Abstract
Study Objectives
Poor sleep-in people with inflammatory bowel disease (IBD) has been associated with worse quality of life, along with anxiety, depression, and fatigue. This meta-analysis aimed to determine the pooled prevalence of poor sleep-in IBD.
Methods
Electronic databases were searched for publications from inception to November 1st 2021. Poor sleep was defined according to subjective sleep measures. A random effects model was used to determine the pooled prevalence of poor sleep-in people with IBD. Heterogeneity was investigated through subgroup analysis and meta-regression. Publication bias was assessed by funnel plot and Egger’s test.
Results
519 Studies were screened with 36 studies included in the meta-analysis incorporating a total of 24 209 people with IBD. Pooled prevalence of poor sleep-in IBD was 56%, 95% CI (51–61%) with significant heterogeneity. The prevalence did not differ based on the definition of poor sleep. Meta-regression was significant for increased prevalence of poor sleep with increase in age and increased of prevalence of poor sleep with objective IBD activity but not subjective IBD activity, depression, or disease duration.
Conclusions
Poor sleep is common in people with IBD. Further research is warranted to investigate if improving sleep quality in people with IBD will improve IBD activity and quality of life.
Keywords: immune function, insomnia, sleep deprivation
Statement of Significance.
This meta-analysis of 36 studies, incorporating over 24 000 people with inflammatory bowel disease (IBD), showed that poor sleep quality is common in those with IBD and more frequent than reported estimates for fatigue and mental health conditions in people with IBD. Meta-regression showed that the differences in poor sleep between IBD populations related in part to IBD activity confirmed by objective measures. The presence of IBD related symptoms alone was not found to be significant.
Introduction
Sleep is an important biologic function with increasing attention turning to its role in overall health. Abnormal sleep has been linked to poor health outcomes including cardiovascular disease [1], metabolic syndrome [2] and increased all-cause mortality in some studies [3], in addition to significant economic cost in the form of decreased productivity and increased health care utilization [4]. Sleep has been shown to regulate a number of gastrointestinal functions including gastrointestinal motility and secretion [5]. Sleep disruption has been associated with increased levels of inflammatory cytokines, such as IL-6, and TNF-α, that have been implicated in the pathogenesis of inflammatory bowel disease [6–8]. Poor sleep has been investigated in some chronic inflammatory diseases [9] and found to be prevalent in rheumatoid arthritis [10] and multiple sclerosis [11].
Inflammatory bowel disease (IBD) is an relapsing-remitting autoimmune disorder that results from a complex interaction between genetics and the environment [12]. IBD leads to a variety of debilitating symptoms such as diarrhea and abdominal pain. It is also associated with so called extra-intestinal manifestations, that include joint pain and skin rashes amongst others [13, 14]. Subjective assessment of the activity of IBD involves patient reported symptoms and utilizes validated scoring systems to ascertain the severity of IBD activity [15]. The reliability of subjective assessment of IBD activity is limited by the high prevalence of so called irritable bowel syndrome (IBS) like symptoms [16]. These IBS-like symptoms are often indistinguishable from the symptoms of active IBD and can occur in the absence of active IBD. Differentiating inactive IBD with IBS-like symptoms from active IBD requires the use of objective measures of IBD activity that directly confirm the presence of inflammation. These objective measures include endoscopic procedures such as colonoscopy, imaging such as magnetic resonance imaging and stool testing for markers of inflammation.
The relationship between IBD activity and sleep quality has been investigated previously with mixed results. A recent meta-analysis on the subject reached the conclusion that subjective sleep quality is worse in those with active IBD [17]. IBD related symptoms themselves, such as diarrhea and abdominal pain, may well disruptive sleep [18], however other studies suggest that endoscopically or histologically active IBD in the absence of any IBD symptoms may be sufficient to disrupt sleep [19, 20]. Extra-intestinal manifestations may also be important with a study suggesting those with enteropathic arthropathy were more likely to have poor sleep than those without [21]. Others suggest that psychosocial factors may be important [22], and in particular depression has been frequently associated with poor sleep [22–29] in an IBD population. Fatigue has also been associated with sleep quality [30–36] and is known to be highly prevalent in people with IBD [37].
Sleep may also be relevant to the development of IBD with data from the Nurses’ Health Study showing that sleep duration was associated with the risk of ulcerative colitis, but not Crohn’s disease [38]. Sleep quality may also have prognostic value in Crohn’s disease with sleep associations seen with increased likelihood of hospitalization and risk of relapse. The effect of IBD therapeutic agents on sleep has been investigated with a prospective study showing improvement in sleep following introduction of biologic therapy [27]—this of course paralleled an improvement in IBD activity. Others have not been able to demonstrate a relationship between the different IBD therapies and sleep quality [39].
In a recent meta-analysis subjective sleep quality was worse in those with IBD than controls [17]. This may be due to IBD associated symptoms, however there is some literature suggesting that those with inactive IBD also appear to have poor sleep [29, 33, 40, 41], although it is unclear if sleep quality in inactive IBD is worse than that of controls. Much of this data relates to subjective sleep quality with few studies incorporating objective sleep quality. Results from studies incorporating objective sleep quality are so far inconsistent noting a recent meta-analysis unable to establish an associated between objective sleep quality and IBD activity [17]. Furthermore, there was significant heterogeneity present in previous meta-analyses that is not well explained [17, 18].
This meta-analysis aimed to extend the work of the previous meta-analyses [17] by establishing the pooled prevalence of poor sleep-in IBD and exploring any heterogeneity that may be present. To the author’s knowledge there has been no previously published estimate of the prevalence of poor sleep-in IBD. An improved understanding of the burden of poor sleep-in IBD may lead to further investigation and interventional studies in this area that may result in improved quality of life for this population.
Methods
This systematic review and meta-analysis was prospectively registered with the International Prospective Register of Ongoing Systematic Reviews [42]. It was performed according to the preferred reporting item for systematic reviews and meta-analyses (PRISMA) guidelines [43].
Search strategy
Pubmed, MEDLINE, and PsychINFO were searched from inception to November 2021, including articles published in the English language using the following search string: (sleep OR circadian OR insomnia OR apnea) AND [(inflammatory bowel disease) OR (crohn’s disease) OR (ulcerative colitis) OR IBD OR crohn’s OR colitis)].
Eligibility criteria
Studies were included if they met the following criteria: (1) cross-sectional, observational, case control, cohort or randomized controlled trial available (2) included a distinct population of people with inflammatory bowel disease (age ≥ 18 years old). Studies with control groups of a healthy population accepted. (3) Sleep quality assessment using a validated subjective patient reported measure of sleep.
Exclusion criteria included: (1) inappropriate study population such a pediatric or adolescent population. (2) Case report or review
Study selection
The first author (AB) performed the literature review and two other authors (PS and JB) independently screened full texts against eligibility criteria, with disagreement resolved by discussion with involvement of another author (RM) when required.
Data collection
Data collection was performed by AB. A pre-defined spreadsheet was used for data collection. Items collected for each study population included type of IBD, age, gender, study design, sample size, sleep assessment, outcome of study, disease activity in terms of subjective scores of disease activity or objective measures of disease activity, IBD disease duration, depression in terms of scores assessing depressive symptoms.
Study quality assessment
Risk of bias in individual studies was assessed according to study design. Cross-sectional or observational studies were assessed according to modified Newcastle-Ottawa Scale. Cohort or case control studies were assessed according to Newcastle-Ottawa Scale [44].
Statistical analysis was performed using Stata SE 16 (StataCorp, College Station, TX, USA) and the ‘metaprop’ [45] command to estimate the pooled prevalence of poor sleep-in people with inflammatory bowel disease. Heterogeneity among studies was assessed using the I2 statistic with I2 > 50% considered to indicate substantial heterogeneity. A random effects model was used [45]. A Forest plot was performed to estimate individual and pooled effect sizes with associated 95% CI. Publication bias was assessed using funnel plots with significant visual asymmetry used to indicate publication bias. Egger’s test with p values less than .05 were considered to indicate significant publication bias. Trim-fill analysis was undertaken. In order to investigate sources of heterogeneity subgroup analysis and meta-regression were conducted.
Results
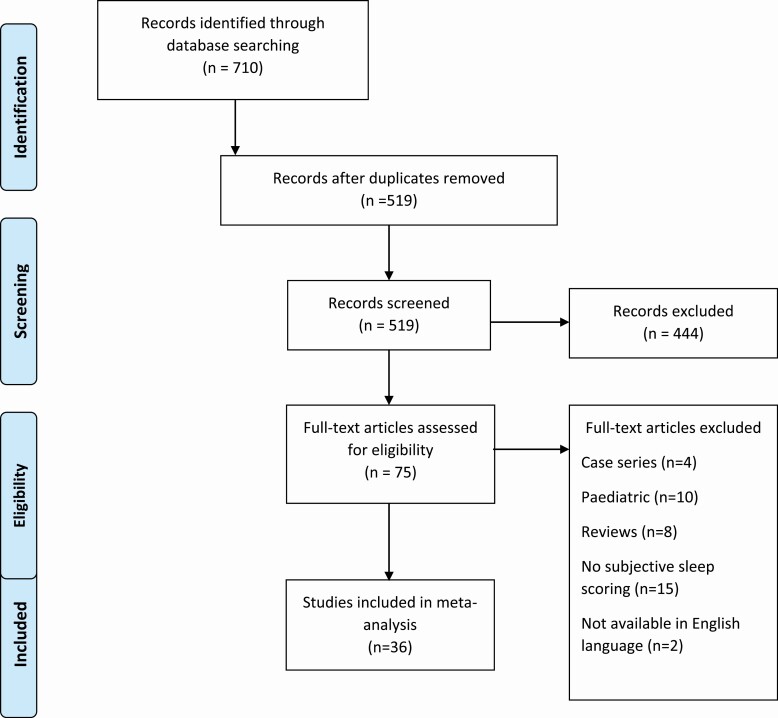
The literature search (see Figure 1) identified 519 records following removal of duplicates, which further reduced to 75 records following screening. Following exclusions 36 records were included in the meta-analysis incorporating 24 209 people with IBD.
Figure 1.
PRISMA flowchart—selection of studies and results of literature search for review and meta-analysis.
Study characteristics
Characteristics of included studies can be seen in Table 1 and further data in Supplementary Table 1. Publication dates ranged from 2011 to 2020. Most of the studies were single centre (n = 20), two were multi-centre, three recruited from an existing IBD registry, two recruited from a longitudinal cohort study, three used internet survey data, and two used data from a nationwide IBD cohort. The majority incorporated a cross-sectional design. No study included sample size calculations for prevalence estimates, and no study incorporated a population sampling regimen. Sample size ranged from 34 to 10 634 participants. The mean age of participants ranged from 25 to 45 years. The proportion of female participants ranged from 42 to 72%.
Table 1.
Studies with people with inflammatory bowel disease with subjective sleep quality assessment
| Study | Year | Country | Poor sleep definition | Study population | Population | Sample size | Percentage female (%) | Age | Number with Crohn’s disease | Proportion with poor sleep | Study summary |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdalla et al. [49] | 2017 | USA | PROMIS-SD t score > 50 | Patients within Crohn’s colitis foundation of America Partners Cohort | IBD | 6309 | 71 | 44 | 3947 | 0.54 | IBD-IBS diagnosis was associated with increased narcotic usage and poor sleep |
| Ali et al. [20] | 2013 | USA | PSQI > 5 | Single centre—clinic | IBD | 41 | 66 | 37 | 23 | 0.87 | Clinically active IBD was associated with poor sleep |
| Ananthakrishnan et al. [29] | 2013 | USA | PROMIS-SD t score > 50 | CCFA partners cohort | IBD | 3173 | 72 | 44 | 2079 | 0.6 | Sleep disturbance was associated with an increased risk of disease flares in Crohn’s disease but not ulcerative colitis |
| Ballou et al. [62] | 2018 | USA | PSQI > 5 | Single centre—clinic | IBD | 44 | 71 | 42 | 22 | 0.54 | IBD patients at a tertiary clinic have poorer sleep than healthy controls |
| Bazin et al. [63] | 2019 | France | PSQI > 5 | Single centre—clinic | Crohn’s disease | 34 | 44 | 40 | 34 | 0.35 | Sleep efficiency is lower in those active Crohn’s disease than in remission |
| Bucci et al. [64] | 2018 | Italy | PSQI > 5 | Single centre—clinic | IBD | 47 | 53 | 38 | 28 | 0.38 | Bruxism was associated with pathological sleep |
| Calvo et al. [26] | 2020 | Spain | PSQI > 5 | Single centre—clinic | IBD | 102 | 43 | 45 | 51 | 0.54 | Poor sleep quality is present in more than half of people with IBD |
| Chakradeo et al. [65] | 2018 | USA | PSQI > 5 | Single centre—clinic | IBD | 115 | 62 | 41 | 0.63 | Later chronotype and markers of circadian misalignment were associated with IBD specific complications and lower quality of life | |
| Chrobak et al. [31] | 2018 | Poland | PSQI > 5 | Single centre—clinic | IBD | 72 | 42 | 42 | 34 | 0.68 | Chronotype preferences contribute to fatigue in IBD |
| Frigstad et al. [32] | 2018 | Norway | BSNQ | Multi-centre | IBD | 405 | 49 | 40 | 227 | 0.19 | Sleep and depressive symptoms were associated with total fatigue scores |
| Gîlc-Blanariu et al. [22] | 2020 | Romania | PSQI > 5 | Single centre—clinic | IBD | 110 | 47 | 44 | 34 | 0.75 | Poor sleep is frequent in IBD and associated with psychological distress |
| Gingold-Belfer et al. [66] | 2014 | Israel | PSQI > 5 | single centre—clinic | Crohn’s disease | 108 | 47 | 40 | 108 | 0.37 | Poor sleep is associated with active Crohn’s disease but not inactive disease |
| Graff et al. [33] | 2011 | USA | PSQI > 5 | Manitoba IBD cohort | IBD | 318 | 60 | 43 | 160 | 0.49 | Poor sleep is prevalent in those with active IBD but also in those with inactive IBD |
| Habibi et al. [67] | 2019 | Iran | PSQI > 5 | Single centre—clinic | IBD | 68 | 63 | 38 | 24 | 0.32 | Poor sleep is prevalent in those with IBD including those in remission |
| Hashash et al. [34] | 2016 | USA | PSQI > 5 | Single centre—registry | IBD | 685 | 53 | 44 | 418 | 0.54 | Fatigue was associated wit poor sleep and psychopathology |
| Hood et al. [24] | 2018 | USA | PSQI > 5 | Multi-centre—clinic | IBD | 47 | 0 | 0.59 | Poor sleep is prevalent in ulcerative colitis and related to depression | ||
| IsHak et al. [48] | 2017 | USA | PROMIS-SD t score > 50 | Single centre—clinic | IBD | 110 | 43 | 42 | 62 | 0.6 | Patient’s with Crohn’s disease demonstrated worse impairments in quality of life and function than those with ulcerative colitis |
| Iskandar et al. [68] | 2020 | USA | PSQI > 5 | Single centre—clinic | Crohn’s disease | 61 | 32 | 61 | 0.57 | Crohn’s disease patients reported more disturbed sleep than controls but this was not confirmed with objective measures | |
| Kani et al. [69] | 2019 | Turkey | PSQI > 5 | Single centre—clinic | IBD | 136 | 58 | 39 | 72 | 0.59 | Dream anxiety may lead to sleep disturbance in patients with IBD |
| Kappelman et al. [47] | 2014 | USA | PROMIS-SD t score > 50 | internet cohort multi-centre | IBD | 10634 | 71 | 44 | 6689 | 0.58 | Health outcomes measures differ between patients with IBD and the general population |
| Keskin et al. [70] | 2020 | Turkey | PSQI > 5 | Single centre—clinic | IBD | 89 | 56 | 37 | 41 | 0.51 | IBD risk factor for sleep disturbance with eveningness more common than in controls |
| Lee et al. [39] | 2018 | USA | PSQI > 5 | Single centre—clinic | IBD | 56 | 66 | 45 | 39 | 0.82 | Treatment with immuno-modulators or biologics does not appear to improve sleep quality |
| Marinelli et al. [25] | 2020 | Italy | PSQI > 5 | Single centre—clinic | IBD | 166 | 47 | 44 | 87 | 0.67 | Sleep quality was not associated with IBD activity but with mood, disability and quality of life |
| Michalopoulos et al. [19] | 2018 | Greece | PSQI > 5 | Single centre—clinic | IBD | 90 | 46 | 40 | 54 | 0.45 | In IBD in clinical remission endoscopic findings was associated with poor sleep |
| Schindlbeck et al. [36] | 2016 | Germany | PSQI > 5 | Single centre—clinic | IBD | 43 | 72 | 47 | 30 | 0.61 | Restless leg syndrome in inflammatory disease with associated with worse quality of life |
| Sobolewska-Włodarczyk et al. [71] | 2018 | Poland | PSQI > 5 | Single centre—clinic | IBD | 65 | 43 | 40 | 30 | 0.69 | Poor sleep-in IBD related to IBD activity |
| Sobolewska-Włodarczyk et al. [72] | 2020 | Poland | PSQI > 5 | Single centre—clinic | IBD | 65 | 47 | 40 | 30 | 0.57 | Specific adipokine profiles are associated with circadian rhythms |
| Sochal et al. [23] | 2020 | Poland | PSQI > 5 | Single centre—clinic | IBD | 133 | 55 | 37 | 68 | 0.43 | Poor sleep-in IBD is common and related to mood |
| Sofia et al. [73] | 2019 | USA | PSQI>5 | Single centre—clinic | IBD | 92 | 62 | 43 | 92 | 0.77 | Poor sleep is common in Crohn’s disease and associated with adverse outcomes |
| Stevens et al. [27] | 2016 | USA | PROMIS-SD t score > 50 | Single centre—registry | IBD | 160 | 48 | 35 | 94 | 0.44 | Vedolizumab and anti-TNF biologics were associated with improvement in sleep quality |
| Takahara et al. [74] | 2016 | Japan | PSQI > 5 | Single centre—clinic | IBD | 80 | 42 | 42 | 34 | 0.4 | Restless leg syndrome occurs frequently in Japanese patients with IBD |
| Uemura et al. [75] | 2016 | Japan | PSQI > 5.5 | Single centre—clinic | IBD | 136 | 44 | 42 | 48 | 0.44 | Sleep disturbance common in Japanese IBD patients and associated with poor quality of life |
| van Langenberg et al. [76] | 2017 | Australia | PSQI > 5 | Single centre—clinic | IBD | 49 | 58 | 44 | 49 | 0.63 | Crohn’s disease patients demonstrated subtle cognitive impairment |
| Wilson et al. [28] | 2014 | USA | PROMIS-SD t score > 50 | single centre—registry | IBD | 131 | 55 | 25 | 78 | 0.44 | High CRP associated with poor sleep irrespective of night-time disruptions |
| Zargar et al. [77] | 2019 | Iran | PSQI > 5 | Single centre—clinic | IBD | 115 | 49 | 38 | 30 | 0.51 | IBS may worsen sleep disturbance in IBD |
| Zhang et al. [21] | 2020 | China | PSQI > 5 | Single centre—clinic | IBD | 120 | 50 | 36 | 39 | 0.99 | Sleep quality in those with peripheral arthropathy and IBD was worse than those without |
Characteristics of studies included in the meta-analysis of poor sleep prevalence. See Supplementary Table 1 for further details.
USA, United States of America; IBD, inflammatory bowel disease; PSQI, Pittsburgh Sleep Quality Index; BSNQ, Basic Nordic Sleep Questionnaire—first question used.
Measurement of sleep quality
The Pittsburgh Sleep Quality Index (PSQI) was reported in the majority of included studies (n = 29) (see Table 2).The PSQI is a validated measure to assess perceived sleep quality [46]. The index consists of subscales on sleep duration, sleep disturbance, sleep latency, daytime dysfunction, sleep efficiency, overall sleep quality and medications for sleep. The score ranges from 0 to 21, with a PSQI > 5 considered to represent poor sleep quality. PSQI sub-scores were reported in seven studies and consequently this was not investigated further.
Table 2.
Meta-regression performed for prevalence of poor sleep
| Number of studies | Coefficient | Standard error | P value | Residual heterogeneity (I2%) | |
|---|---|---|---|---|---|
| Crohn’s disease | 31 | 0.0147 | 0.10 | .88 | 96.8 |
| Age | 36 | 0.017 | 0.006 | .005 | 95.6 |
| Female gender | 33 | 0.002 | 0.002 | .28 | 96.5 |
| IBD disease duration | 18 | 0.005 | 0.007 | .54 | 97.6 |
| Objective IBD activity | 8 | 0.64 | 0.17 | .001 | 30.5 |
| Subjective IBD activity | 25 | 0.013 | 0.21 | .95 | 98.0 |
| Depression | 15 | 0.13 | 0.17 | .43 | 95.6 |
IBD, inflammatory bowel disease.
The Patient Reported Outcomes Measurement Information Systems sleep disturbance (PROMIS-SD) questionnaire was used by six studies [27–29, 47–49]. The PROMIS-SD questionnaire was developed by the National Institute of Health [50]. The PROMIS-SD has comparable performance to the PSQI in identifying poor sleep [51]. A PROMIS-SD t score over 50 is referred to as poor sleep. A single study [32] used the Basic Nordic Sleep Questionnaire [52] (BNSQ), utilizing the first dimension of the BNSQ and a score above 3 considered significant.
Prevalence of poor sleep-in IBD
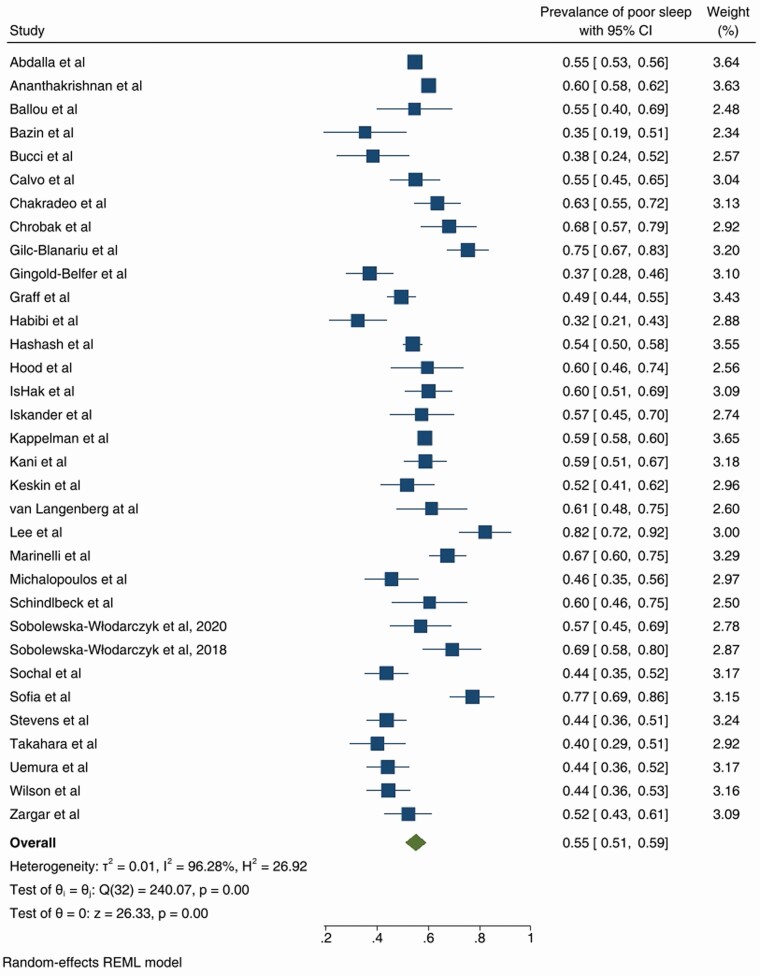
The prevalence of poor sleep varied from 32 to 99%, with random effects model derived pooled prevalence of 55%, 95% CI (51–59) with substantial heterogeneity (Forest plot in Figure 2), outliers were removed [21, 32]. Funnel plot was symmetric (Supplementary Figure 1) and Egger’s test not significant (p = .49). The Trim-fill method did not suggest any additional studies.
Figure 2.
Forest plot of the prevalence of poor sleep-in those with inflammatory bowel disease.
Subgroup analysis
Subgroup analysis was performed for definition of poor sleep, study of origin and publication date. There was no difference in the prevalence of poor sleep by definition of poor sleep (PSQI or PROMIS-SD sleep, p = .75). Most studies were from the United States of America (n = 15), followed by Europe (n = 12), and others including Australia, Japan, Turkey and Iran. The pooled prevalence was similar between Europe (56% [49–63]), and the USA (58% [53–64], both of which were significantly different to other (Australia, Japan, Turkey, Iran) (44% [36–52]) (p = .01)(see Supplementary Figure 2). Publication date subgroups were considered from 2011 to 2016 (n = 10), and 2017 to 2020 (n = 23). The prevalence of poor sleep was higher in the 2017 to 2020 subgroup (p = .03, 58% [53–63] v 50% [44–55]). However, altering the publication date subgroups by a single year resulting in no effect seen, discounting the above result.
Meta-regression
Meta-regression was performed for demographics and IBD related data (see Table 2 and supplementary Table 3). Age was significant (p = .005) with increasing proportion of poor sleep associated with increase in age, however residual heterogeneity remaining significant (I2 95.6%). Meta-regression was not significant for gender (p = .28), IBD type (p = .88), and IBD disease duration (p = .54).
IBD activity
IBD activity was reported in 25 studies (n = 23 229) in the form of subjective disease activity scores such as the Harvey Bradshaw Index [15] or the Crohn’s disease activity index [53] (see Supplementary Table 2). The meta-regression incorporated the number of people with active IBD as per these subjective disease activity scores. Meta-regression for subjective disease activity was not significant (p = .95). Objective IBD activity was reported in eight studies (n = 1931), with objective measures including C-reactive protein, fecal calprotectin, endoscopic findings and histology (see Supplementary Table 3). On meta-regression objective IBD activity was significant (p = .001), increasing proportion of poor sleep was associated with increase in objective disease activity. Residual heterogeneity was I2 30.5%, this is as compared to heterogeneity for these eight studies at I2 of 82.6%, suggesting that objective disease activity may explain much of the inter-study heterogeneity.
Depression
Assessment of depression was performed in 15 studies (n = 10 744) (see Supplementary Table 4). Eight of these studies reported a significant association between poor sleep quality and depression [22–29]. Scoring systems included the Hospital Anxiety and Depression Scale [54] (n = 6), PROMIS [55] depression score (n = 4), Beck’s Depression Inventory II [56] (n = 3), depressive symptoms (n = 1) and depression under treatment (n = 1). On meta-regression depression was not significant (p = .43).
Discussion
This is the largest and only meta-analysis to date providing prevalence estimates for poor sleep-in IBD. The pooled prevalence for poor sleep-in IBD was high (55%), eclipsing that reported in a recent meta-analysis of fatigue (47%) [37], and of symptoms of anxiety (32%) and depression (25%) [4]. The prevalence of poor sleep reported here is of a higher magnitude than the prevalence of sleep disorder in IBS with a recent meta-analysis reporting a pooled prevalence of 37.6% [57]. This highlights the importance of poor sleep-in IBD and suggests further resources should be allocated to investigate this area.
Sources of heterogeneity in the prevalence estimate of poor sleep included age, geographic location, and objective disease activity. Age-related sleep changes have been well described with decreasing sleep quality accepted [58], with a similar association between age and sleep quality seen in a rheumatoid arthritis population [59, 60]. It was considered that the significance of age may also relate to IBD disease duration, however this was not significant on meta-regression.
Objective IBD activity did vary between studies and was a significant source of heterogeneity. A recent meta-analysis was unable to elicit a significant relationship between objective IBD activity and sleep [17], which may in part be due to the small number of studies utilizing objective measures of IBD activity. It did however find that subjective IBD activity was associated with poor sleep quality—a finding not replicated here despite the variance between different studies. This suggests that the underlying inflammatory response may be more significant than the associated symptoms, consistent with studies associated histology activity and endoscopic activity in the absence of symptoms with poor sleep [19, 20]. IBD activity is likely not the only driver of poor sleep quality with several studies reporting frequent poor sleep-in those with inactive disease [29, 33, 40, 41].
Depression was not a significant source of heterogeneity despite varying between studies and despite several positive findings in the literature [22–29]. Low physical activity [31, 61] and the presence of extra-intestinal IBD manifestations [21] have also been associated with poor sleep, unfortunately these were reported a minority of studies making further investigation impractical.
Limitations
As a result of the paucity of studies incorporating objective sleep assessments, we used a definition of poor sleep based on self-reported sleep quality. There is a suggestion in some studies [25] that people with IBD will report significantly worse sleep than can be substantiated objectively, and consequently the true prevalence of poor sleep may be lower. This supports the need for objective sleep assessments in people with IBD. Other limitations include most studies being single centre, although results were similar to multi-centre or nationwide studies. Although we note that prevalence from nationwide studies was similar to other single centre studies. No study incorporated sample size calculations or included a rigorous sampling approach. There was a general lack of demographic data reported in studies, such as race, that may have a significant influence on the prevalence of poor sleep-in this population. The differentiation between gender and sex was not well defined in most studies. Finally, a single reviewer was responsible for data collection.
Future work
Further work should consider studies incorporating objective disease and sleep quality measurements to understand the relationship and type of sleep disorders in this population. There are few interventional studies in this area, with a need to establish if the potential benefit of improving sleep-in people with IBD would extend beyond quality of life to incorporate IBD related outcomes such as IBD activity, and surgery. There is also the lack of simple IBD specific screening tool for use in IBD clinic to identify those with poor sleep who would benefit from referral onto a sleep physician.
Conclusions
This meta-analysis has demonstrated that the prevalence of poor sleep-in IBD is significant, although there was substantial heterogeneity between studies. Meta-regression demonstrated that age and objective IBD activity were significant, with subjective IBD activity not significant. Objective IBD activity explained most of the heterogeneity between studies. Further research is required in this area to establish the relationship between IBD activity and sleep quality and to consider sleep targeted interventions in an IBD population.
Supplementary Material
Contributor Information
Alex Barnes, Department of Gastroenterology, Southern Adelaide Local Health Network (SALHN) Flinders Medical Centre, Bedford Park South Australia, Australia; Adelaide Institute for Sleep Health, Flinders Health and Medical Research Institute, College of Medicine and Public Health, Flinders University, Bedford Park, South Australia, Australia.
Réme Mountifield, Department of Gastroenterology, Southern Adelaide Local Health Network (SALHN) Flinders Medical Centre, Bedford Park South Australia, Australia; Adelaide Institute for Sleep Health, Flinders Health and Medical Research Institute, College of Medicine and Public Health, Flinders University, Bedford Park, South Australia, Australia.
Justin Baker, Department of Gastroenterology, Southern Adelaide Local Health Network (SALHN) Flinders Medical Centre, Bedford Park South Australia, Australia; Adelaide Institute for Sleep Health, Flinders Health and Medical Research Institute, College of Medicine and Public Health, Flinders University, Bedford Park, South Australia, Australia.
Paul Spizzo, Department of Gastroenterology, Southern Adelaide Local Health Network (SALHN) Flinders Medical Centre, Bedford Park South Australia, Australia.
Peter Bampton, Adelaide Institute for Sleep Health, Flinders Health and Medical Research Institute, College of Medicine and Public Health, Flinders University, Bedford Park, South Australia, Australia.
Jane M Andrews, Inflammatory Bowel Disease Service, Department of Gastroenterology and Hepatology, (CAHLN) Royal Adelaide Hospital, Adelaide, South Australia, Australia; School of Medicine, Faculty of Health and Medical Sciences, University of Adelaide, Adelaide, South Australia, Australia.
Robert J Fraser, Department of Gastroenterology, Southern Adelaide Local Health Network (SALHN) Flinders Medical Centre, Bedford Park South Australia, Australia; Adelaide Institute for Sleep Health, Flinders Health and Medical Research Institute, College of Medicine and Public Health, Flinders University, Bedford Park, South Australia, Australia.
Sutapa Mukherjee, Adelaide Institute for Sleep Health, Flinders Health and Medical Research Institute, College of Medicine and Public Health, Flinders University, Bedford Park, South Australia, Australia; Department of Respiratory and Sleep Medicine, Southern Adelaide Local Health Network (SALHN) Flinders Medical Centre, Bedford Park, South Australia, Australia.
Funding
No funding was received for this work.
Disclosure Statement
Peakers fees, and Ad Boards from: Abbott, AbbVie, Allergan, Anatara, AstraZeneca, Bayer, BMS 2020, Celegene, Celltrion, Falk, Ferring, Gilead, Hospira, Immuninc, ImmunsanT, Janssen, MSD, Nestle, Novartis, Progenity, Pfizer, Sandoz, Shire, Takeda, Vifor, RAH research Fund, The Hospital Research Fund 2020-2022, The Helmsley Trust 2020-2023.
Data Availability Statement
The data underlying this article are available in the Harvard Dataverse Digital Repository at https://doi.org/10.7910/DVN/FVLPYA
Author Contributions
A.B.: responsible for study concept and design, data acquisition, analysis and data interpretation, drafting of manuscript, critical revision of the manuscript. P.S.: responsible for data acquisition, data interpretation, drafting of manuscript, critical revision of the manuscript. J.B.: responsible for data acquisition and critical revision of the manuscript. P.B.: responsible for critical revision of the manuscript. J.A.: responsible for study conception. R.J.F.: responsible for study conception. R.M.: responsible for critical revision of the manuscript. S.M.: responsible for study concept and design, critical revision of the manuscript.
References
- 1. Daghlas I, et al. Sleep duration and myocardial infarction. J Am Coll Cardiol. 2019;74(10):1304–1314. doi: 10.1016/j.jacc.2019.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deng H, et al. short sleep duration increases metabolic impact in healthy adults: a Population-Based Cohort Study. Sleep. 2017;40(10). [DOI] [PubMed] [Google Scholar]
- 3. Cappuccio F, et al. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hillman D, et al. The economic cost of inadequate sleep. Sleep. 2018;41(8). doi: 10.1093/sleep/zsy083. [DOI] [PubMed] [Google Scholar]
- 5. Orr WC, et al. The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol Hepatol. 2020;5(6):616–624. [DOI] [PubMed] [Google Scholar]
- 6. Vgontzas AZ, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. [DOI] [PubMed] [Google Scholar]
- 7. Shoham SD, et al. Recombinant tumor necrosis factor and interleukin 1 enhance slowwave sleep. Am J Physiol. 1987;253:R142–R149. [DOI] [PubMed] [Google Scholar]
- 8. Majde JJ. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116:1188–1198. [DOI] [PubMed] [Google Scholar]
- 9. Ranjbaran Z, et al. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm Res. 2007;56(2):51–57. doi: 10.1007/s00011-006-6067-1. [DOI] [PubMed] [Google Scholar]
- 10. Zhang L, et al. Rheumatoid arthritis is associated with negatively variable impacts on domains of sleep disturbances: evidence from a systematic review and meta-analysis. Psychol Health Med. 2021;26(3):267–277. doi: 10.1080/13548506.2020.1764597. [DOI] [PubMed] [Google Scholar]
- 11. Marrie R, et al. A systematic review of the incidence and prevalence of sleep disorders and seizure disorders in multiple sclerosis. Mult Scler. 2015;21(3):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ananthakrishnan AN. Environmental risk factors for inflammatory bowel diseases: a review. Dig Dis Sci. 2015;60(2):290–298. doi: 10.1007/s10620-014-3350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caprilli R; European Crohn's and Colitis Organisation. European evidence based consensus on the diagnosis and management of Crohn’s disease: special situations. Gut. 2006;55(supl 1) : i36–i58. doi: 10.1136/gut.2005.081950c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dignass A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6(10):991–1030. [DOI] [PubMed] [Google Scholar]
- 15. Harvey RF, et al. A simple index of Crohn’s-disease activity. Lancet. 1980;8(1):514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 16. Ozer M, et al. Prevalence of irritable bowel syndrome-like symptoms using Rome IV criteria in patients with inactive inflammatory bowel disease and relation with quality of life. Medicine (Baltim). 2020;99(19):e20067. doi: 10.1097/MD.0000000000020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ballesioa A, et al. A meta-analysis on sleep quality in inflammatory bowel disease. Sleep Med Rev. 2021;60:301–308. [DOI] [PubMed] [Google Scholar]
- 18. Hao G, et al. Sleep quality and disease activity in patients with inflammatory bowel disease: a systematic review and meta-analysis. Sleep Med. 2020;75:301–308. doi: 10.1016/j.sleep.2020.08.032. [DOI] [PubMed] [Google Scholar]
- 19. Michalopoulos G, et al. Association of sleep quality and mucosal healing in patients with inflammatory bowel disease in clinical remission. Ann Gastroenterol. 2018;31(2):211–216. doi: 10.20524/aog.2018.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ali T, et al. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19(11):2440–2443. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, et al. Sleep characteristics and influencing factors of sleep quality in patients with inflammatory bowel disease-peripheral arthritis. Front Med. 2019;6:190. doi: 10.3389/fmed.2019.00190. eCollection 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gîlc-Blanariu G, et al. Sleep impairment and psychological distress among patients with inflammatory bowel disease-beyond the obvious. J Clin Med. 2020;9(7):2304. doi: 10.3390/jcm9072304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sochal M, et al. Determinants of sleep quality in inflammatory bowel diseases. J Clin Med. 2020;9(9):2921–2922. doi: 10.3390/jcm9092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hood M, et al. Sleep quality in ulcerative colitis: associations with inflammation, psychological distress, and quality of life. Int J Behav Med. 2018;25(5):517–525. [DOI] [PubMed] [Google Scholar]
- 25. Marinelli C, et al. Sleep disturbance in inflammatory bowel disease: prevalence and risk factors—a cross-sectional study. Sci Rep. 2020;10(1):507. doi: 10.1038/s41598-020-57460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garcia Calvo E, et al. Prevalence and factors associated with poor sleep quality in inflammatory bowel disease outpatients. Rev Esp Enferm Dig. 2021;113(7):512–518. doi: 10.17235/reed.2020.7202/2020. [DOI] [PubMed] [Google Scholar]
- 27. Stevens B, et al. Vedolizumab therapy is associated with an improvement in sleep quality and mood in inflammatory bowel diseases. Dig Dis Sci. 2017;62(1):197–206. doi: 10.1007/s10620-016-4356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson R, et al. High C-reactive protein is associated with poor sleep quality independent of nocturnal symptoms in patients with inflammatory bowel disease. Dig Dis Sci. 2015;60(7):2136–2143. doi: 10.1007/s10620-015-3580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ananthakrishnan A, et al. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2013;11(8):965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Canakis A, et al. Sleep and fatigue in IBD: an unrecognized but important extra-intestinal manifestation. Curr Gastroenterol Rep. 2020;22(2):8. doi: 10.1007/s11894-020-0746-x. [DOI] [PubMed] [Google Scholar]
- 31. Chrobak A, et al. Associations between chronotype, sleep disturbances and seasonality with fatigue and inflammatory bowel disease symptoms. Chronobiol Int. 2018;35(8):1142–1152. doi: 10.1080/07420528.2018.1463236. [DOI] [PubMed] [Google Scholar]
- 32. Frigstad S, et al. Fatigue is not associated with vitamin D deficiency in inflammatory bowel disease patients. World J Gastroenterol. 2018;24(29):3293–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Graff L, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17(9):1882–1889. [DOI] [PubMed] [Google Scholar]
- 34. Hashash J, et al. Quality of sleep and coexistent psychopathology have significant impact on fatigue burden in patients with inflammatory bowel disease. J Clin Gastroenterol. 2018;52(5):423–430. [DOI] [PubMed] [Google Scholar]
- 35. Paixão D, et al. Evaluation of home polysomnography findings, quality of sleep, and fatigue in inflammatory bowel disease: a case series. J Clin Sleep Med. 2019;15(1):39–45. doi: 10.5664/jcsm.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schindlbeck K, et al. Impact of restless legs syndrome in patients with inflammatory bowel disease on sleep, fatigue, and quality of life. Int J Colorectal Dis. 2017;32(1):125–130. [DOI] [PubMed] [Google Scholar]
- 37. D’Silva A, et al. Prevalence and risk factors for fatigue in adults with inflammatory bowel disease: a systematic review with meta-analysis. Clin Gastroenterol Hepatol. 2021;20(5):995–1009.e7. doi: 10.1016/j.cgh.2021.06.034 [DOI] [PubMed] [Google Scholar]
- 38. Ananthakrishnan A, et al. Sleep duration affects risk for ulcerative colitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2014;12(11):1879–1886. doi: 10.1016/j.cgh.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee A, et al. Immunomodulator and biologic agent effects on sleep quality in patients with inflammatory bowel disease. Oschner J. 2018;18(1):76–80. [PMC free article] [PubMed] [Google Scholar]
- 40. Keefer L, et al. An initial report of sleep disturbance in inactive inflammatory bowel disease. J Clin Sleep Med. 2006;2(4):409–416. [PubMed] [Google Scholar]
- 41. Ranjbaran Z, et al. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. 2007;22(11):1748–1753. doi: 10.1111/j.1440-1746.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 42. Schiavo JH. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q. 2019;38(2):171–180. [DOI] [PubMed] [Google Scholar]
- 43. Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lo C, et al. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nyaga VN, et al. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39–42. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Pyschiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 47. Kappelman M, et al. Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12(8):1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. IsHak W, et al. Patient-Reported Outcomes of Quality of Life, Functioning, and GI/Psychiatric Symptom Severity in Patients with Inflammatory Bowel Disease (IBD). Inflamm Bowel Dis. 2017;23(5):798–803. [DOI] [PubMed] [Google Scholar]
- 49. Abdalla M, et al. Prevalence and impact of inflammatory bowel disease-irritable bowel syndrome on patient-reported outcomes in CCFA partners. Inflamm Bowel Dis. 2017;23:325–331. [DOI] [PubMed] [Google Scholar]
- 50. Buysse DJ, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781–792. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu L, et al. Development of short forms from the PROMIS™ sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med. 2011;10(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Partinen M, et al. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;4:150–155. [DOI] [PubMed] [Google Scholar]
- 53. Best WR, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70(3):439–44. [PubMed] [Google Scholar]
- 54. Zigmond A, et al. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 55. Cella D, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Arnau R, et al. Psychometric evaluation of the Beck Depression Inventory-II with primary care medical patients. Health Psychol. 2001;20(2):112–119. [DOI] [PubMed] [Google Scholar]
- 57. Duan BWRDL. Prevalence of sleep disorder in irritable bowel syndrome: a systematic review with meta-analysis. Saudi J Gastroenterol. 2018;24(3):141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ohayon M, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 59. Sariyildiz M, et al. Sleep quality in rheumatoid arthritis: relationship between the disease severity, depression, functional status and the quality of life. J Clin Med Res. 2014;6(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Son C, et al. Sleep quality in rheumatoid arthritis, and its association with disease activity in a Korean population. Korean J Intern Med. 2015;30(3):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Langenberg D, et al. Sleep and physical activity measured by accelerometry in Crohn’s disease. Aliment Pharmacol Ther. 2015;41(10):991–1004. [DOI] [PubMed] [Google Scholar]
- 62. Ballou S, et al. Sleep disturbances are commonly reported among patients presenting to a gastroenterology clinic. Dig Dis Sci. 2018;63(11):2983–2991. doi: 10.1007/s10620-018-5237-7. [DOI] [PubMed] [Google Scholar]
- 63. Bazin T, et al. Altered sleep quality is associated with Crohn’s disease activity: an actimetry study. Sleep Breath. 2020;24(3):971–977. [DOI] [PubMed] [Google Scholar]
- 64. Bucci C, et al. Prevalence of sleep bruxism in IBD patients and its correlation to other dental disorders and quality of life. Gastroenterol Res Pract. 2018. ;2018:7274318. doi: 10.1155/2018/7274318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chakradeo P, et al. Chronotype, social jet lag, sleep debt and food timing in inflammatory bowel disease. Sleep Med. 2018;52:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gingold-Belfer R, et al. Impaired sleep quality in Crohn’s disease depends on disease activity. 2014;59(1):146–151. doi: 10.1007/s10620-013-2890-8. [DOI] [PubMed] [Google Scholar]
- 67. Habibi F, et al. Sleep quality and associated factors in Iranian inflammatory bowel disease patients. J Res Med Sci. 2019;24:59. doi: 10.4103/jrms.JRMS_14_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Iskandar H, et al. Self-reported sleep disturbance in Crohn’s disease is not confirmed by objective sleep measures. Sci Rep. 2020;10(1):1980–1985. doi: 10.1038/s41598-020-58807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kani H, et al. The effect of dream anxiety on sleep quality in inflammatory bowel diseases. Dig Dis. 2020;38(5):380–389. [DOI] [PubMed] [Google Scholar]
- 70. Keskin E C. Chronotype and sleep quality in patients with inflammatory bowel disease. Med Bull Haseki. 2020;58:72–77. [Google Scholar]
- 71. Sobolewska-Wlodarczyk A, et al. Sleep disturbance and disease activity in adult patients with inflammatory bowel diseases. J Physiol Pharmacol. 2018;69(3). doi: 10.26402/jpp.2018.3.09. [DOI] [PubMed] [Google Scholar]
- 72. Sobolewska-Włodarczyk A, et al. Circadian rhythm abnormalities in patients with inflammatory bowel disease—association with adipokine profile. Scand J Gastroenterol. 2020;55(3):294300–294300. doi: 10.1080/00365521.2020.1737727. [DOI] [PubMed] [Google Scholar]
- 73. Sofia M, et al. Poor sleep quality in Crohn’s disease is associated with disease activity and risk for hospitalization or surgery. 2020;26(8):1251–1259. doi: 10.1093/ibd/izz258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Takahara I, et al. Prevalence of restless legs syndrome in patients with inflammatory bowel disease. Dig Dis Sci. 2017;62(3):761–767. doi: 10.1007/s10620-016-4420-y. [DOI] [PubMed] [Google Scholar]
- 75. Uemura R, et al. Sleep disturbances in Japanese patients with inflammatory bowel disease and their impact on disease flare. Springer Plus. 2016;13(5):1792. doi: 10.1186/s40064-016-3408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van Langenberg D, et al. Cognitive impairment in Crohn’s disease is associated with systemic inflammation, symptom burden and sleep disturbance. United Eur Gastroenterol J. 2017;5(4):579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zargar A, et al. Effect of irritable bowel syndrome on sleep quality and quality of life of inflammatory bowel disease in clinical remission. Int J Prev Med. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the Harvard Dataverse Digital Repository at https://doi.org/10.7910/DVN/FVLPYA