SUMMARY
Altered hematopoietic stem cell (HSC) fate underlies primary blood disorders but microenvironmental factors controlling this are poorly understood. Genetically barcoded genome editing of synthetic target arrays for lineage tracing (GESTALT) zebrafish were used to screen for factors expressed by the sinusoidal vascular niche that alter the phylogenetic distribution of the HSC pool under native conditions. Dysregulated expression of protein kinase C delta (PKC-δ, encoded by prkcda) increases the number of HSC clones by up to 80% and expands polyclonal populations of immature neutrophil and erythroid precursors. PKC agonists such as cxcl8 augment HSC competition for residency within the niche and expand defined niche populations. CXCL8 induces association of PKC-δ with the focal adhesion complex, activating extracellular signal-regulated kinase (ERK) signaling and expression of niche factors in human endothelial cells. Our findings demonstrate the existence of reserve capacity within the niche that is controlled by CXCL8 and PKC and has significant impact on HSC phylogenetic and phenotypic fate.
In brief
Binder et al. show that prkcda, encoding PKC-δ, is transcriptionally regulated in the sinusoidal vascular hematopoietic niche. Dysregulated expression within the niche alters hematopoietic stem cell (HSC) clonal evolution and phenotypic fate. This provides insight into how the microenvironment maintains a reserve capacity of HSCs for regeneration and stress responses.
Graphical abstract

INTRODUCTION
Hematopoiesis is a cellular developmental program influenced by hematopoietic stem and progenitor cell (HSPC)-intrinsic genetic programs and HSPC-extrinsic factors from the surrounding microenvironment.1,2 In hematologic neoplasms, the actions of mutated transcription factors, histone and DNA modifiers, and signaling receptors are appreciated to be the primary forces driving clonal expansion, loss of phylogenetic diversity, and adoption of a malignant cell fate.3–9 However, the mechanisms by which the hematopoietic microenvironment regulates hematopoietic stem cell (HSC) clonal diversity and cell fate remain poorly understood.
The hematopoietic microenvironment can be regarded as a collection of specialized cellular niches with overlapping functions depending on the developmental context or presence of external stresses.2 The sinusoidal vascular niche is found in developing or regenerative microenvironment tissue and is critical for hematopoietic recovery after transplantation because of its expression of Notch ligands, CXCL12, stem cell factor (SCF), and other angiocrine growth factors.10–18 Overexpression of the angiogenic chemokine receptor cxcr1 in embryonic zebrafish increases the three-dimensional volume of the caudal hematopoietic tissue (CHT), a transient sinusoidal vascular niche present from 3 to 7 days post fertilization (dpf), increases expression of cxcl12a, and enhances colonization by phenotypic HSPCs.19,20 Conversely, zebrafish with homozygous null mutations in cxcl8, the ligand for cxcr1, have reduced colonization of the CHT by phenotypic HSPCs.20 Given the importance of the sinusoidal niche in the development and regeneration of the hematopoietic system, we hypothesized that perturbations in downstream components of cxcl8/cxcr1 signaling would alleviate or exacerbate natural evolutionary bottlenecks, thereby altering the fate of the HSC pool. Using genetic lineage tracing techniques and single-cell RNA sequencing (scRNA-seq) analysis to map the fate of native HSC clones, we demonstrate involvement of protein kinase C (PKC) and cxcl8 in regulating the capacity of the sinusoidal endothelial niche to support a phylogenetically and phenotypically diverse hematopoietic system.
RESULTS
Differential expression of prkcda in the vascular niche
We aimed to identify downstream components of cxcl8 signaling with a specific pattern of expression relative to CHT-derived sinusoidal endothelial cells and non-CHT-derived arteriovenous endothelial cells. Kdrl:EGFP;lyve1b:DsRed zebrafish embryos (Figure 1A) were dissociated at 72 h post fertilization (hpf).21,22 Kdrl:EGFP(+) lyve1b:DsRed(+) CHT-derived sinusoidal endothelial cells and kdrl:EGFP(+) lyve1b:dDsRed(−) non-CHT-derived arteriovenous endothelial cells were purified by fluorescenceactivated cell sorting (FACS), bulk RNA sequencing (RNA-seq) was performed, and 219 significantly differentially regulated genes were identified (Figure 1B; Table S1). Genes known to be expressed at high levels in sinusoidal vascular tissues such as kdr (vegfr2b), flt4 (vegfr3), and egfl7 were among the significantly upregulated genes in CHT-derived sinusoidal endothelial cells.18,23 The list of significantly downregulated genes in CHT-derived sinusoidal endothelial cells included prkcda, which encodes the novel PKC isozyme, PKC-δ. Of all annotated zebrafish PKC isoforms, only prkcda was differentially regulated in CHT-derived sinusoidal endothelial cells and non-CHT-derived arteriovenous endothelial cells (Figure 1C). Whole-mount in situ hybridization of 72 hpf embryos confirmed these findings by showing expression of prkcda mRNA in the region of the heart and hindbrain (Figure 1D).24 PKC is a downstream component of cxcl8/cxcr1 signaling, although a role for the delta isoform in this pathway has not been described.25,26 The pattern of expression observed suggested that prkcda expression may be tightly controlled in order to properly regulate responses to cxcl8-expressing cells within the CHT.
Figure 1. Transcription of prkcda is repressed in the vascular niche and dysregulated expression of prkcda expands phenotypic HSPCs.
(A) Representative images of the kdrl:EGFP; lyve1b:DsRed transgenic line. Bar, 100 μm.
(B) Volcano plot indicating genes with significantly differentially regulated genes in red; prkcda and selected CHT-specific genes are shown. Positive absolute log fold change (ALFC) indicates upregulation in CHT-derived sinusoidal endothelial cells compared to non-CHT-derived arteriovenous endothelial cells.
(C) Differential expression of PKC-family genes in CHT-derived and non-CHT-derived endothelial cells. Values represent average fragments per kilobase of exon per million mapped fragments (FPKM) from three biological replicates. For prkcda, p = 0.00035.
(D–F) Whole-mount in situ hybridization for prkcda in 72-hpf zebrafish embryos. (E and F) Flow cytometric analysis of kdrl:EGFP;sele:prkcda-2A-mCherry (E) and Runx1:EGFP;sele:prkcda-2A-mCherry (F) embryos.
(G and H) Representative images of kdrl:EGFP zebrafish embryos injected (G) or not (H) with the sele:prkcda-2A-mCherry expression construct. Bar, 100 μm.
(I) Dysregulated expression of prkcda in Runx1:EGFP;sele:prkcda-2A-mCherry embryos expands HSPCs. Each PKC family member is compared to clutchmates injected with a vector containing an empty multiple cloning site (control). Data are presented as fold change relative to control for each clutch (two or three clutches analyzed per PKC family member; each point represents a biological replicate). Boxes represent mean ± SEM. Prkcda vs. control: 1.94 ± 0.3-fold increase, p = 0.0092, Welch’s t test.
(J) Representative images of CHT colonization in control and prkcda groups. Dotted lines indicate the CHT. Bar, 100 μm.
(K and L) Representative images of kdrl:EGFP(+) sele:prkcda-2A-mCherry(+) (K) and kdrl:EGFP(+); sele:prkcda-2A-mCherry(−) (L) transgenics. Bar, 100 mm.
(M) Phenotypic HSPCs were quantified in stable Runx1:EGFP;sele:prkcda-2A-mCherry transgenics (+) and Runx1:EGFP clutchmates (−) at 72 hpf. Data are pooled from two independent clutches and are represented as fold change relative to (−). Boxes represent mean ± SEM; each point represents a biological replicate. + vs. −: 1.19 ± 0.08-fold increase, p = 0.014, Wilcoxon rank-sum test.
(N) Representative images of CHT colonization for the (−) and (+) animals from Figure 1G. Dotted lines indicate CHT.
(O) Phenotypic HSPCs were quantified in Runx1:EGFP transgenics treated with HA-100 or an equivalent volume of DMSO as a vehicle control. Data are pooled from two independent experiments and are shown as fold change relative to control. Box represents mean ± SEM; each point represents a biological replicate. HA-100 vs. control: 0.38 ± 0.06-fold decrease, p = 8.3 × 10−6, Wilcoxon rank-sum test. See also Figure S1 and Table S1.
Ectopic expression of prkcda in the vascular niche expands phenotypic HSPCs
Given the tight transcriptional control on prkcda expression in the CHT and since upstream cxcl8/cxcr1 signaling is a positive regulator of HSPC colonization, we hypothesized that dysregulated expression of prkcda would alter CHT colonization by HSPCs. E-selectin (sele) is a specific marker for CHT endothelial cells; a 798-bp enhancer/promoter element from sele, was used to drive expression of prkcda or other zebrafish PKC family members in CHT endothelial cells by microinjecting expression constructs into embryos at the single-cell stage to generate F0 mosaic transgenics. Kdrl:EGFP and Runx1:EGFP zebrafish carry reporter transgenes that are highly specific for endothelial cells and embryonic HSPCs, respectively.27,28 Dissociated kdrl: EGFP;sele:prkcda-2A-mCherry F0 transgenics showed co-expression of these transgenes by flow cytometry, whereas Runx1:EGFP;sele:prkcda-2A-mCherry F0 transgenics did not (Figures 1E, 1F, S1A, and S1B). Confocal imaging of the CHT in kdrl:EGFP;sele:prkcda-2A-mCherry F0 transgenics showed mCherry expression in a subset of CHT endothelial cells (Figure 1G), in contrast to uninjected clutchmates (Figure 1H). Together these data show specific expression of the sele-driven transgene in CHT endothelial cells but not HSPCs. To determine the effect of dysregulated prkcda expression by CHT endothelial cells on HSPC numbers, Runx1:EGFP;sele:prkcda-2A-mCherry transgenics were compared to negative control clutchmates microinjected with expression constructs containing an empty multiple cloning site (control). Expression of the prkcda transgene caused significantly greater colonization of the CHT by EGFP(+) HSPCs at 72 hpf compared to clutchmate controls (Figures 1I and 1J). By contrast, there was no significant difference in CHT colonization in prkcaa, prkcdb, prkchb, or prkcz transgenics compared to clutchmate controls (Figure 1I).
A stable sele:prkcda-2A-mCherry transgenic line was generated and crossed to the kdrl:EGFP endothelial cell reporter line; kdrl:EGFP(+) sele:prkcda-2A-mCherry(+) transgenics demonstrated specific expression in a subset of CHT endothelial cells compared to kdrl:EGFP(+) sele:prkcda-2A-mCherry(−) clutchmates (Figures 1K and 1L). The sele:prkcda-2A-mCherry line was crossed to Runx1:EGFP HSPC reporter zebrafish. Sele:prkcda-2A-mcherry(+) zebrafish showed greater CHT colonization by HSPCs compared to sele:prkcda-2A-mcherry(−) clutchmates (Figures 1M and 1N). HA-100 dihydrochloride (HA-100) is a selective inhibitor of PKC enzymes that is soluble in water and does not fluoresce in the emission spectrum of GFP. Runx1:EGFP embryos treated at the maximum tolerated dose of HA-100 (50 mM) had lower CHT colonization by HSPCs compared to control-treated clutchmates (Figure 1O). These data suggest that, as a class, PKC family enzymes positively regulate HSPC colonization of the embryonic vascular niche. Transcriptional dysregulation of PKC family members with non-specific patterns of expression with respect to endothelial cell of origin (sinusoidal versus arteriovenous), including conventional (prkcaa) and atypical (prkcz) subtypes, did not affect CHT engraftment. However, dysregulated expression of prkcda, which is transcribed at a lower level in CHT-derived sinusoidal endothelial cells, was sufficient to increase CHT engraftment. This is consistent with a possible role in regulating responses to cxcl8 signaling within the vascular niche.
Vascular niche expression of prkcda increases the number of long-term HSC clones
The genome editing of synthetic target arrays for lineage tracing (GESTALT) lineage tracing system29 was used to interrogate the effect of dysregulated vascular niche expression of prkcda on long-term HSC fate in the setting of otherwise unperturbed, native hematopoiesis. GESTALT embryos were microinjected with Cas9, barcoding sgRNAs, and either sele:prkcda-2A-mCherry (prkcda) or empty vector (sele:mcs-2a-mCherry, control) expression constructs (Figure 2A). Barcoded zebrafish were grown to 3 months post fertilization (mpf), peripheral blood was sampled, GESTALT barcodes were sequenced, and the number of HSC clones contributing to peripheral blood was quantified using an unsupervised algorithm for selecting informative amplicon barcodes from experimental replicates (SABER).30 Peripheral blood was serially resampled at 6, 9, 12, and 22 mpf. Accounting for repeated measures across all serial samples, animals in the sele:prkcda-2A-mCherry group had more HSC clones contributing to the peripheral blood compared to control clutchmates from 3 to 22 mpf (Figure 2B). Post hoc testing showed significantly more HSC clones in the prkcda group compared to control at 6 and 9 mpf.
Figure 2. Vascular niche expression of prkcda increases the number of long-term HSC clones.
(A) Schematic outline of the long-term fate-mapping experiment.
(B) GESTALT barcodes were amplified from peripheral blood samples and quantified using SABER. The number of HSC clones at each time point is shown for sele:prkcda-2A-mCherry transgenics (prkcda) and clutchmate controls. Representative data from one of two experiments is shown. Boxes indicate mean ± SEM; each point represents a biological replicate. For 3 mpf to 22 mpf, prkcda vs. control: p = 0.0008, robust two-way mixed ANOVA. Post hoc testing for prkcda vs. control at 6 and 9 mpf: 6.5 ± 0.7 vs. 3.6 ± 0.4 HSC clones, p = 0.001 and 7.0 ± 0.8 vs. 4.2 ± 0.8 HSC clones, p = 0.02, Welch’s t test.
(C) Correlation of serial samples to baseline for GESTALT barcode-identified fish. Each row per column represents a biological replicate.
(D) The Pearson correlation for each fish is plotted by group and by time point (6 mpf and later). Boxes represent mean ± SEM; each point represents a biological replicate. For prkcda vs. control: mean Pearson coefficient of 0.58 ± 0.11 vs. 0.90 ± 0.03, p = 0.03, Welch’s t test.
(E) Representative plots showing HSC clonal dynamics in control and prkcda groups. The height of each polygon at the indicated time points is proportional the measured frequency of alleles derived from uniquely-barcoded HSC clones. Only HSC clones with a contribution of 2% or greater are included.
We sought to determine the stability of HSC clonal output over time. Since zebrafish are housed in groups, GESTALT barcodes were used to identify longitudinal blood samples derived from the same fish. Sixteen sele:prkcda-2A-mCherry fish and 14 clutchmate controls could be identified at two or more time points. To measure stability of clonal output in individual fish, GESTALT barcode frequencies at each time point were correlated with baseline (Pearson correlation shown in Figures 2C and 2D). There was no difference in GESTALT barcode correlation at the 6, 9, and 12 mpf time points compared to baseline (Figure 2D). However, at the 22 mpf time point, the GESTALT barcode correlation was significantly lower for sele:prkcda-2A-mCherry transgenics compared to clutchmate controls (Figure 2D). Representative plots of allele frequency over time are shown in Figure 2E. These findings show that dysregulated expression of prkcda expands the capacity of the hematopoietic niche and allows it to support more phylogenetically distinct HSC clones that can contribute to hematopoiesis through at least 12 months of life. From 12 to 22 mpf, the effect of prkcda dysregulation in the vascular niche led to changes in the phylogenetic makeup of the HSC pool that were not observed in control clutchmates.
Polyclonal expansion of erythroid precursors and immature neutrophils in zebrafish with dysregulated expression of prkcda
scRNA-seq was performed on sele:prkcda-2A-mCherry transgenic zebrafish to identify phenotypic changes occurring in the hematopoietic compartment concurrent with the changes observed in HSC phylogenetic diversity. Kidney marrow was harvested at 22 mpf and 10,995 cells (6,346 from sele:prkcda-2A-mCherry and 4,649 from clutchmate controls) were isolated without pre-enrichment using 10X Genomics 3′-capture technology. Unsupervised clustering revealed eight clusters of hematopoietic cells (Figures 3A–3C; see Figure S2A for all cell populations). The identity of individual hematopoietic clusters was assigned by gene module Gene Ontology (GO)-term analysis and inspection of top specific cluster markers (Tables S2–S4). A group of hematologic cell clusters was identified with specific expression of canonical hematopoietic genes (Figure 3C). Initial cluster assignments could be made on the basis of these genes, including myeloid progenitors (csf1rb, gata2b), lymphoid progenitors (ccr9a, cd81a, gata2a), erythroid progenitors (gata1a, lmo2), and three neutrophil populations (lyz, mpx).31–37 A very small cluster was identified that expressed high levels of markers characteristic of HSCs (tal1, lmo2, meis1b, pbx1b) and thrombocyte precursors (mpl, itga2b). To confirm identities to hematopoietic cell clusters, these data were compared to three reference scRNA-seq datasets (two mouse, one zebrafish), using defined zebrafish orthologs for cross-species comparisons (Figures S2B and S2C).38–40 A normalized aggregate expression score was calculated for each reference gene set within each hematopoietic cell cluster and cluster identities assigned according to the highest aggregate scores. Stratification by experimental group showed significant selective expansion of lymphoid, erythroid, and neutrophil 1 clusters and the presence of a highly specific pro-neutrophil cluster in sele:prkcda-2A-mCherry transgenic zebrafish (Figure 3D).
Figure 3. Polyclonal expansion of erythroid precursors and immature neutrophils in zebrafish with dysregulated expression of prkcda.
(A) Uniform Manifold Approximation and Projection (UMAP) plot of hematologic cell clusters. All data points for the indicated clusters are shown; data are from n = 9 (control) and n = 10 (prkcda) pooled kidney marrows.
(B) UMAP plot of hematologic cell clusters stratified by experimental group; colors indicated local cell density in two-dimensional UMAP space.
(C) Expression of selected canonical genes within each hematopoietic cell cluster.
(D) Differential representation of experimental groups by hematologic cell cluster. Values were normalized to the number of cells recovered for each experimental group. For prkcda compared to control by cluster: lymphoid, 1.15-fold expansion, p = 0.049; erythroid, 7.99-fold expansion, p = 3.3 × 10−111; neutrophil 1, 2.68-fold expansion, p = 4.56 × 10−91; and pro-neutrophil, 137-fold expansion, p = 3.82 × 10−88, Fisher’s exact test.
(E) UMAP representation of GESTALT barcodes mapped to single hematopoietic cells. Colored points indicate biological replicates. Gray circles indicate cells without barcoding data.
(F) Clonal contribution to the hematologic cell clusters is indicated by the colored bars. Each color represents a uniquely defined GESTALT clone. See also Figure S2 and Tables S2–S4.
We wished to understand the clonal distribution of expanded cell populations observed in kidney marrow from sele:prkcda-2A-mCherry animals. Expansion of monoclonal cell populations arising from experimentally altered niche tissue have been reported, but polyclonal cell populations have not.41 The latter would support a broad increase in the capacity of the vascular niche to support hematopoiesis. In the GESTALT system, barcode sequences are expressed as poly-adenylated mRNA under control of a ubiquitous enhancer/promoter element.29 Expressed GESTALT barcodes were amplified from 10X cell-barcoded cDNA and mapped onto the scRNA-seq data. Cells from the control group could be assigned to four fish and cells from the prkcda group could be assigned to eight fish. Multiple identifiable fish were represented in each of the hematopoietic cell clusters (Figure 3E). Each cluster contained cells with multiple distinct GESTALT barcodes and most GESTALT barcodes could be identified in multiple cell clusters (Figure 3F). These data show that dysregulated expression of prkcda within the vascular niche expands its capacity to support a polyclonal population of HSPCs, increasing the phylogenetic diversity of the HSC pool and specifically expanding erythroid and immature neutrophil subsets. Together, these findings describe a change in HSC fate resulting from dysregulated expression of prkcda in the vascular niche.
Expression of prkcda in vivo is controlled by defined cis-acting regulatory elements
Observing altered HSC fates in zebrafish with dysregulated expression of prkcda in the vascular niche led us to hypothesize the existence of specific epigenetic mechanisms, i.e., one or more definable cis-acting regulatory elements, controlling prkcda expression. scRNA-seq expression profiles showed specific expression of prkcda in kidney marrow neutrophils (Figure 4A). Single-cell assay for transposase-accessible chromatin with sequencing sequencing (scATAC-seq) performed on kidney marrow cells from the same animals identified five unique cell clusters based on global chromatin accessibility (Figures 4B and S3A). We established cell cluster identities by label transfer from scRNA-seq data obtained from the same samples, using Cicero gene activity scores as a surrogate for gene expression in the scATAC-seq data.42,43 These identities were confirmed by plotting top specific gene activity scores and chromatin accessibility at putative lineage-defining transcription factor binding sites. (Figures S3B–S3G). Top specific gene activity markers for the neutrophil cluster included prkcda. Plotting chromatin accessibility at the prkcda locus identified an intronic peak approximately 7 kb from the transcription start site that was most prominent in the neutrophil cluster and interacted strongly with the prkcda promoter (link score 0.73; Figure 4C). To understand whether this putative regulatory sequence was conserved across species and tissue types, we used a comparative approach and transduced human umbilical vein endothelial cells (HUVEC) with the adenovirus E4ORF1 gene to generate E4-HUVEC cells, a well-defined model of the human sinusoidal vascular niche.44,45 Similar to the zebrafish kidney marrow scATAC-seq data, bulk ATAC sequencing identified a peak of accessible chromatin in an intronic region approximately 6 kb from the PRKCD transcriptional start site (Figures 4D and S3H). Find individual motif occurrences (FIMO)46 analysis of putative transcription factor binding sites in the zebrafish and human enhancer sequences identified nine with q < 0.05 in both datasets (Figures 4E and S3I). These included the pluripotency factor KLF4 and WT1, a zinc-finger transcription factor expressed during hematopoiesis,47 in the endocardium during development and after infarction,48 and in tumor neo-vasculature, where it supports endothelial cell proliferation, migration, and tube formation via binding at the vascular endothelial growth factor (VEGF) promoter.49,50 We tested the function of this intronic sequence by generating an mClover reporter construct and microinjecting this into zebrafish embryos. Fluorescence was observed in bulbus arteriosus endothelial cells of 72-hpf zebrafish embryos using live imaging (Figures 4F; Video 1) and confirmed by immunofluorescence for the vascular marker Fli1 and transgenic mClover (Figure 4G). This indicates the intronic sequence is an enhancer of prkcda expression in neutrophils and endothelial cells and identifies a cis-regulatory element with the potential to influence HSC fate.
Figure 4. Regulation of prkcda expression by an intronic enhancer element.
(A) Expression of PKC isoforms in kidney marrow cell clusters identified in Figure 3.
(B) UMAP representation of global chromatin accessibility in single zebrafish kidney marrow cells. Samples are from the same kidney marrow pools shown in Figure 3.
(C) Chromatin accessibility at the prkcda locus in kidney marrow cells by cluster. Peaks of chromatin accessibility and Cicero links identifying co-accessible peaks are shown.
(D) Bulk ATAC sequencing showing chromatin accessibility at the human PRKCD locus in E4-HUVEC cells. Peaks were identified using MACS2.
(E) FIMO analysis of zebrafish and human intron enhancer peak sequences. The number of putative binding sites with q < 0.05 in each set is indicated.
(F) Expression of an mClover reporter construct driven by the zebrafish prkcda intronic enhancer in uninjected (i) and injected (ii) animals. Arrowhead indicates the bulbus arteriosus. Bar, 25 μm.
(G) Immune fluorescence in a 72 hpf zebrafish embryo expressing the prkcda intronic enhancer reporter. Cryosections were probed with anti-GFP/mClover and anti-Fli1 antibodies and fluorescent secondaries (see STAR Methods). The outline indicates pericardium (P), ventricle (V), and bulbus arteriosus (BA, cardiac outflow tract). Bar, 10 μm. See also Figure S3 and Video S1.
PKC agonists augment HSPC engraftment and niche colonization in competition assays
We wished to understand the cellular and molecular mechanisms by which PKC enzymes, including prkcda, facilitate HSC colonization of the vascular niche. A chemical screen was performed to identify positive regulators of HSC engraftment in a zebrafish kidney marrow transplant assay. The experimental approach has been previously described51; briefly, kidney marrow from ubi:GFP transgenic zebrafish was treated at room temperature for 4 h prior to competitive transplantation into conditioned casper zebrafish recipients. Treated marrow was transplanted in a 1:2 ratio with untreated marrow from ubi:mCherry transgenic zebrafish (40,000 and 80,000 cells, respectively, per recipient). Short- and long-term readouts were performed at 4 and 12 weeks post transplantation (wpt) using two-color fluorescent imaging of the kidney marrow or FACS analysis of peripheral blood chimerism (Figure 5A). Marrow treated with 1,2-didecanoyl-sn-glycerol (DDG, a stabilized analog of diacylglycerol [DAG]; Millipore Sigma) significantly outcompeted control-treated marrow at the 4 wpt time point (Figure 5B). DAG is an activator of PKC family enzymes.52 In particular, the subfamily of novel PKC enzymes (including PKC-δ) are strictly activated by DAG and do not require calcium release for activation.53,54 To confirm this finding, the competitive transplant experiment was repeated using 12-O-tetradecanoylphorbol-13-acetate (TPA, also known as phorbol 12-myristate 13-acetate [PMA]), another DAG structural analog and PKC activator.55 Marrow treated with TPA significantly out-competed control-treated marrow relative to competitor at 4 and 12 wpt (Figure 5C). To identify downstream signaling dependencies for the enhanced engraftment observed after treatment with these PKC agonists, the competitive transplant assay was performed using TPA in combination with inhibitors of PKC (AEB071, PKCi) and mitogen-activated protein kinase kinase (MEK) (AZD6244, MEKi) signaling. Co-treatment with PKCi blunted the competitive advantage in engraftment conferred by TPA at 4 wpt, while co-treatment with MEKi significantly reduced the competitive advantage conferred by TPA (Figure 5D). These data suggested a role for PKC and mitogen-activated protein kinase (MAPK) in responding to cell-extrinsic signaling factors present in the hematopoietic microenvironment. To identify whether HSPCs, endothelial cells, or both respond to PKC stimulation, these cell types were FACS-sorted separately from kdrl:mCherry;Runx1:EGFP dual-reporter transgenics, treated ex vivo with DDG for 15 min, and assayed for ERK phosphorylation by intra-cellular flow cytometry. Kdrl+ endothelial cells showed a 38% increase in p-ERK+ cells, whereas no increase was observed in Runx1+ HSPCs (Figures 5E and S1). Since MEK/ERK inhibition blocked the engraftment advantage conferred by PKC signaling, these data support a model in which the vascular niche mediates HSPC engraftment via PKC and ERK.
Figure 5. PKC agonists augment HSPC engraftment and niche colonization in competition assays.
(A) Schematic illustration of competitive marrow transplant experiments.
(B–D) Competitive transplant data are presented as Log2(green/red) where green/red is the calculated ratio of drug- or control-treated marrow to untreated competitor marrow. Boxes represent mean ± SEM; each point is a biological replicate. (B) DDG vs. vehicle control at 4 wpt: DDG:competitor ratio 6.58 ± 2.18 vs. control:competitor ratio 2.11 ± 1.12, p = 0.026, Welch’s t test. (C) TPA vs. vehicle control at 4 wpt: TPA:competitor ratio 0.99 ± 0.31 vs. control:competitor ratio 0.24 ± 0.07, p = 0.027, Wilcoxon rank-sum test. 12 wpt: TPA:competitor ratio 0.34 ± 0.11 vs. control:competitor ratio 0.03 ± 0.01, p = 0.016, Wilcoxon rank-sum test. (D) TPA treatment in the presence or absence of PKC and MEK inhibitors, vs. control. TPA vs. TPA+MEKi: TPA:competitor ratio 0.71 ± 0.13 vs. TPA+MEKi:competitor ratio 0.22 ± 0.08, p = 0.018, Wilcoxon rank-sum test.
(E) ERK phosphorylation was assessed by intracellular flow cytometry in sorted kdrl+ endothelial cells and Runx1+ HSPCs.
(F) Distribution of CHT residency times for n = 46 HSPCs from Runx1:cxcl8 transgenics and n = 62 HSPCs from control clutchmates. Vertical lines indicate the median for each group. Representative data from one of four similar experiments is shown. For cxcl8 vs. control: median of 2.67 vs. 1.5 h, p = 0.003, Wilcoxon rank-sum test.
(G) Distribution of CHT residency times for Runx1:cxcl8 transgenics and control clutchmates in the prkcda(−/−) mutant background. n = 108 HSPCs for Runx1:cxcl8 and n = 111 HSPCs for control in 10 fish per group.
(H) Distribution of CHT residency times for n = 110 HSPCs from Runx1:cxcl8 transgenics and n = 223 HSPCs from ΔELR-CXC clutchmates. Vertical lines indicate the median for each group. For cxcl8 vs. ΔELR-CXC; median of 4.66 vs. 2.66 h, p = 0.002, Wilcoxon rank-sum test.
(I) Distribution of CHT residency times for n = 404 HSPCs for Runx1:cxcl8 and n = 174 HSPCs for competitor. Vertical lines indicated the median for each group. For cxcl8 vs. competitor: median of 2.75 vs. 1.75 h, p = 1 3 10−9, Wilcoxon rank-sum test.
(J) Violin plots represent the percentage of time individual HSPCs were cuddled by endothelial cells within the CHT. n = 32 HSPCs for Runx1:cxcl8 and n = 108 HSPCs for control. For cxcl8 vs. control: median of 97.7% vs. 79.3% cuddling time, p = 0.0013, Wilcoxon rank-sum test.
(K) Quantification of Runx1:EGFP+ HSPCs in Runx1:EGFP;Runx1:cxcl8–2A-mCherry transgenics (TG) and Runx1:EGFP clutchmates (−). Data are from one of two representative zebrafish clutches. Boxes represent mean ± SEM; each point is a biological replicate. For TG vs. −: 4.2 ± 0.27 vs. 2.1 ± 0.25 HSPCs per CHT, p = 5.5 3 10−7, Welch’s t test.
(L) Quantification of Runx1:EGFP(+) HSPCs in Runx1:cxcl8–2A-mCherrytransgenics (TG) and cxcl8 transgene-negative control clutchmates (−) treated with the indicated drugs or vehicle control. Data are presented as fold change relative to control-treated, transgene-negative clutchmates and are pooled from eight independent experiments. Boxes represent mean ± SEM; each point is a biological replicate. For ibrutinib-treated cxcl8 transgene-negative zebrafish: 0.51 ± 0.08-fold relative to vehicle-treated clutchmates, p = 0.0067, Welch’s t test.
(M) Quantitative RT-PCR for CXCL8 mRNA expression by THP-1 cells treated with ibrutinib or vehicle control. Four biological replicates were performed per condition; each point is the mean of three technical replicates. For ibrutinib-treated cells: 0.11 ± 0.08-fold decrease relative to vehicle control, p = 0.002, Welch’s t test. See also Figure S4.
A genetic approach was used to identify mechanisms by which cxcl8, a PKC agonist, might augment colonization of the vascular niche by HSPCs. Tol2-based zebrafish expression constructs were generated to enforce expression of a zebrafish cxcl8a (ENSDARG00000104795, cxcl8) minigene under control of the Runx1+23 HSC-specific enhancer,27 with either P2A-GFP or P2A-mCherry co-expressed from the same transcript. These constructs (or controls expressing the fluorescent protein alone) were microinjected into single-cell casper zebrafish embryos and time-lapse fluorescence microscopy was used to quantify the dynamics of HSPC colonization of the CHT in F0 transgenics (Figure S4A). Compared to HSPCs from clutchmate controls, HSPCs from Runx1:cxcl8 transgenics had a significantly longer residency time within the CHT (Figure 5F).
We hypothesized that the low level of prkcda expression observed in the sinusoidal endothelial cells of the CHT might be required downstream of cxcl8 signaling in order to prolong HSPC residency time in the CHT. We generated a homozygous zebrafish line with a 4-bp deletion in the third exon of prkcda leading to loss of mRNA expression (prkcda(−/−); Figures S4B and S4C). Prkcda(−/−) embryos microinjected with a Runx1:cxcl8–2A-mCherry expression construct showed no difference in CHT residency time compared with prkcda(−/−) animals microinjected with a control expression construct (Figure 5G). The effect of cxcl8 on HSPC residency time was dependent upon the ELR-CXC amino acid motif, which is responsible for its engagement with cxcr1/cxcr256; Runx1:cxcl8 transgenics had significantly longer CHT residency time compared to clutchmates injected with a similar construct lacking this motif (Figure 5H).
Next, we designed an assay to directly observe in vivo competition between HSPCs with and without enforced expression of cxcl8 within the same animal. Runx1:cxcl8–2A-GFP and Runx1: mCherry (or alternatively Runx1:EGFP and Runx1:cxcl8–2A-mCherry) expression constructs were co-injected into casper zebrafish embryos at a 1:1 molar ratio. HSPCs expressing only the control construct were identified as competitor HSPCs and those expressing the Runx1:cxcl8 construct with or without expression of the control construct were identified as cxcl8-expressing HSPCs within each co-injected embryo. CHT residency time was significantly longer for cxcl8-expressing HSPCs compared to competitor HSPCs (Figure 5I).
It was important to distinguish and quantify two types of HSPC behavior observed in the time-lapse imaging: prolonged interactions between relatively immobile HSPCs and a single pocket of endothelial cells (“cuddling”) and persistent HSPC migration throughout the CHT. HSPCs from control transgenic animals showed a bimodal distribution of these behaviors with many cells migrating through the CHT without cuddling. By contrast, a large majority of HSPCs in Runx1:cxcl8 transgenic animals spent most of their CHT residency time cuddling with endothelial cells (Figures 5J and S4D).
In order to quantify the effect of enforced expression of cxcl8 on HSPC colonization of the CHT in terms of absolute numbers, a stable Runx1:cxcl8–2A-mCherry transgenic line was generated and crossed to the established Runx1:EGFP transgenic reporter line. Enforced expression of cxcl8 by HSPCs in this transgenic line increased CHT colonization 2-fold compared to cxcl8 transgene-negative clutchmates (Figure 5K). Taken together, these findings show that cxcl8 signals through cxcr1/cxcr2 and prkcda, augmenting HSPC residency within the vascular niche via increased endothelial cell cuddling and expanding HSPC numbers.
We aimed to understand the upstream signaling mechanisms driving cxcl8 expression by HSPCs in the zebrafish embryo and screened a panel of small-molecule inhibitors to identify those that reduced CHT colonization. We hypothesized that inhibition of these upstream mechanisms would reduce GFP(+) HSPC numbers in Runx1:EGFP embryos but would be rescued in Runx1:cxcl8–2A-mCherry; Runx1:EGFP embryos in which cxcl8 expression is transgene-driven. Hemizygous Runx1:cxcl8–2A-mCherry transgenics were crossed to homozygous Runx1:EGFP transgenics, clutches were treated from 48 to 72 hpf, embryos were genotyped by mCherry expression, and HSPCs were enumerated by GFP expression. Treatment of zebrafish embryos with dactolisib (phosphoinositide 3-kinase [PI3K]/mammalian target of rapamycin [mTOR]), ly294002 (PI3K), mk2206 (AKT), and sorafenib (multikinase) at maximum tolerated doses did not significantly decrease CHT colonization in zebrafish embryos at 72 hpf, whereas treatment with the BTK/Tec family kinase inhibitor ibrutinib did significantly reduce CHT colonization in Runx1:cxcl8–2A-mCherry(−);Runx1:EGFP(+) transgenics (Figure 5L). However, enforced expression of cxcl8 in Runx1:cxcl8–2A-mCherry(+);Runx1:EGFP(+) transgenics rescued CHT colonization by HSPCs in the presence of ibrutinib. Ibrutinib treatment significantly reduced CXCL8 expression in the human myeloid cell line, THP-1 (Figure 5M). These results implicate BTK/Tec family kinase signaling up-stream of cxcl8 signaling in HSPCs as they colonize the vascular niche.
Cxcl8 expression by HSPCs expands hematopoietic niche populations
In order to understand how cxcl8 signaling by HSPCs modifies the hematopoietic niche, we performed scRNA-seq on Runx1:cxcl8–2A-mCherry zebrafish embryos and clutchmate controls. We recovered 17,699 cells, and gene module analysis was used to define niche cell clusters (Figures 6A and S5A–S5D). Module 3 included many recognized niche genes, including cxcl12a, kitlg, lepr, bmp4, WNT factors, and Notch ligands (Figure S5D). Enriched GO terms for genes in module 3 compared to other modules included cell migration, cell communication, response to growth factor, and developmental processes (Table S3).
Figure 6. Cxcl8 expression by HSPCs expands niche populations and favors early-pseudotime hematopoietic cells.
(A) UMAP plot showing cellular populations from 96-hpf zebrafish embryos. Data are pooled from four samples derived from two clutches of Runx1:cxcl8–2A-mCherry transgenics and clutchmate controls. Each sample was a pool of 15–20 dissociated embryos.
(B) Expression of population-defining niche genes.
(C) Expression of selected hematopoietic and signaling factors by niche cell clusters.
(D) Differential representation of niche cell clusters according to experimental group. Positive values indicate enrichment in the Runx1:cxcl8–2A-mCherry group relative to clutchmate controls. Values were normalized according to the number of cells recovered per group. Sinusoidal, p = 0.03; lepr+ MSC, p = 6.0 3 10−18; osteoblast, p = 0.03; fibroblast, p = 0.0005; Fisher’s exact test.
(E) GSEA enrichment plot for PKC-δ transcriptional targets. Expression data are ranked by differential expression in sinusoidal endothelial cells from Runx1:cxcl8–2A-mCherry embryos compared to control.
(F) The log-transformed ratio of precursor and myeloid cells to lymphoid cells is shown. Boxes indicate mean ± SEM. For cxcl8 vs. control, 3.25 ± 0.19 vs. 2.76 ± 0.16, p = 0.049, Welch’s t test.
(G) Pseudotime trajectory in the hematopoietic cell partition. The root node is indicated.
(H) Stratification of progenitor/myeloid cells by experimental group.
(I) Distribution of progenitor/myeloid cells from Runx1:cxcl8 transgenics and control clutchmates according to pseudotime. For cxcl8 vs. control, p = 3.4 3 10−5, two-sample Kolmogorov-Smirnov test.
(J) Differential representation of niche cell clusters according to experimental group. Positive values indicate enrichment in the Runx1:cxcl8–2A-mCherry group relative to clutchmate controls. Values were normalized according to the number of cells recovered per group. Osteoblast, p = 0.0029; fibroblast, p = 2.7 3 10−5; sinusoidal endothelial cells, p = 0.013; Fisher’s exact test. See also Figures S5 and S6 and Tables S2–S4.
Niche cell clusters were individually identified as sinusoidal endothelial cell, osteoblast, fibroblast, chondrocyte, pericyte, and lepr+ mesenchymal stem cell (MSC) populations based on canonical gene expression and comparison with published datasets (Figures 6A, 6B, and S5E–S5I). To identify pericyte, fibroblast, and chondrocyte populations, murine niche cluster gene sets were converted to zebrafish orthologs and used to calculate aggregate gene scores (Figure S5G).57 Expression of cxcl12a and the proliferative markers cdk1, mki67, cenpf, and myca were mutually exclusive between fibroblasts and pericytes/chondrocytes, respectively (Figures S5H and S5I). Expression of kitlga, cxcl12a, and bmp4 within the six defined niche clusters is shown in Figure 6C. Also shown is expression of marcksl1a, the immediate downstream target of PKC signaling. Sinusoidal endothelial cells had the highest per-cell expression of cxcl12a and marcksl1a. Cluster membership according to experimental group was normalized according to total number of cells recovered and plotted for each niche population (Figure 6D). Sinusoidal endothelial cell, lepr+ MSC, osteoblast, and fibroblast populations were all significantly more abundant in Runx1:cxcl8–2A-mCherry embryos compared to clutchmate controls. To identify a signature of PKC-δ activation, pseudobulk differential expression analysis was performed on sinusoidal endothelial cells identified in Runx1:cxcl8–2A-mCherry and clutchmate control embryos. A list of PKC-δ-specific transcriptional targets has previously been reported.58,59 Gene set enrichment analysis (GSEA) showed significant enrichment of PKC-δ transcriptional targets in genes upregulated in sinusoidal endothelial cells from Runx1:cxcl8–2A-mCherry embryos (normalized enrichment score [NES] 1.97; adjusted p = 0.0003, Figure 6E). This analysis shows that cxcl8 expression by HSPCs leads to selective expansion of important niche cellular subsets with high levels of expression of factors supporting hematopoiesis.
Cxcl8 expression by HSPCs skews hematopoiesis in favor of immature populations and expands marrow niche populations
Next, we wished to understand the impact of long-term enforced cxcl8 expression on the hematopoietic compartment. Kidney marrow was harvested from adult Runx1:cxcl8–2A-mCherry zebrafish at 3 mpf, analyzed by flow cytometry, and the relative proportion of myeloid, lymphoid, and precursor cells determined by forward- and side-scatter characteristics (Figures S6A and S6B).60–62 The ratio of precursor plus myeloid cells to lymphoid cells was significantly greater in adult Runx1:cxcl8–2A-mCherry zebrafish compared to clutchmate controls (Figure 6F). At 12 mpf, kidney marrow was harvested from Runx1:cxcl8–2A-mCherry zebrafish and control clutchmates and scRNA-seq was performed. Progenitor/myeloid cells expressing tal1, gata2b, mpx, and lyz and lymphoid cells expressing gata2a, ccr9a, and cd81a were identified (Figures S6C and S6D). A pseudotime trajectory was plotted for the hematopoietic cells (Figure 6G). Root and leaf nodes were identified by expression of genes characteristic of HSPCs and mature myeloid cells (tal1, spi1b, and mpx; Figure S6E). Compared to control clutchmates, Runx1:cxcl8–2A-mCherry animals showed a relative expansion of early-pseudotime cells (Figures 6H and 6I). Consistent with the FACS experiment, the ratio of progenitor/myeloid cells to lymphoid cells was increased in Runx1:cxcl8–2A-mCherry zebrafish (18.0 vs. 7.8), suggesting the development of a “myeloid bias” seen in aged and diseased hematopoiesis.
We hypothesized that enforced expression of cxcl8 in Runx1:cxcl8–2A-mCherry animals might expand niche-like cell populations as was observed in the embryo scRNA-seq experiments. A group of cells expressing cxcl12a, dcn, fn1a, and col1a1a was identified as the niche partition (Table S4; Figure S6D), and four sub-populations were identified by Louvain clustering (Figure S6F). Identities of these sub-populations were established using aggregate gene scores for the top markers from the embryonic niche (Figure S6G). The osteoblast, fibroblast, and sinusoidal cell populations were significantly over-represented in Runx1:cxcl8–2A-mCherry animals compared to clutchmate controls (Figure 6J). Thus, in concordance with our findings in zebrafish embryos, enforced expression of cxcl8 in Runx1:cxcl8–2A-mcherry transgenics expands specific niche populations in the adult marrow.
CXCL8 links PKC-δ to the focal adhesion complex
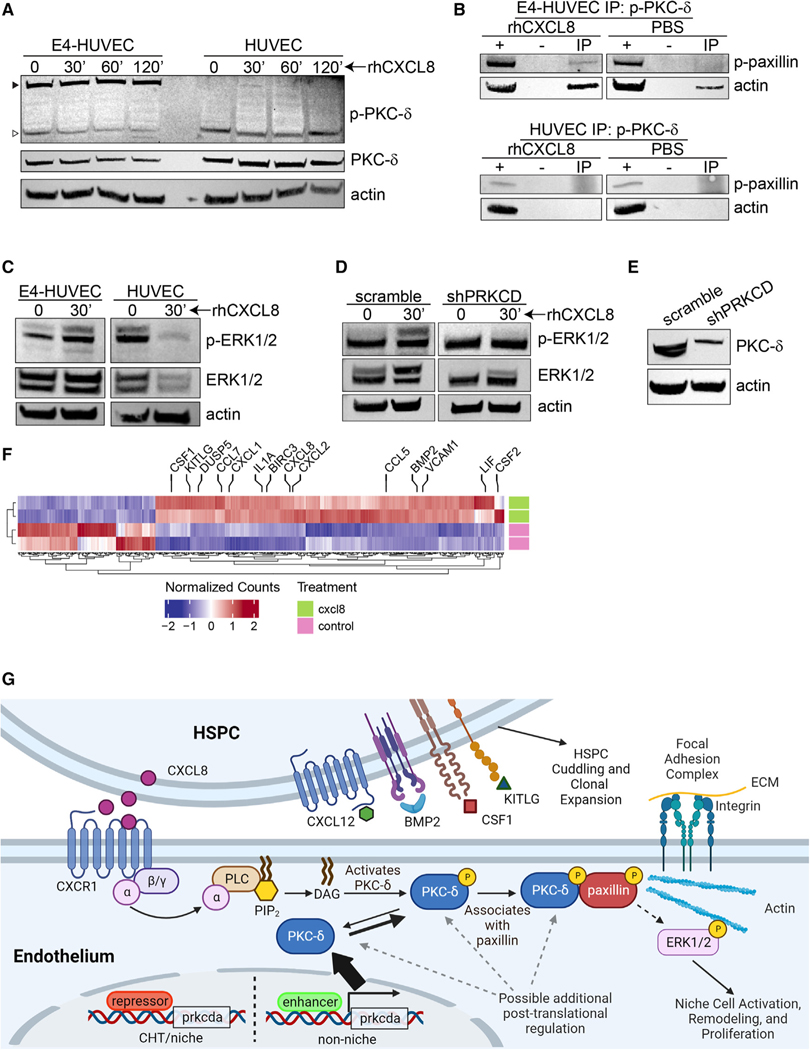
We sought to identify molecular mechanisms by which CXCL8 activates the vascular niche to support hematopoiesis and how these might depend on PKC-δ. Immunoblotting HUVEC and E4-HUVEC cell lysates showed expression of total PKC-δ at the anticipated molecular weight of 78 kDa (middle panel, Figure 7A). Immunoblotting E4-HUVEC cell lysates for p-PKC-δ (phosphorylated Thr-505, Cell Signaling Technology) demonstrated a prominent high-molecular-weight band (approximately 130 kDa) that was not observed in HUVEC cells (black triangle, top panel, Figure 7A). By contrast, p-PKC-δ migrated at 78 kDa in HUVEC cells (white triangle, top panel, Figure 7A). Treatment of E4-HUVECs with recombinant human (rh) CXCL8 changed the relative intensity of the p-PKC-δ bands in favor of the 130-kDa form but had little effect on HUVEC cells (Figures 7A and S7A–S7C). A high-molecular weight form of PKC-δ was previously shown to associate with the signal transduction adapter protein paxillin as part of the focal adhesion complex.63,64 To determine whether this was also the case in the vascular niche-like E4-HUVEC cells, we performed immunoprecipitation for p-PKC-δ, probed for p-paxillin, and found no association in the absence of rhCXCL8. However, in the presence of rhCXCL8, co-immunoprecipitation of p-PKC-δ and p-paxillin was observed (Figures 7B and S7D). Treatment with rhCXCL8 also increased association of p-PKC-δ with actin, the cytoskeletal component of the focal adhesion complex (Figures 7B and S7D). Parental HUVEC cells expressed comparatively low levels of p-paxillin; co-immunoprecipitation of p-PKC-δ and p-paxillin or actin was not observed in either the presence or absence of rhCXCL8 (Figures 7B and S7D). Together, these findings show that CXCL8 treatment preferentially stabilizes the high-molecular-weight form of p-PKC-δ, allowing its recruitment to focal adhesion complexes that may be induced upon HSC-niche interaction.
Figure 7. CXCL8 links PKC-δ to the focal adhesion complex.
(A) E4-HUVEC and parental HUVEC cells were treated with rhCXCL8 (10 ng/mL) for the indicated times. The black triangle indicates the ~130-kDa form of p-PKC-δ and the white triangle indicates the ~78-kDa form.
(B) Immunoprecipitation (IP) for p-PKC-δ with immunoblotting for p-paxillin and actin is shown. Input lysate is indicated by (+); (−) indicates beads-only control. Treatment was with 10 ng/mL rhCXCL8 or PBS for 30 min.
(C) E4-HUVEC or parental HUVEC cells were treated with 10 ng/mL rhCXCL8 for the indicated times and immunoblotting for p-ERK1/2 and ERK1/2 was performed.
(D) E4-HUVEC cells were transduced with lentivirus expressing shRNA against PRKCD or scramble control. Immunoblotting for p-ERK1/2 and ERK1/2 was performed.
(E) shRNA knockdown of PKC-δ expression.
(F) Bulk RNA sequencing was performed in E4-HUVEC cells treated with rhCXCL8 for 6 h or PBS as a control. Significantly differentially regulated genes are shown with key hematopoietic factors highlighted. Each row indicates a biological replicate.
(G) Proposed model for HSPC remodeling of the niche via CXCL8 and PKC-δ. HSPCs produce CXCL8 upon encountering the vascular niche, which signals via CXCR1, releases DAG, and activates PKC family members. Transcription of PKC-δ is normally tightly controlled in order to maintain a reserve capacity to support HSPCs. CXCL8 signaling induces association of PKC-δ with the focal adhesion complex, activating niche functions such as HSPC cuddling and growth factor production. See also Figure S7 and Table S5 and S6.
PKC-δ signals via ERK activation and ERK1/2 have been shown to associate with the focal adhesion complex.65,66 Since our competitive transplant assay had shown that the advantage conferred by PKC agonists was dependent on MEK/ERK signaling (Figure 5D) and intracellular flow cytometry showed ERK phosphorylation in DDG-treated zebrafish endothelial cells (Figure 5E), we hypothesized that there would be induction of p-ERK in E4-HUVEC cells treated with rhCXCL8. This was indeed the case, and, by contrast, parental HUVEC cells treated with rhCXCL8 did not show induction of p-ERK (Figures 7C and S7E). This observation was dependent on PKC-δ since E4-HUVEC cells transduced with PRKCD short hairpin RNA (shRNA) did not show induction of p-ERK upon rhCXCL8 treatment (Figures 7D, 7E, S7F, and S7G). Bulk RNA-seq was performed on rhCXCL8-treated E4-HUVEC cells and showed transcriptional upregulation of anti-apoptotic factors, chemokines, and hematopoietic growth factors (Figures 7F; Table S5). GSEA demonstrated induction of gene programs associated with HSC differentiation, cytokine-cytokine receptor interactions, and ERK phosphorylation (Figures S7H–S7J; Table S6). These findings show that CXCL8 promotes association of p-PKC-δ with the focal adhesion complex, activating the MEK/ERK signaling cascade and expression of key hematopoietic growth factors.
DISCUSSION
Here we have shown that transcriptional dysregulation of PKC-δ increases the number of HSC clones that contribute to hematopoiesis and that PKC agonists such as CXCL8 or TPA enhance the ability for hematopoietic stem cells to compete for colonization or engraftment of the vascular niche. Based on these data, we propose the following model (Figure 7G): HSPCs produce CXCL8 upon encountering the vascular niche, signal via CXCR1, release DAG, and activate PKC family members. CXCL8 itself is likely induced by nuclear factor κB (NF-κB), the activity of which is positively regulated by BTK.67,68 Transcription of PKC-δ is normally tightly regulated to keep the available pool of PKC in a tonic state and thereby maintain a reserve capacity to support additional HSCs. CXCL8 stabilizes association of phosphorylated PKC-δ with the focal cell adhesion complex, facilitating cytoskeletal reorganization, HSPC cuddling, and downstream activation of ERK1/2. This induces niche cell proliferation and expression of chemotactic factors, hematopoietic growth factors, and inflammatory signals that support the cuddled HSPC. The result of this cascade of intra- and intercellular signaling events is a change in fate of the cuddled HSC: HSCs that engage with the niche in this way actively contribute to hematopoiesis and those that do not are maintained in reserve or are pruned altogether from the HSC phylogenetic tree. The network of the epigenetic regulators that limits prkcda transcription in the vascular niche of the CHT but enhances its transcription in other endothelial cells is unclear and awaits further study. Understanding this regulatory network and how CXCL8 and other post-translational regulators of PKC-δ control its activity in the vascular niche may be relevant for hematologic diseases and engraftment after HSCT.
Using genetic barcoding techniques to study native hematopoiesis, we were able to experimentally isolate the effect of PKC-δ overexpression on HSC fate from any potential artifact of transplantation. HSPCs cycle rapidly in the CHT19,20 and its murine cognate, the fetal liver,69–71 in part to meet the needs of the developing organism. This degree of proliferation may be necessary to compensate for a population bottleneck observed in lineage tracing studies related to colonization of the fetal liver.72 It was therefore quite surprising to find that a positive regulator of stem cell colonization (prkcda) is transcriptionally repressed in this tissue. Using ATAC sequencing in humans and zebrafish, we were able to identify intronic enhancer elements within PKC-δ sufficient to drive expression of a fluorescent reporter transgene in zebrafish vascular tissues. This points to a defined reserve capacity within the HSC pool that is maintained by active repression of epigenetic enhancer elements within niche cells and represents a mechanism by which the vascular niche regulates HSC fate. Dysregulation of this mechanism in sele:prkcda-2A-mCherry transgenic zebrafish pulls HSCs from the reserve pool, which may become prematurely exhausted, leading to their replacement by 12 mpf. Alternatively, prkcda expression may permit additional reserve short-term HSCs to survive the transition to adult hematopoiesis and thereby contribute their progeny to the peripheral blood. Expanded marrow cellular populations observed in sele: prkcda-2A-mCherry transgenics show that prkcda expression regulates HSC phenotypic fate as well. Future studies with refined transgenic lines and more sensitive fate recorders will be required to further define the progenitor and precursor populations and epigenetic elements involved.73
Altered HSC fate defines the natural history of hematopoietic disorders such as myelodysplasia and acute myeloid leukemia. Patients whose HSCs acquire a leukemic cell fate through a lengthy process of evolution and clonal selection have a dismal prognosis with all modern therapies.74,75 Single-cell sequencing, phylogenetic barcoding, and time-lapse imaging have allowed insights into the cellular and molecular mechanisms by which the hematopoietic niche shapes long-term HSC fate. Understanding more about the reserve capacity of the hematopoietic niche could provide new therapeutic avenues for preserving HSC clonal diversity in patients with hematopoietic disorders.
Limitations of the study
In order to confirm the cell autonomy of the effects of prkcda and cxcl8 on HSPC colonization and niche interactions, we attempted to make a series of knockouts using a tissue-specific CRISPR system but were unable to do so effectively. As is widely recognized, tissue-specific gene disruption is not easily achieved in the zebrafish model. Tissue-specific CRISPR, in particular, requires a strong transgenic driver. We believe the sele enhancer/promoter element used here is insufficiently strong for this method.
Experiments with enforced expression of cxcl8 showed expansion of sinusoidal endothelial cells but also other well-defined niche cell types. Therefore, we cannot exclude a role for other niche cells in the effects observed in these experiments.
Although our E-selectin-based transgenics showed specific expression in CHT endothelial cells and not in HSPCs, it is possible that some unrecognized off-target expression has occurred in our experiments.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Bradley W. Blaser (bradley.blaser@osumc.edu).
Materials availability
Plasmids and zebrafish lines generated in this study are available upon request and subsequent completion of Uniform Biological Material Transfer Agreement.
Data and code availability
The sequencing data generated during this study is available at GEO: GSE191029. Processed data are available as an R package via Mendeley Data: https://doi.org/10.17632/6s7vy929dc.1.
Analysis code is publicly available at https://github.com/blaserlab/pkc_cxcl8. Raw data processing scripts and additional documentation are available within the R data package.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Zebrafish
The zebrafish, Danio rerio, was used for all studies. GESTALT zebrafish were a kind gift of A. Schier.29 Casper, kdrl:EGFP, Runx1:EGFP, and lyve1b:dsRed zebrafish were previously reported.76 All zebrafish were group housed in recirculating aquatic systems with automated control of temperature (28.5C), pH (7.4) and conductivity (1000 μS) under a 14h:10h light:dark cycle. Animals were fed live rotifers beginning at approximately 5 dpf79 and commercial pelleted diets beginning at approximately 9 dpf (Gemma 75, Gemma 300, Skretting, Oregon, USA). Experiments were performed at the developmental stages indicated in the main text (72–96 hpf for embryos and larvae; 3 mpf and later for adults). Zebrafish sex determination occurs between 25 and 50 days post-fertilization so embryo/larvae experiments were performed on hermaphroditic animals.80 Adult zebrafish experiments were performed on unselected populations of male and female animals. The M:F ratio was approximately 1:1. The OSUCCC Aquatic Facility is AAALAC-accredited and all experiments were performed under IACUC protocol 2018A00000012.
Cell lines and primary cultures
Pooled primary HUVEC cells from male and female donors were purchased from Thermo Fisher Scientific (C01510C), passaged twice and cryopreserved. Early-passage cells were used for all in vitro experiments (2–4 passages total). HUVEC and E4-HUVEC cells were cultured in M200 medium (ThermoFisher Scientific M200500) supplemented with 2% FBS, Hydrocortisone 1 μg/mL, human EGF 10 ng/mL, b-FGF 3ngmL and Heparin 10 μg/mL (LSGS kit, ThermoFisher Scientific). Cells were serum-starved in M200 alone for 8h prior to treatment with rhCXCL8 (RND Systems). 293T cells were cultured in high-glucose Dulbecco’s Modified Eagles Medium supplemented with 10% fetal bovine serum, 4 mM L-glutamine, 1mM sodium pyruvate and penicillin/streptomycin. Cells were cultured in a humidified 5% C02 atmosphere at 37C.
METHOD DETAILS
FACS
Zebrafish embryos were euthanized by tricaine overdose, minced with a razor blade in PBS, and digested in collagenase with agitation (Liberase, Roche). Endothelial cells were isolated after two rounds of cell sorting. In the first round, kdrl:EGFP(+) cells were enriched from total live cells. Enriched endothelial cells were resorted into kdrl:EGFP(+); lyve1b:dsRed(+) and kdrl:EGFP(+); lyve1b:dsRed(−) fractions. For kidney marrow FACS, adult zebrafish were euthanized by ice water immersion, kidney marrow was dissected and triturated ice-cold PBS. Erythrocytes were lysed with NH4Cl. For intracellular phosphoprotein staining, cells were fixed in PFA for 10 min, dehydrated in methanol, and washed before antibody staining in the presence of 0.5% BSA and 0.02% sodium azide. Sorting was performed on a BD FACSAria instrument and analysis was performed on a BD LSRII instrument.
Microscopy
For live zebrafish embryo imaging, animals were anesthetized in tricaine (MS-222, Sigma) and then mounted in 1 mL of 1.2% LMP agarose in E3 embryo medium containing 40 μL of tricaine solution. Fluorescence microscopy was performed using a BZ-X710 (KEYENCE Corp of America, Itasca, IL) digital microscope and maximum intensity projections generated using Keyence BZ-X Analyzer software and ImageJ.78 For some experiments, images were obtained on a Nikon inverted Ti microscope using a Yokogawa spinning disc confocal and Andor iXon 33 EMC cameras or a Nikon A1R live cell imaging microscope. Timelapse images were recorded at 15–20 min intervals and assembled into image stacks using ImageJ. For gross imaging of kidney marrow transplant recipients, anesthetized zebrafish were imaged using a Zeiss Discovery Dissecting Stereoscope using Axiovision v4.6. Fluorescence intensity in red and green channels was measured in the area of the kidney marrow and in an adjacent background area using ImageJ.51 Green:Red ratio was calculated as:.
Quantitative image analysis
For static imaging experiments, HSPCs within the bounds of the CHT (dorsal boundary: ventral aspect of the caudal artery; ventral boundary: dorsal aspect of the caudal vein; rostral boundary: termination of the yolk sac extension, caudal boundary: apex of the angle formed by the caudal artery and vein) were identified and manually counted using the multi-point tool in ImageJ. In order to quantify CHT residency time in time lapse experiments, individual HSPCs were identified and manually tracked through image stacks, noting the total number of frames each HSPC continuously remained in the CHT. To determine cuddling time, each timelapse frame in which an HSPC was in close proximity to the same endothelial cells from the preceding timelapse frame was marked as “cuddled”. Percent cuddling time was calculated for each HSPC as:
All quantitative image analysis was performed in a blinded fashion or with confirmation by a second observer.
Transgenesis and GESTALT barcoding
For transgenesis experiments, 20 pg of expression plasmid DNA was injected with 20 pg Tol2 mRNA in a volume of 1 nL into zebrafish embryos at the single-cell stage. For barcoding experiments, GESTALT sgRNAs 1–10 were synthesized at Horizon Discovery according to published sequences.29 20 pg of equimolar pooled GESTALT sgRNAs and 0.3 nL EnGen Spy Cas9 (20 μM) were added per nanoliter injected.
Single cell RNA sequencing
Raw cell suspensions from zebrafish kidney marrow were generated as for FACS analysis. For zebrafish larval scRNA-seq, animals were anesthetized with tricaine and euthanized on ice. Deyolking was performed in a solution of 55 mM NaCl, 1.8 mM KCl, and 1.25 mM NaHCO3 until complete by visual inspection. After washing, larvae were dissociated in TrypLE Express with pipetting. Kidney marrow or larval cell suspensions were then gently triturated in FACSmax Cell Dissociation Solution (Genlantis, CA, USA) and passed through a 40 μm cell strainer. Cell suspensions were transferred to DNA lo-bind tubes on ice. Cells were filtered twice more with 40 μm flowmi filters. Red blood cells were lysed with NH4Cl and cell suspensions underwent bead-based dead cell depletion using the Dead Cell Depletion kit with MS Columns (Miltenyi Biotec, Germany). Flow-through containing live cells was centrifuged at 500 g for 5 min and cells were resuspended in PBS with 0.04% BSA. Cell suspensions were counted using the Invitrogen LIVE/DEAD Viability/Cytotoxicity Kit using a Keyence BZ-X710 fluorescence microscope. Cell suspensions were loaded into the 10X chip, cDNA was synthesized and amplified, and sequencing libraries were prepared according to the 10X Genomics Chromium Next GEM Single Cell 3′ Reagent Kit v3.1 RevD protocol. Final gene expression libraries were quantified using Qubit, an Agilent DNA High-Sensitivity Bio-analyzer, and KAPA qPCR. Gene expression libraries were pooled and sequenced using the NovaSeq6000 SP PE 150bp kit.
Bulk GESTALT barcode sequencing
Peripheral blood was collected from anesthetized zebrafish by retro-orbital bleeding using a Hamilton syringe. Blood was added to 50 μL blood buffer (250 mg Heparin, 1 mL FBS, 49 mL of H2O) and DNA was extracted with the Zymo Quick DNA Miniprep Kit. 5 μL (approximately 1 μg) genomic DNA was used in a 50 μL PCR reaction using GESTALT F and R primers.29 The PCR products were cleaned up using the Agencourt AMPure XP Purification Kit with 90 μL beads to bind the DNA and 42 μL of Elution Buffer. PCR product was visualized on a 1% agarose gel and 25 μL of a 20 ng/μL concentration was sent for Amplicon EZ sequencing (Genewiz). GESTALT barcode sequencing data was analyzed using SABER.30
Single cell GESTALT sequencing
GESTALT barcodes were amplified from 10X-cell barcoded cDNA using a semi-nested PCR approach adapted from a published protocol.77 The first round of PCR amplification was performed in a 50 μL reaction with NEB Q5 mastermix using between 9.5 ng and 16.5 ng barcoded cDNA and 1.25 μL each of primers raj_GP6 and partial_read_1, each at 10 μM. Thermal cycling was one cycle of 98°C × 30 s (initial denaturation), fifteen cycles of 98°C × 10 s, 61°C × 25 s, 72°C × 30 s with finale extension of 72°C × 2 min. The reaction products were purified using 30 μL AMPure beads (0.6X ratio, Beckman Coulter) and eluted in 20 μL. The second round of amplification was performed in 50 μL with Q5 mastermix using 8 μL of the product from step 1, and 1.25 μL each of primers mod_GP12 and partial_read_1, each at 10 μM. Thermal cycling was one cycle of 98°C × 30 s (initial denaturation), 8 cycles of 98°C × 10 s, 60°C × 25 s, and 72°C × 30 s with a final extension of 72°C × 2 min. The reaction was cleaned up with 30 μL AMPure beads and eluted in 30 μL. This product was used as the input for the sample index PCR (step 5.4) of the 10X genomics Next GEM VDJ reagent kit v1.1 protocol. Single-cell GESTALT libraries were sequenced using a Miseq 300 cycle kit with a 28 × 301 configuration.
Single cell ATAC sequencing
Cells were prepared as described for scRNA-seq and cryopreserved. Nuclei were isolated from freshly thawed cells using the 10X Single Cell ATAC Sequencing Demonstrated Protocol for PBMCs, RevD. Nuclei were loaded onto the 10X Single Cell ATAC Chip E using version 1 Gel Bead and Library reagents. Libraries were prepared according to the manufacturer’s protocol. ScATAC libraries were sequenced at Genewiz using a 2 × 150 configuration.
Bulk RNA sequencing
RNA sequencing libraries were made from zebrafish endothelial subsets sorted from 3 independent clutches using the SMART-Seq v4 Ultra Low Input RNA Kit (Clontech). E4 HUVEC RNA sequencing libraries were made using the Zymo-Seq RiboFree Total RNA Library kit. Libraries were sequenced in a paired-end fashion and data were pre-processed using the ENCODE paired-end/stranded long RNA pipeline for HUVECs (https://www.encodeproject.org/pipelines/ENCPL002LPE/) or TopHat/Cufflinks for zebrafish endothelial cells.81 Differential gene expression was calculated in R using DESeq2 (HUVEC data) or Cuffdiff (zebrafish endothelial cells). For the Cuffdiff analysis, p values had a default lower bound of 10−5.
Bulk ATAC sequencing
E4-HUVEC cells were trypsinized, nuclei isolated, and libraries prepared using the Active Motif ATAC Seq Kit. Libraries were sequenced at the Nationwide Children’s Institute for Genomic Medicine using a 2 × 150 configuration. Data were processed using the ENCODE replicated ATAC seq pipeline with N = 3 biological replicates. Sequences were aligned to the GRCh38 reference sequence. Pooled bigwig track data and MACS2 peaks are shown. Data were visualized using blaseRtools functions (https://github.com/blaserlab) with gene model data from the UCSC hg38 assembly.
Embryo drug treatments
Maximum tolerated doses of HA-100, dactolisib, ibrutinib, ly294002, mk2206 and sorafenib were determined in preliminary dose-finding experiments. The MTD was defined as the maximum concentration where excess mortality and morphologic defects relative to untreated controls were not observed.
Kidney marrow treatments
Kidney marrow was harvested from adult ubi:EGFP and ubi:mCherry zebrafish51 and RBC lysis was performed using NH4Cl. Ubi:EGFP marrow was treated at room temperature with DDG or TPA for 4 h. In experiments using inhibitors, cells were pretreated for 60 min before adding TPA.
Kidney marrow transplantation
Casper recipient zebrafish were conditioned with 35 Gy gamma irradiation over 2 doses on D-1 and D0. Ubi:EGFP (40,000 cells) and ubi:mCherry (80,000) cells were injected into the retro-orbital plexus in anesthetized recipients. After recovery, fish were immediately placed on-flow. Transplant related mortality (recipient death in the first week) was typically less than 10%.
Lentivirus
Lentiviral construct pLV [Exp]-EGFP: T2A: Puro-hPGK>E4ORF1 was synthesized (Vector Builder, USA) and transfected into Lenti-X 293T cells using VSV-G Lenti-X Packaging single shots (Takara Bio, USA). Lentivirus-containing supernatant was harvested and purified using the Lenti-X Maxi Purification Kit. HUVEC cells were transduced in complete M200 medium with polybrene (5 μg/mL) using serial dilutions of purified viral particles. Transient expression of lentiviral constructs was observed using fluorescence microscopy. After 48 h, cell culture medium was exchanged and stably transfected cells were selected using 0.5 μg/mL puromycin. For gene knockdown experiments, commercially prepared lentivirus targeting human PRKCD or scramble control was purchased from Vectorbuilder (Chicago, USA). E4-HUVEC cells were transduced according to the manufacturer’s instructions. Transduced cells were visualized by mCherry expression and selected in G418.
Immunoprecipitation and immunoblotting
HUVEC and E4-HUVEC cultures were harvested, washed with PBS, and lysed using Novex NP40 Cell Lysis Buffer (Invitrogen) and vortex mixing. Lysates were cleared by centrifugation at 10,000g for 10 min at 4°. Supernatants were stored in aliquots at −80C. Protein concentration was determined using the Pierce BCA Protein assay kit (Thermo Fisher Scientific). Protein lysates (20 μg) were loaded on iBolt 4–12% Bis-Tris gels (Invitrogen) and transferred to nitrocellulose membranes. Membranes were blocked in TBST with 5% BSA and then incubated with primary and HRP-conjugated secondary antibodies according to the manufacturers’ recommendations. Detection was with SuperSignal West Pico Plus Chemiluminescent Substrate. Blots were imaged using an iBright imaging system (Thermo Fisher Scientific). Immunoprecipitation experiments were performed using the Pierce Crosslink Magnetic IP/Co-IP kit and anti-phospho PKC-δ.
Whole-mount in situ hybridization
Antisense RNA probe for prkcda was generated by PCR amplification of cDNA using prkcda_probe_F and prkcda_probe_R to generate a 400 bp product. In vitro transcription was performed using the Maxiscript T7 transcription kit (Thermo Fisher AM1314). DIG-labeled probe was generated using the Roche DIG RNA labeling kit (Sigma-Aldrich 11175025910) and resuspended in Hyb+ at a concentration of 50 ng/μL of RNA probe. Embryos were fixed at 72 hpf in 4% formaldehyde overnight and then stored in 100% methanol prior to performing in situ hybridization using standard techniques.82 Embryos were stored in methanol prior to imaging using a Leica Microsystems S9i Stereomicroscope and camera at 53 magnification using Leica Application Suite EZ software (Wetzlar, Germany).
Immunofluorescence
Zebrafish embryos were euthanized and fixed in 4% paraformaldehyde at 4°C overnight, washed with PBS, and incubated with 30% sucrose in PBS until sinking to the bottom of the tube. 7-μm sections were sliced using a cryostat. Free-floating embryo sections were permeabilized in PBS containing 100 μM digitonin for 10 min and incubated with 1% BSA in PBST (PBS with 0.1% Tween 20) for 30 min. Primary antibody incubations were performed with anti-Fli1 (1:50) and anti-GFP/mClover (1:1000) in 1% BSA in PBST at 4°C overnight. After three PBS washes, embryos sections were incubated with following conjugated secondary antibodies: donkey anti-rabbit (AF-594, 1:500) and goat anti-mouse (AF-488, 1:500) for 1 h at room temperature. After three additional PBS washes, the stained embryo sections were mounted using mounting medium on microscope slides. Images were captured using Keyence BZ-X Analyzer software.
QUANTIFICATION AND STATISTICAL ANALYSIS
ScRNA-seq data preprocessing and quality control
10X scRNA-seq libraries were sequenced using a 151bp Read 1, 8 bp Index 1, 151 bp Read 2 configuration on an Illumina Novaseq instrument (Nationwide Children’s Hospital Institute for Genomic Medicine). FASTQs were generated and filtered cell-barcode matrices generated using CellRanger 4.0 (10X Genomics). A minimum of 15,000 sequencing reads per cell were obtained. Alignment was performed to the GRCz11 reference. Low quality cells were identified using scater as those either with a percent of reads mapped to mitochondrial genes more than 2 median absolute deviations (MADs) greater than the median or a number of expressed genes less than 2 MADs below the median for each sample.83 Doubletfinder was used to remove high-confidence doublets with a predicted doublet rate of 4%.84
Dimensionality reduction
Dimensionality reduction was performed in Monocle3 using principal components analysis (PCA) and uniform manifold approximation and projection (UMAP).85,86 The top 100 principal components were used for UMAP dimensionality reduction. Sample alignment was performed using batchelor and UMAP coordinates were recalculated if significant batch effects were noted.87
Cell clustering
Depending on the level of resolution necessary, cell clustering was performed using the partition-based graph abstraction, Leiden and/or Louvain clustering algorithms as implemented in Monocle3 using default parameters.88–90
Dataset reanalysis
Gene expression profiles from mouse single cell data were used to aid identification of zebrafish cell clusters.38,39 To map expression of published murine gene sets onto zebrafish scRNA-seq data, 1:1Mouse:Zebrafish orthologs were identified using the latest data available at https://zfin.org/downloads/mouse_orthos.txt.
Gene expression, gene module analysis, and aggregated gene scores
Size factors were calculated for each cell as the number of UMI counts in that cell divided by the geometric mean of UMI counts for all cells in the dataset. Expression values for single genes were calculated as size factor-normalized UMI counts and log-transformed for plotting. Variable genes were identified in each single cell data set by spatial autocorrelation using Moran’s I test.91 Gene modules were identified using UMAP dimensionality reduction and Louvain clustering on variable genes as implemented in Monocle3.92 Aggregate gene expression scores from gene modules and published gene lists were calculated by taking the sum of size-factor normalized UMI counts within each gene group, scaling this value across gene groups and taking the mean of the scaled aggregate value across groups of cells.
Pseudotime analysis
Reversed graph embedding was used to calculate pseudotime coordinates for cellular partitions. Cellular trajectories were anchored by inspection of canonical gene expression.
GO-term analysis
GO term enrichment for gene lists indicated in Results was determined using topGO by mapping to the org.Dr.eg.db annotation in Bioconductor (https://doi.org/10.18129/B9.bioc.org.Dr.eg.db) and testing for significant associations with the “classicFisher” test.93 GO term redundancy was reduced based on semantic similarity using rrvgo and enrichment was visualized using custom scripts.94
Single cell GESTALT barcode analysis
Adapter and poly-A trimming was performed with cutadapt.95 Custom aligner software was written in the D programming language to identify the best matching GESTALT lineage barcode for each read pair. This software utilizes the dhtslib, HTSlib, dparasail, and parasail software libraries.96,97 The aligner takes in FASTQ files paired reads containing the 10X single cell barcode and UMI (Read 1) and the GESTALT lineage barcode (Read 2). The aligner also takes as input whitelist files of known GESTALT lineage barcodes from bulk peripheral blood sequencing and known 10X single cell barcodes from the scRNA-seq data. For each 10X-GESTALT barcode read pair, a semi-global Needleman-Wunsch alignment algorithm was used to align the GESTALT lineage barcode read to all whitelisted GESTALT sequences using a match score of 3, a mismatch penalty of 4, a gap open penalty of 7, and a gap extension penalty of 4.98 The alignment with the highest semi-global alignment score was chosen as the predicted GESTALT lineage barcode for that read. Similarly, the 10X barcode read was aligned against all whitelisted 10X cell barcodes with a match score of 2, a mismatch penalty of 2, a gap open penalty of 10, and a gap extension penalty of 5. The alignment with the highest semi-global alignment score was chosen as the predicted 10X barcode. Resulting alignments were then filtered to include those that had a similarity score of at least 0.95 for quality control.
Single cell ATAC-seq analysis
ScATAC data were pre-processed using 10X CellRanger-ATAC v1.2 using a custom zebrafish reference. A Seurat object was generated using the filtered peak-barcode matrix and fragment files produced by CellRanger-ATAC. To identify links between co-accessible peaks, the Seurat object was converted to a Cicero object using functions from SeuratWrappers. Links were generated in Cicero and transferred to the Seurat object. UMAP coordinates and cell clusters (Leiden method) were generated using the published Cicero workflow. Cell cluster identities were established by label transfer from scRNA-seq data obtained from the same pools of fish. Briefly, the Seurat GeneActivity function was used to compute counts per cell in the scATAC data within gene bodies and promoters. Gene activity scores were used as a surrogate for expression in the Seurat label transfer algorithm. The resulting individual cell type predictions were grouped according to scATAC Leiden cluster and a consensus label was assigned. ScATAC-seq data were visualized using blaseRtools functions.
General statistical analysis
Summary statistics and hypothesis testing as described in the text was performed using R v4.2. Unless otherwise indicated, data are presented as mean ± S.E.M. P < 0.05 was considered statistically significant.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| PKCδ (D10E2) Rabbit mAb | Cell Signaling Technology | Cat# 9616S; RRID:AB_10949973 |
| Phospho-PKCδ (Thr505) Rabbit Polyclonal Antibody | Cell Signaling Technology | Cat# 9374S; RRID:AB_2168837 |
| Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (E10) Mouse mAb | Cell Signaling Technology | Cat# 9106S; RRID:AB_331768 |
| p44/42 MAPK (Erk1/2) (137F5) Rabbit mAb | Cell Signaling Technology | Cat# 4695S; RRID:AB_390779 |
| Phospho-Paxillin (Tyr118) Polyclonal Antibody | ThermoFisher Scientific | Cat# 44–722G; RRID:AB_2533733 |
| Fli1 Rabbit Polyclonal Antibody | ThermoFisher Scientific | Cat# PA5–13440; RRID:AB_2106098 |
| Gfp Tag Mouse Monoclonal Antibody (GF28R) | ThermoFisher Scientific | Cat# MA5–15256; RRID:AB_10979281 |
| Goat anti-mouse AF-488 | ThermoFisher Scientific | Cat# A32723; RRID:AB_2633275 |
| Donkey anti-rabbit AF-594 | ThermoFisher Scientific | Cat# A-21207; RRID:AB_141637 |
|
| ||
| Biological samples | ||
|
| ||
| Human Umbilical Vein Endothelial Cells, HUVEC Pooled | ThermoFisher Scientific | Cat# C01510C |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| EnGen® Spy Cas9 NLS | New England Biolabs | Cat# m0646 |
| Recombinant Human IL-8/CXCL8 Protein | R&D Systems | Cat# 208-IL-010 |
| HA-100 dihydrochloride | Santa Cruz Biotechnology | Cat# sc-203072 |
| dactolisib | Selleck Chemicals | Cat# S1009 |
| ibrutinib | Selleck Chemicals | Cat# S2680 |
| ly294002 | Selleck Chemicals | Cat# S1005 |
| mk2206 | Selleck Chemicals | Cat# S1078 |
| sorafenib | Millipore Sigma | Cat# SML2653 |
| AEB071 | Cayman Chemical | Cat# 16726 |
| AZD6244 | Cayman Chemical | Cat# 11599 |
| Phorbol 12-myristate 13-acetate (TPA) | Cayman Chemical | Cat# 10008014 |
| 1,2-didecanoyl-sn-glycerol (DDG) | Millipore Sigma | Cat# 800810P |
|
| ||
| Critical commercial assays | ||
|
| ||
| Chromium™ Single Cell 3’ GEM, Library & Gel Bead Kit v3 | 10X Genomics | Cat# 1000092 |
| LIVE/DEAD™ Viability/Cytotoxicity Kit, for mammalian cells | ThermoFisher Scientific | Cat# L3224 |
| Taqman™ Gene Expression Assay (FAM), S (250 reactions/250 μL), assay ID: Hs00174103_m1 | ThermoFisher Scientific | Cat# 4331182 |
| Taqman™ Gene Expression Assay (FAM), S (250 reactions/250 μL), assay ID: Hs03003631_g1 | ThermoFisher Scientific | Cat# 4331182 |
| SMART-Seq v4 Ultra Low Input RNA Kit | Clontech | Cat# 634888 |
| Zymo-Seq RiboFree Total RNA Library Kit | Zymo Research | Cat#R3000 |
| ATAC-seq Kit | Active Motif | Cat# 53150 |
| Pierce™ Crosslink Magnetic IP/Co-IP Kit | ThermoFisher Scientific | Cat# 88805 |
| Chromium™ Single Cell ATAC Library & Gel Bead Kit | 10X Genomics | Cat# 1000111 |
|
| ||
| Deposited data | ||
|
| ||
| Processed source data for analysis scripts | This paper | Mendeley Data: https://doi.org/10.17632/6s7vy929dc.1 |
| FASTQs and cell-barcode matrices for scRNA-seq and scATAC-seq experiments | This paper | GEO: GSE191029 |
| GRCh38 Reference Sequence | Genome Reference Consortium | https://useast.ensembl.org/Homo_sapiens/Info/Index |
| GRCz11 Reference Sequence | Genome Reference Consortium | https://useast.ensembl.org/Danio_rerio/Info/Index |
| Zfin GRCz11 Gene Model | The Zebrafish Information Network | https://zfin.org/downloads |
|
| ||
| Experimental models: cell lines | ||
|
| ||
| E4-HUVEC | This paper | N/A |
| Lenti-X™ 293T cell Line | Takara Bio | Cat# 632180 |
|
| ||
| Experimental models: organisms/strains | ||
|
| ||
| Zebrafish: casper | White et al.76 | N/A |
| Zebrafish: GESTALT | McKenna et al.29 | N/A |
| Zebrafish: kdrl:EGFP: Tg(kdrl:EGFP) | Beis et al.21 | ZDB-ALT-050916–14 |
| Zebrafish: Runx1:EGFP: Tg(Mmu.Runx1:EGFP) | Tamplin et al.27 | ZDB-ALT-150512–1 |
| Zebrafish: lyve1b:dsRed: Tg(−5.2lyve1b:dsRed)nz101 | Okuda et al.22 | ZDB-ALT-120723–3 |
| Zebrafish: prkcda(−/−) | This paper | N/A |
| Zebrafish: Runx1:cxcl8–2A-mCherry: | This paper | N/A |
| Zebrafish: sele:prkcda-2A-mCherry: | This paper | N/A |
|
| ||
| Oligonucleotides | ||
|
| ||
| Primer: raj_GP6: GAGGACTACACCATCGTGGAG | Raj et al.77 | N/A |
| Primer: partial_read_1: GTGACTGGAGTTCAGACGTG TGCTCTTCCGATCTNNNNNNNNNTCGAGCTCAAGC TTCGGAC | This paper | N/A |
| Primer: mod_GP12: CTACACGACGCTCTTCCGATCT | Modified from Raj et al.77 | N/A |
| Primer: GESTALT F: ACACTCTTTCCCTACACGACGCTCTTCCGATCTTCGAGCTCAAGCTTCGG | Modified from McKenna et al.29 | N/A |
| Primer: GESTALT R: GACTGGAGTTCAGACGTGTGCT CTTCCGATCTCTGCCATTTGTCTCGAGGTC | Modified from McKenna et al.29 | N/A |
| Primer: prkcda_probe_F: CACCTCACTATCCTCGCTGG | This paper | N/A |
| Primer: prkcda_probe_R: TAATACGACTCACTATAGGCTCATGGCTAGTTTGGTGG | This paper | N/A |
| HsPRKCD shRNA targeting sequence: CTTCGGAGGGAAATTGTAAAT | This paper | N/A |
|
| ||
| Recombinant DNA | ||
|
| ||
| Plasmid: sele792-prkcda-2a-mcherry-395 | This paper | N/A |
| Plasmid: sele792-mcs-2a-mcherry-395 | This paper | N/A |
| Plasmid: pLV [Exp]-EGFP: T2A: Puro-hPGK> | This paper | N/A |
| E4ORF1 | ||
| Plasmid: prkcda-intron-1-enhancer-clover394 | This paper | N/A |
| Plasmid: pLV[shRNA]-mCherry/Neo-U6>hPRKCD | This paper | N/A |
|
| ||
| Software and algorithms | ||
|
| ||
| Project analysis scripts | This paper | https://github.com/blaserlab/pkc_cxcl8 |
| Data processing scripts | This paper | Mendeley Data: https://doi.org/10.17632/6s7vy929dc.1 |
| Analysis function library | This paper | https://github.com/blaserlab/blaseRtools |
| Supporting data for analysis functions | This paper | https://github.com/blaserlab/blaseRdata |
| Encode RNA seq pipeline | ENCODE Consortium | https://www.encodeproject.org/pipelines/ENCPL002LPE/ |
| Encode ATAC seq pipeline | ENCODE Consortium | https://www.encodeproject.org/pipelines/ENCPL787FUN/ |
| 10X CellRanger v4.0 | 10X Genomics | https://support.10xgenomics.com/ |
| 10X CellRanger-ATAC v1.2 | 10X Genomics | https://support.10xgenomics.com/ |
| ImageJ | Schneider et al.78 | https://ImageJ.nih.gov/ij/ |
| BZ-X700 Viewer Ver. 1.31 | Keyence Corp | N/A |
| BZ-X700 Analyzer Ver. 1.31 BZ-H3AE | Keyence Corp | N/A |
|
| ||
| Other | ||
|
| ||
| OctoMACs Dead Cell Removal Kit | Miltenyi Biotec | Cat#130–090-101 |
| BZ-X710 Fluorescence Microscope | Keyence Corp | N/A |
| Nikon Ti Custom Microscope with Yokogawa Spinning Disk | Boston Microscopes and suppliers | N/A |
| Nikon A1R Live Cell Imaging System | Nikon | N/A |
| S9i stereomicroscope with integrated camera | Leica | N/A |
| VSV-G Lenti-X Packaging single shots | Takara Bio | Cat#631275 |
Highlights.
Lineage tracing reveals microenvironmental control of HSC clonal evolution
Expression of prkcda is regulated to maintain HSC reserve capacity
Cxcl8 signals via prkcda/pkc-δ and promotes HSC competition for niche residency
Microenvironmental control of HSC fate has implications for hematologic disorders
ACKNOWLEDGMENTS
The authors would like to thank S. Cabrera for animal husbandry and members of the Zon Laboratory for critical reading of the manuscript.
This work was supported by NIH R01 DK128238 (B.W.B.), K08 DK111920 (B.W.B.), a Young Investigator Award from the Pelotonia Foundation (B.W.B.), UM1 CA186712 (W.E.C.), U01 HL134812 (L.I.Z.), P01 HL032262 (L.I.Z.), P01 HL131477, R01 HL144780 (L.I.Z.), RC2 DK120535 (L.I.Z.), an Alex’s Lemonade Stand Crazy 8 Initiative Award (L.I.Z.), and research fellowships from the German Research Foundation and the Care-for-Rare Foundation (both to V.B.).
Footnotes
DECLARATION OF INTERESTS
L.I.Z. is a founder and stockholder of Fate Therapeutics, CAMP4 Therapeutics, Amagma Therapeutics, Scholar Rock, and Branch Biosciences. He is a consultant for Celularity and Cellarity.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2023.112528.
REFERENCES
- 1.Perlin JR, Robertson AL, and Zon LI (2017). Efforts to enhance blood stem cell engraftment: recent insights from zebrafish hematopoiesis. J. Exp. Med 214, 2817–2827. 10.1084/jem.20171069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinho S, and Frenette PS (2019). Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol 20, 303–320. 10.1038/s41580-019-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abelson S, Collord G, Ng SWK, Weissbrod O, Mendelson Cohen N, Niemeyer E, Barda N, Zuzarte PC, Heisler L, Sundaravadanam Y, et al. (2018). Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 559, 400–404. 10.1038/s41586-018-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genovese G, Kä hler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, et al. (2014). Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med 371, 2477–2487. 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. (2014). Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med 371, 2488–2498. 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. (2017). Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med 377, 111–121. 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meisel M, Hinterleitner R, Pacis A, Chen L, Earley ZM, Mayassi T, Pierre JF, Ernest JD, Galipeau HJ, Thuille N, et al. (2018). Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature 557, 580–584. 10.1038/s41586-018-0125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagata Y, Makishima H, Kerr CM, Przychodzen BP, Aly M, Goyal A, Awada H, Asad MF, Kuzmanovic T, Suzuki H, et al. (2019). Invariant patterns of clonal succession determine specific clinical features of myelodysplastic syndromes. Nat. Commun 10, 5386. 10.1038/s41467-019-13001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avagyan S, Henninger JE, Mannherz WP, Mistry M, Yoon J, Yang S, Weber MC, Moore JL, and Zon LI (2021). Resistance to inflammation underlies enhanced fitness in clonal hematopoiesis. Science 374, 768–772. 10.1126/science.aba9304. [DOI] [PubMed] [Google Scholar]
- 10.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, et al. (2010). Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251–264. 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Himburg HA, Termini CM, Schlussel L, Kan J, Li M, Zhao L, Fang T, Sasine JP, Chang VY, and Chute JP (2018). Distinct bone marrow sources of pleiotrophin control hematopoietic stem cell maintenance and regeneration. Cell Stem Cell 23, 370–381.e5. 10.1016/j.stem.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, and Lévesque JP (2012). Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med 18, 1651–1657. 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, Chiu VK, Nolan DJ, Shido K, Benjamin L, and Rafii S. (2010). Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat. Cell Biol 12, 1046–1056. 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulos MG, Guo P, Kofler NM, Pinho S, Gutkin MC, Tikhonova A, Aifantis I, Frenette PS, Kitajewski J, Rafii S, and Butler JM (2013). Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep. 4, 1022–1034. 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itkin T, Gur-Cohen S, Spencer JA, Schajnovitz A, Ramasamy SK, Kusumbe AP, Ledergor G, Jung Y, Milo I, Poulos MG, et al. (2016). Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328. 10.1038/nature17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding L, Saunders TL, Enikolopov G, and Morrison SJ (2012). Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462. 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding L, and Morrison SJ (2013). Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235. 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, Kopp HG, Shido K, Petit I, Yanger K, et al. (2009). Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell 4, 263–274. 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin HF, Handin RI, and Herbomel P. (2006). Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 25, 963–975. 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Blaser BW, Moore JL, Hagedorn EJ, Li B, Riquelme R, Lichtig A, Yang S, Zhou Y, Tamplin OJ, Binder V, and Zon LI (2017). CXCR1 remodels the vascular niche to promote hematopoietic stem and progenitor cell engraftment. J. Exp. Med 214, 1011–1027. 10.1084/jem.20161616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beis D, Bartman T, Jin SW, Scott IC, D’Amico LA, Ober EA, Verkade H, Frantsve J, Field HA, Wehman A, et al. (2005). Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 132, 4193–4204. 10.1242/dev.01970. [DOI] [PubMed] [Google Scholar]
- 22.Okuda KS, Astin JW, Misa JP, Flores MV, Crosier KE, and Crosier PS (2012). lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 139, 2381–2391. 10.1242/dev.077701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi A, Kontarakis Z, Gerri C, Nolte H, Hölper S, Krüger M, and Stainier DYR (2015). Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524, 230–233. 10.1038/nature14580. [DOI] [PubMed] [Google Scholar]
- 24.Munson MJ, Mathai BJ, Ng MYW, Trachsel-Moncho L, de la Ballina LR, Schultz SW, Aman Y, Lystad AH, Singh S, Singh S, et al. (2021). GAK and PRKCD are positive regulators of PRKN-independent mitophagy. Nat. Commun 12, 6101. 10.1038/s41467-021-26331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, Chen Y, Han X, and Wu K. (2016). The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 31, 61–71. 10.1016/j.cytogfr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang K, Niggemann B, Zanker KS, and Entschladen F. (2002). Signal processing in migrating T24 human bladder carcinoma cells: role of the autocrine interleukin-8 loop. Int. J. Cancer 99, 673–680. 10.1002/ijc.10424. [DOI] [PubMed] [Google Scholar]
- 27.Tamplin OJ, Durand EM, Carr LA, Childs SJ, Hagedorn EJ, Li P, Yzaguirre AD, Speck NA, and Zon LI (2015). Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 160, 241–252. 10.1016/j.cell.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin SW, Beis D, Mitchell T, Chen JN, and Stainier DYR (2005). Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209. 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- 29.McKenna A, Findlay GM, Gagnon JA, Horwitz MS, Schier AF, and Shendure J. (2016). Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science 353, aaf7907. 10.1126/science.aaf7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teets EM, Gregory C, Shaffer J, Blachly JS, and Blaser BW (2020). Quantifying hematopoietic stem cell clonal diversity by selecting informative Amplicon barcodes. Sci. Rep 10, 2153. 10.1038/s41598-020-59119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baron CS, Barve A, Muraro MJ, van der Linden R, Dharmadhikari G, Lyubimova A, de Koning EJP, and van Oudenaarden A. (2019). Cell type purification by single-cell transcriptome-trained sorting. Cell 179, 527–542.e19. 10.1016/j.cell.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y, Lu Y, He Y, Feng Z, Zhan Y, Huang X, Liu Q, Zhang J, Li H, Huang H, et al. (2019). Ikzf1 regulates embryonic T lymphopoiesis via Ccr9 and Irf4 in zebrafish. J. Biol. Chem 294, 16152–16163. 10.1074/jbc.RA119.009883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawir DF, Iwanami N, Schorpp M, and Boehm T. (2017). A missense mutation in zbtb17 blocks the earliest steps of T cell differentiation in zebrafish. Sci. Rep 7, 44145. 10.1038/srep44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu X, Zhang Y, Liu F, and Wang L. (2020). Rac2 regulates the migration of T lymphoid progenitors to the thymus during zebrafish embryogenesis. J. Immunol 204, 2447–2454. 10.4049/jimmunol.1901494. [DOI] [PubMed] [Google Scholar]
- 35.Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, and Bhandoola A. (2010). CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood 115, 1897–1905. 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter RH, and Barrington RA (2004). Signaling by the CD19/CD21 complex on B cells. Curr. Dir. Autoimmun 7, 4–32. 10.1159/000075685. [DOI] [PubMed] [Google Scholar]
- 37.Oren R, Takahashi S, Doss C, Levy R, and Levy S. (1990). TAPA-1, the target of an antiproliferative antibody, defines a new family of trans-membrane proteins. Mol. Cell Biol 10, 4007–4015. 10.1128/mcb.10.8.4007-4015.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muench DE, Olsson A, Ferchen K, Pham G, Serafin RA, Chutipongtanate S, Dwivedi P, Song B, Hay S, Chetal K, et al. (2020). Mouse models of neutropenia reveal progenitor-stage-specific defects. Nature 582, 109–114. 10.1038/s41586-020-2227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tusi BK, Wolock SL, Weinreb C, Hwang Y, Hidalgo D, Zilionis R, Waisman A, Huh JR, Klein AM, and Socolovsky M. (2018). Population snapshots predict early haematopoietic and erythroid hierarchies. Nature 555, 54–60. 10.1038/nature25741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Q, Iyer S, Lobbardi R, Moore JC, Chen H, Lareau C, Hebert C, Shaw ML, Neftel C, Suva ML, et al. (2017). Dissecting hematopoietic and renal cell heterogeneity in adult zebrafish at single-cell resolution using RNA sequencing. J. Exp. Med 214, 2875–2887. 10.1084/jem.20170976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raaijmakers Marc HGP, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. (2010). Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464, 852–857. 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pliner HA, Packer JS, McFaline-Figueroa JL, Cusanovich DA, Daza RM, Aghamirzaie D, Srivatsan S, Qiu X, Jackson D, Minkina A, et al. (2018). Cicero predicts cis-regulatory DNA interactions from single-cell chromatin accessibility data. Mol. Cell 71, 858–871.e8. 10.1016/j.molcel.2018.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao Y, Hao S, Andersen-Nissen E, Mauck WM 3rd, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, et al. (2021). Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29. 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seandel M, Butler JM, Kobayashi H, Hooper AT, White IA, Zhang F, Vertes EL, Kobayashi M, Zhang Y, Shmelkov SV, et al. (2008). Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc. Natl. Acad. Sci. USA 105, 19288–19293. 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramalingam R, Rafii S, Worgall S, Brough DE, and Crystal RG (1999). E1(−)E4(+) adenoviral gene transfer vectors function as a “prolife” signal to promote survival of primary human endothelial cells. Blood 93, 2936–2944. [PubMed] [Google Scholar]
- 46.Grant CE, Bailey TL, and Noble WS (2011). FIMO: Scanning for occurrences of a given motif. Bioinformatics 27, 1017–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellisen LW, Carlesso N, Cheng T, Scadden DT, and Haber DA (2001). The Wilms tumor suppressor WT1 directs stage-specific quiescence and differentiation of human hematopoietic progenitor cells. EMBO J. 20, 1897–1909. 10.1093/emboj/20.8.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duim SN, Kurakula K, Goumans MJ, and Kruithof BPT (2015). Cardiac endothelial cells express Wilms’ tumor-1: Wt1 expression in the developing, adult and infarcted heart. J. Mol. Cell. Cardiol 81, 127–135. 10.1016/j.yjmcc.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Wagner N, Michiels JF, Schedl A, and Wagner KD (2008). The Wilms’ tumour suppressor WT1 is involved in endothelial cell proliferation and migration: expression in tumour vessels in vivo. Oncogene 27, 3662–3672. 10.1038/sj.onc.1211044. [DOI] [PubMed] [Google Scholar]
- 50.McCarty G, Awad O, and Loeb DM (2011). WT1 protein directly regulates expression of vascular endothelial growth factor and is a mediator of tumor response to hypoxia. J. Biol. Chem 286, 43634–43643. 10.1074/jbc.M111.310128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li P, Lahvic JL, Binder V, Pugach EK, Riley EB, Tamplin OJ, Panigrahy D, Bowman TV, Barrett FG, Heffner GC, et al. (2015). Epoxyeicosatrienoic acids enhance embryonic haematopoiesis and adult marrow engraftment. Nature 523, 468–471. 10.1038/nature14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinberg SF (2008). Structural basis of protein kinase C isoform function. Physiol. Rev 88, 1341–1378. 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kikkawa U, Matsuzaki H, and Yamamoto T. (2002). Protein kinase C delta (PKC delta): activation mechanisms and functions. J. Biochem 132, 831–839. 10.1093/oxfordjournals.jbchem.a003294. [DOI] [PubMed] [Google Scholar]
- 54.Ogita K, Miyamoto S, Yamaguchi K, Koide H, Fujisawa N, Kikkawa U, Sahara S, Fukami Y, and Nishizuka Y. (1992). Isolation and characterization of delta-subspecies of protein kinase C from rat brain. Proc. Natl. Acad. Sci. USA 89, 1592–1596. 10.1073/pnas.89.5.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang G, Kazanietz MG, Blumberg PM, and Hurley JH (1995). Crystal structure of the cys2 activator-binding domain of protein kinase C delta in complex with phorbol ester. Cell 81, 917–924. 10.1016/0092-8674(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 56.Falsone A, Wabitsch V, Geretti E, Potzinger H, Gerlza T, Robinson J, Adage T, Teixeira MM, and Kungl AJ (2013). Designing CXCL8-based decoy proteins with strong anti-inflammatory activity in vivo. Biosci. Rep 33, e00068. 10.1042/BSR20130069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, Kokkaliaris KD, Mercier F, Tabaka M, Hofree M, et al. (2019). A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell 177, 1915–1932.e16. 10.1016/j.cell.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garg R, Caino MC, and Kazanietz MG (2013). Regulation of transcriptional networks by PKC isozymes: identification of c-Rel as a key transcription factor for PKC-regulated genes. PLoS One 8, e67319. 10.1371/journal.pone.0067319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caino MC, von Burstin VA, Lopez-Haber C, and Kazanietz MG (2011). Differential regulation of gene expression by protein kinase C isozymes as determined by genome-wide expression analysis. J. Biol. Chem 286, 11254–11264. 10.1074/jbc.M110.194332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, et al. (2007). Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011. 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, and Zon LI (2009). Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136, 1136–1147. 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, and Zon LI (2003). Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol 4, 1238–1246. 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 63.Robertson J, Jacquemet G, Byron A, Jones MC, Warwood S, Selley JN, Knight D, Humphries JD, and Humphries MJ (2015). Defining the phospho-adhesome through the phosphoproteomic analysis of integrin signalling. Nat. Commun 6, 6265. 10.1038/ncomms7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosales JL, and Isseroff RR (1995). Increased expression of a high molecular weight (130 KD) protein kinase C isoform in a differentiation-defective ras-transfected keratinocyte line. J. Cell. Physiol 164, 509–521. 10.1002/jcp.1041640309. [DOI] [PubMed] [Google Scholar]
- 65.Ueda Y, Hirai S.i., Osada S.i., Suzuki A, Mizuno K, and Ohno S. (1996). Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J. Biol. Chem 271, 23512–23519. 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 66.López-Colomé AM, Lee-Rivera I, Benavides-Hidalgo R, and López E. (2017). Paxillin: a crossroad in pathological cell migration. J. Hematol. Oncol 10, 50. 10.1186/s13045-017-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petro JB, Rahman SM, Ballard DW, and Khan WN (2000). Bruton’s tyrosine kinase is required for activation of IkappaB kinase and nuclear factor kappaB in response to B cell receptor engagement. J. Exp. Med 191, 1745–1754. 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shinners NP, Carlesso G, Castro I, Hoek KL, Corn RA, Woodland RT, Scott ML, Wang D, and Khan WN (2007). Bruton’s tyrosine kinase mediates NF-kappa B activation and B cell survival by B cell-activating factor receptor of the TNF-R family. J. Immunol 179, 3872–3880. 10.4049/jimmunol.179.6.3872. [DOI] [PubMed] [Google Scholar]
- 69.Morrison SJ, Hemmati HD, Wandycz AM, and Weissman IL (1995). The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 92, 10302–10306. 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fleming WH, Alpern EJ, Uchida N, Ikuta K, Spangrude GJ, and Weissman IL (1993). Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J. Cell Biol 122, 897–902. 10.1083/jcb.122.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bowie MB, McKnight KD, Kent DG, McCaffrey L, Hoodless PA, and Eaves CJ (2006). Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J. Clin. Invest 116, 2808–2816. 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ganuza M, Hall T, Finkelstein D, Chabot A, Kang G, and McKinney-Freeman S. (2017). Lifelong haematopoiesis is established by hundreds of precursors throughout mammalian ontogeny. Nat. Cell Biol 19, 1153–1163. 10.1038/ncb3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McKenna A, and Gagnon JA (2019). Recording development with single cell dynamic lineage tracing. Development 146. 10.1242/dev.169730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dohner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, Ebert BL, Fenaux P, Godley LA, Hasserjian RP, et al. (2022). Diagnosis and management of AML in adults: 2022 ELN recommendations from an international expert panel. Blood. 10.1182/blood.2022016867. [DOI] [PubMed] [Google Scholar]
- 75.Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, Lamprecht TL, Shen D, Hundal J, Fulton RS, et al. (2015). Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature 518, 552–555. 10.1038/nature13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, Bourque C, Dovey M, Goessling W, Burns CE, and Zon LI (2008). Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183–189. 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raj B, Wagner DE, McKenna A, Pandey S, Klein AM, Shendure J, Gagnon JA, and Schier AF (2018). Simultaneous single-cell profiling of lineages and cell types in the vertebrate brain. Nat. Biotechnol 36, 442–450. 10.1038/nbt.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lawrence C, Best J, Cockington J, Henry EC, Hurley S, James A, Lapointe C, Maloney K, and Sanders E. (2016). The complete and updated “rotifer polyculture method” for rearing first feeding zebrafish. J. Vis. Exp, e53629. 10.3791/53629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagabhushana A, and Mishra RK (2016). Finding clues to the riddle of sex determination in zebrafish. J. Biosci 41, 145–155. 10.1007/s12038-016-9593-1. [DOI] [PubMed] [Google Scholar]
- 81.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, and Pachter L. (2012). Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc 7, 562–578. 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thisse C, and Thisse B. (2008). High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc 3, 59–69. 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 83.McCarthy DJ, Campbell KR, Lun ATL, and Wills QF (2017). Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 33, 1179–1186. 10.1093/bioinformatics/btw777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McGinnis CS, Murrow LM, and Gartner ZJ (2019). DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337.e4. 10.1016/j.cels.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, and Rinn JL (2014). The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol 32, 381–386. 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, and Newell EW (2018). Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol 37, 38–44. 10.1038/nbt.4314. [DOI] [PubMed] [Google Scholar]
- 87.Haghverdi L, Lun ATL, Morgan MD, and Marioni JC (2018). Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat. Biotechnol 36, 421–427. 10.1038/nbt.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blondel VD, Guillaume JL, Lambiotte R, and Lefebvre E. (2008). Fast unfolding of communities in large networks. J. Stat. Mech 2008, P10008. 10.1088/1742-5468/2008/10/P10008. [DOI] [Google Scholar]
- 89.Wolf FA, Hamey FK, Plass M, Solana J, Dahlin JS, Göttgens B, Rajewsky N, Simon L, and Theis FJ (2019). PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol. 20, 59. 10.1186/s13059-019-1663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Traag VA, Waltman L, and van Eck NJ (2019). From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep 9, 5233. 10.1038/s41598-019-41695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moran PA (1950). Notes on continuous stochastic phenomena. Biometrika 37, 17–23. 10.2307/2332142. [DOI] [PubMed] [Google Scholar]
- 92.Cao J, Spielmann M, Qiu X, Huang X, Ibrahim DM, Hill AJ, Zhang F, Mundlos S, Christiansen L, Steemers FJ, et al. (2019). The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502. 10.1038/s41586-019-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alexa A, and Rahnenfuhrer J. (2020). topGO: Enrichment Analysis for Gene Ontology. [Google Scholar]
- 94.Sayols S. (2020). Rrvgo: A Bioconductor Package to Reduce and Visualize Gene Ontology Terms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. j 17, 10. 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 96.Bonfield JK, Marshall J, Danecek P, Li H, Ohan V, Whitwham A, Keane T, and Davies RM (2021). HTSlib: C library for reading/writing high-throughput sequencing data. GigaScience 10. 10.1093/gigascience/giab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Daily J. (2016). Parasail: SIMD C library for global, semi-global, and local pairwise sequence alignments. BMC Bioinf. 17, 81. 10.1186/s12859-016-0930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Needleman SB, and Wunsch CD (1970). A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol 48, 443–453. 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data generated during this study is available at GEO: GSE191029. Processed data are available as an R package via Mendeley Data: https://doi.org/10.17632/6s7vy929dc.1.
Analysis code is publicly available at https://github.com/blaserlab/pkc_cxcl8. Raw data processing scripts and additional documentation are available within the R data package.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.