Abstract
Melamine stabilizes heterogeneous nucleation of calcium crystals by increasing the retention time and decreasing the rate of dissolution. Stabilization of such mixed crystals limit the efficacy of non-invasive treatment options for kidney stones. Crystalline forms of uric acid (UA) are also involved in urolithiasis or UA kidney stones; however, its interactions with contaminating melamine and the resulting effects on the retention of kidney stones remain unknown. Since melamine augments calcium crystal formation, it provides an avenue for us to understand the stability of UA-calcium phosphate (CaP) crystals. We show here that melamine facilitates UA+CaP crystal formation, resulting in greater aggregates. Moreover, melamine induced mixed crystal retention through a time-dependent manner in presence and/or absence of hydroxycitrate (crystal inhibitor), indicating its abridged effectiveness as conventional remedy. CaP was also shown to modify optical properties of UA+CaP mixed crystals. Differential staining of individual crystals revealed enhanced co-aggregation of UA and CaP. The dissolution rate of UA in presence of melamine was faster than its heterogeneous crystallization form with CaP, although the size was comparatively much smaller, suggesting disparity in regulation between UA and CaP crystallization. While melamine stabilized UA, CaP and mixed crystals in relatively physiological conditions (artificial urine), the retentions of those crystals were further augmented by melamine, even in presence of hydroxycitrate, thus reducing treatment efficacy.
Keywords: A1. Biocrystallization, A2. Heterogeneous Nucleation, B1. Solubility, B2. Kidney stones dissolution, B3. Calcium compounds
1. Introduction:
Kidney stone recurrence has been shown to be as high as fifty percent even after a decade of stone removal1. Inadequate eradication of stone crystals is the main cause in their retention at the renal tubules (nephrocalcinosis), and accumulation into stones (nephrolithiasis), or both1. The mechanism behind the kidney stone formation process starts from several physicochemical events including supersaturation, nucleation, growth, aggregation, and retention of urinary stone constituents2. All these events are controlled by the presence of both promoters and inhibitors of crystallization1–2. Crystal nucleation leading to crystal aggregation is considered to be the most critical step in stone formation3. Aggregation of preformed crystals is essential for stone growth where such process further helps to crystalize within themselves or to form co-aggregates with another type of crystal(s)3. A typical example is the calcium phosphate (CaP) nidus seen in mixed crystals of CaP and calcium oxalate (CaOx)2–3. Similarly, uric acid (UA) crystals were proposed to aid the development of urinary stones by creating a nidus for heterogeneous crystallization4. The most common combination was CaP and CaOx mixed with a small amount of UA5. While the excreted stone composition of UA did not exceed 10% of the total, nearly half of all patient stones were consistent with this composition5. Moreover, 30% of adult patients with calcium urolithiasis suffered from hyperuricosuria with hyperuricemia suggesting that UA abnormalities may have a role in calcium nephrolithiasis6. In addition, UA growth characteristics are influenced by alkali metal ions, exhibiting rod-like shapes or hierarchical structures in presence of Na+ or K+, respectively7. Synthetic non-birefringent ammonium urate crystals were also observed to be comprised of hierarchical structures with clusters of needle-like crystals within its core structures, which has similar characteristic of cetacean renal stones8. Furthermore, the hierarchical structure and stacking defect produced during UA crystallization can trigger a select core-shell phase transition of UA crystals, which can be attributed to the tautomeric nature of UA9,10.
Dissolution therapies are among the most common remedies to kidney stone formers11. Several studies have investigated the effectiveness of such therapies dependent on renal stone types such as cysteine, struvite and UA stones2. Citrates, in recent times have been used as superior dissolution agents for calcium crystals and were more effective in dissolving calcium crystals than water or phosphates alone12–13. The presence or absence of crystal inhibitors correlated much better with stone formation inclination than whether or not a patient had hypercalcuria14. It had also been proposed that the colloidal phase of urate removes certain inhibitors for calcium nucleation from the urine and subsequently promotes crystal nucleation4. The most common treatment option for UA stones is allopurinol, which reduces the amount of UA in the urine by inhibiting the conversion of purines into UA, thus preventing UA stone formation15,16. However, allopurinol has the risk of xanthine accumulation which could lead to xanthine nephropathy or even xanthine urolithiasis17,18. Furthermore, in favorable conditions, UA precipitates readily in the presence of CaP, and vice versa15.
Melamine, a metabolite to cyanuric acid19 has been shown to interact with other calcium crystals to enhance stone formation, while citrate, bicarbonate, and low urine pH were shown to inhibit calcium crystallization12,13. The extensive use of melamine in industries has been implicated in nephrotoxicity resulting from contamination of food products. Previously, we have characterized the mechanism of melamine and calcium crystal interactions and found that melamine allowed for increased crystal attachment and subsequent crystal growth20. Later, we found that melamine also stabilized the CaP and CaOx crystals by strong hydrogen bond interactions, which prevented the dissolution of CaP+CaOx mixed crystals21. However, the interaction between melamine and UA+CaP mixed crystals is unknown. Previous studies have shown that the presence of UA can provoke the crystallization of CaOx monohydrate through a heterogeneous nucleation process, and even act as a nucleation site22,23.
CaP makes up about 10% of all stones with the cause having relation to a high urine pH24 but treatment options have not been tested to prevent recurrence of calcium phosphate stones. By dissolving various preformed mixed crystals, the interactions between crystal complexes could be observed to understand how they behave in aggregation20,25. Therefore, melamine, an environmental factor, poses as an interesting toxin because of its effects on other crystals found in calcium stones. Melamine can induce mixed calcium crystal formation which aggregates with greater concentrations21. We have shown that UA crystals can aggregate in the presence of CaP crystals by forming heterogeneous composites, which yields greater formations at higher levels of CaP or UA concentrations. In presence of melamine, the UA+CaP mixed crystal becomes highly stabilized, preventing rapid dissolution in physiological conditions. Moreover, hydroxycitrate (HCit), a citrate derivative, was shown to favorably dissolute the mixed crystal in presence of melamine21, and rapidly destabilize the mixed crystal formations, which might have therapeutic applications for stone treatments. Our study will elucidate UA and calcium crystal properties through their interactions.
2. Methods and Materials
2.1. Materials
Calcium chloride, monosodium phosphate, disodium phosphate, UA, monosodium urate (MSU), potassium HCit and Hank’s Balanced Salt Solution (HBSS) were purchased from Sigma-Aldrich (St. Louis, MO). A stock solution of UA (12 mM) was prepared by dissolving UA crystals in 1 × HBSS pH 7.4. Melamine was purchased from Sigma-Aldrich and a stock solution of 1 mM was prepared by dissolving melamine in double-distilled water (ddH2O) at room temperature (RT; 25°C).
2.2. Preparation of UA, CaP, and MSU crystals
Preformed CaP crystals were prepared by mixing solutions of 2.4 mM calcium chloride (CaCl2), 0.9 mM disodium phosphate (Na2HPO4), 1X HBSS, and 5.8 mM monosodium phosphate (NaH2PO4). Mixing ratio for calcium chloride, disodium phosphate, and monosodium phosphate is 2.1:1:5.4. UA crystals were prepared by diluting a stock solution to 3 mM using UA crystalline powder in HBSS. MSU crystals were prepared by using UA sodium salt and were dissolved in HBSS to yield a concentration of 3 mM. Crystals were then centrifuged at 10,000 rpm for 5 min, and the supernatant was discarded.
2.3. Differential staining of crystal and measurements
Alizarin Red (AR) pH 4.3, Methylene Blue 0.1% and/or Oil Red O were used to stain CaP, UA, or melamine crystals, respectively, as previously described20. Methylene Blue staining was done to detect UA and MSU crystals. AR (3,4-Dihydroxy-9, 10-dioxo-2-anthracenesulfonic acid sodium) staining was done to detect the presence of CaP crystals. For Oil Red O staining, samples were incubated at 37°C for 72 h. Stained images were obtained using Zeiss Axiovision microscope (Carl Zeiss Microscopy, Germany). Stained crystal sizes were measured and quantified as “crystal index” using ImageJ (NIH, USA) software as previously described21. The freehand tool was used to create irregularly shaped selections in ImageJ to trace the crystals. Type of crystals were identified based upon the staining and categorized accordingly: AR pH 4.3 stained for CaP, methylene blue stained for UA or MSU, and Oil red O stained for melamine crystals. Particle sizes less than 10μm were excluded from the overall crystal size average.
2.4. Polarized light microscopy
Birefringence signal from microscope slide wet mounts of crystals was imaged using polarized light microscopy (MT9930, Meiji Techno, America) as described previously21. Type of crystals were identified based upon birefringent properties of the crystals in polarized light microscopy21.
2.5. Time-dependence in crystal dissolution
Preformed UA, MSU, CaP, and/or melamine crystals were inserted in diH2O or artificial urine with or without HCit as previously described21. Preformed crystals were incubated at euthermia in diH2O or artificial urine and with or without HCit for 0, 1, 3, and 10 min to observe crystal dissolution under microscope. Images were obtained using Zeiss Axiovision microscope and then quantified using ImageJ to find crystal index21.
2.6. Statistical Analysis
The results were reported as the mean ± standard error (SE) and were analyzed with two-tailed test using Origin 6.1 software (version 6.1, Northampton, MA). The significant difference levels were set at *p < 0.05 and **p < 0.01.
3. Results and analysis
3.1. Addition of melamine facilitates CaP+UA mixed crystal formation.
In our previous study, CaP within mixed calcium crystals have aggregated significantly in the presence of melamine20. We used methylene blue staining to stain UA crystals26,27. However, sequential staining of UA and CaP mixed crystals has not been performed previously. First, we performed the differential staining of UA, CaP, and melamine crystals using methylene blue, AR 4.3, and Oil Red O (Figure S1–S2) to confirm the identities of the formed crystals. After implementing UA and CaP in solution, we saw significant augmentations of UA+CaP mixed crystals with each addition of melamine (0 mM, 2.1 mM, 4.2 mM, and 6.25 mM; Figure 1A–E). AR 4.3 and methylene blue staining showed increased CaP and UA crystal sizes in 2.1 mM melamine, exhibiting immediate formations of UA+CaP mixed crystals in lower concentrations (Figure 1A–B, E). The greatest size increase in CaP was from 2.1 mM to 4.2 mM melamine concentration, but the augmentation tapered off between 4.2 and 6.25 mM melamine (Figure 1C, D). Similarly, methylene blue staining revealed increased UA crystals with mixed UA+CaP crystal formations in the presence of melamine (Figure 1A–E). The highest rise in UA crystal size was found with melamine concentrations from 2.1 mM to 4.2 mM (Figure 1B–C, E). We found that in the absence of melamine and with lower CaP and UA concentrations, conditions for heterogeneous crystallization were not favorable, which may be expected if seeding had occurred28. However, even with smaller melamine concentrations, we immediately see crystal interactions taking place between CaP and UA crystals as represented by the bicolored hues of methylene blue and AR 4.3 staining in relatively larger aggregates (Figure 1B, E). By the addition of 2.1 or 4.2 mM melamine, we see nearly complete intermingled crystals (Figure 1B–E). These results suggest that melamine facilitates the interaction between UA and CaP crystals, and aggregation among CaP+UA mixed crystals.
Figure 1.

Uric acid and calcium phosphate (UA+CaP) crystals were formed with (A) 0 mM melamine (Mel), (B) 2.1 mM Mel, (C) 4.2 mM Mel, or (D) 6.25 mM Mel. Blue (UA), purple (CaP), and red (Mel) arrows are depicted in the images. (E) Relative crystal sizes were quantified using ImageJ and were represented as a bar diagram for mean+SEM. *, P<0.05. Scale bar (white) = 50 μm.
3.2. Melamine induced mixed crystal retention through a time dependent manner in the presence and absence of HCit (crystal inhibitor)
Since we found that melamine could increase UA+CaP mixed crystal formation, our goal was to know whether melamine can stabilize those mixed UA crystals. Therefore, we first performed dissolution experiments of CaP, UA, and UA+CaP crystals in the aqueous phase and measured their relative crystal sizes at each time point (0, 1, 3, 10 min; Figure 2A–D; Figure S1). Moreover, we added MSU as another close derivative of UA, which is usually found in joints for gout diseases29. MSU+CaP crystals were less susceptible to dissolution throughout the 10 mins time pass compared to the other crystal groups (Figure 2A, D). UA and CaP crystals experienced the greatest dissolution compared to the other crystal groups, especially during the 1 min marker, with a continual decline throughout the 10 min (Figure 2B–D). Meanwhile, the UA+CaP crystal group experienced greater dissolution compared to the MSU+CaP group, throughout the 10 mins duration (Figure 2A, D). Crystal sizes remained significantly greater for MSU mixed crystals compared to UA mixed crystals, up until the 10-min mark (Figure 2C–D). Interestingly, while the inclusion of melamine had slowed dissolution as expected, MSU+CaP+melamine mixed crystal dissolution rate was slightly greater compared to that of UA+CaP+melamine (Figure 2E–G), which was reversed in the non-melamine conditions in Figure 2A. These results suggest that UA may have a stronger interaction with CaP in presence of melamine compared to MSU and CaP mixed crystals. Since HCit, among other citrate-derivatives, is a preferable agent to dissolute CaP crystals21 we exposed UA+CaP and MSU+CaP crystals in presence/absence of melamine (Figure 2E). Notably, HCit was able to dissolute all crystal groups, even in presence of melamine (Figure 2H–J), and as expected, the UA+CaP mixed crystal has exhibited greater dissolution in presence of HCit than in presence of melamine with HCit. Similarly, MSU+CaP crystals were dissolved faster than MSU+CaP+Mel crystals in presence of HCit (Figure 2H–J). However, the dissolution rate did not differ significantly between both MSU+CaP+HCit and MSU+CaP+HCit groups through the 3 mins duration (Figure 2J). These results suggest that while melamine stabilize formed mixed crystal aggregates, the presence of HCit can facilitate the dissolution of these crystal aggregates. The findings presented may provide a basis for treating melamine-related renal toxicity using HCit. Melamine is at its most toxic when combined with other substances, especially in the case of melamine cyanurate, which is melamine combined with cyanuric acid19; however, does not pose as much of a threat as an individual homogeneous substance. HCit may act on melamine, calcium and UA crystal surfaces by creating defects on their surfaces and lead to reduced growth rate in supersaturated conditions30. However, future studies would need to be performed to show the effectiveness of HCit in dissoluting melamine-related compounds.
Figure 2.

(A) Time-dependent dissolutions of calcium phosphate (CaP), uric acid (UA), monosodium urate (MSU), UA+CaP, or MSU+CaP crystals in diH2O at (B) 1 min, (C) 3 min, and (D) 10 min timepoints. (E) Time-dependent dissolutions of MSU+CaP and melamine (MSU+CaP+Mel) or UA+CaP+Mel crystals in diH2O at (F) 1 min and (G) 3 min timepoints. (H) Time-dependent dissolutions of UA+CaP, MSU+CaP, UA+CaP+Mel, or MSU+CaP+Mel crystals with hydroxycitrate (HCit) in diH2O at (I) 1 min and (J) 3 min timepoints. Bar diagrams depicting relative crystal sizes are represented in mean+SEM. *, P<0.05; **, P<0.01. Relative crystal sizes were measured using ImageJ software.
3.3. CaP crystals within CaP+UA mixed crystals changes its polarized property with the concentration dependent addition of UA
We previously demonstrated that the typically low birefringence signal of CaP crystals becomes higher with the addition of melamine, along with larger aggregates21. While UA has higher birefringence than CaP crystals, we show that each addition of UA (2.1, 4.2, and 6.25 mM) with CaP significantly augments not only aggregate birefringence, but also the relative birefringence intensity of the mixed crystal (Figure 3A). Individual stained and unstained crystals were identified in brightfield and birefringence for UA, MSU, and CaP (Figure S3–S4). Birefringent UA+CaP also corresponded with larger crystal aggregates as shown in the brightfield images (Figure 3A, B). These results show that CaP crystals could aggregate in presence of greater UA concentrations. Previous studies demonstrated that CaP brushite and hydroxyapatite seeds were effective in stabilizing the aggregation of urate crystals31, but further investigation may be required to assess differences in UA crystal aggregation.
Figure 3.

(A) Calcium phosphate (CaP) and uric acid (UA) crystal formations were imaged with brightfield and polarized light microscopy after implementing UA (0, 2.1, 4.2, or 6.25 mM). (B) ImageJ measurements of relative crystal sizes are represented as a bar diagram in mean+SEM. *, P<0.01. Scale bar = 10 μm.
3.4. Melamine prevents the dissolution of UA, CaP and mixed crystals in relatively physiological conditions
Since we found negative effects on the dissolution of UA and mixed crystals in the presence of melamine and HCit in buffered solutions (Figure 2A–C), we examined such effects in a physiological condition by implementing artificial urine (Figure 4A–D). UA+CaP crystals with or without melamine partially dissolved upon the addition of HCit, observable from about 1 min exposure (Figure 4A–B). UA+CaP and UA+CaP+HCit crystals yielded the steepest dissolution from 0 to 3 mins, but HCit continued to dissolve the UA+CaP at a higher rate compared to the other crystal groups (Figure 4A–C). Interestingly, the dissolution rates were lower in the presence of melamine between 3 to 10 mins, perhaps due to the support of melamine interaction and increased stability (Figure 4A, C–D). Therefore, UA+CaP+HCit experienced the greatest dissolution compared to the other the crystal groups (Figure 4A–D). These results suggest that melamine stabilized the UA, CaP and mixed crystals in relatively physiological conditions, and the retention of those crystals were further augmented by melamine, even in presence of HCit. In a previous study, melamine and UA acid clusters were not only broken by potassium citrate, but also makes the disaggregated composite accessible to water32. Moreover, the interaction energy between UA and potassium citrate was found to be greater than that of melamine and potassium, and melamine and UA32. However, while potassium citrate is a commonly prescribed treatment method for calcium or UA stones, it has the risk of promoting CaP kidney stone formation by alkalinizing the urine24. On the other hand, HCit is expected to not alter the pH of the urine making it favorable for CaP crystal treatment30.
Figure 4.
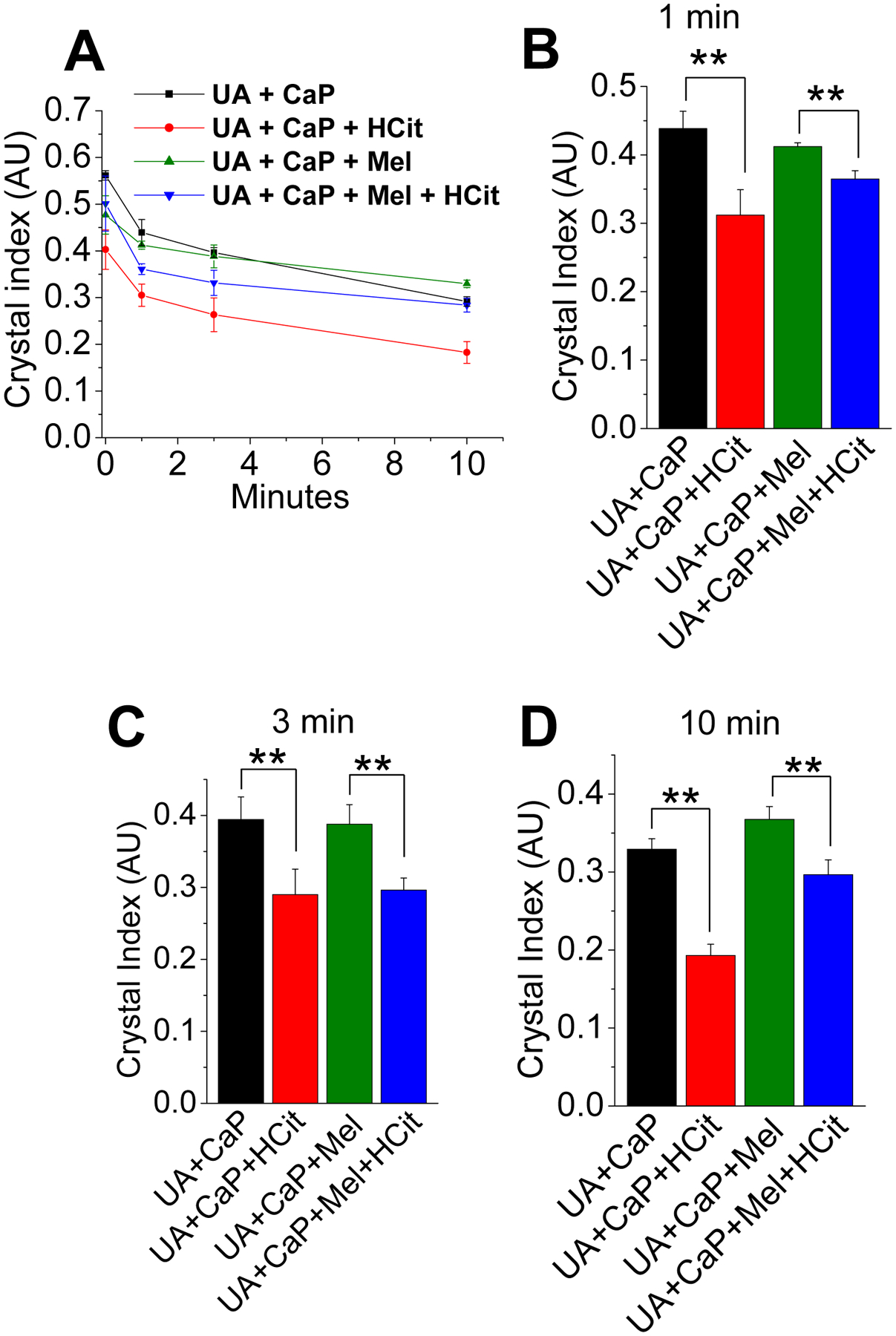
(A) Time-dependent dissolutions of uric acid (UA) and/or calcium phosphate (CaP) crystals in with melamine (Mel) and/or hydroxycitrate (HCit) at (B) 1 min, (C) 3 min, and (D) 10 min timepoints in artificial urine. Bar diagrams depicting relative crystal index are represented in mean+SEM. **, P<0.01. Relative crystal sizes were measured using ImageJ software.
4. Conclusions
Our study demonstrates that CaP crystals form mixed crystal aggregates with the rise in UA concentrations, while melamine can stabilize these heterogeneous crystals and prevent dissolution. These results, however, would need to be replicated in in-vivo settings to observe its efficacy. Whether the crystals formed in our study are co-crystals are up to debate33,34, but we adhered to the definition that “co-crystals are defined multiple component crystals where all components are solid under ambient conditions when in their pure form”35. For the definition of mixed crystals, Villeneuve et al. stated that “mixed crystals (also called solid solutions) can result when structurally related compounds are accommodated at essentially random sites in the lattice in quantities that can be varied continuously over certain ranges”36. In our case, since we are varying the concentrations of melamine or UA within saturated solution mixtures to crystals that are formed, the resulting crystals can fit within the definition. Furthermore, while it is possible that melamine can form a salt with citrate, which may contribute to competitive inhibition of melamine and urate crystallization37, however the interaction between melamine and hydroxycitrate leads to competing crystallization is unknown. Nonetheless, our findings are most interesting in the context of recurrence rate among individuals who have received some form of intervention or treatment remains as high as 45%, for calcium stone or calcium urolithiasis patients38. As stated earlier, several events play vital roles in stone formation, however, crystal growth can be adequately reduced and subsequently eliminated when potential regulators of crystal retention and stabilization are identified and targeted. Thus, it is imperative to understand what might be causing this increased retention of UA leading to UA and CaP mixed crystal recurrences.
Supplementary Material
Figure S1. Schematic diagram for crystal staining of uric acid (UA; methylene blue), calcium phosphate (CaP; AR pH 4.3), and melamine (Mel; Oil Red O); and dissolution of UA, CaP, and/or Mel in diH2O or artificial urine in 1, 3, or 10 min time-lapse intervals.
Figure S2. (A) Uric acid (UA) and melamine (Mel), (B) calcium phosphate (CaP) and UA crystals, and (C) CaP, Mel+UA, CaP+UA, Mel, or UA crystals in diH2O. Crystals were stained with methylene blue (UA and MSU) or CaP (AR pH=4.3). Scale bars = 10 μm.
Figure S3. Brightfield and birefringence images of uric acid (UA), monosodium urate (MSU), and calcium phosphate (CaP) crystals in diH2O. Crystals were stained with methylene blue (UA or MSU) and CaP (AR pH=4.3). Scale bars = 10 μm.
Figure S4. Unstained (A) UA and CaP crystals, (B) UA+Mel crystals, and (C) UA+Mel, UA, and CaP crystals in diH2O are depicted in the images. Scale bars = 10 μm.
Melamine facilitates the interaction and aggregation of UA and CaP crystals
Melamine induced mixed crystal retention in absence of hydroxycitrate
UA crystal birefringence increases with addition of CaP
Melamine prevents dissolution of UA-mixed crystals in physiological conditions
Funding:
We acknowledge the support from National Institute of Diabetes and Digestive and Kidney Diseases (DK102043) and National Institute of Biomedical Imaging and Bioengineering (EB021483) to B.C.B. These funding agencies were not involved in the preparation of the article, study design, collection, analyses, and interpretation of data, writing of the report, or decision to submit this article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Author Statement:
Madison Ezell: Conceptualization, Methodology, Software, Experimentation, Draft of Manuscript, and Reviewing the revised manuscript. Samuel Shin: Conceptualization, Methodology, Software, Draft of Manuscript, Reviewing, and Editing the revised manuscript. Yuyan Chen: Methodology, Software, and Experimentation, and reviewing the revised manuscript. Khanh Ly: Methodology, Software, Reviewing, and Editing and reviewing the revised manuscript. Leron Maddi: Methodology, Software, and Experimentation and reviewing the revised manuscript. Christopher B. Raub: Methodology, Software, Reviewing, and Editing the revised manuscript. Bidhan C. Bandyopadhyay: Conceptualization, Methodology, Software, Draft of Manuscript, Reviewing, and Editing the revised manuscript. All authors discussed the results and commented on the manuscript.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflicts of Interest:
The authors declare no conflict of interest.
References:
- 1.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003. May;63(5):1817–23. Doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 2.Alelign T, Petros B. Kidney Stone Disease: An Update on Current Concepts. Adv Urol. 2018;2018:3068365. Published 2018 Feb 4. Doi: 10.1155/2018/3068365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie B, Halter TJ, Borah BM, Nancollas GH. Aggregation of Calcium Phosphate and Oxalate Phases in the Formation of Renal Stones. Cryst Growth Des. 2015;15(1):204–211. Doi: 10.1021/cg501209h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pak CY, Waters O, Arnold L, Holt K, Cox C, Barilla D. Mechanism for calcium urolithiasis among patients with hyperuricosuria: supersaturation of urine with respect to monosodium urate. J Clin Invest. 1977;59(3):426–431. Doi: 10.1172/JCI108656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirejczyk JK, Porowski T, Filonowicz R, et al. An association between kidney stone composition and urinary metabolic disturbances in children. J Pediatr Urol. 2014;10(1):130–135. Doi: 10.1016/j.jpurol.2013.07.010 [DOI] [PubMed] [Google Scholar]
- 6.Coe FL, Raisen L. Allopurinol treatment of uric-acid disorders in calcium-stone formers. Lancet. 1973;1(7795):129–131. Doi: 10.1016/s0140-6736(73)90197-9 [DOI] [PubMed] [Google Scholar]
- 7.Li M, Han D, & Gong J (2022). What roles do alkali metal ions play in the pathological crystallization of uric acid? CrystEngComm, 24, 3749–3761. [Google Scholar]
- 8.Geng X, Meegan J, Smith C, Sakhaee K, Rimer JD. Crystallization of Hierarchical Ammonium Urate: Insight into the Formation of Cetacean Renal Stones. Cryst Growth Des. 2019;19(11):6727–6735. [Google Scholar]
- 9.Li M, Tang W, Gong J. Unusual shape-preserved pathway of a core-shell phase transition triggered by orientational disorder. IUCrJ. 2023;10(Pt 1):38–51. Published 2023 Jan 1. doi: 10.1107/S2052252522011034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Presores JB, Swift JA. Solution-mediated phase transformation of uric acid dihydrate. CrystEngComm. 2014;16(31):7278–7284. doi: 10.1039/c4ce00574k [DOI] [Google Scholar]
- 11.Tzelves L, Mourmouris P, Skolarikos A. Outcomes of dissolution therapy and monitoring for stone disease: should we do better?. Curr Opin Urol. 2021;31(2):102–108. Doi: 10.1097/MOU.0000000000000844 [DOI] [PubMed] [Google Scholar]
- 12.Chutipongtanate S, Chaiyarit S, Thongboonkerd V. Citrate, not phosphate, can dissolve calcium oxalate monohydrate crystals and detach these crystals from renal tubular cells. Eur J Pharmacol. 2012;689(1–3):219–225. Doi: 10.1016/j.ejphar.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 13.Petrova EV; Gvozdev NV; Rashkovich LNG Growth and dissolution of calcium oxalate monohydrate (COM) Crystals. J. Optoelectron. Adv. Mater 2004, 6 (1), 261–268. [Google Scholar]
- 14.Dent CE, Sutor DJ. Presence or absence of inhibitor of calcium-oxalate crystal growth in urine of normal and of stoneformers. Lancet. 1971;2(7728):775–778. Doi: 10.1016/s0140-6736(71)92737-1 [DOI] [PubMed] [Google Scholar]
- 15.Gröbner W, Zöllner N. Zur Beeinflussung der Purin- und Pyrimidinsynthese durch Allopurinol [The influence of allopurinol on purine- and pyrimidine synthesis (author’s transl)]. Klin Wochenschr. 1978;53(16):255–260. [PubMed] [Google Scholar]
- 16.Suzuki I, Yamauchi T, Onuma M, Nozaki S. Allopurinol, an inhibitor of uric acid synthesis—can it be used for the treatment of metabolic syndrome and related disorders?. Drugs Today (Barc). 2009;45(5):363–378. Doi: 10.1358/dot.2009.45.5.1370460 [DOI] [PubMed] [Google Scholar]
- 17.LaRosa C, McMullen L, Bakdash S, et al. Acute renal failure from xanthine nephropathy during management of acute leukemia. Pediatr Nephrol. 2007;22:132–5. [DOI] [PubMed] [Google Scholar]
- 18.Pais VM Jr, Lowe G, Lallas CD, Preminger GM, Assimos. Xanthine urolithiasis. Urology. 2006;67(5):1084.e9–1084.e11 [DOI] [PubMed] [Google Scholar]
- 19.Prior TJ, Armstrong JA, Benoit DM, et al. The structure of the melamine–cyanuric acid co-crystal. CrystEngComm. 2013;15:5838–5843. [Google Scholar]
- 20.Gombedza F, Evans S, Shin S, et al. Melamine promotes calcium crystal formation in three-dimensional microfluidic device. Sci Rep. 2019;9(1):875. Published 2019 Jan 29. Doi: 10.1038/s41598-018-37191-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boadi EA, Deems NJ, Raub CB, Bandyopadhyay BC. Matting Calcium Crystals by Melamine Improves Stabilization and Prevents Dissolution. Cryst Growth Des. 2019;19(11):6636–6648. Doi: 10.1021/acs.cgd.9b01036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grases F, Sanchis P, Isern B, Perelló J, Costa-Bauzá A. Uric acid as inducer of calcium oxalate crystal development. Scand J Urol Nephrol. 2007;41(1):26–31. Doi: 10.1080/00365590600831571 [DOI] [PubMed] [Google Scholar]
- 23.Grases F, Sanchis P, Perelló J, Costa-Bauzá A. Role of uric acid in different types of calcium oxalate renal calculi. Int J Urol. 2006;13(3):252–256. Doi: 10.1111/j.1442-2042.2006.01262.x [DOI] [PubMed] [Google Scholar]
- 24.Goldfarb DS. A woman with recurrent calcium phosphate kidney stones. Clin J Am Soc Nephrol. 2012;7(7):1172–1178. Doi: 10.2215/CJN.00560112 [DOI] [PubMed] [Google Scholar]
- 25.Wei Z, Amponsah PK, Al-Shatti M, Nie Z, Bandyopadhyay BC. Engineering of polarized tubular structures in a microfluidic device to study calcium phosphate stone formation. Lab Chip. 2012;12(20):4037–4040. Doi: 10.1039/c2lc40801e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sours RE, Fink DA, Swift JA. Dyeing uric acid crystals with methylene blue. J Am Chem Soc. 2002. Jul 24;124(29):8630–6. Doi: 10.1021/ja026083w. [DOI] [PubMed] [Google Scholar]
- 27.Kleeberg J Färbung von Harnsäurekristallen mit Methylenblau [Staining of uric acid crystals with methylene blue]. Z Urol Nephrol. 1972. Aug;65(8):619–29. [PubMed] [Google Scholar]
- 28.Pak CY, Hayashi Y, Arnold LH. Heterogeneous nucleation with urate, calcium phosphate and calcium oxalate. Proc Soc Exp Biol Med. 1976;153(1):83–87. Doi: 10.3181/00379727-153-39485 [DOI] [PubMed] [Google Scholar]
- 29.Martillo MA, Nazzal L, Crittenden DB. The crystallization of monosodium urate. Curr Rheumatol Rep. 2014;16(2):400. Doi: 10.1007/s11926-013-0400-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung J, Granja I, Taylor MG, Mpourmpakis G, Asplin JR, Rimer JD. Molecular modifiers reveal a mechanism of pathological crystal growth inhibition. Nature. 2016;536(7617):446–450. [DOI] [PubMed] [Google Scholar]
- 31.Moe OW, Xu LHR. Hyperuricosuric calcium urolithiasis. J Nephrol. 2018;31(2):189–196. Doi: 10.1007/s40620-018-0469-3 [DOI] [PubMed] [Google Scholar]
- 32.Chattaraj KG, Paul S. Investigation on the Mechanisms of Synchronous Interaction of K3Cit with Melamine and Uric Acid That Avoids the Formation of Large Clusters. J Chem Inf Model. 2020;60(10):4827–4844. [DOI] [PubMed] [Google Scholar]
- 33.Stahly GP. Diversity in single- and multiple-component crystals. the search for and prevalence of polymorphs and cocrystals. Crystal Growth & Design. 2007;7(6):1007–1026. doi: 10.1021/cg060838j [DOI] [Google Scholar]
- 34.Aitipamula S, Banerjee R, Bansal AK, et al. Polymorphs, salts, and cocrystals: What’s in a name? Crystal Growth & Design. 2012;12(5):2147–2152. doi: 10.1021/cg3002948 [DOI] [Google Scholar]
- 35.Shan N, Zaworotko MJ. The role of cocrystals in pharmaceutical science. Drug Discov Today. 2008;13(9–10):440–446. doi: 10.1016/j.drudis.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 36.Villeneuve NM, Dickman J, Maris T, Day GM, Wuest JD. Seeking rules governing mixed molecular crystallization. Crystal Growth & Design. 2022;23(1):273–288. doi: 10.1021/acs.cgd.2c00992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchewka MK, Pietraszko A. Structure and spectra of Melaminium Citrate. Journal of Physics and Chemistry of Solids. 2003;64(11):2169–2181. doi: 10.1016/s0022-3697(03)00218-x [DOI] [Google Scholar]
- 38.Zisman AL. Effectiveness of Treatment Modalities on Kidney Stone Recurrence. Clin J Am Soc Nephrol. 2017;12(10):1699–1708. doi: 10.2215/CJN.11201016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Schematic diagram for crystal staining of uric acid (UA; methylene blue), calcium phosphate (CaP; AR pH 4.3), and melamine (Mel; Oil Red O); and dissolution of UA, CaP, and/or Mel in diH2O or artificial urine in 1, 3, or 10 min time-lapse intervals.
Figure S2. (A) Uric acid (UA) and melamine (Mel), (B) calcium phosphate (CaP) and UA crystals, and (C) CaP, Mel+UA, CaP+UA, Mel, or UA crystals in diH2O. Crystals were stained with methylene blue (UA and MSU) or CaP (AR pH=4.3). Scale bars = 10 μm.
Figure S3. Brightfield and birefringence images of uric acid (UA), monosodium urate (MSU), and calcium phosphate (CaP) crystals in diH2O. Crystals were stained with methylene blue (UA or MSU) and CaP (AR pH=4.3). Scale bars = 10 μm.
Figure S4. Unstained (A) UA and CaP crystals, (B) UA+Mel crystals, and (C) UA+Mel, UA, and CaP crystals in diH2O are depicted in the images. Scale bars = 10 μm.


