平较转染scrambled siRNA显著下调(P<0.001,图4A)。表明通过转染siRNA成功敲低了AK003290基因的表达。
图4.
敲低 AK003290 基因的表达(A)对LDH漏出率(B)及细胞培养液中IL-1β(C)和IL-18(D)含量的影响
Figure 4 Effect of knocking down the expression of AK003290 gene (A) on the LDH leakage rate (B) and content of IL-1β (C) and IL-18 (D) in the cell culture medium
H9C2 cells transfected with AK003290 siRNA were pretreated with 40 μmol/L tanshinone IIA for 12 h, and then treated with OGD for 12 h. Knocking down the expression of AK003290 gene can increase the leakage rate of LDH and the release of inflammatory cytokines in H9C2 cells induced by OGD treatment. Data are represented as the means±standard deviation, n=6. ***P<0.001 vs the scrambled siRNA group, †P<0.05 vs the scrambled siRNA+TAN+OGD group. LDH: Lactate dehydrogenase; IL-1β: Interleukin-1β; IL-18: Interleukin-18; OGD: Oxygen glucose deprivation.
TAN+OGD组与scrambled siRNA+TAN+OGD组在LDH漏出率、细胞培养液中IL-1β和IL-18含量方面的差异均无统计学意义(均P>0.05);与scrambled siRNA+TAN+OGD组相比,AK003290 siRNA+TAN+OGD组细胞LDH漏出率及细胞培养液中IL-1β和IL-18含量增加,差异均有统计学意义(均P<0.05,图4B~4D)。
2.5. 敲低 AK003290 基因表达可增加OGD处理细胞中NF-κB的活性
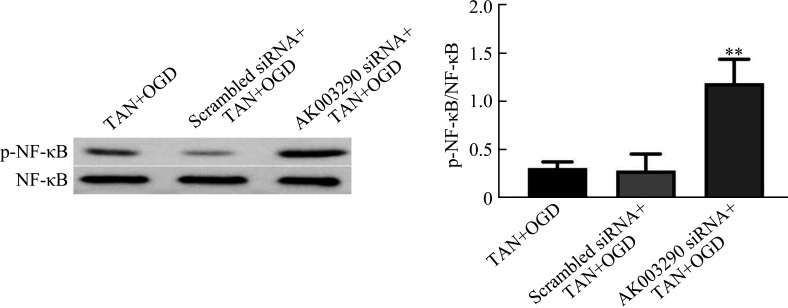
蛋白质印迹法结果显示:TAN+OGD组与scrambled siRNA+TAN+OGD组之间p-NF-κB水平的差异无统计学意义(P>0.05);与scrambled siRNA+TAN+OGD组相比,AK003290 siRNA+TAN+OGD组p-NF-κB水平明显上调(P<0.01,图5)。
图5.
敲低 AK003290 基因的表达对p-NF-κB蛋白质表达水平的影响
Figure 5 Effect of knocking down the expression of AK003290 gene on the protein expression level of of p-NF-κB
The protein expression level of p-NF-κB in H9C2 cells was detected by Western blotting. H9C2 cells transfected with AK003290 siRNA were pretreated with 40 μmol/L tanshinone IIA for 12 h, and then treated with OGD for 12 h. Knocking down the expression of AK003290 gene can increase the protein expression level of p-NF-κB. Data are represented as the means±standard deviation, n=3. **P<0.01 vs the scrambled siRNA+TAN+OGD group. p-NF-κB: Phospho-nuclear factor-κB; OGD: Oxygen glucose deprivation.
3. 讨 论
心肌缺血是心脏血流灌注减少所导致的心肌供氧减少和能量代谢异常,不能满足心脏正常工作的一种病理状态。目前中国心肌缺血的患病率逐年上升,已成为中老年人的常见病,同时由于不健康的生活方式,心肌缺血的发生呈现年轻化趋势[10]。心肌缺血是目前疾病防治的重点,探讨心肌缺血的机制,进而寻找有效的干预方案具有十分重要的临床意义。已有研究[11]证实炎症反应在心肌缺血时细胞损伤中起着重要作用,抑制炎症反应能够有效减轻心肌缺血损伤。抑制炎症级联反应的治疗方案对心肌缺血患者意义重大[12]。
本研究采用的心肌细胞OGD模型,能模拟心肌缺血时的病理生理状态,已广泛运用于心肌缺血领域的研究[13]。结果显示丹参酮ⅡA能抑制OGD所导致的LDH漏出率及细胞培养液中IL-1β和IL-18含量的增加,证实丹参酮ⅡA具有抗炎的心肌保护作用,这与Ren等[14]报道的结果一致。NF-κB作为炎症相关基因关键的转录因子,在炎症因子瀑布级联反应中起重要的调节作用,阻断NF-κB活化和抑制炎症级联反应能减轻细胞炎症损伤[15]。本研究结果显示丹参酮ⅡA抑制OGD所导致的细胞NF-κB磷酸化,其可能通过调控NF-κB发挥抗炎作用。
LncRNA能够调控炎症反应,其中MALAT1、NKILA和miR-146等lncRNA能调控NF-κB的活性[16]。AK003290是一种肌组织中高表达的lncRNA,本团队前期的研究[6]发现丹参酮ⅡA能上调AK003290,因此进一步探究丹参酮ⅡA调控AK003290对心肌细胞炎症损伤的影响及其机制。通过siRNA敲低AK003290基因的表达后,丹参酮ⅡA的心肌细胞保护效果被抑制,且NF-κB磷酸化水平增加,表明AK003290在丹参酮ⅡA的心肌保护中起作用。
综上所述,丹参酮ⅡA促进AK003290表达,从而抑制NF-κB活化,减少炎症因子释放和心肌细胞炎症损伤,为丹参酮ⅡA用于心肌损伤疾病的防治提供了实验基础依据。下一步笔者将在小鼠细胞模型以及原代细胞模型水平进一步证实丹参酮ⅡA的作用和机制。
作者阅读并同意最终的文本。
基金资助
湖南省卫生健康委员会科研计划(202103010510,C20190620);湖南省中医药科研计划(201943,D2022024)。
This work was supported by the Scientific Research Program from the Health Comission of Hunan Province (202103010510, C20190620) and the Traditional Chinese Medicine Scientific Research Program of Hunan Province (201943, D2022024), China.
利益冲突声明
作者声称无任何利益冲突。
作者贡献
徐勤 研究选题与设计,实验操作,数据分析与解释,论文撰写;王朝华、游三丽 数据采集;袁李礼 论文审阅与修改;李贺 研究设计与指导,论文审阅与修改。所有
原文网址
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/202303369.pdf
参考文献
- 1. Pagliaro BR, Cannata F, Stefanini GG, et al. Myocardial ischemia and coronary disease in heart failure[J]. Heart Fail Rev, 2020, 25(1): 53-65. 10.1007/s10741-019-09831-z. [DOI] [PubMed] [Google Scholar]
- 2. 中国心血管健康与疾病报告编写组 . 中国心血管健康与疾病报告2021概要[J]. 中国循环杂志, 2022, 37(6): 553-578. 10.3969/j.issn.1000-3614.2022.06.001. [DOI] [Google Scholar]; The Writing Committee of the Report on Cardiovascular Health and Diseases in China . Report on cardiovascular health and diseases in China 2021: an updated summary[J]. Chinese Circulation Journal, 2022, 37(6): 553-578. 10.3969/j.issn.1000-3614.2022.06.001. [DOI] [Google Scholar]
- 3. Mauersberger C, Schunkert H, Sager HB. Inflammation-related risk loci in genome-wide association studies of coronary artery disease[J]. Cells, 2021, 10(2): 440. 10.3390/cells10020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ansari MA, Khan FB, Safdari HA, et al. Prospective therapeutic potential of Tanshinone IIA: An updated overview[J]. Pharmacol Res, 2021, 164: 105364. 10.1016/j.phrs.2020.105364. [DOI] [PubMed] [Google Scholar]
- 5. Chen WN, Guo SN, Li XM, et al. The regulated profile of noncoding RNAs associated with inflammation by tanshinone IIA on atherosclerosis[J]. J Leukoc Biol, 2020, 108(1): 243-252. 10.1002/JLB.3MA0320-327RRR. [DOI] [PubMed] [Google Scholar]
- 6. 易娜, 李贺, 游三丽, 等. 丹参酮ⅡA通过AK003290减轻H2O2诱导的原代小鼠心肌细胞焦亡[J]. 中国病理生理杂志, 2021, 37(6): 1035-1041. 10.3969/j.issn.1000-4718.2021.06.010. [DOI] [Google Scholar]; YI Na, LI He, YOU Sanli, et al. Tanshinone ⅡA attenuates H2O2-induced primary mouse cardiomyocyte pyroptosis via AK003290[J]. Chinese Journal of Pathophysiology, 2021, 37(6): 1035-1041. 10.3969/j.issn.1000-4718.2021.06.010. [DOI] [Google Scholar]
- 7. Tang Y, Tang Y, Xiang Y, et al. AK003290 protects myocardial cells against apoptosis and promotes cardiac function recovery via miR-539-3p/ErbB4 axis in ischemic-reperfusion injury[J]. DNA Cell Biol, 2021, 40(12): 1528-1538. 10.1089/dna.2021.0323. [DOI] [PubMed] [Google Scholar]
- 8. 闫曙光, 许小凡, 李倩, 等. 基于Notch/NF-κB/NLRP3信号通路的巨噬细胞活化与溃疡性结肠炎的研究进展[J]. 中南大学学报(医学版), 2019, 44(10): 1174-1178. 10.11817/j.issn.1672-7347.2019.180693. [DOI] [PubMed] [Google Scholar]; YAN Shuguang, XU Xiaofan, LI Qian, et al. Progress in the study of macrophage activation and ulcerative colitis from the Notch/NF-κB/NLRP3 signaling pathway[J]. Journal of Central South University. Medical Science, 2019, 44(10): 1174-1178. 10.11817/j.issn.1672-7347.2019.180693. [DOI] [PubMed] [Google Scholar]
- 9. 罗小玲, 邬力祥, 刘发益, 等. 低浓度凝血酶预处理对星形胶质细胞氧糖剥夺损伤的影响[J]. 中南大学学报(医学版), 2007, 32(5): 845-849. 10.3321/j.issn:1672-7347.2007.05.023. [DOI] [PubMed] [Google Scholar]; LUO Xiaoling, WU Lixiang, LIU Fayi, et al. Protective effect of thrombin precondition on astrocytes insulted by oxygen-glucose deprivation[J]. Journal of Central South University. Medical Sciences, 2007, 32(5): 845-849. 10.3321/j.issn:1672-7347.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 10. Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications[J]. Nat Rev Cardiol, 2019, 16(4): 203-212. 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 11. Jones DP, Patel J. Therapeutic approaches targeting inflammation in cardiovascular disorders[J]. Biology (Basel), 2018, 7(4): 49. 10.3390/biology7040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahtta D, Sudhakar D, Koneru S, et al. Targeting inflammation after myocardial infarction[J]. Curr Cardiol Rep, 2020, 22(10): 110. 10.1007/s11886-020-01358-2. [DOI] [PubMed] [Google Scholar]
- 13. Wu XX, Liu L, Zheng QL, et al. Protocatechuic aldehyde protects cardiomycoytes against ischemic injury via regulation of nuclear pyruvate kinase M2[J]. Acta Pharm Sin B, 2021, 11(11): 3553-3566. 10.1016/j.apsb.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ren ZH, Tong YH, Xu W, et al. Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression[J]. Phytomedicine, 2010, 17(3/4): 212-218. 10.1016/j.phymed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 15. Kracht M, Müller-Ladner U, Schmitz ML. Mutual regulation of metabolic processes and proinflammatory NF-κB signaling[J]. J Allergy Clin Immunol, 2020, 146(4): 694-705. 10.1016/j.jaci.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 16. Ghafouri-Fard S, Abak A, Fattahi F, et al. The interaction between miRNAs/lncRNAs and nuclear factor-κB (NF-κB) in human disorders[J]. Biomedecine Pharmacother, 2021, 138: 111519. 10.1016/j.biopha.2021.111519. [DOI] [PubMed] [Google Scholar]