Summary
Statins are a mainstay intervention for cardiovascular disease prevention, yet their use can cause rare severe myopathy. HMG-CoA reductase, an essential enzyme in the mevalonate pathway, is the target of statins. We identified nine individuals from five unrelated families with unexplained limb-girdle like muscular dystrophy and bi-allelic variants in HMGCR via clinical and research exome sequencing. The clinical features resembled other genetic causes of muscular dystrophy with incidental high CPK levels (>1,000 U/L), proximal muscle weakness, variable age of onset, and progression leading to impaired ambulation. Muscle biopsies in most affected individuals showed non-specific dystrophic changes with non-diagnostic immunohistochemistry. Molecular modeling analyses revealed variants to be destabilizing and affecting protein oligomerization. Protein activity studies using three variants (p.Asp623Asn, p.Tyr792Cys, and p.Arg443Gln) identified in affected individuals confirmed decreased enzymatic activity and reduced protein stability. In summary, we showed that individuals with bi-allelic amorphic (i.e., null and/or hypomorphic) variants in HMGCR display phenotypes that resemble non-genetic causes of myopathy involving this reductase. This study expands our knowledge regarding the mechanisms leading to muscular dystrophy through dysregulation of the mevalonate pathway, autoimmune myopathy, and statin-induced myopathy.
Keywords: HMG-CoA reducatase, limb-girdle like muscular dystrophy, rare genetic disease, HMGCR, mevalonate pathway, autoimmune myopathy, statin-induced myopathy
Graphical abstract

We identified rare bi-allelic variants in HMGCR in nine individuals with muscular dystrophy and no genetic diagnosis. Functional and in silico studies implicate HMGCR dysfunction in muscular dystrophy pathogenesis.
Main text
Muscular dystrophies are diverse muscle degenerative disorders that are often inherited. This group of conditions are caused by pathogenic genetic variants in more than 40 protein-encoding genes.1 Still, many individuals with characteristic features of muscular dystrophy remain undiagnosed genetically, indicating that additional genes and genetic mechanisms underlying this disease group may yet be discovered. Muscular dystrophies are characterized by progressive muscle weakness, dystrophic muscle pathology, and often increased creatinine phosphokinase (CPK) levels. Predominantly axial and proximal muscle weakness characterizes limb-girdle muscular dystrophies (LGMDs) and the genetic etiology is identified in only 30%–60%, with autosomal-recessive forms accounting for the majority of probands with definitive diagnosis.2,3
HMGCR (MIM: 142910) encodes 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase and is the rate-limiting enzyme in the mevalonate pathway which catalyzes the conversion of HMG-CoA to mevalonate. HMGCR is located on the chromosomal region 5q13.3 and is constrained for predicted loss-of-function (pLI = 1) and missense variants (z = 3.76) per the gnomAD database.4 HMGCR mRNA is expressed across all tissues and the major isoform encodes for 888 amino acids. HMG-CoA reductase is a widely studied homo-tetramer enzyme, which consists of a dimer of dimers with a sterol-sensing regulatory domain that binds to the endoplasmic reticulum membrane and a catalytic domain that interfaces with adjacent monomers. Proper function of both domains is critical for enzymatic activity5 and disruption of either domain may dysregulate the enzyme. The role of the mevalonate pathway in muscle physiology is highlighted by the discovery of rare bi-allelic variants in GGPS1 (MIM: 606982), which encodes geranylgeranyl diphosphate synthase (GGPPS), that cause congenital muscular dystrophy, sensorineural hearing loss,6 and primary ovarian insufficiency in affected females.7 Herein, we report five males and four females from five unrelated families (Figure 1A) with undiagnosed muscular dystrophy who harbor ultra-rare HMGCR variants identified through clinical and research exome sequencing (ES).
Figure 1.
Bi-allelic variants in HMGCR in five affected families results in an autosomal-recessive progressive limb-girdle muscular dystrophy
(A) Five families with nine affected individuals and bi-allelic variant segregation. Filled symbols indicate affected individuals. Parents of consanguineous family are fourth cousins.
(B) Spatial distribution of muscle weakness in six affected individuals (age during examination) utilizing MRC muscle strength scores generated by MuscleViz denoting: 0, paralysis; 1, trace or minute muscle contraction; 2, muscle movement is possible without gravity; 3, muscle movement is possible against gravity; 4, muscle strength is reduced, but movement against resistance is possible; and 5, preserved normal strength.
(C) T1 axial MRI images (from family 5 II:I, at 31 years of age) show striking replacement of almost all muscles of the upper leg (top image) with fibroadipose tissue (signal artifact in right upper leg due to metallic implant). In the lower leg (bottom image), there is increased signaling in the soleus and gastrocnemius muscles with relative sparing of the anterior muscles (tibialis anterior, extensor hallucis longus, and extensor digitorum longus) and lateral muscles (peroneus longus and brevis).
(D) Histopathologic findings from family 2 II:1 (top row) includes mild variation in myofiber size on H&E (left), increased subsarcolemmal oxidative enzyme activity on NADH stain (middle), and increased subsarcolemmal mitochondria on electron microscopy (right). Findings from family 2 II:2 (bottom row) includes dystrophic myopathic changes with marked variation in myofiber size with atrophic and hypertrophic myofibers, degenerating fibers with phagocytosis, endomysial fibrosis, and many rimmed vacuoles with basophilic granule deposition on H&E (left). Gomori trichrome (middle) highlights the endomysial fibrosis and stains the rimmed vacuoles stain red. Electron microscopy shows myofibrillar disruption and vacuoles containing lysosomal debris (right).
(E) Ultrastructural findings of moderately increased subsarcolemmal mitochondria of the tibialis anterior muscle from family 5 II:I at 35 years of age.
The identified variants segregate in accordance with Mendelian expectations for an autosomal-recessive rare disease. While one family (family 4) was consanguineous by clinical history (parents are fourth cousins), the parents were each heterozygous for different rare HMGCR variants in all families except family 5 in which the mother did not have the identified variant. The clinical and research data acquired was done in accordance with ethical standards, proper informed consent, and as approved by the participating institutions review boards (see supplemental methods). Affected individuals demonstrated progressive proximal weakness and developed significant elevations in CPK (1,000–18,000 U/L) (Table 1). Age at symptom recognition ranged from 4 months to 10 years. First recorded symptoms included hypotonia, delayed motor milestones, prominent calves, and neck weakness. Elevated liver enzymes or incidental CPK elevation prompted a clinical evaluation in some individuals. Proximal muscle weakness was more prominent in the deltoids, hip flexors, and hip abductors. Axial muscle weakness was seen in neck flexors and extensors. Relative sparing of biceps, triceps, and wrist extensors and flexors was seen in most individuals (Figure 1B). Striking replacement of almost all muscles of the upper leg with fibroadipose tissue and relative sparing of the anterior muscles and lateral muscles by MRI is shown in Figure 1C. One individual developed lower facial muscle weakness leading to difficulty chewing and dysphagia. Progression of weakness and atrophy of muscles ranged from insidious to rapid, starting in early childhood to adolescence. Complete loss of ambulation with full-time wheelchair dependence occurred in four individuals, and three individuals needed some assistance with ambulation. Respiratory insufficiency was prominent and required non-invasive ventilation in three individuals with one individual ventilated full-time. There was no evidence of cardiac involvement, symptoms worsening with fever, or craniofacial abnormalities in any of the individuals. Most individuals in our cohort had no brain MRI abnormalities, except family 4 with one sibling (II:1) showing symmetric bilateral basal ganglia calcifications of unclear etiology.
Table 1.
Clinical characteristics of individuals with limb-girdle muscular dystrophy and HMGCR variants
| F1-II:1 | F1-II:2 | F1-II:3 | F2-II:1 | F2-II:2 | F3-II:1 | F4-II:1 | F4-II:2 | F5-II:1 | |
|---|---|---|---|---|---|---|---|---|---|
| Age (yr), Sex | 35, M | 37, M | 39, M | 19, F | 22, M | 14, F | 10, F (D) | 8, M (D) | 35, F |
| cDNA (NM_000859.2) | c.1328G>A, c.1867 G>A | c.365+4A>G, c.2375A>G | c.1544G>C, c.1637T>C | c.1327C>T, c.1517_1519delCTT | c.1401C>G | ||||
| Protein (NP_00850.1) | p.Arg443Gln, p.Asp623Asn | ?, p.Tyr792Cys | p.Arg515Thr, p.Leu546Ser | p.Arg443Trp, p.Ser508del | p.Ile467Met | ||||
| Age of first elevated CK and range values (HP:0003236) | 6 yr, 1,378–4,325 |
8 yr, N/A | 10 yr, N/A | 7 yr 8,500–12,600 |
13 yr 2,000–3,000 |
20 mo 2,263–6,040 |
2 yr 11,551–18,185 |
1 yr 4,203 |
16 yr 7,424 |
| Proximal weakness (HP:0003701) | + | + | + | + | + | + | + | + | + |
| Axial weakness (HP:0003327) | + | + | N/A | − | + | + | + | + | + |
| Muscle atrophy (HP:0003202) | +, proximal | N/A | N/A | − | +, diffuse | − | +, diffuse | N/A | +, diffuse |
| Calf hypertrophy (HP:0008981) | + | − | − | − | − | − | + | + | − |
| Myalgias (HP:0003326) | + | − | − | − | − | + | − | − | + |
| Reduced deep tendon reflexes (HP:0001315) | + | − | N/A | + | + | − | + | + | + |
| Gait disturbance (HP:0001288) | waddling | waddling | N/A | waddling | waddling | − | Tsg, phs | phs | N/A |
| Loss of ambulation (HP:0002505) | −, LIM, AS | − | − | −, LIM, AS | +, WC | − | + | + | +, WC |
| Reduced respiratory function (HP:0002747) | +, NIV | N/A | N/A | nt | + | +, NIV | + | + | +, cNIV |
| Cardiac abnormalities (HP:0001627) | − | − | − | − | − | − | − | − | − |
| Disease progression: Rapid (HP:0003678) Slow (HP: 0003677) |
slow, stable | N/A | N/A | rapid | rapid | slow, stable | rapid | rapid | rapid |
+, present; −, not present; AS, assisted ambulation; cNIV, continuous non-invasive ventilation; D, deceased; F, family; LIM, limited; mo, months; N/A, not available; NIV, non-invasive ventilation; nt, not tested; phs, poor heel strike; Tsg, Trendelenburg and stiff gait; yr, years; WC, wheelchair.
Muscle biopsy was performed in five individuals. Four individuals demonstrated non-specific dystrophic features such as degenerating fibers with phagocytosis, prominent fiber size variation, internalized nuclei, and increased endomysial connective tissue (Figure 1D). Dystrophin staining was normal or focally present. In three individuals with electron microscopy studies, we documented a moderate increase in subsarcolemmal mitochondrial clusters (Figures 1D and 1E). In addition, two participants from different families showed myelin bodies. Detailed pathology report findings for all biopsied individuals are included in Table S1. Anti-HMGCR antibody testing in two families (F1, F5) failed to detect protein.
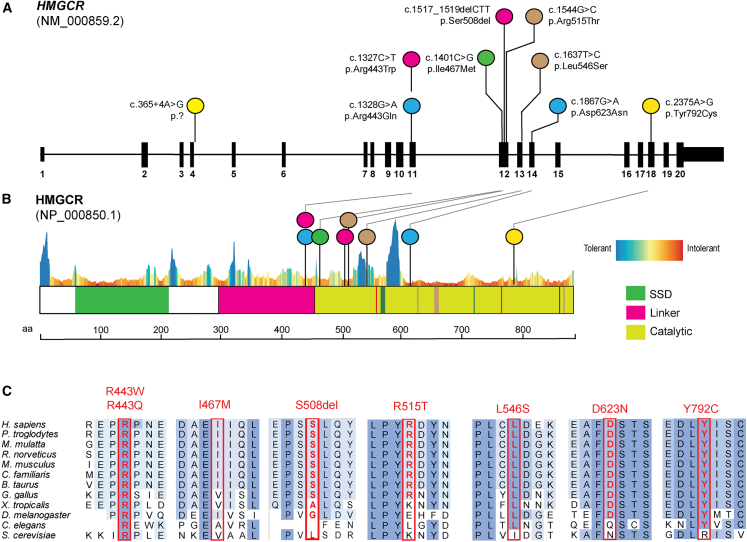
We identified seven missense variants in HMGCR (GenBank: NM_000859.2 and GenBank: NP_000850.1): c.1327C>T (p.Arg443Trp), c.1328G>A (p.Arg443Gln), c.1401C>G (p.Ile467Met), c.1544G>C (p.Arg515Thr), c.1637T>C (p.Leu546Ser), c.1867G>A (p.Asp623Asn), and c.2375A>G (p.Tyr792Cys). In addition, we identified one in-frame deletion c.1517_1519delCTT (p.Ser508del) and one splice site intronic variant (c.365+4A>G). All variants are absent or present at extremely low frequencies (only in heterozygotes) in gnomAD (v.2.1.1) (Table S2). The variant c.1401C>G in F5 appears to be homozygous due to unconfirmed paternal uniparental disomy. Clinical deletion and duplication testing in F5-II:1 did not reveal any findings. To investigate the impact of amino acid substitutions on the structure and function of HMGCR, we mapped the missense variant alleles to the annotated domains and observed clustering in the linker and catalytic domains (Figures 2A and 2B). All missense variants lie in evolutionarily conserved residues (Figure 2C) with conflicted predictions of deleteriousness by CADD and REVEL (Table S2) and intolerant residues per MetaDome. The intron 4 splice variant detected is predicted (by SpliceAI) to cause a cryptic donor gain causing an in-frame insertion of two amino acids and/or with donor loss, and exon 4 skipping leads to an out-of-frame product. RNA sequencing in F2-II:1’s fibroblast cell lines revealed a paternal allele bias with c.1544G>C in 88% of transcripts (Figure S1) and no evidence of altered splicing transcripts.
Figure 2.
Pathogenic variants in HMGCR cluster in linker and catalytic domains in highly conserved residues
(A) Schematic representation of HMGCR transcript map and location of variants identified in cohort. Compound heterozygous variant combinations per family and affected probands are colored the same.
(B) HMGCR domains are highlighted and reveal clustering of variants in the catalytic region and linker domain with a constraint plot by MetaDome. SSD, sterol-sensing domain.
(C) Ortholog alignment of HMG-CoA reductase residues altered by missense or in-frame deletion variants are highlighted with a red rectangle.
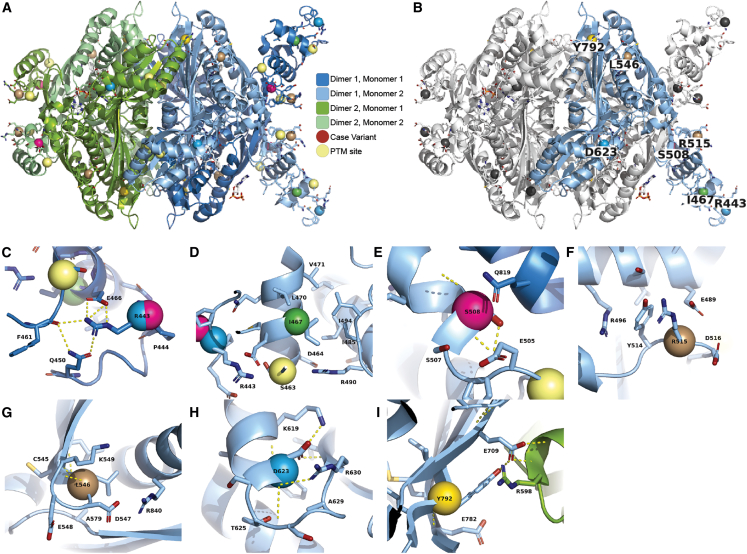
We investigated variants in the overall tetrameric organization of human HMGCR (Figures 3A and 3B) recreating the catalytic and linker domains’ arrangement into a “domain-swapped” configuration (Figures S2 and S3). This results in the linker of one monomer positioned at the catalytic domain of the adjacent monomer.5 The missense variants appear to alter protein stability and energy changes between folded and unfolded structures. They locate close to translational modification sites such as phosphorylation sites, known to impair dimer or tetramer formation. Residues in the linker regions (Figures 3C and 3D) of HMGCR including Arg443 (altered in two unrelated families) have a central role in a local hydrogen bond network that involves Asp623 in a different monomer due to charge complementarity. Furthermore, Asp623 forms specific contacts with residues from multiple local secondary structures and stabilizes them. Finally, p.Ile467Met is moderately stabilizing (negative ΔΔGfold, see Table S2), potentially reducing necessary motions for proper function, despite being in a poorly resolved region. Interestingly, altered residues in the catalytic region (Figures 3E–3I), such as Ser508 and Arg515, are located at the monomer-monomer interface disrupting atomic interactions within or between monomers. Meanwhile, the residues Leu546, Asp623, and Tyr792 occur at important cross-dimer interfaces of the tetramer. Thus, each distinct genetic variant is likely to produce an altered enzyme, yet the altered enzymes have different mechanisms of dysfunction.
Figure 3.
3D molecular structure of HMGCR reveals likely molecular mechanisms
(A) Each monomer of the tetramer is colored distinctly to illustrate the dimer-of-dimers architecture. Each dimer of the tetramer is a domain-swapped structure where the linker of one monomer is adjacent to the catalytic center of another (see Figure S1 for more details).
(B) Three monomers have been colored white and one monomer remains colored as in (A). Sites of case variants are colored gray in three monomers and colored in one monomer with the WT amino acid labeled.
(C and D) Arg443 is within the linker region, which is partially resolved in crystallographic experiments. Residues within the linker that are resolved participate in a hydrogen bond network with Arg443 (C) and moderately stabilizing effects on residue Ile467 (D).
(E and F) Ser508 is a position directly at the monomer-monomer interface, and (F) Arg515 is at the monomer-monomer interface in the domain-swapped region (see Figure S1). Arg515 is flanked by negatively charged residues and may dynamically form inter- and intra-monomer interactions.
(G) Leu546 is at the end of a small loop between the two beta strands of the domain-swapped sheet. It is surrounded by other hydrophobic residues and between the side chains of the adjacent alpha helix.
(H) Asp623 is at the base of a loop that connects a beta sheet to an alpha helix, and its side chain forms multiple specific contacts with residues from both secondary structures.
(I) Tyr792 is directly part of the cross-dimer interface of the tetramer. PTM, post translational modification.
The functional analysis of three missense variants affecting five individuals was performed after protein purification (see supplemental methods). The assay determines HMGCR enzymatic activity by measuring absorbance reduction over time, which is dependent on NADPH consumption in the reaction. All three missense variants exhibit significant enzymatic activity impairment relative to wild-type (WT) HMGCR protein (Figure 4A). p.Asp623Asn had the highest relative activity, maintaining 44.64% of the activity of WT protein. In contrast, p.Tyr792Cys maintained 25.42% activity, while p.Arg443Gln only maintained 1.16% activity (Figure 4B). The specificity of the HMGCR activity of the purified protein was confirmed by blocking the reduction in absorbance using pravastatin as an inhibitor (Figure S4). Next, we measured protein stability with a thermal shift assay (see supplemental methods) using a dye that binds exposed hydrophobic residues as the protein unfolds. Dye binding is measured with increasing temperature over time using a real-time PCR machine, allowing us to estimate the protein melting point (Tm). Fluorescence was plotted with the change in temperature for all four proteins and Tm was calculated by graphing the derivative of the dye signal by the derivative of the temperature over time and averaging the max value from four replicates for each protein (Figure S2). This indicates that there is disruption in the stability of the p.Arg443Gln variant, which was also demonstrated using the protein thermal shift assay, with p.Arg443Gln having the most significant reduction in stability compared to WT protein Tm estimates (Figure 4C).
Figure 4.
Enzymatic activity, size, and stability of purified recombinant HMGCR
(A and B) Enzymatic activity of the protein measured as a reduction in absorbance at 340 nm. The activity was quantified relative to WT HMGCR with all three variants showing significant reductions in activity compared to WT protein. Error bars are mean ± SD.
(C) Fluorescence curves from thermal shift assays over increasing temperature from three missense variants constructs and WT. Thermal shift assay averaged across four replicates show the three missense variants cause significant reductions in stability compared to WT protein Tm estimates.
(D) Estimation of the protein size using the S200 increase column. Two of the variant proteins, p.Asp623Asn and p.Tyr792Cys, eluted at the same volume as WT, while p.Arg443Gln eluted earlier. Acrylamide gels are depicted and stained with Coomassie blue showing only the p.Arg443Gln variant was different in size compared with WT with an approximately 20% increase in size. ∗∗p value 0.0041, ∗∗∗0.0002, and ∗∗∗∗<0.0001.
The oligomerization state of the protein was measured using analytical size exclusion. The catalytic domain of HMGCR alone is approximately 50 kDa and forms a tight tetramer (approximately 200 kDa), with this oligomeric state being important for the enzymatic activity of the protein.5 To determine the protein oligomeric state, we ran the purified protein through an analytical size exclusion chromatography (SEC) column. The complex eluted from the column at approximately 115 kDa, which is smaller than the predicted size of the protein tetramer (200 kda), but this often is a result of the globular shape of control proteins as well as the result of interaction with the column resin, so it is likely that this protein forms the tetrameric state that was observed in the crystal structure.5 The p.Asp623Asn and p.Tyr792Cys variants appeared similar in size and shape to the wild-type protein, while the p.Arg443Gln variant was approximately 20 percent larger (Figure 4D, Table S3). This is most likely caused by disruption in the local structure of the protein at the site of this variant and not changes in oligomerization. Consequently, there is disruption in the stability of the p.Arg443Gln variant, which we then measured using the protein thermal shift assay (Figure 4D).
The bi-allelic hypomorphic variants in HMGCR identified in this cohort are extremely rare and cluster in important regions. Interestingly, two families are affected by different missense variants in the same linker domain residue (Arg443). We hypothesized that variants in this region likely alter the local structure leading to separation from the transmembrane domain and a prodegradatory state. On the other hand, missense variants within the catalytic domain may destabilize the monomeric structure or the monomer-monomer interfaces directly, leading to a greater proportion of monomeric or dimeric states and thereby increasing the rate of HMGCR degradation or affecting enzymatic efficiency. We also demonstrate in vitro the loss of HMGCR activity and decreased stability caused by three hypomorphic missense variants accounting for more than half of the cohort. Complete loss of enzymatic activity was not observed, consistent with embryonic lethality with Hmgcr homozygous knockout.8 We could not appreciate a correlation between variant location and the age of disease onset or severity. However, the phenotypic variability might speak to the degree of hypomorphic impairment resulting from the combinatorial effect of the bi-allelic variants at this locus in the enzyme tetrameric form.
The inhibition of HMG-CoA reductase by statins is widely known to cause dose-dependent myopathic side effects9 in 7%–29% of prescribed individuals with or without CPK elevations, with some overlapping features to the clinical phenotype hereby described. Furthermore, in very rare instances, statin-treated individuals can develop a necrotizing autoimmune myopathy mediated by anti-HMGCR antibodies and present with a limb-girdle muscular dystrophy,10,11 which can initially present as isolated hyper-CPKemia even without prior statin use.12 Myopathic disease caused by HMGCR deficiency is further supported by multiple animal models. Modeling in C. elegans (which lacks the cholesterol-synthesizing branch of the mevalonate pathway) by disrupting hmgcr-1 demonstrated defects in growth, reproduction, mitochondria morphology, and protein prenylation.13 Compromised protein prenylation has also been described in individuals with bi-allelic hypomorphic alleles affecting mevalonate kinase14 (one step downstream of HMG-CoA reductase) impairing proper cytoplasmic traffic and cytoskeleton arrangement. Statin-driven disruption of mitochondrial function through different mechanisms has been widely studied.15 Lovastatin-induced muscle atrophy in zebrafish and human skeletal muscle showed increased muscle protein degradation by the ubiquitin proteasome pathway.16 Complete loss of Hmgcr in mice causes early embryonic lethality.8 Hmgcr muscle-specific knockout mice display myopathic findings and increased CPK levels similar to those induced by statins.17 Oral mevalonate supplementation rescued the myopathic phenotype in these mice. Coincidentally to our report, a consanguineous Bedouin pedigree was reported with a homozygous rare missense variant in HMGCR GenBank: NM_000859.3:c.2465G>A (p.Gly822Asp) reflecting similar limb-girdle muscle dystrophy in six individuals with an onset during the fourth decade. In addition, oral administration of mevalonolactone to mice with statin-induced myopathy and an affected individual with HMGCR-muscular dystrophy showed symptom improvement.18 These individuals, despite showing a later onset (fourth decade) than our cohort (first and second decade), also display only a neuromuscular phenotype, contrary to other enzymatic deficiencies downstream (GGPS1) of HMGCR.
In conclusion, our report presents compelling evidence supporting the contribution of hypomorphic bi-allelic variants in HMGCR to an autosomal-recessive form of muscular dystrophy. Thus, molecular and genomic testing is indicated in the context of the diagnostic odyssey of unexplained muscular dystrophies. Our findings will likely contribute to understanding of the mechanism of statin-induced myopathy. Future studies elucidating the role of mevalonate pathway are required to expand our knowledge regarding the cellular mechanisms leading to muscular dystrophy, autoimmune myopathy, and potential statin-induced neuromuscular complaints.
Acknowledgments
The authors want to thank the families for their willingness to contribute, and Christopher Mendoza, Kia Brooks, Angela Kokkinis, and Gilberto (“Mike”) Averion for their help in clinical evaluations. This work was funded by the Mayo Clinic Center for Individualized Medicine. This study was also supported by the U.S. National Human Genome Research Institute (NHGRI), National Heart, Lung, and Blood Institute (NHBLI), Baylor-Hopkins Center for Mendelian Genomics (BHCMG, grant no. UM1 HG006542), U.S. National Institute of Neurological Disorders and Stroke (NINDS, grant no. R35NS105078), and Muscular Dystrophy Association (MDA, grant no. 512848). Sequencing was also funded through the Clinical Center Genomics Opportunity, sponsored by the NIHGRI, NIH Deputy Director for Intramural Research, and the NIH Clinical Center. Additional sequencing and analysis were provided by the Broad Institute and Harvard Center for Mendelian Genomics, NHBLI and National Eye Institute (grant no. UM1 HG008900) and NIHGRI (grant no. R01 HG009141 and U01 HG0011755). C.G.B.’s laboratory is also supported by intramural funds from NINDS. Data collection for family 4 was supported by the Bernard F. and Alva B. Gimbel Foundation and NIH R01NS080929. This research was completed with technical support by the Medical College of Wisconsin (MCW) Research Computing Center. Finally, this publication was supported in part by The Linda T. and John A. Mellowes Endowed Innovation and Discovery Fund and the Genomic Sciences and Precision Medicine Center of MCW. D.P. is supported by a Clinical Research Training Scholarship in Neuromuscular Disease partnered by the American Brain Foundation (ABF) and Muscle Study Group (MSG).
Declaration of interests
The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing from Baylor Genetics Laboratories. J.R.L. has stock ownership in 23andMe, is a paid consultant for Regeneron Pharmaceuticals, and is a co-inventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases, genomic disorders, and bacterial genomic fingerprinting.
Published: May 10, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2023.04.006.
Web resources
gnomAD Browser, https://www.gnomad.broadinstitute.org/
HPO terms, https://hpo.jax.org/app/
MuscleViz, https://muscleviz.github.io/
OMIM, https://www.omim.org/
Protein Data Bank, https://www.rcsb.org/
Protein Paint, https://proteinpaint.stjude.org/
PyMOL, https://pymol.org/2/
UniProt, https://www.uniprot.org/
Supplemental information
Data and code availability
The published article includes all variant information pertinent to this study. This study did not generate datasets or code.
References
- 1.Mercuri E., Bönnemann C.G., Muntoni F. Muscular dystrophies. Lancet. 2019;394:2025–2038. doi: 10.1016/S0140-6736(19)32910-1. [DOI] [PubMed] [Google Scholar]
- 2.Reddy H.M., Cho K.A., Lek M., Estrella E., Valkanas E., Jones M.D., Mitsuhashi S., Darras B.T., Amato A.A., Lidov H.G., et al. The sensitivity of exome sequencing in identifying pathogenic mutations for LGMD in the United States. J. Hum. Genet. 2017;62:243–252. doi: 10.1038/jhg.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Töpf A., Johnson K., Bates A., Phillips L., Chao K.R., England E.M., Laricchia K.M., Mullen T., Valkanas E., Xu L., et al. Sequential targeted exome sequencing of 1001 patients affected by unexplained limb-girdle weakness. Genet. Med. 2020;22:1478–1488. doi: 10.1038/s41436-020-0840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Istvan E.S., Palnitkar M., Buchanan S.K., Deisenhofer J. Crystal structure of the catalytic portion of human HMG-CoA reductase: insights into regulation of activity and catalysis. EMBO J. 2000;19:819–830. doi: 10.1093/emboj/19.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaiyrzhanov R., Perry L., Rocca C., Zaki M.S., Hosny H., Araujo Martins Moreno C., Phadke R., Zaharieva I., Camelo Gontijo C., Beetz C., et al. GGPS1-associated muscular dystrophy with and without hearing loss. Ann. Clin. Transl. Neurol. 2022;9:1465–1474. doi: 10.1002/acn3.51633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley A.R., Zou Y., Dunford J.E., Rooney J., Chandra G., Xiong H., Straub V., Voit T., Romero N., Donkervoort S., et al. GGPS1 Mutations Cause Muscular Dystrophy/Hearing Loss/Ovarian Insufficiency Syndrome. Ann. Neurol. 2020;88:332–347. doi: 10.1002/ana.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohashi K., Osuga J.i., Tozawa R., Kitamine T., Yagyu H., Sekiya M., Tomita S., Okazaki H., Tamura Y., Yahagi N., et al. Early embryonic lethality caused by targeted disruption of the 3-hydroxy-3-methylglutaryl-CoA reductase gene. J. Biol. Chem. 2003;278:42936–42941. doi: 10.1074/jbc.M307228200. [DOI] [PubMed] [Google Scholar]
- 9.Stroes E.S., Thompson P.D., Corsini A., Vladutiu G.D., Raal F.J., Ray K.K., Roden M., Stein E., Tokgözoğlu L., Nordestgaard B.G., et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohassel P., Landon-Cardinal O., Foley A.R., Donkervoort S., Pak K.S., Wahl C., Shebert R.T., Harper A., Fequiere P., Meriggioli M., et al. Anti-HMGCR myopathy may resemble limb-girdle muscular dystrophy. Neurol. Neuroimmunol. Neuroinflamm. 2019;6:e523. doi: 10.1212/NXI.0000000000000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mammen A.L., Chung T., Christopher-Stine L., Rosen P., Rosen A., Doering K.R., Casciola-Rosen L.A. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63:713–721. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohassel P., Mammen A.L. Anti-HMGCR Myopathy. J. Neuromuscul. Dis. 2018;5:11–20. doi: 10.3233/JND-170282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranji P., Rauthan M., Pitot C., Pilon M. Loss of HMG-CoA reductase in C. elegans causes defects in protein prenylation and muscle mitochondria. PLoS One. 2014;9:e100033. doi: 10.1371/journal.pone.0100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Politiek F.A., Waterham H.R. Compromised Protein Prenylation as Pathogenic Mechanism in Mevalonate Kinase Deficiency. Front. Immunol. 2021;12:724991. doi: 10.3389/fimmu.2021.724991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mollazadeh H., Tavana E., Fanni G., Bo S., Banach M., Pirro M., von Haehling S., Jamialahmadi T., Sahebkar A. Effects of statins on mitochondrial pathways. J. Cachexia Sarcopenia Muscle. 2021;12:237–251. doi: 10.1002/jcsm.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanai J.i., Cao P., Tanksale P., Imamura S., Koshimizu E., Zhao J., Kishi S., Yamashita M., Phillips P.S., Sukhatme V.P., Lecker S.H. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J. Clin. Invest. 2007;117:3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osaki Y., Nakagawa Y., Miyahara S., Iwasaki H., Ishii A., Matsuzaka T., Kobayashi K., Yatoh S., Takahashi A., Yahagi N., et al. Skeletal muscle-specific HMG-CoA reductase knockout mice exhibit rhabdomyolysis: A model for statin-induced myopathy. Biochem. Biophys. Res. Commun. 2015;466:536–540. doi: 10.1016/j.bbrc.2015.09.065. [DOI] [PubMed] [Google Scholar]
- 18.Yogev Y., Shorer Z., Koifman A., Wormser O., Drabkin M., Halperin D., Dolgin V., Proskorovski-Ohayon R., Hadar N., Davidov G., et al. Limb girdle muscular disease caused by HMGCR mutation and statin myopathy treatable with mevalonolactone. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2217831120. e2217831120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all variant information pertinent to this study. This study did not generate datasets or code.