Abstract
Background:
Neuropsychiatric symptoms (NPS) and cognitive impairment are highly prevalent among persons with HIV (PWH). We examined the effect of the most common NPS, depression and anxiety, on cognitive change among PWH and compared these associations to those among persons without HIV (PWoH).
Methods:
Participants included 168 PWH and 91 PWoH who completed baseline self-report measures of depression (Beck Depression Inventory-II) and anxiety (Profile of Mood States [POMS] – Tension-anxiety subscale) and completed a comprehensive neurocognitive evaluation at baseline and at 1-year follow-up. Demographically-corrected scores from 15 neurocognitive tests were used to calculate global and domain-specific T-scores. Linear mixed-effects models examined the effect of depression and anxiety and their interaction with HIV-serostatus and time on global T-scores.
Results:
There were significant depression-by-HIV and anxiety-by-HIV interactions on global T-scores such that, among PWH only, greater depressive and anxiety symptoms at baseline related to worse global T-scores across visits. Non-significant interactions with time suggest stability in these relationships across visits. Follow-up analyses examining cognitive domains revealed that both the depression-by-HIV and the anxiety-by-HIV interactions were driven by learning and recall.
Limitations:
Follow-up was limited to one-year and there were fewer PWoH than PWH, creating a differential in statistical power.
Conclusion:
Findings suggest that anxiety and depression have stronger links to worse cognitive functioning in PWH than PWoH, particularly learning and memory, and that these associations seem to persist for at least one-year.
Keywords: Neuropsychology, Infectious disease, HIV, Anxiety, Depression, Cognitive decline
1. Introduction
Neuropsychiatric symptoms (NPS) including depression and anxiety are more prevalent among persons with HIV (PWH) versus people without HIV (PWoH) (De Francesco et al., 2019; Milanini et al., 2017; Muñoz-Moreno et al., 2021). NPS have adverse effects on quality of life and health-related outcomes, including worse medication adherence and disease progression among PWH (Brandt et al., 2017; Kamat et al., 2012; Springer et al., 2012; Tate et al., 2003), and also relate to poorer cognitive function among PWH (Brandt et al., 2017; Castellon et al., 1998; De Francesco et al., 2019; Muñoz-Moreno et al., 2021; Paul et al., 2005; Shapiro et al., 2013). Most studies that have examined NPS in relation to cognitive function among PWH have focused on depression, reporting associations between depressive symptoms and poorer cognition among PWH (Muñoz-Moreno et al., 2021). In our prior longitudinal study among PWH, greater cumulative burden of depression (i. e., greater severity and chronicity over time) was related to steeper cognitive decline over ten years (Paolillo et al., 2020). How anxiety relates to cognition has been less examined among PWH (Brandt et al., 2017; Muñoz-Moreno et al., 2021) with some, but not all (Milanini et al., 2017), reporting associations between greater anxiety symptoms (Brandt et al., 2017; Muñoz-Moreno et al., 2021) and poorer cognition. Fewer studies have compared the relationship between NPS and cognition between PWH and PWoH; an important comparison given the higher rates of NPS and cognitive impairment among PWH, and the potential compounding effects of NPS and HIV-related disease mechanisms (e.g., chronic inflammation, hyperlipidemia) and/or common comorbidities among PWH (e.g. substance abuse, cardiovascular disease). Cross-sectional studies that have conducted this comparison found that apathy (Castellon et al., 1998) and depressive (De Francesco et al., 2019) symptoms more strongly related to poorer cognitive outcomes in PWH versus PWoH. The current study adds to the minimal research comparing the link between NPS and cognition in PWH versus PWoH and extends prior research by conducting a longitudinal analysis examining how baseline NPS relates to cognitive change over time. Longitudinal analyses such as this will allow for a better understanding of the temporal pattern and stability of these relationships.
We aimed to examine the association of depression and anxiety at baseline with global cognitive function and cognitive change over a one-year period and determine whether HIV-serostatus moderates this relationship. Furthermore, we explored whether any significant associations between depressive or anxiety symptoms and global cognitive function were driven by specific cognitive domains. We hypothesized that greater depressive or anxiety symptoms would relate to worse cognition and greater cognitive decline over one-year, more so among PWH than PWoH.
2. Methods
2.1. Participants
Participants included 168 PWH and 91 PWoH enrolled in various NIH-funded research studies within the UC San Diego (UCSD) HIV Neurobehavioral Research Program (HNRP) between 1999 and 2016. Exclusion criteria for the parent studies were history of non-HIV-related neurological or medical disorders that affect brain function (e.g., epilepsy, schizophrenia), learning disabilities, dementia diagnosis and English not a primary language. Our inclusion criteria for the present study were availability of study variables, including data on at least one NPS (depression, anxiety and/or apathy) and neurocognitive performance at both baseline and one year later. Participants meeting our inclusion criteria (N = 259) were significantly younger, more educated, less depressed with shorter duration of HIV infection, and less AIDS diagnoses compared to excluded participants (N = 172, ps < .05). Less than 7 % of participants were missing depressive and anxiety symptom data at both baseline and follow-up. UCSD’s Human Research Protections Program approved all study procedures and all participants provided written informed consent.
2.2. Neurocognitive and clinical evaluations
Participants completed standardized neuromedical and neurocognitive evaluations at each visit. HIV disease characteristics (e.g., CD4 count, viral load) were determined either by self-report or laboratory testing. DSM-IV diagnoses of current and lifetime substance use disorders (alcohol, cannabis, amphetamine, cocaine, hallucinogens, inhalant, sedatives, opioids and PCP) and major depressive disorder (MDD) were determined based on the fully-structured computer-based Composite International Diagnostic Interview version 2.1. The neurocognitive evaluation measured verbal fluency, working memory, processing speed, learning and delayed recall, executive function, and complex motor function. Specific tests are described elsewhere (Cysique et al., 2011). Raw test scores were transformed into T-scores adjusted for age, education, sex, race/ethnicity and practice effects (Cysique et al., 2011) based on normative samples of PWoH, and then averaged across all tests to generate a global and domain-specific T-scores. For descriptive purposes, deficit scores were derived from individual test T-scores and then averaged as a Global Deficit Score to define neurocognitive impairment at each time point by an established cut-score of ≥0.5 (Cysique et al., 2011).
2.3. Neuropsychiatric symptoms
Depression symptoms were measured by the Beck Depression Inventory-II (BDI-II; score range: 0–63) (Beck et al., 1996). Anxiety symptoms were measured by the Profile of Mood States (POMS) – Tension-Anxiety Subscale (score range: 0–36) (McNair et al., 1981). All symptom scores were examined continuously with higher scores indicating greater symptoms.
2.4. Statistical analysis
Sample characteristics were compared between PWH and PWoH using Student’s t-test for continuous variables and Fisher’s exact test for categorical variables. HIV disease characteristics were summarized among PWH. When needed to improve symmetry or normality of distributions, square-root transformation was applied to variables with skewed distributions (i.e., nadir and current CD4 count and depressive and anxiety symptoms).
Using linear mixed-effects models with subject-specific random intercepts, we examined the relationship between NPS scores and change in Global T-score over a one-year period and the moderating role of HIV serostatus. Depression and anxiety were modelled separately. Models included the 3-way interaction of NPS X HIV-serostatus X time (i.e., visit 2 vs. visit 1) and all lower-order 2-way interactions and main effects. PWoH served as the reference. Any significant interaction with HIV-serostatus was probed via analyses stratified by HIV-serostatus. Any non-significant interactions were removed from models to assess lower order effects. If significant associations were detected, we repeated analyses using domain-specific T-scores to explore whether findings were domain specific. Alpha level was set at 0.05. Statistical analyses were implemented using R version 3.6.0, 2019.
3. Results
PWH were younger and more likely to be male with greater depressive, anxiety and apathy symptoms compared to PWoH (Table 1). The prevalence of clinically-significant depressive, and anxiety symptoms and neurocognitive impairment at baseline were higher in PWH versus PWoH, although not significantly. Linear mixed-effects models revealed no significant 3-way NPS X HIV-serostatus X time interactions for on global T-scores. When removing the 3-way interactions, there were also no significant 2-way NPS X time interactions. There were significant 2-way HIV X depression (coefficient = −0.98; 95%CI = −1.55 to −0.41, p = .001), and HIV X anxiety interactions (coefficient = −0.86; 95%CI = −1.47 to 0.25, p = .006). Fig. 1 shows that greater depressive and anxiety symptoms related to poorer global T-scores among PWH when controlling for visit (depression: coefficient = −0.80, 95%CI = −1.14 to −0.46, p < .001; anxiety: coefficient = −0.57, 95%CI = −0.92 to −0.23, p = .001) but not among PWoH.
Table 1.
Sample characteristics at baseline by HIV serostatus.
| Characteristic | PWoH | PWH | Effect sizef (95 % CI) | p-value |
|---|---|---|---|---|
| (n = 91) | (n = 168) | |||
| Demographic/Clinical Characteristics | ||||
| Age in years, M (SD) | 55.1 (5.37) | 53.3 (4.58) | 0.38 (0.12, 0.64) | 0.004 |
| Education in years, M (SD) | 14.4 (2.46) | 14.1 (2.99) | 0.12 (−0.13, 0.38) | 0.35 |
| Male, n (%) | 57 (62.6 %) | 143 (85.1 %) | 0.29 (0.15, 0.56) | <0.001 |
| Race/Ethnicity, n (%) | 0.94 | |||
| Black/African-American | 11 (12.1 %) | 25 (14.9 %) | ||
| Hispanic | 12 (13.2 %) | 20 (11.9 %) | ||
| Caucasian | 65 (71.4 %) | 115 (68.5 %) | ||
| Other | 3 (3.30 %) | 8 (4.77 %) | ||
| History of illicit substance use diagnosisa, n (%) | 24 (48.0 %) | 29 (39.7 %) | 0.36 | |
| History of alcohol use diagnosisb, n (%) | 27 (39.7 %) | 44 (44.4 %) | 0.54 | |
| Neuropsychiatric Symptoms | ||||
| BDI-II scorec M (SD, range) | 6.63 (7.26, 0–37) | 9.79 (8.31, 0–37) | −0.45 (−0.71, 0.19) | 0.001 |
| Clinically-significant depressive Symptoms, n (%) | 8 (8.89 %) | 28 (17.7 %) | 0.45 (0.17, 1.09) | 0.063 |
| POMS Tension-Anxiety Subscale scorec,d, M (SD, range) | 6.83 (6.07, 0–27) | 8.65 (6.18, 0–30) | −0.33 (−0.59, 0.065) | 0.014 |
| High anxiety symptoms (POMS subscale score >14.5), n (%) | 9 (10.3 %) | 28 (17.7 %) | 0.54 (0.21, 1.25) | 0.14 |
| HIV disease characteristics | ||||
| AIDS diagnosis, n (%) | - | 104 (61.9 %) | ||
| Current CD4, median (IQR) | - | 528 [380, 727] | ||
| Nadir CD4, median (IQR) | - | 191 [58.0, 309] | ||
| On ART, n (%) | - | 125 (75.3 %) | ||
| % undetectable plasma HIV RNA viral load, on ART | - | 105 (88.2 %) | ||
| Neurocognition | ||||
| Global mean T-score at Visit 1, M (SD) | 49.6 (6.31) | 48.8 (6.60) | 0.12 (−0.14, 0.37) | 0.38 |
| Global mean T-score at Visit 2, M (SD) | 49.0 (6.64) | 48.9 (6.64) | −0.019 (−0.27, 0.24) | 0.88 |
| Neurocognitive impairment at Visit 1e n (%) | 19 (20.9 %) | 51 (30.3 %) | 0.61 (0.31, 1.14) | 0.1 |
| Neurocognitive impairment at Visit 2e, n (%) | 30 (33.0 %) | 48 (28.6 %) | 1.23 (0.68, 2.20) | 0.48 |
Note. PWH = people with HIV. PwoH = people without HIV. ART = Antiretroviral therapy. BDI-II = Beck Depression Inventory-II (score range: 0–63). POMS = Profile of Mood States (Tension-anxiety subscale score range: 0–36).
History of illicit substance use diagnosis available in 123 participants.
History of alcohol and marijuana use diagnosis available in 166 participants.
BDI and POMS means and SDs reflect raw scores. BDI-II scores >16 were considered clinically significant33.
Three HIV- participants were missing anxiety symptom (POMS) data.
Cognitive impairment was defined by an established global deficit cut-score of ≥0.531,32.
Effect size is Cohen’s d for continuous variables and odds ratio for binary variables.
Fig. 1.
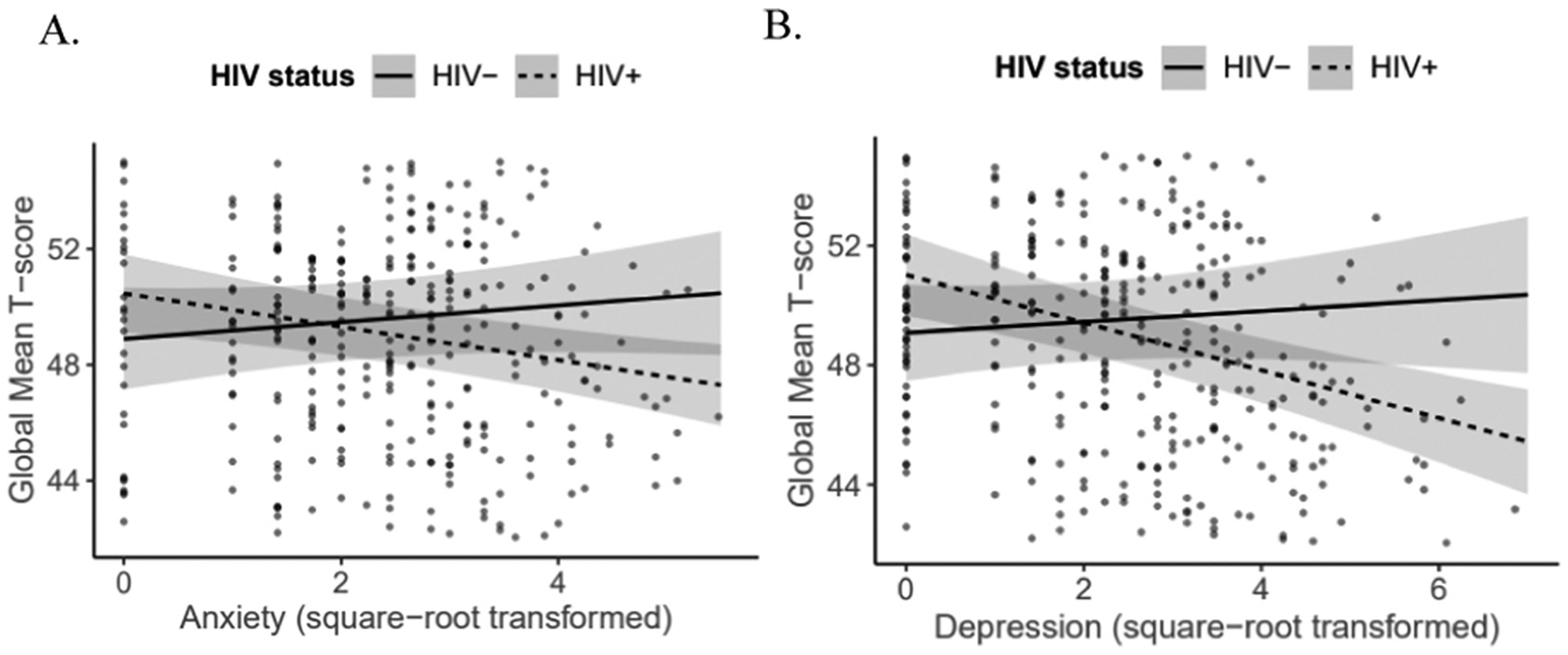
Neuropsychiatric symptoms at baseline in relation to global T-scores across visits 1 and 2 by HIV-serostatus for (A) anxiety and (B) depression symptoms.
When examining domain-specificity of findings, we found that both interactions were significant only in the domains of learning (depression: coefficient = −1.44, 95%CI = −2.61 to −0.26, p = .02; anxiety: coefficient = −2.05, 95%CI = −3.40 to −0.70, p = .003) and recall (depression: coefficient = −1.53, 95%CI = −2.79 to −0.26, p = .02; anxiety: coefficient = −1.77, 95%CI = −3.25 to −0.30, p = .02). Simple slopes revealed that greater depressive symptoms significantly related to poorer learning (coefficient = −1.17, 95%CI = −1.88 to −0.47, p = .001) and recall (coefficient = −1.34, 95%CI = −2.09 to −0.58, p = .001) among PWH but not among PWoH. Similarly, greater anxiety symptoms significantly related to poorer learning (coefficient = −0.96, 95%CI = −1.75 to −0.17, p = .017) and recall (coefficient = −1.22, 95% CI = −2.09 to −0.36, p = .001) among PWH but not among PWoH. Similar to results with global T-scores, time did not relate to learning or recall scores either independently or through interactions with NPS and/or HIV-serostatus, indicating no significant change in learning and recall scores over 1-year regardless of depressive/anxiety symptoms or HIV-serostatus.
Because anxiety and depressive symptoms are highly correlated (r = 0.68), we examined whether they independently relate to global T-scores in a multivariable model including both symptom types and adjusting for visit. This multivariable model indicated very weak collinearity (variance inflation factor < 1.3), and a significant HIV X depressive symptoms interaction (coefficient = −0.92, 95%CI = −1.60 to −0.23, p = .009). Simple slopes revealed that greater depressive symptoms significantly related to poorer global T-scores (coefficient = −0.63, 95%CI = −1.01 to −0.25, p = .001) among PWH but not among PWoH. Anxiety symptoms did not relate to global T-scores either independently or via interactions with HIV.
4. Discussion
We found that baseline depression and anxiety related to worse global cognition across visits among PWH, but not PWoH. This association was specific to learning and recall in domain-specific analyses. However, when depressive and anxiety symptoms were modelled together, anxiety no longer related to cognition among PWH, suggesting that the anxiety and cognition link may be driven by the depressive symptoms that often co-occur with anxiety.
Our results support prior findings of links between depressive and anxiety symptoms and poorer cognition among PWH (Brandt et al., 2017; Castellon et al., 1998; De Francesco et al., 2019; Muñoz-Moreno et al., 2021; Paul et al., 2005; Shapiro et al., 2013), and extend these findings by suggesting that these relationships may be greater in, and possibly specific to PWH, and that the anxiety links may be driven by depression. Our associations cannot establish causality and may be reflective of shared neuropathogenesis between depression/anxiety and cognitive dysfunction. PWH may be particularly susceptible to this shared neuropathogenesis, possibly due to contributions of HIV-related mechanisms. One potential pathway is the effect of chronic depressive and anxiety symptoms on hypothalmic-pituitary-adrenal (HPA) axis resulting in persistent elevated levels of glucocorticoids (GCs), the adrenal steroid hormone secreted during stress. Elevated GC levels have neurotoxic effects over time, particularly in the hippocampus where GC receptors are most dense (Sapolsky, 2000). HPA axis alterations can also affect cognition through the stimulation of proinflammatory cytokines by GCs (Silverman and Sternberg, 2012). GC dysregulation and chronic, low-grade inflammation are also characteristic of HIV (Nixon and Landay, 2010). Thus, HPA axis perturbations and inflammation are two related biological pathways by which depression/anxiety and HIV may have additive effects on cognition. Behavioral pathways may also contribute and interact with biological pathways in linking depression anxiety to cognition among PWH, given similarities in sociodemo-graphic and lifestyle factors that relate to depression/anxiety and HIV-serostatus (e.g., early life adversity, stigma).
Our study has limitations. Although our numbers of PWH and PWoH were quite large, it is possible that we were not adequately powered to detect the 3-way interaction of NPS X HIV serostatus X time. Thus, the lack of an influence of time on the NPS and cognition relationship in our results should be interpreted with caution, and studies with larger samples are needed to better test this effect. Additionally, there were fewer PWoH than PWH in our sample, creating a differential in statistical power by HIV-serostatus. Our follow-up was limited to one-year to maximize our sample size of participants with follow-up data. NPS were only measured at baseline although Paolillo et al. reported that cumulative burden of NPS is a stronger predictor of cognitive trajectories over time than symptoms at a single time-point (Paolillo et al., 2020).
Overall, our findings suggest that depressive symptoms may be particularly likely to associate with poorer cognition in PWH and help us to understand how mood and cognitive disorders cluster together. This understanding could inform more holistic treatment efforts to improve quality of life among PWH.
Acknowledgements
The San Diego HIV Neurobehavioral Research Program [HNRP] is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Gregory Brown, Ph.D.; Neurobiology Component: Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P. I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Scott Letendre, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Bin Tang, Ph.D., Anya Umlauf, M.S.
This work was supported by the National Institute of Health and the National Institute of Mental Health [P30MH062512, N01 MH22005, HHSN271201000036C and HHSN271201000030C, U24 MH100928]. This work was further supported by salary support from National Institute on Aging R01 AG062387 [R.C.M.].
Footnotes
CRediT authorship contribution statement
Erin Sundermann, Raeanne Moore and Michelle Kim conceptualized and developed the study. Bin Tang conducted all statistical analyses. Emily Paolillo and Robert Heaton assisted with the analytical plan and result interpretation. Erin Sundermann was primarily responsible for writing the manuscript; however, all authors contributed. All authors contributed to the editing of the manuscript.
Declaration of competing interest
Dr. Raeanne Moore is a co-founder of KeyWise AI, Inc. and a consultant for NeuroUX. The terms of these arrangements have been reviewed and approved by UC San Diego in accordance with its conflict of interest policies. The remaining authors have no conflicts of interest to declare.
References
- Beck AT, Steer RA, Brown GK, 1996. Manual for the Beck Depressed Mood Inventory-II. Psychological Corporation. [Google Scholar]
- Brandt C, Zvolensky MJ, Woods SP, Gonzalez A, Safren SA, O’Cleirigh CM, 2017. Anxiety symptoms and disorders among adults living with HIV and AIDS: a critical review and integrative synthesis of the empirical literature. Clin. Psychol. Rev 10.1016/j.cpr.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon SA, Hinkin CH, Wood S, Yarema KT, 1998. Apathy, depression, and cognitive performance in HIV-1 infection. J. Neuropsychiatry Clin. Neurosci 10 (3), 320–329. 10.1176/jnp.10.3.320. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Franklin D, Abramson I, Ellis RJ, Letendre S, Collier A, Clifford D, Gelman B, McArthur J, Morgello S, Simpson D, McCutchan JA, Grant I, Heaton RK, 2011. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J. Clin. Exp. Neuropsychol 10.1080/13803395.2010.535504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francesco D, Underwood J, Bagkeris E, Boffito M, Post FA, Mallon PWG, Vera JH, Williams I, Anderson J, Johnson M, Sabin CA, Winston A, Babalis D, Burgess L, Mallon P, Sachikonye M, Asboe D, Garvey L, Pozniak A, Clarke A, Bexley A, Richardson C, Kirk S, Gleig R, Bracchi M, Pagani N, Cerrone M, Bradshaw D, Ferretti F, Higgs C, Seah E, Fletcher S, Anthonipillai M, Moyes A, Deats K, Syed I, Matthews C, Fernando P, Chiwome C, Hardwick S, Mguni S, Clark R, Nevin-Dolan R, Pelluri S, Campbell L, Yurdakul S, Okumu S, Pollard L, Santana-Suarez B, Macken A, Ghavani-Kia B, Maher J, Byrne M, Flaherty A, Babu S, Otiko D, Phillips L, Laverick R, Beynon M, Salz AL, Severn A, Tembo L, Stott M, McDonald L, Dransfield F, Whitehouse A, Ngwu N, Hemat N, Jones M, Carroll A, Kinloch S, Youle M, Madge S, 2019. Depression, lifestyle factors and cognitive function in people living with HIV and comparable HIV-negative controls. HIV Med 10.1111/hiv.12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R, Woods SP, Marcotte TD, Ellis RJ, Grant I, 2012. Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Arch. Clin. Neuropsychol 10.1093/arclin/acs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF, 1981. Manual for the Profile of Mood States Educational and Industrial Testing Service, San Diego, CA. [Google Scholar]
- Milanini B, Catella S, Perkovich B, Esmaeili-Firidouni P, Wendelken L, Paul R, Greene M, Ketelle R, Valcour V, 2017. Psychiatric symptom burden in older people living with HIV with and without cognitive impairment: the UCSF HIV over 60 cohort study. In: AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV 10.1080/09540121.2017.1281877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Moreno JA, Cysique LA, Rourke SB, 2021. Neuropsychiatric Disorders, Emotional Disturbances, and Their Associations with HIV-Associated Neurocognitive Disorder. Curr. Top. Behav. Neurosci 50, 347–366. 10.1007/7854_2021_233. [DOI] [PubMed] [Google Scholar]
- Nixon DE, Landay AL, 2010. Biomarkers of immune dysfunction in HIV. Curr. Opin. HIV AIDS 10.1097/COH.0b013e32833ed6f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolillo EW, Pasipanodya EC, Moore RC, Pence BW, Atkinson JH, Grelotti DJ, Grant I, Heaton RK, Moore DJ, 2020. Cumulative burden of depression and neurocognitive decline among persons with HIV: a longitudinal study. J. Acquir. Immune Defic. Syndr 84, 304–312. 10.1097/QAI.0000000000002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Flanigan TP, Tashima K, Cohen R, Lawrence J, Alt E, Tate D, Ritchie C, Hinkin CJ, 2005. Apathy correlates with cognitive function but not CD4 status in patients with human immunodeficiency virus. Neuropsychiatry Clin. Neurosci 17 (1), 114–118. 10.1176/jnp.17.1.114. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, 2000. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Shapiro ME, Mahoney JR, Zingman BS, Pogge DL, Verghese J, 2013. Apathy correlates with cognitive performance, functional disability, and HIV RNA plasma levels in HIV-positive individuals. J. Clin. Exp. Neuropsychol 35 (9), 934–945. 10.1080/13803395.2013.838941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MN, Sternberg EM, 2012. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer SA, Dushaj A, Azar MM, 2012. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behav 10.1007/s10461-012-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate D, Paul RH, Flanigan TP, Tashima K, Nash J, Adair C, Boland R, Cohen RA, 2003. The impact of apathy and depression on quality of life in patients infected with HIV. AIDS Patient Care STDs 10.1089/108729103763807936. [DOI] [PubMed] [Google Scholar]


