Abstract
Background:
Due to the complexity of chronic kidney disease (CKD) pathophysiology, biomarkers representing different mechanistic pathways have been targeted for the study and development of novel biomarkers. The discovery of clinically useful CKD biomarkers would allow for the identification of those children at the highest risk of kidney function decline for timely interventions and enrollment in clinical trials.
Summary:
Glomerular filtration rate and proteinuria are traditional biomarkers to classify and prognosticate CKD progression in clinical practice but have several limitations. Over the recent decades, novel biomarkers have been identified from blood or urine with metabolomic screening studies, proteomic screening studies, and an improved knowledge of CKD pathophysiology. This review highlights promising biomarkers associated with the progression of CKD that could potentially serve as future prognostic markers in children with CKD.
Key Messages:
Further studies are needed in children with CKD to validate putative biomarkers, particularly candidate proteins and metabolites, for improving clinical management.
Keywords: Chronic kidney disease, Children, Biomarker, Metabolomics, MicroRNA
Introduction
The progression of chronic kidney disease (CKD) to kidney failure is associated with significant morbidity and mortality across all ages [1–3]. Although clinical management is mostly supportive and targeted to prevent complications, early recognition of CKD progression may allow for enrollment in clinical trials, timely interventions, and prompt counseling of patients and their families [4]. Biomarkers that improve clinical risk prediction and optimize prognostication of CKD progression may offer the opportunity for more individualized clinical management and improved outcomes.
The Limitations of Current Clinical Markers of CKD Progression
Currently, the clinically used markers of CKD progression are glomerular filtration rate (GFR) and urine albumin, and both have limitations [5, 6]. Among the many functions of the kidney, GFR reflects the volume of plasma filtered by the kidney per minute. The decline in GFR typically occurs later in the course of CKD after there has been substantial kidney injury, inflammation, and fibrosis which have resulted in nephron loss [7]. Further, the GFR trajectory is nonlinear with a higher rate of GFR decline in later stages of CKD [8], making it more challenging to prognosticate kidney failure from GFR measurements. In CKD, nephrons can develop compensatory hypertrophy and hyperfiltration leading to a normal or elevated GFR that might mislead the treating physician and affect the inferences of CKD research studies [9–11]. In most clinical settings, GFR is estimated using serum creatinine, a readily available and inexpensive marker. However, serum creatinine varies with age, sex, muscle mass, dietary habits, and medications [12]. Similarly, urine albumin is a marker that reflects glomerular injury, glomerular permeability, or tubular injury, which may not appear until the kidney has endured prolonged inflammation, cellular injury, inflammation, fibrosis, and glomerular hyperfiltration [4].
Considerations of CKD Biomarker Research in Children
Most novel biomarkers were first identified in adults and then studied in children. This trend is common in the development of biomarkers for many diseases as there is more incident disease in adults as well as dramatic growth and maturation during childhood, which may require age-specific biomarker reference ranges [13]. After birth, there is a robust increase in kidney blood flow and a neonate’s GFR will double within the first 2 weeks of life, reaching comparable GFR levels to adults by the age of 2 years. Additionally, kidney functions such as tubular reabsorption and urinary concentration abilities are not fully developed at birth and may take months or years to reach adult levels [10, 14]. Lastly, etiologies of CKD are different in children as compared to adults. The leading causes of CKD in children are congenital anomalies of the kidney and urinary tract followed by glomerulonephritis, while in adults, diabetes, and hypertension are the main causes of CKD [2, 10].
Metabolomics in CKD
Metabolites, such as amino acids, sugars, and lipids, are intermediate or end products of cellular metabolism. Metabolomic analysis of biological fluids (e.g., blood and urine) has shown promise as a method for novel biomarker discovery in kidney disease [15, 16]. Two main analytical platforms used are magnetic resonance spectroscopy and mass spectrometry, usually coupled with modern separation techniques (such as liquid/gas chromatography), to provide qualitative and quantitative descriptions of metabolites in biological systems. Targeted metabolomics analyses measure a defined group of metabolites based on prior knowledge, while non-targeted metabolomics includes a comprehensive measurement of a large number of analytes.
Metabolites of the Amino Acid Super Pathway
Tryptophan (Trp) is an essential amino acid needed for protein biosynthesis and serves as a precursor of metabolites along different biologic pathways (Fig. 1) [17]. Kidney diseases are linked to three Trp metabolic pathways: the indole, the kynurenine (Kyn), and the serotonin pathways [18]. Altered Trp and its downstream products were associated with kidney function and CKD progression in both children and adult studies [18]. Activation of Trp metabolism by inflammation leads to changes in levels of metabolites within the Kyn pathway and then the secretion of anti-inflammatory cytokines [17]. In a targeted metabolomic analysis of plasma samples collected in the Chronic Kidney Disease in Children (CKiD) study cohort, Trp, Kyn, and the ratio of Kyn to Trp were significantly associated with the stage of CKD [19]. Kyn and Kyn/Trp were also found to be associated with the onset and progression of CKD in large population-based cross-sectional studies, suggesting the potential to be used as prognostic markers [20, 21]. A study of the Chronic Renal Insufficiency Cohort (CRIC) found lower clearance of kynurenic acid, a degraded metabolite of Kyn, to be strongly associated with CKD progression in adults [22].
Fig. 1.

Novel plasma and urine biomarkers of chronic kidney disease progression. EGF, epidermal growth factor; HDL, high-density lipoprotein; KIM-1, kidney injury molecule-1; Kyn, kynurenine; LDL, low-density lipoprotein; MCP-1, monocyte chemoattractant protein-1; suPAR, soluble urokinase plasminogen activator receptor; TNFR1, TNF-α receptor type 1; TNFR2, TNF-α receptor type 2; Trp, tryptophan; VLDL, very-low-density lipoprotein; YKL-40, chitinase-3-like protein 1.
Some Trp derivatives through the indole and the Kyn pathways are uremic toxins such as indoxyl sulfate, asymmetric dimethylarginine (ADMA), and symmetric dimethylarginine (SDMA). These metabolites are normally excreted in the urine but are commonly observed to be accumulated in patients with CKD [23]. Based on liquid chromatography-quadrupole-time-of-flight mass spectrometry (LC-QTOF-MS), plasma concentrations of glycine, citrulline, ADMA, and SDMA were increased, regardless of creatinine level, in 32 children with CKD (aged 6–18 years) compared to healthy controls (aged 6–19 years) [24]. The ratio of SDMA to ADMA (SDMA/ADMA) was found to increase with greater CKD severity [19]. The accuracy of classifying early CKD samples and healthy controls was improved from 71% based on serum creatinine only to 89% based on SDMA, citrulline, and S-adenosylmethionine in addition to serum creatinine [25].
Metabolites of the Lipid Super Pathway
Lipids are vital to all living cells in that they are the main structural components of biological membranes, function as an energy reserve, are vital in signal transduction, and interact with proteins to modulate their functions. Lipid and lipoprotein abnormalities are frequently present in patients with CKD, including high triglyceride (TG) and TG-rich particle levels, low high-density lipoprotein (HDL) levels, and elevated small dense low-density lipoprotein (LDL) cholesterol levels [26, 27]. Growing evidence supports that lipid metabolic alterations potentially contribute to the progression of CKD, as well as cardiovascular disease [26, 28]. To date, many comprehensive studies have used mass spectrometers to study lipid profiles and provided insights into lipid alterations in CKD. Fatty acid profiles and acylcarnitine profiles are both associated with advancing stages of CKD and are potential predictors of CKD progression [29]. In the Clinical Phenotyping Resource and Biobank Core (CPROBE) cohort of 1,235 adults, CKD progression from stage 2 to stage 5 was associated with a higher abundance of plasma-saturated C16-C20 free fatty acids and a lower abundance of long-chain-to-intermediate-chain acylcarnitine ratio [30]. However, evidence for the pediatric CKD population is sparse. Mass spectrometric analysis of urine from 28 children discovered that patients with focal segmental glomerulosclerosis had increased urinary levels of fatty acid and lysophosphatidylcholines but decreased phosphatidylcholine levels, compared to healthy controls and children with minimal change disease; this likely resulted from increased metabolism of phosphatidylcholine [30]. Additional research is needed to understand the CKD-associated lipids and lipoproteins and to identify diagnostic and prognostic markers in pediatric patients.
Metabolites Identified in Non-Targeted Analyses
Non-targeted metabolomics analysis is not restricted to a limited number of known metabolites and thus can allow for the exploration of a rare biological condition. This approach can be advantageous in order to identify novel metabolites and metabolic pathways; however, it may be challenging to identify metabolites that exist at low concentrations [31].
Using plasma samples from 645 CKiD participants (aged 6 months to 16 years with a baseline eGFR of 30–90 mL/min/1.73 m2), ultra-high performance liquid chromatography-tandem mass spectrometry was used to identify seven metabolites (N6-carbamoylthreonyladenosine, 5,6-dihydrouridine, pseudouridine, C-glycosyltryptophan, lanthionine, 2-methylcitrate/homocitrate, and gulonate) which were associated with progression to kidney failure or a 50% decline in eGFR among those with a baseline eGFR ≥60 mL/min per 1.73 m2. The strongest associations, which were also consistently observed in bootstrapped samples, were for 5,6-dihydrouridine with an adjusted hazard ratio (aHR) of 16.94 (95% confidence interval [CI]: 5.26–54.63), C-glycosyltryptophan (aHR: 24.11; 95% CI: 6.15–94.56), and pseudouridine (aHR: 39.35; 95% CI: 7.73–200.39) [32]. In children with a baseline eGFR <60 mL/min per 1.73 m2, tetrahydrocortisol sulfate was protective for CKD progression [32]. In adults, C-glycosyltryptophan and pseudouridine were found to be strongly negatively correlated with eGFR and significantly associated with a rapid decline of eGFR [33, 34]. A study using published results from three genome-wide association studies (with a sample size >1,500 individuals) and a bidirectional Mendelian randomization approach found that lower eGFR is associated with higher blood metabolite levels of the Trp pathway including Kyn, C-glycosyltryptophan, 3-indoxyl sulfate, and indole-3-lactate [35].
Significant changes in LC-QTOF-MS-based plasma levels of sphingosine-1-phosphate, n-butyrylcarnitine, cis-4-decenoylcarnitine, and bilirubin were observed in pediatric patients with CKD; and overall performance of correctly classifying CKD patients and controls based on these five metabolites was 96%. Performance was even better when only early CKD (stage 2) patients were considered [36]. A large cohort study with 1,288,905 newborns (CKD occurred in 0.16% with a median time of 612 days) identified amino acids and acylcarnitines that were significantly associated with CKD, as well as ratios of amino acids and acylcarnitines including alanine-to-17 hydroxyprogesterone ratio (adjusted odds ratio [aOR]: 1.35; 95% CI: 1.07–1.70 per log unit increase) and phenylalanine-to-glycine ratio (aOR: 1.30; 95% CI: 1.08–1.56 per log unit increase), which together improved the performance of a CKD prediction model [37]. Validation studies are needed to further examine the relationships between the newly identified metabolites and kidney disease in a pediatric patient population.
MICRORNAS in CKD
MicroRNAs (miRNAs) are short noncoding RNAs that play important roles in regulating post-transcriptional gene expression [38]. Studies have identified clusters of miRNAs that are highly expressed in the kidney, and some are specifically involved in kidney developmental and pathological processes, such as podocyte development, kidney fibrotic transformation, and inflammation [39–41]. Circulating exosomal miRNAs in body fluids are usually found in extracellular vesicles (Fig. 1) and thus protected from nuclease degradation [42, 43]. With these features, changes in levels of miRNAs contained in extracellular vesicles may be used to detect kidney disease progression. Reduced levels of circulating miRNAs were observed in patients with advanced CKD (stage 4 CKD and kidney failure) compared to those with normal kidney function or moderate CKD (stage 3 CKD) [44].
Improved methods to isolate, store, and quantify miRNAs have contributed to the expanded knowledge of miRNA sequence and function [45, 46]. Polymerase chain reaction and microarrays (i.e., biochips) are two commonly used detection methods: the former has high sensitivity and specificity, and the latter has the advantage of not being influenced by the preselection of genes and allows high-throughput screening for a larger number of miRNAs [47]. Next-generation RNA sequencing methods enable novel sequence discovery in a high-throughput manner with high sensitivity and specificity, while both polymerase chain reaction and microarrays are limited to known sequences [46, 48].
With a further understanding of CKD etiology and the advent of detection methods, more promising novel biomarker candidates have been discovered. The latest discovery of miRNAs in CKD, with an emphasis on specific etiologies of kidney disease (e.g., diabetic kidney disease, hypertensive nephropathy), have been summarized [49–51]. Of note, existing evidence is mostly from ex vivo or in vivo studies, and there remain major challenges in translating experimental findings to clinical use. Utilizing miR-NAs, particularly those carried by extracellular vesicles in blood and urine, as noninvasive diagnostic, prognostic, and therapeutic biomarkers appear promising, but additional epigenetic and validation studies are needed.
Targeted Biomarkers of CKD Progression
Due to the complexity of CKD pathophysiology, biomarkers representing different mechanistic pathways have been targeted for the study and development of novel biomarkers. Here, we describe blood and urine biomarkers that have been observed to have strong associations with CKD progression in children and adults (Fig. 2). Targeted biomarkers which improve risk prediction could help guide clinical care, resource allocation, and for prognostic enrichment in clinical trial enrollment (Fig. 3). Of note, the literature on targeted CKD biomarkers is in part driven by assay development and therefore the largest bodies of literature are found on biomarkers that have been most studied and not necessarily on markers that show the best prognostic performance.
Fig. 2.
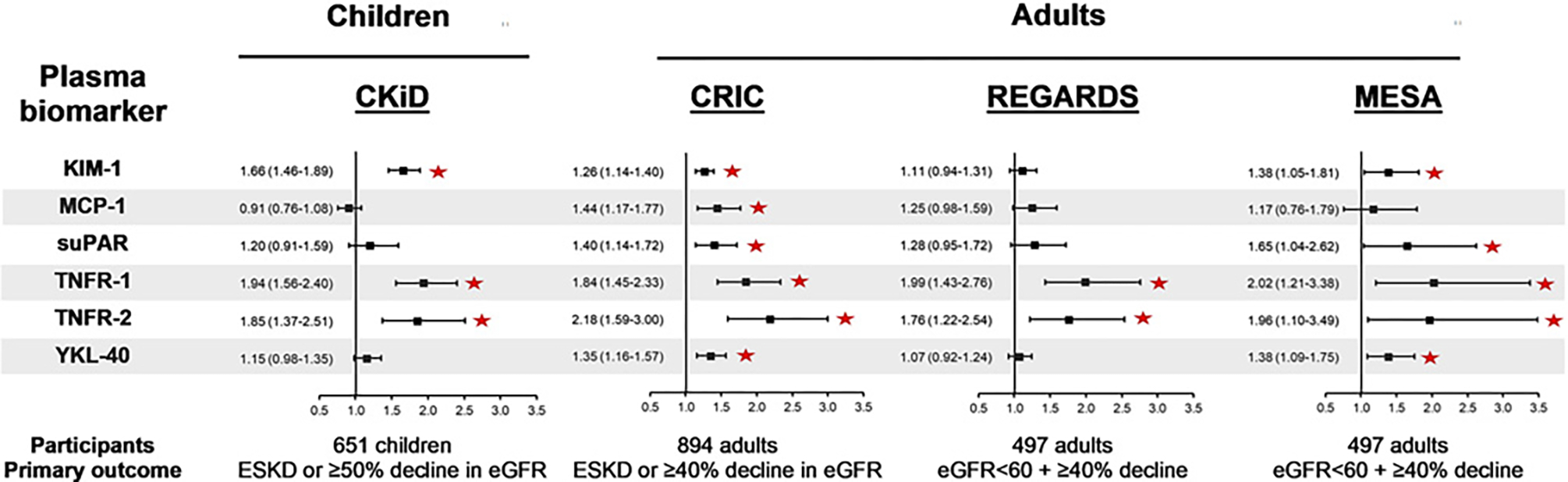
Fully adjusted hazard ratio with 95% confidence interval for CKD progression per doubling of plasma biomarkers in selected cohorts. eGFR unit is mL/min per 1.73 m2. * sign indicates statistically significant association. CKiD, Chronic Kidney Disease in Children cohort study; CRIC, Chronic Renal Insufficiency Cohort study; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; MESA, Multi-Ethnic Study of Atherosclerosis study; REGARDS, Reasons for Geographic and Racial Differences in Stroke study; suPAR, soluble urokinase plasminogen activator receptor; TNFR1, TNF-a receptor type 1; TNFR2, TNF-a receptor type 2; YKL-40, chitinase-3-like protein-1.
Fig. 3.
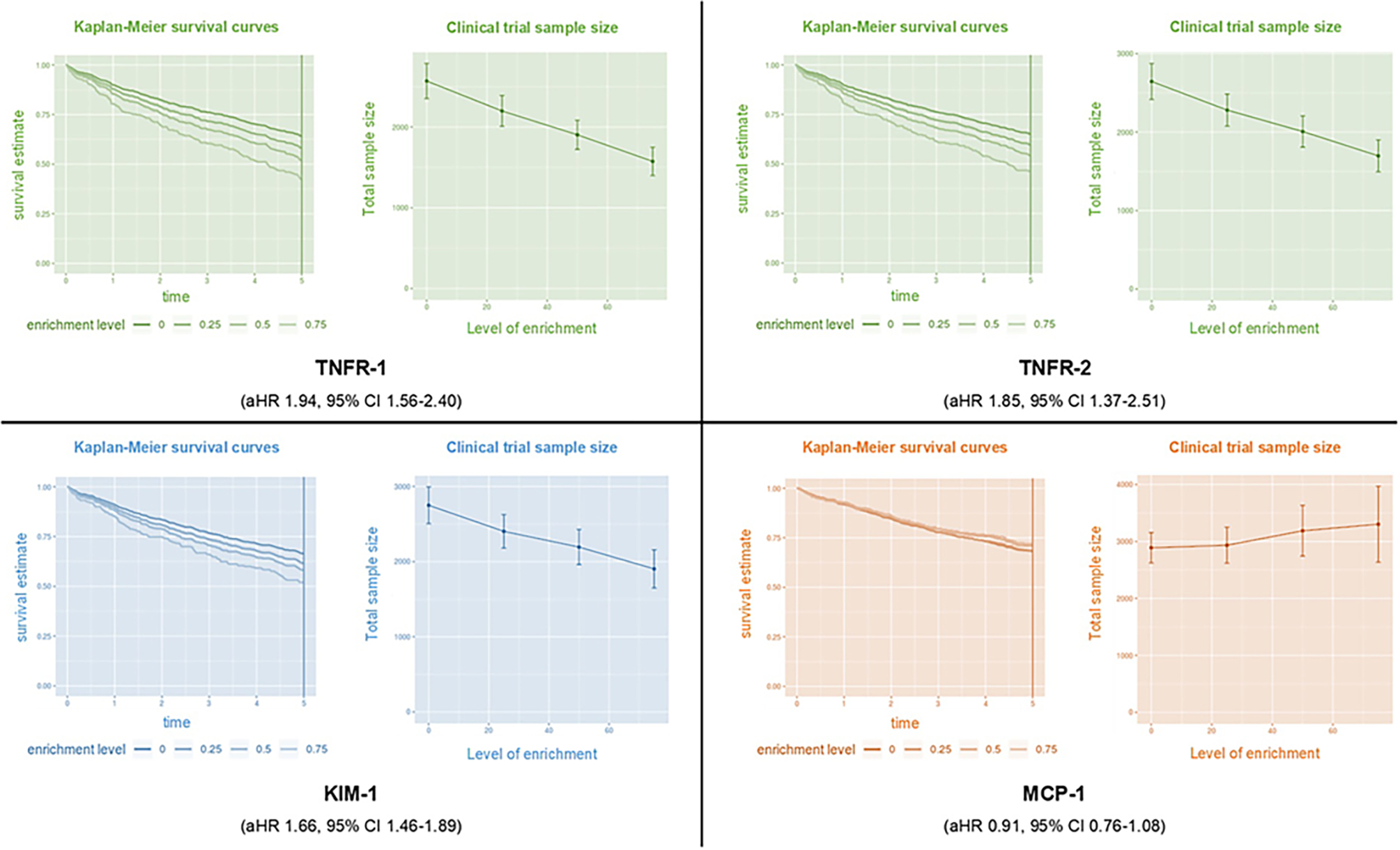
Biomarker prognostic enrichment tool of trials application on selected plasma biomarkers for chronic kidney disease (CKD) progression in children. Based on the rate of events (CKD progression) observed in the Chronic Kidney Disease in Children (CKiD) study and the associations of biomarkers with CKD progression (adjusted hazard ratio per doubling of biomarker), we demonstrate the time in years to develop CKD progression and number of participants needed, based on the level of enrichment. Clinical trial enrichment is an enrollment strategy to enroll participants that are more likely to develop the outcome of interest based on their characteristics. This strategy allows for enrolling fewer patients, not enrolling patients who are unlikely to develop the primary outcome, and reducing the cost of a clinical trial. Parameters used in the biomarker prognostic enrichment tool for survival outcomes: simulated sample size 1,000, 5 years observation period, survival rate 0.66 (34% of children developed CKD progression within 5 years), effect size (hazard ratio per doubling of plasma biomarker), enrichment levels 0.25 steps between 0 and 0.75, trial length of 5 years, treatment hazard ratio for sample size calculation of 0.8.Using TNFR-1 as an example, with a 75% level of enrichment, 1,500 participants would be enrolled and 60% would have reached CKD progression by 5 years compared to 40% of 2,500 participants without enrichment. Using MCP-1 as an example, we are unable to increase the percentage of participants who reach the primary outcome and we are unable to reduce the sample size of the clinical trial. The prognostic enrichment tools can be accessed at prognosticenrichment.com. KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; TNFR1, TNF-a receptor type 1; TNFR2, TNF-a receptor type 2.
Biomarkers of Tubular Injury
Kidney Injury Molecule-1
Kidney injury molecule-1 (KIM-1) is a transmembrane glycoprotein expressed in kidney proximal tubule cells and immune cells which are released upon tissue injury or ischemia [52, 53]. In CKD, KIM-1 levels are elevated in both blood and urine due to chronic tubular injury [52, 53].
Higher plasma and urine KIM-1 levels have been associated with an increased risk of CKD progression in adults and children [54, 55]. In the CKiD cohort, after multivariable adjustment, doubling of plasma and urine KIM-1 concentrations were associated with CKD progression (defined as development of kidney failure or ≥50% decline in eGFR) with an aHR of 1.66 (95% CI: 1.46–1.89) and 1.38 (95% CI: 1.26–1.51), respectively [54, 55]. Likewise, in two adult CKD cohorts using identical assays to measure plasma KIM-1, higher concentrations of plasma KIM-1 were associated with CKD progression [56, 57]. However, no significant associations were found between KIM-1 levels and CKD progression in two other studies of CKD in adults: Reasons for Geographic and Racial Differences in Stroke (REGARDS) (plasma KIM-1) and CRIC (urine KIM-1) [56, 58]. This variability in results could possibly be related to the varying etiologies of CKD in the different cohorts, as KIM-1 concentrations are influenced by the etiology of kidney disease [54]. In children with glomerular diseases in the Nephrotic Syndrome Study Network, urine KIM-1 was associated with disease activity and time to remission [59]. Histologically, higher tubulointerstitial expression of KIM-1 was associated with interstitial fibrosis, tubular atrophy, and glomerulosclerosis [59]. Thus, KIM-1 could be an important biomarker of CKD progression and should be further studied in pediatric kidney disease.
Biomarkers of Inflammation
Tumor Necrosis Factor Receptor Type 1 and Type 2
Tumor necrosis factor receptor type 1 (TNFR1) and tumor necrosis factor receptor type 2 (TNFR2) are cell receptors that mediate local inflammation when stimulated by the pro-inflammatory cytokine, TNFα [60]. TNFR1 is located on glomerular and peritubular endothelial cells and helps mediate a response to inflammation [54, 60]. TNFR2 is located on lymphocytes and plays an important role in the regeneration and proliferation of immune cells [60].
Plasma TNFR1 and TNFR2 were found in pediatric and adult CKD cohorts to be independent predictors of CKD progression. In the CKiD study, 651 children were followed for >6 years, and doubling of plasma TNFR1 and TNFR2 was associated with CKD progression with an aHR of 1.94 (95% CI: 1.56–2.40) and 1.85 (95% CI: 1.37–2.51), respectively [54]. Similar performance was noted in adult cohorts; the Joslin Study of Type 2 Diabetes and Kidney Complications, Multi-Ethnic Study of Atherosclerosis (MESA), CRIC, and REGARDS (Fig. 2) [56, 57, 61]. The significant associations of TNFR1 and TNFR2 with CKD progression regardless of age and CKD etiology underscore the potential utility of these biomarkers to identify individuals at high risk for CKD progression.
Soluble Urokinase Plasminogen Activator Receptor
Soluble urokinase plasminogen activator receptor (suPAR) is a protein released in the circulation in states of inflammation after cleavage of the urokinase plasminogen activator receptor on podocytes and endothelial cells [62]. SuPAR was initially identified to be a marker of disease activity in focal segmental glomerulosclerosis but was subsequently studied in patients with acute kidney injury, diabetic nephropathy, and CKD [10, 62].
Higher plasma suPAR concentrations have been found to be associated with an increased risk of CKD progression in adult and pediatric CKD cohorts. In two European pediatric cohorts of 898 children with CKD, higher serum suPAR was associated with a rapid decline in kidney function in children with a baseline eGFR of 40–80 mL/min per 1.73 m2 [63]. Notably, in the CKiD cohort, using a different suPAR assay and methods of analysis yielded different results. Using an enzyme-linked immunosorbent assay (R&D systems) and lognormal regression models, a significant association was observed between plasma suPAR with CKD progression, whereas using an electrochemiluminescence multiplex assay (Meso Scale Discovery platform) and Cox regression models, suPAR did not have a significant association with CKD progression after multivariable adjustment [54, 64, 65]. These different results in the same group of children highlight the importance of the biomarker assay used to measure biomarker concentrations, the consideration of quality control attributes, and biomarker validation studies.
In adult studies, an independent association between doubling of plasma suPAR with CKD progression was observed in the MESA and CRIC cohorts with aHRs of 1.96 (95% CI: 1.10–3.49) and 1.40 (95% CI: 1.14–1.72), respectively, and aHR of 1.40 (95% CI: 1.26–1.55) per quartile increment in the Emory Cardiovascular Biobank [56, 57, 62]. In the REGARDS cohort, suPAR was associated with a ≥40% decline in eGFR, however, after multivariable adjustment, this association was not significant [56]. The variation in suPAR performance highlights the importance of unifying assays and methodologies in the search for promising biomarkers with transparency of findings [65].
Monocyte Chemoattractant Protein-1
Monocyte chemoattractant protein-1 (MCP-1) is a potent chemotactic factor secreted by immune cells and, in the kidney, by endothelial cells and podocytes to recruit monocytes/macrophages in the setting of inflammation [66]. MCP-1 is present in the glomeruli and tubulointerstitium and can be detected in the urine [66]. Although MCP-1 has been studied in both plasma and urine, plasma MCP-1 did not show a significant association with CKD progression in several adult and pediatric studies [54, 56]. However, urine MCP-1 was independently associated with eGFR decline in several CKD studies. In the CKiD study, doubling of urine MCP-1 was associated with CKD progression with an aHR of 1.29 (95% CI: 1.20–1.39) in the fully adjusted model [55]. In the Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) study of 1,538 adult patients, higher baseline urine MCP-1 was independently associated with a increased risk of the composite kidney outcome (incident CKD, ≥50% drop in eGFR, or development of kidney failure) with an aHR of 1.32 (95% CI: 1.18–1.46) per doubling of MCP-1 [67]. Urine MCP-1 concentrations were also observed to correlate with disease activity and response to therapies for several kidney diseases such as diabetic nephropathy, IgA nephropathy, and crescentic glomerulonephritis, and thus it could be a promising biomarker of CKD progression [66].
Biomarkers of Repair
Chitinase-3-Like Protein 1
Chitinase-3-like protein 1 (YKL-40) is a glycoprotein secreted by macrophages in response to injury to promote tissue repair and limit apoptosis [68]. After multivariable adjustment, there was no significant association between urine YKL-40 and CKD progression in children with CKD [55]. However, urine YKL-40 was found to be associated with CKD outcomes in adults in other clinical settings. In adult participants of the ASSESS-AKI cohort, higher urine YKL-40 was associated with the composite kidney outcome of incident CKD, ≥50% decline in eGFR, or development of kidney failure (aHR per doubling 1.15, 95% CI: 1.09–1.22) [67]. In the Systolic Blood Pressure Intervention Trial (SPRINT), adults with YKL-40 in the highest quartile had a HR of 1.95 (95% CI: 1.08–3.51) for developing CKD progression, compared to the lowest quartile [69]. Thus, YKL-40 might have a potential role in prognosticating kidney disease in adult populations.
Biomarkers of Tubular Health
Epidermal Growth Factor
Epidermal growth factor (EGF) is expressed in kidney tubular cells and promotes regeneration and cellular proliferation [70]. Low urine EGF is associated with interstitial fibrosis and tubular atrophy [71]. Pediatric and adult studies have demonstrated a strong relationship between lower urine EGF and an increased risk of CKD progression. In the prospective, multicenter Cardiovascular Comorbidity in Children with CKD (4C) study, lower urinary EGF had a higher risk of CKD progression with aHR of 0.76 (95% CI: 0.69–0.84) even after adjustment for known risk factors [72]. Similarly, in adjusted models in the CKiD study, halving of urine EGF was associated with aHR of 2.27 (95% CI: 2.0–2.56) for CKD progression [55]. Studies of adult patients with CKD have shown similar associations in three separate cohorts where urine EGF was correlated with eGFR, eGFR slope, and chronic histologic changes [71]. Findings from pediatric and adult studies demonstrate that higher urine EGF is protective of kidney function decline and can be a promising prognostic marker for CKD progression.
Conclusion
Biomarkers that help us better characterize kidney disease or improve clinical risk prediction are needed to improve CKD management, inform clinical decisions around the timely initiation of therapies, and to guide clinical trial enrollment. Plasma KIM-1, TNFR1, and TNFR2, and urine KIM-1, MCP-1, and EGF have demonstrated significant associations with CKD progression in children with comparable results in adults. In the era of machine learning applications in precision medicine, creating prognostic models using novel blood or urine biomarkers in addition to clinical variables could be a promising strategy for improving clinical care. An example of such an approach is the recently CLIA-approved KidneyIntelX model, which is a composite score of plasma TNFR1, TNFR2, and KIM-1 with clinical variables predicting CKD progression in adults with diabetic kidney disease [6]. This model has a negative predictive value of 90% and an AUC of 0.77 (95% CI: 0.76–0.79) for CKD progression, compared to an AUC of 0.61 (95% CI: 0.60–0.63) for the clinical model alone [73].
Metabolite and miRNA profiling has emerged as a promising noninvasive method for novel biomarker discovery. Continued use of high-throughput profiling methods allows for the discovery of biomarker panels for diagnostic and prognostic purposes. It is possible that a panel of multiple biomarkers can better capture CKD-related biological processes and outperform a single biomarker. Overall, the discovery of biomarkers, if advanced and validated in the future, may substantially improve clinical management and adverse outcomes in CKD.
Acknowledgments
We would like to thank Christi Piper, MLIS, AHIP for her extensive search of the CKD biomarker and metabolomic literature.
Funding Sources
This research was supported by NIH career development grant K08DK110536 (to J. Greenberg). This research was also supported by the CKD Biomarkers Consortium (NIDDK grant U01 DK106982) to S. Furth and M. Denburg. M. Denburg was also supported by R21 AT009752. S. Furth is supported by the NIH K24DK078737 and U01-DK-66174. The CKiD study is funded by the NIDDK, with additional funding from the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01-DK-082194, U01-DK-66116). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK, the National Institutes of Health, the Department of Health and Human Services, or the government of the USA.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Ali I, Ibrahim ST, Chinnadurai R, Green D, Taal M, Whetton TD, et al. A paradigm to discover biomarkers associated with chronic kidney disease progression. Biomark Insights. 2020;15:1177271920976146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harada R, Hamasaki Y, Okuda Y, Hamada R, Ishikura K. Epidemiology of pediatric chronic kidney disease/kidney failure: learning from registries and cohort studies. Pediatr Nephrol. 2022. Jun;37(6):1215–29. [DOI] [PubMed] [Google Scholar]
- 3.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LYC, Ayanian J, et al. US renal data system 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016. Mar;67(3 Suppl 1): Svii, S1–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S, Smyth B. From proteinuria to fibrosis: an update on pathophysiology and treatment options. Kidney Blood Press Res. 2021;46(4):411–20. [DOI] [PubMed] [Google Scholar]
- 5.Waikar SS, Rebholz CM, Zheng Z, Hurwitz S, Hsu C-Y, Feldman HI, et al. Biological variability of estimated GFR and albuminuria in CKD. Am J Kidney Dis. 2018. Oct;72(4):538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zabetian A, Coca SG. Plasma and urine biomarkers in chronic kidney disease: closer to clinical application. Curr Opin Nephrol Hypertens. 2021. Nov;30(6):531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magalhães P, Pejchinovski M, Markoska K, Banasik M, Klinger M, Švec-Billá D, et al. Association of kidney fibrosis with urinary peptides: a path towards non-invasive liquid biopsies? Sci Rep. 2017. Dec;7(1):16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong Y, Muñoz A, Schwartz GJ, Warady BA, Furth SL, Abraham AG. Nonlinear trajectory of GFR in children before RRT. J Am Soc Nephrol. 2014. May;25(5):913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonneijck L, Muskiet MHA, Smits MM, van Bommel EJ, Heerspink HJL, van Raalte DH, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017. Apr;28(4):1023–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandokji I, Greenberg JH. Plasma and urine biomarkers of CKD: a review of findings in the CKiD study. Semin Nephrol. 2021. Sep;41(5):416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng DK, Jacobson LP, Brown TT, Palella FJ, Martinson JJ, Bolan R, et al. HIV therapy, metabolic and cardiovascular health are associated with glomerular hyperfiltration among men with and without HIV infection. AIDS. 2014. Jan;28(3):377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992. Oct;38(10):1933–53. [PubMed] [Google Scholar]
- 13.Greenberg JH, Parikh CR. Biomarkers for diagnosis and prognosis of AKI in children: one size does not fit all. CJASN. 2017. Sep;12(9):1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez RA, Sequeira Lopez ML, Fernandez L, Cherñavvsky DR, Norwood VF. The maturing kidney: development and susceptibility. Ren Fail. 1999. Jul;21(3–4):283–91. [DOI] [PubMed] [Google Scholar]
- 15.Weiss RH, Kim K. Metabolomics in the study of kidney diseases. Nat Rev Nephrol. 2012. Jan;8(1):22–33. [DOI] [PubMed] [Google Scholar]
- 16.Riccio S, Valentino MS, Passaro AP, Izzo M, Guarino S, Miraglia del Giudice E, et al. New insights from metabolomics in pediatric renal diseases. Children. 2022. Jan;9(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorgdrager FJH, Naudé PJW, Kema IP, Nollen EA, Deyn PPD. Frontiers in immunology; 2019. [cited 2022 Jul 28].;10. Available from: 10.3389/fimmu.2019.02565. Tryptophan metabolism in inflammaging: from biomarker to therapeutic target [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu C-N, Tain Y-L. Developmental programming and reprogramming of hypertension and kidney disease: impact of tryptophan metabolism. Int J Mol Sci. 2020. Nov;21(22):8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks ER, Lin DC, Langman CB, Thompson JW, St John-Williams L, Furth SL, et al. Metabolomic patterns in adolescents with mild to moderate CKD. Kidney Int Rep. 2019. Jan;4(5):720–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhee EP. Metabolomics and renal disease. Curr Opin Nephrol Hypertens. 2015. Jul;24(4):371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goek O-N, Prehn C, Sekula P, Römisch-Margl W, Döring A, Gieger C, et al. Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol Dial Transpl. 2013. Aug;28(8):2131–8. [DOI] [PubMed] [Google Scholar]
- 22.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019. Oct;322(13):1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallée M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins. 2014. Mar;6(3):934–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benito S, Sánchez A, Unceta N, Andrade F, Aldámiz-Echevarria L, Goicolea MA, et al. LC-QTOF-MS-based targeted metabolomics of arginine-creatine metabolic pathway-related compounds in plasma: application to identify potential biomarkers in pediatric chronic kidney disease. Anal Bioanal Chem. 2016. Jan;408(3):747–60. [DOI] [PubMed] [Google Scholar]
- 25.Benito S, Sánchez-Ortega A, Unceta N, Jansen JJ, Postma G, Andrade F, et al. Plasma biomarker discovery for early chronic kidney disease diagnosis based on chemometric approaches using LC-QTOF targeted metabolomics data. J Pharm Biomed Anal. 2018. Feb;149:46–56. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y-Y, Vaziri ND, Lin R-C. Lipidomics. Advances in clinical chemistry Elsevier; 2015. p. 153–75. [DOI] [PubMed] [Google Scholar]
- 27.Barbagallo CM, Cefalù AB, Giammanco A, Noto D, Caldarella R, Ciaccio M, et al. Lipoprotein abnormalities in chronic kidney disease and renal transplantation. Life. 2021. Apr;11(4):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noels H, Lehrke M, Vanholder R, Jankowski J. Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat Rev Nephrol. 2021. Aug;17(8):528–42. [DOI] [PubMed] [Google Scholar]
- 29.Baek J, He C, Afshinnia F, Michailidis G, Pennathur S. Lipidomic approaches to dissect dysregulated lipid metabolism in kidney disease. Nat Rev Nephrol. 2022. Jan;18(1):38–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erkan E, Zhao X, Setchell K, Devarajan P. Distinct urinary lipid profile in children with focal segmental glomerulosclerosis. Pediatr Nephrol. 2016. Apr;31(4):581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tracz J, Luczak M. Applying proteomics and integrative “omics” strategies to decipher the chronic kidney disease-related Atherosclerosis. Int J Mol Sci. 2021. Jan;22(14):7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denburg MR, Xu Y, Abraham AG, Coresh J, Chen J, Grams ME, et al. Metabolite biomarkers of CKD progression in children. CJASN. 2021. Aug;16(8):1178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekula P, Goek O-N, Quaye L, Barrios C, Levey AS, Römisch-Margl W, et al. A metabolome-wide association study of kidney function and disease in the general population. J Am Soc Nephrol. 2016. Apr;27(4):1175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinbrenner I, Schultheiss UT, Kotsis F, Schlosser P, Stockmann H, Mohney RP, et al. Urine metabolite levels, adverse kidney outcomes, and mortality in CKD patients: a metabolome-wide association study. Am J Kidney Dis. 2021. Nov;78(5):669–77.e1. [DOI] [PubMed] [Google Scholar]
- 35.Cheng Y, Li Y, Benkowitz P, Lamina C, Köttgen A, Sekula P. The relationship between blood metabolites of the tryptophan pathway and kidney function: a bidirectional Mendelian randomization analysis. Sci Rep. 2020. Jul;10(1):12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benito S, Sánchez-Ortega A, Unceta N, Andrade F, Aldámiz-Echevarria L, Goicolea MA, et al. Untargeted metabolomics for plasma biomarker discovery for early chronic kidney disease diagnosis in pediatric patients using LC-QTOF-MS. Analyst. 2018. Sep;143(18):4448–58. [DOI] [PubMed] [Google Scholar]
- 37.Sood MM, Murphy MSQ, Hawken S, Wong CA, Potter BK, Burns KD, et al. Association between newborn metabolic profiles and pediatric kidney disease. Kidney Int Rep. 2018. Feb;3(3):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ambros V The functions of animal microRNAs. Nature. 2004. Sep;431(7006):350–5. [DOI] [PubMed] [Google Scholar]
- 39.Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. CJASN. 2009. Jul;4(7):1255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li JY, Yong TY, Michael MZ, Gleadle JM. Review: the role of microRNAs in kidney disease. Nephrology. 2010;15(6):599–608. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020. May;16(5):269–88. [DOI] [PubMed] [Google Scholar]
- 42.Jeyaram A, Jay SM. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2017. Nov;20(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018. Sep;379(10):958–66. [DOI] [PubMed] [Google Scholar]
- 44.Neal CS, Michael MZ, Pimlott LK, Yong TY, Li JYZ, Gleadle JM. Circulating microRNA expression is reduced in chronic kidney disease. Nephrol Dial Transpl. 2011. Nov;26(11):3794–802. [DOI] [PubMed] [Google Scholar]
- 45.Sanz-Rubio D, Martin-Burriel I, Gil A, Cubero P, Forner M, Khalyfa A, et al. Stability of circulating exosomal miRNAs in healthy subjects. Sci Rep. 2018. Jul;8(1):10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koshiol J, Wang E, Zhao Y, Marincola F, Landi MT. Strengths and limitations of laboratory procedures for MicroRNA detection. Cancer Epidemiol Biomarkers Prev. 2010. Apr;19(4):907–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Govindarajan R, Duraiyan J, Kaliyappan K, Palanisamy M. Microarray and its applications. J Pharm BioAllied Sci. 2012. Aug;4(Suppl 2):S310–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motameny S, Wolters S, Nürnberg P, Schumacher B. Next generation sequencing of miRNAs – strategies, resources and methods. Genes. 2010. Jun;1(1):70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters LJF, Floege J, Biessen EAL, Jankowski J, van der Vorst EPC. MicroRNAs in chronic kidney disease: four candidates for clinical application. Int J Mol Sci. 2020. Jan;21(18):6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Earle A, Bessonny M, Benito J, Huang K, Parker H, Tyler E, et al. Urinary exosomal MicroRNAs as biomarkers for obesity-associated chronic kidney disease. J Clin Med. 2022. Jan;11(18):5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.SunIO LermanLO. Urinary microRNA in kidney disease: utility and roles. Am J Physiol Renal Physiol. 2019. May 1;316(5):F785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karmakova TA, Sergeeva NS, Kanukoev LY, Alekseev BY, Kaprin AD. Kidney injury molecule 1 (KIM-1): a multifunctional glycoprotein and biological marker (review). Sovrem Tekhnologii Med. 2021;13(3):64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin C, Wang N. Kidney injury molecule-1 in kidney disease. Ren Fail. 2016. Nov;38(10):1567–73. [DOI] [PubMed] [Google Scholar]
- 54.Greenberg JH, Abraham AG, Xu Y, Schelling JR, Feldman HI, Sabbisetti VS, et al. Plasma biomarkers of tubular injury and inflammation are associated with CKD progression in children. J Am Soc Nephrol. 2020;31(5):1067–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenberg JH, Abraham AG, Xu Y, Schelling JR, Feldman HI, Sabbisetti VS, et al. Urine biomarkers of kidney tubule health, injury, and inflammation are associated with progression of CKD in children. J Am Soc Nephrol. 2021. Oct;32(10):2664–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarnak MJ, Katz R, Ix JH, Kimmel PL, Bonventre JV, Schelling J, et al. Plasma biomarkers as risk factors for incident CKD. Kidney Int Rep. 2022. Jul;7(7):1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schrauben SJ, Shou H, Zhang X, Anderson AH, Bonventre JV, Chen J, et al. Association of multiple plasma biomarker concentrations with progression of prevalent diabetic kidney disease: findings from the chronic renal insufficiency cohort (CRIC) study. J Am Soc Nephrol. 2021. Jan;32(1):115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu C-Y, Xie D, Waikar SS, Bonventre JV, Zhang X, Sabbisetti V, et al. Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. Kidney Int. 2017. Jan;91(1):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Q, Troost JP, Dai T, Nast C, Eddy S, Wei B, et al. Kidney injury molecule-1 and periostin urinary excretion and tissue expression levels and association with glomerular disease outcomes. Glomerular Dis. 2021. Jun;1(2):45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehaffey E, Majid DSA. Tumor necrosis factor-α, kidney function, and hypertension. Am J Physiol Ren Physiol. 2017. Oct;313(4):F1005–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012. Mar;23(3):507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayek SS, Sever S, Ko Y-A, Trachtman H, Awad M, Wadhwani S, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015. Nov;373(20):1916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaefer F, Trachtman H, Wühl E, Kirchner M, Hayek SS, Anarat A, et al. Association of serum soluble urokinase receptor levels with progression of kidney disease in children. JAMA Pediatr. 2017. Nov;171(11):e172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weidemann DK, Abraham AG, Roem JL, Furth SL, Warady BA. Plasma soluble urokinase plasminogen activator receptor (suPAR) and CKD progression in children. Am J Kidney Dis. 2020. Aug;76(2):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abraham AG, Xu Y, Roem JL, Greenberg JH, Weidemann DK, Sabbisetti VS, et al. Variability in CKD biomarker studies: soluble urokinase plasminogen activator receptor (suPAR) and kidney disease progression in the chronic kidney disease in children (CKiD) study. Kidney Med. 2021. Oct;3(5):712–21.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim MJ, Tam FWK. Urinary monocyte chemoattractant protein-1 in renal disease. Clin Chim Acta. 2011. Nov;412(23–24):2022–30. [DOI] [PubMed] [Google Scholar]
- 67.Puthumana J, Thiessen-Philbrook H, Xu L, Coca SG, Garg AX, Himmelfarb J, et al. Biomarkers of inflammation and repair in kidney disease progression. J Clin Invest. 2021. Feb;131(3):139927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt IM, Hall IE, Kale S, Lee S, He C-H, Lee Y, et al. Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol. 2013. Feb;24(2):309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malhotra R, Katz R, Jotwani V, Ambrosius WT, Raphael KL, Haley W, et al. Urine markers of kidney tubule cell injury and kidney function decline in SPRINT trial participants with CKD. Clin J Am Soc Nephrol. 2020. Mar;15(3):349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng F, Harris RC. Epidermal growth factor, from gene organization to bedside. Semin Cell Dev Biol. 2014. Apr;28:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015. Dec;7(316):316ra193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Azukaitis K, Ju W, Kirchner M, Nair V, Smith M, Fang Z, et al. Low levels of urinary epidermal growth factor predict chronic kidney disease progression in children. Kidney Int. 2019. Jul;96(1):214–21. [DOI] [PubMed] [Google Scholar]
- 73.Chan L, Nadkarni GN, Fleming F, McCullough JR, Connolly P, Mosoyan G, et al. Derivation and validation of a machine learning risk score using biomarker and electronic patient data to predict progression of diabetic kidney disease. Diabetologia. 2021. Jul;64(7):1504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


