Abstract
Systemic effects associated with hormones and cytokines secreted by tumor cells can cause paraneoplastic syndrome. Leukemoid reactions and hypercalcemia are relatively common manifestations of paraneoplastic syndrome. Here, we describe the case of a 90-year-old woman who presented with leukocytosis and hypercalcemia and was diagnosed with granulocyte-colony stimulating factor (G-CSF)-producing cervical cancer with elevated levels of parathyroid hormone-related protein (PTHrP). The patient visited our hospital complaining of general fatigue and anorexia. On admission, she presented with marked leukocytosis, hypercalcemia, and an increase in C-reactive protein level. On the basis of abdominal magnetic resonance imaging and histopathological examination, the patient was diagnosed with cervical cancer. Additional tests confirmed elevated plasma levels of G-CSF, PTHrP, and serum interleukin-6. Immunostaining of pathological specimens of the uterine cervix showed expression of G-CSF in tumor cells. The patient was diagnosed with G-CSF-producing cervical cancer accompanied by elevation of PTHrP levels. As a treatment for hypercalcemia, discontinuation of oral vitamin D derivative and administration of saline and elcatonin were ineffective, and therapeutic intervention with zoledronic acid hydrate was required. Considering the patient’s advanced age, surgical resection of cervical cancer was not performed. She died from congestive heart failure approximately 3 months after hospitalization. This case was indicated to be a paraneoplastic syndrome in which G-CSF and PTHrP-induced leukocytosis and hypercalcemia. To the best of our knowledge, there have been no reports of G-CSF-producing cervical cancer with elevated PTHrP levels, and our case is the first report.
Keywords: Granulocyte-colony stimulating factor, Parathyroid hormone-related protein, Hypercalcemia, Paraneoplastic syndrome, Cervical cancer
Introduction
Leukocytosis is a common paraneoplastic syndrome reported in various organs, particularly the lungs [1, 2]. Serum granulocyte-colony stimulating factor (G-CSF) concentrations are elevated in many cases, and it is assumed that G-CSF-producing tumors are involved in pathogenesis [2]. Hypercalcemia is another common paraneoplastic syndrome. Malignancy-associated hypercalcemia (MAH) can be broadly classified into four types [3]. The first is humoral hypercalcemia of malignancy (HHM) caused by parathyroid hormone-related protein (PTHrP), which is overproduced by tumor cells. Hypercalcemia is induced through mechanisms such as the promotion of bone resorption and increased reabsorption of calcium in the kidneys. The second type is local osteolytic hypercalcemia (LOH), which is an osteolytic metastasis with the local release of cytokines. The third type, which is rarer than the two above, is a pattern in which the tumor releases 1, 25-(OH)2 vitamin D. The fourth type recently proposed is ectopic parathyroid hormone secretion. Although rare, concomitant manifestations of hypercalcemia and leukocytosis have been observed in the same tumor. They have been proposed to be classified as a single clinical entity, termed hypercalcemia-leukocytosis syndrome [4].
In this paper, we report a case of cervical cancer presenting with leukocytosis and hypercalcemia, which was finally diagnosed as a G-CSF-producing tumor accompanied by elevation of PTHrP levels. To date, very few articles have been published on G-CSF-producing tumors with elevated levels of PTHrP. In particular, no case reports on cervical cancer have been published. We present an extremely rare case with a clinical course and a review of the literature.
Case report
A 90-year-old woman visited our facility with chief complaints of general malaise and anorexia. As part of her medical history, she was treated for hypertension, osteoporosis, and reflux esophagitis. On admission, she had a blood pressure of 133/61 mmHg, a heart rate of 80 beats/min, and a body temperature of 36.2 °C. The Glasgow coma scale score was E4V5M6, with no apparent disturbance of consciousness. Physical examination revealed a slight tenderness of the upper abdomen. No abnormal findings were observed in the head, neck, lungs, or heart. No obvious skin rashes, edema, or bedsores were observed in the extremities or trunk. Hematological tests indicated leukocytosis with a white blood cell (WBC) count of 27,000/μL. The hemoglobin level and platelet counts were 11.3 g/dL and 30.5 × 104/μL, respectively. Laboratory investigations revealed hypercalcemia and elevated levels of C-reactive protein (CRP), with a corrected calcium concentration of 14.0 mg/dL and CRP level of 7.69 mg/dL. As a test related to hypercalcemia, parathyroid hormone levels were suppressed at 12 pg/mL (normal range 15–65 pg/mL), and 1, 25-(OH)2 vitamin D concentration was 29 pg/mL (normal range 20–60 pg/mL), which were within the normal range. Elevation of serum angiotensin I-converting enzyme levels and monoclonal protein was not detected. Tuberculosis-specific interferon-γ assay was negative. No acute renal injury or liver dysfunction was observed. Details of the clinical laboratory data are presented in Table 1.
Table 1.
Clinical laboratory findings on admission
| Hematological test | ALT | 15 | U/L | ACE | 8.7 | ||
| WBC | 27,000 | /uL | γGTP | 23 | U/L | Serum immunoelectrophoresis | Negative |
| Neutro | 90 | % | ALP | 100 | U/L | Infection-related survey | |
| Lympho | 6 | % | LDH | 144 | U/L | Blood culture (vein) | Negative |
| Mono | 3 | % | Amy | 37 | U/L | Sputum culture | Negative |
| Eosino | 1 | % | CK | 23 | U/L | HBs-Ag | Negative |
| RBC | 356 | × 104/uL | CRP | 7.69 | mg/dL | HCV-Ab | Negative |
| Hb | 11.3 | g/dL | Glu | 177 | mg/dL | IFN-γ release assay | negative |
| Plt | 30.5 | × 104/uL | 1,25-(OH)2vitaminD | 29 | pg/mL | β-D glucan | 8.5 |
| Laboratory investigation | Endocrine study | Tumor markers | |||||
| TP | 6.9 | g/dL | PTH intact | 12 | pg/mL | CEA | 2.3 |
| Alb | 2.8 | g/dL | TSH | 0.55 | uIU/mL | CA19-9 | 7 |
| UN | 10.9 | mg/dL | Free T3 | 1.08 | pg/mL | CA125 | 14 |
| Cre | 0.73 | mg/dL | Free T4 | 1.46 | ng/dL | IL2R | 623 |
| eGFR | 56 | mL/min/1.73 m2 | Immunological analysis | Urinalysis | |||
| UA | 5.2 | mg/dL | IgG | 1735 | mg/dL | pH | 6.0 |
| Na | 139 | mmol/L | IgA | 240 | mg/dL | Protein | ( −) |
| K | 2.7 | mmol/L | IgM | 148 | mg/dL | Occult blood | (+ / −) |
| Cl | 97 | mmol/L | C3 | 127 | mg/dL | Glu | ( −) |
| Ca | 12.9 | mg/dL | C4 | 28 | mg/dL | WBC | (+ / −) |
| Corrected Ca | 14.0 | mg/dL | CH50 | 49 | U/mL | Urinary sediment | |
| P | 2.8 | mg/dL | ANA | 40 > | RBC | 1–4 | |
| T-bil | 0.50 | mg/dL | MPO-ANCA | 0.6 | IU/mL | WBC | 5–9 |
| AST | 22 | U/L | PR3-ANCA | 0.5 > | IU/mL | 24-h urinary protein | 0.17 |
WBC white blood cell, Neutro neutrophil, Lympho lymphocyte, Mono monocyte, Eosino eosinophil, RBC red blood cell, Hb hemoglobin, Plt platelet, TP total protein, Alb albumin, UN urea nitrogen, Cre creatinine, eGFR estimated glomerular filtration rate, UA uric acid, Na sodium, K potassium, Cl chloride, Ca calcium, P phosphorus, T-bil total bilirubin, AST aspartate aminotransferase, ALT alanine aminotransferase, γGTP gamma glutamyl transpeptidase, ALP alkaline phosphatase, LDH lactate dehydrogenase, Amy amylase, CK creatine kinase, CRP C-reactive protein, Glu glucose, PTH parathyroid hormone, TSH thyroid stimulating hormone, T3 triiodothyronine, T4 thyroxine, Ig immunoglobulin, C3 complement component 3, C4 complement component 4, CH50 complement activity, ANA anti-nuclear antibody, MPO myeloperoxidase, PR3 proteinase3, ANCA anti-neutrophil cytoplasmic antibody, ACE angiotensin converting enzyme, HBs hepatitis B surface, Ag antigen, HCV hepatitis C virus, Ab antibody, IFN interferon, CEA carcinoembryonic antigen, CA carbohydrate antigen, IL2R interleukin-2 receptor, HPF high power field
Drug-induced hypercalcemia was suspected due to an oral vitamin D derivative (eldecalcitol), which was administered for osteoporosis, and the drug was discontinued. Furthermore, saline infusion and elcatonin were administered, but therapeutic efficacy was insufficient and hypercalcemia persisted. A bacterial infection with leukocytosis and increased CRP level was assumed, and antibiotic treatment (tazobactam/piperacillin hydrate) was started. However, its response to antimicrobial treatment is poor. Pathogenic bacteria were not detected in blood and sputum cultures prior to treatment, and fever and infection focus on imaging findings were not detected. Therefore, bacterial infection was ruled out and the antibacterial agent was discontinued. An abdominal computed tomography (CT) scan was performed as a screening test to find the cause of the high inflammatory response and showed a diffuse low absorption area in the uterus. Abdominal magnetic resonance imaging demonstrated diffuse thickening of the uterine wall and high signal intensity on diffusion-weighted imaging (Fig. 1). Imaging findings suggested gynecological malignancy, and biopsy confirmed cervical cancer. Hematoxylin and eosin staining of the specimen revealed necrotic tissue and multinucleated squamous cell carcinoma (Fig. 2). Systemic CT tomography revealed no bone metastasis of the malignancy. The clinical staging of cervical cancer corresponded to stage III.
Fig. 1.
Magnetic resonance imaging of the uterus. a T2-weighted fat-suppressed image showed diffuse thickening of the uterus. Arrows indicate the uterus. b The diffusion-weighted image revealed a diffuse high signal of the uterus
Fig. 2.
Histopathological findings of the cervix. a Necrotic findings are observed in cervical tissue. Hematoxylin and eosin (HE) staining, original magnification 100 × . Scale bar = 200 μm. b Arrow heads indicate squamous cell carcinoma of the cervix, showing multinucleated cell morphology. HE staining, original magnification 400 × . Scale bar = 50 μm
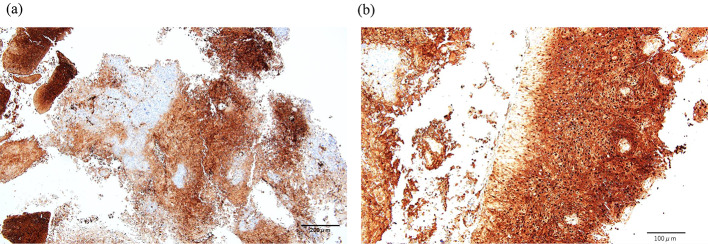
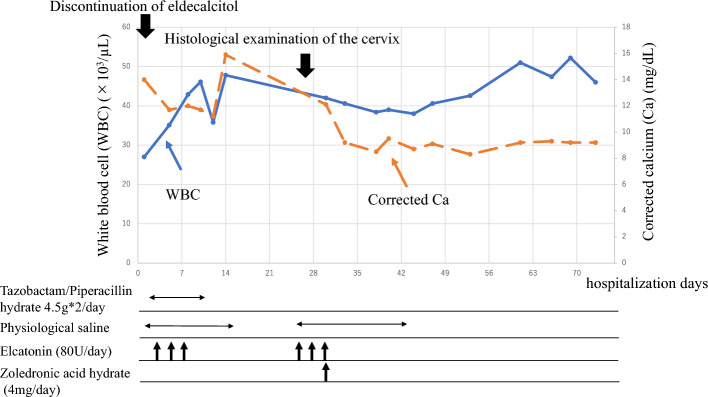
Refractory leukocytosis, hypercalcemia, and elevated CRP levels indicated a paraneoplastic syndrome based on cervical cancer as a differential diagnosis. Plasma G-CSF and PTHrP and serum interleukin (IL)-6 concentrations were measured at 106 pg/mL (normal range 10.5–57.5 pg/mL), 9.3 pmol/L (normal range 1.1 ≧ pmol/L), and 85 pg/mL (normal range 7.0 ≧ pg/mL), respectively, with abnormally high values. Furthermore, immunostaining of the uterocervical specimen showed G-CSF expression in tumor cells (Fig. 3). Based on these findings, a diagnosis of G-CSF-producing cervical cancer with leukocytosis and hypercalcemia due to paraneoplastic syndrome was made. Although hypercalcemia improved after the administration of zoledronic acid hydrate, no radical treatment for cervical cancer was performed and the patient died approximately 3 months after admission. The clinical course of this case is shown in Fig. 4.
Fig. 3.
Immunostaining of the cervix with antigranulocyte colony stimulating factor (G-CSF) antibody [CSF3/3166R] (Abcam, United Kingdom). a The expression of G-CSF (dark brown area) is observed in the cervical specimen. Original magnification 100 × , Scale bar = 200 μm. b Cervical tumor cells are strongly stained. Original magnification 200 × , Scale bar = 100 μm
Fig. 4.
Clinical course in this case. The antibacterial drug was ineffective and leukocytosis persisted. Hypercalcemia was refractory to the withdrawal of vitamin D derivative and administration of saline and elcatonin. Bisphosphonate (zoledronic acid hydrate) was effective and normalized the corrected calcium level
Discussion
Here, we describe a rare case of cervical cancer with leukocytosis and hypercalcemia. Immunohistological staining demonstrated G-CSF secretion in cervical cancer, with elevated plasma levels of G-CSF and PTHrP. These findings indicate that the main pathophysiology, in this case, was paraneoplastic syndrome. To our knowledge, this is the first report of a G-CSF-producing cervical cancer with elevated levels of PTHrP.
G-CSF is a glycoprotein that promotes the proliferation and differentiation of neutrophil progenitor cells in the bone marrow. Paraneoplastic production of G-CSF was first reported in a patient with lung cancer [5]. Since then, patients with various types of nonhematologic cancers have been reported [6, 7]. Transplantation of tumor cells into mouse models has been reported to induce neutrophilia caused by tumor cell-derived cytokines [8]. Furthermore, inflammatory cytokines, including IL-6, have been suggested to be released from tumor cells along with G-CSF [9]. Production of inflammatory substances, such as G-CSF and IL-6, causes leukocytosis and an increase in the inflammatory response, and there are cases in which it is misdiagnosed as an infectious disease. Therefore, the diagnosis of malignant neoplasms can be delayed. Early diagnosis of tumors is important to improve treatment strategies and prognosis. The following diagnostic criteria for G-CSF-producing cancer have been proposed: (a) abnormally high WBC count in peripheral blood without evidence of infection, (b) increased concentration of G-CSF in blood samples, (c) decreased WBC count or G-CSF level after cancer treatment, and (d) expression of G-CSF in tumor tissues [5]. G-CSF-producing squamous cell carcinoma often has multinucleated cell morphology [10]. The findings observed in the present case are as follows: (1) leukocytosis without infectious diseases, (2) high plasma levels of G-CSF with elevated serum IL-6 concentration, and (3) expression of G-CSF in cervical tissue with a pathological image of multinucleated squamous cell carcinoma. In our case, the transition of the WBC count and the G-CSF level after cancer treatment was unknown, but was finally considered consistent with G-CSF-producing cervical cancer.
MAH is classified into four types: HHM associated with the production of PTHrP, LOH mainly composed of osteolysis, 1, 25-(OH)2 vitamin D secretion caused by tumors, and ectopic hyperparathyroidism [3]. The frequency of HHM has been reported to be high, accounting for up to 80% of MAH [11]. Patients with HHM associated with PTHrP often present with tumors of head and neck, lung, breast, kidney, bladder, and adult T-cell leukemia [12]. These patients generally have advanced conditions and are reported to have poor prognosis [13]. The diagnosis of HHM caused by PTHrP requires the exclusion of LOH, 1, 25- (OH) 2 vitamin D-producing tumors, primary or ectopic hyperparathyroidism, drug-induced hypercalcemia, and granulomatous diseases. In this case, the improvement in hypercalcemia was insufficient even with discontinuation of vitamin D derivative (eldecalcitol), and no imaging studies suggesting bone metastasis, tuberculosis, and sarcoidosis were observed. Furthermore, based on clinical laboratory findings, HHM caused by elevated PTHrP levels was diagnosed. PTHrP-related HHM cases generally have higher serum calcium levels and more severe hypercalcemic-related clinical symptoms than primary hyperparathyroidism [14]. In our case, the patient was refractory to discontinuation of vitamin D derivative, saline infusion, and the use of calcitonin; however, hypercalcemia improved after administration of bisphosphonate (zoledronic acid hydrate). Bisphosphonates, which inhibit osteoclast differentiation and function, are an effective treatment option for MAH. In particular, zoledronic acid has been shown to be useful for MAH in randomized-controlled clinical trials [15].
G-CSF-producing tumors with elevated PTHrP levels have been reported in the glossopharynx, lung, bile duct, gallbladder, pancreas, and urinary organs [16–23]. Although G-CSF or PTHrP production has been reported in gynecological malignancies [2, 24], there have been no case reports of coproduction. Yoneda et al. reported that of 225 patients with oral malignancies, five (2.2%) presented with leukocytosis and hypercalcemia. Unfortunately, G-CSF and PTHrP levels have not yet been reported. The occurrence of these two distinct paraneoplastic syndromes in the same patient was greater than expected by chance alone. Although its frequency is relatively uncommon, its association with hypercalcemia–leukocytosis syndrome has been reported [4]. In patients with lung cancer, both G-CSF and PTHrP-producing cancer cells have K-ras codon12 mutations, suggesting that this gene regulates the secretion of these two cytokines [25]. A literature review of case reports on G-CSF-producing tumors with elevated levels of PTHrP has been performed in the last decade (Table 2). Including our case, only eight cases have been reported [17–23]. Sex differences were more common in women (62.5%), with a mean age of 65.4 ± 15.9 years. The mean of each clinical laboratory data set was 36,650 ± 30,217 /μL of WBC, 15.1 ± 2.0 mg/dL of serum calcium, 140.8 ± 89.0 pg/mL of plasma G-CSF, and 12.1 ± 5.3 pmol/L of plasma PTHrP. Our case is the oldest compared to other reports, and the clinical laboratory findings were similar. Bisphosphonates are administered in most cases of hypercalcemia. All patients died during the clinical course, and the causes of death were primarily related to cancer progression. The prognosis for G-CSF- producing tumors with elevation of PTHrP levels is extremely poor. MAH associated with PTHrP is common in patients with advanced cancer [12], and it has been suggested that the tumor growth effect mediated by the autocrine mechanism of G-CSF may be associated with a poor prognosis [17]. Cachexia may also contribute to the poor prognosis. In addition to inflammatory cytokines, such as IL-6, PTHrP has been suggested to induce browning of white adipocytes, resulting in increased cachexia [26, 27].
Table 2.
Literature review of case reports on G-CSF-producing tumors with elevated levels of PTHrP
| No [reference] | Sex | Age | Primary tumor | Pathology | WBC (/µL) | Ca (mg/dL) | CRP (mg/dL) | G-CSF (pg/mL) (normal range) | PTHrP (pmol/L) (≦ 1.1) | Therapeutic intervention for hypercalcemia | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 [17] | Female | 76 | Gallbladder | SCC | 13,700 | 11.8 | 12.2 | 126 (≦ 39.0) | 6.9 | Fluid infusion, diuretic, and bisphosphonate | Progression of primary disease |
| 2 [18] | Male | 78 | Bile duct | Adeno carcinoma | 14,190 | 14.6 | 9.3 | 333.4 (≦ 39) | 6.0 | Bisphosphonate | Carcinomatous lymphangiomatosis |
| 3 [19] | Male | 65 | Pancreas | Anaplastic carcinoma | 13,000 | 14.3 | N/A | N/A | 13.0 | Fluid infusion, diuretic, steroid, elcatonin, and bisphosphonate | Cancerous peritonitis |
| 4 [20] | Male | 57 | Tongue | SCC | 22,770 | 16.3 | N/A | 60 (< 39) | 12.2 | Fluid infusion, steroid, elcatonin, and bisphosphonate | Cancerous pleuritis |
| 5 [21] | Female | 38 | Bladder | UC | 107,000 | 14.4 | 15.2 | 77.1 (≦ 39) | 11.1 | Elcatonin and bisphosphonate | Hemorrhagic cerebral infarction |
| 6 [22] | Female | 71 | Bladder | UC | 37,000 | 18.6 | N/A | 198 (< 30) | 24.4 | Denosumab | N/A |
| 7 [23] | Female | 48 | Pancreas | Adeno carcinoma | 58,540 | 17.1 | 13.8 | 85.1 (< 39) | 13.7 | Fluid infusion, diuretic, elcatonin, and bisphosphonate | Progression of primary disease |
| This case | Female | 90 | Uterine cervix | SCC | 27,000 | 14.0 | 7.7 | 106 (10.5–57.5) | 9.3 | Fluid infusion, elcatonin, and bisphosphonate | Congestive heart failure |
G-CSF granulocyte-colony stimulating factor, PTHrP parathyroid hormone-related protein, WBC white blood cells, Ca calcium, CRP C-reactive protein, SCC squamous cell carcinoma, UC urothelial carcinoma, N/A not available
In this paper, we reported a rare case of G-CSF-producing cervical cancer with elevated levels of PTHrP. In refractory and unidentified leukocytosis and hypercalcemia, it is important to make a differential diagnosis considering the possibility of paraneoplastic syndrome. Immediate initiation of therapeutic strategies, including surgical resection, chemotherapy, and radiotherapy, is required based on early diagnosis to improve prognosis.
Acknowledgements
The authors acknowledge Editage for providing editorial and publication support.
Declarations
Conflict of interest
The authors declare they have no competing interests in relation to this work.
Human and animal rights
This article does not contain studies with human participants or animals performed by any of the authors.
Informed consent
No information identifying individual patients was published, and personal information was protected. The patient’s family provided informed consent for the publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ascensao JL, Oken MM, Ewing SL, Goldberg RJ, Kaplan ME. Leukocytosis and large cell lung cancer. A frequent association. Cancer. 1987;60:903–905. doi: 10.1002/1097-0142(19870815)60:4<903::AID-CNCR2820600431>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Clark LH, Moll S, Houghton D, O'Connor S, Soper JT. Leukocytosis due to markedly elevated granulocyte-colony stimulating factor levels in a patient with endometrial cancer: case report and literature review. Gynecol Oncol Rep. 2017;20:5–8. doi: 10.1016/j.gore.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guise TA, Wysolmerski JJ. Cancer-associated hypercalcemia. N Engl J Med. 2022;386:1443–1451. doi: 10.1056/NEJMcp2113128. [DOI] [PubMed] [Google Scholar]
- 4.Yoneda T, Nishimura R, Kato I, Ohmae M, Takita M, Sakuda M. Frequency of the hypercalcemia-leukocytosis syndrome in oral malignancies. Cancer. 1991;68:617–622. doi: 10.1002/1097-0142(19910801)68:3<617::AID-CNCR2820680329>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Asano S, Urabe A, Okabe T, Sato N, Kondo Y. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977;49:845–852. doi: 10.1182/blood.V49.5.845.845. [DOI] [PubMed] [Google Scholar]
- 6.Granger JM, Kontoyiannis DP. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: a retrospective, single-institution study. Cancer. 2009;115:3919–3923. doi: 10.1002/cncr.24480. [DOI] [PubMed] [Google Scholar]
- 7.Nasser SM, Choudry UH, Nielsen GP, Ott MJ. A leukemoid reaction in a patient with a dedifferentiated liposarcoma. Surgery. 2001;129:765–767. doi: 10.1067/msy.2001.109498. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 9.Inoue M, Minami M, Fujii Y, Matsuda H, Shirakura R, Kido T. Granulocyte colony-stimulating factor and interleukin-6-producing lung cancer cell line. LCAM J Surg Oncol. 1997;64:347–350. doi: 10.1002/(SICI)1096-9098(199704)64:4<347::AID-JSO18>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Tohyama A, Kurita T, Shibahara M, Harada H, Ueda T, Matsuura Y, Hisaoka M, Yoshino K. Poorly differentiated cervical squamous cell carcinoma resembling giant cell carcinoma of the lung: extreme morphology of this tumor and its clinical course. J UOEH. 2022;44:263–267. doi: 10.7888/juoeh.44.263. [DOI] [PubMed] [Google Scholar]
- 11.Bhandari S, Kumar R, Tripathi P, Chan A, Mudra S, Redman R. Outcomes of hypercalcemia of malignancy in patients with solid cancer: a national inpatient analysis. Med Oncol. 2019;36:90. doi: 10.1007/s12032-019-1315-8. [DOI] [PubMed] [Google Scholar]
- 12.Stewart AF. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373–379. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 13.Gastanaga VM, Schwartzberg LS, Jain RK, Pirolli M, Quach D, Quigley JM, Mu G, Scott Stryker W, Liede A. Prevalence of hypercalcemia among cancer patients in the United States. Cancer Med. 2016;5:2091–2100. doi: 10.1002/cam4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grill V, Martin TJ. Hypercalcemia of malignancy. Rev Endocr Metab Disord. 2000;1:253–263. doi: 10.1023/A:1026597816193. [DOI] [PubMed] [Google Scholar]
- 15.Major P, Lortholary A, Hon J, Abdi E, Mills G, Menssen HD, Yunus F, Bell R, Body J, Quebe-Fehling E, Seaman J. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19:558–567. doi: 10.1200/JCO.2001.19.2.558. [DOI] [PubMed] [Google Scholar]
- 16.Furihata M, Sonobe H, Iwata J, Ido E, Ohtsuki Y, Asahi Y, Kubonishi I, Miyoshi I. Lung squamous cell carcinoma producing both parathyroid hormone-related peptide and granulocyte colony stimulating factor. Pathol Int. 1996;46:376–379. doi: 10.1111/j.1440-1827.1996.tb03624.x. [DOI] [PubMed] [Google Scholar]
- 17.Ueda K, Kinoshita A, Akasu T, Hagiwara N, Yokota T, Imai N, Iwaku A, Fushiya N, Koike K, Nishino H. A case of adenosquamous cell carcinoma of the gallbladder with markedly elevated PTHrP and G-CSF levels. Nihon Shokakibyo Gakkai Zasshi. 2016;113:1564–1571. doi: 10.11405/nisshoshi.113.1564. [DOI] [PubMed] [Google Scholar]
- 18.Ozawa N, Doi S, Tsujikawa T, Mabuchi M, Kajiyama Y, Sato K, Kikuchi K, Takahashi M, Kawamoto M, Yasuda I. Intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor and parathyroid hormone-related protein. Nihon Shokakibyo Gakkai Zasshi. 2017;114:1285–1292. doi: 10.11405/nisshoshi.114.1285. [DOI] [PubMed] [Google Scholar]
- 19.Seki H, Yasui N, Shimada A, Matsumoto H, Domoto H. Resection of a granulocyte colony-stimulating factor-producing anaplastic carcinoma of the pancreas, associated with humoral hypercalcemia of malignancy. Jpn J Cancer Chemother. 2018;45:859–862. [PubMed] [Google Scholar]
- 20.Kaneko N, Kawano S, Matsubara R, Goto Y, Jinno T, Maruse Y, Sakamoto T, Hashiguchi Y, Iida M, Nakamura S. Tongue squamous cell carcinoma producing both parathyroid hormone-related protein and granulocyte colony-stimulating factor: a case report and literature review. World J Surg Oncol. 2016;14:161. doi: 10.1186/s12957-016-0918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato T, Yasuda K, Iida H, Watanabe A, Fujiuchi Y, Miwa S, Imura J, Komiya A. Trousseau's syndrome caused by bladder cancer producing granulocyte colony-stimulating factor and parathyroid hormone-related protein: a case report. Oncol Lett. 2016;12:4214–4218. doi: 10.3892/ol.2016.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshi S, Numahata K, Morozumi K, Katumata Y, Kuromoto A, Takai Y, Hoshi K, Bilim V, Sasagawa I. Bladder cancer metastasis producing beta-human chorionic gonadotropin, squamous cell carcinoma antigen, granulocyte-colony stimulating factor, and parathyroid hormone-related protein. IJU Case Rep. 2018;2:47–50. doi: 10.1002/iju5.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada R, Tanaka K, Inoue H, Sakuno T, Harada T, Yoshizawa N, Miura H, Takeuchi T, Nakamura M, Katsurahara M, Hamada Y, Horiki N, Takei Y. Pancreatic adenocarcinoma producing parathyroid hormone-related protein. Case Rep Oncol Med. 2017;2017:5656130. doi: 10.1155/2017/5656130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savvari P, Peitsidis P, Alevizaki M, Dimopoulos M, Antsaklis A, Papadimitriou CA. Paraneoplastic humorally mediated hypercalcemia induced by parathyroid hormone-related protein in gynecologic malignancies: a systematic review. Onkologie. 2009;32:517–523. doi: 10.1159/000226209. [DOI] [PubMed] [Google Scholar]
- 25.Oshika Y, Nakamura M, Hatanaka H, Abe Y, Tokunaga T, Ohnishi Y, Kijima H, Yamazaki H, Tamaoki N, Ueyama Y. A human lung cancer xenograft producing granulocyte-colony stimulating factor and parathyroid hormone-related protein. Oncol Rep. 1998;5:359–362. doi: 10.3892/or.5.2.359. [DOI] [PubMed] [Google Scholar]
- 26.Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14:754–762. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi S, Hakuta M, Aiba K, Ito Y, Horikoshi N, Miura M, Hatake K, Ogata E. Elevation of circulating plasma cytokines in cancer patients with high plasma parathyroid hormone-related protein levels. Endocr Relat Cancer. 2003;10:403–407. doi: 10.1677/erc.0.0100403. [DOI] [PubMed] [Google Scholar]