ABSTRACT
Fluoropyrimidines remain some of the most used chemotherapeutics, despite the appearance in the therapeutic arsenal of targeted therapy and immunotherapy. Fluropyrimidines related cardiotoxicity is an undesirable adverse event and affects almost 20% of patients. The mechanisms of fluoropyrimidine toxicity are closely related to deficient allelic variants of DPYD, but considering the low penetrance and interindividual variability, not all adverse reactions are explained by their presence. In this case, we report a patient with recurrent fluoropyrimidine toxicity without a deficient allelic variant and how this case was managed by the oncologist and cardiologist, considering the need to use fluoropyrimidine in the treatment.
Keywords: DPYD, colon cancer, fluoropyrimidines, cardiotoxicity, mutation testing
INTRODUCTION
Fluoropyrimidines remain one of the most used chemotherapy drugs worldwide due to their multiple indications. It is predicted that two million patients are treated yearly with fluoropyrimidine-based treatment [1]. The indications for these chemotherapy drugs are head and neck, breast, gastro-intestinal, and vulvar cancers [2]. Dihydropyrimidine dehydrogenase (DPD) deficiency is incriminated in adverse events following fluoropyrimidine chemotherapy. The fluoropyrimidines used in clinical practice are 5-fluorouracil (5-FU), Capecitabine, Tegafur and TAS-102 or S-1, the last two especially in Asia. 5-FU and Capecitabine cardiotoxicity is well known, and most often manifests as chest pain, but severe incidents such as sudden death have been reported [3]. Here we report a case of a patient who developed recurrent cardiac events after fluoropyrimidine treatment.
CASE REPORT
A 42-year-old Caucasian woman presented in our service in January 2016 with clinical and imaging suspicion of bowel obstruction. The surgery revealed the presence of a mass obstructing the intestinal lumen, for which a segmental sigmoid resection and a colostomy were performed. The histopathological and imaging examinations established the diagnosis of stage IIIB sigmoid colon adenocarcinoma (pT3N1M0V1R0) and the indication of adjuvant chemotherapy. The cardiac examination before the beginning of chemotherapy showed ECG and ultrasound parameters within normal limits and did not reveal any associated cardiovascular pathologies.
The patient began CAPOX chemotherapeutic protocol in February 2016, and the first cycle was without events. Still, during the first days of the second cycle, the patient presented intense chest pain associated with Capecitabine intake, so Capecitabine was discontinued. The oral treatment with Capecitabine was then replaced with 5-FU. We started therapy with a 25% dose reduction due to the poor tolerance of the treatment with Capecitabine, but during the continuous infusion of 5-FU, the patient presented chest pain with irradiation in the left upper limb up to the fingertips. We immediately stopped the infusion, performed an ECG that showed no ischemic changes, and the cardiac enzymes of myocardial injury (troponin and creatinine-kinase) were normal. Genetic testing was performed to determine the allelic variant DPYD*2A (IVS14+1G>A, c.1905+1G>A), but it was not detected in this patient. Subsequently, the patient returned for a new cycle of chemotherapy when she experienced high-intensity chest pain again. Cardiac enzymes and ECG were still normal; therefore, we requested a cardiological consultation. The ultrasound parameters were kept within normal limits at the cardiological examination, and the coronary angiography showed no pathological aspects.
The patient received seven cycles of low-dose chemotherapy due to cardiac symptoms associated with fluoropyrimidine treatment but without any cardiac morphological or physiological alteration at routine examinations. After one year of disease-free survival, in June 2018, a CT scan showed the presence of multiple liver metastases, and we needed to restart chemotherapy. Given the presence of the G12D mutation in codon 12 of the KRAS gene which was tested when the patient became metastatic, the patient's history as well as the response to the prior therapy, we restarted the treatment with a fluoropyrimidine-based protocol (FOLFOX 4), with 25% dose reductions because of the cardiac toxicity during the adjuvant setting, associated with VEGF inhibitor, Bevacizumab. Due to the previous toxicity, we tested the patient for other three frequent allelic variants of DPYD, DPYD*9B (c.2846A>T), DPYD*13 (c.1679T>G), and HapB3 (c.1129-5923C>G), but none of them were detected.
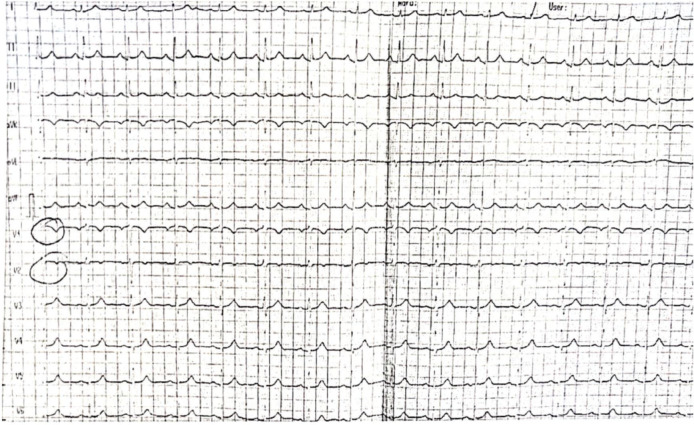
During the second cycle in the metastatic setting, shortly after initiating continuous infusion of 5-FU, the patient experienced mild chest pain lasting for several seconds. An ECG was performed but with no specific modifications (Figure 1). Subsequently, the treatment was resumed with the reappearance of chest pain but with increased intensity, constrictive character and irradiation at the left upper limb and mandible level. We administered Nitroglycerine with slight pain relief. An ECG showed negative T waves in the lower territory (Figures 2, 3 and 4), and troponin T showed an increase but within the normal range (4.31 ng/ml vs. < 0.04 ng/ml). Despite treatment with Nitroglycerine, the pain persisted, and an emergency coronary angiography was performed that showed no changes in the coronary arteries. Also, the left ventricle ejection fraction was within normal limits. The diagnosis of unstable angina pectoris was established, and treatment with Verapamil, Trimetazidine and Nitroglycerine, as needed, was recommended.
Fig. 1.
The first ECG during the second chemotherapy cycle.
Fig. 2.
The ECG during a crisis, after the treatment was resumed.
Fig. 3.
The persistence of ECG changes 1 hour after the onset symptoms, despite treatment with Nitroglycerine.
Fig. 4.
Maintenance of ECG changes 3 hours after the onset of symptoms.
Three weeks later, the patient returned to our hospital to continue treatment, and we decided to reduce the dose of 5-FU by another 25%. Under therapy with antianginal drugs and periodic cardiological examinations, the patient followed various chemotherapeutic protocols based on 5-FU with clinical response and local treatment, consisting of radiofrequency ablation of the liver metastases until October 2019. Afterwards, the patient stopped the treatment by choice, dying in February 2020 in a palliative care centre.
DISCUSSION
Fluoropyrimidines are antimetabolites used in many cancers, such as head and neck, oesophagal, gastric, pancreatic, colorectal, breast, and vulvar [2-4]. 5-FU and Capecitabine, the oral prodrug of 5-FU, are catabolized to inactive metabolites in the liver by DPD. Severe toxicity can occur in patients with low DPD activity. Fluoropyrimidines induce grade 3-4 toxicity in 10-30% of patients and life threatening toxicity in 0.3-2% of cases [4]. DPD deficiency is detected in almost 39-61% of cases with severe toxicity, which makes it a risk factor for fluoropyrimidine toxicity [5].
DPD activity shows significant inter-individual variations that are partly explained by the presence of multiple genetic variants of the DPYD gene, which encodes the DPD enzyme [5]. Up to now, more than 2000 known variants of the DPYD gene exist. The most studied deleterious DPYD variants associated with altered DPD activity and fluoropyrimidine-based chemotherapy are DPYD*2A, DPYD*9B, DPYD*13 and Haplotype B3 [6]. It is estimated that almost 3 to 15% of patients possess a partial deficiency and 0.1 to 5% a complete DPD deficiency [7].
Given the available data on the association between DPYD gene variants and fluoropyrimidine toxicity, several international guidelines recommend phenotypic and genotypic testing before starting fluoropyrimidine treatment and dose adjustment accordingly. From March 2020, the European Medicines Agency's Pharmacovigilance Risk Assessment Committee recommends testing all patients before undergoing fluoropyrimidine treatment. Furthermore, patients who have a complete DPD deficiency should not be treated with fluoropyrimidines. For those with a partial deficiency, it is necessary to reduce doses from the beginning of the treatment and adjust doses later, depending on the toxicities.
In our case, we tested the patient for the presence of the DPYD*2A variant in the adjuvant setting and the result was negative. Unfortunately, it is the only test available in Romania and is not reimbursed. Later, when we needed to decide the treatment for the metastatic disease, and considering the previous events, we tested, in a research project, the presence of the following three most frequent variants (DPYD*9B, DPYD*13, and Haplotype B3), but the presence of none of the above variants was identified. The fact that the genetic mutation was not detected was not of high significance, given the interindividual variability of the DPYD mutation and the high number of mutational variants.
Fluoropyrimidines are the second most cardiotoxic chemotherapy agents after anthracyclines and are estimated to affect up to 19% of patients [8]. Fluoropyrimidine-associated cardiotoxicity manifests through chest pain, acute coronary syndrome, coronary dissection, arrhythmias, QT prolongation, cardiogenic shock, myopericarditis, heart failure and sudden death [8,9]. Another manifestation of cardiotoxicity is the development of silent cardiac ischemia (≥1 mm negative deviation of ST segment). Numerous prospective and retrospective studies have reported ECG changes of silent ischemia ranging from 4% to 88% [10]. Several hypotheses have been formulated regarding the pathogenesis of fluoropyrimidine cardiotoxicities, such as coronary vasospasm, direct myocardial toxicity, metabolites accumulation leading to ischemia, vascular endothelial dysfunction, hypercoagulable status, erythrocyte membranes alterations leading to a diminished ability to deliver and release oxygen and an allergenic reaction with the release of an inflammatory mediator's storm that can break the atherosclerotic plague [8-11].
The most recognized mechanism of cardiotoxicity is coronary vasospasm which can lead to ischemic events. Patients may present manifestations of the acute coronary syndrome; sometimes, ECG can show ST segment and T wave changes [12]. Also, cardiac biomarkers such as troponin T can increase. Even in symptomatic patients with ECG changes, coronary angiography is usually normal, without signs of thrombotic events [12]. Studies have proven that high levels of protein kinase C and endothelin-1 can induce endothelium vasoconstriction in case of overexposure to 5-FU [13,14]. This evidence supports the theory of coronary vasospasm as a pathophysiological mechanism of fluoropyrimidine cardiotoxicity and, therefore, the benefit that antianginal therapy may have.
Classically, symptoms occur during the first two cycles of 5-FU infusion, and if treatment is continued during the following cycles, symptoms reappear and are more intense. As for capecitabine, although its intake is oral, its metabolism is similar to that of intravenous 5-FU. Therefore, the pattern of cardiotoxicity symptoms is like the infusion pattern [15]. Many studies displayed that continuous infusion of 5-FU compared with bolus intravenous administration is associated with an increased risk of cardiotoxicity [16].
In front of a patient with significant fluoropyrimidine cardiotoxicity, we have the possibility of rechallenging with the same drug, the use of an alternative fluoropyrimidine or non-fluoropyrimidine agents. Clasen et al., based on their case series, have made some recommendations regarding rechallenge with the same drug. They suggest the following: 1) switching from continuous infusion to bolus administration of the drug, 2) pre-treatment with extended-release calcium channel blockers and nitrates three to four hours before 5-FU infusion, 3) treatment during the infusion of 5-FU with short-acting calcium channel blockers and Nitroglycerine as needed, 4) posttreatment with extended-release calcium channel blockers and Nitrates 12 or 24 hours after the first dose of pre-treatment with the same drugs [15]. In cases of life-threatening adverse events, in 2015 FDA approved uridine triacetate (Vistogard) as an antidote for fluoropyrimidine toxicity [17].
Our patient presented several episodes of chest pain, starting right from the first cycle of treatment. After each such episode, we performed cardiac enzymes testing and ECGs that were normal. When we changed Capecitabine with 5-FU, we reduced with 25% the dose of 5-FU from the beginning and maintained the decreased dose. Later, when the disease recurred, out of the desire to give our patient the best treatment and considering that she responded well to the adjuvant regimens, we decided to rechallenge with fluoropyrimidine treatment with a further 25% reduction in the continuous infusion dose. During the second cycle of the metastatic setting, the chest pain reappeared, but it was more intense than before, and Nitroglycerine did not improve it.
Moreover, there were ischemic changes on the ECG, and the biochemical tests showed increased troponin levels, but without exceeding the upper limit of normal. The next step was to perform an emergency coronary angiography, which did not show any pathological alterations. Considering the symptomatology and evaluations, the diagnosis of unstable angina pectoris due to coronary vasospasm was established, and long-term treatment with Verapamil and Nitroglycerine as needed was recommended. Considering the cardiologist's advice and therapy schedule, we continued the fluoropyrimidine treatment of the patient, with the total dose reduced by 50%, without other acute episodes.
CONCLUSION
In conclusion, we present a complicated case of fluoropyrimidine cardiotoxicity with recurrent episodes of chest pain caused by coronary vasospasm, which led to cardiac ischemia certified by ECG changes and increased troponin levels. The management of such a patient, besides a specific cardiological treatment, requires a therapeutic decision regarding the continuation or discontinuation of the oncological treatment. Treatment individualization by detecting certain genetic mutations can prevent situations in which chemotherapy, even for curative purposes, can endanger the patient's life through the severe side effects it can cause. Even though we did not find an allelic variant of DPYD, we strongly recommend pre-therapeutic mutation testing or in case of severe or recurrent toxicity. We also emphasize the collaboration with the cardiologists with the help of their recommendations, the patient being able to continue the treatment, in doses adapted to the previous events.
Declaration of interest
The authors have no conflict of interests.
Informed consent
Informed consent was obtained from the patient next of kin for publication of this case report and accompanying images.
REFERENCE
- 1.Meulendijks D, Cats A, Beijnen JH, et al. Improving safety of fluoropyrimidine chemotherapy by individualizing treatment based on dihydropyrimidine dehydrogenase activity - Ready for clinical practice. Cancer Treat Rev. 2016;50:23–34. doi: 10.1016/j.ctrv.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Deac AL, Burz CC, Bocşe HF, et al. A review on the importance of genotyping and phenotyping in fluoropyrimidine treatment. Med Pharm Rep. 2020;93(3):223–230. doi: 10.15386/mpr-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan C, Parekh H, Allegra C, et al. 5-FU induced cardiotoxicity: case series and review of the literature. Cardio-Oncology. 2019;5:13. doi: 10.1186/s40959-019-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quaranta S, Thomas F. Pharmacogenetics of anti-cancer drugs: state of the art and implementation – recommendations of the French National Network of Pharmacogenetics. Therapie. 2017;72(2):205–215. doi: 10.1016/j.therap.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 5.van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer. 2004;40(7):939–950. doi: 10.1016/j.ejca.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Meulendijks D, Henricks LM, Sonke GS, et al. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16(16):1639–1650. doi: 10.1016/S1470-2045(15)00286-7. [DOI] [PubMed] [Google Scholar]
- 7.Boisdron-Celle M, Capitain O, Faroux R, et al. Prevention of 5-fluorouracil-induced early severe toxicity by pre-therapeutic dihydropyrimidine dehydrogenase deficiency screening: Assessment of a multiparametric approach. Semin Oncol. 2017;44(1):13–23. doi: 10.1053/j.seminoncol.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Kanduri J, More LA, Godishala A, et al. Fluoropyrimidine-Associated Cardiotoxicity. Cardiol Clin. 2019;37(4):399–405. doi: 10.1016/j.ccl.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Sara JD, Kaur J, Khodadadi R, et al. 5-fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol. 2018;10:1758835918780140. doi: 10.1177/1758835918780140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polk A, Vaage-Nilsen M, Vistisen K, et al. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev. 2013;39(8):974–984. doi: 10.1016/j.ctrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Karabay CY, Gecmen C, Aung SM, et al. Is 5-fluorouracil-induced vasospasm a Kounis syndrome? A diagnostic challenge. Perfusion. 2011;26(6):542–545. doi: 10.1177/0267659111410347. [DOI] [PubMed] [Google Scholar]
- 12.Shoemaker LK, Arora U, Rocha Lima CM. 5-fluorouracil-induced coronary vasospasm. Cancer Control. 2004;11(1):46–9. doi: 10.1177/107327480401100107. [DOI] [PubMed] [Google Scholar]
- 13.Mosseri M, Fingert HJ, Varticovski L, et al. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res. 1993;53(13):3028–3033. [PubMed] [Google Scholar]
- 14.Thyss A, Gaspard MH, Marsault R, et al. Very high endothelin plasma levels in patients with 5-FU cardiotoxicity. Ann Oncol. 1992;3(1):88. doi: 10.1093/oxfordjournals.annonc.a058084. [DOI] [PubMed] [Google Scholar]
- 15.Clasen SC, Ky B, O'Quinn R, et al. Fluoropyrimidine-induced cardiac toxicity: challenging the current paradigm. J Gastrointest Oncol. 2017;8(6):970–979. doi: 10.21037/jgo.2017.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosmas C, Kallistratos MS, Kopterides P, et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol. 2008;134(1):75–82. doi: 10.1007/s00432-007-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ison G, Beaver JA, McGuinn WD, Jr, et al. FDA Approval: Uridine Triacetate for the Treatment of Patients Following Fluorouracil or Capecitabine Overdose or Exhibiting Early-Onset Severe Toxicities Following Administration of These Drugs. Clin Cancer Res. 2016;22(18):4545–9. doi: 10.1158/1078-0432.CCR-16-0638. [DOI] [PubMed] [Google Scholar]