Key Points
Question
In infants and children treated for growth failure or faltering, moderate or severe wasting, or edema, which postdischarge interventions are helpful in improving outcomes?
Findings
This systematic review, which included 8 intervention studies from 7 countries with 5965 participants, found that biomedical, cash transfer, and integrated interventions may improve certain outcomes in children after treatment for acute malnutrition.
Meaning
Given limited evidence on the efficacy of interventions to improve postdischarge outcomes following nutritional treatment for moderate or severe acute malnutrition, additional research on the effectiveness and operational feasibility is warranted to inform global guidance.
This systematic review evaluates the evidence on postdischarge interventions to improve outcomes among children treated for acute malnutrition within 6 months after discharge.
Abstract
Importance
Children treated for acute malnutrition remain at increased risk of relapse, infection, and mortality after programmatic recovery. Global guidelines for the management of acute malnutrition currently provide no recommendations to sustain recovery following treatment discharge.
Objective
To inform guideline development by evaluating the evidence on postdischarge interventions to improve outcomes within 6 months after discharge.
Evidence Review
In this systematic review, 8 databases were searched from inception through December 2021 and included randomized and quasi-experimental studies investigating interventions delivered after discharge from nutritional treatment for children aged 0 to 59 months. Outcomes were relapse, deterioration to severe wasting, readmission, sustained recovery, anthropometry, all-cause mortality, and morbidity within 6 months after discharge. The risk of bias was assessed using Cochrane tools, and the certainty of the evidence was evaluated with the GRADE approach.
Findings
Of 7124 records identified, 8 studies, conducted in 7 countries between 2003 and 2019 with 5965 participants, were included. The study interventions included antibiotic prophylaxis (n = 1), zinc supplementation (n = 1), food supplementation (n = 2), psychosocial stimulation (n = 3), unconditional cash transfers (n = 1), and an integrated biomedical, food supplementation, and malaria prevention package (n = 1). Risk of bias was moderate or high for half the studies. Only unconditional cash transfers were associated with reduced relapse, while the integrated package was associated with improved sustained recovery. Zinc supplementation, food supplementation, psychosocial stimulation, and unconditional cash transfers were associated with improvements in postdischarge anthropometry, while zinc supplementation was associated with reductions in multiple postdischarge morbidities.
Conclusions and Relevance
In this systematic review of postdischarge interventions to reduce relapse and improve other postdischarge outcomes among children treated for acute malnutrition, evidence was limited. Biomedical, cash, and integrated interventions showed promise in improving certain postdischarge outcomes for children treated for moderate or severe acute malnutrition in single studies. Further evidence on the efficacy, effectiveness, and operational feasibility of postdischarge interventions in other contexts is needed to inform global guidance development.
Introduction
Wasting (ie, a child being too thin for their height) affects at least 45 million children worldwide1 and is associated with increased child morbidity and mortality.2 Despite the effectiveness of community-based management of acute malnutrition, children successfully discharged from treatment continue to face increased risks of relapse, infection, and death following nutritional recovery.3,4,5,6 A recent systematic review showed that the proportion of children who relapse after successful discharge from nutritional treatment for severe acute malnutrition (SAM) may be as high as 37%.3 Other studies indicate postdischarge mortality risk could be as high as 9%.4,7,8 An increased burden of morbidities (eg, fever, cough, diarrhea) has also been documented following successful discharge from nutritional treatment.9,10,11,12
Despite increased risks of adverse outcomes following nutritional recovery, current World Health Organization (WHO) guidelines provide no or limited recommendations for interventions to improve postdischarge outcomes.13 A recent systematic review on postdischarge interventions following hospitalization for complicated SAM concluded that medical and psychosocial interventions showed promise in reducing postdischarge mortality following hospitalization in this specific subset of children with acute malnutrition.14 An important knowledge gap exists in our understanding of which interventions are effective to improve postdischarge outcomes in most children treated on an outpatient basis for uncomplicated SAM, moderate acute malnutrition (MAM), growth failure or faltering, or edema. In addition, little is known about the subgroups of children who may benefit from postdischarge interventions. The identification of effective interventions and subgroups of children who may benefit should be used to inform the development of guidelines for postdischarge interventions to prevent relapse and other adverse outcomes after discharge and to improve targeting and the longer-term impact of standard treatment. In 2021, the WHO identified this as a priority area for guideline development and commissioned a systematic review.15 The current review aimed to evaluate the evidence on the effectiveness of postdischarge interventions for infants and children treated for moderate or severe wasting, growth failure or faltering, or edema to improve outcomes and to identify subgroups of children who may benefit from postdischarge interventions.
Methods
This systematic review was conducted following Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) reporting guideline16 and Cochrane Handbook for Systematic Reviews of Interventions17 guidelines. The protocol was registered with Prospero (CRD42022308380).
Search Strategy and Selection Criteria
We searched PubMed, Cochrane Library, Embase, Web of Science Index Medicus, CINAHL, Latin American and Caribbean Health Sciences Literature (LILACS), e-Library of Evidence for Nutrition Actions (eLENA; WHO), and Index Medicus for the Eastern Mediterranean Region (IMEMR) from inception through December 2021 without language or geographical restrictions. The search strategy was developed using index terms along the following themes: child or infant, wasting, intervention, and discharge (eAppendix 1 in Supplement 1). References of extracted articles were reviewed for additional studies to include.
Peer-reviewed articles were included if they (1) assessed children aged 0 to 59 months treated for complicated or uncomplicated MAM or SAM, growth faltering or failure, or edema; (2) examined any intervention delivered partially or completely after discharge from nutritional treatment, where after discharge was defined as following exit from all phases of nutritional treatment; (3) study design was individually randomized clinical trial (RCT), cluster RCT, quasi-randomized study, controlled before-after study, or interrupted time series; and (4) assessed relapse, deterioration to severe wasting, readmission, anthropometric measures, all-cause mortality, or morbidity up to 6 months after discharge. Outcome definitions are provided in eAppendix 2 in Supplement 1.
Data Analysis
Two reviewers (L.B. and S.M.R.) independently screened titles, abstracts, and full texts for inclusion. Disagreements were resolved through discussion with a third reviewer (S.I.). Data were extracted and reviewed using a standardized form. The risk of bias was assessed by 2 reviewers (L.B. and S.M.R.) using the Cochrane Risk of Bias 2 tool for RCTs and cluster RCTs18 and the Cochrane Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool for nonrandomized studies,19 and the certainty of the evidence using the GRADE approach.20,21 Disagreements were resolved through discussion with a third reviewer (S.I.). Authors of the original studies were not contacted for clarification or additional information.
Data were synthesized narratively by study characteristics, intervention characteristics, and outcomes assessed. Included interventions were classified by type based on the hypothesized mechanisms of action: biomedical, food supplementation, psychosocial stimulation, cash transfers, or integrated packages operating through multiple mechanisms. Results were summarized narratively and presented by intervention type. Considering that food availability is a key driver of child wasting and the provision of food supplementation likely affected outcomes, we summarized results on psychosocial stimulation alone in studies providing psychosocial stimulation with and without food supplementation. Meta-analysis was planned when at least 2 studies assessed similar interventions and predefined outcomes. Meta-analysis did not pool experimental and quasi-experimental studies.17 Subgroup meta-analysis to examine effect modification by child and study characteristics was planned (eAppendix 3 in Supplement 1) but not conducted as only 1 study reported subgroup analyses.
Results
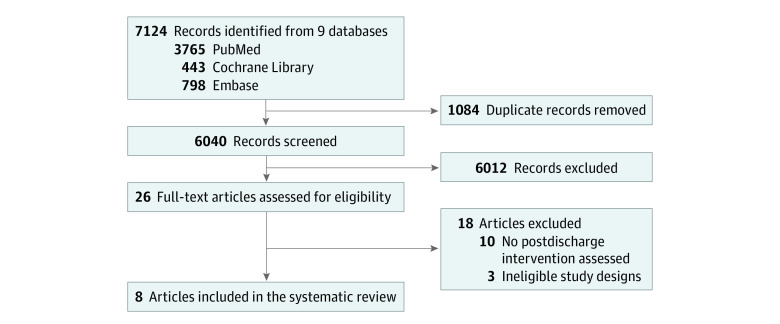
We identified 7124 records (Figure). After removing duplicates and screening titles and abstracts, we evaluated 26 full texts, of which 18 were excluded (eTable 1 in Supplement 1). We included 8 studies comprising 5 RCTs,22,23,24,25,26 2 cluster-RCTs,27,28 and 1 quasi-experimental study29 from 7 countries published between 2003 and 2019 (Table 1). Analytic sample sizes ranged from 80 to 1778 children per study (pooled population, 5965 participants). Six studies enrolled children admitted in hospital,22,23,24,25,26,29 1 in an outpatient therapeutic program setting,27 and 1 in a community-based supplementary feeding program setting.28 In 4 of the 6 studies that enrolled children admitted in hospital,22,23,24,29 there was no outpatient treatment at the time of the studies and discharge from inpatient treatment constituted complete exit from nutritional treatment. In the remaining 2 studies that enrolled children admitted in hospital,25,26 children were discharged from inpatient treatment to outpatient treatment for continued follow-up. Study populations included children treated for SAM (n = 6),22,24,25,26,27,29 MAM (n = 1),28 and protein energy malnutrition (n = 1).23 The review identified no studies of children treated for growth faltering, growth failure, or edema.
Figure. Study Flow Diagram.
Table 1. Characteristics of the 8 Studies Included in the Systematic Review.
| Source | Country, setting, and study design | Length of follow-up | Summary of population assessed | Sample size | Summary of intervention | Summary of comparator | Intervention duration | Outcomes assessed | Summary of findings | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Abessa et al,25 2019 | Ethiopia, hospital, RCT | 6 mo | Children aged 6-60 mo with uncomplicated SAM. | 339: 169 intervention and 170 control | Psychosocial support stimulation: 2 phases of inpatient and outpatient of play-based psychosocial stimulation. | Biomedical and dietary treatment | Up to 6 mo after discharge from inpatient treatment | Anthropometric measures | No association with improved anthropometric measures by end of follow-up | Some concerns |
| Berkley et al,26 2016 | Kenya, hospital, RCT | 12 mo | Children aged 60 d to 59 mo diagnosed with SAM. | 1778: 887 intervention and 891 control | Biomedical: 6 mo of daily oral co-trimoxazole prophylaxis. | Placebo | 6 mo from discharge from inpatient treatment. | Relapse to SAM, sustained recovery, anthropometric measure, all-cause mortality, morbidity or recovery from morbidity | No association with reduced mortality or adverse events or improved anthropometric outcomes | Low |
| Chauhan et al,22 2019 | India, hospital, RCT | 1.5 mo | Children aged 6 mo to 5 y with SAM | 80: 40 intervention and 40 control | Food supplementation: nonmilk based LTF provided at discharge and 3 follow-ups every 2 weeks. | Advice on home-based diet, no LTF received | 6 weeks starting at discharge from inpatient treatment | Anthropometric measures | The intervention was associated with improved weight gain over 6 weeks of follow-up and anthropometric outcomes; a greater number of children in the intervention group were labeled as cured. | Some concerns |
| Grellety et al,27 2017 | Democratic Republic of the Congo, OTP, cluster RCT | 6 mo | Children aged 6-59 mo in outpatient treatment for uncomplicated SAM | 1481: 734 intervention and 747 control | Cash transfer: all caregivers with ≥1 children with SAM received an unconditional cash transfer of US $40 per month during treatment and follow-up for a total of 6 mo. | Standard of care. | A total of 6 mos starting at admission to outpatient treatment and continuing after discharge | Relapse, sustained recovery, anthropometric measures | Intervention associated with decreased risk of MAM and SAM relapse and increased weight and MUAC gain. Changes in WAZ, WHZ, BMIZ, MUACZ, and MUAC-for-height z score were all positive in the intervention group. | Low |
| Makonnen et al,23 2003 | Lesotho, hospital, RCT | 3 mo | Children aged 6 mo to 5 y with signs of protein energy malnutrition based on the 1999 Wellcome Classification or signs and symptoms of kwashiorkor | 300: 150 intervention and 150 control | Biomedical: daily dose of 10 mg of zinc was administered from the first day of admission to 90 d postdischarge | Placebo | Starting at admission to inpatient treatment and continuing until 90 d after discharge from inpatient treatment. | Anthropometric measures, all-cause mortality, morbidity or recovery from morbidity. | Intervention associated with reduced morbidity at 30, 60 and 90 d after discharge as well as improved WAZ at 30, 60 and 90 d after discharge and MUAC<5% at 60 and 90 d after discharge. | Low |
| Nahar et al,29 2009 | Bangladesh, hospital, time-lagged controlled study | 6 mo | Children aged 6 to 24 mo hospitalized with SAM | 97: 54 intervention and 43 control | Psychosocial stimulation: 18 total sessions in hospital, at home, and the hospital for follow-up visits. | Routine nutritional and health care and health and nutrition education | From admission to 6 mo after discharge from inpatient treatment | Anthropometric measures | Intervention associated with improved WAZ at 6 mo after discharge. | Serious |
| Nahar et al,24 2012 | Bangladesh, hospital, RCT | 6 mo | Children aged 6-24 mo hospitalized with severe underweight without acute infections. | 507: 102 psychosocial stimulation, 101 food supplementation, 103 psychosocial stimulation and food supplementation, 99 clinic control, 102 hospital control | Psychosocial stimulation: individual play sessions and parental education; food supplementation: food packets for 3 mo. | Fortnightly follow-up at clinic or hospital with growth monitoring, health education, and micronutrient supplementation | 6 mo | Anthropometric measures | No intervention effect on anthropometric outcomes after 6 mo of intervention. Any psychosocial stimulation improved WAZ compared with no stimulation at 6 mo. | Low |
| Stobaugh et al,28 2017 | Malawi, community-based supplementary feeding program, cluster RCT | 12 mo | Children aged 6-62 mos discharged as recovered from community-based treatment for MAM. | 1383: 769 intervention and 718 control | Intervention package consisting of the following interventions: food supplementation, biomedical support, and malaria prevention | Standard of care and routine nutrition and health counselling | Up to 1 y after discharge from outpatient treatment | MAM relapse, deterioration to severe wasting among children recovered from moderate wasting, sustained recovery, all-cause mortality | The intervention was associated with improved sustained recovery at 1, 3, and 6 mo after discharge, but had no effect on sustained recovery at 12 mo (primary outcome). | High |
Abbreviations: BMIZ, body mass index z score; LTF, local therapeutic food; MAM, moderate acute malnutrition; MUAC, mid–upper arm circumference; MUACZ, mid–upper arm circumference z score; OTP, outpatient therapeutic program; RCT, randomized clinical trial; SAM, severe acute malnutrition; WAZ, weight-for-age z score; WHZ, weight-for-height/length z score.
Evaluated interventions included biomedical interventions (n = 2),23,26 food supplementation (n = 1),22 psychosocial stimulation (n = 2),25,29 food supplementation and/or psychosocial stimulation (n = 1),24 unconditional cash transfers (n = 1),27 and an integrated package providing biomedical intervention, food supplementation, and malaria prevention (n = 1).28 Mean duration of postdischarge follow-up was 9.3 months (range: 1.5 months22 to 12 months26,28). Analyzed outcomes are summarized in Table 2. Risk of bias was low for 4 of the included studies,23,24,26,27 moderate for 2,22,25 and high for 2,28,29 with bias arising from the randomization process, potential selection of the reported results, and missing data (Table 1; eTable 2 and eFigures 1-3 in Supplement 1).
Table 2. Outcomes Analyzed by the 8 Studies Included in the Systematic Review.
| Outcome | Studies reporting outcome, No. (%) | Specific studies reporting each outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Abessa et al,25 2019 | Berkley et al,26 2016 | Chauhan et al,22 2019 | Grellety et al,27 2017 | Makonnen et al,23 2003 | Nahar et al,29 2009 | Nahar et al,24 2012 | Stobaugh et al,28 2017 | ||
| Relapse | |||||||||
| Relapse to moderate acute malnutrition | 2 (25) | No | No | No | Yes | No | No | No | Yes |
| Relapse to severe acute malnutrition | 2 (25) | No | Yes | No | Yes | No | No | No | No |
| Deterioration to severe wasting among children recovered from moderate wasting | 1 (13) | No | No | No | No | No | No | No | Yes |
| Readmission | 0 | No | No | No | No | No | No | No | No |
| Sustained recovery | 0 | No | Yes | No | No | No | No | No | No |
| Sustained recovery | 3 (38) | No | Yes | No | Yes | No | No | No | Yes |
| Anthropometric measures | |||||||||
| Body mass index z score | 1 (13) | No | No | No | Yes | No | No | No | No |
| Height-for-age z score | 4 (50) | Yes | Yes | No | Yes | No | No | Yes | No |
| Head circumference-for-age z score | 1 (13) | No | Yes | No | No | No | No | No | No |
| Height | 1 (13) | No | No | No | Yes | No | No | No | No |
| Mid–upper arm circumference | 4 (50) | No | Yes | Yes | Yes | Yes | No | No | No |
| Mid–upper arm circumference for height z score | 1 (13) | No | No | No | Yes | No | No | No | No |
| Mid–upper arm circumference z score | 2 (25) | Yes | No | No | Yes | No | No | No | No |
| Weight-for-age z score | 6 (75) | Yes | Yes | No | Yes | Yes | Yes | Yes | No |
| Weight | 1 (13) | No | No | No | Yes | No | No | No | No |
| Weight gain | 1 (13) | No | No | Yes | No | No | No | No | No |
| Weight-for-height z score | 5 (63) | Yes | Yes | Yes | Yes | No | No | Yes | No |
| All-cause mortality | 2 (25) | No | Yes | No | No | No | No | No | Yes |
| Morbidity or recovery from co-morbidity | |||||||||
| Incidence of acute respiratory infection | 1 (13) | No | No | No | No | Yes | No | No | No |
| Incidence of diarrhea | 2 (25) | No | Yes | No | No | Yes | No | No | No |
| Incidence of fever | 1 (13) | No | No | No | No | Yes | No | No | No |
| Incidence of lower respiratory tract infection | 1 (13) | No | Yes | No | No | No | No | No | No |
| Incidence of malaria | 1 (13) | No | Yes | No | No | No | No | No | No |
| Incidence of edema | 1 (13) | No | No | No | No | Yes | No | No | No |
| Incidence of outpatient clinical episodes | 1 (13) | No | Yes | No | No | No | No | No | No |
| Incidence of pallor | 1 (13) | No | No | No | No | Yes | No | No | No |
| Incidence of pneumonia | 1 (13) | No | Yes | No | No | No | No | No | No |
| Incidence of skin infection | 2 (25) | No | Yes | No | No | Yes | No | No | No |
| Incidence of upper respiratory tract infection | 1 (13) | No | Yes | No | No | No | No | No | No |
| Incidence of urinary tract infection | 1 (13) | No | Yes | No | No | No | No | No | No |
| Incidence of vomiting | 1 (13) | No | No | No | No | Yes | No | No | No |
One study assessed daily antibiotic prophylaxis with oral co-trimoxazole compared with placebo for 6 months starting at discharge from inpatient treatment and continuing during and after outpatient treatment.26 Estimates for associations after discharge from nutritional treatment could not be extracted, as the authors did not report postdischarge estimates from all nutritional treatment (only postdischarge estimates of inpatient treatment, which included follow-up during subsequent outpatient treatment and postoutpatient discharge). Associations at 6 months and 12 months after discharge from inpatient treatment for antibiotic prophylaxis with oral co-trimoxazole are summarized in eAppendix 4 and eTable 3 in Supplement 1.
Daily supplementation with 10 mg of zinc starting at admission to inpatient treatment and continuing until 90 days after discharge was associated with higher mid–upper arm circumference (MUAC) and weight-for-age z score (WAZ) at 90 days after discharge; lower prevalence of diarrhea, skin infections, vomiting, fever, acute respiratory infection, and pallor at 30, 60, and 90 days after discharge; and lower prevalence of edema at 30 and 60 days after discharge.23 The certainty of the evidence was downgraded to moderate for all outcomes due to imprecision, ie, small sample size, few events, and wide confidence intervals (eTable 4 in Supplement 1).
Two studies assessed food supplementation.22,24 Daily food supplementation (150 kcal/d) for 3 months starting at discharge from inpatient SAM treatment was not associated with postdischarge anthropometric z scores.24 Supplementation with a nonmilk based local therapeutic food (833 kcal/d) for 6 weeks starting at discharge from inpatient SAM treatment was associated with lower proportion of children with low MUAC or low weight-for-height z score (WHZ) at 6 weeks after discharge.22 The certainty of the evidence was downgraded to moderate for anthropometric z scores due to imprecision (small sample size and wide confidence intervals) and to low for weight gain and MUAC due to high risk of bias and imprecision (small sample size and no measure of uncertainty reported) (eTable 5 in Supplement 1).
Of the 3 studies that assessed psychosocial stimulation, 2 provided psychosocial stimulation alone25,29 and 1 provided psychosocial stimulation with and without food supplementation.24 Meta-analysis of the 2 studies that provided psychosocial stimulation alone was not conducted, as 1 study was quasi-experimental.29 Estimates for associations after discharge from nutritional treatment could not be extracted from 1 study,25 as the authors did not provide postdischarge estimates from all nutritional treatment (only postdischarge estimates of inpatient treatment, which included follow-up during subsequent outpatient treatment and post–outpatient discharge). Associations at 6 months after discharge from inpatient treatment are summarized in eAppendix 5 and eTable 6 in Supplement 1.
Psychosocial stimulation delivered to children aged 6 to 24 months hospitalized with severe underweight (WAZ <−3 SD) through biweekly 1-hour sessions for 6 months was not associated with anthropometric z scores at 6 months after discharge.24 In contrast, psychosocial stimulation for hospitalized, severely underweight children aged 6 to 24 months starting at admission and continuing for 6 months after discharge (delivered through individual and group sessions in hospital and home visits after discharge) was associated with higher mean (SD) WAZ vs children who did not receive the intervention after 6 months of follow-up: −3.1 (0.9) vs −3.6 (1.2); P = .03.29 The certainty of evidence on the effect of psychosocial stimulation was very low for all anthropometric z scores due to downgrades for risk of bias, inconsistency, and imprecision (eTable 6 and eTable 7 in Supplement 1).
Unconditional cash transfers among children aged 6 to 59 months of age in an outpatient SAM treatment program (US $40/mo for 6 months starting at admission) was associated with lower risk of MAM relapse (hazard ratio [HR], 0.21; 95% CI, 0.11-0.41) and SAM relapse (HR, 0.30; 95% CI, 0.16-0.58) and positive changes in weight, MUAC, and weight-related anthropometric z scores.27 The certainty of the evidence was downgraded to moderate due to indirectness, related to limited generalizability of the study (eTable 8 in Supplement 1).
One study assessed an integrated package providing biomedical support (a single dose of albendazole and zinc supplementation for 14 days), food supplementation (40 g/d of lipid-based nutrient supplement, providing 200 kcal and 1 recommended daily allowance of micronutrients for 8 weeks), and malaria prevention (provision of an insecticide-treated bed net and a monthly dose of 25 mg/kg of sulfadoxine-pyrimethamine for malaria chemoprophylaxis during the peak of the rainy season) starting at discharge from MAM treatment.28 The integrated package was not associated with MAM relapse, deterioration to severe wasting, or all-cause mortality after 1, 3, or 6 months of follow-up. The intervention was associated with higher prevalence of sustained recovery after 1 month (intervention vs control: 78% vs 74%; P = .04), 3 months (intervention vs control: 69% vs 63%; P = .02), and 6 months (intervention vs control: 64% vs 59%; P = .04).28 The certainty of the evidence was downgraded 2 levels for all outcomes due to risk of bias and imprecision (eTable 9 in Supplement 1).
Discussion
In this systematic review aiming to analyze postdischarge interventions for children successfully treated for complicated or uncomplicated MAM or SAM, growth failure or faltering, or edema, we included 8 studies conducted from 2003 to 2019 that evaluated biomedical, food supplementation, psychosocial stimulation, cash transfer, and integrated interventions delivered after discharge from nutritional treatment. One zinc supplementation trial was associated with improved MUAC and WAZ and reduced morbidities within 90 days after discharge.23 One of the 2 food supplementation interventions, which provided a nonmilk-based local therapeutic food, was associated with improved postdischarge weight gain, MUAC, and WHZ.22 Psychosocial stimulation was associated with improved WAZ at 6 months after discharge in one quasi-experimental study.29 One unconditional cash transfer intervention was associated with reduced MAM and SAM relapse and improved anthropometric measures.27 The integrated biomedical, nutrition, and malaria package following MAM treatment showed some evidence of benefits on sustained recovery and protection from deterioration to severe wasting within 6 months of discharge.28 None of the included interventions reduced the risk of all-cause mortality. The review did not identify studies among children treated for growth failure or faltering or edema.
Infections are a main cause of mortality during SAM recovery.30 Growing evidence demonstrates elevated morbidity in the postdischarge period despite anthropometric recovery.9,10,11,12,31 Zinc supplementation is effective for reducing diarrhea risk in children,32,33 including in those recovering from protein energy malnutrition34 and particularly in areas with high prevalence of malnutrition and zinc deficiency.35 Preventive zinc supplementation might also reduce the incidence of pneumonia.36 The postdischarge zinc supplementation intervention included in this review was associated with reduced risk of multiple morbidities, including diarrhea and acute respiratory infection, and improvement in anthropometry after discharge,23 suggesting zinc supplementation may be an effective strategy to reduce the risk of other morbidities beyond diarrhea. While having a low risk of bias, this was a single, older study that applied a now outdated nutrition treatment protocol and discharge criterion (ie, recovery defined at WHZ >80% of expected weight). These findings should be replicated in other contemporary settings while exploring questions related to the effective dose and duration of zinc supplementation.
Current WHO guidelines for inpatient SAM treatment recommend child play activities during treatment to continue after program discharge.37 Our review included 2 RCTs24,25 and 1 quasi-experimental study29 of psychosocial stimulation interventions. The psychosocial stimulation interventions were of varying intensity and duration and overall showed small to no association with anthropometric measures after discharge from all nutritional treatment. When follow-up from discharge from inpatient treatment and from all nutritional treatment were pooled in an ad hoc meta-analysis, the intervention was associated with WAZ and HAZ (eTable 7 in Supplement 1). More research could be beneficial to assess the effectiveness and cost-effectiveness of combining psychosocial stimulation with other interventions to improve postdischarge nutritional and child development outcomes. Additional work is also needed to understand and minimize any potential increased burden for caregivers and health workers’ from attending or delivering psychosocial stimulation sessions.
We found limited evidence of benefit of direct food supplementation. One randomized study showed no benefit of food supplementation (150 kcal/d for 3 months) on postdischarge anthropometric measures.24 Another indicated some benefits of food supplementation (833 kcal/d for 6 weeks) on postdischarge anthropometric measures.22 However, the latter was a relatively small study at serious risk of bias. International organizations, like the World Food Programme, have increasingly been shifting toward the provision of cash in emergency and humanitarian settings, as studies indicate that cash distribution can be more efficient than direct food aid, allowing more people to be reached with cash at no extra cost.38,39 A single study showed that unconditional cash transfers was associated with reduced MAM and SAM relapse and improved anthropometric measures within 6 months from admission.27 The unconditional cash transfer was hypothesized to improve child outcomes by several mechanisms, including reducing sharing of therapeutic foods during nutritional treatment, increasing household food accessibility and consumption, reducing morbidities by providing money for health care, and increasing income generating opportunities by serving as investment capital.27 However, evidence on the effectiveness of a cash intervention in one setting may not directly generalize to other contexts. The size of the cash transfer in the included study was relatively large, corresponding to 70% of the monthly household income of very poor households in this setting,27 which likely contributed to its success in improving a range of nutritional outcomes. More research is needed on the optimal amount, duration, and any potential unintended behavioral and market consequences of postdischarge cash transfers in different settings.
Our findings also showed that an integrated package of interventions may be a promising strategy to improve certain postdischarge outcomes in children treated for MAM. The package included food and zinc supplementation, antibiotic prophylaxis, and malaria prevention and was associated with sustained recovery within 6 months after discharge, but effect sizes were overall small. The integrated package had no association with relapse, deterioration to severe wasting, or all-cause mortality,28 which may be due to its short duration (longest intervention was food supplementation provided for 8 weeks). Although the intervention improved sustained recovery within 6 months after discharge, the authors reported that intervention effect sizes were no longer significant after 12 months of follow-up. More work is needed to understand long-term outcomes and why recovery was not sustained over the longer period of follow-up and to determine which intervention components of the package may be beneficial. Replication in other settings is also warranted to assess the feasibility and effectiveness of packaging these same interventions in different contexts.
Limitations
This study has limitations. First, included studies assessed various interventions that differed in intensity and duration. Some interventions started at discharge from nutritional treatment and continued for a fixed duration after discharge, while others started during treatment and continued for a varying duration after discharge, which led to variable duration of exposure. It is possible that the duration of some interventions might have been insufficient to meaningfully improve postdischarge outcomes. Second, results from some studies could not be summarized when authors reported postdischarge estimates from inpatient treatment and not postdischarge estimates from all nutritional treatment. Future studies should provide more precise documentation of all phases of nutritional treatment and the number of outcomes by phase. Third, outcomes were measured at different points, with some measured only shortly after discharge while others measured after 6 months of follow-up. The length of follow-up in some studies might have been insufficient to detect changes in the examined outcomes. Fourth, author-reported definitions of outcomes like relapse and sustained recovery varied across studies, making it difficult to draw conclusions. Fifth, only 1 study reported subgroup analysis, and we were therefore unable to identify specific groups of children who may benefit more from postdischarge interventions. Given these limitations, our findings should be interpreted with caution until more evidence on postdischarge interventions is generated.
Conclusions
This systematic review included 8 studies of postdischarge interventions among children being treated for acute malnutrition. Despite the known risk for postdischarge mortality and poor clinical and nutritional outcomes for children successfully treated in acute malnutrition programs, this review confirms a paucity of evidence on the effectiveness of postdischarge interventions at present. The limited existing evidence suggests that biomedical, cash transfer, and integrated interventions may be promising strategies to improve outcomes following treatment for moderate or severe wasting. Consideration should be extended to assess associations with all-cause mortality and readmission following discharge. Rigorous evidence on the efficacy, effectiveness, and programmatic feasibility of postdischarge interventions is urgently needed to inform global policy and program implementation.
eAppendix 1. Search Terms Used in PubMed
eAppendix 2. Outcome Definitions
eAppendix 3. Prespecified Subgroups for Subgroup Analyses
eAppendix 4. Effect of Antibiotic Prophylaxis With Co-trimoxazole
eAppendix 5. Effect of Psychosocial Stimulation Postdischarge From Inpatient Treatment
eFigure 1. Risk of Bias for Individually Randomized Clinical Studies Included in the Review
eFigure 2. Risk of Bias for Cluster Randomized Clinical Studies Included in the Review
eFigure 3. Risk of Bias for Observational Studies Included in the Review
eTable 1. Excluded Records During Full-Text Review and Reasons for Exclusion
eTable 2. Detailed Summary of Included Studies
eTable 3. GRADE Evidence Profile for the Effect of Daily Oral Co-trimoxazole Prophylaxis Compared With Routine Care (at 12 Months After Discharge From Inpatient Treatment)
eTable 4. GRADE Evidence Profile for the Effect of Zinc Supplementation Compared With Placebo
eTable 5. GRADE Evidence Profile for the Effect of Food Supplementation Compared With Routine Care
eTable 6. GRADE Evidence Profile for the Effect of Psychosocial Stimulation Compared With Routine Care (at 6 Months After Discharge From All Nutritional Treatment)
eTable 7. GRADE Evidence Profile for the Effect of Psychosocial Stimulation Compared With Routine Care (at 6 Months After Discharge From Inpatient Treatment)
eTable 8. GRADE Evidence Profile for the Effect of Unconditional Cash Transfers Compared With Routine Care
eTable 9. GRADE Evidence Profile for the Effect of an Integrated Package of Medical Care, Food Supplementation, and Malaria Prevention Compared With Routine Care
Data Sharing Statement
References
- 1.World Health Organization . Levels and trends in child malnutrition: key findings of the 2021 edition of the joint child malnutrition estimates. May 5, 2021. Accessed April 18, 2023. https://www.who.int/publications/i/item/9789240025257
- 2.Black RE, Victora CG, Walker SP, et al. ; Maternal and Child Nutrition Study Group . Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427-451. doi: 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- 3.Stobaugh HC, Mayberry A, McGrath M, et al. Relapse after severe acute malnutrition: a systematic literature review and secondary data analysis. Matern Child Nutr. 2019;15(2):e12702. doi: 10.1111/mcn.12702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahwere P, Mtimuni A, Sadler K, Banda T, Collins S. Long term mortality after community and facility based treatment of severe acute malnutrition: analysis of data from Bangladesh, Kenya, Malawi and Niger. J Public Health Epidemiol. 2012;4(8):215-225. [Google Scholar]
- 5.Stobaugh HC, Rogers BL, Rosenberg IH, et al. Children with poor linear growth are at risk for repeated relapse to wasting after recovery from moderate acute malnutrition. J Nutr. 2018;148(6):974-979. doi: 10.1093/jn/nxy033 [DOI] [PubMed] [Google Scholar]
- 6.Chang CY, Trehan I, Wang RJ, et al. Children successfully treated for moderate acute malnutrition remain at risk for malnutrition and death in the subsequent year after recovery. J Nutr. 2013;143(2):215-220. doi: 10.3945/jn.112.168047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bwakura-Dangarembizi M, Dumbura C, Amadi B, et al. ; the HOPE-SAM study team . Risk factors for postdischarge mortality following hospitalization for severe acute malnutrition in Zimbabwe and Zambia. Am J Clin Nutr. 2021;113(3):665-674. doi: 10.1093/ajcn/nqaa346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hitchings MDT, Berthé F, Aruna P, et al. Effectiveness of a monthly schedule of follow-up for the treatment of uncomplicated severe acute malnutrition in Sokoto, Nigeria: A cluster randomized crossover trial. PLoS Med. 2022;19(3):e1003923. doi: 10.1371/journal.pmed.1003923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashraf H, Alam NH, Chisti MJ, et al. A follow-up experience of 6 months after treatment of children with severe acute malnutrition in Dhaka, Bangladesh. J Trop Pediatr. 2012;58(4):253-257. doi: 10.1093/tropej/fmr083 [DOI] [PubMed] [Google Scholar]
- 10.Bahwere P, James P, Abdissa A, et al. Use of tuberculin skin test for assessment of immune recovery among previously malnourished children in Ethiopia. BMC Res Notes. 2017;10(1):570. doi: 10.1186/s13104-017-2909-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanum S, Ashworth A, Huttly SR. Growth, morbidity, and mortality of children in Dhaka after treatment for severe malnutrition: a prospective study. Am J Clin Nutr. 1998;67(5):940-945. doi: 10.1093/ajcn/67.5.940 [DOI] [PubMed] [Google Scholar]
- 12.Bliznashka L, Grantz KH, Botton J, et al. Burden and risk factors for relapse following successful treatment of uncomplicated severe acute malnutrition in young children: secondary analysis from a timulate trial in Niger. Matern Child Nutr. 2022;18(4):e13400. doi: 10.1111/mcn.13400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . Guideline: updates on the management of severe acute malnutrition in infants and children. August 9, 2013. Accessed April 18, 2023. https://www.who.int/publications/i/item/9789241506328 [PubMed]
- 14.Noble CCA, Sturgeon JP, Bwakura-Dangarembizi M, Kelly P, Amadi B, Prendergast AJ. Postdischarge interventions for children hospitalized with severe acute malnutrition: a systematic review and meta-analysis. Am J Clin Nutr. 2021;113(3):574-585. doi: 10.1093/ajcn/nqaa359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Call for authors—systematic reviews on prevention and treatment of wasting. April 30, 2021. Accessed February 28, 2022. https://www.who.int/news-room/articles-detail/call-for-authors-systematic-reviews-on-prevention-and-treatment-of-wasting
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane; 2022. Accessed April 18, 2023. http://www.training.cochrane.org/handbook
- 18.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in timulate trials. BMJ. 2019;366(August):l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 19.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355(October):i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schünemann H, Brożek J, Guyatt G, Oxman A, eds; GRADE Working Group . Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. 2013. Accessed April 18, 2023. https://gdt.gradepro.org/app/handbook/handbook.html
- 21.GRADEpro GDT . GRADEpro guideline development tool. Accessed April 18, 2023. https://www.gradepro.org/
- 22.Chauhan HH, Javadekar BB, Jayswal AV, Thakkar PA, Parmar NT. Non-milk based local therapeutic feed plus home-based diet as compared to homebased diet alone for nutritional rehabilitation of severe acute malnutrition following discharge: a timulate clinical trial. J Clin Diagnostic Res. 2019;13(2):SC05-SC08. doi: 10.7860/JCDR/2019/29669.12568 [DOI] [Google Scholar]
- 23.Makonnen B, Venter A, Joubert G. A randomized controlled study of the impact of dietary zinc supplementation in the management of children with protein-energy malnutrition in Lesotho—I: mortality and morbidity. J Trop Pediatr. 2003;49(6):340-352. doi: 10.1093/tropej/49.6.340 [DOI] [PubMed] [Google Scholar]
- 24.Nahar B, Hossain MI, Hamadani JD, et al. Effects of a community-based approach of food and psychosocial stimulation on growth and development of severely malnourished children in Bangladesh: a timulate trial. Eur J Clin Nutr. 2012;66(6):701-709. doi: 10.1038/ejcn.2012.13 [DOI] [PubMed] [Google Scholar]
- 25.Abessa TG, Worku BN, Wondafrash M, et al. Effect of play-based family-centered psychomotor/psychosocial stimulation on the development of severely acutely malnourished children under six in a low-income setting: a randomized controlled trial. BMC Pediatr. 2019;19(1):336. doi: 10.1186/s12887-019-1696-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkley JA, Ngari M, Thitiri J, et al. Daily co-trimoxazole prophylaxis to prevent mortality in children with complicated severe acute malnutrition: a multicentre, double-blind, timulate placebo-controlled trial. Lancet Glob Health. 2016;4(7):e464-e473. doi: 10.1016/S2214-109X(16)30096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grellety E, Babakazo P, Bangana A, et al. Effects of unconditional cash transfers on the outcome of treatment for severe acute malnutrition (SAM): a cluster-randomised trial in the Democratic Republic of the Congo. BMC Med. 2017;15(1):87. doi: 10.1186/s12916-017-0848-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stobaugh HC, Bollinger LB, Adams SE, et al. Effect of a package of health and nutrition services on sustained recovery in children after moderate acute malnutrition and factors related to sustaining recovery: a cluster-randomized trial. Am J Clin Nutr. 2017;106(2):657-666. doi: 10.3945/ajcn.116.149799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahar B, Hamadani JD, Ahmed T, et al. Effects of psychosocial stimulation on growth and development of severely malnourished children in a nutrition unit in Bangladesh. Eur J Clin Nutr. 2009;63(6):725-731. doi: 10.1038/ejcn.2008.44 [DOI] [PubMed] [Google Scholar]
- 30.Jones KD, Thitiri J, Ngari M, Berkley JA. Childhood malnutrition: toward an understanding of infections, inflammation, and antimicrobials. Food Nutr Bull. 2014;35(2)(suppl):S64-S70. doi: 10.1177/15648265140352S110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aprameya H, Kamath SP, Kini PK, et al. Socioepidemiological determinants of severe acute malnutrition and effectiveness of nutritional rehabilitation center in its management. Int J Health Allied Sci. 2015;4(3):148-153. doi: 10.4103/2278-344X.160873 [DOI] [Google Scholar]
- 32.Sazawal S, Black RE, Bhan MK, Bhandari N, Sinha A, Jalla S. Zinc supplementation in young children with acute diarrhea in India. N Engl J Med. 1995;333(13):839-844. doi: 10.1056/NEJM199509283331304 [DOI] [PubMed] [Google Scholar]
- 33.Ruel MT, Rivera JA, Santizo M-C, Lönnerdal B, Brown KH. Impact of zinc supplementation on morbidity from diarrhea and respiratory infections among rural Guatemalan children. Pediatrics. 1997;99(6):808-813. doi: 10.1542/peds.99.6.808 [DOI] [PubMed] [Google Scholar]
- 34.Khanum S, Alam AN, Anwar I, Akbar Ali M, Mujibur Rahaman M. Effect of zinc supplementation on the dietary intake and weight gain of Bangladeshi children recovering from protein-energy malnutrition. Eur J Clin Nutr. 1988;42(8):709-714. [PubMed] [Google Scholar]
- 35.Lazzerini M, Wanzira H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst Rev. 2016;12(12):CD005436. doi: 10.1002/14651858.CD005436.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lassi ZS, Moin A, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev. 2016;12(12):CD005978. doi: 10.1002/14651858.CD005978.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashworth A, Khanum S, Jackson A, Schofield EC. Guidelines for the Inpatient Treatment of Severely Malnourished Children. World Health Organization. 2003. Accessed April 18, 2023. https://apps.who.int/iris/handle/10665/42724 [Google Scholar]
- 38.Margolies A, Hoddinott J. Costing alternative transfer modalities. J Dev Effect. 2015;7(1):1-16. doi: 10.1080/19439342.2014.984745 [DOI] [Google Scholar]
- 39.Center for Global Development. Doing cash differently: how cash transfers can transform humanitarian aid. September 2015. Accessed April 18, 2023. https://cdn.odi.org/media/documents/9828.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Terms Used in PubMed
eAppendix 2. Outcome Definitions
eAppendix 3. Prespecified Subgroups for Subgroup Analyses
eAppendix 4. Effect of Antibiotic Prophylaxis With Co-trimoxazole
eAppendix 5. Effect of Psychosocial Stimulation Postdischarge From Inpatient Treatment
eFigure 1. Risk of Bias for Individually Randomized Clinical Studies Included in the Review
eFigure 2. Risk of Bias for Cluster Randomized Clinical Studies Included in the Review
eFigure 3. Risk of Bias for Observational Studies Included in the Review
eTable 1. Excluded Records During Full-Text Review and Reasons for Exclusion
eTable 2. Detailed Summary of Included Studies
eTable 3. GRADE Evidence Profile for the Effect of Daily Oral Co-trimoxazole Prophylaxis Compared With Routine Care (at 12 Months After Discharge From Inpatient Treatment)
eTable 4. GRADE Evidence Profile for the Effect of Zinc Supplementation Compared With Placebo
eTable 5. GRADE Evidence Profile for the Effect of Food Supplementation Compared With Routine Care
eTable 6. GRADE Evidence Profile for the Effect of Psychosocial Stimulation Compared With Routine Care (at 6 Months After Discharge From All Nutritional Treatment)
eTable 7. GRADE Evidence Profile for the Effect of Psychosocial Stimulation Compared With Routine Care (at 6 Months After Discharge From Inpatient Treatment)
eTable 8. GRADE Evidence Profile for the Effect of Unconditional Cash Transfers Compared With Routine Care
eTable 9. GRADE Evidence Profile for the Effect of an Integrated Package of Medical Care, Food Supplementation, and Malaria Prevention Compared With Routine Care
Data Sharing Statement