Abstract
There is no consensus on the use of hormone replacement therapy (HRT) after treatment of advanced corpus cancer. We report a case of advanced corpus cancer at a young age, in which HRT was initiated 7 years after surgery, and regional lymph node recurrence was later detected. The patient was 35 years old at the time of initial treatment in X year, when she was diagnosed with stageIIIC2 corpus cancer and underwent a hysterectomy with bilateral salpingo-oophorectomy and a retroperitoneal lymphadenectomy. HRT was started at X + 7 years, and at X + 9 years, a 25 × 12-mm-sized mass was found in the hilum of the right kidney. A laparoscopic resection revealed regional lymph node recurrence of the corpus cancer. A retrospective study further revealed that a tumor measuring 12 × 3 mm was found at X + 3 years and grew to 18 × 7 mm in X + 6 years, just before the start of the HRT. We hypothesize that HRT did not induce tumor recurrence; instead, it allowed for long-term follow-up and early diagnosis.
Keywords: Advanced corpus cancer, Late recurrence, Hormone replacement therapy
Introduction
Uterine corpus cancer is the most common gynecological malignancy [1], and it is not uncommon for patients to be < 40 years of age [2]. The main treatment strategy for corpus cancer is surgical resection, which usually includes a hysterectomy and salpingo-oophorectomy. The challenge, especially in the case of premenopausal, early-onset corpus cancer, is dealing with medically induced early menopause associated with oophorectomy and the resultant strong menopausal symptoms.
Menopausal symptoms are a major concern that cannot be ignored as they can sometimes cause a marked decline in activities of daily living, which can cause an unintended strain on social wellbeing. Hormone replacement therapy (HRT) is considered the most effective treatment for menopausal symptoms that develop after the surgical removal of the adnexa [3]. However, there is concern as to whether it increases the frequency of cancer recurrence and contributes to the progression of recurrent tumors in corpus cancer. Menopausal symptoms are painful for patients and should be treated; however, the use of HRT to treat menopausal symptoms should not induce cancer recurrence and exacerbate the disease progression [4]. This is a continuing problem in clinical practice.
There have been several reports on HRT after treatment for corpus cancer. Clinical studies, including randomized controlled trials, have been conducted in low-risk groups (endometrioid carcinoma grade 1 or 2 with less than half myometrial invasion), and a significant number of these studies show HRT to be acceptable in most cases [5–8]. However, HRT is not actively used in advanced corpus cancer which is at intermediate or high risk of recurrence because a consensus has not been reached yet on whether HRT is acceptable [6, 8, 9].
Additionally, although corpus cancer sometimes develops late recurrence more than 10 years after treatment, regular follow-up is often completed in about 5–6 years. We are therefore looking for good strategies on how to extract such cases clinically.
Herein, we report a case of postoperative HRT for advanced corpus cancer with multiple lymph node metastases that developed at the young age of 35 years.
Case presentation
The patient was 35 years old (gravida 0, para 0).
In year X, she visited previous doctor with the complaint of abnormal genital bleeding. She was suspected of having a corpus cancer and was referred to our department. The patient’s family history was as follows: mother (lung cancer), grandfather (lung cancer), grandmother (breast cancer and colon cancer), and uncle (gastric cancer).
Upon examination at our department, the depth of myometrial invasion was more than half, lymph nodes in the pelvic and para-aortic regions were enlarged, and advanced corpus cancer was strongly suspected. As for tumor marker, CA19-9 and CA125 was elevated to 95.2 U/mL, 110.6 U/mL, respectively. A total hysterectomy and bilateral salpingo-oophorectomy with retroperitoneal lymphadenectomy were also performed.
After pathological examination, she was diagnosed with grade 1 endometrioid carcinoma. Myometrial invasion exceeded more than half, and there were many inflammatory cells around the tumor (Fig. 1A). Estrogen receptor was positive in nucleus (Fig. 1B). Multiple lymph node metastases were observed, including the obturator (Fig. 1C) and para-aortic lymph nodes (Fig. 1D). A diagnosis of pT1bN1,2M0, International Federation of Gynecology and Obstetrics stage IIIC2 was made.
Fig. 1.
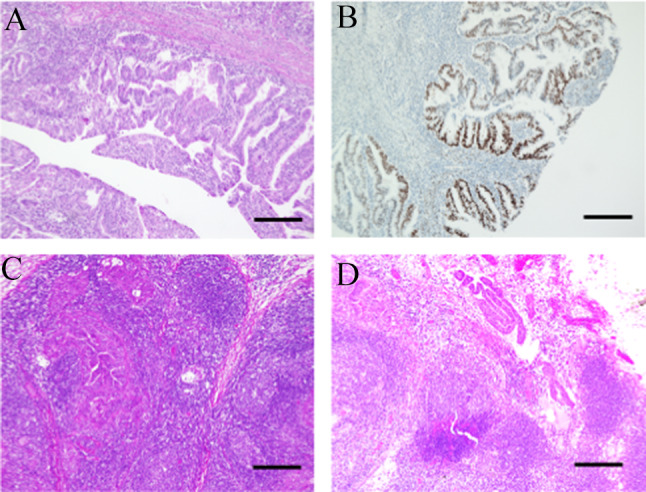
Pathological findings and images at initial treatment. a Microscopic findings of the uterus corpus. Cancer cells displayed a glandular architecture. Nuclear atypia was mild (× 100 magnification, bar equals 200 μm). b Immunohistochemistry (IHC) of estrogen receptor (ER). It is positive in cancer cells (× 100 magnification, bar equals 200 μm). c Microscopic findings of metastasis in obturator lymph node (× 100 magnification, bar equals 200 μm). d Microscopic findings of metastasis in para-aortic lymph node (× 100 magnification, bar equals 200 μm)
A postoperative chemotherapy (six courses of doxorubicin hydrochloride + cisplatin) was administered, and a computed tomography (CT) scan taken at the end of chemotherapy showed no apparent tumor recurrence.
Initiation of HRT
During regular follow-up after completion of treatment, she frequently complained of symptoms of hot flashes and palpitations, which were thought to be menopausal symptoms. Due to concerns about tumor recurrence, symptomatic therapies other than HRT were initially chosen. However, improvement in the symptoms was only partial.
A CT scan of the cervical specimen performed at X + 6 years revealed no obvious recurrence. As for tumor marker, CA19-9 and CA125 was within normal range; 6.7 U/mL, 12.4 U/mL, respectively. After thorough discussion with the patient, HRT (application of 0.72 mg estradiol via a skin patch every other day) was initiated. After that, the patient’s complaints of uncomfortable menopausal symptoms decreased dramatically.
Identification of tumor and diagnosis of corpus cancer recurrence
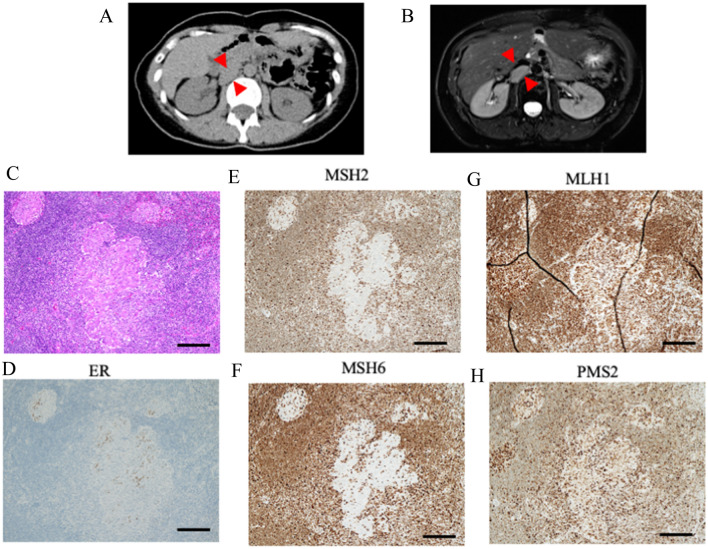
A CT scan performed at X + 9 years revealed a 25-mm-sized mass in the right renal hilum (Fig. 2A). A tumor around the adrenal gland was initially suspected, and the patient was referred to our urology department.
Fig. 2.
Images and pathological findings at treatment of recurrent tumor. a Image of computed tomography around the right renal hilum. Red arrow indicated the recurrent tumor between renal hilum and IVC. b Image of magnetic resonance imaging. Enhance-contrasted T1-weighted image. Red arrow indicated the recurrent tumor between renal hilum and IVC. c–h Microscopic findings of recurrent tumor (× 100 magnification, bar equals 200 μm). c Cancer cells displayed a glandular architecture, surrounded by lymph node structure. d Immunohistochemistry (IHC) of estrogen receptor (ER). Partialy positive in cancer cells. e IHC of MSH2. Complete loss in cancer cells. f IHC of MSH6. Complete loss in cancer cells. g IHC of MLH1. Expression is retained in cancer cells. h IHC of PMS2. Expression is retained in cancer cells
Magnetic resonance imaging was performed, and a 25 × 37-mm nodule was found near the right renal hilum upon the inferior vena cava (Fig. 2B). There were no obvious abnormalities in tumor markers (CEA; 0.9 ng/mL, CA19-9; 11.3 U/mL, CA125; 26.3 U/mL).
Based on these results, a tumor of adrenal origin was suspected. A laparoscopic resection of the tumor was performed. The lesion was located under the right renal vein dorsal to the inferior vena cava, and there was no invasion of the tumor into the surrounding tissue.
Pathological examination revealed that the estrogen-receptor positive cells with nuclei atypia proliferated in a back-to-back cribriform pattern (Fig. 2C, D). A diagnosis of late recurrence of corpus cancer was made. Based on her medical history, the possibility of mismatch repair (MMR) factor abnormality in the background was considered. Consequently, MSH2/6 loss was observed (Fig. 2E, F). MLH1/PMS2 were retained (Fig. 2G, H). Finally, dMMR corpus cancer was diagnosed. No pathological mutations suggestive of Lynch syndrome were found in germline genetic testing. Therefore, this case was considered a corpus cancer with sporadic MMR deficiency.
After a thorough consultation with the patient, we decided to administer postoperative chemotherapy. Six courses of docetaxel and carboplatin were administered. After chemotherapy, we restarted HRT. One and half year following chemotherapy, there was no apparent recurrence.
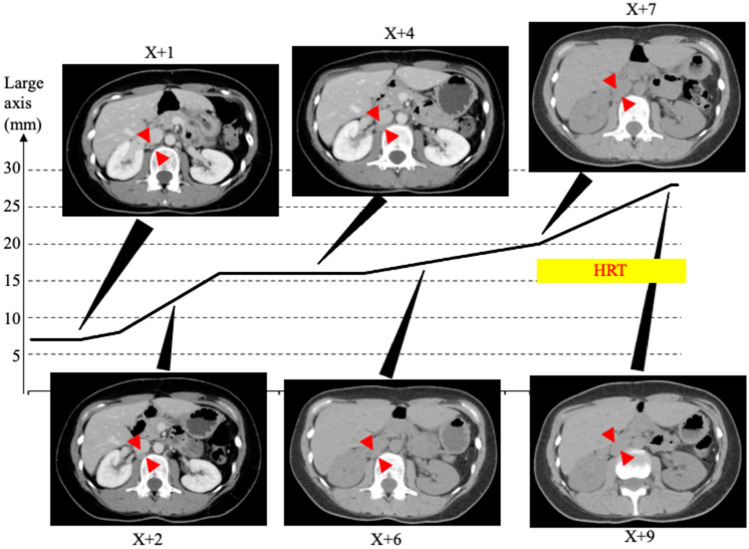
Once the patient was diagnosed with late recurrence of corpus cancer, we retrospectively reviewed the CT images obtained at our hospital after treatment. The results are shown in Fig. 3.
Fig. 3.
Retrospective study of recurrent tumor from the end of initial treatment to just before the identification of tumor recurrence. Two years after the initial treatment, the presence of tumor was confirmed, and there was a trend toward gradual enlargement over the years
At the time of surgery, no obvious tumor was observed at the same site. At X + 1 years (2 years after the end of chemotherapy), a tumor 12 × 3 mm in size was identified. Two years later (X + 4), the tumor was 16 × 7 mm in size, and 2 years later (X + 6, just before the start of HRT), it grew to 18 × 7 mm in size. After start of HRT, the tumor grew to 20 × 12 mm in size in X + 7 year, and finally it had grown to 28 × 13 mm in X + 9 years.
Discussion
In this study, we report a case of solitary lymph node recurrence of advanced corpus cancer with sporadic MMR dificiency, which was identified in the late postoperative period (9 years after surgery).
The controversy in this case is how HRT, initiated 6 years after the completion of initial postoperative chemotherapy, contributed to the disease course of the tumor. A retrospective study of this case revealed that the lymph nodes had already enlarged to approximately 10 mm on CT images 3 years after the completion of postoperative chemotherapy. Furthermore, the tumor had grown to approximately 15 mm on the images before the start of the HRT. Since no tumor was seen at the initial treatment, it is likely that the tumor metastasis had occurred prior to HRT, and it is unlikely that HRT caused recurrence. The next question was whether HRT contributed to disease progression.
Although we can only speculate on this issue, we believe that HRT is unlikely to have contributed to disease progression based on the following reasoning. First, we did not observe a marked change in the rate of tumor growth. In addition, at the time of the second surgery, there was no evidence of invasion into the surrounding tissues (kidney, inferior vena cava, etc.), and we could not find an extracapsular spread pattern. No other obvious metastases were observed. Based on the above, we speculated that HRT was involved to a very low degree in disease progression as well. However, it is regrettable that the decision to start HRT was based solely on simple CT screening. If a PET-CT scan, for example, had been added, recurrence could have been identified at this point and the patient could have been treated earlier.
However, in this case, we believe that HRT ultimately led the diagnosis of recurrence, because the patient continued to undergo follow-up. In other words, we can expect an indirect benefit of HRT, which may encourage behavioral changes in both patients and health providers to care for recurrence over time. It has been reported that corpus cancer can recur even after a long interval of time, sometimes for more than 10 years [10–13]. In cases of recurrent corpus cancer, if the tumor is solitary and can be surgically resected, surgical treatment is known to markedly improve prognosis [13]. After several cases of late recurrence at about 10 years post-treatment, including this case, we basically follow-up for 10 years after treatment. However, it is sometimes difficult to explain to patients that they need to come to our institute for such a long period of time. We believe that HRT will not only prevent the decline in quality of life caused by estrogen deficiency, but also give patients the active motivation of visiting an institution for a long period of time.
In this case study of a recurrent tumor, we found that the tumor had MMR deficiency, which is known to have a slightly higher recurrence rate [14, 15]. However, the effect of HRT on MMR-deficient subtype remains unknown. The present case suggests that HRT does not significantly affect the tumor progression of MMR-deficient corpus cancer; however, further case series are needed to confirm this.
In conclusion, we encountered a young patient with advanced corpus cancer who developed solitary recurrence during HRT. HRT is not only effective in treating menopausal symptoms caused by early surgical menopause, but its administration may also lead to a change in patient and healthcare provider awareness, which may facilitate early detection of recurrent disease.
Funding
None.
Data Availability
The data shown in the report are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Written informed consent was obtained from the patient for the publication of this case report and its accompanying images.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Soliman PT, Oh JC, Schmeler KM, Sun CC, Slomovitz BM, Gershenson DM, Burke TW, Lu KH. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol. 2005;105(3):575–580. doi: 10.1097/01.AOG.0000154151.14516.f7. [DOI] [PubMed] [Google Scholar]
- 3.Brennan A, Hickey M. The use of menopausal hormone therapy after cancer. Best Pract Res Clin Obstet Gynaecol. 2022;81:22–30. doi: 10.1016/j.bpobgyn.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama Y, Ito K, Takamatsu K, Takehara K, Nakanishi T, Harano K, Watari H, Susumu N, Aoki D, Saito T, Disease Committee of Uterine Cancer JGOG How do Japanese gynecologists view hormone replacement therapy for survivors of endometrial cancer? Japanese Gynecologic Oncology Group (JGOG) survey. Int J Clin Oncol. 2015;20(5):997–1004. doi: 10.1007/s10147-015-0808-5. [DOI] [PubMed] [Google Scholar]
- 5.Barakat RR, Bundy BN, Spirtos NM, Bell J, Mannel RS, Gynecologic Oncology Group Study Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24(4):587–592. doi: 10.1200/JCO.2005.02.8464. [DOI] [PubMed] [Google Scholar]
- 6.Edey KA, Rundle S, Hickey M. Hormone replacement therapy for women previously treated for endometrial cancer. Cochrane Database Syst Rev. 2018;5:CD008830. doi: 10.1002/14651858.CD008830.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho HW, Ouh YT, Lee JK, Hong JH. Effects of hormone therapy on recurrence in endometrial cancer survivors: a nationwide study using the Korean Health Insurance Review and Assessment Service database. J Gynecol Oncol. 2019;30(4):e51. doi: 10.3802/jgo.2019.30.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Donato V, Palaia I, D'Aniello D, Musacchio L, Santangelo G, Di Mauro F, Di Pinto A, Musella A, Fischetti M, Tomao F, Perniola G, Benedetti Panici P. Does hormone replacement therapy impact the prognosis in endometrial cancer survivors? A systematic review. Oncology. 2020;98(4):195–201. doi: 10.1159/000505427. [DOI] [PubMed] [Google Scholar]
- 9.Londero AP, Parisi N, Tassi A, Bertozzi S, Cagnacci A. Hormone replacement therapy in endometrial cancer survivors: a meta-analysis. J Clin Med. 2021;10(14):3165. doi: 10.3390/jcm10143165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masuda M, Sato H, Ozaki T, Matsui K, Miyoshi S, Takaishi Y, Tsuji S, Takei T, Horiuchi T, Ikeda M, Yasuda M, Morishita H, Ando Y, Oida K, Taguchi N, Hirose M. Late peritoneal relapse of endometrial carcinoma: a case report. Eur J Gynaecol Oncol. 2021;42(2):2274. doi: 10.31083/j.ejgo.2021.02.2274. [DOI] [Google Scholar]
- 11.Kim A, Nguyen L, Kalir T, Chuang L. Pelvic recurrence of stage 1a well-differentiated endometrial carcinoma after 13 years: a case report. J Turk Ger Gynecol Assoc. 2016;17(1):51–54. doi: 10.5152/jtgga.2015.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yechieli R, Robbins JR, Schultz D, Munkarah A, Elshaikh MA. Vaginal recurrence more than 17 years after hysterectomy and adjuvant treatment for uterine carcinoma with successful salvage brachytherapy: a case report. Case Rep Oncol. 2011;4(1):242–245. doi: 10.1159/000328076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi A, Matsuura M, Matoda M, Nomura H, Okamoto S, Kanao H, Kondo E, Omatsu K, Kato K, Utsugi K, Takeshima N. Clinicopathological features of early and late recurrence of endometrial carcinoma after surgical resection. Int J Gynecol Cancer. 2017;27(5):967–972. doi: 10.1097/IGC.0000000000000984. [DOI] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Momeni-Boroujeni A, Nguyen B, Vanderbilt CM, Ladanyi M, Abu-Rustum NR, Aghajanian C, Ellenson LH, Weigelt B, Soslow RA. Genomic landscape of endometrial carcinomas of no specific molecular profile. Mod Pathol. 2022 doi: 10.1038/s41379-022-01066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data shown in the report are available from the corresponding author on reasonable request.