Abstract
Innovative therapeutic strategies are urgently needed for Alzheimer’s disease (AD) due to the increasing size of the aging population and the lack of effective drug treatment. Here, we report the therapeutic effects of extracellular vesicles (EVs) secreted by microglia, including macrosomes and small EVs, on AD-associated pathology. Macrosomes strongly inhibited β-amyloid (Aβ) aggregation and rescued cells from Aβ misfolding–induced cytotoxicity. Furthermore, macrosome administration reduced Aβ plaques and ameliorated cognitive impairment in mice with AD. In contrast, small EVs slightly promoted Aβ aggregation and did not improve AD pathology. Proteomic analysis of small EVs and macrosomes revealed that macrosomes harbor several important neuroprotective proteins that inhibit Aβ misfolding. In particular, the small integral membrane protein 10–like protein 2B in macrosomes has been shown to inhibit Aβ aggregation. Our observations provide an alternative therapeutic strategy for the treatment of AD over conventional ineffective drug treatments.
Microglial macrosomes inhibit Aβ fibrillation and Aβ-associated cytotoxicity and ameliorate cognitive impairments in AD mice.
INTRODUCTION
Alzheimer’s disease (AD) is expected to affect about 150 million individuals worldwide by 2050, resulting in considerable personal and public health burden (1, 2), and at a critical juncture for drug discovery with a 99.6% clinical failure rate of most developed drug interventions (3). The failure of drugs, including verubecestat [β-amyloid (Aβ) secretase inhibitors] and solanezumab (monoclonal antibody for Aβ), to improve cognitive impairment in patients is mainly attributed to unsatisfactory inhibition effect on the target, drug-associated side effects, inability to cross the blood-brain barrier, and neuroinflammatory responses (3–5). Thus, innovative strategies are urgently needed to develop effective therapeutics for AD compared to traditional drug–based therapies (6). Recent microglia-based therapeutic strategies have yielded promising results in several studies. Martorell et al. (7) showed that auditory and visual gamma entrainment induced microglial clustering around Aβ plaques, which ameliorated AD-associated pathology and improved cognition in AD mice. A nasal proteosome-based adjuvant (protollin), which can activate microglia to prevent Aβ deposition, is being prepared for human clinical trials (8). However, Aβ stimulation–induced microglial inflammation can contribute to neurotoxicity through the activation of several proinflammatory cascades, which might be detrimental to AD treatment (9, 10). These mixed outcomes indicate that effective cell-free therapies needed to be explored.
Several studies have demonstrated that microglia eliminate Aβ plaques primarily through phagocytosis (11). Apart from phagocytic mechanisms, cells also release extracellular vesicles (EVs), which contain information related to a broad range of cellular behaviors and physiological functions (12, 13). EVs are divided into exosomes and microvesicles (referred to as macrosomes in this study) according to their size and biogenesis and carry cargo material including proteins, lipids, and microRNAs. Exosome biogenesis starts with the formation of a multivesicular body via endocytosis and ends with exocytosis (14, 15). Exosomes are characterized by dimensions ranging from 30 to 150 nm in diameter, as well as the classical marker CD36 and endosomal sorting complexes required for transport (ESCRT) proteins TSG101 and Alix (14). Macrosomes are shed by outward vesiculation of the plasma membrane, which represents an active element of the cell secretome, and typically have dimensions ranging from 150 to 1000 nm in diameter (14, 16). Flotillin-2 is enriched in macrosomes (17), and adenosine diphosphate ribosylation factor 6 (ARF6) regulates the abscission and shedding of macrosomes (18) and can be regarded as macrosomal markers. Proteins including apolipoproteins A1/2 (APOA1/2), apolipoprotein B (APOB), and albumin (ALB) are major non-EV contaminants that are often coisolated with EVs (19), and evaluating their presence helps to assess the purity of EVs.
Microglial EVs mediate the complex and coordinated communication in the central nervous system and exert both beneficial and detrimental effects in many pathophysiological states (20). Thus, elucidating the EV components that affect disease pathogenesis has important implications for the development of therapeutic interventions for neurological diseases. In this study, we investigated the effect of microglia-derived EVs on AD-related pathology in vitro and in an AD mouse model. As shown in Fig. 1A, EVs released by microglia were extracted through gradient centrifugation at 11,000g to isolate the macrosomes and at 100,000g to isolate the small EVs (21). We used Aβ40 peptide as the objective pathological target, as its misfolding and aggregation are widely prevalent in the brains of patients with AD (22) and initiate neurotoxic events including neuroinflammation, synaptic and neuronal loss, and tau-associated pathology (23–25).
Fig. 1. Characterization of small EVs and macrosomes, and their influence on Aβ40 fibrillation.
(A) Flowchart of this study. (B to E) Size distribution profiles (B) and (C) and representative TEM images (D) and (E) of small EVs (B) and (D) and macrosomes (C) and (E), detected by NanoSight tracking analysis (NTA) or transmission electron microscopy (TEM). (F) Measurement of the hydrodynamic diameters of small EVs or macrosomes. n = 26 particles measured. (G) Western blot analysis of representative signature proteins in small EVs or macrosomes. This experiment was carried out thrice. (H and I) Thioflavin T (ThT)–monitoring kinetic curves of Aβ40 (25 μM in 10 mM PBS buffer solution) fibrillation at 37°C in the absence and presence of small EVs or macrosomes. Black lines: Aβ40 alone; red or blue lines: Aβ40 incubated with small EVs (red) or macrosomes (blue), respectively. The concentration of small EVs or macrosomes is 50 (H) or 2.5 (I) μg ml−1, respectively. The error bars represent SDs from the mean of at least three independent experiments. (J to L) Atomic force microscopy (AFM) images of Aβ40 before (J) and after incubation with small EVs (K) or macrosomes (L) at 37°C for 70 hours, and the corresponding section profile along the green line. AFM samples were prepared by dropping 10 μl of test solutions collected in (H) on mica film. Error bars, means ± SD, one-way analysis of variance (ANOVA), Tukey’s test. ***P < 0.001.
Here, we report that small EV treatment promoted fibril formation and did not improve spatial learning and memory deficits and Aβ plaque generation in AD mice. Macrosomes had a strong inhibitory effect on Aβ fibrillation and rescued Aβ-mediated cell death. This result is proven by several pathogenic cellular cascades, such as Ras-related C3 botulinum toxin substrate (Rac1) activation experiments, dynamic detection of Ca2+ dyshomeostasis, and reactive oxygen species (ROS) assays. Furthermore, macrosome treatment for 1 month substantially ameliorated cognitive ability, attenuated neuroinflammation, and reduced Aβ plaques in AD mice brains, suggesting their use as a potential therapeutic for AD.
In addition, small EV and macrosome proteomic analysis revealed that macrosomes integrate multiple neuroprotective proteins into one system, displaying an advanced platform for AD combination therapy that is distinct from conventional small-molecule inhibitors. In particular, reduction of small integral membrane protein 10–like protein 2B (SIL2B) expression in microglia with small interfering RNA (siRNA) decreased the inhibitory effect of macrosomes on Aβ fibrillation, providing a potential endogenous inhibitor for the treatment of AD. The notable discovery of the inhibitory effect of endogenous macrosomes on AD progression suggests that they could be used in potential AD therapies and may provide a treatment paradigm for neurodegenerative diseases.
RESULTS
Macrosomes prevent Aβ fibrillation
We used conventional differential centrifugation to isolate small EVs and macrosomes from human microglial clone 3 (HMC3) cell (see Materials and Methods) and measured individual fractions using NanoSight tracking analysis (NTA) to validate a homogeneous particle size concentrated at 126 nm for small EVs (Fig. 1B). The NTA test showed that the isolated macrosome size was in the range of 200 to 600 nm (Fig. 1C). Transmission electron microscopy (TEM) with negative small EV staining further revealed their size and morphology, with numerous small EV fractions with an average size of 103 nm (Fig. 1D, fig. S1A, and red dots in Fig. 1F). In comparison, the morphology of macrosomes was different from that of small EVs (Fig. 1E and fig. S1B), with observed macrosomes ranging from 161 to 545 nm (blue dots in Fig. 1F) and an average size of 276 nm, which was substantially larger than that of small EVs. Western blot analysis demonstrated that isolated small EVs were enriched for the markers CD63, TSG101, and Alix. Macrosomes were enriched for Flotillin-2 and ARF6 (Fig. 1G and fig. S2). Notably, both isolated small EVs and macrosomes were devoid of APOA1/2, APOB, and ALB.
Given the intrinsic heterogeneity of EVs, we investigated the effect of small EVs and macrosomes on Aβ40 fibrillation using an amyloid-specific dye–thioflavin T (ThT) fluorescence assay (26). Fresh Aβ40 (25 μM) was incubated with different concentrations [measured using bicinchoninic acid (BCA) reagent] of small EVs or macrosomes for 70 hours at 37°C. Individual Aβ40 fibril formation was assessed by an increase in ThT fluorescence after 42 hours (Fig. 1H, black line). Treatment with small EVs (50 μg ml−1) advanced the time of fibril formation from 42 to 32 hours (Fig. 1H, red line) compared with Aβ40 incubation alone. In contrast, macrosome (50 μg ml−1) treatment completely prevented fibril formation, with no detected increase in ThT fluorescence even after an incubation time of more than 70 hours (Fig. 1H, blue line). EVs also affected fibril formation in a concentration-dependent manner. A low concentration of small EVs (2.5 μg ml−1) still accelerated the fibrillation process, as reflected by a clear fibril formation time of 25 hours and a remarkable increase in ThT intensity (Fig. 1I, red line). Macrosomes inhibited fibril formation time even at a low concentration (2.5 μg ml−1) to 50 hours (Fig. 1I, blue line) with the ThT intensity closer to that of Aβ40 incubation alone.
Atomic force microscopy (AFM) analyses further confirmed the fibril inhibitory effect of macrosomes. Numerous long and dense fibrils intertwined with each other were detected for Aβ40 alone (Fig. 1J), which became denser when small EVs were added (Fig. 1K). In sharp contrast, mature amyloid fibrils were rarely observed after macrosome treatment; occasionally, a short fibril could be found with a height of 7 nm (Fig. 1L), which is less than half of that detected for Aβ40 alone (18 nm) or with small EV addition (19 nm). Further study demonstrated that macrosomes could also inhibit Aβ42 fibrillation, a relatively minor constituent (∼9%) of Aβ species associated with AD progression (figs. S3 and S4). These results demonstrated that small EVs and macrosomes showed notable differences in preventing Aβ fibrillation and that macrosomes exhibited therapeutic potential for AD.
Macrosomes inhibit Aβ40-mediated cytotoxicity
Because macrosomes inhibited Aβ40 fibrillation, they could also mediate cytoprotective effects against Aβ40-mediated cell death. Therefore, we investigated the influence of small EVs and macrosomes on Aβ40-mediated cytotoxicity using the cell counting kit-8 (CCK-8) assay in SHSY5Y cells, which is a typical cell line for neurodegenerative disease studies. Small EVs and macrosomes themselves did not cause evident toxicity in SHSY5Y cells at different concentrations, and the cell survival rates were higher than 90% (Fig. 2A). Aβ40 addition to SHSY5Y cells resulted in notable cell toxicity, with cell viability of 49.7 ± 9.7% and 44.7 ± 9.4% after Aβ40 incubation for 48 and 72 hours, respectively (Fig. 2, B and C). Encouragingly, macrosome addition to SHSY5Y cells at the beginning protected them from Aβ40-mediated cell death, and the cell viability recovered to 94.9 ± 8.4% and 86.3 ± 1.3% after 48 and 72 hours, respectively (Fig. 2, B and C). Under the same conditions, small EVs failed to rescue cell viability (43.9 ± 6.4% and 43.8 ± 1.4%). Furthermore, combining Aβ40 with different concentrations of small EVs or macrosomes for 48 hours provided a half-inhibitory concentration (IC50) (Fig. 2, D to F), which indicated that macrosomes could rescue cells from Aβ40-induced death with an IC50 of 5 ± 1 μg ml−1, whereas small EVs had no therapeutic effects.
Fig. 2. Effect of small EVs or macrosomes on Aβ40-mediated cytotoxicity.
(A) SHSY5Y cytotoxicity when different concentrations of small EVs or macrosomes were added for 48 hours, measured by CCK-8 assay (n = 3). (B and C) Comparison of CCK-8 assay detected cytotoxicity treated with Aβ40 (25 μM) and Aβ40 combined with small EVs or macrosomes (50 μg ml−1) for 48 (B) and 72 (C) hours, respectively (n = 3). (D and E) Cytotoxicity of Aβ40 addition to SHSY5Y cells in the presence of different concentrations of small EVs (D) (the concentrations are 0.02, 0.07, 0.21, 0.62, 1.86, 5.57, 16.7 and 50 μg ml−1, respectively) or macrosomes (E) for 48 hours (n = 3). (F) Dose-dependent effect of small EVs (red) and macrosomes (blue) on Aβ40-induced cytotoxicity. (G to J) Flow cytogram detected cell apoptosis before (G) and after treatments with Aβ40 (25 μM) (H) and Aβ40 combined with small EVs (I) or macrosomes (J) (50 μg ml−1) for 48 hours; the cells were stained by annexin V–FITC/PI (n = 3). Error bars, means ± SD. **P < 0.01, indicating a notable difference, one-way ANOVA.
We quantified apoptosis in SHSY5Y cells treated with Aβ40 and small EVs or macrosomes for 48 hours by staining with annexin V–fluorescein isothiocyanate (annexin V–FITC)/propidium iodide (PI) and flow cytometry analysis. As shown in Fig. 2 (G to J) and fig. S5, treatment with Aβ40 alone and Aβ40 incubated with small EVs resulted in 40.08 ± 0.57% and 31.74 ± 5.03% PI+ cells, respectively. Macrosome treatment decreased PI+ cells to 2.16 ± 1.41%, which is closer to that of the control group. These results demonstrate that macrosomes could reduce Aβ40 fibrillation–induced cell death.
Macrosomes rescue apoptotic signals after Aβ deposition in SHSY5Y cells
Rac1, a small guanosine triphosphatase, regulates forgetting behavior in mice (27). Aβ can induce Rac1 activation in cultured cells (28). Cytosolic Ca2+ level is another important signaling molecule responsible for learning and memory, and Aβ aggregates have been shown to increase Ca2+ levels in nerve cells (29). The activation of Rac1 and Ca2+ dyshomeostasis could promote mitochondria to produce more ROS, leading to neuronal loss (29–31) (Fig. 3A). Therefore, detection of Rac1 activity, cytosolic Ca2+ levels, and ROS production in cells could be used to evaluate the learning and memory ability and neuronal loss–related pathology in vitro.
Fig. 3. Impact of macosomes on Aβ40 aggregation–related cellular signaling pathway.
(A) Pathogenic cellular cascades activated by Aβ40 aggregation. Incubated Aβ40 (24 hours) could induce the activation of Rac1 through the signaling pathway phosphatidylinositol 3-kinase (PI3K)/phosphoinositol-dependent kinase (PDK1)/novel protein kinase C (nPKC) axis via N-methyl-d-aspartic acid (NMDA) receptor. It can lead to Ca2+ dyshomeostasis through NMDA and α-amino-3-hydroxy-5-methyl-4-isoxa-zolep-propionate (AMPA) receptors. Then Rac1 activation and Ca2+ accumulation induce ROS production released from mitochondria. (B to G) Representative morphological images and time course of normalized fluorescence intensity of SHSY5Y cells after addition of Aβ40 (incubated for 24 hours) (B) and (C) (n = 7) and their mixtures with small EVs (D) and (E) (n = 6) and with macrosomes (F) and (G) (n = 6). The cells were transfected with PAK-PBD-GFP biosensor to indicate the Rac1 level, in which color bar indicates fluorescence intensity; red and blue represent the high and low Rac1 level, respectively. (H to M) Fluorescence ratio images and time course of normalized YPet/ECFP ratio of SHSY5Y cells after treatment with Aβ40 (incubated for 24 hours) (H) and (I) (n = 6) and their mixtures with small EVs (J) and (K) (n = 6) and macrosomes (L) and (M) (n = 8). The cells were transfected with Cyto-Ca2+ biosensor to represent the Ca2+ levels. The color bar indicates the YPet/ECFP ratio; red and blue colors represent the high and low concentrations of Ca2+, respectively. Scale bars, 20 μm. (N) Continuous detection of ROS production for 6 hours in SHSY5Y cells after treatment with Aβ40 (incubated for 48 hours) and their mixtures with small EVs or macrosomes (n = 3). The intensity of the blank group was taken as I0 and the intensity of the other group was taken as I. Error bars, means ± SD. In these tests, the concentrations of Aβ40 small EVs or macrosomes are 25 μM or 50 μg ml−1, respectively.
First, we assessed the effect of small EVs and macrosomes on Aβ40-induced Rac1 activity in SHSY5Y cells using a genetically encoded PAK (p21-activated kinase)–PBD (p21-binding domain)–GFP (green fluorescent protein) reporter (32) to visualize real-time Rac1 activation under a fluorescence microscope. Initially, Aβ40 was incubated at 37°C for 24 hours to allow oligomers formation, whereas small EVs or macrosomes were added at the beginning of peptide incubation. When Aβ40 oligomers or their mixture with small EVs were added to the cells, the normalized fluorescence intensity of the cell images increased gradually from 1.0 to 1.2, corresponding to several red signals appearing in the cell membrane (Fig. 3, B to E). By comparison, there was no change in the fluorescence intensity when the mixture of Aβ40 with macrosomes was added to SHSY5Y cells, and red signals were hardly observed in the cell membrane (Fig. 3, F and G), indicating that macrosomes rescued Aβ40 aggregation–induced Rac1 activation.
Subsequently, we investigated whether macrosomes could rescue the excess Ca2+ production triggered by Aβ misfolding using a fluorescence resonance energy transfer (FRET)–based Cyto-Ca2+ biosensor (33) at the single-cell level. The treatment of cells with Aβ40 oligomers or their mixture with small EVs increased the YPet/enhanced cyan fluorescent protein (ECFP) ratio from 1.0 to 1.2, corresponding to increased cytoplasmic Ca2+, with numerous red dots (indicating the location of excessive Ca2+) appearing in the FRET ratio images, as shown in Fig. 3 (H to K). However, the YPet/ECFP ratio was unchanged after the addition of Aβ40 mixture with macrosomes in SHSY5Y cells, and visible red dots could not be found in the FRET ratio image (Fig. 3, L and M). This result indicates that macrosomes rescued Ca2+ dyshomeostasis induced by Aβ40 aggregation. Furthermore, the ROS production was monitored by using the dichlorofluorescein diacetate assay (34). As shown in Fig. 3N, a large amount of cellular ROS was generated after the introduction of Aβ40 oligomers, short fibrils (monomers were incubated at 37°C for 48 hours), or their mixture with small EVs (black or red columns in Fig. 3N). Under the same conditions, macrosomes suppressed the Aβ40 aggregation–induced increased ROS levels (blue columns in Fig. 3N), which were similar to that of the control group. Together, these results indicate that macrosomes could substantially rescue Aβ40 aggregation–induced cell damage.
Macrosomes rescue cognitive ability and memory deficits in AD model mice
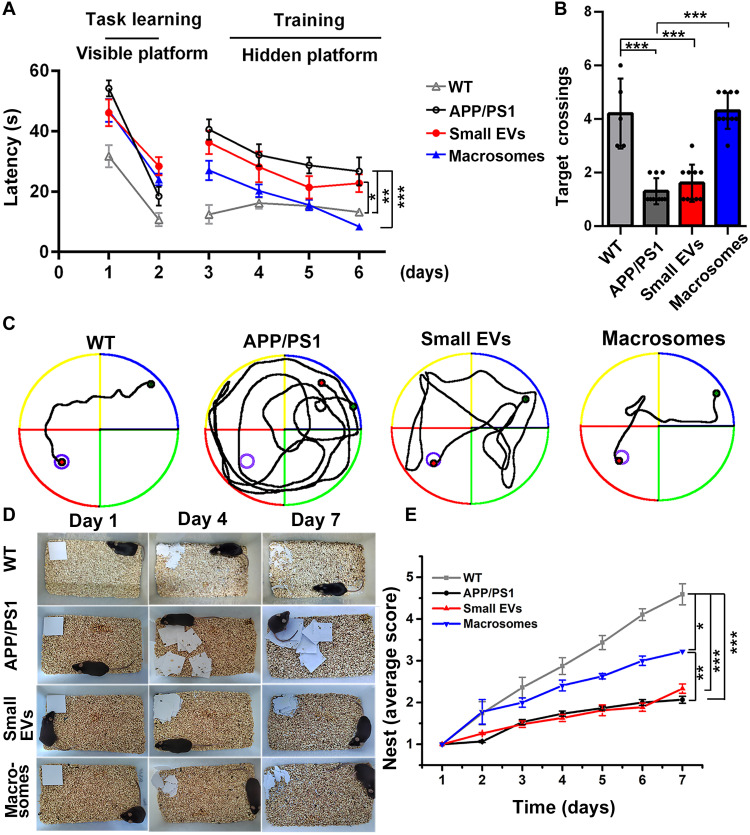
The strong inhibitory effect of macrosomes on Aβ40 aggregation in vitro encouraged us to investigate their therapeutic effect on AD. Thereafter, 5-month-old Aβ precursor protein/presenilin 1 (APP/PS1) double-transgenic (Tg) male mice, which are characterized by Aβ deposition and cognitive impairment (35), were used to evaluate the therapeutic effects of macrosomes in vivo. The mice were daily administrated through nasal feeding for 4 weeks with small EVs or macrosomes (200 μg kg−1), respectively. Mice treated with phosphate-buffered saline (PBS) were used as the control group. Here, nasal feeding was adopted to avoid the participation of EVs in other signaling events and autonomous biochemical reactions in vivo. Subsequently, we evaluated the therapeutic effect of macrosomes on learning and spatial memory in mice using the Morris water maze and nesting construction tests (36, 37). The visible platform tests (during the first 2 days of the learning stage) showed that APP/PS1 Tg mice and mice treated with small EVs or macrosomes had almost the same motility or visual acuity, with no statistical differences (Fig. 4A). The latency of different groups of mice to search for the hidden platform was measured for another 5 days, and the probe trial was conducted after removal of the hidden platform on day 6. Compared with wild-type (WT) mice (gray line and column in Fig. 4, A and B), APP/PS1 Tg mice showed obvious deficits in spatial memory, as evidenced by longer escape latency time and shorter target crossings (black line and column in Fig. 4, A and B). However, APP/PS1 Tg mice treated with macrosomes were largely protected from spatial memory impairment and exhibited a shorter latency to escape onto the platform, more platform crossings, and better-focused search strategies for the platform in the quadrant (blue line and column in Fig. 4, A and B). In comparison, there was no obvious therapeutic effect of small EVs on APP/PS1 Tg mice (red line and column in Fig. 4, A and B). Representative trajectories of mice on day 5 in the place navigation component of the Morris water maze are shown in Fig. 4C. In addition, similar phenomena were observed in the nest building tests. Compared with WT mice, APP/PS1 Tg mice and those treated with small EVs failed to make the nest; in contrast, after macrosome treatment, APP/PS1 Tg mice shredded the paper into small fragments, with a substantially improved nest score (Fig. 4, D and E).
Fig. 4. Assessment of cognitive decline in APP/PS1 Tg mice through Morris water maze and nesting building tests.
Five-month-old APP/PS1 Tg mice were treated with small EVs or macrosomes (200 μg kg−1) for 4 weeks through nasal feeding daily. (A) Latency for escape to visible platform and hidden platform. (B) Numbers of the target crossings in the test. (C) Representative images of track of probe test. (D and E) Representative images of nesting building and scores observed for seven consecutive days. n = 6 mice: WT group; n = 10 mice: APP/PS1, small EV, and macrosome groups. Error bars, means ± SD, one-way ANOVA, Tukey’s test. *P < 0.05, **P < 0.01, and ***P < 0.001.
The therapeutic effect of macrosomes on female AD mice was further evaluated. Identical behavioral tests were performed on 5-month-old APP/PS1 Tg female mice. The Morris water maze and nesting building tests indicated that macrosomes rescued spatial learning and memory impairments in female AD mice as well, and the rescuing effect of small EVs was weaker (fig. S6). Together, these results demonstrate that a 4-week daily treatment with macrosomes largely rescued cognitive ability and memory deficits in AD model mice.
Toxicity evaluation of small EVs and macrosomes in vivo
To evaluate the biosafety of small EVs and macrosomes for the treatment of AD, we performed hematoxylin and eosin staining of major organs after nasal feeding of small EVs or macrosomes for 1 month. The results revealed that there was no noticeable damage to the major organs, including the heart, liver, kidney, and lungs (fig. S7). In summary, both small EVs and macrosomes were safe at the current dose in vivo.
Macrosomes reduced Aβ burden and further microglia activation in the brains of AD mice
Accumulation of Aβ plaques in brain regions is one of the most remarkable pathological features of AD. With AD progression, the accumulation of Aβ produces cytotoxicity, which leads to neuronal dysfunction, cell death, and eventually cognitive dysfunction (23, 24, 38). First, we performed Aβ plaque immunohistochemistry staining of brain regions, including the cortex and hippocampus, to evaluate whether small EV or macrosome treatment affected Aβ accumulation in APP/PS1 Tg mice. We observed a large number of Aβ plaques as brown clumps scattered in APP/PS1 Tg mice. Immunohistochemical staining for Aβ revealed that the senile plaques were spherical in the hippocampus and cortex of the control group (Fig. 5, A and B). Although the plaques of the small EV–treated group were reduced compared with those of the control group, the shapes of senile plaques were also solid spheres. Macrosomes treatment substantially decreased the number and size of Aβ-positive plaques, with the plaques becoming looser, indicating that macrosomes reduced Aβ aggregation in APP/PS1 Tg mice (Fig. 5, A and B).
Fig. 5. Macrosomes reduce Aβ accumulation and inflammatory responses in the brain of AD mice.
(A) Immunohistochemistry staining of Aβ plaques in the brain of APP/PS1 Tg mice, mice treated with small EVs, or mice treated with macrosomes (n = 4). (B) Quantification of Aβ plaques loads in the images of immunohistochemistry in the cortex and hippocampus tissue of mice samples (n = 8 slices from four mice per group). (C to E) Aβ plaques and CLEC7A+ microglia in the brain region of APP/PS1 Tg mice (C), mice treated with small EVs (D), or mice treated with macrosomes (E). DAPI, 4′,6-diamidino-2-phenylindole. (F) Number of CLEC7A+ microglia around the Aβ plaques in the brain region of mice samples in (C) to (E) (n = 3 to 6). Error bars, means ± SD, one-way ANOVA, Tukey’s test. *P < 0.05 and ***P < 0.001.
Continuous stimulation of microglia by Aβ leads to neurotoxicity via several immune responses (10). Thus, we evaluated whether small EV or macrosome treatment could reduce Aβ plaques and subsequent inflammation in the brain tissue sections of APP/PS1 Tg mice, by performing immunofluorescence staining for microglia neurodegenerative phenotype (MGnD) marker C-type lectin domain containing 7A (CLEC7A) (10). CLEC7A+ microglia were found around Aβ plaques in the brains of the vehicle- and small EV–treated group. In contrast, CLEC7A+ microglia were rarely found in the brains of macrosome-treated mice (Fig. 5, C to F). The above result demonstrates that the reduction in Aβ accumulation by macrosomes can decrease microglial activation and prevent excessive inflammation.
Binding affinity and possible binding site in Aβ40
We explored the mechanisms underlying macrosome-mediated Aβ40 aggregation inhibition by measuring the binding affinity between Aβ40 and small EVs or macrosomes using microscale thermophoresis (MST), a biophysical assay particularly appropriate for quantifying the interaction between proteins and vesicles (39). Fluorescein-labeled Aβ40 was used as the target molecule, whereas nonlabeled small EVs or macrosomes were chosen as the ligand in this assay. As shown in Fig. 6 (A and B), MST time traces of fluorescence intensity changes revealed that the equilibrium dissociation constant (Kd) between Aβ40 and macrosomes was 14.3 nM, with a smaller Kd corresponding to a stronger binding affinity. Under the same conditions, the interaction of Aβ40 with small EVs was substantially weaker, and a reliable Kd could not be obtained owing to the irregular fluorescence variation (fig. S8, A and B).
Fig. 6. Binding affinity and sites of Aβ40 with macrosomes.
(A) Time dependence of relative fluorescent intensity of fluorescein-labeled Aβ40 (100 nM) interacted with macrosomes with different concentrations, ranging from 9.16 × 10−12 M to 0.3 μM (from bottom to top). Inset of (A) displays the measurement principle. (B) Changes of the fluorescence values, which are plotted as ΔFnorm (Change of normalized fluorescence values) to yield a binding curve, which could be fitted to derive dissociation constant (Kd) based on a one-site binding model. ‰, per mil. (C) Comparison of 1H–15N heteronuclear single quantum coherence nuclear magnetic resonance (NMR) spectra of 15N–Aβ40 peptide (75 μM) before (blue) and after (red) interaction with macrosomes (20 μg ml−1) in D2O:H2O (1:9, v:v) at 25°C. (D and E) A plot of the NMR chemical shift changes for 15N (D) atoms or 1H (E) protons of Aβ40 induced by macrosomes. ppm, parts per million.
Furthermore, we verified the possible binding sites of Aβ40 with small EVs or macrosomes by performing two-dimensional (2D) 15N–1H heteronuclear single quantum coherence nuclear magnetic resonance (NMR) analysis, determining the chemical shift attribution of each N–H signal based on a published report (40). Changes in the chemical shift in the backbone amide groups of 15N-labeled Aβ40 induced by macrosomes are presented in Fig. 6 (C to E), in which remarkable variations in both the 1H and 15N dimensions were detected for the residues Phe19, Ala21, Asp23, Leu34, Gly37, Val39, and Val40, which have been reported to be involved in Aβ fibrillation (41, 42). In comparison, small EVs induced only weak changes in the chemical shifts of Phe19, Ala21, and Gly37 (fig. S8, C to E). These results provide clear evidence for a strong interaction between Aβ40 and macrosomes, which might restrict the free movement of Aβ40 monomers, thus preventing the formation of Aβ40 oligomers and fibrils (43). From the viewpoint of EVs, macrosomes and small EVs displayed different binding modes with Aβ40, which led us to explore the difference between macrosomes and small EVs in their protein composition.
Proteomic analysis
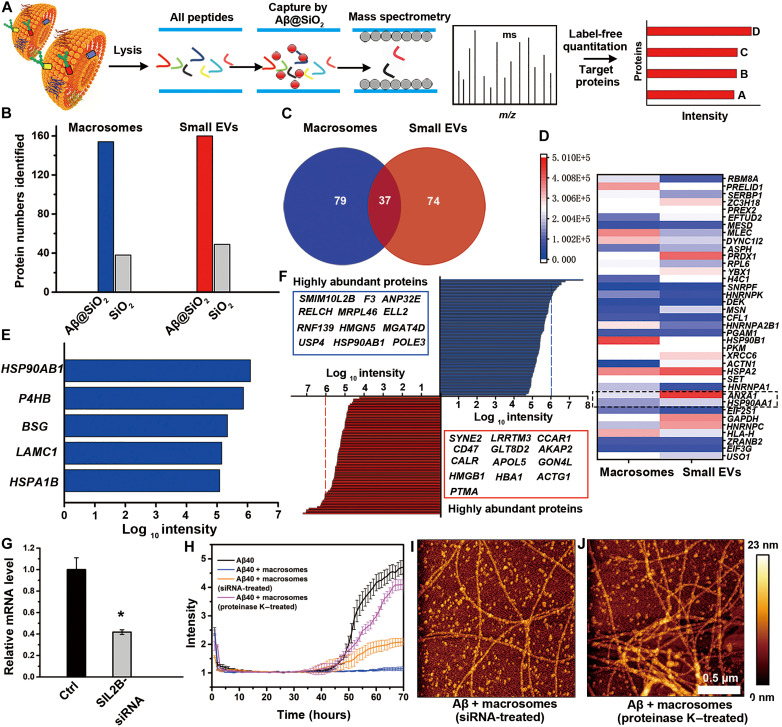
An in-depth analysis of EV composition is required to understand their functions and to design future drugs (14, 44). Therefore, we performed a comprehensive proteomic analysis to identify the crucial components of macrosomes that might contribute to the inhibition of Aβ fibrillation. First, small EVs and macrosomes were lysed and digested into peptides using trypsin, and then, the peptides were desalted using a Sep-Pak C18 column. To identify possible Aβ40-interacting proteins, the digested peptides were enriched by Aβ40-modified silica microspheres (abbreviated as Aβ40@SiO2), which were prepared by grafting Aβ40 onto the silica microsphere surface via the ring-opening reaction of epoxy (fig. S9A) (45). The detailed material characterization of Aβ40@SiO2, including x-ray photoelectron spectroscopy and thermal gravimetric analyzer, is shown in fig. S9 (B to D) and tables S1 and S2.
Using a dispersive solid phase extraction method including “loading, washing, and elution” procedures, a large number of peptides were enriched by Aβ40@SiO2, and they were identified using liquid chromatography–tandem mass spectrometry (LC-MS/MS) and analyzed based on a UniProt human database (see Materials and Methods) (Fig. 7A). As shown in Fig. 7B, 154 and 160 proteins were identified from macrosomes and small EVs, respectively, and 38 and 49 proteins were excluded due to overlap with the proteins enriched by silica gel alone due to physical adsorption. Further analysis (Fig. 7C) revealed that 116 and 111 types of proteins belonged to macrosomes and small EVs, respectively (detailed information is listed in tables S3 and S4 in two Excel spreadsheets), while 37 kinds of proteins could be found in both macrosomes and small EVs. Figure 7D shows a heat diagram of a distinct difference in the abundance of these 37 proteins, in which annexin A1 (gene ANXA1) (table S5) was reported to play a role in Aβ clearance (46). In addition, HSP90 (HSP90AA1) (table S5) has been reported to prevent Aβ aggregation (47). However, their expression levels in small EVs were relatively higher than those in macrosomes, indicating that these two proteins did not contribute to the inhibitory effect of macrosomes on Aβ40 aggregation, suggesting that other proteins in macrosomes strongly prevent Aβ40 aggregation.
Fig. 7. Proteomics of macrosomes and small EVs.
(A) Flow chart of the proteomic analysis of macrosomes or small EVs by Aβ40@SiO2 as the capture probe, including protein lysis, peptide capture, LC-MS, and data analysis. m/z, mass/charge ratio. (B) Identified protein numbers from macrosomes and small EVs, captured by Aβ40@SiO2 or bare SiO2 microspheres. (C) Venn diagram representing the number of unique and overlapping proteins between macrosomes and small EVs. (D) Heatmap of overlapping proteins simultaneously existed in macrosomes and small EVs. Scale bar shown is the protein abundance determined by label-free quantification (LFQ). (E) List of proteins closely related to the aggregation of Aβ and their abundance (log10 LFQ value is defined as the protein abundance), identified from macroscomes. (F) List of proteins and their abundance identified in macrosomes (blue) or small EVs (red). (G) Transfection of HMC3 cells with SIL2B-siRNA reduced SIL2B mRNA expression levels, determined by quantitative reverse transcription polymerase chain reaction (n = 3). (H) ThT-monitoring kinetic curves of Aβ40 (25 μM) fibrillation at 37°C in the absence (black) and presence of macrosomes (blue) or macrosomes lack of SIL2B (orange) or proteinase K–treated macrosomes (purple). The error bars represent SDs from the mean of at least three independent experiments. (I and J) AFM images of Aβ40 after incubation with macrosomes derived from SIL2B-reduced HMC3 cells (I) or proteinase K–treated macrosomes (J) at 37°C for 70 hours. Error bars, means ± SD, one-way ANOVA, Tukey’s test. *P < 0.05.
Subsequently, we analyzed the 79 unique proteins found in macrosomes. Five kinds of proteins (table S5), including HSP70 member (HSPA1B) (47, 48), laminin subunit gamma-1 (LAMC1) (49), basigin (BSG, known as CD147) (50), protein disulfide-isomerase (P4HB) (51), and HSP90 (HSP90AB1) (47), have already been reported to inhibit Aβ aggregation and reduce Aβ-mediated cytotoxicity. These proteins were highly abundant in macrosomes (Fig. 7E) and could be involved in their inhibitory effects.
We further analyzed several other highly abundant small EV and macrosome proteins in addition to the established AD-associated proteins. Figure 7F lists the proteins exclusive to macrosomes or small EVs in order of their abundance, such as SIL2B (SMIM10L2B) and tissue factor (F3), which were highly abundant in macrosomes but were not found in small EVs. Among these proteins, SIL2B (table S5) was the most abundant protein in macrosomes; thus, we investigated its role in Aβ fibrillation by knocking down its expression using SIL2B-siRNA in HMC3 cells. SIL2B-siRNA successfully degraded SIL2B as demonstrated by reduced SIL2B mRNA levels by quantitative reverse transcription polymerase chain reaction (Fig. 7G) and proteomic analysis (fig. S10). Subsequently, macrosomes were extracted from SIL2B-siRNA HMC3 cells, and their effect on Aβ40 fibrillation was investigated. As shown in Fig. 7H (orange line), macrosomes derived from SIL2B-siRNA HMC3 cells had a greatly reduced inhibitory effect on Aβ40 fibrillation. Because of the lack of a specific proteinase to remove SIL2B protein, proteinase K (100 μg ml−1) was used to cleave surface proteins of macrosomes. ThT growth curve (purple line in Fig. 7H) indicated that the inhibitory effect on Aβ40 aggregation was largely reduced when proteinase K–treated macrosomes were added, which further proved the inhibitory effect of SIL2B on Aβ40 aggregation. These were further confirmed by the AFM tests: Many loose, long, and mature amyloid fibrils appeared when Aβ40 was incubated with the SIL2B slienced macrosomes (Fig. 7I) or proteinase K–treated macrosomes (Fig. 7J), which were different from Aβ40 incubated with normal macrosomes (Fig. 1L). Moreover, we evaluated the effect of a key peptide fragment (AASAALSAAAAAAALSGLAVR) in the SIL2B protein that was identified by proteomics analysis on Aβ40 aggregation. Both the ThT assay and AFM tests showed that this peptide inhibited Aβ40 fibrillation in a concentration-dependent manner (fig. S11). Together, these results demonstrate that macrosomes integrate multiple neuroprotective proteins, particularly SIL2B, into one system, thus providing a synergistic therapy for AD. In contrast, Alix, the specific marker of small EVs, was seen around the Aβ neuritic plaques (52). Previous work has also shown that another small EV marker, flotillin-1, is associated with the part of Aβ within the senile plaques and suggested flotillin-1 to be an indicator of the progression of AD pathology (53), which might explain the unsatisfactory rescuing effect of small EVs on Aβ aggregation.
DISCUSSION
Although there have been notable advances in the understanding of AD pathobiology, disease-modifying therapies remain elusive for clinical treatment of AD. In this study, we showed that macrosomes derived from microglia have a strong inhibitory effect on Aβ aggregation and rescue cells from Aβ misfolding–induced death in comparison with small EVs. Macrosome treatment robustly reduced Aβ plaques and improved cognitive impairment in AD mice. However, small EVs failed to improve memory deficits. Mechanistically, our proteomic analyses revealed that macrosomes were enriched in multiple neuroprotective proteins that can prevent Aβ aggregation compared with small EVs, providing an advanced platform against AD. The application of macrosomes in AD represents a promising cell-free therapeutic approach, which is fundamentally different from previous therapies. The identification of endogenous inhibitor proteins in macrosomes reminds us that the combination of these inhibitor proteins into one supporter is an effective strategy, which can be used more widely in AD or other neurodegenerative clinical trials. We should also take advantage of the complex cargo of macrosomes or small EVs, allowing for a multicomponent therapeutic or diagnostic window into disease cure and detection (54, 55).
Despite the great potential of EV-based therapies, the poor yield of EVs remains big major challenges in translating this type of treatment into clinical practice (56). Therefore, strategies such as physical, biological, or chemical means could be used to increase EV production yield efficiently without inducing unwanted responses in host cells (57). Moreover, the constant evolvement of EV isolation methods and further identification of heterogeneous nanoparticle subpopulations will enable a better understanding of the role of different nanoparticles in disease treatment and detection. Current biological markers may only recognize a subpopulation of EVs, and more specific markers of EVs, especially macrosomes, need to be refined in the future. Furthermore, although EVs are thought to be safe, biocompatible, and nonimmunogenic, additional studies are needed to fully evaluate their composition and potential toxicity (56).
In addition, EVs derived from immortalized HMC3 cells were used in this study. Actually, the influence of EVs from primary cultured microglia on the progression of AD should be conducted in the future. The proteomics analysis presented here is only a preliminary finding: Other proteins whether inhibited or promoted Aβ aggregation in macrosomes or small EVs are determined to be studied. Furthermore, it is unclear how these EV proteins influence Aβ aggregation and how the introduction of EVs influences signaling pathways in cells or animals. More comprehensive and careful proteomic analysis should be performed in the future through cutting-edge MS technology and optimization of proteomic methods.
After a deep understanding of how EVs or proteins in EVs are involved in signaling pathways, appropriate approaches could be selected to promote the generation of more macrosomes in vivo, which might achieve the endogenous treatment for AD. Besides, the overexpression of SIL2B and HSP70 (identified inhibitory proteins) in macrosomes might be another strategy that has therapeutic potential in AD.
Briefly, despite the challenges in bringing EVs to practical clinical use, this endogenous vesicle has tremendous amazing medicinal value and can be used in the development of drug delivery and therapy.
MATERIALS AND METHODS
Extraction and chracterization of macrosomes or small EVs
Small EVs or macrosomes were isolated from HMC3 cells. HMC3 cells were cultured in minimum essential medium (MEM) with 10% fetal bovine serum (FBS) (v/v) and 1% penicillin/streptomycin (v/v). For small EV or macrosome extraction, HMC3 cells (80% density) were plated in 150-mm dishes with small EV- or macrosome-depleted media (MEM with 6% FBS depleted of small EVs or macrosomes by ultracentrifugation for 18 hours at 100,000g). After continuous incubation for 24 hours, the supernatant was centrifuged at 500 and 4000g for 20 min at 4°C to remove cells and debris, respectively. Subsequently, the supernatant was centrifuged at 10,000 or 100,000g for 1 or 1.5 hours at 4°C in a type ultracentrifuge to obtain macrosomes or small EVs, respectively. The macrosomes pellet was resuspended in PBS and ultracentrifuged again at 10,000g for 30 min to obtain macrosomes. The small EV pellet was resuspended in PBS and ultracentrifuged again at 100,000g for 1 hour. Before use, the pelleted macrosomes or small EVs were resuspended in PBS and analyzed using a BCA Protein Assay kit. The particle size and the number of macrosomes or small EVs were characterized by NTA. The morphology and shape were determined by TEM. To prepare the TEM specimen, a 10-μl sample was dropped onto a 300-mesh copper grid. After 15 min of adsorption, the excess solution was soaked up from the edge of the grid using filter paper. Then, the above sample was stained with 10 μl, 1.5% (w/v) phosphotungstic acid solution for 30 s, and the staining solution was drawn away from the edge of the grid with filter paper. Last, the sample was observed by TEM.
Protein extraction from small EVs and macrosomes
Small EVs or macrosomes were solubilized in lysis buffer [50 mM tris-HCl (pH 7.4), 8.0 M urea, 65 mM dithiothreitol (DTT), 1 mM ethylene diamine tetraacetic acid, 1% (v/v) protease inhibitor cocktail, 1% (v/v) Triton X-100, and 1% (v/v) phosphatase inhibitor cocktail 3]. Lysed samples were incubated on ice for 30 min and cleared by centrifugation at 14,000g for 10 min. The protein contents of lysates were quantified by a BCA reagent.
Western blot analysis
Proteins (12 μg) from each group were separated by SDS–polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride (PVDF) membranes. PVDF membranes were blocked for 1 hour at room temperature and then incubated with primary antibody overnight at 4°C. The following antibodies were used for Western blot analysis: anti-CD63 (1:1000; Abcam, ab134045), anti-TSG101 (1:1000; Abcam, ab125011), anti-Alix (1:1000; Abcam, ab186429), anti–Flotillin-2 (1:1000; Abcam, ab181988), anti-ARF6 (1:1000; Abcam, ab13126), anti-APOA1 (1:1000; Abcam, ab52945), anti-APOA2 (1:1000; Abcam, ab92478), anti-APOB (1:1000; Abcam, ab139401), and anti-ALB (1:1000; Abcam, ab207327). Horseradish peroxidase–conjugated goat anti-rabbit and goat anti-mouse antibodies were used to detect the bound primary antibodies. The signals were detected by the enhanced chemiluminescence reagent. Data from the bands were determined through ImageJ software.
ThT assay and AFM experiment
ThT assay was performed in PBS (pH 7.4) with 25 μM in 96-well plates (optical bottom, black sides, Thermo Fisher Scientific). A stock solution of Aβ was diluted to desired concentrations immediately before the assay, and ThT fluorescence was monitored at 485 nm with 450-nm excitation at 37°C on a microplate reader. For AFM experiment, each sample was plated on freshly cleaved mica substrate and then dried with nitrogen gas. Imaging was performed using an NanoWizard Ultra Speed, with an Muti75 AFM probe in QI mode at room temperature. Images were captured at 2 μm by 2 μm and 5 μm by 5 μm, respectively. Image analysis was performed using JPKSPM Data Processing software.
Cell viability assay and flow cytometry experiments
The effect of small EVs or macrosomes on Aβ aggregation–induced cytotoxicity was determined using CCK-8 assay. SHSY5Y cells were plated at a concentration of 1 × 104 cells in 96-well plates with 200 μl of medium and incubated for 24 hours. Then, small EVs or macrosomes as wells as Aβ40 monomers alone or together with small EVs or macrosomes were added into the medium and further incubated with cells for 48 or 72 hours at 37°C in a 5% CO2 incubator. Cells treated with PBS were taken as a control. Cell viability was measured by a CCK-8 assay. Simply, 10 μl of CCK-8 solution was added to each well, and the absorbance at 450 nm was measured after 2 hours of incubation using a microplate reader.
The apoptosis experiment was detected by flow cytometry through a double staining with annexin V–FITC and PI. After incubation with Aβ40 alone, the complex of Aβ40 and small EVs, and the complex of Aβ40 and macrosomes for 48 hours at 37°C in 5% CO2, cells were collected and stained with 5 μl of annexin V–FITC solution and 10 μl of PI solution at room temperature in the dark for 15 min. Then, the data were conducted on a flow cytometer.
Morris water maze and Nest building test
All animal procedures were conducted in accordance with the Guidelines for Care and Use of Laboratory Animals of Northeastern University and approved by the Animal Ethics Committee of College of Life and Health Sciences of Northeastern University (58). C57BL/6 mice treated with PBS were taken as a blank control group. Male or female APP/PS1 Tg mice were divided into three groups including AD group and two sample (small EVs or macrosomes) groups. All APP/PS1 Tg mice were 5 months old, which were nasal feeding with PBS, small EVs, and macrosomes (200 μg kg−1), respectively, every day for 4 weeks. For Morris water maze test, all mice were trained and tested in a circular water tank. Before the task, the water was added with some milk. Briefly, the mice were subjected to pretraining for 2 days (visible platform) and given 1 min to find the visible platform. Then, the platform was placed opposite to the location used in the visible platform task and immobilized to 2 cm under the water surface. The mice performed five trials per day, and the releasing locations were selected randomly. The escape latency and the path length before the mice found the hidden platform were recorded to evaluate their spatial learning ability. On the sixth day, the platform was removed, and the number of times that the mice crossed the platform region was recorded for 1 min. Last, the recorded data were analyzed through a computer program (Panlab, SMART 3.0).
The social behaviors of the mice were assessed by the nest building test. In this test, mice were placed in separate cages, and eight identical pieces of paper (5 cm by 5 cm) were placed in the same corner of the cage. The changes in the size of the paper were recorded by photography, and the related scores were evaluated according to a previous work (37).
Immunohistochemistry
Serially sectioned brain slices were deparaffinized and hydrated. Then, these slices were placed at 100°C in an incubator for 10 min and blocked at room temperature for 1 hour in 5% bovine serum albumin (BSA; Sigma-Aldrich). Then, the mouse anti-Aβ antibody (1:400; Santa Cruz Biotechnology, sc-28,365) was added, and slices were kept overnight at 4°C. An UltraSensitive SAP kit (MXB) was used on processed slices after washing, according to the manufacturer’s instructions. Last, the slices were sufficiently washed the next day and stained with diaminobenzidine. Then, the images were captured using a light microscope (DM4000B; Leica) and analyzed by ImageJ software and GraphPad Prism 6 software.
Immunofluorescence staining
The slices or cells were fixed with 4% paraformaldehyde for 10 min and subsequently permeabilized with 0.2% Triton X-100 for 5 min at room temperature. Following blocking with 5% BSA for 1 hour, the slices or cells were incubated with mouse anti-Aβ (1:400; Santa Cruz Biotechnology, sc-28,365) and rabbit anti–Dectin-1 (1:200; Abcam, ab300497) reagents overnight at 4°C. Then, the slices or cells were incubated with the secondary antibodies, including goat anti-mouse immunoglobulin G (IgG) Alexa 488 (1:300; Thermo Fisher Scientific, A32723), goat anti-rabbit IgG Alexa 555 (1:300; Thermo Fisher Scientific, A32732), and rabbit anti-goat IgG Alexa 555 (1:300; Thermo Fisher Scientific, A27017). The sections of brains were observed, and photos were taken by using Pannoramic Desk.
MST test and NMR spectroscopy
Fluorescein-labeled Aβ40 peptide solution was prepared with PBS containing 0.009% Tween 20 at a concentration of 100 nM. A series of two times diluted and unlabeled small EVs or macrosomes were prepared in the PBS buffer, with the highest concentration of small EVs or macrosomes of 0.3 μM. Then, fluorescein-labeled Aβ40 solution and small EVs or macrosomes were completely mixed at a volume ratio of 1:1. After that, the samples were then pulled into capillaries and subjected to MST analysis at 25°C.
2D NMR experiments were conducted with a Bruker AVAVCE III HD 700 Hz instruments. 15N-labeled Aβ40 peptide was dissolved in D2O:H2O (1:9, v:v) according to a previously published protocol at a concentration of 75 μM (42). Small EVs or macrosomes were prepared at a concentration of 20 μg ml−1. 2D NMR spectra were recorded at 25°C immediately when the samples were prepared.
Proteomic analysis
Small EVs or macrosomes (0.07 mg) were dissolved in 8 M urea lysis buffer, then lysed in an ice bath for 30 min, and further sonicated 10 min. Then, the above buffer was exchanged with 50 mM NH4HCO3, and these lysates were reduced by 10 mM DTT at 50°C for 1 hour, alkylated by 25 mM iodoacetamide at room temperature for 30 min in the dark. Then, the proteins were digested with trypsin (enzyme:protein ratio, 1:40) at 37°C overnight. Next, these peptides were desalted with a Sep-Pak C18 column (Waters, Milford, MA, USA) and stored at −20°C until further application.
Then, a loader tip was packed with 2 mg of Aβ40@SiO2 and washed with the PBS solution twice. The above peptides were added to the loader tip and incubated overnight at 4°C to ensure the sufficient interaction of Aβ40@SiO2 with the peptides extracted from small EVs or macrosomes. Subsequently, the solution was centrifuged and washed with PBS again. Last, the precipitation was washed with 80% acetonitrile (ACN)/0.1% formic acid (FA), and the eluent was collected for LC-MS analysis. For the identification of proteins in small EVs or macrosomes, the detailed parameters of MS data processing were set according to a published work (59). For label-free quantification (LFQ) MS, the raw files from small EVs or macrosomes were analyzed using MaxQuant (60) and Perseus software (61) searching against a UniProt human database. Protein abundance is expressed as an LFQ value derived from MaxQuant.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (21922411, 22174138, and 32071252), DICP Innovation Funding (DICP-RC201801 and I202008), Dalian Outstanding Young Scientific Talent (2020RJ01), Sichuan Sci-Tech Support Plan (22SYSX0177), and CAS Key Laboratory of Nano-Bio Interface (no. 22NBI01).
Author contributions: C.W., B.L., and G.Q. conceived the study and designed the experiments. C.W. performed most of experiments. Y.Y. performed the Ca2+ experiment. H.G., M.Z., and Y.F. performed animal experiments. X.Z., Z.S., and H.Z. helped the data analysis. C.W., B.L., and G.Q. wrote the manuscripts.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S11
Tables S1, S2 and S5
Legends for tables S3 and S4
References
Other Supplementary Material for this manuscript includes the following:
Tables S3 and S4
REFERENCES AND NOTES
- 1.Alzheimer’s Association , 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 12, 459–509 (2016). [DOI] [PubMed] [Google Scholar]
- 2.P. Esteve, J. Rueda-Carrasco, M. I. Mateo, M. J. Martin-Bermejo, J. Draffin, G. Pereyra, A. Sandonís, I. Crespo, I. Moreno, E. Aso, P. Garcia-Esparcia, E. Gomez-Tortosa, A. Rábano, J. Fortea, D. Alcolea, A. Lleo, M. T. Heneka, J. M. Valpuesta, J. A. Esteban, I. Ferrer, M. Domínguez, P. Bovolenta, Elevated levels of secreted-frizzled-related-protein 1 contribute to Alzheimer’s disease pathogenesis. Nat. Neurosci. 22, 1258–1268 (2019). [DOI] [PubMed] [Google Scholar]
- 3.J. Cummings, H. H. Feldman, P. Scheltens, The “rights” of precision drug development for Alzheimer’s disease. Alz. Res. Therapy 11, 76 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Y.-H. Liu, B. Giunta, H.-D. Zhou, J. Tan, Y.-J. Wang, Immunotherapy for Alzheimer disease—The challenge of adverse effects. Nat. Rev. Neurol. 8, 465–469 (2012). [DOI] [PubMed] [Google Scholar]
- 5.L. S. Schneider, F. Mangialasche, N. Andreasen, H. Feldman, E. Giacobini, R. Jones, V. Mantua, P. Mecocci, L. Pani, B. Winblad, M. Kivipelto, Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 275, 251–283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.E. Karran, J. Hardy, A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann. Neurol. 76, 185–205 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.A. J. Martorell, A. L. Paulson, H.-J. Suk, F. Abdurrob, G. T. Drummond, W. Guan, J. Z. Young, D. N.-W. Kim, O. Kritskiy, S. J. Barker, V. Mangena, S. M. Prince, E. N. Brown, K. Chung, E. S. Boyden, A. C. Singer, L.-H. Tsai, Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Cell 177, 256–271.e22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D. Frenkel, L. Puckett, S. Petrovic, W. Xia, G. Chen, J. Vega, A. Dembinsky-Vaknin, J. Shen, M. Plante, D. S. Burt, H. L. Weiner, A nasal proteosome adjuvant activates microglia and prevents amyloid deposition. Ann. Neurol. 63, 591–601 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.M. T. Heneka, M. P. Kummer, A. Stutz, A. Delekate, S. Schwartz, A. Vieira-Saecker, A. Griep, D. Axt, A. Remus, T.-C. Tzeng, E. Gelpi, A. Halle, M. Korte, E. Latz, D. T. Golenbock, NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O. Butovsky, H. L. Weiner, Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 19, 622–635 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Y. Huang, K. E. Happonen, P. G. Burrola, C. O’Connor, N. Hah, L. Huang, A. Nimmerjahn, G. Lemke, Microglia use TAM receptors to detect and engulf amyloid β plaques. Nat. Immunol. 22, 586–594 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.B. Liu, B. W. Lee, K. Nakanishi, A. Villasante, R. Williamson, J. Metz, J. Kim, M. Kanai, L. Bi, K. Brown, G. D. Paolo, S. Homma, P. A. Sims, V. K. Topkara, G. Vunjak-Novakovic, Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2, 293–303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.M. Riazifar, M. R. Mohammadi, E. J. Pone, A. Yeri, C. Lässer, A. I. Segaliny, L. L. McIntyre, G. V. Shelke, E. Hutchins, A. Hamamoto, E. N. Calle, R. Crescitelli, W. Liao, V. Pham, Y. Yin, J. Jayaraman, J. R. T. Lakey, C. M. Walsh, K. V. Keuren-Jensen, J. Lotvall, W. Zhao, Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano 13, 6670–6688 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D. K. Jeppesen, A. M. Fenix, J. L. Franklin, J. N. Higginbotham, Q. Zhang, L. J. Zimmerman, D. C. Liebler, J. Ping, Q. Liu, R. Evans, W. H. Fissell, J. G. Patton, L. H. Rome, D. T. Burnette, R. J. Coffey, Reassessment of exosome composition. Cell 177, 428–445.e18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.G. van Niel, G. D’Angelo, G. Raposo, Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018). [DOI] [PubMed] [Google Scholar]
- 16.M. Mathieu, L. Martin-Jaular, G. Lavieu, C. Théry, Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019). [DOI] [PubMed] [Google Scholar]
- 17.M. Prudent, J. Delobel, A. Hübner, C. Benay, N. Lion, J.-D. Tissot, Proteomics of stored red blood cell membrane and storage-induced microvesicles reveals the association of flotillin-2 with band 3 complexes. Front. Physiol. 9, 421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.V. Muralidharan-Chari, J. Clancy, C. Plou, M. Romao, P. Chavrier, G. Raposo, C. D’Souza-Schorey, ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 19, 1875–1885 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.N. Karimi, A. Cvjetkovic, S. C. Jang, R. Crescitelli, M. A. H. Feizi, R. Nieuwland, J. Lötvall, C. Lässer, Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell. Mol. Life Sci. 75, 2873–2886 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R. C. Paolicelli, G. Bergamini, L. Rajendran, Cell-to-cell communication by extracellular vesicles: Focus on microglia. Neuroscience 405, 148–157 (2019). [DOI] [PubMed] [Google Scholar]
- 21.L. Ceccarelli, L. Marchetti, M. Rizzo, A. Moscardini, V. Cappello, E. D. Pozzo, M. Romano, C. Giacomelli, P. Bergese, C. Martini, Human microglia extracellular vesicles derived from different microglia cell lines: Similarities and differences. ACS Omega 7, 23127–23137 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.I. Kuperstein, K. Broersen, I. Benilova, J. Rozenski, W. Jonckheere, M. Debulpaep, A. Vandersteen, I. Segers-Nolten, K. V. D. Werf, V, Subramaniam, D. Braeken, G. Callewaert, C. Bartic, R. D’Hooge, I. C. Martins, F. Rousseau, J. Schymkowitz, B. D. Strooper, Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 29, 3408–3420 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R.-Y. Pan, J. Ma, X.-X. Kong, X.-F. Wang, S.-S. Li, X.-L. Qi, Y.-H. Yan, J. Cheng, Q. Liu, W. Jin, C.-H. Tan, Z. Yuan, Sodium rutin ameliorates Alzheimer’s disease–like pathology by enhancing microglial amyloid-β clearance. Sci. Adv. 5, eaau6328 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.J. Park, H.-J. Ha, E. S. Chung, S. H. Baek, Y. Cho, H. K. Kim, J. Han, J. H. Sul, J. Lee, E. Kim, J. Kim, Y. R. Yang, M. Park, S. H. Kim, T. V. Arumugam, H, Jang, S. W. Seo, P.-G. Suh, D.-G. Jo, O-GlcNAcylation ameliorates the pathological manifestations of Alzheimer’s disease by inhibiting necroptosis. Sci. Adv. 7, eabd3207 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Y. Ying, J.-Z. Wang, Illuminating neural circuits in Alzheimer’s disease. Neurosci. Bull. 37, 1203–1217 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.C. Xue, T. Y. Lin, D. Chang, Z. Guo, Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. Roy. Soc. Open Sci. 4, 160696 (2107). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.W. Wu, S. Du, W. Shi, Y. Liu, Y. Hu, Z. Xie, X. Yao, Z. Liu, W. Ma, L. Xu, C. Ma, Y. Zhong, Inhibition of Rac1-dependent forgetting alleviates memory deficits in animal models of Alzheimer’s disease. Protein Cell 10, 745–759 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.L. Manterola, M. Hernando-Rodríguez, A. Ruiz, A. Apraiz, O. Arrizabalaga, L. Vellón, E. Alberdi, F. Cavaliere, H. M. Lacerda, S. Jimenez, L. A. Parada, C. Matute, J. L. Zugaza, 1–42 β-amyloid peptide requires PDK1/nPKC/Rac 1 pathway to induce neuronal death. Trans. Psychiatry 3, e219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.M. Calvo-Rodriguez, S. S. Hou, A. C. Snyder, E. K. Kharitonova, A. N. Russ, S. Das, Z. Fan, A. Muzikansky, M. Garcia-Alloza, A. Serrano-Pozo, E. Hudry, B. J. Bacskai, Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 11, 2146 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.T.-I. Peng, M.-J. Jou, Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 1201, 183–188 (2010). [DOI] [PubMed] [Google Scholar]
- 31.A. Acevedo, C. González-Billault, Crosstalk between Rac1-mediated actin regulation and ROS production. Free Radic. Bio. Med. 116, 101–113 (2018). [DOI] [PubMed] [Google Scholar]
- 32.S. Shao, C. Xiang, K. Qin, A. ur Rehman Aziz, X. Liao, B. Liu, Visualizing the spatiotemporal map of Rac activation in bovine aortic endothelial cells under laminar and disturbed flows. PLOS ONE 12, e0189088 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.T. Qian, S. Lu, H. Ma, J. Fang, W. Zhong, Y. Wang, FRET imaging of calcium signaling in live cells in the microenvironment. Integr. Biol. 5, 431–438 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.N. Gao, Z. Du, Y. Guan, K. Dong, J. Ren, X. Qu, Chirality-selected chemical modulation of amyloid aggregation. J. Am. Chem. Soc. 141, 6915–6921 (2019). [DOI] [PubMed] [Google Scholar]
- 35.E. Drummond, T. Wisniewski, Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 133, 155–175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.C. V. Vorhees, M. T. Williams, Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R. M. J. Deacon, Assessing nest building in mice. Nat. Protoc. 1, 1117–1119 (2006). [DOI] [PubMed] [Google Scholar]
- 38.J. Wang, B. J. Wu, C. L. Masters, Y.-J. Wang, A systemic view of Alzheimer disease—Insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 13, 612–623 (2017). [DOI] [PubMed] [Google Scholar]
- 39.M. R. Warren, C. Zhang, A. Vedadghavami, K. Bokvist, P. K. Dhal, A. G. Bajpayee, Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA. Biomater. Sci. 9, 4260–4277 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.M. Richman, S. Wilk, M. Chemerovski, S. K. T. S. Wärmländer, A. Wahlström, A. Gräslund, S. Rahimipour, In vitro and mechanistic studies of an antiamyloidogenic self-assembled cyclic D,L-α-peptide architecture. J. Am. Chem. Soc. 135, 3474–3484 (2013). [DOI] [PubMed] [Google Scholar]
- 41.T. Lührs, C. Ritter, M. Adrian, D. Riek-Loher, B. Bohrmann, H. Döbeli, D. Schubert, R. Riek, 3D structure of Alzheimer’s amyloid-β(1–42) fibrils. Proc. Natl. Acad. Sci. U.S.A. 102, 17342–17347 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D. Maity, M. Howarth, M. C. Vogel, M. Magzoub, A. D. Hamilton, Peptidomimetic-based vesicles inhibit amyloid-β fibrillation and attenuate cytotoxicity. J. Am. Chem. Soc. 143, 3086–3093 (2021). [DOI] [PubMed] [Google Scholar]
- 43.K. Hou, J. Zhao, H. Wang, B. Li, K. Li, X. Shi, K. Wan, J. Ai, J. Lv, D. Wang, Q. Huang, H. Wang, Q. Cao, S. Liu, Z. Tang, Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat. Commun. 11, 4790 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.J. Kowal, G. Arras, M. Colombo, M. Jouve, J. P. Morath, B. Primdal-Bengtson, F. Dingli, D. Loew, M. Tkach, C. Théry, Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. U.S.A. 113, E968–E977 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.X. Dong, H. Qin, J. Mao, D. Yu, X. Li, A. Shen, J. Yan, L. Yu, Z. Guo, M. Ye, H. Zou, X. Liang, In-depth analysis of glycoprotein sialylation in serum using a dual-functional material with superior hydrophilicity and switchable surface charge. Anal. Chem. 89, 3966–3972 (2017). [DOI] [PubMed] [Google Scholar]
- 46.M. Ries, R. Loiola, U. N. Shah, S. M. Gentleman, E. Solito, M. Sastre, The anti-inflammatory Annexin A1 induces the clearance and degradation of the amyloid-β peptide. J. Neuroinflammation 13, 234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.C. G. Evans, S. Wisén, J. E. Gestwicki, Heat shock proteins 70 and 90 inhibit early stages of amyloid β-(1–42) aggregation in vitro. J. Biol. Chem. 281, 33182–33191 (2006). [DOI] [PubMed] [Google Scholar]
- 48.N. V. Bobkova, D. G. Garbuz, I. Nesterova, N. Medvinskaya, A. Samokhin, I. Alexandrova, V. Yashin, V. Karpov, M. S. Kukharsky, N. N. Ninkina, A. A. Smirnov, E. Nudler, M. Evgen'ev, Therapeutic effect of exogenous hsp70 in mouse models of Alzheimer’s disease. J. Alzheimer Dis. 38, 425–435 (2014). [DOI] [PubMed] [Google Scholar]
- 49.C. Morgan, N. C. Inestrosa, Interactions of laminin with the amyloid beta peptide. Implications for Alzheimer’s disease. Braz. J. Med. Biol. Res. 34, 597–601 (2001). [DOI] [PubMed] [Google Scholar]
- 50.L. J. Kanyenda, G. Verdile, S. Boulos, S. Krishnaswamy, K. Taddei, B. P. Meloni, F. L. Mastaglia, R. N. Martins, The dynamics of CD147 in Alzheimer’s disease development and pathology. J. Alzheimers Dis. 26, 593–605 (2011). [DOI] [PubMed] [Google Scholar]
- 51.H. Lehew, B. Evangelista, L. Cilenti, K. Teter, Protein disulfide isomerase disrupts and detoxifies aggregates of amyloid beta peptide. FASEB J. 33, 464.5 (2019). [Google Scholar]
- 52.L. Rajendran, M. Honsho, T. R. Zahn, P. Keller, K. D. Geiger, P. Verkade, K. Simons, Alzheimer’s disease β-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 103, 11172–11177 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.H. Kokubo, C. A. Lemere, H. Yamaguchi, Localization of flotillins in human brain and their accumulation with the progression of Alzheimer’s disease pathology. Neurosci. Lett. 290, 93–96 (2000). [DOI] [PubMed] [Google Scholar]
- 54.R. Kalluri, V. S. LeBleu, The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.L. Yuan, J.-Y. Li, Exosomes in Parkinson’s disease: Current perspectives and future challenges. ACS Chem. Nerosci. 10, 964–972 (2019). [DOI] [PubMed] [Google Scholar]
- 56.I. Kimiz-Gebologlu, S. S. Oncel, Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control. Release 347, 533–543 (2022). [DOI] [PubMed] [Google Scholar]
- 57.L. Debbi, S. Guo, D. Safina, S. Levenberg, Boosting extracellular vesicle secretion. Biotechnol. Adv. 59, 107983 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.X. Zhang, X. Zhang, Y. Li, M. Zhong, P. Zhao, C. Guo, H. Xu, T. Wang, H. Gao, Brain targeting and Aβ binding bifunctional nanoparticles inhibit amyloid protein aggregation in APP/PS1 transgenic mice. ACS Chem. Nerosci. 12, 2110–2121 (2021). [DOI] [PubMed] [Google Scholar]
- 59.H. Zhang, J. Peng, X. Li, S. Liu, Z. Hu, G. Xu, R. Wu, A nano-bio interfacial protein corona on silica nanoparticle. Colloids Surf. B Biointerfaces 167, 220–228 (2018). [DOI] [PubMed] [Google Scholar]
- 60.J. Cox, M. Mann, MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008). [DOI] [PubMed] [Google Scholar]
- 61.S. Tyanova, T. Temu, P. Sinitcyn, A. Carlson, M. Y. Hein, T. Geiger, M. Mann, J. Cox, The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016). [DOI] [PubMed] [Google Scholar]
- 62.C. Wang, X. Wang, D. Wang, S. Qian, F. Zhang, M. Li, M. Li, W. Lu, B. Liu, G. Qing, Remarkable difference of phospholipid molecular chirality in regulating PrP aggregation and cell responses. Chinese Chem. Lett. 34, 107332 (2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S11
Tables S1, S2 and S5
Legends for tables S3 and S4
References
Tables S3 and S4