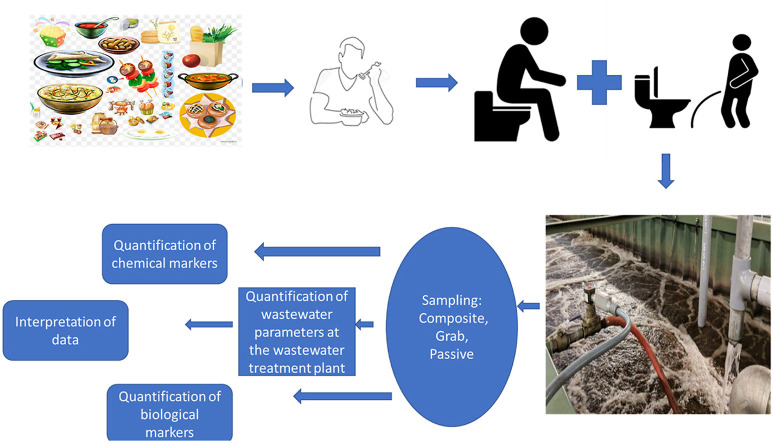
Abstract
Wastewater monitoring and epidemiology have seen renewed interest during the recent COVID-19 pandemic. As a result, there is an increasing need to normalize wastewater-derived viral loads in local populations. Chemical tracers, both exogenous and endogenous compounds, have proven to be more stable and reliable for normalization than biological indicators. However, differing instrumentation and extraction methods can make it difficult to compare results. This review examines current extraction and quantification methods for ten common population indicators: creatinine, coprostanol, nicotine, cotinine, sucralose, acesulfame, androstenedione 5-hydroindoleacetic acid (5-HIAA), caffeine, and 1,7-dimethyluric acid. Some wastewater parameters such as ammonia, total nitrogen, total phosphorus, and daily flowrate were also evaluated. The analytical methods included direct injection, dilute and shoot, liquid/liquid, and solid phase extraction (SPE). Creatine, acesulfame, nicotine, 5-HIAA and androstenedione have been analysed by direct injection into LC-MS; however, most authors prefer to include SPE steps to avoid matrix effects. Both LC-MS and GC-MS have been successfully used to quantify coprostanol in wastewater, and the other selected indicators have been quantified successfully with LC-MS. Acidification to stabilize the sample before freezing to maintain the integrity of samples has been reported to be beneficial. However, there are arguments both for and against working at acidic pHs. Wastewater parameters mentioned earlier are quick and easy to quantify, but the data does not always represent the human population effectively. A preference for population indicators originating solely from humans is apparent. This review summarises methods employed for chemical indicators in wastewater, provides a basis for choosing an appropriate extraction and analysis method, and highlights the utility of accurate chemical tracer data for wastewater-based epidemiology.
Keywords: Wastewater, Chemical indicator, Population biomarker, LC-MS, GC-MS, Extraction, Wastewater parameter
Graphical abstract
1. Introduction
Wastewater is a promising source for determining which pathogens are prevalent in any given community (Baalbaki et al., 2017; Ratola et al., 2012; Rico et al., 2017). It has been efficiently used to monitor illicit drugs (Centazzo et al., 2019), population biomarkers (Chen et al., 2014), viral concentrations (Haramoto et al., 2020; Lapolla et al., 2020) and significant pollutants (Ratola et al., 2012). Since 2020, wastewater-based epidemiology (WBE) has gained renewed attention due to wastewater monitoring for RNA of the SARS-CoV-2 virus that causes COVID-19. However, in many cases, direct comparison of viral loads to other studies and clinical testing cases has been demonstrated to be uninformative. Although some scientists have successfully correlated WBE results with clinical measures of infections in people, many could not because of inappropriate estimates of population size to which to normalize their data and inherent limitations in clinical results (Oloye et al., 2022; Wu et al., 2020). Normalization of results for viral loads with wastewater indicators of the population can improve comparisons temporally and between and among cities and nations. Hence, normalization with appropriate population size estimators has become essential for accurately interpreting WBE results.
Indicators in wastewater can either be of biological or chemical origin, with chemical tracers, such as steroids considered the most suitable tracers because of their stability in wastewater (Hatcher and McGillivary, 1979; Kaiser and Lerch, 2022; Reynolds et al., 2022). However, there are concerns about steroids having an affinity to particulate matter in wastewater or filter paper (Chen et al., 2014). One study found that coprostanol was more stable than crAssphage (a bacteriophage), pepper mild mottle virus, and HF183 (Reynolds et al., 2022). Although concentrations of coprostanol were less than those of crAssphage, pepper mild mottle virus, and HF183, possibly related to the affinity to the particulate matter, its stability and demonstrated utility in the monitoring of the SARS-CoV-2 RNA in the city of Dublin make coprostanol a promising population indicator (Reynolds et al., 2022). A negative relationship between per capita crAssphage RNA and service area size was reported, which was associated with RNA degradation over long transit times (Wilder et al., 2021). Another group reported that only 5-HAA, cotinine and caffeine reveal possibilities to be used as an indicator (Rico et al., 2017). However, sampling and analysis are some of the sources of uncertainty (<11%) in the application of tracer for wastewater-based epidemiology (Q. Zheng et al., 2019). Surrogate markers of the size of populations discharging to a sewershed, either overestimate or underestimate populations, with no consensus on the best indicator to use for any location or time. However, to correct for seasonal variations or differences in loadings and treatments among locations and times, normalizing the pathogen loads to the population contributing waste to the wastewater influent is important. Since population size could vary from day to day, it is necessary to normalize the concentrations of chemicals or load of virus particles to the population discharging the waste. The challenge has always been knowing the best indicator and using the appropriate method to extract the indicator from wastewater. Some of the requirements that need to be fulfilled for a substance to be used for normalization of viral load include: stability in wastewater, with low or no variability in excretion rate; unique to human metabolism; and quantifiable quickly and safely in wastewater (Senta et al., 2015).
The current health status of an urban population served by a central wastewater treatment facility can be monitored through surveillance of constituents in wastewater when different biases introduced are reduced or removed by normalization with appropriate indicators. Therefore, this review examined at some common indicators applied in the literature, emphasizing their extraction, analysis, and justification for their selection as indicators. Pepper mild mottle virus and wastewater parameters will be discussed briefly because they will be used as basis yardstick for determining the usefulness of chemical indicators. Any chemical indicator that performed less than either wastewater parameters or pepper mild mottle virus should not be consider as a normalization chemical.
2. Discussion
4 Pepper mild mottle virus.
Virologists have often normalized monitoring results for viruses associated with human disease to other viruses typically found in wastewater. This likely results from a familiarity with the molecular techniques and instrumentation used for monitoring the target pathogens, as most virologists lack the background and instrumentation to quantify small organic molecules using mass spectrometry. A pervasive approach to normalization is to measure the amount of the Pepper mild mottle virus (PMMoV), a plant pathogenic virus that occurs worldwide in various field-grown bell, hot and ornamental peppers (Jafferali et al., 2021; Torii et al., 2022). It is caused by members of the plant virus genus Tobamovirus, otherwise known as the tobacco mosaic viruses that contain positive-sense RNA genomes that infect plants. This virus has been found to occur in the diet of humans worldwide and is shed into wastewaters in relatively constant amounts for a specific culture. The persistence and the large concentration ratio of PMMoV in WWTP influents to the method detection limit accounts for its usage as a normalization agent (Kuroda et al., 2015). PMMoV was found to be most persistent compared to ten different viruses of environmental relevance (genogroup I, II and IV noroviruses, sapovirus, enterovirus, group A rotavirus, Aichi virus, pepper mild mottle virus, adenovirus, and JC and BK polyomaviruses) in a twelve-month study (Kitajima et al., 2014). Similarly, it showed no significant changes and low reduction due to activities of treatment plants. It was reported to positively correlate with a population (r = 0.92) (HSu et al., 2022) and it has been reported to have similar efficiency has caffeine and sucralose as a tracer (Cantwell et al., 2019). Thus, they can be used as biomarkers of sizes of human populations (Torii et al., 2022). However, PMMoV can vary between cultures and nations because of the variability in the consumption and excretion rate of pepper (Medema et al., 2020), its extraction rate, and its relatively short half-life (7–10 days) (HSu et al., 2022), so this indicator should not be the only indicator to assess the temporal population size variations. Another disadvantage of using PMMoV is the possibility of contribution from other sources that are different from humans (HSu et al., 2022).
3. Wastewater parameters
Some standard parameters that wastewater facilities routinely monitor to gain insight into the condition of the wastewater and estimates of the population they are servicing include flow rate, biochemical oxygen demand (BOD), total nitrogen (TN), total phosphate (TP), volatile suspended solids (VSS), total suspended solids (TSS), pH, and temperature. These parameters offer advantages over chemical population indicators because they are routinely monitored in wastewater facilities. A strong association (>0.7) had been reported between WBE estimations and actual Covid-19 cases, which showed that WBE data normalized with wastewater parameters could identify temporal trends of the diseases' spread (Koureas et al., 2021). However, hydro-chemical-based population estimates generally have high RSDs (>44%) for biochemical oxygen demand, chemical oxygen demand (COD), and ammonium between sites, suggesting that their applicability for use in population estimation may not be appropriate for every WWTP (Tscharke et al., 2019). Also, population estimates from hydro-chemical parameters varied significantly compared with chemical tracers such as codeine, acesulfame, atenolol, naproxen and creatinine (Rico et al., 2017). When wastewater treatment plants receive industrial effluents or other foreign effluents, hydro-chemical parameters such as BOD and COD are greatly affected (Rico et al., 2017). Also, BOD and COD only reflect human activities rather than population size (Chen et al., 2014). Given the above considerations, the normalization of viral load in wastewater should consider more than one parameter.
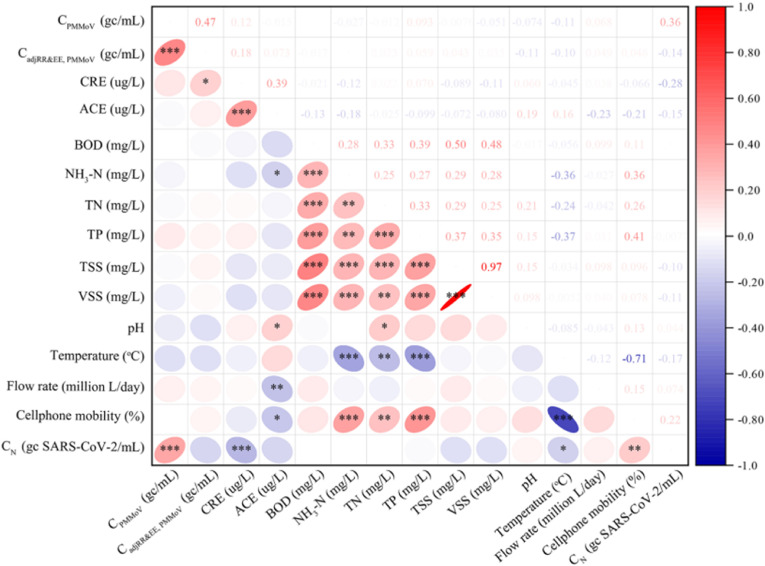
Daily flow rate and population size of a given area are two of the many factors that make it difficult to compare results between WWTP, as they have a pronounced diluting or concentrating effect on the concentration or amount of pathogen (Melvin et al., 2021). High flow rates have been reported to have only a tiny effect on PMMOV, which is always abundant in wastewater (Melvin et al., 2021). For many facilities, because of rainstorms, precipitation, melting of snow, and industrial inputs for comparative purposes, rates of flow, are not stable (Tolouei et al., 2019; Wu et al., 2020). The abundance of PMMoV makes it a better candidate than the flow rate, which can easily be altered by the factors mentioned earlier. It has been reported that RNA concentration normalized for COD achieved slightly better performance for population size compared to TSS, BOD or total inflow (Koureas et al., 2021). Average daily flow correlated negatively with most parameters (Fig. 1 ). Thus, in most case, using the average daily flow rate alone as a means of normalization is not sufficient.
Fig. 1.
Correlation of some parameters measured in wastewater (adapted from (Xie et al., 2022), Permission and license granted by ACS).
Concentrations of TSS are generally greater in raw influents than effluents and result in a matrix effect in raw influents that can significantly negatively impact the pathogen recovery ratio from raw influents (Tolouei et al., 2019). TSSs represent a wide variety of suspended materials and do not separate by settling but must be filtered to remove. Therefore, pathogens or lipophilic chemicals may bind to them and thus become difficult to recover. It has been estimated that about 23% of total SARS-CoV-2 RNA might be discarded due to adsorption during debris removal (Forés et al., 2021). TSS is representative of the soluble organic materials; however, TSS cannot differentiate between living and dead cells (Hwang and Hansen, 1998), so it cannot differentiate between human waste coming from the intestine or domestic waste. TSS correlates well with other wastewater parameters but does not correlate with other population indicators (Fig. 1). TSS has also been reported to influence SARS-CoV-2 concentration because increases in total solids correspond to increasing SARS-CoV-2 RNA (Amoah et al., 2022). TSS should not be consider for normalization because solids from each plant may vary based on other factors different from human factors.
Volatile suspended solids (VSS) have been a better normalizer than TSS, especially when industrial waste, which can contain a reasonable number of inorganic constituents, contributes to the wastewater. Care must be applied in using VSS when soluble organic materials from agricultural and domestic waste contribute to the wastewater (Hwang and Hansen, 1998). VSS correlated well with other wastewater parameters but did not correlate with any population indicator (Fig. 1); hence, the use of VSS to normalize pathogens might not be ideal.
Nutrients such as total nitrogen and phosphorus are routinely measured in wastewater to estimate populations. The possibilities of other sources of the contribution nutrients to is a concern and therefore nutrients not frequently considered for normalization of wastewater data (Q.-D. Zheng et al., 2019).
Ammonium is the primary form in which ammonia is found in wastewater. It is an indirect marker of urine, which is a product of urea hydrolysis. Ammoniums are mainly monitored as ammonium nitrogen (NH4–N) in wastewater measurement. Ammonium is sensitive to commuter and summer holiday-related fluctuations in the size of populations (Been et al., 2014). However, dilution factors significantly impact ammonium concentration because of surface runoff (Been et al., 2014). Nevertheless, it is less affected by non-human sources compared to COD, TP, and BOD (Gracia-Lor et al., 2017). Ammonia showed significant association with the concentrations of SARS-CoV-2 RNA assessed using an Adaptive Neuro-Fuzzy Inference System (ANFIS) (Amoah et al., 2022). Both Spatial and temporal accuracy of the population model showed a good correlation with the NH4–N population (Yu et al., 2021). NH4–N and TN were recorded to have high variance inflation factors, which for the predictor of SARS-CoV-2 RNA concentration in wastewater were low (<2); therefore, multicollinearity was observed with TN and NH4–N (Koureas et al., 2021). Similarly, Fig. 1 showed that TN and NH4–N correlated with each other. NH4–N estimated population has been reported to be lower compared to census data (Been et al., 2014). Therefore, caution must be exercise in employing ammonia load for the population estimate.
pH correlated well with ammonium and SARS-CoV-2 RNA determined by ANFIS (Amoah et al., 2022). pH values between 7.1 and 7.4, slightly alkaline, were associated with the highest SARS-CoV-2 concentration (Amoah et al., 2022). This pH range is in the region where pathogens could survive and be stable. At more alkaline pHs, the stability of the viral particle will be reduced. Therefore, instability in the detectable viral load might occur (Amoah et al., 2022). A slight decrease in viral load (<1) was reported at pH 9 or 10 when the pH was kept constant for 10 min; the decrease was over 5 at pH 11. However, SARS-CoV-2 decreased by less than 1 at pH 12 (Varbanov et al., 2021). Thus, alkaline pH significantly inactivates or reduces SARS-CoV-2.
4. chemical tracers
The goal of normalization is to correct loads of viruses detected in wastewater to the number of people discharging to a location. The most straightforward normalizations are to generate the population size of an area from the volume of influent. Indicators of population, which are either endogenous or exogenous substances, are significant for interpreting WBE data. Chemicals such as pharmaceuticals and personal care products, artificial sweeteners, fluorescent whitening agents, sterols/stanols, and nitrate have potential use as a marker because they are widely present in domestic wastewater (Lim et al., 2017). However, sources of compounds play significant roles in their utility as indicators of population size (Gilli et al., 2006). For example, creatinine is a breakdown product of muscle tissue, making it an excellent candidate for representing a specific population (Table 1 ) (Chen et al., 2014; Chiaia et al., 2008). Other endogenous compounds of interest are the serotonin metabolite (5-hydroxyindoleacetic acid) and the androgenic steroid hormone (androstenedione) (Table 1). Various stanols have been used as indicators of fecal masses in wastewaters. Cholesterol and coprostanol are good candidates, although cholesterol can come from non-mammal sources, while coprostanol originates from the metabolism of cholesterol by gut microorganisms (Reynolds et al., 2022). Caffeine and one of its metabolites (1,7 -dimethyluric acid) have also been used because coffee is widely consumed directly or indirectly (Chen et al., 2014). Therefore caffeine, nicotine, the stimulant drug in tobacco, and cotinine are typical examples of exogenous indicators of the population (Chen et al., 2014). Other notable exogenous compounds are sucralose and acesulfame, which are examples of artificial sweeteners.
Table 1.
Common chemical tracers with Log Kow (National Center for Biotechnology Information).
| Tracer | Description | Log Kow | Uses | References |
|---|---|---|---|---|
| Acesulfame | Artificial sweetener | −1.3 | Marker/Normalization | Rico et al. (2017); Xie et al. (2022) |
| Sucralose | Artificial sweetener | −1.0 | Biomarker | Cantwell et al., 2019 |
| Caffeine | A stimulant drug in coffee | −0.1 | Biomarker/Normalization |
Chen et al. (2014); Rico et al. (2017); Cantwell et al., 2019; Chakraborty et al. (2021); HSu et al., 2022 |
| 1,7 -dimethyluric acid | Caffeine metabolite | −0.2 | Biomarker | Chen et al. (2014) |
| Nicotine | A stimulant drug in tobacco | 1.2 | Biomarker/Normalization | Chen et al. (2014); Senta et al. (2015) |
| Cotinine | Nicotine metabolite | 0.1 | Biomarker/Normalization | Chen et al. (2014); Senta et al. (2015); Rico et al. (2017) |
| 5-Hydroxyindoleacetic acid | Serotonin metabolite | 1.10 | Biomarker/Normalization | Chen et al. (2014); Rico et al. (2017); Choi et al. (2018) |
| Creatinine | Creatine breakdown | −1.8 | Biomarker/Normalization |
Chiaia et al. (2008); Chen et al. (2014); Thai et al. (2014); Rico et al. (2017); Choi et al. (2018); HSu et al., 2022; |
| Coprostanol | Cholesterol metabolite | 8.8 | Biomarker/Normalization | Choi et al. (2018); Reynolds et al. (2022) |
| Androstenedione | Androgen steroid hormone | 2.8 | Biomarker | Baalbaki et al. (2017); Esperanza et al. (2007) |
Estimating population indirectly from hydro-chemical parameters, cell phone, chemical or biology tracers come with some limitations, which might result in misrepresentation of the true population. Using more than one chemical indicator or combination of chemical indicators with any other indicator, such as biology indicator or hydrochemical parameters, has been adjudged the best way to overcome some of the common challenges inherent in using tracers as population indicators. Also, designing a robust and reliable method for chemical indicators extraction and analysis will eliminate some of the biases introduced by the tracer's indicator population-based model.
Identification and quantification of chemical indicators are typically made using gas or liquid chromatography (GC or LC) coupled to various detectors, including flame ionization, ion conductivity, total conductivity, electron capture or mass spectrometry. Both GC- and LC-chromatography come with advantages and disadvantages. For example, extraction and analysis of some stanols have been done with liquid chromatography coupled with triple quadrupole, tandem mass spectrometry (LC/MS/MS) without the need for derivatization, which would otherwise be needed to make the compound more volatile for GC-MS (Bataglion et al., 2015). LC/MS/MS does not usually require clean-up, which is required for GC-MS (Bataglion et al., 2015; Beck and Radke, 2006). In another work, LC/MS/MS was not considered sufficiently sensitive for stanol because stanols are mainly lipophilic (Chen et al., 2014). The lack of sensitivity might be related to the electrospray ionization (ESI) method employed. Thus, careful selection and optimization of the instrumental method are essential for accurate chemical indicators analysis.
The extraction method and the extraction reagents must be carefully selected to ensure maximum recovery of analytes. Ethanol and acetone were found to have more extractive power based on their physical and chemical properties than hexane and dichloromethane to extract some lipids (Olumekun et al., 2021). Therefore, the nature of the extraction solvents (Table S1) and extraction conditions will also be crucial to providing meaningful concentrations above the detection limit and the limit of quantification in both GC-MS and LC-MS. Ethyl acetate was used to extract coprostanol from wastewater by Reynolds et al. (2022), while a 9:1 (v:v) mixture of hexane and ethyl acetate was used by Chen et al. (2014). Also, a hexane/acetone (1:1, v/v) mixture was used to extract coprostanol and other stanols and sterols (Beck and Radke, 2006). Dichloromethane had also been employed for coprostanol extraction from wastewaters (Chou and Liu, 2004). Therefore, different solvents with different chemical properties have been employed for stanol extraction from wastewater, which might have significant effect on the yield of the extract.
Each property of solvents (Table S1) plays a significant role in their ability to extract chemical compounds from wastewater. For example, cholesterol and coprostanol are lipophilic compounds (Table 1) (Kaiser and Lerch, 2022); therefore, it will be more prudent to extract it with compounds that are non-polar and have greater log Kow values. Hence, based on their solubility and polarity, methanol, acetone, and ethanol might not be good candidates for extracting lipophilic compounds. However, since alcohols are valuable in disrupting particle adsorption, it can be of considerable assistance in extraction. Dipole moments vary among solvents, which is indicative of polarity, hence dipole moment of solvent should also be considered before a solvent is chosen for extraction. However, a combination of properties determines the strength of solvents for extraction (Olumekun et al., 2021).
In addition to liquid-liquid extraction (LLE), SPE has been employed to extract stanols and other chemical tracers from wastewater. The step involved in LLE and SPE differs, for instance, LLE does not use cartridge, while the success of SPE relied on choosing appropriate cartridge (Fig. S1). SPE has been performed with different types of cartridge (Table 2 ). Each of this cartridge and the corresponding solvents will have effect on the amount of the analyte eluted through the column. It is worth noting that choosing a proper SPE cartridge is critical for the effective extraction of chemical compounds from wastewater.
Table 2.
Summary of solvents and SPE cartridge employed for solid phase extraction of tracers from wastewater.
| Solvents | Catridge | Target | Recovery | Reference |
|---|---|---|---|---|
| Methanol Dichloromethane |
200 mg of Chromabond-NH2 over 300 mg of Chromabond-C18ec | Stanol | 84 | Beck and Radke (2006) |
| Methanol Formic acid Water |
Oasis MCX | Caffeine | 7–36 | Baker and Kasprzyk-Hordern (2011) |
| 1,7-Dimethylxanthine | 3–15 | |||
| Nicotine | 76–82 | |||
| Cotinine | 37–48 | |||
| n-hexane dichloromethane | Bond Elut C18 (Varian) | Stanol | Not provided | Bataglion et al. (2015) |
| n-hexane dichloromethane | Oasis HLB (Waters) | Stanol | Not provided | Bataglion et al. (2015) |
| 500 mg of StrataX | Stanol | Sodré et al. (2010) | ||
| Acetone, methanol, and water | 500 mg of StrataX, a surface-modified styrene-divinylbenzene polymer sorbent | Coprostanol | 99 | Sodré et al. (2010) |
| Caffeine | 83 | |||
| C-18 discs | androstenedione | Kolodziej et al. (2003) | ||
| Not provided | Strata-X, Chroabond C18, Chromabond Hr-P, AccuBOND C18 |
5-HIAA | 0–76 | Jang et al. (2019) |
| 96% Dichloromethane:iso-propanol (80:20)/4% ammonia Methanol |
UCT™ XRDAH | Androstenedione and cotinine | Androstenedione (44) Cotinine (34) |
Chen et al. (2014) |
4.1. Creatinine
Creatinine is a chemical by-product of energy-producing processes in muscles. Creatinine is excreted into the urine and is therefore present in wastewater. Creatinine is often used as a marker for estimating the total amount of urine in wastewater samples, as the excretion of creatinine is relatively constant across individuals. It is common in wastewater and is easy to quantify, but it degrades readily, so the stability of creatinine has been a concern. Creatinine can be monitored using LC-MS after removing particulate matter and spiking with internal standard (Creatinine-d3). Creatinine might be useful as a biomarker for infection in clinical samples (Pourakbari et al., 2021), but the ease at which it degrades in wastewater makes it worrisome to be used as a population indicator (Choi et al., 2018). Stability issues with creatinine can be solved by acidification, preservation, and even filtration (Chen et al., 2014).
Patients with elevated creatinine kinase have been diagnosed with SARS-CoV-2 positive cases through reverse-transcription polymerase chain reaction (RT-PCR) (Chan et al., 2020), which shows a relationship between the presence of increased creatinine in wastewater and SARS-CoV-2.
Concentrations of creatine in wastewater were found to have a very low correlation (Pearson coefficients, r = 0.06) with population concentration in wastewater compared to caffeine (r = 0.810) (HSu et al., 2022). The log transformation of the daily flow rate also confirmed that the correlation of the creatinine load showed low correlation (r = 0.33) with population when compared with paraxanthine (r = 0.97), caffeine (r = 0.97), 5-HAA (r = 0.87), and PMMoV load (r = 0.92) (HSu et al., 2022). However, another study found a good correlation between creatinine load and a nominal number of connected residents to the wastewater plants (Westhaus et al., 2021). A laboratory study find that the order of degradation of creatinine was first order kinetics and that it did not correlate with the populations across the sampled wastewater treatment plants (Thai et al., 2014). Since, the concentration of creatinine are generally low due to degradation, it is not suitable biomarker for population normalization.
Creatinine is hydrophilic; therefore, a larger proportion will be associated with the aqueous phase of wastewaters; therefore, large volume injection is a common quantification method (Centazzo et al., 2019; Chen et al., 2014). Although some groups have used filtration to remove suspended matter, some have employed centrifugation followed by a dilute and shoot approach (Baker and Kasprzyk-Hordern, 2011; Rico et al., 2017). This approach overcame the challenges with minimal extraction observed with the SPE method (Baker and Kasprzyk-Hordern, 2011; Rico et al., 2017). Therefore, creatinine will be best analysed by simply direct injection after removing suspended matter. Creatinine extracted from wastewater can easily be quantified through LC-MS.
4.2. Artificial sweeteners
Artificial sweeteners are generally abundant in wastewater because they are widely used as substitutes for sucrose and fructose in food, beverages, pharmaceuticals, and personal-care products (Fu et al., 2020; Liu et al., 2014). They are synthetic compounds and do not occur in nature and are associated solely with human activity. Since they are consumed in predictable amounts over extended periods in particular cultures, they can be correlated with the numbers of people in the population. Sucralose and acesulfame were the most abundant artificial sweeteners reported for Wuhan surface water, with 0.33–18.0 μg/L and 0.40–2.78 μg/L concentrations, respectively which accounted for 90% ± 8% of the detected artificial sweeteners (Liu et al., 2014). Acesulfame is a non-nutritive sweetener that is often used in combination with other artificial sweeteners to enhance the sweetness of food and beverages. Sucralose is a chlorinated sucrose derivative that is often used as a sugar substitute in low-calorie foods and beverages. Acesulfame and sucralose have been reported in the range of (6.316–10.513 μg/L) and (2.107–6.500 μg/L) in raw influent wastewater, respectively (Tran et al., 2015). The excretion factor has been reported to be 1.00 (Rico et al., 2017).
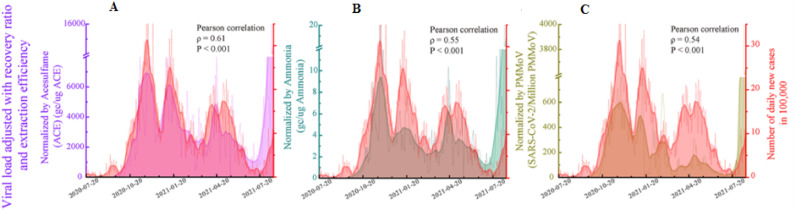
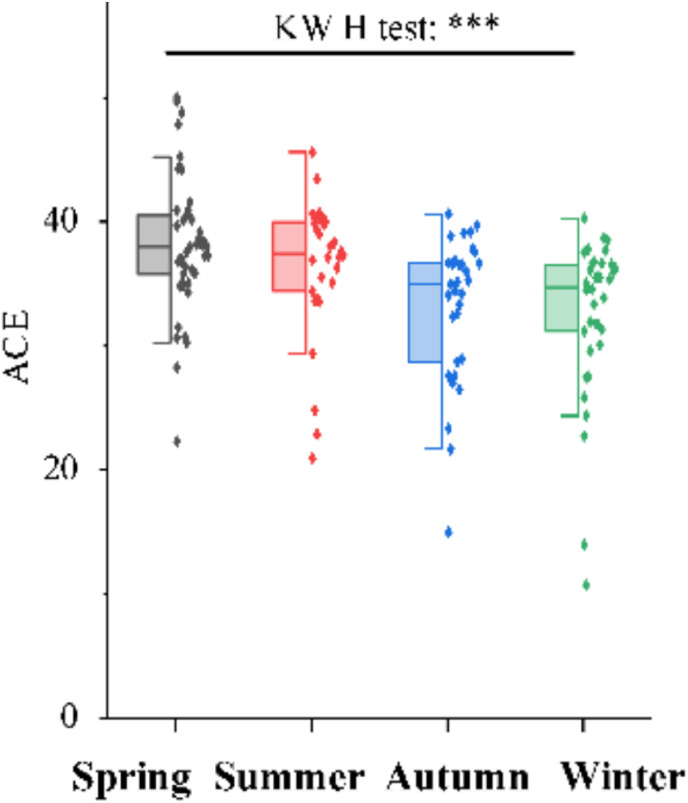
Sucralose and acesulfame are conservative markers since they have been reported to be persistent in biological wastewater treatment processes. Therefore, concentrations and loads of acesulfame and sucralose were comparable in untreated and treated wastewater (Buerge et al., 2009; Tran et al., 2015). Acesulfame was better for normalizing viral load compared to ammonia and PMMoV (Fig. 2 ) (Xie et al., 2022). However, concentrations of artificial sweeteners have been reported to vary with seasons (Liu et al., 2014), and it is therefore not stable throughout the year in regions or countries with varied weather conditions (Fig. 3 ); hence it was used to account for the seasonal population in Saskatoon (Xie et al., 2022). Most artificial sweeteners are highly water-soluble. For instance, sucralose has a high solubility in water with a log Kow of −1 (Table 1) (Baalbaki et al., 2017). The persistent of sucralose and acesulfame and their ability to be detected in wastewater make them a candidate for population normalization.
Fig. 2.
Normalization of SARS-CoV-2 RNA viral load with (A) acesulfame, (B) Ammonia, (C) PMMoV (adapted from Xie et al., 2022, Permission and license granted by ACS).
Fig. 3.
Seasonal stability of acesulfame (adapted from Xie et al., 2022, Permission and license granted by ACS).
Different method has been used to extract artificial sweeteners from wastewater matrix. Artificial sweeteners have been extracted by briefly centrifuging wastewater samples at 16,000 g for 30 min in 2 mL polypropylene micro-centrifuge tubes to remove particulate matter (Tran et al., 2015). Then the supernatant was transferred into an amber auto-sampler vial and spiked with methanol and internal standard (sucralose-d6 and acesulfame-d4). Amber vials were used to avoid photo-degradation of the targeted artificial sweeteners. In some cases, the filtration step has been avoided by adding 15 mM sodium EDTA to the wastewater sample and then adjusting the sample pH to 2.5 and 8.5 using formic acid (FA) and ammonium hydroxide (NH4OH) for acidic and basic extractions, respectively (Van Stempvoort et al., 2013). Internal standards were employed to account for extraction efficiency. The method by Van Stempvoort et al. (Van Stempvoort et al., 2013) is more complex than that of Tran et al. (2015) because Tran et al. (2015) simply precipitated the particulate matter out and worked with the supernatant. Another more complex method involves filtration using 1.2 μm glass fibre filters (GF/C, Whatman, UK), followed by 0.45 μm membrane filters (PALL Corporation, US), subsequently separating dissolved and particulate phases and then SPE extraction ((Tran et al., 2013). The disadvantage of the latter method is the volume of wastewater used and the time needed for the extraction (Table 3 ). In another method, a single filtration step was used, using 0.45 μm cellulose nitrate membrane filters (0.45 μm, 47 mm, Whatman, UK) to filter the wastewater at room temperature, followed by SPE (Gan et al., 2013). So, Tran et al. (2013) and Gan et al. (2013) used different wash buffers because Tran et al. (2013) used Milli-Q-water, while Gan et al. (2013) used Acetic acid–sodium acetate wash buffer with varied pH and 60.6 mg TRIS in 500 mL of Milli-Q water. Xie et al. (Oloye et al., 2022; Xie et al., 2022) avoided complex extraction method by simply using direct injection after filtration. Therefore, since artificial sweeteners could be analysed by simple direct injection, then direct injection would be best method, since no chemical is involved in such method.
Table 3.
Summary table showing advantage and limitation of common method of sample preparation before quantification.
| Type | Methods | Merits | Limitation | Practicality | References |
|---|---|---|---|---|---|
| Direct injection | This method introduces the sample directly into an analytical instrument without any prior sample preparation. | Fast, convenient, and inexpensive. No training is special training or equipment needed. No solvent needed. |
Lower sensitivity and accuracy due to the presence of matrix components and contaminants. Cannot be used with less sensitive analytical instruments. Cannot be used when the target concentration is very low. Recovery is always low compared to other methods. |
Can be used with caution. | Thai et al. (2019); Xie et al. (2022). |
| aDilute and shoot | In the dilute and shoot method, a sample is first diluted to reduce matrix interference and then introduced into an analytical instrument. | Improve the sensitivity and accuracy of the analysis. Avoid matrix effect observable with traditional direct injection. |
Require dilution with water. Requires careful optimization of the dilution factor to avoid loss of target analytes. Compounds need to show sufficient ionization to be successfully determined by the dilute and shoot method. |
It can be used with caution. | Rico et al. (2017). |
| aLarge Volume Injection | A large volume of sample is introduced into an analytical instrument | The number of target analytes present in the sample can be increased. It is a valuable method for trace analysis and the detection of low-concentration analytes. |
It can result in interference from matrix components and contaminants. | It can be used with caution. | Cantwell et al., 2019. |
| Liquid-Liquid | In liquid-liquid extraction, target analytes are separated from a sample matrix by partitioning them between two immiscible liquids | Simple and cost-effective method. No special instrument needed. Avoid matrix effect observable with traditional direct injection. Faster than the SPE method. |
Needs extraction solvents. Requires careful optimization of solvent and extraction time to achieve optimal yield and selectivity. |
It can be used with the proper selection of extraction solvent. | Chen et al. (2014). |
| Solid phase | a sample is passed through a solid sorbent material, where the target analytes are selectively adsorbed onto the sorbent based on their physicochemical properties. The sorbent can be made of different materials, such as silica, polymer, or resin | Solid phase extraction method is a selective and efficient method for sample preparation. Reduce the effect of the matrix effect. It can be used with both sensitive and less sensitive analytical instruments. Different adsorbents can be used, which provides flexibility. |
Needs extraction solvents. Needs clean up. High operational cost. It requires more time and resources than other methods. The sorbents could poorly or strongly retain analyte due to their polarity. |
It can be used with a proper selection of adsorbent. | Esperanza et al. (2004); Chen et al. (2014); Pandopulos et al. (2021). |
Types of direct injection.
The typical method of separation of artificial sweeteners is liquid chromatography. The choice of liquid chromatography is because of the low log Kow of −1.33 for acesulfame (Table 1) (Van Stempvoort et al., 2013), which makes them very mobile. Also, the typical detector for quantification is MS/MS (Fu et al., 2020; Van Stempvoort et al., 2013).
6.3. Caffeine/1,7 -dimethyluric acid/paraxanthine
Caffeine is one of the constituents of coffee, tea, chocolate, energy drinks, and certain medicines (Baz-Lomba et al., 2016). Caffeine is rapidly absorbed and metabolized by the body and is excreted into the urine in its unchanged form. Caffeine consumption in a location can be monitored through wastewater, representing the caffeine intake in that geographical location (Gracia-Lor et al., 2017). Caffeine and its metabolites demonstrate low variability, accuracy, and precision because of their stability in wastewater (HSu et al., 2022). The concentration of caffeine in wastewater varies depending on its consumption in each community, so different amounts have been reported from 12 to 33 μg/L (Chakraborty et al., 2021) to (23.3 ± 8–20.2 ± 10 μg/L) (Viviano et al., 2017), and 53–325 μg/L (Oliveira et al., 2015). The excretion factor of caffeine had been pegged at 0.03 (Rico et al., 2017).
Caffeine and its metabolite (paraxanthine) loads showed a high correlation of r = 0.99 and r = 0.97 with population, respectively (HSu et al., 2022). Caffeine and its metabolites have proved to be effective in estimating the population serving a particular serwer system, thus could serve as tool for normalization (HSu et al., 2022). The SARS-CoV-2 viral loads normalized by the paraxanthine estimated population significantly improved the correlation (rho = 0.5878, p < 0.05) between SARS-CoV-2 load per capita and case numbers per capita (HSu et al., 2022). Caffeine helped to fully understand SARS-CoV-2 viral load because it was used to estimate the population size and the concurrence percentage between caffeine and SARS-CoV-2 viral load for N1 and N2 was greater than 70% (Chakraborty et al., 2021). Interestingly >80% removal of caffeine has also been linked to 100% removal of SARS-CoV-2 viral load (Chakraborty et al., 2021), which suggests a strong correlation and thus could be employed as a normalization chemical for SARS-CoV-2 viral load in wastewater. Here, the association between SARS-CoV-2 RNA and caffeine does not mean that caffeine is an indicator of the virus, but only correlated with the population which excrete the caffeine.
Caffeine has been extracted from wastewater with different types of solvents and combinations of steps without any rationale for selecting the solvents. For example, caffeine and its metabolites were extracted by passing wastewater through 1.6 μm GF/A glass microfiber filters and 0.45 μm mixed cellulose membrane filters (Baz-Lomba et al., 2016). Then an aliquot (3 mL) was spiked with a labelled internal standard solution (Caffeine-C13) and loaded onto the Oasis HLB SPE cartridge. For analysis, the extract was evaporated to dryness and reconstituted in 100 μL methanol-ultrapure water (20:80, v/v), centrifuged and transferred into glass vials for instrumental analysis. One μL of the final extracts was injected into the LC-MS/MS system. Chromatographic separation was performed using an HPLC XTerra C18 column (3.5 μm, 1 mm × 100 mm) (Waters, Milford, MA, USA), and analytes were ionized using electrospray ionization in positive mode. A group avoided the filtration step and only used SPE (Hillebrand et al., 2012). However, 500 mL was used, a relatively large volume compared to 3 mL, which was loaded to SPE by the other group (Baz-Lomba et al., 2016). pH adjustment was made with a buffer to 7.00 (neutral) (Hillebrand et al., 2012), which was avoided by another group (Baz-Lomba et al., 2016). pH adjustment was made because wastewater samples can contain humic and fulvic acids. Co-extraction of humic and fulvic acids with target compounds might be significantly less at neutral pH (Nödler et al., 2010). Prior to extraction, internal standards were added as required. After extraction, the sorbent was rinsed with ultrapure water and dried. The cartridge was wrapped in aluminium foil and kept frozen (−18 °C) until analysis. The analytes were eluted with methanol and ethyl acetate consecutively. The solvents were evaporated, and the dry residue was re-dissolved in an aqueous 5 mM ammonium acetate solution containing 4% methanol. More chemicals were employed for the analysis by Hillebrand et al. (2012) compared with Baz-Lomba et al. (2016). Hence, any method that employs fewer chemicals is better since the science community is moving towards sustainability and a green revolution. Another method that avoided filtration was done by allowing the sample to settle, and the supernatant was taken without allowing resuspension of the settled particulates (Nödler et al., 2010). Samples were extracted in duplicate. Prior to extraction, the sorbent (OASIS HLB) was conditioned with 4 mL of methanol and rinsed twice with 4 mL of ultrapure water. Conditioning the cartridge before loading the sample is essential to avoid the analyte of interest from being strongly sorbed to the cartridge. Generally, samples were spiked with internal standards and pH adjusted appropriately. All sample matrices had pH 7.0 ± 0.2 after buffering. Extraction was done with a flow rate of 15 mL/min. After extraction, the sorbent was rinsed with 2 × 1.5 mL of ultrapure water to remove the inorganic salt matrix. Afterwards, the sorbent was dried by drawing air through the cartridges under a vacuum for 30 min. Analytes were eluted with 2 × 2 mL of methanol, followed by 2 × 2 mL of ethyl acetate. The extract was evaporated to dryness at 40 °C with a gentle stream of nitrogen and re-dissolved in 1 mL of aqueous 5 mM ammonium acetate solution containing 4% methanol (Nödler et al., 2010). The most popular methods for analysing caffeine and its metabolites are SPE with OASIS HLB cartridge. However, some SPE approaches were made online with LC-MS (Viviano et al., 2017), and some were offline (Baz-Lomba et al., 2016).
Interestingly some groups avoided SPE by simply filtering the sample, fortifying it, and injecting it into LC-MS (HSu et al., 2022; Oliveira et al., 2015). This method is simply the best because it is cheaper and more environmentally viable. The recovery from this method is >100% (Oliveira et al., 2015), which is >90% (Baz-Lomba et al., 2016) reported for those that involve the use of SPE step.
Caffeine can be analysed with liquid chromatography because of its low log Kow (−0.13 ± 0.37) (Nödler et al., 2010). The caffeine recovery in wastewater was greater than 90% (Rimoldi et al., 2020). It can be detected in both positive and negative electrospray ionization mode. Ionization could be carried out in electrospray at ± 3.5 kV and detected in MS/MS mode using the 195/110 and 195/138 amu transition ions (Oliveira et al., 2015; Viviano et al., 2017). Caffeine and its metabolite can easily be separated for analysis using most of the typical mobile phases, such as water containing traces of formic acid and methanol or acetonitrile as phases A and B (Chakraborty et al., 2021). Identification can be made by comparison with the retention time, extracted accurate mass (m/z 195.0882 amu) of [M + H]+ ion and MS/MS spectrum of pure standard solutions (Rimoldi et al., 2020).
4.4. Nicotine/cotinine
Many people worldwide widely consume tobacco, and its main stimulant ingredient is the alkaloid nicotine. Nicotine is a plant-derived drug in everyday use worldwide, while cotinine is a by-product of the oxidative enzymatic metabolism of nicotine in the liver (Buerge et al., 2008; Mackuľak et al., 2015). Nicotine metabolites might give an accurate representation of the population than nicotine itself because the higher concentration of nicotine in wastewater has been reported to have originated from multiple sources rather than human waste (Senta et al., 2015). Similarly, Cotinine (one of the nicotine metabolites) has a longer half-life (18–20 h) than nicotine (half-life, 1–2 h) and is therefore a more reliable chemical tracer for tracking tobacco use. An agreement has been reported between nicotine metabolites and the population size (Senta et al., 2015). However, no attempt has been made to normalize SARS-CoV-2 RNA with nicotine or its metabolites.
Consumption of nicotine can be estimated from cigarette consumption determined by tobacco sales, and the excretion rate can thereby be calculated from molecular mass ratios and the percentages of excretion in wastewater (Rico et al., 2017). The excretion factor of cotinine was determined to be 0.14 (Rico et al., 2017), and it is found to be stable in wastewater (Senta et al., 2015). Since, the excretion rate is known and it is stable in water, then nicotine can be used to estimate population size. However, it will not account for population not consuming nicotine related products. Thus, it may under estimate the actual population size of an area.
The SPE method involves filtration to remove suspended matter, spiking with internal standard (cotinine d3 or nicotine-D4), and pH adjustment before loading on an equilibrated cartridge (Chen et al., 2014; Senta et al., 2015; Tscharke et al., 2016). However, another study avoided the initial filtration step (Buerge et al., 2008). Both reusable glass columns containing ∼10 mL of a macroporous polystyrene divinylbenzene adsorbent (BioBeads SM-2, 20–50 mesh, Bio-Rad Laboratories, Hercules, CA) (Buerge et al., 2008) and Oasis HLB cartridges (Senta et al., 2015) had been employed for the extraction. Before extraction, the equilibration column was done with dichloromethane, methanol, and water by one group (Buerge et al., 2008), while another avoided dichloromethane as a preconditioning solvent (Senta et al., 2015). However, no justification for using or not using dichloromethane was provided. Analytes were recovered with methanol, removing residual water from the SPE material and dichloromethane (Buerge et al., 2008). However, only methanol was employed when the Oasis HLB cartridge was employed (Senta et al., 2015) and a mixture of 96% dichloromethane: isopropanol (80:20)/4% ammonia was employed when the cartridge was UCT™ XRDAH (500 mg/6 mL) (Tscharke et al., 2016). Extracts from untreated wastewater contained some suspended material and were filtered (Buerge et al., 2008); therefore, filtration is essential for SPE to avoid suspended matters). However, the effect of filtration after SPE and before SPE needs to be examined.
In other methods, the pH of samples was not adjusted, samples were filtered, and the extraction was performed in one step, using online SPE liquid chromatography coupled with a hybrid mass spectrometer (MS) Q-Exactive (quadrupole coupled to high-resolution orbital trap MS, Thermo Scientific) (Fedorova et al., 2013; Mackuľak et al., 2015). The effect of pH adjustment has not been studied for nicotine and its derivatives; however, pH adjustment might be necessary since ionizable compounds acidification at extraction is used to modify the ionization state of the compounds (and matrix) to ensure they are appropriate for the extraction technique being used.
A direct injection method that avoided the SPE step and solvents was determined to be appropriate for nicotine and its metabolites (Lai et al., 2018; Z. Wang et al., 2021). Samples were only filtered and spiked with internal standards such as nicotine-D4, cotinine-D3, and hydroxycotinine-D3. The amount of nicotine detected by direct injection varied between 0.13 and 3.90 × 106 μg/day (Lai et al., 2018), which is higher compared to 0.5–8 × 103 μg/day (Gao et al., 2020), but it must be noted that the source of the samples was different, hence not directly comparable. Another group had shown nicotine concentration in the range of 0.77–0.93 μg/L (Z. Wang et al., 2021), which was less than 1.36–6.87 μg/L reported for Italian wastewater (Senta et al., 2015). The concentration of nicotine and its metabolite were determined to be in a similar range of 0.65–3.12 μg/L and 2.14–7.00 μg/L for cotinine and trans-3′-hydroxycotinine, respectively (Senta et al., 2015). No significant variation was observed in the mean concentration of nicotine on weekdays and weekend, because changes was less than 10% (Senta et al., 2015). Nicotine and its metabolite extracted from different countries with different methods are difficult to compare to determine the best extraction method because of the varied consumption rate of tobacco. So, when sample enrichment was done by employing the SPE protocol in areas where concentrations were low, the concentration of nicotine obtained from samples extracted with SPE (1.9 × 106 μg/day) was within the range of those processes through direct injection in other areas (0.13–3.90 × 106 μg/day) (Lai et al., 2018). In another study, the SPE step was avoided but acidified to pH 2 at the sampling site using 2 M HCl and frozen before pre-treatment (Gao et al., 2020). It has been justified that an SPE step is not needed because most analytical instruments are sufficiently sensitive to analyze trace amounts of chemicals in complex matrices (Gao et al., 2018, 2020). However, in some cases avoiding SPE has been associated with reduction in the concentration of detected analytes due to matrix effects (Rico et al., 2017).
Liquid chromatography-mass spectrometry (LC-MS) methods are commonly used to detect nicotine and cotinine in wastewater. Different solvents have been employed for mobile phases in LC-MS depending on the stationary phase employed in the column. For example, 5 mM ammonium formate (Lai et al., 2017) was added to formic acid used in some situations, while another group avoided its addition (Z. Wang et al., 2021). Using solvent different from the mobile phase in LC-MS may require reconstitution with a solvent compatible with the mobile phase. For example, fractions recovered with a mixture of 96% dichloromethane, isopropanol and ammonia were reconstituted with 20 μL of methanol followed by mixing with 180 μL of 0.1% formic acid in water (Tscharke et al., 2016). The composition of the mobile phase will have to do with individual experience and the columns.
Separation can be done with most reverse phase columns such as C8 and C18 (Q.-D. Zheng et al., 2019). The column commonly used for separation is the Phenomenex Kinetex Biphenyl column (50 × 2 mm, 2.6 mm Phenomenex, California, US) (Z. Wang et al., 2021), and 100 × 1 mm X-Terra C18 column (Senta et al., 2015).
Positive electrospray has been reported to show better performance than the negative mode for quantification of nicotine because of higher responses (Q. Zheng et al., 2019). So many groups use the positive mode to quantify nicotine (Gao et al., 2018; Lai et al., 2017; Senta et al., 2015; Z. Wang et al., 2021). The recovery of nicotine and its metabolite had been reported to be >70% (Senta et al., 2015), which is reasonable.
4.5. Cholesterol/coprostanol
Cholesterol is a sterol, which is a type of lipid. The body uses cholesterol as the starting point for synthesizing biomolecules, including the hormones estrogen, testosterone and vitamin E, vitamin D, and other vital compounds (Juste and Gérard, 2021). It is an essential structural component of animal cells, which the cells are capable of synthesizing; therefore, all organisms are capable of excreting cholesterol. Cholesterol can be metabolized, or reduced by microbial action, to coprostanol although coprostanol can be formed from other compounds such as stigmasterol in the intestinal trait of various vertebrates (Gilli et al., 2006; Kaiser and Lerch, 2022). Coprostanol is found in domestic raw wastewater due to cholesterol decomposition from a population served by the wastewater treatment plant.
Coprostanol concentration in wastewater was reported to be between 393.92 and 913.68 μg/L (Furtula et al., 2012). The considerable difference between the upper concentration limit and the lower concentration limit could be because cholesterol and coprostanol are diet-dependent (Chen et al., 2014). The concentration of coprostanol and cholesterol range from 36 to 183 μg/L, respectively (Beck and Radke, 2006), which also showed a high standard deviation. Coprostanol is the main human fecal sterol in wastewater, and it constitutes about 60% of the total sterols found in human feces (Gilli et al., 2006; Hagedorn and Weisberg, 2009; Kaiser and Lerch, 2022). Thus, coprostanol is a valuable indicator of feces in wastewater. Coprostanol exhibited the lowest variation in composite influent samples compared with crAssphage, pepper mild mottle virus (Reynolds et al., 2022). Coprostanol is relatively stable in wastewater, but the concentration reported have large standard deviation.
Interestingly, the strongest correlations were observed between SARS-CoV-2 levels and national and Dublin COVID-19 cases when levels were normalized to coprostanol (Reynolds et al., 2022). Coprostanol is a valuable marker of the population to normalize when making measurements of viruses shed in feces. However, the rate at which its sorbs to the particulate matter has reduced its use as a normalization chemical (Choi et al., 2018).
Coprostanol has been extracted using four methods: (1) filtration using glass fibre followed by LLE, (2) LLE without saponification step, (3) LLE including saponification step, and (4) SPE method. Each of these methods has advantages and limitations. For example, Chen et al. (2014) determined cholesterol and coprostanol from wastewater samples by first filtering the sample using glass microfibre filters. Samples were adjusted to acidic pH using either acetic acid (Chen et al., 2014), sulfuric acid (Furtula et al., 2012) or basic pH using NaOH (Gilli et al., 2006). After which, the acidified sample was extracted using the LLE protocol, where the mixture of hexane and ethyl acetate was employed. Another method only employed ethyl acetate and avoided the filtration step (Reynolds et al., 2022), while another group used methanol, chloroform, and hexane (Gilli et al., 2006). The justification for choosing solvents or their ratio for coprostanol extraction is not well explained. Nevertheless, using one solvent is preferable to using multiple solvents.
To gain insight into adsorption to the surface of the glass microfibre filters and the particulate matter in wastewater, the experiment was conducted simultaneously to avoid filtration. The glass microfibre filters generally retain particulate matter in wastewater (Gilli et al., 2006). It has been observed that cholesterol and coprostanol bind to the particulate matter and glass microfibre because unfiltered samples were 625 times greater (p < 0.001) than those from filtered samples (Chen et al., 2014). Challenges with binding sterols to particulate matter can be avoided by skipping the filtration step or extracting the residue. Since sterols bind to particulate matter, LLE without filtration is advantageous because it enables the evaluation of bound and unbound sterol compounds, capturing more sterol compounds than the SPE method (Gilli et al., 2006). A study extracted stanol bonded to particulate matter in the filter using supercritical fluid extraction (SFE) on a Dionex Model 703 (Dionex, USA) SFE extractor with 10 mL stainless steel extraction cells (Jayasinghe et al., 1998). The cost of SFE and the additional step make this method not feasible.
Extraction without filtration overcomes some of the challenges of adsorption of coprostanol and cholesterol to particulate matter or filters. However, the volume of the wastewater to use as a starting material to give sufficient detection limits is a challenge. For example, Gilli et al. (2006) used a large volume of 250 mL volume of wastewater sample compared to 35 mL (Chen et al., 2014) and 10 mL (Reynolds et al., 2022), which are considered small volumes. The lesser the sample volume employed, the faster the extraction and the less the solvents employed.
Another group avoided the filtration step but included the clean-up step (Furtula et al., 2012). The sample pH was first adjusted with sulfuric acid, then extracted with dichloromethane before cleaning up in a column packed with deactivated silica gel. The column was later eluted with hexane and acetone, followed by hexane (Furtula et al., 2012). This method combines LLE with SPE, therefore taking more time than methods that only employed LLE or SPE.
The saponification step allowed the esterified coprostanol to be converted to free coprostanol, making quantification easier. The issue with this method is that it is time-consuming and involves using additional solvents. However, a modified version was reported by Chou and Liu (Chou and Liu, 2004), in which the sample was thoroughly mixed with 0.5 g of anhydrous sodium sulphate in a 60 mL screw-capped vial, and 0.5 N methanolic KOH was added. Then, the mixture was directly saponified by incubation in a water bath at 60 °C for 60 min with periodic shaking. After saponification, samples were cooled to room temperature, deionized water was added, and the pH was adjusted to 7. The extract was then extracted with dichloromethane (3 × 20 mL). Then passed through column chromatography. This method skips the first stage of extraction before saponification. However, the addition of column chromatography for clean-up is an added time. Esterified coprostanol reduces the amount of coprostanol that could be measured.
Quantification with LC-MS does not require the derivatization of the extracted coprostanol. Derivatization sterol components are more volatile and require gas chromatography with MS, FID or ECD detection. The recovery of coprostanol with GC-FID was >90% (Gilli et al., 2006), while the recovery was 84 ± 18% with LC-MS (Beck and Radke, 2006). The difference might be related to the extraction protocol or the analytical methods. GC will generally be good to separate stanol considering the different isomers. However, GC requires a derivatization step, which is necessary for insufficiently volatile and thermally unstable substances. So, using LC-MS could be more advantageous than GC-MS because the former does not require a derivatization step. An LC-MS method, which offers considerable performance compared with traditional GC−MS methods, was developed and applied for the analysis of sterols (Bataglion et al., 2015). However, selecting the right ionization source is essential; for example, an Atmospheric Pressure Chemical Ionization (APCI) source was observed to have signals one order of magnitude better than those from the ESI detection (Baila-Rueda et al., 2013). The APCI ionization technique can ionize compounds with various polarities while remarkably tolerant of matrix additives (de Melo et al., 2019; C. Wang and Gardinali, 2012). Therefore, APCI can benefit coprostanol and cholesterol analysis because these compounds are difficult to ionize (C. Wang and Gardinali, 2012). Other ionization sources that could be tested for sensitivity to sterol/stanol are atmospheric pressure photoionization (APPI), ion Max atmospheric pressure ionization (API) and nanospray ionization (NSI). The advantage of the Ion Max API source is that it can be operated in any of the several API modes, including APCI, ESI and APPI.
4.6. 5-Hydroxyindoleacetic acid
5-hydroxyindoleacetic acid (5-HIAA) is a serotonin metabolite with high daily excretion rates and therefore was proposed as a biomarker of the population for data normalization (Chen et al., 2014). SARS-CoV-2 RNA normalized with 5-HIAA has been found to have a strong correlation (r > 0.9) with the cumulative incidence of COVID19 cases (Gudra et al., 2022).
5-HIAA was stable in wastewater and can readily be analysed with LC-MS (Thai et al., 2019). However, another group reported that it degrades rapidly in untreated wastewater (Chen et al., 2014). It reduced 3 times a week in wastewater at room temperature but stable at pH 2 when stored below 4 °C (Jang et al., 2019). Another study reported a day-to-day variation in the load of 5-HIAA (Gudra et al., 2022). The variation in the day-to-day load of 5-HIAA might not be due to the stability of the compound but to population dynamics or the variability in the excretion rate by the discharging population (Gracia-Lor et al., 2017). Furthermore, 5 -HIAA excretion might vary because of disease or diet, such as nuts or salts consumption (Gracia-Lor et al., 2017).
However, there is an argument that 5-HIAA concentration excreted is not habit or lifestyle-dependent (Gudra et al., 2022). It is important to note that an average healthy individual has a consistent excretion rate of 3 μg/day (Choi et al., 2018). Another study showed that 5-HIAA excreted varied from 103 × 103 μg/day to 17 × 103 μg/day in two cities (Gudra et al., 2022). A similar observation was reported in Valencia, where the concentration varied between 5.5 and 14.3 μg/L (Jang et al., 2019). Since the excretion rate might vary because of infection, the variation in the weekly load of 5-HIAA might not accurately represent the discharging population. Interestingly 5-HIAA varied between weekdays and weekends, which correlates moderately with the movement of people (Gudra et al., 2022).
The common method for extraction of 5-HIAA are SPE, LLE and dilute and shoot methods. The SPE method resulted in minimal extraction of 5-HAA, so a “dilute and shoot” method of direct injection was developed (Rico et al., 2017). The dilute and shoot method involves spiking with internal standards (such as 5-HIAA-d5 or salbutamol-d3) and dilution with uncontaminated, distilled water (Rico et al., 2017). Direct injection when samples were acidified to pH 2 had also been linked with low recovery (Thai et al., 2019). However, reasonable recovery was achieved when wastewater samples were not diluted nor centrifuged but pH adjusted to 2 with hydrochloric acid and spiked with 5-HIAA-d5 prior to extraction with ethyl acetate (Chen et al., 2014; Pandopulos et al., 2021). A group adjusted the pH to 2.7 with formic acid (Jang et al., 2019); the amount of formic acid needed for the adjustment will be higher than HCl. Thus, higher amount of chemical will be used which will end up in the environment. Therefore, LL extraction of 5-HIAA with ethylacetate and acidified with HCl is better than with formic acid.
The solvent used for LLE also plays a significant effect on the recovery. For instance, ethyl acetate and tert-butyl methyl ether gave better reproducibility and extent of extraction for 5-HIAA from wastewater than hexane, chloroform, dichloromethane, hexane/diethyl ether (50: 50) (Jang et al., 2019).
The role of acidification in improving yields was not fully discussed by Chen et al. (2014) nor Jang et al. (2019). If acidifying prevents microbial degradation, samples are best acidified at the sampling point, not during extraction. However, pH adjustment modifies the ionization state of the compounds (and matrix) to ensure they are appropriate for the extraction technique being used. It has been demonstrated that low temperature and acidification improved the stability of 5-HIAA (Chen et al., 2014). The effect of acidification on wastewater samples was investigated by Thai et al. (2019), and it was found that acidification had a negative effect on 5-HIAA detection. Acidification of 5-HIAA to pH 2 prevented it from being detectable, but 5-HIAA measurement can be done by direct injection at neutral pH (Thai et al., 2019). However, Jang et al. (2019) found that extraction recovery of 5-HIAA depends on the pH of solutions and is inversely proportional to pH. A maximum recovery of 74% was obtained by using ethyl acetate at pH 2.7. Hence, differences observed by Thai et al. (2019) and Jang et al. (2019) might be related to the extraction method rather than pH alone.
Changes in pH have a significant effect on extraction with SPE (Jang et al., 2019). However, different cartridges have varied patterns with acidification or pH adjustment. For example, 5-HIAA recovery on Chromabond C18 and AccuBOND C18 cartridges decreased with increased pH, but it has no trend on Chromabond HR-P (Jang et al., 2019). Hence, more work is needed to understand the effects of low pH and high pH on the stability of fecal indicators.
5-HIAA is relatively hydrophilic because its log Kow is 1.1 (Pandopulos et al., 2021) but has been reported to bind into extraction material, resulting in low recovery and poor reproducibility (Jang et al., 2019; Rico et al., 2017). However, it has been pointed out that careful selection of the adsorbent for SPE could improve recovery (Jang et al., 2019). Strata X performed better than Chromabond C18, Chromabond HR-P, and AccuBOND C18, where the recovery was 63 ± 13, 31 ± 1, 5 ± 1, and 24 ± 3, respectively (Jang et al., 2019). 5-HIAA strongly sorbed to some materials, therefore challenging to desorb from them, while weakly sorbed to some at a particular pH. This observation was supported by authors who found no loss of 5-HIAA upon filtration (Chen et al., 2014; Pandopulos et al., 2021).
The residue obtained after extraction is generally reconstituted with the LC-mobile phase solvent (such as acetonitrile), followed by mixing with water prior to LC-MS/MS analysis (Chen et al., 2014). 5-HIAA is commonly identified in mass spectrometer using positive ESI and setting the actual mass and monitoring the following transition m/z (192 → 146) and (192 → 117) (Jang et al., 2019). However, the precursor ion changed to 282 when the extract was derivatized (Pandopulos et al., 2021). It can also be identified in negative mode, with modification to accommodate both the precursor ions and productions (190 → 146 and (195 → 151) (Pandopulos et al., 2021). The limit of detection (LOD) and limit of quantification (LOQ) were improved by > 100-fold when derivatized after the extraction step (Pandopulos et al., 2021). Although derivatization improves both LOD and LOQ, it increases the time and cost of sample preparation. Instead of derivatization, the ion source could have been changed to APCI, which is more sensitive to highly polar compounds while remarkably tolerant of matrix additives (C. Wang and Gardinali, 2012).
4.7. Androstenedione
Androstenedione is a sex steroid hormone and has been reported in wastewater at a concentration from 15 ± 5–55 ± 7 (Baalbaki et al., 2017), 80 ± 6.3–93.5 ± 6.8 ng/L (Esperanza et al., 2007), 270 ± 132 ng/L and (Chang et al., 2011). However, it is not stable in untreated wastewater and degrades rapidly within 24 h (Chen et al., 2014; Thai et al., 2019). Its concentration was reported to be close to the method detection limit of the analytical instrument and thus could not be quantified with reasonable certainty (Esperanza et al., 2007).
It is lipophilic (log Kow 2.75 (Baalbaki et al., 2017),); hence direct injection into LC-MS is probably not the most useful or practical analytical approach. So, a study used large-volume injection LC-MS (Backe et al., 2011). In brief, wastewater samples were centrifuged at 1625 x G for 15 min. Supernatant aliquots were transferred into glass autosampler vials and were spiked with stable-isotope internal standard (D3- Stan). Injection volumes of 1800 μL were employed for wastewater influent and effluent (Backe et al., 2011).
Steroid hormones are frequently extracted with SPE; for example, C-18 SPE disks were employed for androstenedione extraction after filtration and spiking with mesterolone (surrogate standard). Then extracts were subjected to the derivatization step and analysed by gas chromatography-tandem mass spectrometry (GC/MS/MS) (Kolodziej et al., 2003). However, Chen et al. (2014) employed the UCT™ XRDAH cartridge in SPE/LC-MS method for quantification of androstenedione. Both cotinine and androstenedione were extracted following the same protocol, but methanol was used to elute androstenedione and androstenolone-d5 (neutral fraction), while cotinine and cotinine-d3 (basic fraction) was eluted with a mixture of 96% dichloromethane: isopropanol (80:20) and 4% ammonia (Chen et al., 2014). Still, another group used the Oasis HLB cartridge and preconditioned it with ethyl acetate, acetonitrile, and distilled water (Chang et al., 2011). Rinse with distilled water and dry after loading the wastewater spiked with surrogate standard, then extracted with ethyl acetate. The ethyl acetate extracts were dried and resuspended with ethyl acetate and hexane. Clean-up was employed in this research despite the targeted quantification instrument being LC-MS. This clean-up was done on silica cartridges, preconditioned with water-saturated ethyl acetate and hexane/ethyl acetate, and rinsed with hexane/ethyl acetate. The reconstituted extract was loaded on the cartridge and eluted with hexane/ethyl acetate. The final extract was then dried and reconstituted with methanol. This additional step employed in this research is unnecessary with LC-MS because most of the latest equipment is very sensitive.
In another study, androstenedione was extracted from 1 L of wastewater by solid-phase extraction (SPE) (Esperanza et al., 2004). Cartridges used were superclean Envi-18 (Supelco) prepacked with 500 mg of solid phase material. The cartridges were preconditioned with methanol and distilled water. A total of 1% methanol was added to all samples. Surrogate standards were spiked prior to extraction. Samples were loaded onto the cartridges at a flow rate between 5 and 10 mL/min. Cartridges were then washed with distilled water and dried for 15 min by applying a vacuum. Androstenedione was eluted with methanol and collected in a silanized conical bottom culture tube. The extract was cleaned up using SPE neutral alumina (Supelco) cartridges containing 1 g of the adsorbent to remove interferences during GC analysis (Esperanza et al., 2004). Alumina cartridges were preconditioned with 30% methanol in acetone and 20% dichloromethane (DCM) in isooctane. C-18 extracts were taken to dryness using a gentle nitrogen stream while the tubes were submerged in a bath at 40 °C. Extracts were reconstituted in 20% DCM in isooctane and quantitatively transferred to the cartridges. The cartridges were washed with hexanes and eluted with 30% methanol in acetone. The cleaned-up extracts were then concentrated to approximately 1 mL under nitrogen and transferred quantitatively to 2-mL reaction vials for derivatization.
LC-MS is a faster method of quantification because derivatization step can be avoided. For example, before injection in the GC-MS, androstenedione was derivatized in two steps (Esperanza et al., 2004, 2007). First, the extracts were taken to dryness under a gentle nitrogen stream at 40 °C. The dried residue was reconstituted with 15% (w/v) MOX in pyridine. The reaction occurs in a heating block maintained at 70 °C for 4 h. After completion of the first reaction, vials were cooled, and excess reactant was evaporated under a nitrogen stream. Samples of pyridine and 100 μL of 10% TMCS in BSTFA were added, and vials were returned to the reaction block. The reaction was left for a long time over the reaction block because conversion of the hydroxyl groups requires a long hour of heating (15 h at 70 °C). The contents of the vials were taken once more to dryness, and the derivatized target compounds were reconstituted in injection solvent, 20% DCM in hexanes plus 5% (v/v) BSTFA. Before injection, 250 ng of each internal standard, 5 R-androstane and 5 R-cholestane, was added to each vial. The derivatization step involves using additional chemicals, which are costly and waste time. Nevertheless, one-step derivatization using MTBS-TFA is becoming popular because it is quicker than 2-steps, but it still involves additional cost.
Using appropriate stationary and mobile phase is essential to avoid mass loss because of instrumentation errors. The recovery was greater than 78% when the C18 column was employed as a stationary phase, with methanol and water containing 0.1% formic acid chosen as mobile phases (Chang et al., 2011). Another group had replaced formic acid with acetic acid as the second mobile phase (Thai et al., 2019).
It can be detected in positive ion mode; the precursor ion was 482, and the daughter ions were 467, 268 and 253 (Kolodziej et al., 2003, 2004). The LOD and LOQ are 0.1 and 0.3 ng/L, indicating GC/MS/MS is a good instrument for its detection since the signal-to-noise ratio was greater than 6 (Kolodziej et al., 2004). It has been reported that no significant ionization suppression was observed from the analysis of androstenedione (Chang et al., 2011). Thus, MS/MS will be a good detector for androstenedione.
5. Simultaneous extraction and quantification
The nature of wastewater changes from day to day; therefore, the matrix in wastewater varies with time. There have been concerns about variation in data obtained from WWTP of comparable size (Baalbaki et al., 2017). However, some of these concerns can be addressed by using standardized extraction protocols and appropriate QA/QC procedures. Several multi-residue methods using high-performance liquid chromatography (HPLC) with mass spectrometric (HPLC-MS) detection and different ionization techniques have been reported in the literature (Baalbaki et al., 2017). These approaches are more economical and efficient by allowing analysis of more than one analyte of interest at the same time.
Filtration has been criticized by some researchers because it was thought to decrease concentrations of coprostanol but has no effect on the concentrations of 5-HIAA, androstenedione, cotinine, and creatinine (Chen et al., 2014). This observation was supported by other authors who found no loss of 5-HIAA upon filtration (Pandopulos et al., 2021). Thus, when considering sample filtration, the lipophilicity of target analytes needs to be considered. For example, all samples were filtered and spiked before extraction with SPE for five analytes, of which three of them are caffeine, sucralose and androstenedione, and the recoveries were greater than 70% (Baalbaki et al., 2017). Thus, caffeine, artificial sweetners, androstenedione and other analytes with little or no binding affinity to the composition of the filters to be employed can be filtered without reduction in concentrations.
Quantitation of androstenedione and 5-HIAA was achieved using the same chromatography system with an Agilent™ ZORBAX Eclipse XDB-C18 column (1.8 μm, 50 × 4.6 mm) fitted with a C18 guard cartridge (SecurityGuard™; 4 × 3.0 mm; Phenomenex Inc., Torrance, CA) (Chen et al., 2014). This method efficiently determines these two compounds. Similarly, Oasis MAX cartridges were used to extract androstenedione, caffeine, and sucralose when the pH was adjusted to 8 using sodium hydroxide (Baalbaki et al., 2017). An SPE method using Oasis MCX sorbent and subsequent HPLC-ESI-MS had been used cotinine, nicotine, caffeine, creatinine, 1,7 dimethylxanthine, and other 60 chemicals. There has been evidence that the cartridges matter for the SPE method; however, Oasis HLB and Oasis MCX were found to give good recovery (70%) for the majority of compounds (46) from selected 65 analytes with different physical and chemical properties (Baker and Kasprzyk-Hordern, 2011). Nevertheless, the recovery for some of the analytes of interest in this study was low. For example, the reported recovery was 7 ± 3–36 ± 17, 3 ± 3–15 ± 26, 82 ± 31–76 ± 9 and 48 ± 8–37 ± 2 for caffeine, 1,7 dimethylxanthine, nicotine and cotinine, respectively (Baker and Kasprzyk-Hordern, 2011). With Oasis MCX, acidification to pH 2 is needed for ionizable basic compounds since this is a pH that promotes stability of most compounds, whereas Oasis HLB works best at neutral pH (Baker and Kasprzyk-Hordern, 2011). Hence, understanding the best condition to work is as good as selecting the right cartridge.
SPE and dilute and shoot methods were used by Rico et al. (2017) to quantitate 14 and 9 analytes, respectively. Each of these methods has its own advantages and disadvantages (Table 3), and the best method for a particular application depends on the nature of the analytes, sample matrix, and the desired sensitivity and accuracy of the analysis. For one of the communities studied for cotinine, caffeine and creatinie, concentrations recovered using SPE and injection of diluted samples are 1.10–2.10 μg/L and 1.63–4.13 μg/L, 30.08–53.80 μg/L and 49.04–98.73 μg/L, and 0.298–3.27 μg/L and 0.49–2.72 μg/L, respectively (Rico et al., 2017). However, concentrations were less than those reported by Chiaia et al. (2008), using large-volume injection. Cotinine, caffeine, and creatinine were all prepared for LC-MS using large volume injection (Chiaia et al., 2008). Concentrations ranged from 11.5 to 120 μg/L, 0.13–2.7 μg/L, and 220–1500 μg/L for caffeine, cotinine, and creatinine, respectively. The difference in the data might not be because of the extraction methods because the population of the cities served by the WWTP from these two reports differed.
Multiple reaction monitoring (MRM) is becoming popular because of its ability to analyze multiple analytes simultaneously (Backe et al., 2011). The precursor and products ion of each analyte of interest can be determined from literature or standards, then set at positive or negative mode, depending on the mode that gives the best sensitivity. The Q-Exactive mass spectrometer has been adjudged to have better sensitivity than MS/MS quadrupole (Fedorova et al., 2013). Hence careful selection of analysis equipment will improve selection when using MRM.
Using more than one tracer has been adjudged best for a population-based model (O'Brien et al., 2014). Prediction of the population with acesulfame alone was reported to have a 7% error, while no error was observed when the prediction was based on 14 chemical tracers. Hence using multiple chemical tracers to normalize viral load will be more prudent. Therefore, identifying chemicals that could easily quantify without LLE or SPE will be beneficial. Otherwise, identifying multiple chemicals which could be extracted with a single SPE or LLE approach will be better than developing methods for each chemical tracer of interest. Creatinine, artificial safeners, caffeine, androstenedione, 5-HIAA and their metabolites have been quantified without extraction using direct injection with reasonable recovery (80%). A recent review has highlighted methods for chemicals in wastewater, from where it is evident that more analytes could be quantified using the same method (Picó and Barceló, 2021). So, it is feasible to extract and quantify multiple analytes simultaneously.
6. Summary
5-HIAA and caffeine were reported to be in agreement with population estimated from hydro-chemical parameters (Rico et al., 2017). While creatinine was too unstable to be used for population estimation (Rico et al., 2017). One of the important criteria for accepting an indicator for population estimation is stability. Hence, any chemical tracers that are not stable in sewer system could not be used for population estimate for normalization purposes.
Excretion rates play a significant role in estimating population size from chemical or biological indicators. For instance, if the excretion rate is constant, it will be easier to estimate the population size from an indicator. This is evident where the concentration of analyte, flowrate, excretion rate and daily dose intake of the analytes were considered to estimate the population size, and excretion rate was found to be directly proportional to the population size (equation (1)) (Rico et al., 2017). The consistency of biomarkers throughout different populations is another criterion that is essential for their selection as population markers. Artificial sweetener, nicotine and caffeine are example of exogenous markers meeting this criteria, while 5-HIAA is the only endogenous marker that met it (Choi et al., 2018).
| equation 1 |
The ease of quantification and the costs of materials needed for quantification are other things that are important consideration for selection of population marker. This is important, since one marker will not accurately capture the actual population size comparing population size estimated from two or more analytes become essential. Thus, quantifying more than one analyte at the same time using the same method is the ideal way to go. Hence, any marker requiring special preparation method such as cholesterol and coprostanol are not idea since, they affinity for particulate matter (Choi et al., 2018). Analytes such as caffeine, acesulfame, sucralose, nicotine, creatinine, 5-HIAA and androstenedione could be analysed using direct injection (or large volume injection). They might similarly be extracted using either LLE or SPE methods. Interestingly, they can all be analysed using LC-MS.
7. Conclusions
Extraction is significant in the recovery of analytes from wastewater matrix. The extraction solvents must be carefully selected considering the analytes of interest. Methanol and ethyl acetate are the most common solvents for LLE. The pH of the extraction solution significantly affects the yield of the analyte recovered from the wastewater samples when the compounds are ionizable. Some of the indicators discussed here can be quantitated using simple direct injection e.g., creatinine, acesulfame, sucralose, nicotine, cotinine, 5-HIAA, androstenedione. However, dilute and shoot methods give better sensitivity than direct injection. LLE is the best method for sterol and stanol because it avoids binding to SPE materials. However, SPE is more beneficial for extracting androstenedione, 5-HIAA, acesulfame, sucralose, nicotine, and cotinine, but not creatinine. The nature of the SPE cartridge used also matters, as some analytes may be difficult to elute from the adsorbents. Selecting methods that can extract more than one analyte of interest is beneficial and should be considered. Androstenedione and creatinine are not stable without fortification and thus, should be extracted immediately or kept at a pH capable of improving their stability. Caffeine and its metabolites, artificial sweeteners, and 5-HIAA are the best markers to normalized SARS-CoV-2 viral load in wastewater because they best estimate population size since their stability in wastewater and recovery with most analytical methods are good. Although a good indicator, coprostanol often requires a special extraction or analytical method; hence cannot be extracted and analysed with most tracers. LC-MS has become popular for analysing all the analytes reviewed in this paper. APCI overcomes challenges with sterol and low sensitivity with ESI and hence might be the ionization source when ESI is not very sensitive. Since no single indicator meets all the requirements needed as a normalization agent for viral load, it is recommended that both biological and chemical tracers be employed simultaneously for normalization of viral load.
Credit author statement
All authors wrote the first draft, formatted, and edited the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study is part of the project titled “Next generation solutions to ensure healthy water resources for future generations” funded by the Global Water Futures program, Canada First Research Excellence Fund (#419205; Additional information is available at www.globalwaterfutures.ca) and the Public Health Agency of Canada. The research was supported by a Discovery Grant from the Natural Science and Engineering Research Council of Canada (Project # 326415–07). Prof. Giesy was supported by the Canada Research Chairs Program of the Natural Sciences and Engineering Research Council of Canada (NSERC) and a distinguished visiting professorship of Environmental Sciences from Baylor University in Waco, Texas, USA.
Handling Editor: Chang-Ping Yu
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2023.138682.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Amoah I.D., Abunama T., Awolusi O.O., Pillay L., Pillay K., Kumari S., Bux F. Effect of selected wastewater characteristics on estimation of SARS-CoV-2 viral load in wastewater. Environ. Res. 2022;203 doi: 10.1016/j.envres.2021.111877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baalbaki Z., Sultana T., Metcalfe C., Yargeau V. Estimating removals of contaminants of emerging concern from wastewater treatment plants: the critical role of wastewater hydrodynamics. Chemosphere. 2017;178:439–448. doi: 10.1016/j.chemosphere.2017.03.070. [DOI] [PubMed] [Google Scholar]
- Backe W.J., Ort C., Brewer A.J., Field J.A. Analysis of androgenic steroids in environmental waters by large-volume injection liquid chromatography tandem mass spectrometry. Anal. Chem. 2011;83(7):2622–2630. doi: 10.1021/ac103013h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baila-Rueda L., Cenarro A., Cofán M., Orera I., Barcelo-Batllori S., Pocoví M., Ros E., Civeira F., Nerín C., Domeno C. Simultaneous determination of oxysterols, phytosterols and cholesterol precursors by high performance liquid chromatography tandem mass spectrometry in human serum. Anal. Methods. 2013;5(9):2249–2257. [Google Scholar]
- Baker D.R., Kasprzyk-Hordern B. Multi-residue analysis of drugs of abuse in wastewater and surface water by solid-phase extraction and liquid chromatography–positive electrospray ionisation tandem mass spectrometry. J. Chromatogr. A. 2011;1218(12):1620–1631. doi: 10.1016/j.chroma.2011.01.060. [DOI] [PubMed] [Google Scholar]
- Bataglion G.A., Meurer E., de Albergaria-Barbosa A.C.R., Bícego M.C., Weber R.R., Eberlin M.N. Determination of geochemically important sterols and triterpenols in sediments using ultrahigh-performance liquid chromatography tandem mass spectrometry (UHPLC–MS/MS) Anal. Chem. 2015;87(15):7771–7778. doi: 10.1021/acs.analchem.5b01517. [DOI] [PubMed] [Google Scholar]
- Baz-Lomba J.A., Salvatore S., Gracia-Lor E., Bade R., Castiglioni S., Castrignanò E., Causanilles A., Hernandez F., Kasprzyk-Hordern B., Kinyua J. Comparison of pharmaceutical, illicit drug, alcohol, nicotine and caffeine levels in wastewater with sale, seizure and consumption data for 8 European cities. BMC Publ. Health. 2016;16(1):1–11. doi: 10.1186/s12889-016-3686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M., Radke M. Determination of sterols, estrogens and inorganic ions in waste water and size-segregated aerosol particles emitted from waste water treatment. Chemosphere. 2006;64(7):1134–1140. doi: 10.1016/j.chemosphere.2005.11.063. [DOI] [PubMed] [Google Scholar]
- Been F., Rossi L., Ort C., Rudaz S., Delémont O., Esseiva P. Population normalization with ammonium in wastewater-based epidemiology: application to illicit drug monitoring. Environ. Sci. Technol. 2014;48(14):8162–8169. doi: 10.1021/es5008388. [DOI] [PubMed] [Google Scholar]
- Buerge I.J., Buser H.-R., Kahle M., Muller M.D., Poiger T. Ubiquitous occurrence of the artificial sweetener acesulfame in the aquatic environment: an ideal chemical marker of domestic wastewater in groundwater. Environ. Sci. Technol. 2009;43(12):4381–4385. doi: 10.1021/es900126x. [DOI] [PubMed] [Google Scholar]
- Buerge I.J., Kahle M., Buser H.-R., Müller M.D., Poiger T. Nicotine derivatives in wastewater and surface waters: application as chemical markers for domestic wastewater. Environ. Sci. Technol. 2008;42(17):6354–6360. doi: 10.1021/es800455q. [DOI] [PubMed] [Google Scholar]
- Cantwell M.G., Katz D.R., Sullivan J.C., Lyman M. Evaluation of wastewater tracers to predict pharmaceutical distributions and behavior in the Long Island Sound estuary. Chemosphere. 2019;220:629–636. doi: 10.1016/j.chemosphere.2018.12.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centazzo N., Frederick B.-M., Jacox A., Cheng S.-Y., Concheiro-Guisan M. Wastewater analysis for nicotine, cocaine, amphetamines, opioids and cannabis in New York City. Forensic Sciences Research. 2019;4(2):152–167. doi: 10.1080/20961790.2019.1609388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P., Pasupuleti M., Shankar M.J., Bharat G.K., Krishnasamy S., Dasgupta S.C., Sarkar S.K., Jones K.C. First surveillance of SARS-CoV-2 and organic tracers in community wastewater during post lockdown in Chennai, South India: methods, occurrence and concurrence. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Farouji I., Hanoud A.A., Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19) Am. J. Emerg. Med. 2020;38(7):1548-e1. doi: 10.1016/j.ajem.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H., Wan Y., Wu S., Fan Z., Hu J. Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: comparison to estrogens. Water Res. 2011;45(2):732–740. doi: 10.1016/j.watres.2010.08.046. [DOI] [PubMed] [Google Scholar]
- Chen C., Kostakis C., Gerber J.P., Tscharke B.J., Irvine R.J., White J.M. Towards finding a population biomarker for wastewater epidemiology studies. Sci. Total Environ. 2014;487:621–628. doi: 10.1016/j.scitotenv.2013.11.075. [DOI] [PubMed] [Google Scholar]
- Chiaia A.C., Banta-Green C., Field J. Eliminating solid phase extraction with large-volume injection LC/MS/MS: analysis of illicit and legal drugs and human urine indicators in US wastewaters. Environ. Sci. Technol. 2008;42(23):8841–8848. doi: 10.1021/es802309v. [DOI] [PubMed] [Google Scholar]
- Choi P.M., Tscharke B.J., Donner E., O'Brien J.W., Grant S.C., Kaserzon S.L., Mackie R., O'Malley E., Crosbie N.D., Thomas K.V. Wastewater-based epidemiology biomarkers: past, present and future. TrAC, Trends Anal. Chem. 2018;105:453–469. [Google Scholar]
- Chou C.-C., Liu Y.-P. Determination of fecal sterols in the sediments of different wastewater outputs by GC-MS. Int. J. Environ. Anal. Chem. 2004;84(5):379–388. [Google Scholar]
- de Melo M.G., da Silva B.A., de Souza Costa G., da Silva Neto J.C.A., Soares P.K., Val A.L., da Silva Chaar J., Koolen H.H.F., Bataglion G.A. Sewage contamination of Amazon streams crossing Manaus (Brazil) by sterol biomarkers. Environ. Pollut. 2019;244:818–826. doi: 10.1016/j.envpol.2018.10.055. [DOI] [PubMed] [Google Scholar]
- Esperanza M., Suidan M.T., Marfil-Vega R., Gonzalez C., Sorial G.A., McCauley P., Brenner R. Fate of sex hormones in two pilot-scale municipal wastewater treatment plants: conventional treatment. Chemosphere. 2007;66(8):1535–1544. doi: 10.1016/j.chemosphere.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Esperanza M., Suidan M.T., Nishimura F., Wang Z.-M., Sorial G.A., Zaffiro A., McCauley P., Brenner R., Sayles G. Determination of sex hormones and nonylphenol ethoxylates in the aqueous matrixes of two pilot-scale municipal wastewater treatment plants. Environ. Sci. Technol. 2004;38(11):3028–3035. doi: 10.1021/es0350886. [DOI] [PubMed] [Google Scholar]
- Fedorova G., Randak T., Lindberg R.H., Grabic R. Comparison of the quantitative performance of a Q‐Exactive high‐resolution mass spectrometer with that of a triple quadrupole tandem mass spectrometer for the analysis of illicit drugs in wastewater. Rapid Commun. Mass Spectrom. 2013;27(15):1751–1762. doi: 10.1002/rcm.6628. [DOI] [PubMed] [Google Scholar]
- Forés E., Bofill-Mas S., Itarte M., Martínez-Puchol S., Hundesa A., Calvo M., Borrego C.M., Corominas L., Girones R., Rusiñol M. Evaluation of two rapid ultrafiltration-based methods for SARS-CoV-2 concentration from wastewater. Sci. Total Environ. 2021;768 doi: 10.1016/j.scitotenv.2020.144786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K., Wang L., Wei C., Li J., Zhang J., Zhou Z., Liang Y. Sucralose and acesulfame as an indicator of domestic wastewater contamination in Wuhan surface water. Ecotoxicol. Environ. Saf. 2020;189 doi: 10.1016/j.ecoenv.2019.109980. [DOI] [PubMed] [Google Scholar]
- Furtula V., Liu J., Chambers P., Osachoff H., Kennedy C., Harkness J. Sewage treatment plants efficiencies in removal of sterols and sterol ratios as indicators of fecal contamination sources. Water, Air, Soil Pollut. 2012;223(3):1017–1031. [Google Scholar]
- Gan Z., Sun H., Wang R., Feng B. A novel solid-phase extraction for the concentration of sweeteners in water and analysis by ion-pair liquid chromatography–triple quadrupole mass spectrometry. J. Chromatogr. A. 2013;1274:87–96. doi: 10.1016/j.chroma.2012.11.081. [DOI] [PubMed] [Google Scholar]
- Gao J., Li J., Jiang G., Yuan Z., Eaglesham G., Covaci A., Mueller J.F., Thai P.K. Stability of alcohol and tobacco consumption biomarkers in a real rising main sewer. Water Res. 2018;138:19–26. doi: 10.1016/j.watres.2018.03.036. [DOI] [PubMed] [Google Scholar]
- Gao J., Zheng Q., Lai F.Y., Gartner C., Du P., Ren Y., Li X., Wang D., Mueller J.F., Thai P.K. Using wastewater-based epidemiology to estimate consumption of alcohol and nicotine in major cities of China in 2014 and 2016. Environ. Int. 2020;136 doi: 10.1016/j.envint.2020.105492. [DOI] [PubMed] [Google Scholar]
- Gilli G., Rovere R., Traversi D., Schilirò T., Pignata C. Faecal sterols determination in wastewater and surface water. J. Chromatogr. B. 2006;843(1):120–124. doi: 10.1016/j.jchromb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Gracia-Lor E., Rousis N.I., Zuccato E., Bade R., Baz-Lomba J.A., Castrignanò E., Causanilles A., Hernández F., Kasprzyk-Hordern B., Kinyua J. Estimation of caffeine intake from analysis of caffeine metabolites in wastewater. Sci. Total Environ. 2017;609:1582–1588. doi: 10.1016/j.scitotenv.2017.07.258. [DOI] [PubMed] [Google Scholar]
- Gudra D., Dejus S., Bartkevics V., Roga A., Kalnina I., Strods M., Rayan A., Kokina K., Zajakina A., Dumpis U. Detection of SARS-CoV-2 RNA in wastewater and importance of population size assessment in smaller cities: an exploratory case study from two municipalities in Latvia. Sci. Total Environ. 2022;823 doi: 10.1016/j.scitotenv.2022.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn C., Weisberg S.B. Chemical-based fecal source tracking methods: current status and guidelines for evaluation. Rev. Environ. Sci. Biotechnol. 2009;8(3):275–287. doi: 10.1007/s11157-009-9162-2. [DOI] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher P., McGillivary P. Sewage contamination in the New York Bight. Coprostanol as an indicator. 1979;13(10):1225–1229. [Google Scholar]
- Hillebrand O., Nödler K., Licha T., Sauter M., Geyer T. Caffeine as an indicator for the quantification of untreated wastewater in karst systems. Water Res. 2012;46(2):395–402. doi: 10.1016/j.watres.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Hsu S.-Y., Muhamed B.B., Chenhui L., Hsin-Yeh H., Anthony B., Klutts J., Zemmer S.A. MedRxiv; 2022. Biomarkers Selection for Population Normalization in SARS-CoV-2 Wastewater-Based Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S., Hansen C.L. Evaluating a correlation between volatile suspended solid and adenosine 5′-triphosphate levels in anaerobic treatment of high organic suspended solids wastewater. Bioresour. Technol. 1998;63(3):243–250. doi: 10.1016/S0960-8524(97)00131-4. [DOI] [Google Scholar]
- Jafferali M.H., Khatami K., Atasoy M., Birgersson M., Williams C., Cetecioglu Z. Benchmarking virus concentration methods for quantification of SARS-CoV-2 in raw wastewater. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M., Chernyshov V.D., Pirogov A.V., Tataurova O.G., Rozhanets V.V., Shpigun O.A. Determination of 5-hydroxyindole-3-acetic acid in wastewater by high performance liquid chromatography coupled with tandem mass spectrometric detection. Inorg. Mater. 2019;55(14):1352–1358. doi: 10.1134/S002016851914005X. [DOI] [Google Scholar]
- Jayasinghe L.Y., Marriott P.J., Carpenter P.D., Nichols P.D. Application of pentafluorophenyldimethylsilyl derivatization for gas chromatography–electron-capture detection of supercritically extracted sterols. J. Chromatogr. A. 1998;809(1):109–120. doi: 10.1016/S0021-9673(98)00160-5. [DOI] [Google Scholar]
- Juste C., Gérard P. Cholesterol-to-Coprostanol conversion by the gut microbiota: what we know, suspect, and ignore. Microorganisms. 2021;9(9) doi: 10.3390/microorganisms9091881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J., Lerch M. Sedimentary faecal lipids as indicators of Baltic Sea sewage pollution and population growth since 1860 AD. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.112305. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Iker B.C., Pepper I.L., Gerba C.P. Relative abundance and treatment reduction of viruses during wastewater treatment processes—identification of potential viral indicators. Sci. Total Environ. 2014;488:290–296. doi: 10.1016/j.scitotenv.2014.04.087. [DOI] [PubMed] [Google Scholar]
- Kolodziej E.P., Gray J.L., Sedlak david L. Quantification of steroid hormones with pheromonal properties in municipal wastewater effluent. Environ. Toxicol. Chem.: Int. J. 2003;22(11):2622–2629. doi: 10.1897/03-42. [DOI] [PubMed] [Google Scholar]
- Kolodziej E.P., Thoma H., Sedlak david L. Dairy wastewater, aquaculture, and spawning fish as sources of steroid hormones in the aquatic environment. Environ. Sci. Technol. 2004;38(23):6377–6384. doi: 10.1021/es049585d. [DOI] [PubMed] [Google Scholar]
- Koureas M., Amoutzias G.D., Vontas A., Kyritsi M., Pinaka O., Papakonstantinou A., Dadouli K., Hatzinikou M., Koutsolioutsou A., Mouchtouri V.A., Speletas M., Tsiodras S., Hadjichristodoulou C. Wastewater monitoring as a supplementary surveillance tool for capturing SARS-COV-2 community spread. A case study in two Greek municipalities. Environ. Res. 2021;200 doi: 10.1016/j.envres.2021.111749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., Nakada N., Hanamoto S., Inaba M., Katayama H., Do A.T., et al. Pepper mild mottle virus as an indicator and a tracer of fecal pollution in water environments: comparative evaluation with wastewater-tracer pharmaceuticals in Hanoi, Vietnam. Sci. Total Environ. 2015;506:287–298. doi: 10.1016/j.scitotenv.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Lai F.Y., Been F., Covaci A., Nuijs A. L. van. Novel wastewater-based epidemiology approach based on liquid chromatography–tandem mass spectrometry for assessing population exposure to tobacco-specific toxicants and carcinogens. Anal. Chem. 2017;89(17):9268–9278. doi: 10.1021/acs.analchem.7b02052. [DOI] [PubMed] [Google Scholar]
- Lai F.Y., Gartner C., Hall W., Carter S., O'Brien J.W., Tscharke B.J., Been F. Measuring spatial and temporal trends of nicotine and alcohol consumption in Australia using wastewater‐based epidemiology. Addiction. 2018;113(6):1127–1136. doi: 10.1111/add.14157. [DOI] [PubMed] [Google Scholar]
- Lapolla P., Lee R., Andrea M. Wastewater as a red flag in COVID-19 spread. Publ. Health. 2020;185:26. doi: 10.1016/j.puhe.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F.Y., Ong S.L., Hu J. Recent advances in the use of chemical markers for tracing wastewater contamination in aquatic environment: a review. Water. 2017;9(2) doi: 10.3390/w9020143. [DOI] [Google Scholar]
- Liu Y., Blowes D.W., Groza L., sabourin M.J., Ptacek C.J. Acesulfame-K and pharmaceuticals as co-tracers of municipal wastewater in a receiving river. Environ. Sci. J. Integr. Environ. Res.: Process. Impacts. 2014;16(12):2789–2795. doi: 10.1039/c4em00237g. [DOI] [PubMed] [Google Scholar]
- Mackuľak T., Birošová L., Grabic R., Škubák J., Bodík I. National monitoring of nicotine use in Czech and Slovak Republic based on wastewater analysis. Environ. Sci. Pollut. Control Ser. 2015;22(18):14000–14006. doi: 10.1007/s11356-015-4648-7. [DOI] [PubMed] [Google Scholar]
- Medema G., Been F., Heijnen L., Petterson S. Implementation of environmental surveillance for SARS-CoV-2 virus to support public health decisions: opportunities and challenges. Environ. Health: COVID-19. 2020;17:49–71. doi: 10.1016/j.coesh.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin R.G., Hendrickson E.N., Chaudhry N., Georgewill O., Freese R., Schacker T.W., Simmons G.E. A novel wastewater-based epidemiology indexing method predicts SARS-CoV-2 disease prevalence across treatment facilities in metropolitan and regional populations. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-00853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nödler K., Licha T., Bester K., Sauter M. Development of a multi-residue analytical method, based on liquid chromatography–tandem mass spectrometry, for the simultaneous determination of 46 micro-contaminants in aqueous samples. J. Chromatogr. A. 2010;1217(42):6511–6521. doi: 10.1016/j.chroma.2010.08.048. [DOI] [PubMed] [Google Scholar]
- O'Brien J., Jake W., Thai P.K., Eaglesham G., Ort C., Scheidegger A., Carter S., Lai F.Y., Mueller J.F. A model to estimate the population contributing to the wastewater using samples collected on census day. Environ. Sci. Technol. 2014;48(1):517–525. doi: 10.1021/es403251g. [DOI] [PubMed] [Google Scholar]
- Oliveira T.S., Murphy M., Mendola N., Wong V., Carlson D., Waring L. Characterization of Pharmaceuticals and Personal Care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci. Total Environ. 2015;518:459–478. doi: 10.1016/j.scitotenv.2015.02.104. 519. [DOI] [PubMed] [Google Scholar]
- Oloye F.F., Xie Y., Asadi M., Cantin J., Challis J.K., Brinkmann M., McPhedran K.N., Kristian K., Keller M., Sadowski M., Jones P.D., Landgraff C., Mangat C., Fuzzen M., Servos M.R., Giesy J.P. Rapid transition between SARS-CoV-2 variants of concern Delta and Omicron detected by monitoring municipal wastewater from three Canadian cities. Sci. Total Environ. 2022;841 doi: 10.1016/j.scitotenv.2022.156741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olumekun V.O., Oloye F.F., Olajide M., Femi-Oloye O.P., Oludaisi O.B. Biomass Conversion and Biorefinery; 2021. Selection of an Efficient Solvent for Palm Oil Recovery from Waste Mesocarp Fiber. [DOI] [Google Scholar]
- Pandopulos A.J., Bade R., Tscharke B.J., O'Brien J.W., Simpson B.S., White J.M., Gerber C. Application of catecholamine metabolites as endogenous population biomarkers for wastewater-based epidemiology. Sci. Total Environ. 2021;763 doi: 10.1016/j.scitotenv.2020.142992. [DOI] [PubMed] [Google Scholar]
- Picó Y., Barceló D. Identification of biomarkers in wastewater-based epidemiology: main approaches and analytical methods. TrAC, Trends Anal. Chem. 2021;145 doi: 10.1016/j.trac.2021.116465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourakbari B., Mahmoudi S., Mahmoudieh Y., Eswhaghi H., Navaeian A., Rostamyan M., Mamishi S. SARS‐CoV‐2 RNAaemia in children: an Iranian referral hospital‐based study. J. Med. Virol. 2021;93(9):4452–5457. doi: 10.1002/jmv.27065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratola N., Cincinelli A., Alves A., Katsoyiannis A. Occurrence of organic microcontaminants in the wastewater treatment process. A mini review. Occurrence and Fate of Emerging Contaminants in Municipal Wastewater Treatment Systems. 2012;239–240:1–18. doi: 10.1016/j.jhazmat.2012.05.040. [DOI] [PubMed] [Google Scholar]
- Reynolds L.J., Sala-Comorera L., Khan M.F., Martin N.A., Whitty M., Stephens J.H., Nolan T.M., Joyce E., Fletcher N.F., Murphy C.D., Meijer W.G. Coprostanol as a population biomarker for SARS-CoV-2 wastewater surveillance studies. Water. 2022;14(2) doi: 10.3390/w14020225. [DOI] [Google Scholar]
- Rico M., Andrés-Costa M.J., Picó Y. Estimating population size in wastewater-based epidemiology. Valencia metropolitan area as a case study. Special Issue on Emerging Contaminants in Engineered and Natural Environment. 2017;323:156–165. doi: 10.1016/j.jhazmat.2016.05.079. [DOI] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senta I., Gracia-Lor E., Borsotti A., Zuccato E., Castiglioni S. Wastewater analysis to monitor use of caffeine and nicotine and evaluation of their metabolites as biomarkers for population size assessment. Water Res. 2015;74:23–33. doi: 10.1016/j.watres.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Sodré F.F., Locatelli M.A.F., Jardim W.F. Occurrence of emerging contaminants in Brazilian drinking waters: a sewage-to-tap issue. Water Air Soil Pollut. 2010;206(1):57–67. doi: 10.1007/s11270-009-0086-9. [DOI] [Google Scholar]
- Thai P.K., O'Brien J., Jiang G., Gernjak W., Yuan Z., Eaglesham G., Mueller J.F. Degradability of creatinine under sewer conditions affects its potential to be used as biomarker in sewage epidemiology. Water Res. 2014;55:272–279. doi: 10.1016/j.watres.2014.02.035. [DOI] [PubMed] [Google Scholar]
- Thai P.K., O'Brien J.W., Banks A.P.W., Jiang G., Gao J., Choi P.M., Yuan Z., Mueller J.F. Evaluating the in-sewer stability of three potential population biomarkers for application in wastewater-based epidemiology. Sci. Total Environ. 2019;671:248–253. doi: 10.1016/j.scitotenv.2019.03.231. [DOI] [PubMed] [Google Scholar]
- Tolouei S., Burnet J.-B., Autixier L., Taghipour M., Bonsteel J., Duy S.V., Sauvé S., Prévost M., Dorner S. Temporal variability of parasites, bacterial indicators, and wastewater micropollutants in a water resource recovery facility under various weather conditions. Water Res. 2019;148:446–458. doi: 10.1016/j.watres.2018.10.068. [DOI] [PubMed] [Google Scholar]
- Torii S., Oishi W., Zhu Y., Thakali O., Malla B., Yu Z., Zhao B., Arakawa C., Kitajima M., Hata A., Ihara M., Kyuwa S., Sano D., Haramoto E., Katayama H. Comparison of five polyethylene glycol precipitation procedures for the RT-qPCR based recovery of murine hepatitis virus, bacteriophage phi6, and pepper mild mottle virus as a surrogate for SARS-CoV-2 from wastewater. Sci. Total Environ. 2022;807 doi: 10.1016/j.scitotenv.2021.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran N.H., Gan J., Nguyen V.T., Chen H., You L., Duarah A., Zhang L., Gin K.Y.-H. Sorption and biodegradation of artificial sweeteners in activated sludge processes. Bioresour. Technol. 2015;197:329–338. doi: 10.1016/j.biortech.2015.08.083. [DOI] [PubMed] [Google Scholar]
- Tran N.H., Hu J., Ong S.L. Simultaneous determination of PPCPs, EDCs, and artificial sweeteners in environmental water samples using a single-step SPE coupled with HPLC–MS/MS and isotope dilution. Talanta. 2013;113:82–92. doi: 10.1016/j.talanta.2013.03.072. [DOI] [PubMed] [Google Scholar]
- Tscharke B.J., O'Brien J.W., Ort C., Grant S., Gerber C., Bade R., Thai P.K., Thomas K.V., Mueller J.F. Harnessing the power of the census: characterizing wastewater treatment plant catchment populations for wastewater-based epidemiology. Environ. Sci. Technol. 2019;53(17):10303–10311. doi: 10.1021/acs.est.9b03447. [DOI] [PubMed] [Google Scholar]
- Tscharke B.J., White J.M., Gerber J.P. Estimates of tobacco use by wastewater analysis of anabasine and anatabine. Drug and Testing. 2016;8(7):702–707. doi: 10.1002/dta.1842. [DOI] [PubMed] [Google Scholar]
- Van Stempvoort D.R., Roy J.W., Grabuski J., Brown S.J., Bickerton G., Sverko E. An artificial sweetener and pharmaceutical compounds as co-tracers of urban wastewater in groundwater. Sci. Total Environ. 2013;461–462:348–359. doi: 10.1016/j.scitotenv.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Varbanov M., Bertrand I., Philippot S., Retourney C., Gardette M., Hartard C., Jeulin H., Duval R.E., Loret J.-F., Schvoerer E., Gantzer C. Somatic coliphages are conservative indicators of SARS-CoV-2 inactivation during heat and alkaline pH treatments. Sci. Total Environ. 2021;797 doi: 10.1016/j.scitotenv.2021.149112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviano G., Valsecchi S., Polesello S., Capodaglio A., Tartari G., Salerno F. Combined use of caffeine and turbidity to evaluate the impact of CSOs on river water quality. Water, Air, Soil Pollut. 2017;228(9):330. doi: 10.1007/s11270-017-3505-3. [DOI] [Google Scholar]
- Wang C., Gardinali P.R. Comparison of multiple API techniques for the simultaneous detection of microconstituents in water by on‐line SPE‐LC‐MS/MS. J. Mass Spectrom. 2012;47(10):1255–1268. doi: 10.1002/jms.3051. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zheng Q., Gartner C., Chan G.C.K., Ren Y., Wang D., Thai P.K. Comparison of tobacco use in a university town and a nearby urban area in China by intensive analysis of wastewater over one year period. Water Res. 2021;206 doi: 10.1016/j.watres.2021.117733. [DOI] [PubMed] [Google Scholar]
- Westhaus S., Weber F.-A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T., Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany – suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder M.L., Middleton F., Larsen D.A., Du Q., Fenty A., Zeng T., Insaf T., Kilaru P., Collins M., Kmush B., Green H.C. Co-quantification of crAssphage increases confidence in wastewater-based epidemiology for SARS-CoV-2 in low prevalence areas. Water Res. X. 2021;11 doi: 10.1016/j.wroa.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhang Jianbo, Xiao Amy, Gu Xiaoqiong, Lin Lee Wei, Armas Federica, Kauffman Kathryn, William Hanage, Mariana Matus, Newsha Ghaeli, Endo Noriko, Claire Duvallet, Mathilde Poyet, Katya Moniz, Washburne Alex D., Erickson Timothy B., Chai Peter R., Thompson Janelle, Alm Eric J., Gilbert Jack A. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5(4) doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Challis J.K., Oloye F.F., Asadi M., Cantin J., Brinkmann M., McPhedran K.N., Hogan N., Sadowski M., Jones P.D., Landgraff C., Mangat C., Servos M.R., Giesy J.P. RNA in municipal wastewater reveals magnitudes of COVID-19 outbreaks across four waves driven by SARS-CoV-2 variants of concern. ACS ES&T Water. 2022 doi: 10.1021/acsestwater.1c00349. [DOI] [PubMed] [Google Scholar]
- Yu H., Shao X.-T., Liu S.-Y., Pei W., Kong X.-P., Wang Z., Wang D.-G. Estimating dynamic population served by wastewater treatment plants using location-based services data. Environ. Geochem. Health. 2021;43(11):4627–4635. doi: 10.1007/s10653-021-00954-7. [DOI] [PubMed] [Google Scholar]
- Zheng Q., Tscharke B., O'Brien J., Gerber C., Mackie R., Gao J., Thai P. Uncertainties in estimating alcohol and tobacco consumption by wastewater-based epidemiology. Environ. Chem.: Sewage Epidemiology. 2019;9:13–18. doi: 10.1016/j.coesh.2019.03.004. [DOI] [Google Scholar]
- Zheng Q.-D., Wang Z., Liu C.-Y., Yan J.-H., Pei W., Wang Z., Wang D.-G. Applying a population model based on hydrochemical parameters in wastewater-based epidemiology. Sci. Total Environ. 2019;657:466–475. doi: 10.1016/j.scitotenv.2018.11.426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.