Summary
Background
Most cancer drugs enter the US market first. US Food and Drug Administration (FDA) approvals of new cancer drugs may influence regulatory decisions in other settings. The study examined whether characteristics of available evidence at FDA approval influenced time-to-marketing authorisation (MA) in Brazil, and price differences between the two countries.
Methods
All new FDA-approved cancer drugs from 2010 to 2019 were matched to drugs with MA and prices approved in Brazil by December 2020. Characteristics of main studies, availability of randomised controlled trials (RCTs), overall survival (OS) benefit, added therapeutic benefit, and prices were compared.
Findings
Fifty-six FDA-approved cancer drugs with matching indications received a MA at the Brazilian Health Regulatory Agency (Anvisa) after a median of 522 days following US approval (IQR: 351–932). Earlier authorisation in Brazil was associated with availability of RCT (median: 506 vs 760 days, p = 0.031) and evidence of OS benefit (390 vs 543 days, p = 0.019) at FDA approval. At Brazilian marketing authorisation, a greater proportion of cancer drugs had main RCTs (75% vs 60.7%) and OS benefit (42.9% vs 21.4%) than that in the US. Twenty-eight (50%) drugs did not demonstrate added therapeutic benefit over drugs for the same indication in Brazil. Median approved prices of new cancer drugs were 12.9% lower in Brazil compared to the US (adjusted by Purchasing Power Parity). However, for drugs with added therapeutic benefit median prices were 5.9% higher in Brazil compared to the US, while 17.9% lower for those without added benefit.
Interpretation
High-quality clinical evidence accelerated the availability of cancer medicines in Brazil. The combination of marketing and pricing authorisation in Brazil may favour the approval of cancer drugs with better supporting evidence, and more meaningful clinical benefit albeit with variable degree of success in achieving lower prices compared to the US.
Funding
None.
Keywords: Brazil, United States, Pharmaceutical preparations, Health technology assessment, Cancer, Drug legislation
Research in context.
Evidence before this study
From 2002 to 2021, over 215 new cancer drugs were launched globally. There is considerable uncertainty in the clinical benefits of cancer drugs at the time of regulatory marketing authorisation. In addition, high prices of cancer drugs are not always commensurate with their clinical benefits. While most new cancer drugs (∼95%) are first approved by the US Food and Drug Administration (FDA), the implications for regulatory agencies in low- and middle-income countries have not been documented. Literature searches were conducted on Pubmed and Lilacs with search terms “cancer drugs”, “approval”, “food and drug administration”, “price”, and “Brazil”; “cancer drugs”, “approval”, “Food and Drug Administration”, and “Brazil”; “cancer drugs”, “Brazil”, and “price”, without time or language restrictions. Articles were also identified through searches of the authors’ own files. A total of 107 articles were identified. Only one study compared regulatory outcomes for cancer drugs approved and one study compared pricing of cancer drugs in Brazil with other Latin American countries and high-income countries. No studies comparing study characteristics or regulatory outcomes and prices between United States and Brazil were identified.
Added value of this study
This is the first comparative study analysing the evidence supporting regulatory decisions and prices of cancer drugs in Brazil compared to the US. Brazil is a middle-income country with a large pharmaceutical market where clinical evidence is not only considered for marketing authorisation but also later for pricing approval. Our study pioneers by providing an in-depth analysis of the quantity and quality of evidence available on new cancer drugs and their impact on approval times and prices in Brazil. Cancer drugs approved in the US from 2010 to 2019 with better evidence and more meaningful clinical benefit were approved earlier in Brazil than those without these characteristics. These findings highlight the relevance of robust evidence, as it accelerated the availability of cancer medicines in Brazil. Median cancer drug prices were overall lower in Brazil than in the US after adjusting for Purchasing Power Parity (PPP).
Implications of all the available evidence
Available evidence suggests that the coordination of marketing authorisation and pricing decisions in the Brazilian drug regulatory system leads to faster availability of drugs with better clinical evidence and rewards evidence of added clinical benefit. The study corroborates the importance of comparative evidence for informing marketing authorisation and pricing decisions. Policy implications include aligning the evidence requirements for marketing authorisation and pricing with health technology assessment, introducing PPP-adjustment for external reference pricing, and adopting a life-cycle approach for pricing. Brazil’s activities based on regulatory reliance may also be beneficial for other countries, in particular those that use Brazil in their reference basket.
Introduction
Cancer drugs comprise the single largest category of new drug approvals. The United States (US) Food and Drug Administration (FDA) approved over 184 new cancer drugs between 2002 and 2021.1 At the time of FDA approval, cancer drugs have uncertainties in their evidence base.2 A growing proportion of new cancer drugs are approved on the basis of single-arm studies instead of randomised controlled trials (RCTs), which are accepted as the gold standard for evaluating new drugs. Fewer than a fifth of new cancer drugs had main studies with active comparators.3 Most main studies measured the effect of cancer drugs on surrogate endpoints, such as disease progression or tumour shrinkage, which may not be reliable predictors of longer overall survival (OS).3 Between 2006 and 2016, fewer than half of RCTs supporting FDA approvals of cancer drugs met the threshold for demonstrating clinically meaningful benefit.4
Despite uncertainties of their clinical benefits, pharmaceutical companies charge high prices for new cancer drugs. In the US, an average treatment course cost US$150,000 per patient in 2018, and spending on newly-approved cancer drugs was estimated at $39.5 billion.5 Previous evidence found no meaningful relationship between clinical benefit, and drug prices in the US and Europe.6,7 High drug prices can result in financial toxicity for individuals and financial pollution in health systems.8
Decision making for marketing authorisation, pricing and reimbursement for new medicines based on limited clinical evidence is a common challenge for many health authorities around the world, particularly for high-priced cancer drugs.9,10 Pharmaceutical companies decide where and when to seek marketing authorisation for their new products. Cancer drugs typically enter the US market first: approximately 95% of cancer drugs were first launched in the US between 2002 and 2021.1,11 US evidence standards can have consequences for other countries as companies subsequently submit near-identical datasets to other regulatory agencies, and many countries rely on regulatory decisions of authorities like the FDA, without further assessments.12 Uncertainties in the evidence base at the time of US cancer drug approvals therefore pose challenges for stakeholders in other health care systems. Also, prices set in the US can either directly or indirectly influence prices in other countries due to commonly used external reference pricing policies, in which prices in one country are guided by those in other countries.13,14
We assessed the marketing authorisation and pricing of FDA-approved cancer drugs in Brazil—an upper-middle income country with a universal national Unified Healthcare System (SUS), and a fast-growing pharmaceutical market. Brazil has an established drug regulatory system, which dates to 1999. In addition to evaluating the quality, safety, and efficacy of new medicines, Brazilian authorities also regulate prices, based on comparative efficacy, as a condition for market entry (Supplementary Fig. S1). By contrast, only marketing authorisation is required for market entry in the US. In theory, the combination of marketing and pricing authorisation requirements in Brazil would incentivise drug manufacturers to preferentially seek marketing authorisation in Brazil for products with demonstrated clinical benefits.
Marketing authorisation (MA) in Brazil is granted by the Brazilian Health Regulatory Agency (Anvisa), which regulates healthcare products and services, ranging from authorisation of clinical trials to post-marketing surveillance. MA by Anvisa establishes that drugs are safe, effective and of good quality and is valid for 10 years.15 Companies are required to apply for MA renewal, prior MA expiration, when additional regulatory reviews are conducted. Medicines treating rare diseases (affecting up to 65 per 100,000 individuals), including several cancers, are eligible for shorter regulatory reviews and conditional approvals, based on less robust evidence, with MA valid for shorter periods of time.15 As member of several regulatory harmonisation fora, Anvisa’s evidence standards have become broadly similar to those of other stringent regulatory agencies but with important differences. Anvisa also has non-binding guidelines for cancer drug trial endpoints, which recommends the use of a clinically relevant primary endpoint such as OS as “the most reliable method for demonstrating efficacy” in RCTs.16
To be allowed to enter the Brazilian market, companies must also apply for maximum pricing approval from the Drug Market Regulatory Chamber (CMED). CMED uses health technology assessment (HTA) to determine if a new drug has added therapeutic benefit in relation to drugs authorised for the same condition in Brazil. New patented drugs with added therapeutic benefit are classified as “category I” if at least one of the following is demonstrated: a) greater efficacy, b) equivalent efficacy with a significant decrease in risk of adverse effects, or c) equivalent efficacy with potential for a significant reduction in the total cost of treatment. New drugs without patent or added therapeutic benefit are classified as “category II”.17 For category I, the maximum approved price is determined using external reference pricing (ERP), as the lowest price charged in a basket of 10 countries. For category II, the price is defined as the lower price of the ERP and internal reference price (IRP). IRP anchors the price of a new drug using the cost of treatment with authorised drugs for the same condition.15 Actual prices paid can only be lower, not higher, than the maximum prices approved by CMED.
Our objectives in this paper were threefold. First, we sought to characterise the evidence supporting marketing authorisation of new cancer drugs in the US and Brazil. Second, we examined whether the characteristics of available evidence at the time of FDA approval influenced time-to-marketing authorisations of matched cancer drug indications in Brazil. Third, we compared cancer drug prices in the US and Brazil.
Methods
Sample of drugs
All new cancer drugs approved by the US FDA from January 1, 2010 to December 31, 2019, were identified and matched with approvals in Brazil (with marketing authorisation up to July 31, 2020, and pricing authorisation by December 31, 2020) (Supplementary Table S1).
Only new chemical entities, and biologicals with single active ingredient/moiety with cancer indications were eligible for inclusion. We matched cancer drugs in the two settings using their first-approved indications. We excluded drugs for preventive or palliative care, or fixed dose combinations, and other products such as vaccines, radiopharmaceuticals (radiotherapies), supportive therapies, cellular, and gene therapy products, consistent with previous studies.18,19
Information sources
Publicly available information for FDA-approved cancer drugs was obtained from the “Compilation of CDER New Molecular Entity (NME) Drug and New Biologic Approvals from 1985 to 2019”, FDA annual reports “New Drug Therapy Approval”, labelling and regulatory review reports available in the Drugs@FDA database. Corresponding information in Brazil was obtained from public medicines assessment reports, and labelling at the Brazilian Health Regulatory Agency (Anvisa)’s website. Pricing approval reports in Brazil were obtained from the Executive Secretariat of Drug Market Regulation Chamber (SCMED). US prices were obtained from the Federal Supply Schedule Service of the US Department of Veteran Affairs (available at https://www.va.gov/), which are used as benchmark prices in external reference pricing in Brazil (Supplementary Table S2).20 When more detailed information was needed in Brazil, regulatory information systems, and databases were also consulted (Supplementary Table S1).
Data extraction
We first collected data on FDA-approved cancer drugs. For each drug in our sample, we identified its first approved indication. We extracted information on the characteristics and results of the main clinical studies supporting regulatory decisions, including study phase (I-III), randomisation (Randomised Controlled Trial, RCT or not), comparator (none, active comparator, placebo, or add-on therapy), control arm, masking (open, blinded), endpoints, and dates of manufacturer submission, and FDA marketing authorisation. We also recorded the regulatory pathway (i.e., priority review, fast-track designation, accelerated approval pathway, breakthrough therapy designation), or Orphan Drug Act designation. For all matched cancer drug indications, we extracted corresponding data from Anvisa (Supplementary Table S1).
Next, we reviewed information on the Brazilian pricing approval reports, listing dates of submission, and approval, factors leading to price decisions, including external reference prices, and approved category (with or without added therapeutic benefit) and price, at the time of price approval in Brazil. We subsequently collected data on drugs’ supporting evidence, comparative efficacy in their approved indications, as determined by the Brazilian authority (Supplementary Table S1).
Categorisation of evidence
Cancer drugs with marketing authorisation at FDA and Anvisa were categorised according to the availability of RCTs, classified as high quality evidence by the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) (https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/), documented evidence of statistically significant OS benefit, and substantial clinical benefit supporting the approved indication. In the RCTs, participants were randomly allocated to receive either the study drug, or an active comparator, standard therapy, or placebo. Non-comparative randomised trials, or studies comparing different doses of the same drug, without a control group, were not considered as RCTs.
We classified the control arm as optimal, or sub-optimal.21,22 The quality of a control arm in RCTs was considered suboptimal when (a) there were restrictions on the choice of control that excluded other potentially equivalent agents, or (b) the control arm was specified but the recommended agent was potentially inferior.21,22
Consistent with previous studies, we systematically coded the availability of documented evidence of OS benefit (Supplementary Table S3).19,23 We also documented the availability of statistically significant Progression Free Survival (PFS) result, when PFS was the primary endpoint in main studies.
We defined the availability of substantial clinical benefit using the European Society of Medical Oncology—Magnitude of Clinical Benefit Scale (ESMO–MCBS), which is a publicly available validated and reproducible scale to assess clinical benefit of cancer drugs for solid tumours. Scores A or B in the curative setting and 4 or 5 in the non-curative setting indicate a “substantial magnitude of clinical benefit”.24 We used scorecards available on the ESMO-MCBS website. We also noted if new cancer drugs were categorised as having added therapeutic benefit (category I) or not (category II) at the time of pricing approval in Brazil.
Analysis
Duration of regulatory review by the FDA and Anvisa was defined as the number of days between submission and marketing authorisation. For the FDA, the marketing authorisation was the date of the decision communication and for Anvisa, the date of publication in the official gazette (Diário Oficial da União, DOU).
In Brazil, the duration of pricing review was defined as the number of days between submission to CMED and pricing approval. Medicines are allowed to enter the market only after marketing authorisation and maximum pricing approval.
To evaluate a possible association between time-to-marketing authorisation in Brazil since FDA approval and strength of available evidence (i.e., availability of RCT, OS benefit, substantial clinical benefit and added therapeutic benefit), we performed a log-rank test using the Kaplan Meier method. We repeated this analysis for time from FDA approval to Anvisa application and review time at Anvisa. To compare the characteristics of evidence supporting regulatory approval of new cancer drugs in the US and Brazil, we used Fisher-exact tests.
For each drug, we identified maximum ex-factory prices for all approved presentations (pack sizes, dosage form, concentration), and corresponding US reference prices. Inclusion for price analysis depended on availability of price in the US and Brazil, and categorisation of added therapeutic benefit. The median price per concentration unit (mg, mg/ml, mg/dose) was obtained for each drug in the US and in Brazil (in BRL). Brazilian maximum authorised ex-factory prices were converted into US PPP (Purchasing Power Parity) with the exchange rate of the Organisation for Economic Cooperation and Development (OECD) (https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm) in the year of approval and the price difference between the two countries was calculated. A comparison of median price differences according to (i) availability of RCTs, (ii) evidence of documented OS benefit, (iii) substantial clinical benefit (at FDA approval), and (iv) added therapeutic benefit (at the time of price approval in Brazil) was calculated. All differences were expressed in percentages. Medians were compared using Wilcoxon sum rank test. We considered p < 0.05 as statistically significant in all analyses. Analyses were performed using Stata 14 and SAS 9.4.
Ethics statement
No confidential information was used in this research. Data not publicly available was obtained under the provisions of the Brazilian Law of Access to Information. Access to these resources was approved by Anvisa and SCMED. According to Brazilian legislation, this research did not involve human subjects and Institutional Review Board approval was not required.
Role of the funding source
No funding was received for this study.
Results
Sample characteristics
From 2010 to 2019, 377 new medicines received marketing authorisation by the FDA. Of 101/377 (26.8%) new cancer drugs, 93/101 (24.7%) had a single active ingredient/moiety. After identifying matching indications in Brazil, our study sample included 56/101 cancer drugs with 58 indications, corresponding to 60.2% of cancer drug approvals during the study period in the US (Table 1).
Table 1.
Sample drugs with common indication, and time to approval at FDA and Anvisa.
| Active Ingredient/Moiety | Common indication FDA/Anvisaa | Time for MA approval at FDA (days) | Time for MA approval Anvisa (days) |
|---|---|---|---|
| Cabazitaxel | Hormone-refractory metastatic prostate cancer | 78 | 158 |
| Eribulin mesylate | Metastatic breast cancer | 230 | 523 |
| Ipilimumab | Unresectable or metastatic melanoma | 273 | 423 |
| Vandetanib | Irresectable or metastatic locally advanced medullary thyroid cancer | 273 | 355 |
| Abiraterone acetate | Metastatic castration-resistant prostate cancer | 129 | 166 |
| Vemurafenib | Unresectable or metastatic melanoma with BRAFV600E mutation | 111 | 213 |
| Brentuximab vedotin | Hodgkin lymphoma/systemic anaplastic large cell lymphoma | 172 | 577 |
| Crizotinib | Locally advanced or metastatic non-small cell lung cancer (NSCLC) that is anaplastic lymphoma kinase (ALK)-positive | 149 | 1146 |
| Ruxolitinib | Intermediate or high-risk myelofibrosis, including primary myelofibrosis, post-polycythemia vera myelofibrosis and post-essential thrombocythemia myelofibrosis | 166 | 581 |
| Axitinib | Advanced renal cell carcinoma | 288 | 1487 |
| Vismodegib | Metastatic basal cell carcinoma, or locally advanced basal cell carcinoma | 144 | 1312 |
| Pertuzumab | Growth factor receptor 2 (HER2)-positive metastatic breast cancer | 183 | 454 |
| Carfilzomib | Multiple myeloma | 297 | 278 |
| Enzalutamide | Metastatic castration-resistant prostate cancer | 101 | 676 |
| Cabozantinib | Advanced renal cell carcinoma (RCC) | 125 | 172 |
| Ponatinib | 1) Chronic phase, accelerated phase, or blast phase chronic myeloid leukemia (CML), resistant or intolerant to prior tyrosine kinase inhibitor therapy or 2) philadelphia chromosome positive acute lymphoblastic leukemia (Ph + ALL) | 78 | 208 |
| Obinutuzumab | Chronic lymphocytic leukemia | 193 | 605 |
| Afatinib | Metastatic non-small cell lung cancer (NSCLC) whose tumuors have epidermal growth factor receptor (EGFR) | 239 | 193 |
| Trametinib | Unresectable or metastatic melanoma with BRAF V600 mutations | 299 | 258 |
| Dabrafenib | Unresectable metastatic melanoma with BRAF V600E mutation | 303 | 1147 |
| Ado-trastuzumab emtansine | HER2-positive, metastatic breast cancer | 179 | 374 |
| Nivolumab | Unresectable or metastatic melanoma | 145 | 325 |
| Olaparib | BRCA mutated advanced ovarian cancer | 319 | 473 |
| Blinatumomab | Philadelphia chromosome-negative relapsed or refractory Bcell precursor acute lymphoblastic leukemia (ALL). | 75 | 853 |
| Pembrolizumab | Unresectable or metastatic melanoma | 189 | 353 |
| Ramucirumab | Gastric Cancer (Advanced gastric cancer or gastro-esophageal junction adenocarcinoma) | 241 | 542 |
| Siltuximab | Multicentric Castleman’s disease (MCD) | 236 | 361 |
| Belinostat | relapsed or refractory peripheral T-cell lymphoma (PTCL). | 206 | 397 |
| Alectinib | Anaplastic lymphoma kinase (ALK)-positive, metastatic non-small cell lung cancer (NSCLC) | 158 | 209 |
| Elotuzumab | Multiple myeloma | 154 | 535 |
| Ixazomib | Multiple myeloma | 133 | 642 |
| Daratumumab | Multiple myeloma | 130 | 404 |
| Cobimetinib | Unresectable or metastatic melanoma with a BRAF V600 mutation | 334 | 402 |
| Lenvatinib | Differentiated thyroid cancer | 183 | 567 |
| Palbociclib | Postmenopausal women with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer | 174 | 522 |
| Osimertinib | Metastatic epidermal growth factor receptor (EGFR) T790M mutation positive non-small cell lung cancer (NSCLC) | 161 | 362 |
| Olaratumab | Advanced soft tissue sarcoma (STS) | 238 | 312 |
| Atezolizumab | Locally advanced or metastatic urothelial carcinoma | 127 | 495 |
| Venetoclax | Chronic lymphocytic leukemia (CLL) with 17p deletion | 165 | 825 |
| Acalabrutinib | Mantle cell lymphoma (MCL) | 140 | 213 |
| Abemaciclib | Hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer | 146 | 238 |
| Inotuzumab ozogamicin | Relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL) | 240 | 157 |
| Durvalumab | Locally advanced or metastatic urothelial carcinoma | 200 | 305 |
| Midostaurin | Acute myeloid leukemia (AML) that is FLT3 mutation positive | 242 | 285 |
| Brigatinib | Anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) | 242 | 383 |
| Avelumab | Metastatic Merkel cell carcinoma (MCC) | 181 | 313 |
| Ribociclib | Postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer. | 196 | 487 |
| Gilteritinib | Relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation | 244 | 228 |
| Larotrectinib | Solid tumuors with neurotrophic receptor tyrosine kinase (NTRK) gene fusion | 245 | 327 |
| Lorlatinib | Anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) | 332 | 192 |
| Cemiplimab-rwlc | Metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC | 212 | 145 |
| Apalutamide | Non-metastatic castration-resistant prostate (nm-CRPC) | 127 | 242 |
| Darolutamide | Non-metastatic castration-resistant prostate cancer (nm-CRPC) | 154 | 208 |
| Polatuzumab vedotin-piiq | Relapsed or refractory diffuse large B-cell lymphoma | 173 | 235 |
| Alpelisib | Hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer in postmenopausal women, and in men. | 157 | 167 |
| Erdafitinib | Locally advanced or metastatic urothelial carcinoma, with susceptible FGFR genetic alteration | 206 | 277 |
| Median | 182 | 358 | |
| IQR | (145.5–240.5) | (231.5–529) |
Notes: FDA: Food and Drug Administration; Anvisa: Brazilian Health Regulatory Agency; MA: marketing authorisation; IQR: interquartile; time indicated in days.
The common indication at FDA and Anvisa corresponds to the specific indication explored in the present study.
Sample characteristics are summarised in Table 2. The sample included 19/56 (33.9%) new chemical entities and 37/56 (66.1%) new biologicals. All drugs were included in at least one FDA expedited development or review programme. The main cancer sites, according to the matched indications, were lymphatic and hematopoietic systems, and related tissues (11/58, 18.9%), followed by skin (10/58, 17.2%) breast (7/58, 12.1%), and lung cancer (6/58, 10.5%) (Supplementary Table S4).
Table 2.
Regulatory classification, pathway of the sample drug approvals at the time of marketing authorisation of the first indication at FDA from 2010 to 2019.
| Characteristics (US FDA approvals) | Drugs in the cohort (n = 56) |
|
|---|---|---|
| Numbera | % (in sample) | |
| Regulatory classification | ||
| New chemical entity | 19/56 | 33.9 |
| New biological application | 37/56 | 66.1 |
| Regulatory pathway | ||
| Priority review | 52/56 | 92.9 |
| Orphan drug designation | 37/56 | 66.1 |
| Accelerated approval | 24/56 | 42.9 |
| Breakthrough therapy | 30/56 | 53.6 |
| Fast track designation | 29/56 | 51.8 |
| First-in-class | 23/56 | 41.1 |
| First approved in the US | 46/56 | 82.1 |
Total number of drugs in the sample n = 56. More information on FDA special approval pathways can be found at: https://www.fda.gov/patients/learn-about-drug-and-device-approvals/fast-track-breakthrough-therapy-accelerated-approval-priority-review.
Marketing authorisation times and maturity of evidence
The median duration of time between marketing authorisation at FDA and Anvisa of the matched cancer drug indications was 522 days (IQR: 351–932 days) (Supplementary Fig. S2).
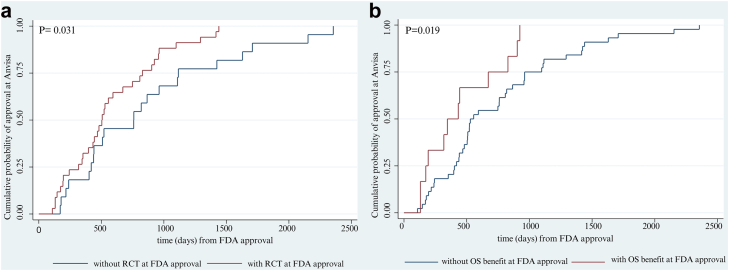
Thirty-four (34/56, 60.7%) cancer drugs had RCTs as main clinical studies at the time of FDA approval. The time between FDA and Anvisa marketing authorisation was 506 days (IQR: 425–672) for drugs with RCTs as main clinical studies, and 760 days (IQR: 423.8–1116) for drugs without RCTs as main clinical studies (p-value = 0.031) (Fig. 1 panel a).
Fig. 1.
Probability of approval at Anvisa according to existence of RCT (panel a) and overall survival benefit at FDA approval (panel b). Notes: Anvisa: Brazilian Health Regulatory Agency; FDA: Food and Drug Administration; RCT: randomised controlled trial; OS benefit: overall survival benefit.
At the time of FDA approval, 12/56 (21.4%) drugs had documented evidence of OS benefit. Median time to Anvisa marketing authorisation was 390 days (IQR: 188.3–711.3) for drugs with OS benefit at the time of FDA approval and 543 days (IQR: 14.8–996) for drugs without such benefit (p-value = 0.019) (Fig. 1 panel b). There were no statistically significant differences for time to Anvisa marketing authorisation according to availability of substantial clinical benefit (ESMO-MCBS scores four or five at the non-curative settings).
Prices were approved in Brazil a median of 630 days (IQR: 459.8–1054) after FDA marketing authorisation, and a median of 95 days (IQR: 88–135) after Anvisa marketing authorisation (Supplementary Fig. S2).
Comparison of the evidence submitted for regulatory approval in the US and Brazil
Characteristics of main studies supporting marketing authorisation in the US and Brazil are summarised in Table 3. A greater proportion of cancer drugs had RCTs (75% vs 60.7%, p = 0.16), RCTs with active comparators (42.9% vs 21.4%, p = 0.025), and evidence of documented OS benefit (42.9% vs 21.4%, p = 0.025) at the time of Brazil marketing authorisation compared to the US. The proportion of drugs with a statistically significant PFS result was similar in the US and Brazil (25.0% vs 35.7%, p = 0.30). At the time of FDA approval, 11/56 (19.6%) drugs had substantial clinical benefit (ESMO-MCBS score ≥4, non-curative setting) compared to 16/56 (28.6%) at the time of Brazil marketing authorisation. Data on ESMO-MCBS was available for 26/56 drugs at the FDA, with 15 drugs classified as MCBS one to three. At Anvisa, data was available for 29/56 drugs, with 13 of them classified as MCBS two to three.
Table 3.
Characteristics of main studies supporting marketing authorisation of cancer medicines in the US and in Brazil.
| Main clinical trial characteristics | US/FDA |
Brazil/Anvisa |
p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Drugs with Randomised Controlled Trial (RCT) as main studies | 34/56 (60.7) | 42/56 (75.0) | 0.16 |
| Primary endpointa | |||
| Overall survival (OS) | 10/56 (17.9) | 16/56 (28.6) | 0.27 |
| Progression free-survival (PFS) | 17/56 (30.4) | 23/56 (41.1) | 0.34 |
| Other | 3256 (57.1) | 23/56 (41.1) | 0.016 |
| Type of control arm of the RCTs | |||
| Active comparator | 12/56 (21.4) | 24/56 (42.9) | 0.025 |
| Placebo controlled | 16/56 (28.6) | 19/56 (33.9) | 0.064 |
| Add-on therapy | 6/56 (10.7) | 6/56 (10.7) | 1 |
| Adequacy of control arm | |||
| Optimal | 13/56 (23.2) | 19/56 (33.9) | 0.30 |
| Suboptimal | 9/56 (16.1) | 13/56 (23.2) | 0.48 |
| Clinically relevant outcome | |||
| Overall Survival Benefit | 12/56 (21.4) | 24/56 (42.9) | 0.025 |
| Substantial Clinical Benefitb | 11/56 (19.6) | 16/56 (28.6) | 0.42 |
Bold p-value indicates that the correspondent difference is statistically significant.
Some studies had more than one primary endpoint.
European Society of Medical Oncology—Magnitude of Clinical Benefit Scale (ESMO-MCBS) ≥4, data available with ESMO-MCBS classification n = 26 drugs at the FDA and n = 29 drugs at Anvisa.
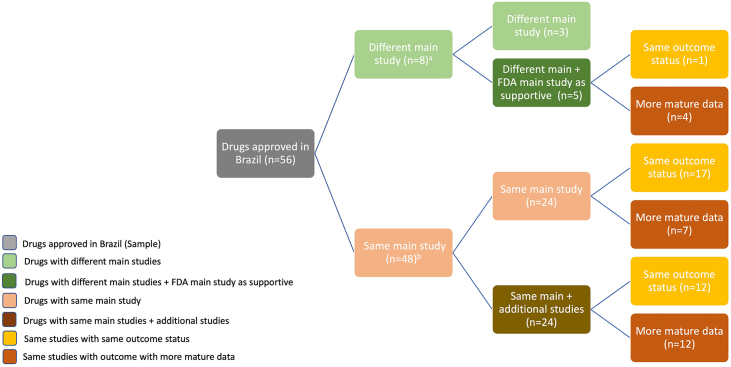
One-third (17/56) of drugs were approved in Brazil based on the same main studies with same outcome status as those supporting FDA approvals. When the same main study was used, 23/56 (41.1%) had more mature data by the time of Anvisa marketing authorisation (Fig. 2).
Fig. 2.
Comparison of main studies supporting marketing authorisation in the US and Brazil and their outcome status. Notes: n = number of drugs; (a) 8/56 drugs were approved in Brazil (Anvisa) with different main studies, five of them had the US (FDA) main studies as supportive in Brazil; (b) 48/56 drugs were approved in Brazil with the same main studies as in the US, half of them (24/48) had the same main study and 24 were accompanied by additional studies.
Difference in cancer drug prices in the US and Brazil
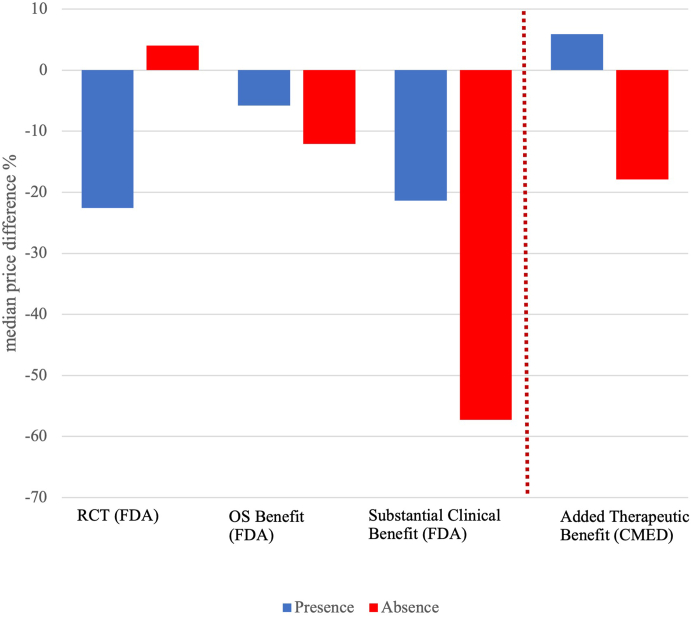
Overall, PPP-adjusted median prices were 12.1% (IQR: −50.3%; 13.9%) lower in Brazil compared with median prices in the US, with substantial variation. The median price difference was larger for the subset of drugs with RCT at the time of FDA approval compared to those without such evidence (−22.6% vs 4.0%; p = 0.043). However, there was no statistically significant association between median price differences according to documented evidence of OS benefit (−5.8% vs −12.1%) p = 0.46), or availability of substantial clinical benefit (−21.4% vs −57.3%; p = 0.10), Fig. 3.
Fig. 3.
Median price difference between cancer drugs approved in the US (2010–2019) and Brazil. Notes: RCT: randomised controlled trial; OS Benefit: overall survival benefit; and Substantial Benefit: substantial clinical benefit according to the European Society of Medical Oncology—Magnitude of Clinical Benefit Scale (ESMO-MCBS) ≥4] at the time of marketing authorisation—parameters measured at Food and Drug Administration (FDA); Added Therapeutic Benefit at price approval—parameter measured at Drug Market Regulatory Chamber (CMED). Prices are PPP-adjusted.
Following a comparative effectiveness assessment conducted by the Brazilian regulator, 27/56 (48.2%) cancer drugs had their prices authorised in category I (added therapeutic benefit) while 28/56 (50%) were authorised in category II (no added therapeutic benefit). PPP-adjusted median prices for drugs with added therapeutic benefit were 5.9% higher in Brazil compared to US prices, while prices for those without added benefit were 17.9% lower in Brazil than in the US (p = 0.034).
Discussion
In this study, we compared the evidence supporting regulatory decisions and prices of new cancer drugs in the US and Brazil. Approximately 60% (56/101) of US-approved cancer drugs from 2010 to 2019 received marketing and pricing authorisation for matching indications in Brazil by December 2020. Cancer drugs with stronger evidence and more meaningful clinical benefit at FDA approval received earlier marketing authorisation in Brazil. By the time of the Brazilian marketing authorisation, most cancer drugs had more mature evidence, with a greater proportion of drugs with RCTs as main studies and documented (statistically significant) evidence of OS benefit than those approved in the US. However, half of the drugs did not demonstrate added therapeutic benefit over other authorised drugs for the same indication in Brazil.
Maximum approved PPP-adjusted median prices were 12.1% lower in Brazil compared with those in the US. For drugs with added therapeutic benefit, PPP-adjusted median prices were 5.9% higher in relation to US prices, while for those without added benefit, median prices were 17.9% lower (p = 0.034). This finding is consistent with those from earlier studies, which revealed large price differences across different countries. Ex-factory price differences (not adjusted for PPP) of 31 originator cancer drugs in 16 European countries, Australia, and New Zealand ranged from 28% to 388%.25
Our finding that drugs with stronger evidence of benefit at the time of FDA approval were available earlier in the Brazilian market deserves further comment. No statistically significant difference was found in the duration of regulatory review for products with stronger evidence at the time of FDA approval (results not shown). Instead, pharmaceutical companies appeared to prioritise regulatory submissions in Brazil for products with better evidence. The median duration of time between FDA approval and Anvisa submission was shorter for drugs with RCTs as main studies vs those without (49 vs 246 days), and drugs with documented evidence of OS benefit (44 vs 155 days).
A prevailing argument made by pharmaceutical companies, and supported by some patient groups and clinicians, is that greater uncertainty of clinical benefit is an acceptable trade-off for faster access to promising cancer treatments. Drugs approved with less complete data than what is traditionally required (for example on the basis of surrogate endpoints or without RCTs) do not reliably demonstrate meaningful clinical benefit for patients.26 According to earlier studies, ineffective treatments can remain on the market for long periods of time.27 Although there was a time lag between cancer drug indication approvals in the US and Brazil, more drugs that received marketing authorisation in Brazil had more mature clinical evidence.
Our findings highlight the need to incentivise pharmaceutical companies to generate comparative evidence on new cancer drugs.2,10 Incorporating elements of health technology assessment into drug development could help improve the timely provision of robust comparative data for regulatory decision.28,29 Doing so may require revising regulatory evidence requirements. Early, frequent, and transparent interactions between regulators and drug manufacturers, for example through joint scientific advice from regulatory clinical trials, marketing authorisation, post-marketing surveillance, and pricing authorities could clarify evidence standards, and send a strong signal to manufacturers about the type of evidence that will be rewarded with more favourable regulatory and pricing outcomes. Crucially, such reforms in individual countries may have a limited effect, considering that most clinical trials are conducted in multiple countries, and regulations are usually agreed under harmonisation and reliance mechanisms.
The current pricing mechanism in Brazil relies on health technology assessment to identify the added therapeutic benefit of new drugs in relation to existing therapeutic options and combines this with internal and external reference pricing. During our study period, Brazilian authorities authorised lower prices for cancer drugs with no evidence of added therapeutic benefit over existing alternatives. Our finding that drugs with evidence of RCTs and substantial clinical benefit had higher prices in Brazil can suggest the value prioritisation of drugs with these characteristics. Nevertheless, pricing is only conducted at market entry and is not subsequently revised (even when new therapeutic alternatives or generic versions are available). It does not distinguish among different grades of evidence or magnitudes of clinical benefit, and neither employs PPP adjustment.
Although the Brazilian regulatory framework is designed to prevent drugs without added therapeutic benefit costing more than existing alternatives, making regulatory decisions based only on nominal price references may be misleading. Therefore the use of PPP as part of the pricing methodology could be considered.30 In addition, adopting a lifecycle approach and revising the price when new evidence or new therapeutic alternatives (or generic versions) emerge could be considered. A regulatory performance assessment of the existing regulatory framework and regulatory impact assessment of potential reforms could help inform future policy decisions.
Policymakers in other settings should consider whether a combination of approval and pricing regulations can achieve a balance between timely access to new cancer drugs and a minimum clinical evidence threshold for market entry.2,10 The evidence provided by this study suggests that regulatory requirements and a comprehensive review, combined with the assessment of added therapeutic benefit as a criterion for price setting in Brazil, may contribute to prioritising drugs with better evidence and more meaningful clinical benefit by pharmaceutical companies.
Study limitations
There are some limitations to this study. Study characteristics and outcomes reported in FDA, Anvisa and CMED assessment reports were occasionally ambiguous, or incomplete, and it was sometimes difficult to establish if the reported information corresponded to the same study in different settings. To address this issue, we consulted Clinicaltrials.gov and published studies, as needed. The ESMO-MCBS information was limited to cancer drugs targeting solid tumours and was only available for part of the sample. The study reviewed maximum approved prices in Brazil and “list” prices in the US, which are used in external reference pricing in Brazil. These prices may differ from actual prices.
Conclusions
Compared to the US, Brazil’s pharmaceutical regulatory framework, which combines marketing and pricing authorisation, favours the faster availability of drugs with better clinical evidence and rewards evidence of added clinical benefit. Brazilian pricing regulation ensures that drugs without documented evidence of added therapeutic benefit are not allowed to have higher prices than existing alternatives on the market. However, our findings suggest that the Brazilian system shows variable degrees of success in securing lower prices, as cancer drug with added therapeutic benefit were more expensive in Brazil than in the US (after PPP adjustment).
Contributors
AMIB: conceptualisation, data curation, formal analysis, investigation, writing original draft, writing—review, and editing.
FLM: data curation, formal analysis, and writing—review.
AKW: formal analysis, writing—review, and editing.
CGSOC: formal analysis, writing—review, and editing.
SV: investigation, writing—review, and editing.
EM: investigation, writing—review, and editing.
CLTA: formal analysis, and writing—review.
HN: conceptualisation, data curation, formal analysis, investigation, writing original draft, writing—review, and editing.
All authors reviewed and approved the final version of the work, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data sharing statement
The relevant websites accessed for obtaining the publicly available data used in this manuscript are listed in the Supplementary Table S2. Other non-confidential complementary information was obtained based on the provisions of the Brazilian Law of Access to Information (LAI). For obtaining data not publicly available, which are non-confidential or non-restricted, based on the provisions of the LAI, the Integrated Ombudsman and Access to Information Platform from the Brazilian Federal Government “Fala.br” (https://falabr.cgu.gov.br/publico/Manifestacao/SelecionarTipoManifestacao.aspx?ReturnUrl=%2f) can be used. No additional data are available.
Declaration of interests
The authors declare that there are no conflicts of interest regarding this article. HN reports grants from the Health Foundation, the National Institute for Health and Care Research, and UK Research and Innovation outside the submitted work, and consulting fees from the World Health Organization and Pharmaceutical Group of the European Union; HN also reports being an adviser to the Analysis section of The BMJ.
Acknowledgments
Funding sources: No funding was received for the writing for this article.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100506.
Contributor Information
Adriana M. Ivama-Brummell, Email: Adriana.ivama@anvisa.gov.br.
Anita K. Wagner, Email: anita_wagner@hms.harvard.edu.
Claudia G.S. Osorio-de-Castro, Email: claudia.osorio@ensp.fiocruz.br.
Sabine Vogler, Email: Sabine.Vogler@goeg.at.
Elias Mossialos, Email: e.a.mossialos@lse.ac.uk.
Carla L. Tavares-de-Andrade, Email: carla.andrade@fiocruz.br.
Huseyin Naci, Email: h.naci@lse.ac.uk.
Appendix A. Supplementary data
References
- 1.IQVIA Institute for Human Data Science . IQVIA; 2022. Global oncology trends 2022; p. 65. [Google Scholar]
- 2.Naci H., Salcher-Konrad M., Kesselheim A.S., et al. Generating comparative evidence on new drugs and devices before approval. Lancet. 2020;395:986–997. doi: 10.1016/S0140-6736(19)33178-2. [DOI] [PubMed] [Google Scholar]
- 3.Downing N.S., Aminawung J.A., Shah N.D., Krumholz H.M., Ross J.S. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA. 2014;311:368–377. doi: 10.1001/jama.2013.282034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tibau A., Molto C., Ocana A., et al. Magnitude of clinical benefit of cancer drugs approved by the US food and drug administration. J Natl Cancer Inst. 2018;110:486–492. doi: 10.1093/jnci/djx232. [DOI] [PubMed] [Google Scholar]
- 5.Demartino P.C., Miljković M.D., Prasad V. Potential cost implications for all US food and drug administration oncology drug approvals in 2018. JAMA Intern Med. 2020;181(2):120–122. doi: 10.1001/jamainternmed.2020.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vokinger K.N., Hwang T.J., Grischott T., et al. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost–benefit analysis. Lancet Oncol. 2020;21:664–670. doi: 10.1016/S1470-2045(20)30139-X. [DOI] [PubMed] [Google Scholar]
- 7.Salas-Vega S., Shearer E., Mossialos E. Relationship between costs and clinical benefits of new cancer medicines in Australia, France, the UK, and the US. Soc Sci Med. 2020;258 doi: 10.1016/j.socscimed.2020.113042. [DOI] [PubMed] [Google Scholar]
- 8.Wagner A.K., Ubel P.A., Wharam J.F. Financial pollution in the US Health care system. JAMA Heal Forum. 2021;2 doi: 10.1001/JAMAHEALTHFORUM.2021.0195. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO) 2018. Pricing of cancer medicines and its impacts.https://apps.who.int/iris/bitstream/handle/10665/277190/9789241515115-eng.pdf?ua=1 Geneva. Available at: [Google Scholar]
- 10.Trapani D., Tay-Teo K., Tesch M.E., et al. Implications of oncology trial design and uncertainties in efficacy-safety data on health technology assessments. Curr Oncol. 2022;29:5774–5791. doi: 10.3390/curroncol29080455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downing N.S., Aminawung J.A., Shah N.D., Braunstein J.B., Krumholz H.M., Ross J.S. Regulatory review of novel therapeutics — comparison of three regulatory agencies. N Engl J Med. 2012;366:2284–2293. doi: 10.1056/nejmsa1200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durán C.E., CañáS M., Urtasun M., Elseviers M., Stichele R.V., Christiaens T. Potential negative impact of reputed regulators decisions on the approval status of new cancer drugs in Latin American countries: a descriptive analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0254585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rand L.Z., Kesselheim A.S. International reference pricing for prescription drugs in the United States: administrative limitations and collateral effects. Value Heal. 2021;24(4):473–476. doi: 10.1016/j.jval.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Mosegui G.B.G., Villar F.A. Availability of biological cancer drugs under research: registration and price in Brazil, Colombia, and Mexico. Physis Rev Saúde Coletiva. 2020;30:1–22. doi: 10.1590/S0103-73312020300413. [DOI] [Google Scholar]
- 15.Ivama-Brummell A.M., Pingret-Kipman D., Louly P.G., Andrade R.R. Medicines regulation, pricing and reimbursement in Brazil. Rev Bras Farmácia Hosp e Serviços Saúde. 2022;13:769. doi: 10.30968/rbfhss.2022.131.0769. [DOI] [Google Scholar]
- 16.Governo do Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária (Anvisa) Guia para desfechos para estudos clínicos de medicamentos oncológicos. 2015. https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/medicamentos/publicacoes-sobre-medicamentos/desfechos-para-estudos-clinicos-de-medicamentos-oncologicos.pdf/view Available at:
- 17.Government of Brazil . DOU; 2004. Drug Market Regulation Chamber (CMED). Resolution No. 2, dated 5 March 2004. Establishes the criteria for defining prices of new products and new pharmaceutical presentations.https://www.gov.br/anvisa/pt-br/assuntos/medicamentos/cmed/legislacao/arquivos/arquivos-resolucoes/6325json-file-1 Available at: [Google Scholar]
- 18.Davis C., Naci H., Gurpinar E., Poplavska E., Pinto A., Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ. 2017;359 doi: 10.1136/BMJ.J4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naci H., Guan X., Woloshin S., Xu Z., Wagner A.K. Communication of survival data in US food and drug administration-approved labeling of cancer drugs. JAMA Intern Med. 2021;181:1521–1522. doi: 10.1001/jamainternmed.2021.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secretaria Executiva da Câmara de Regulação do Mercado de Medicamentos (SCMED) DOU; 2006. Comunicado no 15, de 27 de dezembro de 2006, sobre a definição do preço inicial de medicamentos novos. Brasil. 29 dez: 4. [Google Scholar]
- 21.Hilal T., Sonbol M.B., Prasad V. Analysis of control arm quality in randomized clinical trials leading to anticancer drug approval by the US food and drug administration. JAMA Oncology. 2019;5:887–892. doi: 10.1001/jamaoncol.2019.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilal T., Gonzalez-Velez M., Prasad V. Limitations in clinical trials leading to anticancer drug approvals by the US Food and Drug Administration. JAMA Intern Med. 2020;180:1108–1115. doi: 10.1001/jamainternmed.2020.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu M., Naci H., Booth C.M., et al. Real-world use of and spending on new oral targeted cancer drugs in the US, 2011-2018. JAMA Intern Med. 2021;181(12):1596–1604. doi: 10.1001/JAMAINTERNMED.2021.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Society for Medical Oncology (ESMO) Evidence-based standards for patient care: a tool to assist in the prioritisation of medicines in cancer care. ESMO-MCBS Factsheet. https://www.esmo.org/content/download/288502/5736211/1 Available at:
- 25.Vogler S., Vitry A., Babar Z.U.D. Cancer drugs in 16 European countries, Australia, and New Zealand: a cross-country price comparison study. Lancet Oncol. 2016;17:39–47. doi: 10.1016/S1470-2045(15)00449-0. [DOI] [PubMed] [Google Scholar]
- 26.Cipriani A., Ioannidis J.P.A., Rothwell P.M., et al. Generating comparative evidence on new drugs and devices after approval. Lancet. 2020;395:998–1010. doi: 10.1016/S0140-6736(19)33177-0. [DOI] [PubMed] [Google Scholar]
- 27.Beaver J.A., Pazdur R. “Dangling” accelerated approvals in oncology. N Engl J Med. 2021;384:e68. doi: 10.1056/NEJMP2104846. [DOI] [PubMed] [Google Scholar]
- 28.Eichler H.-G., Enzmann H., Rasi G. Added therapeutic benefit and drug licensing. Nat Rev Drug Discov. 2019;18(9):651–652. doi: 10.1038/d41573-019-00068-x. published online April 11. [DOI] [PubMed] [Google Scholar]
- 29.Maignen F., Osipenko L., Pinilla-Dominguez P., Crowe E. Integrating health technology assessment requirements in the clinical development of medicines: the experience from NICE scientific advice. Eur J Clin Pharmacol. 2017;73:297–305. doi: 10.1007/s00228-016-2174-2. [DOI] [PubMed] [Google Scholar]
- 30.Moye-Holz D., Vogler S. Comparison of prices and affordability of cancer medicines in 16 countries in Europe and Latin America. Appl Health Econ Health Policy. 2021;20(1):67–77. doi: 10.1007/S40258-021-00670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.