Abstract
Background
Despite scale-up of seasonal malaria chemoprevention (SMC) with sulfadoxine-pyrimethamine and amodiaquine (SP-AQ) in children 3–59 months of age in Burkina Faso, malaria incidence remains high, raising concerns regarding SMC effectiveness and selection of drug resistance. Using a case-control design, we determined associations between SMC drug levels, drug resistance markers, and presentation with malaria.
Methods
We enrolled 310 children presenting at health facilities in Bobo-Dioulasso. Cases were SMC-eligible children 6–59 months of age diagnosed with malaria. Two controls were enrolled per case: SMC-eligible children without malaria; and older (5–10 years old), SMC-ineligible children with malaria. We measured SP-AQ drug levels among SMC-eligible children and SP-AQ resistance markers among parasitemic children. Conditional logistic regression was used to compute odds ratios (ORs) comparing drug levels between cases and controls.
Results
Compared to SMC-eligible controls, children with malaria were less likely to have any detectable SP or AQ (OR, 0.33 [95% confidence interval, .16–.67]; P = .002) and have lower drug levels (P < .05). Prevalences of mutations mediating high-level SP resistance were rare (0%–1%) and similar between cases and SMC-ineligible controls (P > .05).
Conclusions
Incident malaria among SMC-eligible children was likely due to suboptimal levels of SP-AQ, resulting from missed cycles rather than increased antimalarial resistance to SP-AQ.
Keywords: amodiaquine, antimalarial resistance, malaria, seasonal malaria chemoprevention, sulfadoxine-pyrimethamine
In this case-control study assessing drug levels and resistance markers for seasonal malaria chemoprevention (SMC) with sulfadoxine-pyrimethamine-amodiaquine in children in Burkina Faso, suboptimal drug levels and not increased prevalence of resistance markers was associated with incident malaria in SMC-eligible children.
In the Sahel subregion of Africa, where malaria transmission is seasonal and resistance to sulfadoxine-pyrimethamine (SP) is relatively low, seasonal malaria chemoprevention (SMC) with monthly SP and amodiaquine (SP-AQ) is widely used to prevent malaria in children during the malaria transmission season [1]. Since its recommendation by the World Health Organization (WHO) in 2012, SMC has been scaled up in 13 countries across Africa and distributed to nearly 45 million children in 2021 [2]. In Burkina Faso, SMC is provided to children 3–59 months of age for 4–5 months during the malaria transmission season, with evidence of excellent adherence [3].
Despite the apparent success of SMC, which has demonstrated 88% protection against malaria incidence under programmatic conditions [4, 5], children <5 years of age continue to make up a substantial proportion of the malaria burden in Burkina Faso. In the 2021 Demographic Health Survey, 28% of children aged 6–59 months were parasitemic by rapid diagnostic test (RDT) [6]. Several reasons may explain this phenomenon. First, operational challenges may result in suboptimal intervention coverage or limited adherence to the 3-day regimen of SP-AQ [7]. Second, current dosing guidelines may not be optimal for chemoprevention in certain groups, such as malnourished children who are at risk of subprotective drug concentrations [8, 9]. Third, continued use of SMC may have increased the selection of parasites highly resistant to SP or AQ.
In most of eastern and southern Africa, the antimalarial efficacy of SP is limited by 5 common mutations in the Plasmodium falciparum target enzymes dihydrofolate reductase (PfDHFR) and dihydropteroate synthase (PfDHPS; PfDHFR N51I, C59R, and S108N; PfDHPS A437G and K540E) [10, 11], with the emergence of additional mutations, in particular PfDHFR I164L and PfDHPS A581G, mediating higher-level resistance in some regions [12–15]. In most of western Africa, where SMC is used, 4 of the 5 common mutations are seen at high prevalence, mediating a moderate level of resistance, but the additional resistance mediator PfDHPS K540E is very uncommon [16]. AQ resistance, most notably characterized by mutations in the P falciparum chloroquine resistance transporter (PfCRT K76T) and multidrug resistance 1 (PfMDR1 N86Y) proteins, has decreased across Africa in recent years [16, 17]. However, with continued SMC, selection of additional parasite mutations may lead to decreased preventive efficacy of SMC with SP-AQ.
Considering concerns regarding SMC drug exposure and selection of drug resistance, we conducted a case-control study that evaluated SP-AQ drug concentrations in children presenting with and without malaria and compared the prevalence of resistance markers in SMC-eligible children with malaria to that in older children ineligible for SMC in Burkina Faso.
METHODS
Study Setting
The study was conducted in Bobo-Dioulasso, Burkina Faso, between 16 August and 4 November 2021. Malaria transmission in this area is intense and highly seasonal, with the peak incidence from July to November. From 2015 to 2021, the national malaria control program provided SMC to children 3–59 months of age, delivered through door-to-door campaigns by community health workers, for 4 cycles, beginning each year in July. The first dose of AQ and the single dose of SP was supervised, while the second and third doses of AQ were provided to the parent/guardian with instructions on how to administer the drug at home.
Study Design
Following a case-control design, subjects were recruited from Colsama and Sakaby health facilities in Bobo-Dioulasso. Recruitment took place following the second through fourth cycles of SMC (5–8 August, 3–6 September, and 2–5 October).
Cases and Controls
Cases were defined as children aged 6–59 months presenting with fever or history of fever in the past 24 hours and diagnosed with uncomplicated P falciparum malaria by histidine-rich protein 2–based RDT (MALARIA Pf, Advy Chemical, Thane, India). For each case, 2 controls were enrolled from the same health facility within 0–2 days of case identification. The first set of controls included children aged 6–59 months presenting at the health facility with a nonmalarial diagnosis, with malaria ruled out by RDT (SMC-eligible controls). The second set of controls included children aged 5–10 years presenting with fever or history of fever in the past 24 hours and diagnosed with uncomplicated P falciparum malaria by RDT (SMC-ineligible controls).
For cases and controls, children were excluded if they (1) resided outside of the health facility catchment area; (2) received antimalarials other than for SMC in the past 14 days; (3) exhibited signs of severe malaria or a nonmalarial illness that would prevent necessary study procedures; or (4) were severely malnourished, defined as a mid-upper arm circumference (MUAC) <115 mm. To ensure uniform recruitment, an average of 8.5 (range, 5–16) cases were enrolled per week; 99% of controls were enrolled on the same day as cases (Supplementary Figure 1).
Ethical Approvals
The study was approved by the Burkina Faso Comité National d’Ethique pour la Recherche en Santé, the Comité d’Éthique Institutionnel de l’Institut de Recherche en Sciences de la Santé, and the University of California, San Francisco Committee on Human Research (ClinicalTrials.gov NCT04969185).
Study Procedures
After obtaining written informed consent, parents/guardians were asked about participant demographics, recent receipt of SMC, and bednet use. Anthropometric measures, including weight, MUAC, and height (recumbent length for children <2 years of age) were collected. Approximately 2 mL of venous blood was collected to measure hemoglobin (HemoCue, Brea, California), prepare thick and thin smears, and spot onto filter paper for subsequent molecular studies. The remaining blood was stored at room temperature for a maximum of 4 hours and centrifuged to separate plasma, which was stored at −80°C in an ethylenediaminetetraacetic acid–coated microtainer.
Assessment of Nutrition Status
Given that children with severe malnutrition are ineligible to receive SMC, we restricted our analyses to children with moderate malnutrition. A child was considered to be moderately malnourished if they had a MUAC of 115–125 mm or a height-for-age, weight-for-age, or weight-for-length/height z score 2–3 standard deviations below the mean according to WHO Child Growth Standards [18]. The z scores were calculated using the zscorer package in R [19]. Sensitivity analyses were conducted to assess whether associations differed between acute and chronic forms of moderate malnutrition, and no differences were found (data not presented).
Characterization of SMC Exposure
Receipt of SMC within the prior month was characterized through parent/guardian recall. Concentrations of sulfadoxine (SDX), pyrimethamine (PYR), amodiaquine (AQ), and N-desethylamodiaquine (DEAQ), the active metabolite of AQ, were quantified using ultra-high-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS), consisting of a Waters UPLC (I class) system and a Sciex Triple Quad 6500+ system. For SDX and PYR, 5 µL of plasma was mixed with 175 µL acetonitrile and centrifuged, supernatants were diluted 5-fold with water, and 3 µL was injected into the UPLC-MS/MS. For AQ and DEAQ, 10 µL of plasma underwent solid-phase extraction using a hydrophilic-lipophilic balanced microelution 96-well plate. After washing with 100 µL water and 200 µL methanol-water (1:9, v/v), samples were eluted twice with 25 µL acetonitrile-water (1:1, v/v) containing 0.5% formic acid, the eluent was mixed with 50 µL water, and 1 µL was injected into the UPLC-MS/MS. The calibration ranges were 1–200 µg/mL for SDX, 2–1000 ng/mL for PYR, 0.1–100 ng/mL for AQ, and 1–1000 ng/mL for DEAQ. The coefficients of variation of quality controls during sample analysis were <9% for SDX (n = 29), <12% for PYR (n = 29), <9% for AQ (n = 10), and <11% for DEAQ (n = 10). Drug levels below the lower limit of quantification were considered undetectable.
Characterization of Drug Resistance Markers
DNA was extracted from dried blood spots with Chelex-100 [20]. Sequences were characterized using molecular inversion probe (MIP) methods, using a previously described MIP panel targeting drug resistance loci [21], followed by next-generation sequencing as previously described [14]. Targeted loci included full sequences of the pfcrt, pfmdr1, pfdhfr, pfdhps, and pfK13 genes. MIPTools software (version 0.19.12.13) was used to organize raw sequencing data and to perform variant calling (https://github.com/bailey-lab/MIPTools). Individual genotypes were assigned for polymorphic sites that were covered by a minimum of 5 unique molecular identifiers (UMIs), and variants were required to have a genotype allele count ≥3 UMIs for alternate alleles and ≥2 UMIs for reference alleles. Sequencing reads are available in the National Center for Biotechnology Information under accession number PRJNA918715.
Statistical Analysis
Descriptive Statistics
These were summarized and compared using the Pearson χ2 test or Fisher exact test (when the frequency of any cell value was <5) for categorical variables and the Student t test or Mann–Whitney test for continuous variables, depending on the degree of normality of underlying distributions. Agreement between receipt of SMC and detectable drug levels was assessed using the κ coefficient.
Associations Between Drug Concentrations and Presentation With Malaria
Unadjusted and multivariable conditional logistic regression models were used to compute odds ratios (ORs) quantifying differences in the presence of and concentrations of drugs between cases and SMC-eligible controls. For models in which drug concentration was the primary exposure, drug levels were log transformed to normalize their right-skewed distribution, and undetectable values were substituted with the lowest calibration value. Both unadjusted and adjusted ORs were reported, with adjusted models including the following covariates: age, sex, weight-for-age z score, and maternal education. To test whether week after SMC administration or moderate malnutrition modified associations between drug concentrations and malaria presentation, 2-way interaction terms were included in adjusted models. Interaction terms with a P value <.10 were considered statistically significant.
Association Between SMC Exposure and Drug Resistance Markers
The χ2 or Fisher exact test was used to compare prevalences of markers between cases and controls and to determine whether the prevalence of markers among cases differed across the 4-month SMC campaign period. Alleles with a pure mutant or mixed genotype were considered mutant.
All analyses were performed using Stata 16.0 (StataCorp, College Station, Texas). P values <.05 were considered statistically significant unless otherwise stated.
RESULTS
Characterization of Cases and Controls
A total of 310 children were enrolled, including 104 cases (children 6–59 months of age diagnosed with malaria), 103 SMC-eligible controls (children 6–59 months of age without malaria), and 103 SMC-ineligible controls (children 5–10 years diagnosed with malaria) (Table 1). Compared to SMC-eligible controls, cases were slightly older (3.1 vs 2.8 years; P = .073), more likely to be febrile (100% vs 70%; P < .001), and less likely to report receiving SMC in the previous month (76% vs 91%; P = .003). Compared to SMC-ineligible controls, cases were more likely to report having slept under a bednet in the previous night (86% vs 76%; P = .067) and having received SMC in the previous month (76% vs 5%; P < .001). The prevalence of moderate malnutrition was similar between cases and SMC-eligible controls (21%) but lower in older, SMC-ineligible controls (12%; P = .065).
Table 1.
Characteristics of Study Population
| Characteristics | Eligible for SMC (Age 6–59 mo) | Age 5–10 y | |||
|---|---|---|---|---|---|
| Cases With Malaria (n = 104) |
SMC-Eligible Controls (n = 103) |
P Valuea | SMC-Ineligible Controls (n = 103) |
P Valueb | |
| Demographic characteristics | |||||
| Facility | .97 | .97 | |||
| Colsama | 30 (29%) | 30 (29%) | 30 (29%) | ||
| Sakaby | 74 (71%) | 73 (71%) | 73 (71%) | ||
| Age of child, y, mean (SD) | 3.1 (1.1) | 2.8 (1.0) | .073 | 7.63 (1.6) | <.001 |
| Female sex | 57 (55%) | 51 (50%) | .45 | 55 (43%) | .84 |
| Fever or history of fever within 24 h | 104 (100%) | 71 (70%) | <.001 | 103 (98%) | .57 |
| Nutritional indicators | |||||
| MUAC, mm, mean (SD) | 150 (11.5) | 148 (10.3) | .23 | 164 (13.8) | <.001 |
| WAZ, mean (SD) | −0.75 (0.98) | −0.70 (0.90) | .70 | −0.85 (0.87) | .42 |
| Malnourished | 22 (21%) | 22 (21%) | .97 | 12 (12%) | .065 |
| Malaria prevention measures | |||||
| Slept under bednet last night | 89 (86%) | 90 (89%) | .56 | 78 (76%) | .067 |
| Self-reported receiving recent SMC cycle | 75 (76%) | 93 (91%) | .003 | 5 (5%) | <.001 |
| Maternal characteristics | |||||
| Maternal age, y, mean (SD) | 29.3 (8.1) | 29.0 (8.3) | .79 | 33.1 (9.3) | .0017 |
| Highest education attained by mother | .036 | .14 | |||
| None | 54 (52%) | 38 (37%) | 47 (46%) | ||
| Literacy or Koranic school | 13 (13%) | 8 (8%) | 13 (13%) | ||
| Primary | 18 (17%) | 21 (21%) | 11 (11%) | ||
| Secondary or higher | 19 (18%) | 36 (35%) | 32 (31%) | ||
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: MUAC, mid-upper arm circumference; SD, standard deviation; SMC, seasonal malaria chemoprevention; WAZ, weight-for-age z score.
P value compares cases to controls without malaria.
P value compares cases to controls with malaria.
SMC Drug Concentrations
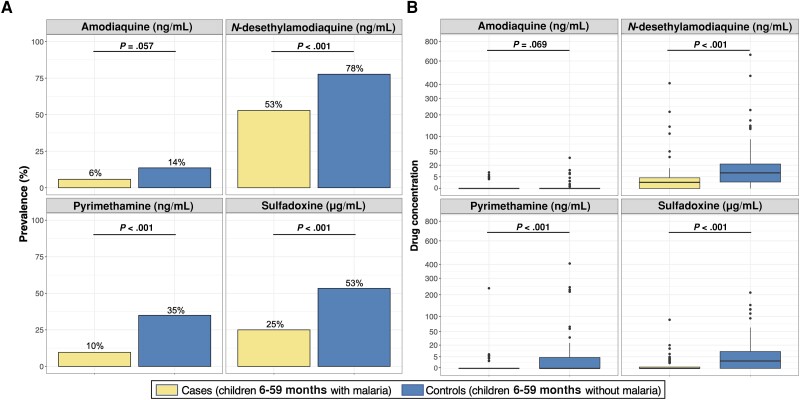
Circulating levels of SDX, PYR, AQ, and DEAQ were quantified for all children eligible for SMC (n = 207). Detectable levels of at least 1 SMC component were seen in 55% of cases compared to 79% of uninfected controls (Table 2). DEAQ was most commonly detected (53% in cases and 78% in controls) (Figure 1A). There was good agreement between detection of at least 1 SP-AQ component and reported receipt of SMC in the prior month (κ agreement = 68%; P = .005) (Supplementary Table 1).
Table 2.
Associations Between Seasonal Malaria Chemoprevention (SMC) Exposure and Malaria Diagnosis in Children Eligible for SMC
| SMC Exposure | No. (%) or Median (IQR) | Unadjusted | Adjusteda | |||
|---|---|---|---|---|---|---|
| Cases (n = 104) | Controls (n = 103) | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Guardian recall of child's receipt of SMC | ||||||
| Received the most recent cycleb | 75 (76%) | 93 (91%) | 0.22 (.08–.66) | .007 | 0.26 (.09–.82) | .021 |
| Children with detectable levels of drugs | ||||||
| Any drug | 57 (55%) | 81 (79%) | 0.35 (.19–.66) | .001 | 0.33 (.16–.67) | .002 |
| Amodiaquine | 6 (6%) | 14 (14%) | 0.38 (.14–1.08) | .069 | 0.51 (.17–1.59) | .25 |
| N-desethylamodiaquine | 55 (53%) | 80 (78%) | 0.34 (.18–.64) | .001 | 0.31 (.15–.64) | .002 |
| Pyrimethamine | 10 (10%) | 36 (35%) | 0.19 (.08–.45) | <.001 | 0.18 (.07–.47) | <.001 |
| Sulfadoxine | 23 (25%) | 55 (53%) | 0.29 (.15–.56) | <.001 | 0.30 (.15–.59) | <.001 |
| Drug concentrationc | ||||||
| Amodiaquine (ng/mL) | 0 (0–0) | 0 (0–0) | 0.85 (.64–1.12) | .24 | 0.89 (.67–1.21) | .48 |
| N-desethylamodiaquine (ng/mL) | 1.4 (0–4.2) | 9.0 (1.5–23.1) | 0.55 (.42–0.72) | <.001 | 0.55 (.42–.73) | <.001 |
| Pyrimethamine (ng/mL) | 0 (0–0) | 0 (0–4.6) | 0.43 (.26–.71) | .001 | 0.44 (.26–.74) | .002 |
| Sulfadoxine (μg/mL) | 0 (0–0.5) | 2.0 (0–11.3) | 0.52 (.38–.70) | <.001 | 0.51 (.37–.70) | <.001 |
Abbreviations: IQR, interquartile range; OR, odds ratio; SMC, seasonal malaria chemoprevention.
Adjusted for maternal education and child age, sex, and weight-for-age z score.
Missing values for 5 cases and 1 control.
For regression models, drug levels were log transformed, with values below the lower limit of quantification (LLOQ) replaced with the lowest calibration value of the assay. For median (IQR) calculation, values below the LLOQ were replaced with zero.
Figure 1.
Prevalence of detectable drug levels (A) and distribution of drug concentrations (B) in children presenting with and without malaria. A, P values comparing prevalence of children with detectable drug levels between cases and controls were computed using Pearson χ2 test. B, In the boxplots, the thick black horizontal line indicates median, the upper and lower bounds of the box indicate the 25th and 75th percentiles, the upper and lower bounds of the whiskers indicate the value 1.5 times the interquartile range (IQR), and black points indicate outliers. Median (IQR) of concentrations is provided in Table 2. Mann–Whitney tests were used to compute P values comparing differences in drug distributions between cases and controls.
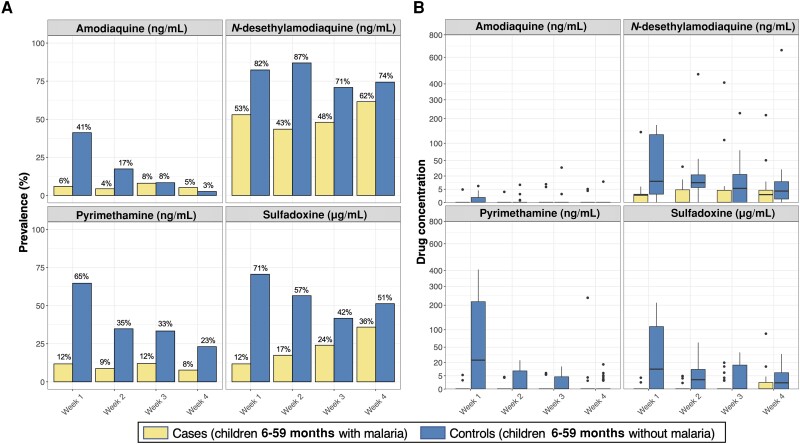
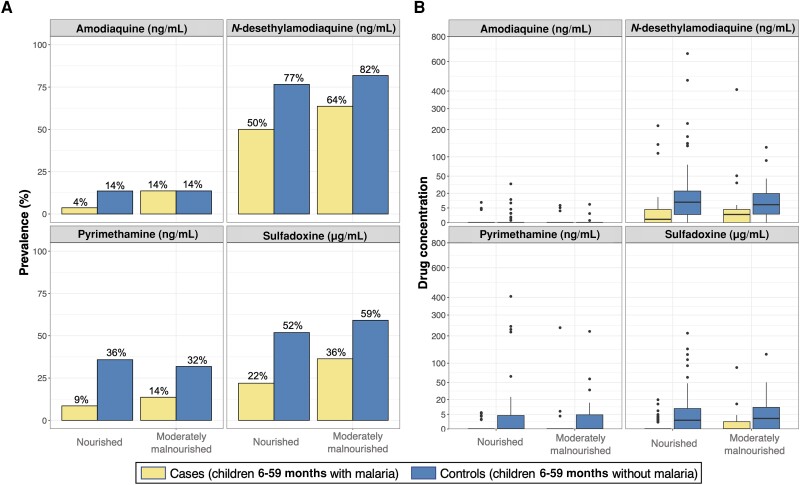
In the crude analysis, both reported receipt of SMC and detection of at least 1 SP-AQ component were associated with decreased odds of malaria (Table 2). These relationships were maintained after controlling for age, sex, weight-for-age, and maternal education (adjusted OR for reported receipt, 0.26 [95% confidence interval {CI}, .09–.82], P = .021; adjusted OR for detectable SP-AQ, 0.33 [95% CI, .16–.67], P = .002). Based on our adjusted analyses, cases were significantly less likely to have detectable DEAQ (OR, 0.31 [95% CI, .15–.64]; P = .002), PYR (OR, 0.18 [95% CI, .07–.47]; P < .001), or SDX (OR, 0.30 [95% CI, .15–.59]; P < .001) (Table 2). There was little evidence to suggest that these associations differed between weeks after SMC administration (P value for interaction term of any SP-AQ*week = .45) (Figure 2A) or between malnourished and well-nourished children (P value for interaction term of any SP-AQ*malnutrition = .66) (Figure 3A).
Figure 2.
Prevalence of detectable drug levels (A) and distribution of drug concentrations (B) in children presenting with and without malaria, stratified by time since the most recent seasonal malaria chemoprevention drug administration. A, Prevalence of cases and controls with detectable drug levels is provided above each barplot. B, In each boxplot, the thick black horizontal line indicates median, the upper and lower bounds of the box indicate the 25th and 75th percentiles, the upper and lower bounds of the whiskers indicate the value 1.5 times the interquartile range, and black points indicate outliers.
Figure 3.
Prevalence of detectable drug levels (A) and distribution of drug concentrations (B) in children presenting with and without malaria, stratified by malnutrition status. A, Prevalence of cases and controls with detectable drug levels is provided above each barplot. B, For each boxplot, the thick black horizontal line indicates median, the upper and lower bounds of the box indicate the 25th and 75th percentiles, the upper and lower bounds of the whiskers indicate the value 1.5 times the interquartile range, and black points indicate outliers.
Median plasma concentrations of DEAQ and SDX were markedly lower in cases than in SMC-eligible controls (Table 2; Figure 1B). These relationships were consistent across each of the 4 weeks after SMC drugs were administered (P value for concentration of SP-AQ components*week > .10) (Figure 2B) and between malnourished and well-nourished children (P value for concentration of SP-AQ components*malnutrition > .10) (Figure 3B). Restricting our analyses to children with detectable levels of SMC drugs, median (interquartile range [IQR]) concentrations of DEAQ, PYR, and SDX were approximately 2–3 fold lower in cases than controls (DEAQ, 3.7 ng/mL [1.8–7.2] vs 12.5 ng/mL [5.1–30.7]; PYR, 4.9 ng/mL [3.7–6.2] vs 8.6 ng/mL [4.2–23.7]; SDX, 2.9 μg/mL [1.3–4.7] vs 9.4 μg/mL [4.7–31.7]).
SP Levels Soon After Scheduled SMC
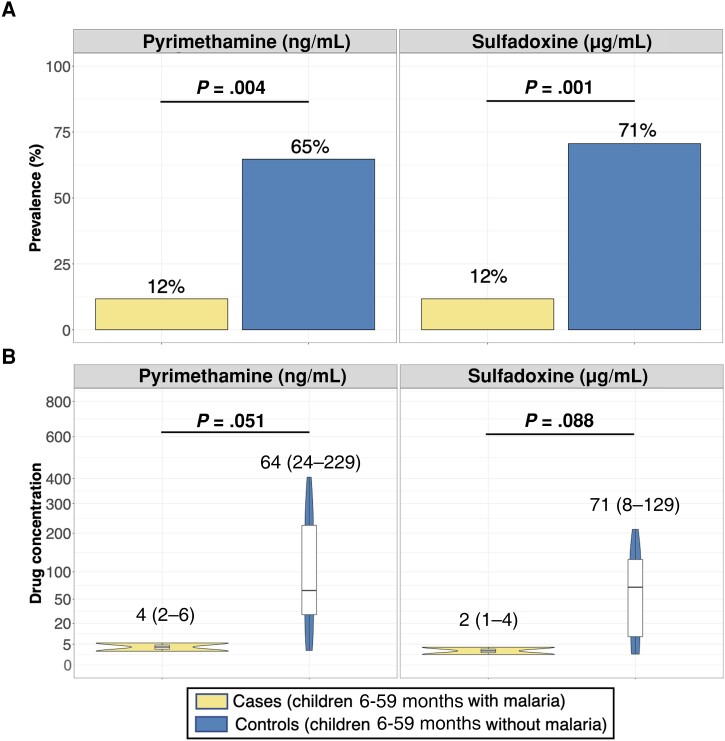
To further explore reasons for lower drug exposure among cases, we quantified SP levels among case-control combinations who presented in the first week after SMC (n = 34), when detection of these drugs would be most likely. During this period, 82% (14/17) of cases versus 29% (5/17) of controls had undetectable levels of either SDX or PYR, suggesting that the majority of these cases did not receive SMC during this cycle (Figure 4A). Only 3 cases (12%) had detectable levels of either drug during this period, all of whom had markedly lower concentrations of SDX (range, 1–4 μg/mL) and PYR (range, 2–6 ng/mL) compared to controls (SDX range, 8–129 μg/mL; PYR range, 24–229 ng/mL) (Figure 4B).
Figure 4.
Prevalence of detectable drug levels (A) and distribution of concentrations in those with detectable levels of sulfadoxine and pyrimethamine (B) in children presenting with and without malaria within the first week after scheduled seasonal malaria chemoprevention administration. A, Prevalence of cases and controls with detectable drug levels is provided above each barplot and reported P values were computed using Pearson χ2 test. B, Only children with detectable levels of sulfadoxine-pyrimethamine contributed to the data presented on the distributions of concentrations. B, For each boxplot, the thick black horizontal line indicates median, the upper and lower bounds of the box indicate the 25th and 75th percentiles, the upper and lower bounds of the whiskers indicate the value 1.5 times the interquartile range, and black points indicate outliers. Median (range) is provided in text above each boxplot. Mann–Whitney tests were used to compute P values comparing differences in drug distributions between cases and controls.
Prevalence of Drug Resistance–Mediating Mutations
We compared prevalences of key mutations between parasites isolated from cases (children 6–59 months years with malaria) and SMC-ineligible controls (children 5–10 years with malaria) (Table 3). The most important markers of aminoquinoline resistance were very uncommon (PfCRT K76T, 6% and PfMDR1 N86Y, 2%). The PfMDR1 Y184F mutation, which appears to play a role in parasite fitness but not amodiaquine resistance [22], was present at 66% prevalence. Prevalences of 4 of the 5 common mutations associated with resistance to SP (PfDHFR N51I, C59R, and S108N; PfDHPS A437G) ranged from 85% to 100%, with 78% of isolates harboring all 4 mutations. Median SDX and PYR concentrations were similar between those with and without these 4 mutations (P > .05). The PfDHPS A613S mutation, which mediates a higher level of resistance [23], was seen in 21% of samples, but prevalences of additional mutations predicting high-level resistance (PfDHFR I164L; PfDHPS K540E and A581G) were very low (0%–1%). We also assessed sequences encoding the P falciparum kelch (PfK13) protein, for which propeller domain mutations are primary mediators of artemisinin partial resistance [24]; no propeller domain mutations were identified. For all studied drug resistance markers, prevalences of mutations did not differ significantly between parasites isolated from cases and SMC-ineligible controls regardless of whether cases had detectable levels of SP-AQ (Table 3), arguing against marked selection of resistance by SMC exposure. Moreover, the prevalence of drug resistance markers did not differ between SMC cycles (P > .05).
Table 3.
Drug Resistance Markers in Parasites Isolated From Children Eligible (Cases) and Ineligible (Controls) for Seasonal Malaria Chemoprevention
| Mutation | Prevalence, no./No. (%) | P Valuea | P Valueb | |||
|---|---|---|---|---|---|---|
| Overall | All Cases (n = 103) |
Cases With Detectable SP-AQ Levels (n = 57) | SMC-Ineligible Controls (n = 103) |
|||
| PfDHPS | ||||||
| S436A | 81/119 (68%) | 43/66 (65%) | 20/31 (65%) | 38/53 (71%) | .45 | .33 |
| A437G | 105/119 (88%) | 59/66 (89%) | 29/31 (94%) | 46/53 (87%) | .66 | .28 |
| K540E | 0/137 (0%) | 0/72 (0%) | 0/32 (0%) | 0/65 (0%) | NA | NA |
| A581G | 2/147 (1%) | 1/74 (1%) | 0/33 (0%) | 1/73 (2%) | 1.00 | .69 |
| A613S | 28/138 (20%) | 16/69 (23%) | 11/32 (34%) | 12/69 (17%) | .40 | .053 |
| A613T | 0/138 (0%) | 0/69 (0%) | 0/32 (0%) | 0/69 (0%) | NA | NA |
| PfDHFR | ||||||
| N51I | 107/128 (84%) | 60/68 (88%) | 27/31 (87%) | 47/60 (78%) | .13 | .24 |
| C59R | 120/120 (100%) | 66/66 (100%) | 30/30 (100%) | 54/54 (100%) | NA | NA |
| S108N | 103/106 (97%) | 62/62 (100%) | 24/24 (100%) | 41/44 (93%) | .069 | .26 |
| I164L | 0/76 (0%) | 0/50 (0%) | 0/20 (100%) | 0/26 (0%) | NA | NA |
| PfCRT | ||||||
| K76T | 9/101 (9%) | 3/57 (5%) | 2/25 (8%) | 6/44 (13%) | .17 | .70 |
| PfMDR1 | ||||||
| N86Y | 3/142 (2%) | 1/70 (1%) | 0/31 (0%) | 2/72 (3%) | 1.00 | .49 |
| Y184F | 99/145 (68%) | 47/75 (63%) | 23/35 66%) | 52/70 (74%) | .13 | .24 |
| S1034C | 0/145 (0%) | 0/73 (0%) | 0/33 (0%) | 0/72 (0%) | NA | NA |
| N1042D | 0/145 (0%) | 0/73 (0%) | 0/33 (0%) | 0/72 (0%) | NA | NA |
| D1246Y | 0/94 (0%) | 0/58 (0%) | 0/24 (0%) | 0/36 (0%) | NA | NA |
Abbreviations: NA, Not applicable; PfCRT, Plasmodium falciparum chloroquine resistance transporter; PfDHFR, Plasmodium falciparum target enzymes dihydrofolate reductase; PfDHPS, Plasmodium falciparum dihydropteroate synthase; PfMDR1, Plasmodium falciparum multidrug resistance 1; SMC, seasonal malaria chemoprevention; SP-AQ, sulfadoxine-pyrimethamine and amodiaquine.
P value comparing frequency of drug resistance markers between all available SMC-eligible cases to SMC-ineligible controls was computed using Pearson χ2 test or Fisher exact test (if frequency of any cell value was <5).
P value comparing frequency of drug resistance markers between cases with any detectable levels of SP-AQ to SMC-ineligible controls was computed using Pearson χ2 test or Fisher exact test (if frequency of any cell value was <5).
DISCUSSION
We set out to determine if, in Burkina Faso, episodes of malaria despite SMC were associated with inadequate exposure to SMC drugs or the presence of key mediators of drug resistance. We compared SP-AQ plasma concentrations between SMC-eligible children with and without malaria and prevalence of SP-AQ resistance markers between parasitemic children eligible and ineligible for SMC. Children presenting with malaria were significantly more likely to have undetectable or markedly lower plasma concentrations of SP-AQ than children presenting with other medical problems. Genotyping revealed no differences in the prevalence of P falciparum resistance markers, which were similarly low in cases and controls, suggesting limited selection of high-level resistance by SMC. Taken together, our results suggest that the major factor driving breakthrough malaria infections was limited SMC exposure rather than drug resistance.
In our study, median levels of SDX, PYR, and DEAQ were significantly lower in cases compared to controls; 45% of cases had undetectable levels of SMC drugs at malaria diagnosis. There may be several reasons why drug levels were lower among cases (eg, suboptimal dosing, incomplete adherence, or missed SMC cycles) [25]. Based on a prior report suggesting high adherence to the 3-day regimen of SP-AQ in Burkina Faso [3] and our exploratory analyses which demonstrated that 82% of cases diagnosed with malaria in the week following scheduled SMC lacked any detectable SP drug, it is most likely that the majority of incident malaria cases missed the most recent cycle of SMC. Assuming all children with undetectable levels of SMC drugs missed their most recent SMC cycle, missing an SMC cycle would equate to a 3-fold (1/OR for detectable SP-AQ, 1/0.33) higher odds of malaria, a finding comparable to a previously reported result [7]. Thus, SMC programs should prioritize reaching high and consistent SMC coverage.
SMC efficacy is limited by drug resistance. With SP, PfDHPS 540E, a mutation that is highly prevalent in other parts of Africa, has remained uncommon in regions receiving SMC, facilitating continued good preventive efficacy. Consistent with other reports [16], we demonstrated little evidence of the emergence of additional mutations associated with high-level resistance to SP or AQ, and existing mutations were observed at similar prevalences in SMC-eligible and ineligible children. Importantly, no parasites from study subjects harbored the PfDHPS K540E mutation, which mediates decreased SP efficacy in other parts of Africa, and the PfDHFR I164L (0%) and PfDHPS A581G (1%) mutations were very uncommon. Notably, the PfDHPS A613S mutation was seen at higher prevalence (21%) than that previously seen in Burkina Faso and most other countries implementing SMC [16], but the role of this mutation in mediating SP resistance is uncertain [26, 27]. Considering AQ, the PfCRT K76T mutation (the primary mediator of resistance to chloroquine and amodiaquine) was also uncommon, with no difference in prevalence between isolates from cases and controls. In summary, despite its prolonged use in the sub-Sahel region of Africa, we found little evidence to suggest that SMC selected for parasites with decreased sensitivity to SP-AQ.
Prior reports have shown that malnutrition can substantially affect the pharmacology of antimalarials [8, 28–30], including SP-AQ [8]. However, in our study, we found that moderate malnutrition, which was common, had limited associations with SP-AQ concentrations or malaria incidence. This suggests that the current age-based dosing guidelines provided adequate drug exposures in moderately malnourished children [8]. However, further studies with larger sample sizes are needed to confirm our findings. Notably, we did not evaluate these associations in children with severe malnutrition, as these children were not eligible for SMC.
Our study had some limitations. First, we utilized a test-negative design to sample controls. While test-negative studies are efficient [31–33], a limitation is that the exposure distribution of controls may not represent the source population, which may have biased our effect estimates toward the null. Second, the validity of receipt of SMC was based on parent/guardian recall and may be prone to measurement error. Third, we did not have more detailed data on SMC utilization, including how many of the 3 doses were taken by each subject or whether children vomited any of the 3 doses. Such information would have provided more insight into associations between drug levels and incident malaria. Fourth, statistical power may have been limited for some secondary analyses, including association between receipt of SMC and selection of drug resistance markers and effect modification by malnutrition. Last, due to safety concerns, we did not enroll SMC-eligible children between 3 and 5 months of age, limiting generalizability to this age group.
Our study demonstrated that the most plausible reason for malaria episodes in children eligible for SMC in Burkina Faso was suboptimal concentrations of SMC drugs, likely due to missed SMC cycles, rather than increasing resistance to SP-AQ. In addition to limiting preventive efficacy, suboptimal concentrations of SMC drugs may facilitate selection of drug resistance. Thus, continued efforts to achieve high and consistent SMC coverage and to monitor drug resistance markers and parasite susceptibility to SMC components should remain important priorities.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Michelle E Roh, Institute for Global Health Sciences, Malaria Elimination Initiative, University of California, San Francisco.
Issaka Zongo, Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso.
Alassane Haro, Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso.
Liusheng Huang, Department of Clinical Pharmacy, University of California, San Francisco.
Anyirékun Fabrice Somé, Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso.
Rakiswendé Serge Yerbanga, Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso.
Melissa D Conrad, Department of Medicine, University of California, San Francisco.
Erika Wallender, Department of Clinical Pharmacy, University of California, San Francisco.
Jennifer Legac, Department of Medicine, University of California, San Francisco.
Francesca Aweeka, Department of Clinical Pharmacy, University of California, San Francisco.
Jean-Bosco Ouédraogo, Institut de Recherche en Sciences de la Santé, Bobo-Dioulasso, Burkina Faso; Institut des Sciences et Techniques, Bobo-Dioulasso, Burkina Faso.
Philip J Rosenthal, Department of Medicine, University of California, San Francisco.
Notes
Acknowledgments . We thank study children and their parents/guardians for their participation in the study; the study teams at the study clinic and the Institut de Recherche en Sciences de la Santé for supporting this work; and Shreeya Garg (University of California, San Francisco) and Rebecca DeFeo, David Giesbrecht, and Jeffrey Bailey (Brown University) for their assistance and guidance with DNA sequencing.
Financial support . This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant number 5R01 AI117001).
References
- 1. World Health Organization (WHO) . WHO guidelines for malaria. Geneva, Switzerland: WHO, 2022.
- 2. World Health Organization (WHO) . World malaria report 2022. Geneva, Switzerland: WHO, 2022.
- 3. Somé AF, Zongo I, Sagara I, et al. Factors influencing second and third dose observance during seasonal malaria chemoprevention (SMC): a quantitative study in Burkina Faso, Mali and Niger. Trop Med Infect Dis 2022; 7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cairns M, Ceesay SJ, Sagara I, et al. Effectiveness of seasonal malaria chemoprevention (SMC) treatments when SMC is implemented at scale: case–control studies in 5 countries. PLoS Med 2021; 18:e1003727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baba E, Hamade P, Kivumbi H, et al. Effectiveness of seasonal malaria chemoprevention at scale in West and Central Africa: an observational study. Lancet 2020; 396:1829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Institut National de la Statistique et de la Démographie (INSD) and ICF International . Enquête démographique et de santé du Burkina Faso 2021. Ouagadougou, Burkina Faso and Rockville, MD: INSD/ICF, 2022. [Google Scholar]
- 7. Cairns ME, Sagara I, Zongo I, et al. Evaluation of seasonal malaria chemoprevention in two areas of intense seasonal malaria transmission: secondary analysis of a household-randomised, placebo-controlled trial in Houndé District, Burkina Faso and Bougouni District, Mali. PLoS Med 2020; 17:e1003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Kock M, Tarning J, Workman L, et al. Population pharmacokinetic properties of sulfadoxine and pyrimethamine: a pooled analysis to inform optimal dosing in African children with uncomplicated malaria. Antimicrob Agents Chemother 2018; 62:e01370-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oshikoya KA, Sammons HM, Choonara I. A systematic review of pharmacokinetics studies in children with protein-energy malnutrition. Eur J Clin Pharmacol 2010; 66:1025–35. [DOI] [PubMed] [Google Scholar]
- 10. Picot S, Olliaro P, de Monbrison F, Bienvenu A-L, Price RN, Ringwald P. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar J 2009; 8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okell LC, Griffin JT, Roper C. Mapping sulphadoxine-pyrimethamine-resistant Plasmodium falciparum malaria in infected humans and in parasite populations in Africa. Sci Rep 2017; 7:7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lynch C, Pearce R, Pota H, et al. Emergence of a dhfr mutation conferring high-level drug resistance in Plasmodium falciparum populations from southwest Uganda. J Infect Dis 2008; 197:1598–604. [DOI] [PubMed] [Google Scholar]
- 13. Kavishe RA, Kaaya RD, Nag S, et al. Molecular monitoring of Plasmodium falciparum super-resistance to sulfadoxine-pyrimethamine in Tanzania. Malar J 2016; 15:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tumwebaze P, Tukwasibwe S, Taylor A, et al. Changing antimalarial drug resistance patterns identified by surveillance at three sites in Uganda. J Infect Dis 2017; 215:631–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kateera F, Nsobya SL, Tukwasibwe S, et al. Molecular surveillance of Plasmodium falciparum drug resistance markers reveals partial recovery of chloroquine susceptibility but sustained sulfadoxine-pyrimethamine resistance at two sites of different malaria transmission intensities in Rwanda. Acta Trop 2016; 164:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beshir KB, Muwanguzi J, Nader J, et al. Prevalence of Plasmodium falciparum haplotypes associated with resistance to sulfadoxine–pyrimethamine and amodiaquine before and after upscaling of seasonal malaria chemoprevention in seven African countries: a genomic surveillance study. Lancet Infect Dis 2023; 23:361–70. [DOI] [PubMed] [Google Scholar]
- 17. Nayebare P, Asua V, Conrad MD, et al. Associations between malaria-preventive regimens and Plasmodium falciparum drug resistance-mediating polymorphisms in Ugandan pregnant women. Antimicrob Agents Chemother 2020; 64:e01047-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Onis M, Martorell R, Garza C, Lartey A; WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr 2006; 95:76–85. [DOI] [PubMed] [Google Scholar]
- 19. Myatt M, Guervarra E. Package ‘zscorer.’2019. https://cran.r-project.org/web/packages/zscorer/zscorer.pdf. Accessed 19 September 2022.
- 20. Tumwebaze P, Conrad MD, Walakira A, et al. Impact of antimalarial treatment and chemoprevention on the drug sensitivity of malaria parasites isolated from Ugandan children. Antimicrob Agents Chemother 2015; 59:3018–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aydemir O, Janko M, Hathaway NJ, et al. Drug resistance and population structure of Plasmodium falciparum across the Democratic Republic of Congo using high-throughput molecular inversion probes. J Infect Dis 2018; 218:946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duvalsaint M, Conrad MD, Tukwasibwe S, et al. Balanced impacts of fitness and drug pressure on the evolution of PfMDR1 polymorphisms in Plasmodium falciparum. Malar J 2021; 20:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gregson A, Plowe CV. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev 2005; 57:117–45. [DOI] [PubMed] [Google Scholar]
- 24. Ariey F, Witkowski B, Amaratunga C, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014; 505:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ding J, Coldiron ME, Assao B, et al. Adherence and population pharmacokinetic properties of amodiaquine when used for seasonal malaria chemoprevention in African children. Clin Pharm Therap 2020; 107:1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naidoo I, Roper C. Mapping ‘partially resistant,’ ‘fully resistant,’ and ‘super resistant’ malaria. Trends Parasitol 2013; 29:505–15. [DOI] [PubMed] [Google Scholar]
- 27. Chauvin P, Menard S, Iriart X, et al. Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in pregnant women in Yaoundé, Cameroon: emergence of highly resistant pfdhfr/pfdhps alleles. J Antimicrob Chemother 2015; 70:2566–71. [DOI] [PubMed] [Google Scholar]
- 28. Wallender E, Ali AM, Hughes E, et al. Identifying an optimal dihydroartemisinin-piperaquine dosing regimen for malaria prevention in young Ugandan children. Nature Commun 2021; 12:6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chotsiri P, Denoeud-Ndam L, Baudin E, et al. Severe acute malnutrition results in lower lumefantrine exposure in children treated with artemether-lumefantrine for uncomplicated malaria. Clin Pharm Therap 2019; 106:1299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Worldwide Antimalarial Resistance Network (WWARN) AL Dose Impact Study Group . The effect of dose on the antimalarial efficacy of artemether–lumefantrine: a systematic review and pooled analysis of individual patient data. Lancet Infect Dis 2015; 15:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vandenbroucke JP, Pearce N. Test-negative designs: differences and commonalities with other case–control studies with “other patient” controls. Epidemiol 2019; 30:838–44. [DOI] [PubMed] [Google Scholar]
- 32. Vandenbroucke JP, Brickley EB, Pearce N, Vandenbroucke-Grauls CM. The evolving usefulness of the test-negative design in studying risk factors for COVID-19. Epidemiol 2022; 33:e7–8. [DOI] [PubMed] [Google Scholar]
- 33. Sullivan SG, Feng S, Cowling BJ. Potential of the test-negative design for measuring influenza vaccine effectiveness: a systematic review. Expert Rev Vaccines 2014; 13:1571–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.