Abstract
The Transforming Growth Factor beta (TGF-β) family consists of numerous secreted peptide growth factors that play significant roles in cell function, tissue patterning, and organismal homeostasis, including wound repair and immunity. Typically studied as homodimers, these ligands have the potential to diversify their functions through ligand interactions that are synergistic, cooperative, additive, and/or antagonistic. In the nematode Caenorhabditis elegans, there are only five TGF-β ligands, providing an opportunity to dissect ligand interactions in fewer combinations than in vertebrates. As in vertebrates, these ligands can be divided into bone morphogenetic protein (BMP) and TGF-β/Activin subfamilies that predominantly signal through discrete signaling pathways. The BMP subfamily ligand DBL-1 has been well studied for its role in the innate immune response in C. elegans. Here we show that all five TGF-β ligands play a role in the immune response. We also demonstrate that multiple TGF-β ligands act cooperatively as part of this response. We show that the two BMP-like ligands – DBL-1 and TIG-2 – function independently of each other in the immune response, while TIG-2/BMP and the TGF-β/Activin-like ligand TIG-3 function cooperatively. Structural modeling supports the potential for TIG-2 and TIG-3 to form heterodimers. Finally, we show that canonical DBL-1/BMP receptor and Smad signal transducers function in the response to bacterial pathogens, while components of the DAF-7 TGF-β/Activin signaling pathway do not play a role in survival. These results demonstrate a novel potential for BMP and TGF-β/Activin subfamily ligands to interact, and may provide a mechanism for distinguishing the developmental and homeostatic functions of these ligands from an acute response such as the innate immune response to bacterial pathogens.
Introduction
TGF-β signaling is a highly conserved mechanism that plays significant roles in development in organisms ranging from invertebrates to humans (Attisano and Wrana, 1996; Derynck et al., 1998; Massagué et al., 1998; Morita et al., 2002). This signaling family also plays an important role in the post-developmental adult in maintaining homeostasis and repair (Massagué et al., 1998; Blobe et al., 2000; Massagué, 2000; Massagué, 2012), as well as serving as one of the highly conserved signaling mechanisms involved in the immune response (Medzhitov and Janeway, 1998; Hoffman et al., 1999; Kim et al., 2002; Schulenburg et al., 2004; Millet and Ewbank, 2004; Irazoqui et al., 2008; Partridge et al., 2010). The canonical TGF-β family signaling cascade is initiated by binding of a dimerized ligand to a heterotetrametric receptor complex and mediated through activation of the Smad proteins to regulate transcription (Wrana et al., 1994; Massagué et al., 1998). The human genome contains 33 TGF-β ligands, seven type I receptors, and five type II receptors (Massagué et al., 1998; Huminiecki et al., 2009; Wrana, 2013; Aykul and Martinez-Hackert, 2016). Currently, active research is focused on the mechanisms that mediate specific or promiscuous interactions between ligands and receptors, and the consequences for Smad signaling. These mechanisms have profound implications for normal development and disease (Silberstein and Daniel, 1987; Akhurst and Derynck, 2001; Li et al., 2006; Massagué et al., 2008; Pohlers et al., 2009; Zamarron and Chen, 20011; Principe et al., 2014). The number of potential interactions, however, increases the difficulty of these analyses. The reduced repertoire of ligands and receptors in invertebrate organisms provides an opportunity to study ligand-receptor functional interactions more completely. Due to the conservation of TGF-β signaling pathways, studies in the genetically tractable organisms Drosophila and C. elegans have resulted in universal insights into conserved signaling mechanisms.
In C. elegans, there are five ligands associated with TGF- β signaling: DBL-1, DAF-7, TIG-2, TIG-3, and UNC-129. DAF-7 and TIG-3 are most appropriately classified as TGF-β/Activin-like ligands, whereas DBL-1 and TIG-2 share the most similarity with BMP ligands in mammals (Harpin, 2004; Patterson and Padgett, 2000). Of the five TGF-β ligands in C. elegans, only two have well-characterized signaling pathways through which they signal (Patterson and Padgett, 2000; MacNeill et al., 2009). The TGF-β-like ligand DAF-7 is the regulator of the dauer pathway and its expression in favorable conditions prevents entry into the dauer larval stage (Ren et al., 1996; Schackwitz et al., 1996; Inoue and Thomas, 2000). The BMP-like ligand DBL-1 activates the BMP-like pathway in the worm and plays a significant role in development (Suzuki et al., 1999; Yoshida et al., 2001; Wang et al., 2002). UNC-129, TIG-2, and TIG-3 have been classified (as BMP-like or TGF-β-like) based on their structural characteristics, but have not been fully associated with all members of either the BMP-like or TGF-β-like signaling pathways in C. elegans (Harpin, 2004; Patterson and Padgett, 2000).
Previous work from our lab and others has demonstrated that the BMP homolog DBL-1 functions in the innate immune response of C. elegans to pathogenic bacteria and fungi by inducing the expression of antimicrobial peptide genes in response to pathogen exposure. (Ciccarelli et al., 2023; Mallo et al., 2002; Zugasti and Ewbank, 2009; Zhang and Zhang, 2012). Here we demonstrate that the DBL-1 ligand is only one of four TGF-β family ligands with a significant and consistent role in the immune response to two gram-negative bacterial pathogens. We show that in response to either Serratia marcescens or Photorhabdus luminescens, multiple TGF-β family ligand mutants have a significant reduction in survival compared to control animals. We also demonstrate that two BMP-like ligands, DBL-1 and TIG-2, act independently of each other. In contrast, we have shown a non-additive relationship between TIG-2/BMP and the TGF-β-like ligand TIG-3, suggesting a cooperative role for these two distinct ligands in the immune response. Finally, we have identified signaling components with similar phenotypes to TIG-2 and TIG-3 in response to bacterial pathogen. Our studies thus uncover novel signaling paradigms that may act more broadly to distinguish distinct physiological outcomes regulated by TGF-β ligands.
Materials and Methods
Nematode Strains and Growth Conditions
C. elegans were maintained on E. coli (DA837) using EZ worm plates containing streptomycin. Worms were maintained at 20°C, except in experiments using dauer pathway mutants. These strains were maintained at 15°C to prevent entry into dauer. The N2 strain is used as a control. All strains used in this study are: N2, daf-1(m40), daf-7(m62), daf-8(e1398), daf-14(m77), sma-2(e502), sma-3(wk30), sma-4(jj278), sma-6(wk7), tig-2(ok3336), tig-2(ok3416), tig-3(tm2092), unc-129(ev554), daf-7(m62);dbl-1(wk70), tig-2(ok3416)dbl-1(wk70), tig-2(ok3416);unc-129(ev554), tig-3(tm2092);tig-2(ok3416), tig-3(tm2092);unc-129(ev554), tig-3(tm2092);tig-2(ok3416);unc-129(ev554), pdbl-1::GFP.
Bacteria
Control bacteria in all experiments is E. coli strain DA837, cultured at 37°C. S. marcescens strain Db11 (cultured at 37°C) and P. luminescens (cultured at 30°C) were used for bacterial pathogen in survival analyses. S. marcescens (Db11) is seeded on EZ worm plates containing streptomycin and grown overnight at 37°C. P. luminescens is seeded on EZ worm plates with no antibiotic and grown overnight at 30°C.
Survival Analysis
Survival plates were prepared at least one day prior to use. Each plate was seeded with 500μl pathogenic bacteria in a full lawn. FuDR, at a concentration of 50 μM per plate, was used to prevent reproduction and reduce the incidence of matricide during survival analysis. Survivals were conducted at 20°C, except survivals using dauer pathway mutants, which were conducted at 15°C to minimize dauer entry (unless otherwise stated). Survival analyses were repeated for all ligand experiments and DBL-1 pathway experiments. All graphs made using GraphPad Prism and statistical analysis performed using Logrank/Mantel Cox test.
Fluorescence Imaging
Fluorescence imaging of the dbl-1p::GFP transcriptional reporter strain was done using a Zeiss ApoTome with AxioVision software and a (20X) objective. Exposure times were kept consistent. Image analysis and fluorescence intensity measurements were done using ImageJ software. Fluorescence intensity was measured for ten nuclei in each of four worms per bacterial exposure condition.
qRT-PCR Analysis
qRT-PCR analysis was performed on collected samples of N2 control animals with or without 24-hour P. luminescens pathogen exposure. RNA was obtained by a previously described protocol (Yin et al., 2015) and followed by use of the Qiagen RNeasy miniprep kit (Cat. No. 74104). cDNA was made using SuperScript IV VILO Master Mix (Cat. No.11756050) from Invitrogen, Waltham, MA. qRT-PCR analysis was done using Power SYBR Green PCR Master Mix (Cat. No. 4367659) from Applied Biosystems, Waltham, MA. qRT-PCR was repeated on separate biological replicates. Delta delta Ct analysis was done using Applied Biosystems and StepOne software. Graphs made using GraphPad Prism software.
Structural Modeling
SignalP 6.0 was used to predict the signal sequence for TGF-β family members. No signal sequence for TIG-3 isoform A could be determined, and this potential isoform was not considered further. For TIG-3 isoform B, the prodomain and mature domain are defined using a consensus cleavage site (Newfeld and Wisotzkey, 2020). The TIG-2 cleavage site was determined by manual sequence analysis using the (R/K)-Xn-(R/K) motif as a reference where X is an amino acid sequence of length n such that n is even and 0 ≤ n ≤ 6 (Hinck et al., 2016). The existence of multiple functional cleavage sites is common (Hinck et al., 2016), and two such sites (RSRR and KVKR) were identified. Both cleavage sites are supported by structural modeling and preserve the cystine knot domain. The main distinction between the two sites is the length of the initial disordered region of the mature ligand (RSRR: 134-residue vs. KVKR: 116-residue mature form). Previously reported cleavage sites were used for the remaining TGF-β family members (Hinck et al., 2016). The full-length domain (Fig. 7C) is defined as the entire monomer sequence, excluding the signal sequence. Dimeric structures were modeled using the ColabFold (version 1.5.2) implementation of AlphaFold2-multimer (version 3). The AlphaFold2_mmseqs2 notebook was run under the default settings, and amber relaxation was applied to all five structural models. The model assigned rank one was selected for visualization and analysis. Interchain residue contacts (Fig. 6, Fig. 7C) within 4 Å and their predicted aligned error (PAE) value were identified using UCSF ChimeraX (version 1.5). One-dimensional amino acid sequence tracks (Fig. 6) include only dimer-interface residues that are within a distance of 4 Å and have a low positional error (PAE < 5 Å). Images of three-dimensional structures were rendered using PyMOL (version 2.5.4). pLDDT color coding and secondary structure annotation of one-dimensional amino acid sequences were generated using iCn3D (version 3.24.2) and reformatted. The alpha helices used in secondary structure labeling are modified vectors from BioRender.com.
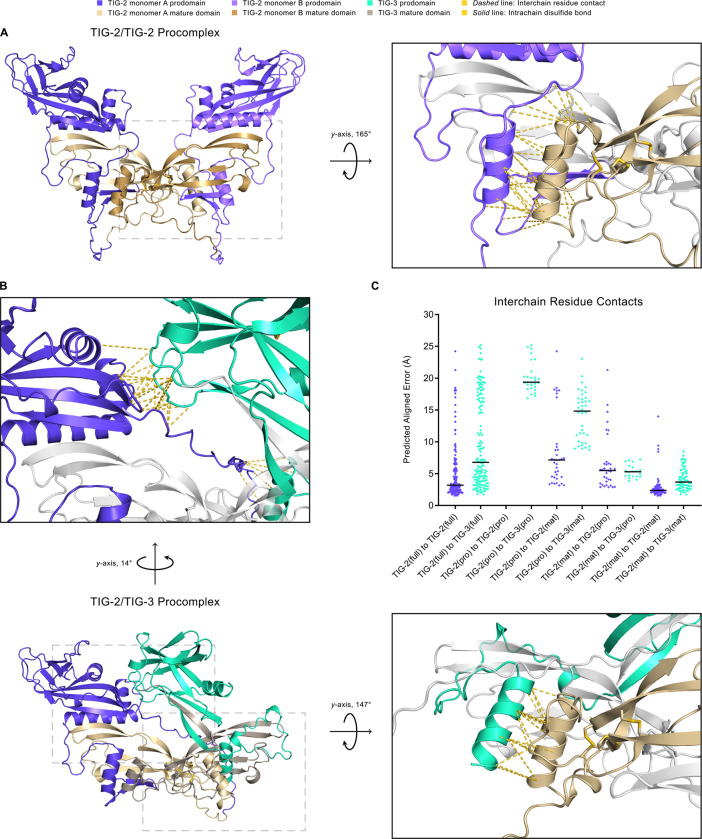
Figure 7. Structural Modeling of TIG-2/TIG-2 and TIG-2/TIG-3 Procomplexes Using AlphaFold2.
A. The TIG-2/TIG-2 procomplex exhibits a symmetric open-arm conformation with monomer A in blue-violet (prodomain) and khaki (mature domain) and monomer B in light blue-violet (prodomain) and dark khaki (mature domain). The right panel is the magnified and rotated area defined by the gray dashed box in the left panel. Interchain residue contacts between the mature domain (khaki) of pro-TIG-2 monomer A to the prodomain (light blue-violet) of proTIG-2 monomer B are shown as gold dashed lines. As is true for the mature form, the TIG-3 homodimer procomplex (not shown) has significantly lower multimer metrics (pLDDT: 66.5, pTM: 0.354, ipTM: 0.281) and thus a reduced predicted likelihood to homodimerize. B. The TIG-2/TIG-3 procomplex adopts an asymmetric conformation, with pro-TIG-2 forming an open-arm conformation and pro-TIG-3 (prodomain in green-cyan, mature domain in gray-beige) presenting a crossed-arm conformation. Pro-TIG-2 contains no prodomain cysteines and hence is not prodomain disulfide-linked to pro-TIG-3. The only two cysteines in the pro-TIG-3 prodomain are paired in an intra-prodomain 60–70 disulfide bond (solid gold line). The enlarged upper panel corresponds to the upper-left dashed box following rotation to emphasize the interchain residue contacts resulting from the prodomain of pro-TIG-3 (green-cyan) crossing over to interact with the prodomain of pro-TIG-2 (blue-violet). Similarly to A, the right panel corresponds to the lower-right gray dashed box and features interchain residue contacts between the mature domain of pro-TIG-2 (khaki) and the prodomain of pro-TIG-3 (green-cyan). C. A comparison of interchain residue contacts in TIG-2/TIG-2 (blue-violet diamonds) and TIG-2/TIG-3 (green-cyan diamonds) procomplexes between combinatorial interacting monomer regions (full: full-length, pro: prodomain, mat: mature domain) plotted with their predicted aligned error value and column mean (black horizontal line). For example, the column TIG-2(full) to TIG-3(full) contains all interchain residue contacts between pro-TIG-2 and pro-TIG-3. Correspondingly, the column TIG-2(mat) to TIG-3(pro) includes all interchain residue contacts between the mature domain of pro-TIG-2 to the prodomain of pro-TIG-3. The TIG-2/TIG-3 procomplex compares similarly to the TIG-2/TIG-2 procomplex with respect to full-length to full-length (columns 1 and 2), mature domain to prodomain (columns 7 and 8), and mature domain to mature domain contacts (columns 9 and 10).
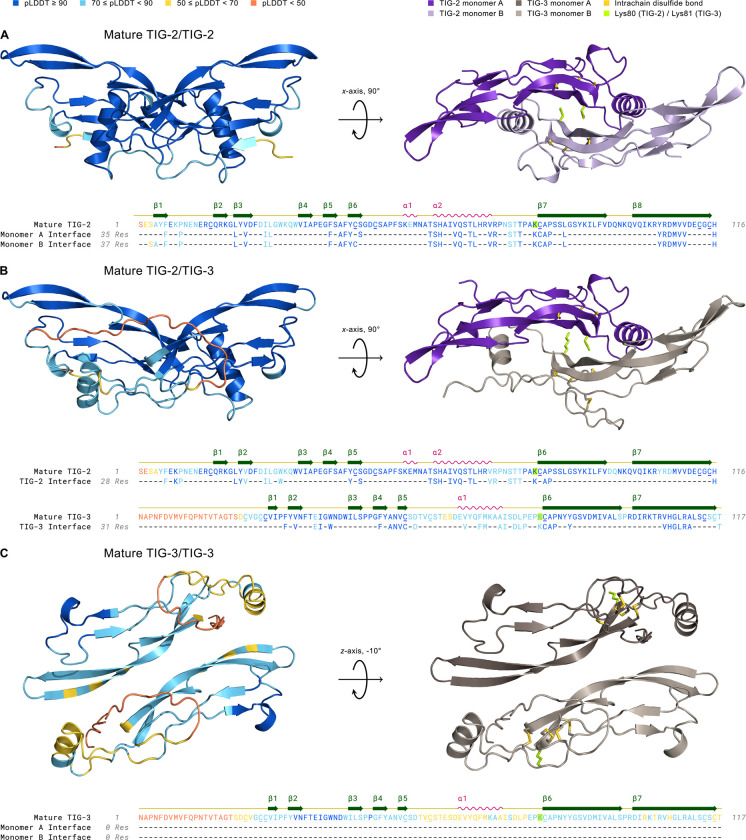
Figure 6. Structural Modeling of TIG-2 and TIG-3 Mature Ligand Homo- and Heterodimer Complexes Using AlphaFold2.
Left complex: 3D structures are colored by pLDDT confidence score with deep blue corresponding to regions of very high confidence and orange representing regions of very low confidence or disorder. Right complex: The mature dimer as in the left panel, but colored by monomer and following geometric rotation. All cysteine residues (underlined) in mature TIG-2 (with 15–81, 44–113, 48–115 disulfides) and TIG-3 (with 22–26, 25–82, 54–114, 59–116 disulfides) homo- and heterodimers are paired in intrachain disulfide bonds (gold) within the cystine knot domain. The cysteine residue that forms the interchain disulfide bond in the mature dimer is absent in TIG-2 and TIG-3, similar to GDF-3, GDF-9, and BMP-15 (Hinck et al., 2016). Interestingly, lysine (yellow-green), with its reactive ε-amino group upon deprotonation, is instead substituted for this cysteine residue in both TIG-2 (Lys80) and TIG-3 (Lys81). Amino Acid Sequence: Residues are colored by pLDDT score. The monomer interface tracks consist of confidently-predicted dimer-interface contacts (see Methods). A. Mature TIG-2(purple)/TIG-2(lavender) exhibits high confidence with respect to per-residue structural modeling (pLDDT: 92.7), pairwise residue alignment confidence (pTM: 0.868), and dimeric interaction confidence (ipTM: 0.875). B. Mature TIG-2(purple)/TIG-3(gray-beige) is confidently modeled regarding its structure and potential for dimerization (pLDDT: 84.9, pTM: 0.798, ipTM: 0.783). C. Mature TIG-3(brown)/TIG-3(gray-beige) falls slightly below (pLDDT: 68.8) the threshold for a confident structure prediction (pLDDT ≥ 70), and its predicted ability to homodimerize is very low (ipTM: 0.288). Additionally, no confidently-predicted (pTM: 0.522) interface residues exist between TIG-3 monomers.
Results
The BMP-like Ligands DBL-1 and TIG-2 are Independently Required for C. elegans Survival on Bacterial Pathogen
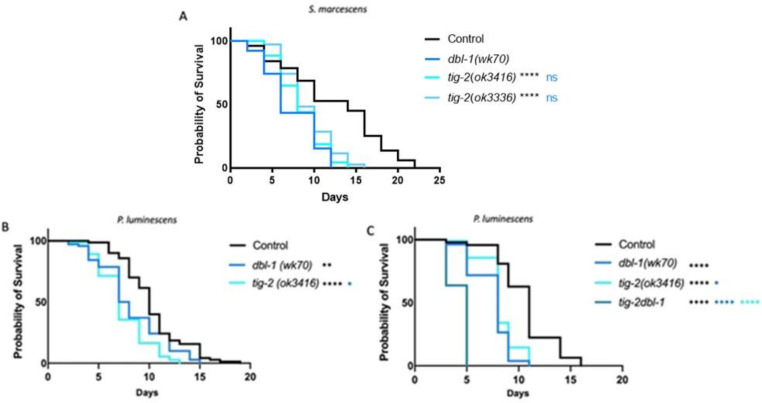
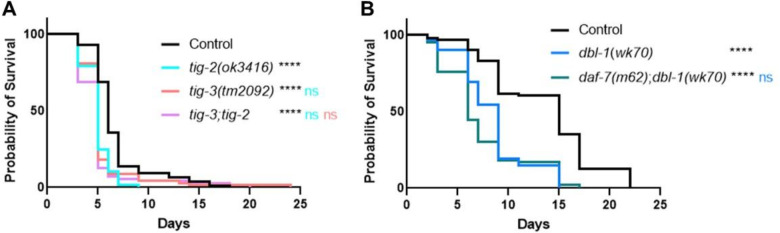
C. elegans has five TGF-β like ligands: DBL-1, DAF-7, UNC-129, TIG-2, and TIG-3. It is established that loss of DBL-1, one of two BMP-like ligands in C. elegans, results in reduced survival of animals on bacterial pathogen (Ciccarelli et al., 2023; Mallo, 2002). In a variety of physiological contexts, heterodimers of BMP ligands are functionally required or outperform homodimers (Tillet et al., 2018; Little and Mullins, 2009; Kim et al., 2019; Shimmi et al., 2005). We therefore considered the possibility that DBL-1/BMP functions with the other BMP-like ligand in the worm, TIG-2. The DBL-1 pathway has been thoroughly studied in response to exposure to the gram-negative bacterium Serratia marcescens, where the worm’s survival is significantly hindered by gut colonization as a consequence of the pathogen supplanting the normal bacterial food source (Mallo, 2002). To test the role of TIG-2/BMP, we quantified survival of two tig-2 mutant strains on S. marcescens and compared these survival rates to dbl-1 and N2 (wild type) control. We used tig-2(ok3336), a 500 bp deletion allele, and tig-2(ok3416), an 800 bp deletion allele, and found that both tig-2 mutant strains resulted in reduced survival against bacterial infection (Fig. 1A). Survival rates for both tig-2 mutant strains were significantly different compared to control. The larger deletion allele resulted in a survival defect similar to that of dbl-1 mutants and more pronounced than the survival defect caused by the 500 bp deletion. We further tested the effect of mutating tig-2 on immunity by analyzing the survival of tig-2 mutant animals on the more virulent P. luminescens bacteria. We found that tig-2 mutants survived significantly worse than control animals (Fig. 1B). Interestingly, we found that tig-2 mutants had an even more pronounced susceptibility to P. luminescens infection than dbl-1 mutants, suggesting a more significant role for TIG-2 in response to P. luminescens infection.
Figure 1. The BMP-like Ligands DBL-1 and TIG-2 are Required for C. elegans Survival on Bacterial Pathogen.
A. Survival analysis of dbl-1(wk70) and two tig-2 mutants (tig-2(ok3416) and (tig-2(ok3336)) on S. marcescens bacteria. n values: Control (51), dbl-1 (39), tig-2 (43), tig-2 (35). B. Survival analysis of dbl-1(wk70) and tig-2(ok3416) on P. luminescens bacteria. n values: Control (70), dbl-1 (70), tig-2 (73). C. Survival analysis of tig-2dbl-1 double mutant on P. luminescens. n values: Control (94), dbl-1 (53), tig-2 (97), tig-2dbl-1 (55). Statistical analysis done using Log-rank (Mantel-Cox) Test. ns p > 0.05; * p ≤ 0.05; ** p ≤ 0.01; **** p < 0.0001. Black asterisks denote significance relative to control, blue is significance relative to dbl-1, and teal is significance relative to tig-2.
Given that both BMP ligands function in the C. elegans immune response, we next considered whether they act independently or together by determining the phenotype of double mutants. If these ligands act independently, then we expect the double mutant to have a more severe phenotype than the single mutants, reflecting the disruption of two independent pathways. If they act together, such as in a heterodimer, then we expect the double mutant to have the same phenotype as the single mutants due to the failure of individual ligands to provide physiological function. When grown on P. luminescens, tig-2dbl-1 double mutant animals demonstrated a more pronounced reduction in survival compared to either the tig-2 or dbl-1 single mutant animals (Fig. 1C). Our survival analysis shows an additive effect of these two mutations combined. These results indicate that the two BMP-like ligands – DBL-1 and TIG-2 – act independently of each other in the response to P. luminescens.
At the time of these experiments, no phenotype had been reported for tig-2 mutants, so we were encouraged to have identified a biological function for this ligand. To rule out a general effect on lifespan, we followed our survival analysis with a lifespan on control E. coli bacteria. Both tig-2 mutants displayed a lifespan similar to control animals (Fig. S1). Animals mutant for dbl-1 also had an unaffected lifespan on control bacteria, as expected. These results indicate that the reduced survival of tig-2 on bacterial pathogen is specific to its susceptibility to infection and not reflective of a lifespan phenotype. Together these results demonstrate significant roles for the two BMP-like ligands – DBL-1 and TIG-2 – in the response to pathogenic bacterial infection, although independent of each other.
Five TGF-β Ligands Demonstrate Involvement in Survival Against Bacterial Infection
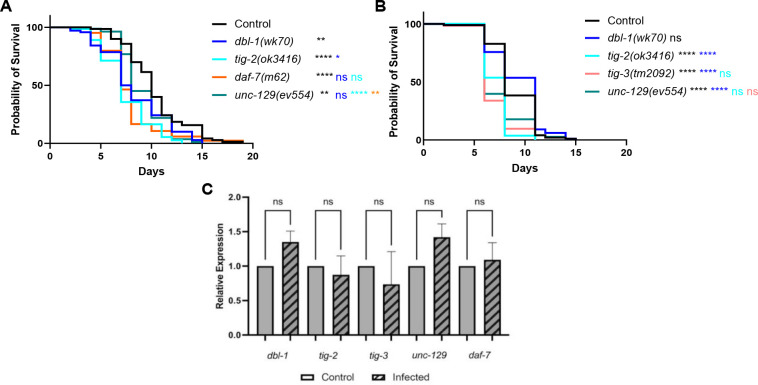
We next were interested in determining if the remaining TGF-β ligands – DAF-7, UNC-129, and TIG-3 – play a role in the C. elegans immune response. UNC-129 and TIG-3 are “orphan” ligands without known receptors and signaling components. DAF-7 is well characterized as the ligand in the dauer pathway, and daf-7 mutants demonstrate a high incidence of dauer when grown at 20°C. For this reason, the strains analyzed alongside daf-7 were all grown to the fourth larval stage (L4) at 15°C and shifted to 20°C when moved to pathogen plates.
We found that in mutants of any of the five TGF-β ligands, survival against pathogenic P. luminescens infection is significantly reduced, with variability between trials for some ligands (Fig. 2A and Fig. 2B). In particular, unc-129 mutants have variable survival outcomes. Surprisingly, dbl-1 mutant animals also have a variable survival pattern on P. luminescens, in spite of their well-characterized susceptibility to a variety of bacterial and fungal pathogens. In contrast, TIG-2, TIG-3, and DAF-7 show consistently significant susceptibility in response to P. luminescens, concordant with the hypothesis that distinct signaling mechanisms are responsible for the specific responses against different bacterial pathogens.
Figure 2. Five TGF-β Ligands Demonstrate Involvement in Survival Against Bacterial Infection.
A. Survival of TGF-β ligand mutants on P. luminescens bacteria. For this trial, strains were grown at 15°C to avoid dauer formation by daf-7 mutants and shifted to 20°C at L4 when exposed to pathogen. n values: Control (70), dbl-1 (70), tig-2 (73), daf-7 (84), unc-129 (82). B. Survival of TGF-β ligand mutants on P. luminescens bacteria. n values: Control (99), dbl-1 (99), tig-2 (108), tig-3 (62), unc-129 (73). C. qRT-PCR analysis showing relative expression of TGF-β ligand genes upon 24-hour exposure to P. luminescens in Control animals. qRT-PCR data represents repeated analyses of two biological replicates. Statistical analysis done using One-way ANOVA with multiple comparison test. For survivals, statistical analysis done using Log-rank (Mantel-Cox) Test. ns p > 0.05; * p ≤ 0.05; ** p ≤ 0.01; **** p < 0.0001. Black asterisks denote significance relative to control, blue is significance relative to dbl-1, teal is significance relative to tig-2, orange is significance relative to daf-7, and salmon is significance relative to tig-3.
To determine whether expression of any of these ligands is induced in response to pathogen, we used qRT-PCR to evaluate expression levels of genes encoding TGF-β ligands in wild-type animals. Upon 24-hour exposure to P. luminescens bacteria, the relative expression of all five TGF-β ligand genes demonstrated no significant alteration compared to control conditions (Fig. 2C). Additionally, analysis of a dbl-1 transcriptional reporter showed no change in fluorescence levels on pathogen compared to control bacteria (Fig. S2). We conclude that although these ligands function in response to pathogen, they are not subject to widespread transcriptional induction.
Multiple TGF-β Ligand Pairs Demonstrate Cooperative Interaction in the C. elegans Response to Bacterial Infection
We next looked for interactions between the remaining C. elegans TGF-β ligands upon exposure to bacterial infection by testing double mutants as previously described for tig-2dbl-1. We analyzed the tig-3;tig-2 double mutant, deficient in both the BMP-like ligand TIG-2 and the TGF-β/Activin-like ligand TIG-3, for survival on P. luminescens. We found that when grown on bacterial pathogen, the tig-3;tig-2 double mutants demonstrated a survival pattern not significantly different from either the tig-2 or the tig-3 single mutants (Fig. 3A). This result contrasts with our analysis of the BMP-like ligands DBL-1 and TIG-2, and suggests that TIG-2/BMP and TIG-3/(TGF-β/Activin) are acting in the immune response in a cooperative manner.
Figure 3. Multiple TGF-β Ligands Demonstrate Cooperative Interaction in the C. elegans Response to Bacterial Infection.
A. Survival analysis of tig-3(tm2092);tig-2(ok3416) double mutants on P. luminescens bacteria. n values: Control (73), tig-2 (68), tig-3 (67), tig-3;tig-2 (71). B. Survival analysis of daf-7(m62);dbl-1(wk70) double mutants on P. luminescens bacteria. n values: Control (94), dbl-1 (94), daf-7 (83). For this trial, strains were grown at 15°C to avoid dauer formation by daf-7 mutants and shifted to 20°C at L4 when exposed to pathogen. Statistical analysis done using Log-rank (Mantel-Cox) Test. ns p > 0.05; **** p < 0.0001. Black asterisks show significance relative to control, blue is significance relative to dbl-1, teal is significance relative to tig-2, and salmon is significance relative to tig-3.
We also assessed the survival pattern of daf-7;dbl-1 double mutants on pathogenic bacteria. For this experiment, animals were grown at 15°C to minimize dauer entry in daf-7 mutants. Survival of daf-7;dbl-1 double mutants was not significantly different compared to dbl-1 single mutants (Fig. 3B). The survival pattern of daf-7;dbl-1 double mutant animals indicates a potential relationship in which both ligands function in a single pathway. This cooperativity is intriguing as the BMP-like ligand DBL-1 is known to signal through a different Type I receptor and different Smads than the TGF-β/Activin-like ligand DAF-7.
Finally, we compared the survival pattern of double and triple mutants for unc-129. Although unc-129 mutants have weak and variable effects on survival, the unc-129 gene did show some potential interactions with other ligand genes. Survival of unc-129;tig-2 double mutants are not significantly different from tig-2 single mutants (Fig. S1). In contrast, in tig-3;unc-129 double mutants, the decreased survival of tig-3 mutants is suppressed (Fig. S2), suggesting a potential antagonistic interaction between UNC-129 and TIG-3. Overall, our results from these survival analyses indicate a complex relationship between the TGF-β family ligands in the immune response. Most surprisingly, ligands that demonstrate cooperative relationships are those from different classes of TGF-β ligands – BMP-like TIG-2 with TGF-β/Activin-like TIG-2; and possibly BMP-like DBL-1 with TGF-β/Activin-like DAF-7.
Canonical BMP Signaling Components are Involved in the C. elegans Immune Response
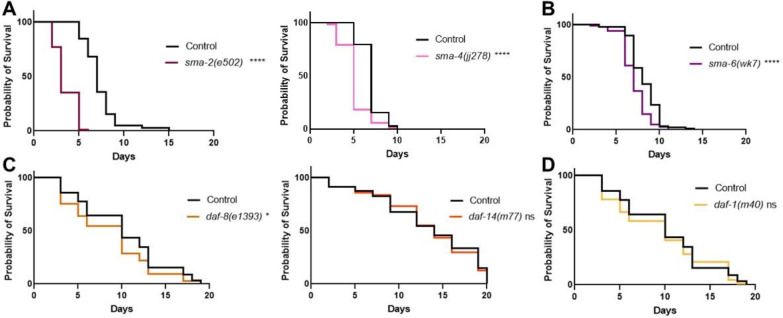
The two TGF-β pathways in C. elegans are known to signal through canonical mechanisms upon activation. DBL-1 signals through the BMP-like pathway with ligand binding to the single Type II receptor DAF-4 and the Type I receptor SMA-6. The BMP pathway Smads – SMA-2, SMA-3, and SMA-4 – transduce the intracellular signal allowing for gene regulation. The TGF-β/Activin-like ligand DAF-7 signals through the TGF-β/Activin-like pathway components, including the Type I receptor DAF-1 and the Smads DAF-8, DAF-14, and DAF-3 (Estevez et al., 1993; Savage et al., 1996; Suzuki et al., 1999; Krishna et al., 1999; Savage-Dunn and Padgett, 2017). Both pathways converge on the single Type II receptor DAF-4. Previous work has shown that in response to fungal infection of the hypodermis, DBL-1 signals in a pathway utilizing only one receptor-regulated Smad (R-Smad SMA-3) without its typical partner R-Smad SMA-2 or the Co-Smad SMA-4 (Zugasti and Ewbank, 2009). Signaling without a Co-Smad is considered non-canonical. We were therefore interested in which components of the BMP-like and TGF-β/Activin-like pathways are required during bacterial infection and whether non-canonical mechanisms are invoked.
Our survival analysis of DBL-1 pathway components shows a consistently reduced survival pattern for R-Smad mutants sma-2 and sma-3 as well as for Co-Smad mutant sma-4 (Fig. 4A). This result is consistent with a significant role for the BMP pathway Smads in the C. elegans response to bacterial pathogen, unlike the results previously shown on fungal infection. Survival analysis of BMP pathway receptors showed a reduced survival pattern for the BMP Type I receptor mutant sma-6 (Fig. 4B). We observe consistently reduced survival for sma-6 mutants, with slight variability in the significance of the survival rate across trials.
Figure 4. Canonical BMP Signaling Components are Involved in the C. elegans Immune Response.
A. Survival of DBL-1 R-Smad mutant sma-2(e502) and Co-Smad mutant sma-4(jj278) on P. luminescens bacteria. n values: Control (103), sma-2 (88), Control (78), sma-4 (72). B. Survival analysis of Type I receptor sma-6(wk7) on P. luminescens. n values: Control (88), sma-6 (100). C. Survival of DAF-7 pathway R-Smad mutants daf-8(e1393) and daf-14(m77) on P. luminescens bacteria. n values: Control (90), daf-8 (77), Control (81), daf-14 (78). D. Survival analysis of Type I receptor daf-1(m40) on P. luminescens. n values: Control (90), daf-1 (86). Statistical analysis done using Log-rank (Mantel-Cox) Test. ns p > 0.05; * p ≤ 0.05; **** p < 0.0001.
We analyzed the survival patterns of the DAF-7 pathway mutants grown at 15°C on P. luminescens bacteria and found either no phenotype or a mild survival deficit. In particular, R-Smad mutant daf-8 demonstrated a slightly reduced rate of survival (Fig. 4C), while neither R-Smad mutant daf-14 (Fig. 4C) nor Type I receptor daf-1(Fig. 4D) had significantly altered survival compared to control animals. Thus, although TGF-β/Activin-like ligands DAF-7 and TIG-3 play a role in the immune response against bacterial pathogen, they are unlikely to signal through the canonical TGF-β/Activin signaling components.
tig-2 and tig-3 Animals Share Reduced Survival and Pumping Rate Phenotypes with sma-3 Mutants
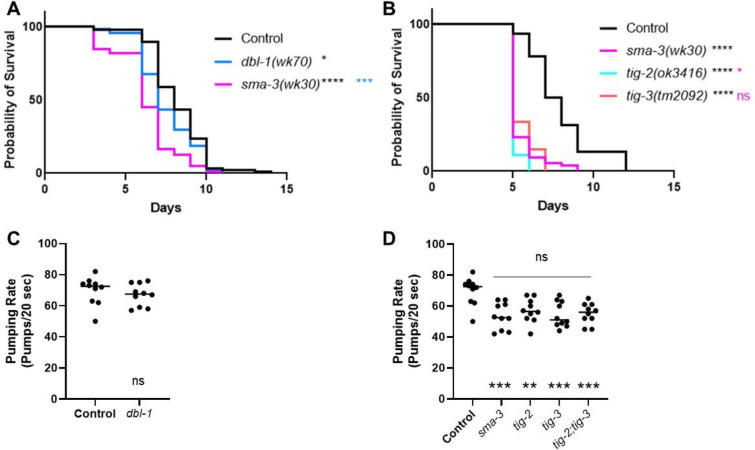
Thus far, we have shown that TIG-2 (BMP) and TIG-3 (TGF-β/Activin) play more significant roles in survival on P. luminescens bacteria than DBL-1/BMP, and that BMP signaling components play a more significant role than TGF-β/Activin signaling components. These observations suggest a model in which TIG-2 and TIG-3 signal through components previously presumed to respond to DBL-1. To test this model, we compared tig-2 and tig-3 phenotypes with those of sma-3 and dbl-1. Survival analysis directly comparing dbl-1 and sma-3 mutants on P. luminescens demonstrates that while both strains have a decreased survival pattern as compared to control, sma-3 mutants have a significantly reduced survival rate compared to dbl-1 mutants (Fig. 5A), suggesting that SMA-3 is responding to other signaling ligands instead of or in addition to DBL-1. Notably, tig-2 and tig-3 survival phenotypes are highly similar to those of sma-3 mutants (Fig. 5B), consistent with a model in which TIG-2 and TIG-3 signal through R-Smad SMA-3 in response to P. luminescens pathogen.
Figure 5. tig-2 and tig-3 Animals Share Reduced Survival and Pumping Rate Phenotypes with sma-3 Mutants.
A. Survival of dbl-1(wk70) and sma-3(wk30). n values: Control (88), dbl-1 (74), sma-3 (78). Black asterisks are compared to control. Blue asterisks are compared to dbl-1. B. Survival of sma-3(wk30) mutants compared to tig-2(ok3416) and tig-3(tm2092). Black asterisks are compared to control. Pink asterisks are compared to sma-3. n values: Control (92), sma-3 (74), tig-2 (55), tig-3 (54). Statistical analysis for all survivals done using Log-rank (Mantel-Cox) test. Survivals were repeated. C. Pumping rate per 20 seconds for dbl-1(wk70) compared to Control. Statistical analysis done using t test. D. Pumping rate per 20 seconds for sma-3(wk30), tig-2(ok3416), tig-3(tm2092), and tig-3;tig-2. Black asterisks are compared to Control. Pink asterisks are compared to sma-3. Statistical analysis done using One-way ANOVA with Multiple Comparison Test. n values for all pumping rate experiments: ten worms per strain. Pumping rate experiments were repeated on independent biological samples. ns p > 0.05; * p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001; **** p < 0.0001.
To test this model further, we analyzed an additional phenotype associated with response to P. luminescens, reduced pharyngeal pumping rate (Ciccarelli et al., 2023). Pharyngeal pumping rate analysis revealed sma-3, but not dbl-1, mutants have a significant reduction in pharyngeal pumping rate, and this reduction is indistinguishable from that of tig-2 and tig-3 mutants, as well as of the tig-3;tig-2 double mutant (Fig. 5C–D). This similarity further strengthens the model that TIG-2 and TIG-3 signal through SMA-3 in this context.
Structural Modeling of Potential Protein-Protein Interaction Between TIG-2/BMP and TIG-3/(TGF-β/Activin)
The cooperative action observed between TIG-2/BMP and TIG-3/(TGF-β/Activin) in the C. elegans immune response could be evidence of TIG-2/TIG-3 heterodimers. Alternatively, homodimers of TIG-2 and of TIG-3 could act together through a receptor clustering mechanism (Ramachandran et al., 2018). To determine whether heterodimers of TIG-2 and TIG-3 are feasible, we performed structural modeling in silico. Using the ColabFold (Mirdita et al., 2022) implementation of AlphaFold2 (Jumper et al., 2021), we asked whether confident structural predictions could be made for TIG-2 homodimers, TIG-3 homodimers, and TIG-2/TIG-3 heterodimers. We first analyzed interactions between mature bioactive domains, using consensus proteolytical cleavage sites (see Materials and Methods). Using the mature domain of TIG-2, we were able to generate a strongly supported model for the TIG-2 homodimer that recapitulates the well-known “butterfly” structure of TGF-β family ligand dimers (Fig. 6). In contrast, the potential for the TIG-3 mature bioactive domain to form homodimers was not supported by ColabFold, with a poor interface predicted template modeling (ipTM) score of 0.288. Using TIG-2 and TIG-3 for modeling, however, resulted in a heterodimer with a high ipTM score of 0.783, similar to that of the TIG-2 homodimer (0.875), as well as to those of known heterodimers (Fig. S6). Intriguingly, both TIG-2 and TIG-3 lack the conserved cysteine residue that mediates interchain disulfide bridges in most TGF-β family ligands (Fig. S4). Two other TGF-β family members that also lack these cysteines are BMP15 and GDF9 (Mottershead et al., 2015). These ligands form stable homo- and heterodimers without interchain disulfide bridges. In fact, GDF9/BMP15 heterodimers, known as cumulin, are capable of forming from homodimers by subunit exchange (Mottershead et al., 2015).
In vivo, prodomains present in the uncleaved precursors have been shown to be required for heterodimer formation between BMP4 and BMP7 (Neugebauer et al., 2015). We therefore performed structural modeling with full-length proteins (prodomain + mature domain). In these models, additional potential contacts between TIG-2 and TIG-3 are identified (Fig. 7). The TIG-2/TIG-2 procomplex resembles a BMP dimer with the prodomains remaining separate from each other, but making contacts with the mature domains (Fig. 7A, C). In the TIG-2/TIG-3 predicted procomplex, the TIG-3 prodomain makes contact with the TIG-2 prodomain and mature domain (Fig. 7B, C). Thus, structural modeling of the full-length proteins also supports the possibility of heterodimer formation consistent with our functional genetic analysis. This model can now be tested with biochemical analysis of protein-protein interactions.
Discussion
The multiplicity of TGF-β family ligands, receptors, and Smad signal transducers may support the generation of diverse context-dependent outcomes through combinatorial interactions. Biochemical and computational approaches are useful to identify the principles mediating the potential protein-protein interactions between these components, but these approaches must be complemented by functional in vivo analyses. The nematode C. elegans has five TGF-β ligands and two characterized TGF-β family signaling pathways (Patterson and Padgett, 2000; MacNeill et al., 2009), providing a powerful in vivo system in which to study TGF-β ligand functions in the context of the intact organism. Critically, TGF-β signaling mechanisms are conserved in this organism. Here we demonstrate that all five TGF-β ligands play a role in the immune response against bacterial pathogen. Furthermore, two ligand pairs consisting of a BMP subfamily and a TGF-β/Activin subfamily member (TIG-2/TIG-3 and DBL-1/DAF-7) show evidence of acting together in the response to gram-negative bacterium P. luminescens.
These studies were initiated with the well characterized BMP-like DBL-1 signaling pathway, which is associated with significant roles in development and body size regulation but has also been shown to play an important role in the immune response against both bacterial and fungal pathogens (Mallo, 2002; Tenor and Aballay, 2008; Zugasti and Ewbank, 2009). Comparing the response to the gram-negative bacteria S. marcescens and P. luminescens, we obtained evidence implicating a level of specificity of ligand responses for particular bacteria. Although both BMP-like ligands, DBL-1 and TIG-2, play a significant role in the response against S. marcescens, TIG-2 has a more significant role than DBL-1 in the response against P. luminescens. These results suggest that the function of DBL-1 in the immune response is not a general one-size-fits-all response to bacterial pathogen but rather a more nuanced response with some level of pathogen specificity. Furthermore, we show that DBL-1/BMP and TIG-2/BMP have additive roles in the response to P. luminescens, suggesting that they trigger independent responses. The orphan ligand UNC-129 shows moderate and variably significant changes in survival on P. luminescens, suggesting a minor or modulatory role. The TGF-β/Activin-like ligands DAF-7 and TIG-3 demonstrated consistently significant reduced survival patterns on P. luminescens suggesting involvement of these ligands in the response to this bacterial pathogen. qRT-PCR analysis of TGF-β ligand expression shows that with 24-hour exposure to bacterial infection, expression levels of the ligands do not significantly change from control conditions. A dbl-1p::GFP transcriptional reporter validates these results for dbl-1. Taken together, we conclude that all five of the TGF-β ligands play a role in the immune response, but not at the level of transcriptional induction.
Analysis of the survival patterns of double mutant animals produced results indicating interactions between multiple TGF-β family ligands. Interestingly, the interactions between pairs of BMP-like and TGF-β/Activin-like ligands suggest cooperative interaction in a common pathway. Specifically, the tig-3;tig-2 double mutant survival pattern is no more severe than either single mutant alone. Similarly, the daf-7;dbl-1 double mutant survival is indistinguishable from that of dbl-1 single mutants. Of these pairs, TIG-2 and TIG-3 play a more major role in the response to P. luminescens. We therefore used computational modeling to determine whether these ligands could feasibly function as a heterodimer. Using ColabFold, we demonstrated that a TIG-2/TIG-3 heterodimer is better supported than the TIG-3 homodimer. Furthermore, tig-2 and tig-3 expression overlaps in neurons such as AFD and M2. These neurons are located adjacent to the pharynx. We have shown that SMA-3/Smad is required in pharyngeal muscle for survival on bacterial pathogens (Ciccarelli et al., 2023), so TIG-2 and TIG-3 are produced in a location appropriate for signaling to SMA-3/Smad in the pharynx.
We also identify the canonical BMP-like pathway as required for response to P. luminescens, including the Type I receptor (SMA-6), the R-Smads (SMA-2 and SMA-3) and Co-Smad (SMA-4). In contrast, TGF-β/Activin pathway components have minimal effects. Unlike dbl-1 mutants, tig-2 and tig-3 mutants have very similar survival phenotypes to sma-3 mutants. Furthermore, tig-2, tig-3, and sma-3 mutants share a pharyngeal pumping defect that is not seen in dbl-1 mutants. These similarities provide evidence that TIG-2 and TIG-3 signal through the canonical BMP signaling pathway consisting of SMA-6 Type I receptor, and SMA-2, SMA-3, and SMA-4 Smads in the context of bacterial pathogen survival. There is precedence for TGF-β/Activin ligands signaling through BMP-like signaling pathways in vertebrates. For example, in endothelial cells, TGF-β can phosphorylate presumptive BMP Smads Smad1/5 through interaction with type I receptor ALK1 rather than ALK5 (Lebrin et al., 2005). Furthermore, the BMP receptor ALK2 (ACVR1) can be activated by Activin to phosphorylate Smad1/5, and this activity is increased by pathogenic FOP mutations (Ramachandran et al., 2021). However, cooperative action between a BMP and TGF-β/Activin ligand, including potential cross-subfamily heterodimers, has not yet been described in vertebrates to our knowledge, and warrants further consideration.
Interestingly, nonredundant roles for TIG-2, TIG-3, and UNC-129 have recently been identified in neuronal guidance in C. elegans (Baltaci et al., 2022). In neuronal guidance, TIG-3/UNC-129 heterodimers are implicated, with TIG-2 being released from a different tissue. In this context, the ligands depend on the BMP signaling components SMA-6 Type I Receptor and SMA-2, SMA-3, and SMA-4 Smads. Relatedly, in Drosophila, three Activin ligands are required nonredundantly to regulate photoreceptor identity through the canonical Activin signaling components (Wells et al., 2017). In addition to heterodimers, nonredundant functions of TGF-β ligands can be explained by a receptor clustering mechanism that generates higher-order signaling complexes (Ramachandran et al., 2018). Our system provides a platform for studying the functional interactions between multiple TGF-β ligands. Cooperativity between BMP and TGF-β/Activin subfamily members may provide a mechanism for distinguishing the developmental and homeostatic functions of these ligands from an acute response such as the innate immune response to bacterial pathogens.
Supplementary Material
Acknowledgements
This work was funded by R15GM112147 to CSD (National Institutes of Health/NIGMS). Some strains were provided by the Caenorhabditis Genetics Center, which is supported by the National Institute of Health – Office of Research Infrastructure Programs (P40 OD010440). We are grateful to the lab of Roger Pocock for sharing double and triple mutants of TGF-β ligand genes. We thank Jan Christian for helpful comments on the manuscript. This work was carried out in partial fulfillment of the requirements for the Ph.D. degree from the Graduate Center of City University of New York (EJC).
References
- Attisano L, Wrana JL. 1996. Signal transduction by members of the transforming growth factor-beta superfamily. Cytokine Growth Factor Rev 7:327–339. doi: 10.1016/s1359-6101(96)00042-1 [DOI] [PubMed] [Google Scholar]
- Aykul S, Martinez-Hackert E. 2016. Transforming Growth Factor-β Family Ligands Can Function as Antagonists by Competing for Type II Receptor Binding. J Biol Chem 291:10792–10804. doi: 10.1074/jbc.M115.713487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltaci O, Pedersen ME, Sherry T, Handley A, Snieckute G, Cao W, Haas M, Archer S, Pocock R. 2022. Atypical TGF-β signaling controls neuronal guidance in Caenorhabditis elegans. iScience 25:103791. doi: 10.1016/j.isci.2022.103791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. 2000. Role of transforming growth factor beta in human disease. N Engl J Med 342:1350–1358. doi: 10.1056/NEJM200005043421807 [DOI] [PubMed] [Google Scholar]
- Ciccarelli EJ, Bendelstein M, Savage-Dunn C. 2023. BMP signaling to pharyngeal muscle in the C. elegans immune response to bacterial pathogen regulates anti-microbial peptide expression and pharyngeal pumping. doi: 10.1101/2023.03.06.531324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A. 2001. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 29:117–129. doi: 10.1038/ng1001-117 [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang Y, Feng XH. 1998. Smads: transcriptional activators of TGF-beta responses. Cell 95:737–740. doi: 10.1016/s0092-8674(00)81696-7 [DOI] [PubMed] [Google Scholar]
- Estevez M, Attisano L, Wrana JL, Albert PS, Massagué J, Riddle DL. 1993. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature 365:644–649. doi: 10.1038/365644a0 [DOI] [PubMed] [Google Scholar]
- Gray AM, Mason AJ. 1990. Requirment for activin A and transforming growth factor-β1 pro-regions in homodimer assembly. Science. 247:1328–1330. doi: 10.1126/science.2315700 [DOI] [PubMed] [Google Scholar]
- Georgi LL, Albert PS, Riddle DL. 1990. Daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell 61:635–645. doi: 10.1016/0092-8674(90)90475-t [DOI] [PubMed] [Google Scholar]
- Herpin A, Lelong C, Favrel P. 2004. Transforming growth factor-beta-related proteins: an ancestral and widespread superfamily of cytokines in metazoans. Dev Comp Immunol 28:461–485. doi: 10.1016/j.dci.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Hinck AP, Mueller TD, Springer TA. 2016. Structural biology and evolution of the TGF-β family. Cold Spring Harb Perspect Biol. 8:a022103. doi: 10.1101/cshperspect.a022103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313–1318. doi: 10.1126/science.284.5418.1313 [DOI] [PubMed] [Google Scholar]
- Huminiecki L, Goldovsky L, Freilich S, Moustakas A, Ouzounis C, Heldin C-H. 2009. Emergence, development and diversification of the TGF-βsignalling pathway within the animal kingdom. BMC Evolutionary Biology 9:28. doi: 10.1186/1471-2148-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazoqui JE, Ng A, Xavier RJ, Ausubel FM. 2008. Role for beta-catenin and HOX transcription factors in Caenorhabditis elegans and mammalian host epithelial-pathogen interactions. Proc Natl Acad Sci U S A 105:17469–17474. doi: 10.1073/pnas.0809527105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. 2021. Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589. doi: 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan M-W, Ausubel FM. 2002. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297:623–626. doi: 10.1126/science.1073759 [DOI] [PubMed] [Google Scholar]
- Kim H-S, Neugebauer J, McKnite A, Tilak A, Christian JL. 2019. BMP7 functions predominantly as a heterodimer with BMP2 or BMP4 during mammalian embryogenesis. eLife 8:e48872. doi: 10.7554/eLife.48872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S, Maduzia LL, Padgett RW. 1999. Specificity of TGFbeta signaling is conferred by distinct type I receptors and their associated SMAD proteins in Caenorhabditis elegans. Development 126:251–260. doi: 10.1242/dev.126.2.251 [DOI] [PubMed] [Google Scholar]
- Lebrin F, Deckers M, Bertolino P, Ten Dijke P. 2005. TGF-beta receptor function in the edothelium. Cardiovasc Res 65:599–608. doi: 10.1016/j.cardiores.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Li M, Wan Y, Sanjabi S, Robertson A-K, Flavell R. 2006. Transforming growth factor-regulation of immune responses. Annual Review of Immunology 24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737 [DOI] [PubMed] [Google Scholar]
- Little SC, Mullins MC. 2009. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol 11:637–643. doi: 10.1038/ncb1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil LT, Hardy WR, Pawson T, Wrana JL, Culotti JG. 2009. UNC-129 regulates the balance between UNC-40 dependent and independent UNC-5 signaling pathways. Nat Neurosci 12:150–155. doi: 10.1038/nn.2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo GV, Kurz CL, Couillault C, Pujol N, Granjeaud S, Kohara Y, Ewbank JJ. 2002. Inducible antibacterial defense system in C. elegans. Curr Biol 12:1209–1214. doi: 10.1016/s0960-9822(02)00928-4 [DOI] [PubMed] [Google Scholar]
- Massagué J. 2012. TGFβ signalling in context. Nat Rev Mol Cell Biol 13:616–630. doi: 10.1038/nrm3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. 2008. TGFbeta in Cancer. Cell 134:215–230. doi: 10.1016/j.cell.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. 1998. TGF-beta signal transduction. Annu Rev Biochem 67:753–791. doi: 10.1146/annurev.biochem.67.1.753 [DOI] [PubMed] [Google Scholar]
- Massagué J, Chen YG. 2000. Controlling TGF-beta signaling. Genes Dev 14:627–644. [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA. 1998. Innate immune recognition and control of adaptive immune responses. Semin Immunol 10:351–353. doi: 10.1006/smim.1998.0136 [DOI] [PubMed] [Google Scholar]
- Millet ACM, Ewbank JJ. 2004. Immunity in Caenorhabditis elegans. Curr Opin Immunol 16:4–9. doi: 10.1016/j.coi.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. 2022. ColabFold: making protein folding accessible to all. Nat Methods 19:679–682. doi: 10.1038/s41592-022-01488-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Flemming AJ, Sugihara Y, Mochii M, Suzuki Y, Yoshida S, Wood WB, Kohara Y, Leroi AM, Ueno N. 2002. A Caenorhabditis elegans TGF-β, DBL-1, controls the expression of LON-1, a PR-related protein, that regulates polyploidization and body length. EMBO J 21:1063–1073. doi: 10.1093/emboj/21.5.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottershead DG, Sugimura S, Al-Musawi SL, Li JJ, Richani D, White MA, Martin GA, Trotta AP, Ritter LJ, Shi J, Mueller TD, Harrison CA, Gilchrist RB. 2015. Cumulin, an oocyte-secreted heterodimer of the transforming growth factor- β family, is a potent activator of granulosa cells and improves oocyte quality. J Biol Chem 290:24007–20. doi: 10.1074/jbc.M115.671487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer JM, Kwon S, Kim HS, Donley N, Tilak A, Sopory S, Christian JL. 2015. The prodomain of BMP4 is necessary and sufficient to generate stable BMP4/7 heterodimers with enhanced bioactivity in vivo. Proc Natl Acad Sci USA 112:E2307–16. doi: 10.1073/pnas.1501449112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge FA, Gravato-Nobre MJ, Hodgkin J. 2010. Signal transduction pathways that function in both development and innate immunity. Developmental Dynamics 239:1330–1336. doi: 10.1002/dvdy.22232 [DOI] [PubMed] [Google Scholar]
- Patterson GI, Padgett RW. 2000. TGF beta-related pathways. Roles in Caenorhabditis elegans development. Trends Genet 16:27–33. doi: 10.1016/s0168-9525(99)01916-2 [DOI] [PubMed] [Google Scholar]
- Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. 2013. Growth differentiation factor 9: bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc. Natl. Acad. Sci. U.S.A. 110:E777–E785. doi: 10.1073/pnas.1218020110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. 2009. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta 1792:746–756. doi: 10.1016/j.bbadis.2009.06.004 [DOI] [PubMed] [Google Scholar]
- Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, Pasche B, Lee C, Grippo PJ. 2014. TGF-β: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst 106:djt369. doi: 10.1093/jnci/djt369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Vizán P, Das D, Chakravarty P, Vogt J, Rogers KW, Müller P, Hinck AP, Sapkota GP, Hill CS. 2018. TGF-β uses a novel mode of receptor activation to phosphorylate SMAD1/5 and induce epithelial-to-mesenchymal transition. eLife 7:e31756. doi: 10.7554/eLife.31756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Mehić M, Wasim L, Malinova D, Gori I, Blaszczyk BK, Carvalho DM, Shore EM, Jones C, Hyvönen M, Tolar P, Hill CS. 2021. Pathogenic ACVR1R206H activation by activin A-induced receptor clustering and autophosphorylation. EMBO J 40:e106317. doi: 10.15252/embj.2020106317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C, Das P, Finelli AL, Townsend SR, Sun CY, Baird SE, Padgett RW. 1996. Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor beta pathway components. Proc Natl Acad Sci U S A 93:790–794. doi: 10.1073/pnas.93.2.790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage-Dunn C, Padgett RW. 2017. The TGF-β Family in Caenorhabditis elegans. Cold Spring Harb Perspect Biol 9:a022178. doi: 10.1101/cshperspect.a022178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenburg H, Kurz CL, Ewbank JJ. 2004. Evolution of the innate immune system: the worm perspective. Immunol Rev 198:36–58. doi: 10.1111/j.0105-2896.2004.0125.x [DOI] [PubMed] [Google Scholar]
- Shimmi O, Umulis D, Othmer H, O’Connor MB. 2005. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 120:873–886. doi: 10.1016/j.cell.2005.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. 1987. Reversible inhibition of mammary gland growth by transforming growth factor-beta. Science 237:291–293. doi: 10.1126/science.3474783 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yandell MD, Roy PJ, Krishna S, Savage-Dunn C, Ross RM, Padgett RW, Wood WB. 1999. A BMP homolog acts as a dose-dependent regulator of body size and male tail patterning in Caenorhabditis elegans. Development 126:241–250. doi: 10.1242/dev.126.2.241 [DOI] [PubMed] [Google Scholar]
- Tenor JL, Aballay A. 2008. A conserved Toll-like receptor is required for Caenorhabditis elegans innate immunity. EMBO Rep 9:103–109. doi: 10.1038/sj.embor.7401104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillet E, Ouarné M, Desroches-Castan A, Mallet C, Subileau M, Didier R, Lioutsko A, Belthier G, Feige J-J, Bailly S. 2018. A heterodimer formed by bone morphogenetic protein 9 (BMP9) and BMP10 provides most BMP biological activity in plasma. J Biol Chem 293:10963–10974. doi: 10.1074/jbc.RA118.002968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, Isaacs MJ, Kawakami Y, Izpisua Belmonte JC, Choe S. 2010. BMP2/6 heterodimer is more effective than BMP-2 or BMP-6 homodimers as inductor of differentiation of human embryonic stem cells. PLoS One. 5:e11167. doi: 10.1371/journal.pone.0011167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KL, Makanji Y, Wilce MC, Chan KL, Robertson DM, Harrison CA. 2009. A common biosynthetic pathway governs the dimerization and secretion of inhibin and related transforming growth factor b (TGFb) ligands. J Biol Chem 284: 9311–9320. doi: 10.1074/jbc.M808763200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tokarz R, Savage-Dunn C. 2002. The expression of TGFbeta signal transducers in the hypodermis regulates body size in C. elegans. Development 129:4989–4998. doi: 10.1242/dev.129.21.4989 [DOI] [PubMed] [Google Scholar]
- Wells BS, Pistillo D, Barnhart E, Desplan C. 2017. Parallel Activin and BMP signaling coordinates R7/R8 photoreceptor subtype pairing in the stochastic Drosophila retina. eLife 6:e25301. doi: 10.7554/eLife.25301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisotzkey RG, Newfeld SJ. 2020. TGF-β prodomain alignments reveal unexpected cysteine conservation consistent with phylogenetic predictions of cross-subfamily heterodimerization. Genetics 214:447–465. doi: 10.1534/genetics.119.302255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL. 2013. Signaling by the TGF-β Superfamily. Cold Spring Harb Perspect Biol 5:a011197. doi: 10.1101/cshperspect.a011197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. 1994. Mechanism of activation of the TGF-β receptor. Nature 370:341–347. doi: 10.1038/370341a0 [DOI] [PubMed] [Google Scholar]
- Yin J, Madaan U, Park A, Aftab N, Savage-Dunn C. 2015. Multiple cis elements and GATA factors regulate a cuticle collagen gene in Caenorhabditis elegans. Genesis 53:278–284. doi: 10.1002/dvg.22847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Morita K, Mochii M, Ueno N. 2001. Hypodermal expression of Caenorhabditis elegans TGF-beta type I receptor SMA-6 is essential for the growth and maintenance of body length. Dev Biol 240:32–45. doi: 10.1006/dbio.2001.0443 [DOI] [PubMed] [Google Scholar]
- Zamarron BF, Chen W. 2011. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 7:651–658. doi: 10.7150/ijbs.7.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Braun EL, Kohno S, Antenos M, Xu EY, Cook RW, Lin SJ, Moore BC, Guillette LJ Jr, Jardetzky TS, Woodruff TK. 2010. Phylogenomic analyses reveal the evolutionary origin of the inhibin α-subunit, a unique TGFβ superfamily antagonist. PLoS ONE 5: e9457. doi: 10.1371/journal.pone.0009457 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.