Abstract
Purpose of Review
Glenoid bone loss presents distinct challenges in reverse total shoulder arthroplasty (rTSA) which, if unaddressed, can cause complications including poor outcomes and early implant failure. The purpose of this review is to discuss the etiology, evaluation, and management strategies of glenoid bone loss in primary rTSA.
Recent Findings
Three-dimensional computed tomography (3D CT) imaging and preoperative planning software have revolutionized the understanding of complex glenoid deformity and wear patterns from bone loss. With this knowledge, a detailed preoperative plan can be created and implemented for a more optimal management strategy. When appropriately indicated, deformity correction techniques with biologic or metal augmentation are successful in addressing the glenoid bone deficiency, creating optimal implant position, and thus providing stable baseplate fixation and improving outcomes.
Summary
Thorough evaluation and characterization of the degree of glenoid deformity with 3D CT imaging is necessary prior to treatment with rTSA. Eccentric reaming, bone grafting, and augmented glenoid components have shown promising results in correcting glenoid deformity due to bone loss, but long-term outcomes are currently unknown.
Keywords: Reverse total shoulder arthroplasty, Glenoid bone loss, Glenoid augments, Glenoid bone grafting
Introduction
Reverse total shoulder arthroplasty (rTSA) is a reliable treatment option for patients with glenohumeral arthritis and concomitant rotator cuff pathology that precludes the use of an anatomic style prosthesis. Despite the expanding indications for this procedure, glenoid bone loss and the resulting deformity remain challenging problems that can create multiple potential issues if not properly evaluated and managed [1–3]. Failure to recognize the pattern and degree of glenoid bone loss can lead to improper positioning and fixation of the glenoid component, which can cause significantly increased stress forces across the implant and subsequent implant micromotion and loosening [3–5]. Ultimately, this can lead to complications such as implant failure, imbalanced soft tissue tensioning, dislocation and instability, scapular notching, poor functional outcomes, and decreased bone stock for future revision procedures [2, 6•, 7]. Understanding the degree and location of glenoid bone loss prior to rTSA can help surgeons determine the appropriate treatment, ultimately providing the best opportunity for an optimal outcome [8]. Multiple techniques for treating glenoid bone loss in the setting of rTSA have been described including eccentric reaming, bone grafting, and augmented glenoid components—the goals of which are to establish a stable glenoid baseplate in the correct position [9]. The purpose of this review is to highlight the etiology, evaluation and patterns of glenoid bone loss, and to discuss the current treatment options to manage glenoid bone loss in the rTSA setting and their related outcomes to date.
Etiology and Classification of Glenoid Bone Loss
Glenoid bone loss in the native shoulder may be encountered in a variety of settings, including rotator cuff tear arthropathy, failed rotator cuff repairs with superior humeral head migration, osteoarthritis, osteoarthritis with rotator cuff failure, fracture sequalae, or chronic glenohumeral dislocation [9]. The etiology of glenoid bone loss and the resulting wear patterns are characteristic of the underlying cause of the degenerative joint disease.
In the setting of rotator cuff arthropathy, the superior migration of the humerus relative to the glenoid results in a superior glenoid bone loss pattern with the deformity in the coronal plane. Failure to address superior glenoid erosion can lead to excessive superior tilt of the glenosphere. This increases the risk of component failure from scapular notching, or from failure of the fixation between the baseplate and the glenoid due to the shear forces across the glenosphere during deltoid firing [10, 11]. Furthermore, Tashjian et al. demonstrated that instability after rTSA is strongly associated with greater superior baseplate inclination [12].
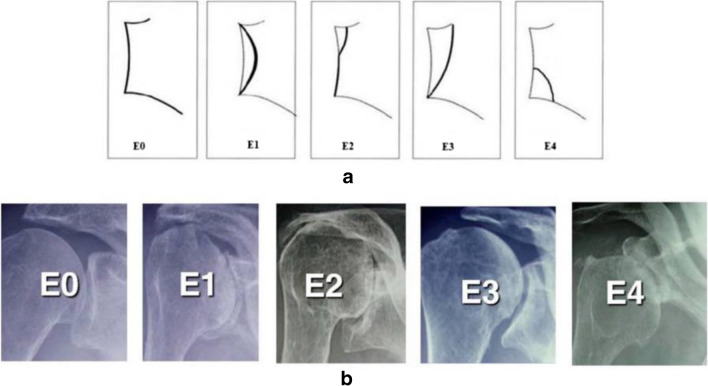
The most common classification system used to describe the degree and location of glenoid erosion in rotator cuff arthropathy is the Favard classification (Fig. 1). Type E0 glenoids have superior head migration without erosion of the glenoid. Type E1 glenoids demonstrate concentric and centralized erosion. Erosion of the glenoid limited to the superior aspect is classified as E2, and glenoid erosion extending from the superior glenoid to the inferior glenoid rim is classified as type E3. Type E4, added to the original classification, is defined as bone loss limited to the inferior aspect of the glenoid [13].
Fig. 1.
Schematic (a) and radiographic (b) representation of the Favard classification of glenoid erosion in rotator cuff arthropathy. Adapted from: Walch et al. [14]
Hamada et al. described an alternative method to classify bony abnormalities associated with rotator cuff arthropathy on standard radiographs (Fig. 2) [15, 16]. Hamada grade 1 is defined as maintenance of the acromiohumeral interval. Grade 2 is defined as narrowing of the acromiohumeral interval to ≤ 5 mm. Grade 3 demonstrates superior migration of the humerus on x-ray with acetabularization of the acromion. Narrowing of the glenohumeral joint space without acromial acetabularization is described as grade 4A, while grade 4B is described as narrowing of the glenohumeral joint space with acetabularization of the acromion [17]. Grade 5 demonstrates collapse of the humeral head.
Fig. 2.
Schematic (A/C) and radiographic (B/D) representation of the Hamada classification of rotator cuff arthropathy. Adapted from: Hamada et al. [15]
Shoulders with primary glenohumeral osteoarthritis in the setting of an intact rotator cuff demonstrate primarily posterior glenoid wear, with an axial plane deformity. Failure to address posterior glenoid erosion can lead to excessive retroversion of the glenoid component causing loosening, instability, and posterior subluxation. Glenoid deformity and bone loss in this setting is primarily evaluated using axial plane computed tomography (CT) images and classified via the Walch classification (Fig. 3). This classification, revised in 2016, focuses on the position of the humeral head relative to the glenoid as well as the degree of bony erosion [18–20]. Type A glenoids retain a centralized humeral head with A1 classified as minor central glenoid erosion and A2 as marked central erosion. Type B glenoid morphology is characterized by posterior subluxation of the humeral head. B1 glenoids have posterior subluxation without significant bony erosion, whereas B2 glenoids are characterized by posterior bony erosion resulting in a biconcave glenoid face. B3 glenoids are monoconcave and demonstrate severe posterior glenoid wear resulting in at least 15° of retroversion and/or 70% humeral head subluxation. Type C is defined as monoconcave glenoids that have over 25° of retroversion without bony erosion, and type D glenoids are defined as those with any level of anteversion or anterior subluxation of the humeral head.
Fig. 3.
Modified Walch classification of glenoid deformity and bone loss. Note that a line drawn from the anterior to posterior native glenoid rim transects the humeral head in the A2 glenoid but not in the A1 glenoid. Reprinted from: Bercik et al. (Copyright 2016), with permission from Elsevier [20]
Inflammatory arthropathy is characterized by concentric, central glenoid erosion with medialization of the joint line. Understanding the degree of central wear is important to prevent the biomechanical disadvantages in rTSA conferred by excessive medialization and loss of offset. Lévigne et al. identified 3 stages of glenoid wear in the setting of rheumatoid arthritis: stage 1 is defined by intact or minimally deformed subchondral bone, stage 2 defined by wear to the level of the coracoid base, and stage 3 characterized by centralized wear extending medial to the coracoid base [21].
Diagnosis and Evaluation of Glenoid Bone Loss
Preoperative evaluation of glenoid bone loss prior to performing rTSA should begin with a standard series of radiographs, including AP, Grashey, scapular Y, and axillary lateral views. The AP view is useful to evaluate for superior migration of the humeral head or central wear that leads to joint line medialization. The axillary lateral view is useful to assess for anterior, posterior, or central glenoid wear patterns that may predispose to abnormal version. However, Nyffeler et al. showed that the axillary view can overestimate glenoid retroversion in up to 86% of patients [22]. Accordingly, advanced imaging in the form of CT is necessary in nearly all cases to characterize the degree and pattern of glenoid bone loss in greater detail, define the bony architecture of the glenoid, and further evaluate the glenoid bone stock. Three-dimensional (3D) CT has been shown to be the most accurate and reliable modality to assess glenoid morphology [22–24]. 3D CT images can account for glenoid inclination or scapular rotation which, often with humeral head subtraction, can give a more complete picture of bone loss and overall osseous morphology of the glenoid than seen on 2D axial imaging [25]. In our practice, 3D CT is routinely obtained in every patient indicated for rTSA for preoperative planning purposes.
3D CT imaging can also be used in conjunction with digital templating software to create a reference model for preoperative planning or intraoperative use. In cases of severe deformity, this may be useful to estimate the size of the new glenoid components, the need for an augment or bone graft, and optimal component position (Fig. 4). Patient-specific instrumentation (PSI) can also be produced from the 3D CT-based preoperative plan.
Fig. 4.
Representative preoperative plan of a primary rTSA using digital templating software based on 3D CT imaging. This tool can be used to anticipate the need for an augmented glenoid component and gauge the optimal component size and position
There are various techniques to quantify parameters such as glenoid version, inclination, vault depth, and humeral head subluxation. These measurements aid in the proper placement of the baseplate and in deciding on the appropriate management strategy for the particular bone loss pattern. Friedman et al. described a method of measuring glenoid version as it relates to the scapular body [26]. This method uses an axial cut of a 2D CT scan to measure the angle between a line connecting the anterior and posterior margins of the glenoid and a line drawn from the tip of the medial border of the scapula to the midpoint of the glenoid fossa. This line, known as the Friedman line, is meant to represent the line of neutral version. This concept has been adapted by numerous authors to account for the biconcave deformity in Walch type B2 glenoids, or by re-thinking the reference for reconstructive planning to be the 3-dimensional face plane of the deformity [27–29]. Furthermore, there is a trend to use 3D CT to measure glenoid version, as this has been shown to be more accurate than using 2-dimensional imaging [24].
Interestingly, restoring anatomic glenoid version has not been shown to lead to improved motion or decreased failure after rTSA. Restoring inclination, on the other hand, is incredibly important to achieving an optimal outcome. Glenoid inclination is most commonly measured using the β angle as described by Maurer et al. [30]. This measurement is defined as the angle between a line connecting the superior and inferior glenoid rim to a line on the floor of the supraspinatus fossa. Because this measurement can be easily obtained on a high-quality AP radiograph, it has high clinical utility in evaluating the magnitude of superior glenoid bone loss preoperatively as well as postoperatively to assess the degree of baseplate inclination. Furthermore, because most surgeons do not obtain routine postoperative CT scans, the β angle can be particularly helpful in evaluating implant position.
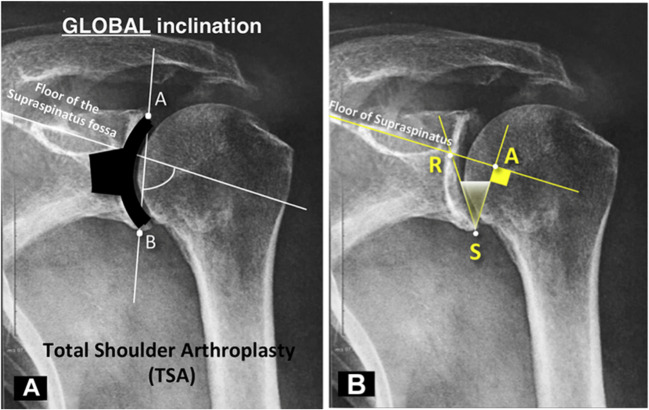
Boileau et al. described a modification of the β angle, termed the reverse shoulder arthroplasty (RSA) angle, that is, defined as the angle between a line perpendicular to the floor of the supraspinatus fossa and a line from the inferior rim to the glenoid face at the junction of the supraspinatus floor line (Fig. 5). This angle is entirely in the inferior aspect of the glenoid vault [31••]. Some surgeons prefer this measurement as it helps assess for inclination and wear at the inferior two-thirds of the glenoid, where the glenoid baseplate is implanted.
Fig. 5.
Traditional β angle measurement (A) versus reverse shoulder arthroplasty (RSA) angle (B) as described by Boileau et al. [31••] Note that unlike the β angle, the RSA angle is completely in the inferior portion of the glenoid vault, where the glenoid baseplate is implanted in rTSA. Adapted from: Boileau et al. [31••]
Techniques to Manage Bone Loss and Their Outcomes
Eccentric Reaming
The goals of glenoid resurfacing include correcting glenoid version, addressing glenoid tilt and providing adequate bony support to the implant. In cases of mild glenoid bone loss (i.e., < 15° retroversion), the simplest way this can be accomplished is by eccentric reaming of the anterior paleoglenoid to the level of the posterior surface. This creates a new neutral articulation to centralize the humeral head and maximizes contact area between the bone and implant, facilitating osteointegration. Care must be taken to avoid excessive reaming as it can remove too much bone from the subchondral plate, which is important for achieving stable baseplate fixation [32, 33]. Removing too much bone can also lead to medialization of the joint, which can result in poor soft tissue tension, impingement, and instability after rTSA, and may necessitate using a lateralized component [34]. The conventional guidelines for the limits to this technique are 15–18° of glenoid retroversion and 5–8 mm of glenoid bone loss, as multiple authors have shown that attempting to correct larger degrees of deformity with eccentric reaming led to significant rates of central peg penetration and inadequate bone stock [33, 35–37].
Few studies have examined the outcomes of rTSA after glenoid deformity correction with eccentric reaming alone. Klein et al. used 3D CT imaging to identify 56 patients with abnormal glenoids undergoing rTSA with eccentric reaming, 39% of which required augmentation with bulk allograft [38]. The authors noted improvement in pain and all clinical outcomes scores at 31 months follow-up, with no difference between patients who received augments and those who did not. Mizuno et al. evaluated a cohort of 27 patients undergoing rTSA with asymmetric reaming to correct posterior glenoid defects [39]. Bone graft was added in 37% of patients. At 54 months follow-up, only one glenoid component failure was identified, and the authors noted significant improvements in range of motion and all patient-reported outcomes (PROs).
McFarland et al. examined 42 patients with intact rotator cuffs who underwent rTSA with glenoid reaming without bone grafting [40]. Of their cohort, 19 were Walch type A1, 18 were type C, and 5 were type B2. The authors reported significant improvement in pain, range of motion, and PROs at a mean of 3 years postoperatively with only one baseplate failure.
Rojas et al. investigated the effect of medialization on postoperative outcomes in a retrospective analysis of 91 patients who underwent primary rTSA with eccentric reaming. All patients received lateralized components [41•]. At a mean of 30 months postoperatively, the authors found no evidence of baseplate failure or loosening, and noted no effect of medialization on PROs. Increasing medialization was associated with decreased postoperative external rotation at the side.
Glenoid Bone Grafting
In cases of more severe glenoid bone loss, complete correction of the deformity may not be possible with corrective asymmetric reaming and standard components. This is primarily due to the resultant medialization after extensive reaming and the risk of suboptimal fixation in poor-quality cancellous bone. In these situations, abnormal glenoid anatomy is typically corrected using posterior bone grafting or metal augments, which will be discussed later.
Indications for glenoid bone grafting during primary rTSA include inability to correct glenoid wear with eccentric reaming, glenoid retroversion greater than 15°, insufficient glenoid bone stock, < 50% contact between the glenoid and the baseplate, and central defects with joint line medialization [3, 34].
Advantages of glenoid bone grafting include cost-effectiveness, preservation and restoration of native glenoid bone stock, and restoration of a more normal joint line that avoids the biomechanical disadvantages of medialization. This theoretically permanent solution to glenoid bone loss can be especially important for potential future glenoid reconstructions in cases of implant failure. On the other hand, glenoid bone grafting is technically challenging and lacks reproducibility as graft contouring can be variable [3]. The most notable disadvantages of glenoid bone grafting are failure of the graft to incorporate, graft resorption, non-union and subsequent baseplate fixation failure, and poor functional outcomes. Graft resorption can be difficult to identify on CT scan, with an accuracy of only 46% in a recent cadaver study [42].
Humeral head autograft is the currently preferred bone grafting technique to correct severe glenoid bone loss. The burr and cut technique uses a preoperative template to contour the humeral head to an appropriately sized graft. If using a baseplate with a central peg, the thickness of the graft should be such that the peg can traverse the graft and gain purchase in at least 10 mm of native glenoid bone [43]. To accommodate the bone graft, the deficient side of the glenoid is prepared to bleeding bone with a burr. The graft is placed with the subchondral bone facing the glenoid and can be temporarily fixed with Kirshner wires, which are replaced by countersunk screws. The baseplate is then impacted, trapping and compressing the graft behind it [10].
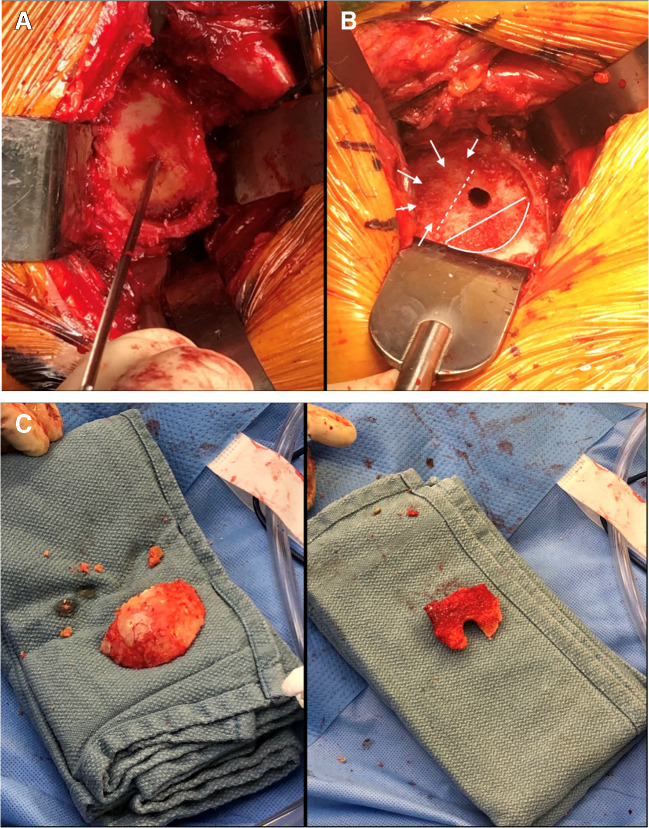
In our preferred technique, the goal is to place the baseplate at the inferior margin of the glenoid. Because the glenoid is concave with a natural superior inclination of 5–8°, the inferior glenoid will come into contact with the reamer in nearly all cases. After guide pin placement, we ream by hand to create a “subchondral smile” of bone reaming at the inferior glenoid, without violating the subchondral bone (Fig. 6A). This is excellent bone and we do not want to remove it or medialize the baseplate from this position. Next, we assess the wear pattern deformity that exists behind the baseplate that will need to be filled (Fig. 6B). The resected humeral head is then contoured as bone graft to fill the defect, and is placed with the cortical surface of the graft facing the cortical surface of the glenoid bone defect. (Fig. 6C). The final glenoid component is then implanted.
Fig. 6.
A Intraoperative representation of the “subchondral smile” created by hand reaming the inferior glenoid to the level of the subchondral bone. B The posterosuperior glenoid defect was assessed (arrows) in relation to the subchondral smile below. C The resected humeral head was contoured to create bone graft to fill the size of the glenoid defect
It is important to note that in the majority of glenoid bone loss patterns, the wear pattern is not symmetric [44•]. In a study utilizing the humeral head as a structural “compensation” graft behind the baseplate with the cortical surface of the graft facing the cortical surface of the glenoid wear deficit, 47% of the defects were posterior–superior. A superior defect was seen in 37%, and a direct posterior wear pattern was seen in 16%. All cases were patients with osteoarthritis with a Walch B2 type glenoid [45].
In our experience, this technique reliably places the glenoid baseplate in 3–12° of inferior inclination by β angle measurement. In a cohort of 38 patients undergoing the bone graft technique previously described, the β angle increased from a mean of 76.3° preoperatively to 93.9° postoperatively, representing approximately 10° of inferior baseplate inclination. The β angle did not change at 2 years postoperatively, all implants were stable, and all grafts had incorporated [45]. We now utilize this technique when preoperative planning software reveals a wear pattern that may be too large for an augmented baseplate to adequately correct. Figure 7 demonstrates a case in which the β angle was successfully corrected using this technique, with stable implants and incorporation of the bone graft at 1 year postoperatively.
Fig. 7.
A Preoperative AP radiographs demonstrating end-stage glenohumeral osteoarthritis of the right shoulder with a β angle of 63°. The patient was indicated for reverse total shoulder arthroplasty. B After exposure of the glenoid, the central guide pin was placed. Hand reaming was performed to create a “subchondral smile” on the inferior aspect of the glenoid. In this case, an anterioinferior glenoid bone defect was noted and the dimensions were assessed. The resected humeral head was used to create a corticocancellous bone graft of corresponding size, and was placed with the cortex of the graft facing the cortical surface of the glenoid wear deficit. The final baseplate was then implanted. C One-year postoperative radiographs demonstrating stable implants, bone graft incorporation and a β angle of 94°
Structural glenoid bone grafting has shown overall good results in the literature, though few studies have focused specifically on this technique in primary rTSA with severe glenoid bone loss. In a cohort of 14 patients undergoing primary rTSA, Tashjian et al. demonstrated reliable correction of defects up to 35° with structural bone grafting, with 100% graft incorporation, 93% survival, and improved pain and function at 2.6 years follow-up [2]. Ernstburnner et al. examined a cohort of 41 primary rTSA procedures with bone grafting, 83% of whom received corticocancellous grafts and with the remaining 17% receiving structural grafts. At an average of 2.8-year follow-up, the authors reported a 78% graft incorporation rate and a glenoid lucency rate of 18%, with no patients requiring revision.
Boileau et al. described a technique termed bony increased offset-reverse shoulder arthroplasty (BIO-RSA) in which humeral head autograft is placed between the glenoid baseplate and reamed glenoid vault, and fixed with a 25-mm central peg and 4 peripheral screws [46]. This technique employs bone graft to effectively lengthen the scapular neck and achieve biological glenoid lateralization while maintaining the center of rotation at the interface between the glenoid prosthesis and native bone [47]. Trapezoidal bone graft can also be used to simultaneously correct glenoid version and tilt [46]. The use of BIO-RSA has demonstrated predictable correction of severe multiplanar glenoid deformity > 25°, as well as high rates of graft incorporation, low revision rates, and excellent functional outcomes up to 10 years postoperatively [46, 48••, 49••].
If there is insufficient bone in the humeral head, structural allograft has demonstrated good results in correcting glenoid deformity with high rates of bony incorporation with low rates of loosening or revision, and equivalent outcomes to autograft [43, 50••, 51].
Augmented Glenoid Component
Introduced in 2011 for rTSA, metal-augmented glenoid components have become an attractive alternative to bone grafting for the management of severe glenoid deformity (Fig. 8). The indications for augmented glenoid components have evolved to become similar to those for bone grafting as previously described. Augments are available as full wedge or half wedge shapes and can be rotated on a circular baseplate to address posterior, superior, and posterosuperior glenoid wear configurations. Augmented baseplates can also be used for lateralization or soft tissue tensioning [34].
Fig. 8.
Preoperative and postoperative radiographs demonstrating rotator cuff arthropathy with a posterosuperior glenoid bone defect addressed with rTSA with a posterosuperior metal baseplate augment
Commercially available metal-augmented glenoid baseplates are becoming increasingly popular because they are less technically challenging than glenoid bone grafting, require less operative time, and do not carry the risks of resorption, non-union, and fixation failure [6•, 34]. These components also carry the advantage of preserving native glenoid bone stock, particularly the structurally robust subchondral bone, by minimizing the amount of reaming required. As such, augmented components decrease the number of interfaces between the component and the intact glenoid and reduce the area required for bony incorporation, which may be useful for elderly patients with a lower capacity for healing. One concern of augmented glenoid components is increased stress on the glenoid vault of a large metal construct in osteopenic bone. These components also carry the potential for peg or screw perforation if there is insufficient native bone, and potentially may fail at modular junctions [34]. Augments are also costly and not universally available, limiting their utility.
Outcomes of augmented glenoid components for rTSA are promising, with low complication and failure rates [52, 53••, 54•]. However, there is a paucity of long-term data regarding their effectiveness. Kirsch et al. conducted a retrospective review of 44 patients undergoing primary rTSA with an augmented baseplate and found significantly improved glenoid version and inclination, improved patient-reported outcomes, and no evidence of loosening or failure at an average of 16 months postoperatively [53••]. Virk et al. also reported a low complication rate with no failures with an 8° posteriorly augmented baseplate in a cohort of patients with Walch B2, B3, and C glenoids undergoing primary rTSA [54•]. Liuzza et al. reported good short-term outcomes with superior or posterior–superior augments for Walch type E glenoids, finding that for each of the PROs and range of motion, 88% of patients achieved the minimal clinically important difference (MCID) threshold and 75% of patients exceeded the substantial clinical benefit (SCB) threshold [55••].
Augmented baseplates have also been found to have comparable or improved short-term outcomes and complication rates to standard components [56, 57•]. Levin et al. conducted a multicenter retrospective analysis of 171 patients undergoing primary rTSA with either standard components or an augment baseplate. The authors reported that the augmented cohort demonstrated significantly greater postoperative improvements in range of motion and PROs at greater than 5 years follow-up. There was no difference in revision rate between cohorts [57•].
In a cohort of 76 augmented baseplates utilized for glenoid bone loss in rTSA, we found that augmented baseplates resulted in a change in β angle from an average of 79.8° to an average of 92.7°. Similarly, there was no significant change at one year postoperatively, indicating that augmented baseplates could address significant glenoid wear equivalently to humeral head bone grafting [58]. These results also corroborate previous literature showing similar outcomes between the two techniques, with augmented components demonstrating a lower rate of complications and scapular notching [59].
Additional Techniques for Managing Glenoid Bone Loss
In cases of glenoid deformity, eccentric placement of glenoid components may be necessary to maximize bony purchase in a compromised glenoid vault. This can be achieved by placing the central guide pin along an “alternative center line,” aligned with the axis of the junction of the scapular spine and scapular body, in order to place the glenoid component in the area of best fixation [6•]. Despite this theoretical advantage, Colley et al. reported no difference in outcomes, range of motion, or complications between rTSAs utilizing the alternative centerline technique and those utilizing the anatomic center line [60•].
Intraoperative navigation uses an optical tracking system to place the central guide pin in the optimal position within the glenoid vault, which can be helpful in cases of glenoid bone loss where it is important to implant the baseplate in the area of maximal bony support. Navigation also provides real-time intraoperative feedback on glenoid version and inclination. Though navigation has been shown to be effective in correcting glenoid deformity, there is little robust outcomes data to fully elucidate its advantages [3]. In a recent study, Holzgrefe et al. found that navigated and non-navigated rTSA demonstrated similar rates of improvement in functional outcomes scores and range of motion at short-term follow-up [61]. Scapular notching and revision were more common in non-navigated shoulders, but this did not reach statistical significance.
PSI uses 3D CT imaging to create surgical instrumentation based on a patient’s unique anatomy, in order achieve optimal positioning of the baseplate. Proponents of PSI cite its reliability and ease of use as the main advantage, avoiding the technical challenges of computer-assisted navigation or using the alternative centerline technique [62]. In a cadaveric study, Levy et al. demonstrated that patient-specific glenoid drill guides were highly accurate in reproducing a 3D CT-based preoperative surgical plan [62]. Through the results of this technology are promising, the literature is conflicting and distinct advantages over standard techniques have yet to be borne out [6•, 63, 64].
Dines et al. introduced a patient-specific approach to correction of glenoid bone loss by using computer-assisted design and manufacturing to develop a custom glenoid baseplate unique to a patient’s anatomy [65]. Bodendorfer et al. reported on a series of 11 patients undergoing 12 rTSAs with custom glenoid components, and noted improved PROs and range of motion at 30 months post-op, with no complications [66•].
Conclusion
Glenoid bone loss is a challenging problem for surgeons performing rTSA. Thorough evaluation and characterization of the degree of glenoid deformity with 3D CT imaging is necessary prior to treatment. Eccentric reaming, bone grafting, and augmented glenoid components have shown promising results in correcting glenoid deformity due to bone loss, but long-term data are needed.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Dr. Nabil Mehta declares that he has no conflict of interest. Dr. Gregory Nicholson reports royalties from Wright Medical Technology, Inc., Innomed, and Arthrosurface; Consultancy with Wright Medical Technology, Inc.; Research support from Arthrex, Inc. and Wright Medical Inc.; Fellowship education support from Wright Medical Inc., and Smith & Nephew; Paid Presenter for Arthrosurface; Board Membership for American Shoulder and Elbow Surgeons.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Seidl AJ, Williams GR, Boileau P. Challenges in reverse shoulder arthroplasty: addressing glenoid bone loss. Orthopedics. 2016;39(1):14–23. doi: 10.3928/01477447-20160111-01. [DOI] [PubMed] [Google Scholar]
- 2.Tashjian RZ, Granger E, Chalmers PN. Structural glenoid grafting during primary reverse total shoulder arthroplasty using humeral head autograft. J Shoulder Elbow Surg. 2018;27(1):e1–e8. doi: 10.1016/j.jse.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Sears BW, Johnston PS, Ramsey ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. JAAOS J Am Acad Orthop Surg. 2012;20(9):604-13. 10.5435/jaaos-20-09-604. [DOI] [PubMed]
- 4.Farron A, Terrier A, Büchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg. 2006;15(4):521–526. doi: 10.1016/j.jse.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85(2):251–258. doi: 10.2106/00004623-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 6.• Friedman LGM, Garrigues GE. Management of Humeral and glenoid bone defects in reverse shoulder arthroplasty. JAAOS J Am Acad Orthop Surg. 2021;29(17):e846-e59. 10.5435/jaaos-d-20-00964. Review article providing an overview of the etiology, management and treatment of humeral and glenoid bone defects in primary and revision rTSA. [DOI] [PubMed]
- 7.Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear. Comparison of Posterior Augments to Superior Augments. Bull Hosp Jt Dis (2013). 2015;73 Suppl 1:S124–8. [PubMed]
- 8.Stephens SP, Paisley KC, Jeng J, Dutta AK, Wirth MA. Shoulder arthroplasty in the presence of posterior glenoid bone loss. J Bone Joint Surg Am. 2015;97(3):251–259. doi: 10.2106/jbjs.N.00566. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Thussbas C, Koch M, Seebauer L. Management of glenoid bone defects with reverse shoulder arthroplasty-surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2018;27(5):853–862. doi: 10.1016/j.jse.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Laver L, Garrigues GE. Avoiding superior tilt in reverse shoulder arthroplasty: a review of the literature and technical recommendations. J Shoulder Elbow Surg. 2014;23(10):1582–1590. doi: 10.1016/j.jse.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(5):732–739. doi: 10.1016/j.jse.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Tashjian RZ, Martin BI, Ricketts CA, Henninger HB, Granger EK, Chalmers PN. Superior baseplate inclination is associated with instability after reverse total shoulder arthroplasty. Clin Orthop Relat Res. 2018;476(8):1622–9. 10.1097/corr.0000000000000340. [DOI] [PMC free article] [PubMed]
- 13.Lévigne C, Boileau P, Favard L, Garaud P, Molé D, Sirveaux F, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17(6):925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Walch G, Collotte P, Raiss P, Athwal GS, Gauci MO. The characteristics of the Favard E4 glenoid morphology in cuff tear arthropathy: a CT study. J Clin Med. 2020;9(11). 10.3390/jcm9113704. [DOI] [PMC free article] [PubMed]
- 15.Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452–2460. doi: 10.1007/s11999-011-1896-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res. 1990(254):92–6. [PubMed]
- 17.Walch G, Edwards TB, Boulahia A, Nové-Josserand L, Neyton L, Szabo I. Arthroscopic tenotomy of the long head of the biceps in the treatment of rotator cuff tears: clinical and radiographic results of 307 cases. J Shoulder Elbow Surg. 2005;14(3):238–246. doi: 10.1016/j.jse.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756–760. doi: 10.1016/s0883-5403(99)90232-2. [DOI] [PubMed] [Google Scholar]
- 19.Walch G, Boulahia A, Boileau P, Kempf JF. Primary glenohumeral osteoarthritis: clinical and radiographic classification. The Aequalis Group. Acta Orthop Belg. 1998;64 Suppl 2:46-52. [PubMed]
- 20.Bercik MJ, Kruse K, 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601–1606. doi: 10.1016/j.jse.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Lévigne C, Franceschi JP, editors. Rheumatoid arthritis of the shoulder: radiological presentation and results of arthroplasty. 1999; Berlin, Heidelberg: Springer Berlin Heidelberg.
- 22.Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493–496. doi: 10.1016/s1058-2746(03)00181-2. [DOI] [PubMed] [Google Scholar]
- 23.Scalise JJ, Bryan J, Polster J, Brems JJ, Iannotti JP. Quantitative analysis of glenoid bone loss in osteoarthritis using three-dimensional computed tomography scans. J Shoulder Elbow Surg. 2008;17(2):328–335. doi: 10.1016/j.jse.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Hoenecke HR, Jr, Hermida JC, Flores-Hernandez C, D'Lima DD. Accuracy of CT-based measurements of glenoid version for total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(2):166–171. doi: 10.1016/j.jse.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Bryce CD, Davison AC, Lewis GS, Wang L, Flemming DJ, Armstrong AD. Two-dimensional glenoid version measurements vary with coronal and sagittal scapular rotation. J Bone Joint Surg Am. 2010;92(3):692–699. doi: 10.2106/jbjs.I.00177. [DOI] [PubMed] [Google Scholar]
- 26.Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032–1037. doi: 10.2106/00004623-199274070-00009. [DOI] [PubMed] [Google Scholar]
- 27.Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G. Glenoid version: how to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg. 2010;19(8):1230–1237. doi: 10.1016/j.jse.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 28.Lewis GS, Armstrong AD. Glenoid spherical orientation and version. J Shoulder Elbow Surg. 2011;20(1):3–11. doi: 10.1016/j.jse.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Berhouet J, Gulotta LV, Dines DM, Craig E, Warren RF, Choi D et al. Preoperative planning for accurate glenoid component positioning in reverse shoulder arthroplasty. Orthop Traumatol Surg Res OTSR. 2017;103(3):407-13. 10.1016/j.otsr.2016.12.019. [DOI] [PubMed]
- 30.Maurer A, Fucentese SF, Pfirrmann CW, Wirth SH, Djahangiri A, Jost B, et al. Assessment of glenoid inclination on routine clinical radiographs and computed tomography examinations of the shoulder. J Shoulder Elbow Surg. 2012;21(8):1096–1103. doi: 10.1016/j.jse.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 31.•• Boileau P, Gauci MO, Wagner ER, Clowez G, Chaoui J, Chelli M et al. The reverse shoulder arthroplasty angle: a new measurement of glenoid inclination for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(7):1281-90. 10.1016/j.jse.2018.11.074. Describes the reverse shoulder arthroplasty angle and demonstrates that it is more accurate than the beta angle in assessing glenoid tilt in rTSA. [DOI] [PubMed]
- 32.Smith MJ, Loftis CM, Skelley NW. Eccentric reaming for B2 glenoids: history, preoperative planning, surgical technique, and outcome. J Shoulder Elb Arthroplast. 2019;3. 10.1177/2471549219870348. [DOI] [PMC free article] [PubMed]
- 33.Gilot GJ. Addressing glenoid erosion in reverse total shoulder arthroplasty. Bull Hosp Jt Dis. 2013;2013(71 Suppl 2):S51–S53. [PubMed] [Google Scholar]
- 34.Holt AM, Throckmorton TW. Reverse shoulder arthroplasty for B2 glenoid deformity. J Shoulder Elb Arthroplast. 2019;3:2471549219897661. doi: 10.1177/2471549219897661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: what are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843–848. doi: 10.1016/j.jse.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Gillespie R, Lyons R, Lazarus M. Eccentric reaming in total shoulder arthroplasty: a cadaveric study. Orthopedics. 2009;32(1):21. doi: 10.3928/01477447-20090101-07. [DOI] [PubMed] [Google Scholar]
- 37.Nowak DD, Bahu MJ, Gardner TR, Dyrszka MD, Levine WN, Bigliani LU, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: the amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680–688. doi: 10.1016/j.jse.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1144–1154. doi: 10.2106/jbjs.I.00778. [DOI] [PubMed] [Google Scholar]
- 39.Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297–1304. doi: 10.2106/jbjs.L.00820. [DOI] [PubMed] [Google Scholar]
- 40.McFarland EG, Huri G, Hyun YS, Petersen SA, Srikumaran U. Reverse total shoulder arthroplasty without bone-grafting for severe glenoid bone loss in patients with osteoarthritis and intact rotator cuff. J Bone Joint Surg Am. 2016;98(21):1801–1807. doi: 10.2106/jbjs.15.01181. [DOI] [PubMed] [Google Scholar]
- 41.• Rojas J, Meshram P, Srikumaran U, McFarland EG. Clinical and radiographic results of eccentric glenoid reaming in reverse total shoulder arthroplasty. Semin Arthroplasty: JSES. 2022;32(2):405-14. 10.1053/j.sart.2021.12.005. Case series showing that medialization due to eccentric reaming after rTSA was not associated with worse outcomes or increased rates of loosening at short-term follow-up, but was associated with a decrease in postoperative external rotation at the side.
- 42.Ferreira LM, Knowles NK, Richmond DN, Athwal GS. Effectiveness of CT for the detection of glenoid bone graft resorption following reverse shoulder arthroplasty. Orthop Traumatol Surg Res OTSR. 2015;101(4):427–430. doi: 10.1016/j.otsr.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Lopiz Y, García-Fernández C, Arriaza A, Rizo B, Marcelo H, Marco F. Midterm outcomes of bone grafting in glenoid defects treated with reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(9):1581–1588. doi: 10.1016/j.jse.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 44.• Otto A, Scheiderer B, Murphy M, Savino A, Mehl J, Kia C et al. Biconcave glenoids show 3 differently oriented posterior erosion patterns. J Shoulder Elbow Surg. 2021;30(11):2620-8. 10.1016/j.jse.2021.04.028. Defines three distinct glenoid wear patterns via 3D CT imaging: posterior-superior, posterior-central, and posterior-inferior. [DOI] [PubMed]
- 45.Thorsness RJ, Griffin JW, Virk MS, Kupfer NY, O’Donnell P. Glenoid bone grafting in reverse total shoulder: graft size and position classification. American Shoulder and Elbow Surgeons Specialty Day; Orlando, FL; 2016.
- 46.Boileau P, Morin-Salvo N, Gauci MO, Seeto BL, Chalmers PN, Holzer N, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management of glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133–2142. doi: 10.1016/j.jse.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 47.Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558–2567. doi: 10.1007/s11999-011-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.•• Boileau P, Morin-Salvo N, Bessière C, Chelli M, Gauci MO, Lemmex DB. Bony increased-offset-reverse shoulder arthroplasty: 5 to 10 years’ follow-up. J Shoulder Elbow Surg. 2020;29(10):2111-22. 10.1016/j.jse.2020.02.008. Case series demonstrating excellent functional outcomes of the BIO-RSA implant at 75 months follow-up, with low revision rate and consistent graft healing. [DOI] [PubMed]
- 49.•• Franceschetti E, Ranieri R, Giovanetti de Sanctis E, Palumbo A, Franceschi F. Clinical results of bony increased-offset reverse shoulder arthroplasty (BIO-RSA) associated with an onlay 145° curved stem in patients with cuff tear arthropathy: a comparative study. J Shoulder Elbow Surg. 2020;29(1):58–67. 10.1016/j.jse.2019.05.023. Comparative cohort study showing similar outcomes between standard rTSA and BIO-RSA at 2 year follow-up. [DOI] [PubMed]
- 50.•• Tashjian RZ, Broschinsky K, Stertz I, Chalmers PN. Structural glenoid allograft reconstruction during reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2020;29(3):534-40. 10.1016/j.jse.2019.07.011. Case series demonstrating restoration of glenoid anatomy, high rates of bony incorporation and low revision rates of rTSA with allograft reconstruction of severe glenoid defects. [DOI] [PubMed]
- 51.Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg. 2016;25(9):1425–1432. doi: 10.1016/j.jse.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Michael RJ, Schoch BS, King JJ, Wright TW. Managing glenoid bone deficiency-the augment experience in anatomic and reverse shoulder arthroplasty. Am J Orthop (Belle Mead, NJ). 2018;47(3). 10.12788/ajo.2018.0014. [DOI] [PubMed]
- 53.•• Kirsch JM, Patel M, Singh A, Lazarus MD, Williams GR, Namdari S. Early clinical and radiographic outcomes of an augmented baseplate in reverse shoulder arthroplasty for glenohumeral arthritis with glenoid deformity. J Shoulder Elbow Surg. 2021;30(7s):S123-s30. 10.1016/j.jse.2020.12.010. Retrospective case series demonstrating that primary rTSA with augmented baseplate yields excellent clinical outcomes and deformity correction at minimum 1 year follow-up. [DOI] [PubMed]
- 54.• Virk M, Yip M, Liuzza L, Abdelshahed M, Paoli A, Grey S et al. Clinical and radiographic outcomes with a posteriorly augmented glenoid for Walch B2, B3, and C glenoids in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2020;29(5):e196-e204. 10.1016/j.jse.2019.09.031. Study demonstrating excellent clinical and radiographic outcomes of primary rTSA with 8º posterior augmented glenoid baseplate at 40 months follow-up. [DOI] [PubMed]
- 55.•• Liuzza L, Mai DH, Grey S, Wright TW, Flurin PH, Roche CP et al. Reverse total shoulder arthroplasty with a superior augmented glenoid component for favard type-E1, E2, and E3 glenoids. J Bone Joint Surg Am Vol. 2020;102(21):1865-73. 10.2106/jbjs.19.00946. Retrospective review demonstrating successful short-term results of augmented glenoid baseplates for superior glenoid wear patterns. [DOI] [PubMed]
- 56.Gulotta LV, Grey SG, Flurin P-H, Wright TW, Zuckerman JD, Roche CP. Clinical outcomes of augmented rTSA glenoid baseplates. Semin Arthroplasty JSES. 2021;31(4):810–815. doi: 10.1053/j.sart.2021.05.010. [DOI] [Google Scholar]
- 57.• Levin JM, Bokshan S, Roche CP, Zuckerman JD, Wright T, Flurin PH et al. Reverse shoulder arthroplasty with and without baseplate wedge augmentation in the setting of glenoid deformity and rotator cuff deficiency-a multicenter investigation. J Shoulder Elbow Surg. 2022. 10.1016/j.jse.2022.04.025. Multicenter study comparing augmented versus enonaugmented baseplates in 4TSA, demonstrating greater postoperative range of motion improvements in the augmented group. [DOI] [PubMed]
- 58.Charles MD, Naylor AJ, Chan W, Cvetanovich GL, Nicholson GP. Reliable correction of beta-angle with augmented glenoid baseplates in reverse total shoulder arthroplasty. European Society for Surgery of the Shoulder and Elbow Annual Congress; Virtual; 2020.
- 59.Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis. 2013;2015(73 Suppl 1):S129–S135. [PubMed] [Google Scholar]
- 60.• Colley R, Polisetty TS, Levy JC. Mid-term outcomes of reverse shoulder arthroplasty using the alternative center line for glenoid baseplate fixation: a case-controlled study. J Shoulder Elbow Surg. 2021;30(2):298-305. 10.1016/j.jse.2020.05.012. Retrospective case control study demonstrating similar patient outcomes and lower complication rate of rTSA implanted using the alternative center line compared to the anatomic center line. [DOI] [PubMed]
- 61.Holzgrefe RE, Hao KA, Panther EJ, Schoch BS, Roche C, King JJ, et al. Early clinical outcomes following navigation-assisted baseplate fixation in reverse total shoulder arthroplasty: a matched cohort study. J Shoulder Elbow Surg. 2022 doi: 10.1016/j.jse.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Levy JC, Everding NG, Frankle MA, Keppler LJ. Accuracy of patient-specific guided glenoid baseplate positioning for reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(10):1563–1567. doi: 10.1016/j.jse.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 63.Throckmorton TW, Gulotta LV, Bonnarens FO, Wright SA, Hartzell JL, Rozzi WB, et al. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: a multi-surgeon study in 70 arthritic cadaver specimens. J Shoulder Elbow Surg. 2015;24(6):965–971. doi: 10.1016/j.jse.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Lau SC, Keith PPA. Patient-specific instrumentation for total shoulder arthroplasty: not as accurate as it would seem. J Shoulder Elbow Surg. 2018;27(1):90–95. doi: 10.1016/j.jse.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Dines DM, Gulotta L, Craig EV, Dines JS. Novel solution for massive glenoid defects in shoulder arthroplasty: a patient-specific glenoid vault reconstruction system. Am J Orthop (Belle Mead NJ) 2017;46(2):104–108. [PubMed] [Google Scholar]
- 66.• Bodendorfer BM, Loughran GJ, Looney AM, Velott AT, Stein JA, Lutton DM et al. Short-term outcomes of reverse shoulder arthroplasty using a custom baseplate for severe glenoid deficiency. J Shoulder Elbow Surg. 2021;30(5):1060-7. 10.1016/j.jse.2020.08.002. Case series demonstrating the short-term safety and efficacy of rTSA using the glenoid vault reconstruction system. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.