Abstract
Background & Aims:
At least 20 to 30% of patients with intestinal failure receiving long-term parenteral nutrition will develop intestinal failure-associated liver disease (IFALD), for which there are few therapeutic options. SEFA-6179 is a first-in-class structurally engineered medium chain fatty acid analogue that acts through GPR84, PPARα, and PPARγ agonism. We hypothesized that SEFA-6179 would prevent biochemical and histologic liver injury in a preterm piglet model of IFALD.
Methods:
Preterm Yorkshire piglets were delivered by caesarean section and parenteral nutrition was provided for 14 days via implanted central venous catheters. Animals were treated with either medium chain triglycerides (MCT) vehicle control or SEFA-6179.
Results:
Compared to MCT vehicle at day of life 15, SEFA-6179 prevented biochemical cholestasis (direct bilirubin: 1.9 vs. <0.2 mg/dL, P=0.01; total bilirubin: 2.7 vs. 0.4 mg/dL, P=0.02; gamma glutamyl transferase 172 vs. 30 U/L, P=0.01). SEFA-6179 also prevented steatosis (45.6 vs. 13.9 mg triglycerides/g liver tissue, P=0.009), reduced bile duct proliferation (1.6 vs. 0.5% area cytokeratin 7 positive, P=0.009), and reduced fibrosis assessed by a masked pathologist (median Ishak score 3 vs. 1, P=0.007). RNA-Seq of liver tissue demonstrated that SEFA-6179 broadly impacted inflammatory, metabolic, and fibrotic pathways, consistent with its in vitro receptor activity (GPR84/PPARα/PPARγ agonist).
Conclusions:
In a preterm piglet model of IFALD, SEFA-6179 treatment prevented biochemical cholestasis and steatosis, and reduced bile duct proliferation and fibrosis. SEFA-6179 is a promising first-in-class therapy for the prevention and treatment of IFALD that will be investigated in an upcoming phase II clinical trial.
Keywords: Parenteral Nutrition, Cholestasis, Liver Diseases / Drug Therapy, Intestinal Failure, Models, Animal
Graphical Abstract

Lay summary
In preterm piglets receiving intravenous nutrition for two weeks, a medium chain fatty acid analogue prevented cholestasis and fatty liver (steatosis), and reduced liver scarring (fibrosis).
INTRODUCTION
Parenteral nutrition is lifesaving for patients who cannot absorb sufficient nutrition and fluids through the gastrointestinal tract. However, long-term parenteral nutrition can result in progressive cholestatic liver disease known as intestinal failure-associated liver disease (IFALD).1,2 Premature neonates are at particularly high risk due to an immature liver poorly able to handle oxidative stress and inflammation, which is compounded by frequent sepsis and surgical gastrointestinal conditions such as necrotizing enterocolitis.3
The number of intestinal failure patients surviving years or decades has increased markedly in recent years as advances in management have transitioned IFALD into a chronic liver disease.4–6 For pediatric patients requiring long-term parenteral nutrition, persistently elevated liver enzymes and abnormal liver histology are common findings, particularly in patients for whom enteral autonomy is not achieved.7–9 The long-term consequences of these abnormalities are unknown. There remains an urgent need for development of new therapeutics for the prevention and treatment of IFALD.
Medium chain fatty acids (MCFAs) are easily absorbed from the gastrointestinal tract into the portal circulation and thus are often used for nutritional support–in the form of medium chain triglycerides (MCT)–for patients with impaired gastrointestinal absorption.10 As a potential therapeutic, MCFAs are established ligands for G protein-coupled receptors (GPRs) and nuclear receptors that regulate metabolism, inflammation, and fibrogenesis, including GPR84, peroxisome proliferator-activated receptor α (PPARα), and peroxisome proliferator-activated receptor γ (PPARγ).11–13 However, MCFA’s rapid ‘glucose-like’ metabolism, in combination with relatively low affinity and micromolar potency towards their established receptors, likely limits the utility of MCFAs as oral drugs in an unmodified form.14 SEFA-6179 is an orally administered MCFA analogue that is designed to resist β-oxidation for use as a fuel source and achieve in vivo concentrations necessary for engagement of the aforementioned MCFA receptors. Here, we utilize a preterm piglet model of IFALD to investigate whether SEFA-6179 prevents biochemical and histologic IFALD.
MATERIALS AND METHODS
Experimental Design
The aim of the study was to determine whether SEFA-6179 prevented biochemical cholestasis and histologic liver disease in a preterm piglet model of IFALD compared to MCT vehicle control. Previous studies have evaluated the preterm piglet model of IFALD and demonstrated biochemical cholestasis, similar to active IFALD seen in human neonates prior to progression of steatotic-fibrotic liver disease.15,16 Thus, the prespecified primary outcome assessed was plasma total and direct bilirubin. Secondary outcomes of interest included nutritional markers (weight, albumin), other biochemical markers of cholestasis (total bilirubin, gamma glutamyl transferase, plasma bile acids), steatosis (both histology and tissue triglyceride content), and fibrosis (Ishak fibrosis score).
All procedures were approved by the Boston Children’s Hospital Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals.17 ARRIVE guidelines were followed for reporting. The study design is shown in Fig. 1. A pregnant Yorkshire sow (Sus scrofa domesticus) were obtained (Parson’s Farm, Hadley, MA) and housed in our facility five days prior to caesarean section. Piglets were delivered at five days prior to full term (111-days gestation) via caesarean section. Following resuscitation, single-lumen (3Fr × 8cm) central venous catheters (Cook Medical, Bloomington, IN) were placed. Detailed procedural information is provided in the Supplemental Information.
Fig. 1. Study design.

Figure created with Biorender.com.
Parenteral Nutrition
Total parenteral nutrition (TPN) was initiated on day of life (DOL) 1, advanced to goal over five days, and continued until sacrifice on DOL 15. The goal daily volume was 162 ml/kg/d, dextrose 16 g/kg/d, amino acids 9 g/kg/d, fat 5.7 g/kg/d, and caloric content 143 kcal/kg/g. The parenteral nutrition composition was based upon previously published work, with the volume decreased after the pilot cohort demonstrated intolerance of a higher dextrose infusion rate (see Fig. S1).15 TPN was compounded daily. All intravenous tubing, including inline 1.2 micron air-eliminating filters (B. Braun, Bethlehem, PA), was changed daily. Detailed compounding and advancement information is provided in the Supplemental Information.
Group Allocation
Following placement of central venous catheters on DOL 1, piglets were allocated into the treatment group (SEFA-6179) or control group (MCT) using stratified randomization by sex utilizing the Excel software RandBetween function by SF (Microsoft, Redmond, WA). Half of each group was female (50%). Group sizes (MCT n=6, SEFA-6179 n=8) were predetermined for a target final sample size of 5–6 piglets per group, based upon a power of 0.80 to detect a two standard deviation difference with alpha 0.05 on a two-sample t-test. Recent work using a neonatal piglet model with soybean oil lipid emulsion had a total bilirubin of approximately 1.7 ± 0.4 mg/dL (mean ± SD based upon n = 8), which would allow a significance difference of 0.8 mg/dL in total bilirubin to be determined.16
Intervention – MCT Vehicle Control or Study Drug
Piglets were treated daily by orogastric gavage using a temporarily placed 8 Fr nasogastric feeding tube with Dobhoff tip (Cardinal Health, Dublin, OH). The control group received isovolumetric (3 ml/kg/d) MCT vehicle. The treatment group received SEFA-6179 at 48mg/kg/d dissolved in MCT vehicle.
Routine Medications
Whole sow blood was collected in citrate phosphate double dextrose (CP2D) solution (Haemonetics, Braintree, MA), sterilely aliquoted in a class II biosafety cabinet into 50ml conical tubes, and centrifuged to obtain plasma at 1200g × 60 min at 20°C. Maternal plasma was provided intravenously over the first 24 hours of life for passive immunity (5 ml/kg every eight hours for three doses). Cefazolin (20mg/kg) was provided twice daily for central line-associated bloodstream infection prophylaxis. No additional antibiotics were necessary. Daily intravenous famotidine (1 mg/kg) was administered for gastric acid hypersecretion prophylaxis.
Animal Care, Monitoring, and Humane Endpoints
Piglets were cohoused with two piglets per incubator with a 12 h light/dark cycle and monitored for 24 hours per day. To minimize confounding, piglets were numbered and housed according to birth order regardless of treatment group and order of treatment was alternated daily. Piglets were assessed daily for weight, temperature, activity, hydration, diarrhea, and skin breakdown. Additional blood chemistries were obtained for suspected hypovolemia or lethargy. At the discretion of the large animal veterinarian, supplemental fluids (e.g., normal saline or sodium bicarbonate 150 mEq/l) were provided for hypovolemia or acidosis. Early euthanasia was at the discretion of the large animal veterinarian (AN) for refractory signs of distress including fever (>40°C), hypoxia, respiratory distress, persistent lethargy, or immobility. Veterinary staff were blinded to group allocation.
Blood Collection and Biochemical Assessment
Blood was collected via central venous catheters on DOL 1 (prior to initiation of parenteral nutrition), DOL 8, and DOL 15 (immediately prior to sacrifice) into lithium heparin plasma separator tubes (BD Microtainer, Franklin Lakes, NJ, USA). Plasma was obtained by centrifugation at 2000g × 15 min at 20° C. Plasma total bilirubin and direct bilirubin were performed by the Boston Children’s Hospital clinical laboratory. Other biochemical markers were assessed using the VetScan VS2 (Zoetis, Parsippany, NJ, USA). Serum adiponectin was assessed using a commercially available enzyme-linked immunosorbent assay according to manufacturer instructions (Biomatik EKN43266, Kitchener, Ontario, Canada).
Sacrifice and Tissue Collection
Piglets were sacrificed on DOL 15 with sodium pentobarbital (110mg/kg). Formalin-fixed paraffin embedded liver tissue was stained for hematoxylin & eosin (H&E) and Masson’s Trichrome. Frozen liver tissue embedded in optimal cutting temperature compound (Tissue-Tek, Torrance, CA) was stained with oil red O. Formalinfixed paraffin embedded liver sections were stained using DAB immunohistochemistry (see Supporting Information) by HistoWiz Inc. (Brooklyn, NY, USA) for αSMA (Abcam ab5694, Cambridge, UK) and cytokeratin 7 (Abcam ab181598, Cambridge, UK). Quantification of tissue staining (oil red O: ten randomly selected 200x fields; αSMA/cytokeratin 7: five randomly selected 0.2×0.2cm fields) was performed using ImageJ (Version 1.53a, National Institutes of Health, Bethesda, MD) and the IHCToolbox plugin.18,19 Tissue triglyceride content of liver tissue flash frozen in liquid nitrogen was quantified using a commercially available triglyceride assay according to the manufacturer’s instructions (Abcam ab65336, Cambridge, MA). Liver fibrosis was assessed on Masson’s Trichrome-stained slides by a masked veterinary pathologist using the Ishak fibrosis score.
mRNA-Seq and Transcriptomic Analysis
Liver tissue was collected at necropsy, flash frozen in liquid nitrogen, and stored at −80° C until analysis. mRNA-Seq was performed by Novogene Co. (Sacramento, CA, USA). Differentially expressed genes were determined using the DESeq2 R package 1.20.0.20 Adjusted P value < 0.05 using the Benjamini and Hochberg approach for multiple testing were used for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis using clusterProfiler.21 Further functional pathway analysis and investigation of upstream regulators was performed using Ingenuity Pathway Analysis (Qiagen Inc.) using differentially expressed genes with adjusted P < 0.1 and log2foldchange > |0.5|.22 RNA-Seq data are filed in the National Center for Biotechnology Information Gene Expression Omnibus.23 Detailed methods are provided in the Supporting Information.
GPR84, PPARα, and PPARγ activation assays
The activity of SEFA-6179 towards GPR84 was determined in vitro using a Hit Hunter® cAMP assay (Eurofins DiscoverX, CA, US) with embelin acting as a positive control. The activity of SEFA-6179 towards human PPARα and PPARγ was determined in vitro using PathHunter® nuclear hormone receptor (NHR) cell lines (Eurofins DiscoverX, CA, US) with GW7647 or troglitazone acting as respective positive controls. Full methodology is provided in the Supporting Information.
Preliminary Cohort
A preliminary cohort was used to establish the preterm piglet model. Piglets were delivered four days preterm and provided 14 days of parenteral nutrition, resulting in cholestasis and liver fibrosis in control piglets receiving MCT vehicle treatment. Piglets receiving SEFA-6179 (24 mg/kg/d) did not develop cholestasis. Two of four MCT piglets (50%) and five of seven SEFA-6179 piglets (71%) survived to DOL 15. Modifications to the study design were made to prevent infectious complications (daily cefazolin instead of cycled) and acute renal failure (increased TPN volume) encountered in this cohort (Fig. S1, Table S1)
Statistical Analysis
The distribution of each outcome was assessed by Shapiro-Wilk (S-W) test. Continuous outcomes were assessed using nonparametric methods as the central limit theorem could not be relied upon due to the small sample size. Due to the sample size, the analysis was not stratified by sex. At prespecified time points, comparison between two groups was performed with the exact Wilcoxon rank-sum test. Animals with early euthanasia were excluded from the analysis as mortality occurred prior to the DOL 8 timepoint; all other data were included. Mean change in continuous outcomes, adjusted for baseline values, was reported from analysis of covariance (ANCOVA) with P value from rank-based analysis of covariance (rANCOVA). To determine the robustness of the results, parametric tests were also used (t-test and ANCOVA, as appropriate) and in all cases the interpretation of the result was consistent for both tests. Assessment of parenteral nutrition volume delivered was performed via area under the curve (AUC) calculated using the trapezoidal method as AUC = 0.5 × Σ [(Ti+1 – Ti)*(Yi+1 + Yi)]. When divided by the number of observation days, this can be interpreted as a weighted average of the outcome (mAUC). Group comparisons of mAUC were made by the exact Wilcoxon rank-sum test. Assessment of the categorical outcome of Ishak fibrosis score was performed using the Fisher exact test. All tests of significance are two-sided with P<0.05 considered statistically significant. Continuous outcomes are reported as median [range]. Statistical analysis was conducted in SAS by PM (Version 9.4, Cary, NC).
RESULTS
SEFA-6179 activates GPR84, PPARγ and PPARα in vitro
The chemical structure of SEFA-6179 is shown in comparison to decanoic acid, a ten-carbon MCFA (Fig. 2A–B). The activity of SEFA-6179 towards GPR84, PPARγ, and PPARα was assessed in vitro compared to full agonists embelin, GW7647, and troglitazone, respectively. (Fig. 2C–H). Based on these assays, SEFA-6179 is a partial GPR84 agonist (69% efficacy, EC50=12μM), a partial PPARα agonist (59% efficacy, EC50=4.4μM), and a full PPARγ agonist (100% efficacy, EC50=40μM).
Fig. 2. In vitro, SEFA-6179 is a GPR84 partial agonist, PPARα partial agonist, and PPARγ full agonist.

Chemical structure of SEFA-6179 (A) is shown in comparison to decanoic acid (B). GPR84 activity of SEFA6179 was determined using a Hit Hunter® cAMP assay with Embelin acting as a positive control (C, D). PPARα activity of SEFA-6179 was determined using PathHunter® nuclear hormone receptor cell lines with GW7647 as a positive control (E, F). PPARγ activity of SEFA-6179 was determined using PathHunter® nuclear hormone receptor cell lines with troglitazone as a positive control (G, H).
Preterm piglets treated with medium chain triglycerides or SEFA-6179 received similar parenteral nutrition and gained similar weight
A preliminary cohort of piglets was used to establish the model (Fig. S1, Table S1). Yorkshire piglets were delivered five days preterm (111-days gestation). Following resuscitation, all piglets had central venous catheters inserted on DOL 1 and parenteral nutrition was initiated. Piglets were randomized to isovolumetric MCT vehicle control (3 ml/kg/d) or SEFA-6179 treatment (48 mg/kg/d).
Nutritional outcomes are shown in Fig. 3. Piglets in each group received similar parenteral nutrition volume (Fig. 3A). Piglets in the MCT and SEFA-6179 groups gained similar weight (Fig. 3B; 0.44 [0.31–0.55] vs. 0.59 [0.52–0.68] kg, P = 0.15). In both groups, plasma albumin increased from largely undetectable at birth through DOL 15. Compared to the MCT group, SEFA-6179 piglets had higher plasma albumin at DOL 8 and 15 (Fig. 3C; DOL 8: 1.5 [<1–1.6] vs. 1.9 [1.8–2.1] g/dL, P = 0.004; DOL 15: 2.7 [1.7–3.5] vs. 3.7 [3.2–3.9] g/dL, P =0.02). Six of six (100%) MCT piglets and five of eight (63%) SEFA-6179 piglets survived to DOL 15. The primary cause of mortality was renal failure. Necropsy findings are shown in Table S2.
Fig. 3. Nutritional outcomes in preterm piglets receiving 14 days of parenteral nutrition.

Piglets receiving medium chain triglyceride (MCT) vehicle received similar parenteral nutrition (A) and gained similar weight (B) as piglets receiving SEFA-6179 (median with interquartile range). Compared to MCT, piglets receiving SEFA-6179 had higher plasma albumin at day of life 8 and 15 (C, boxplot with range). Comparisons of weight gain assessed using rank-based analysis of covariance (rANCOVA); Comparison of parenteral nutrition volume assessed by mean area under the curve and exact Wilcoxon rank sum. Comparisons of plasma albumin were done with exact Wilcoxon rank-sum tests. * P < 0.05; ** P<0.01.
SEFA-6179 prevented biochemical cholestasis at day of life 15
The primary study outcome was biochemical cholestasis. Biochemical markers of cholestasis are shown in Fig. 4. Piglets in the MCT group developed a direct hyperbilirubinemia consistent with biochemical cholestasis at DOL 15, while the direct bilirubin remained normal in SEFA-6179 piglets (1.9 [0.3–2.8] vs. <0.2 [<0.2–0.5] mg/dL, P = 0.01). Similarly, total bilirubin at DOL 15 was markedly elevated in the MCT group compared to the SEFA-6179 group (2.7 [0.8–4.9] vs. 0.4 [0.4–1.3] mg/dL, P = 0.02). Plasma bile acids were elevated in MCT piglets at DOL 15 compared to SEFA-6179 (15 [<1–38] vs. <1 [<1–8] μmol/L, P = 0.03). Gamma glutamyl transferase (GGT), a marker of cholangiocyte injury,24 was also elevated at DOL 15 in the MCT piglets compared to SEFA-6179 piglets (172 [36–370] vs. 30 [29–44] U/L, P=0.01).
Fig. 4. SEFA-6179 prevents biochemical cholestasis and increases serum adiponectin.
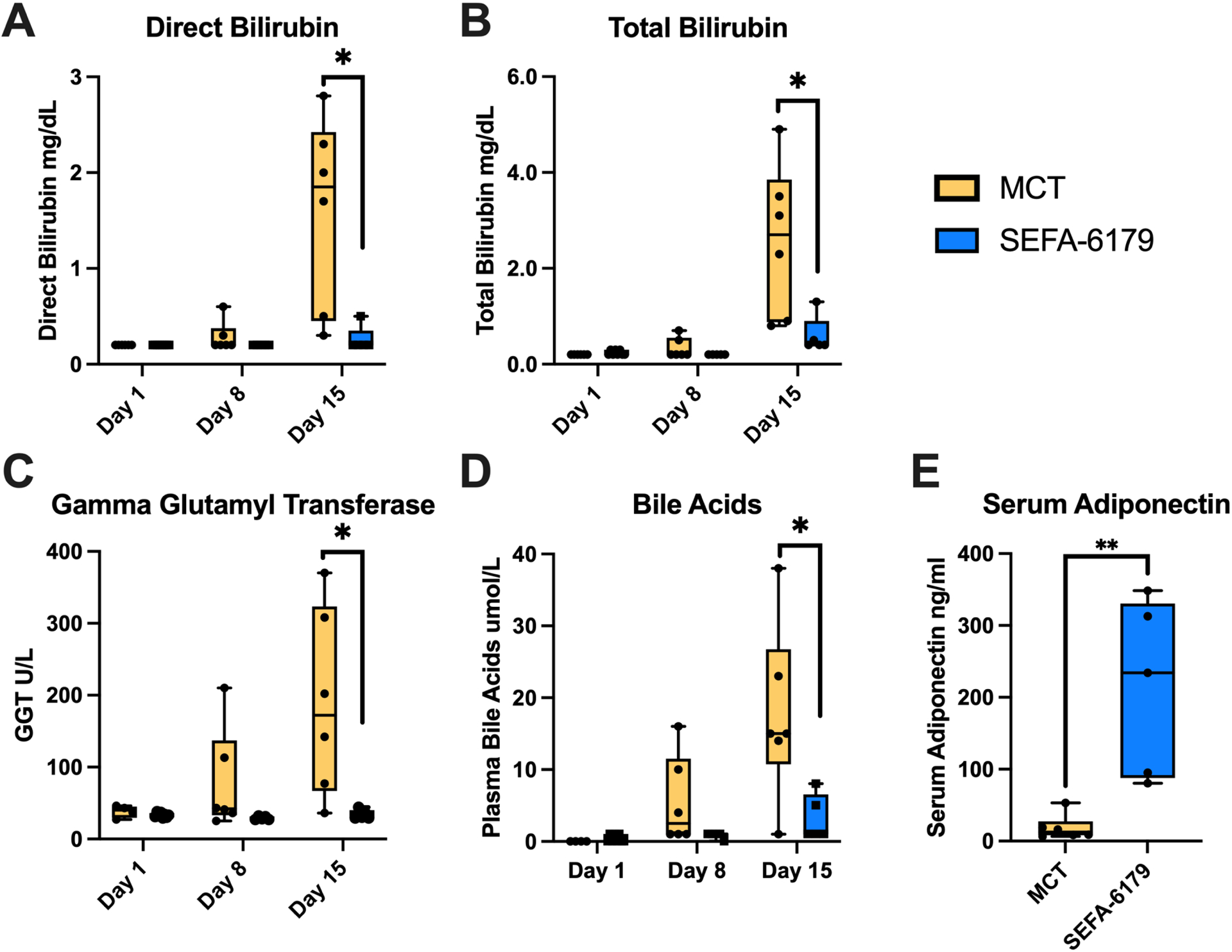
Piglets received daily orogastric gavage of medium chain triglyceride (MCT) vehicle or SEFA-6179. Serum adiponectin was assessed at DOL 15. Boxplots with range. Comparisons between MCT and SEFA-6179 piglets at DOL 1, 8, and 15 were performed with exact Wilcoxon rank-sum tests. *P < 0.05 **P < 0.01
SEFA-6179 prevented liver steatosis, prevented bile duct proliferation, and reduced the Ishak fibrosis stage
Secondary outcomes included histologic biomarkers of progressive and/or chronic IFALD. Representative histology is shown in Fig. 5. MCT piglets demonstrated steatosis, bile duct proliferation, and bridging portalportal and portal-central vein fibrosis. SEFA-6179 piglets demonstrated minimal steatosis, only mild bile duct proliferation, and minimal fibrotic portal expansion. Steatosis was quantified first by staining frozen liver sections with oil red O; MCT piglets demonstrated increased oil red O staining compared to SEFA-6179 piglets (Fig. 5C–D, Fig. 6A; 4.8 [1.0–9.3] vs. 0.3 [0.0–2.6] % area stained, P = 0.02). Liver tissue triglyceride content was also substantially higher in the MCT piglets compared to SEFA-6179 piglets (Fig. 6B; 45.6 [20.4–55.9] vs. 13.9 [12.821.1] mg triglycerides / g liver tissue, P = 0.009). Bile duct proliferation was assessed using immunohistochemical staining for cytokeratin 7, a cholangiocyte marker. MCT piglets demonstrated increased cytokeratin 7 staining compared to SEFA-6179 piglets, consistent with increased bile duct proliferation (Fig. 5E–F, Fig. 6C; 1.6 [1.1–2.1] vs. 0.5 [0.4–1.1] % area stained, P = 0.009).
Fig. 5. Medium chain triglyceride (MCT) piglets show steatosis, bile duct proliferation, and fibrosis on representative liver histology, which is reduced by SEFA-6179 treatment.
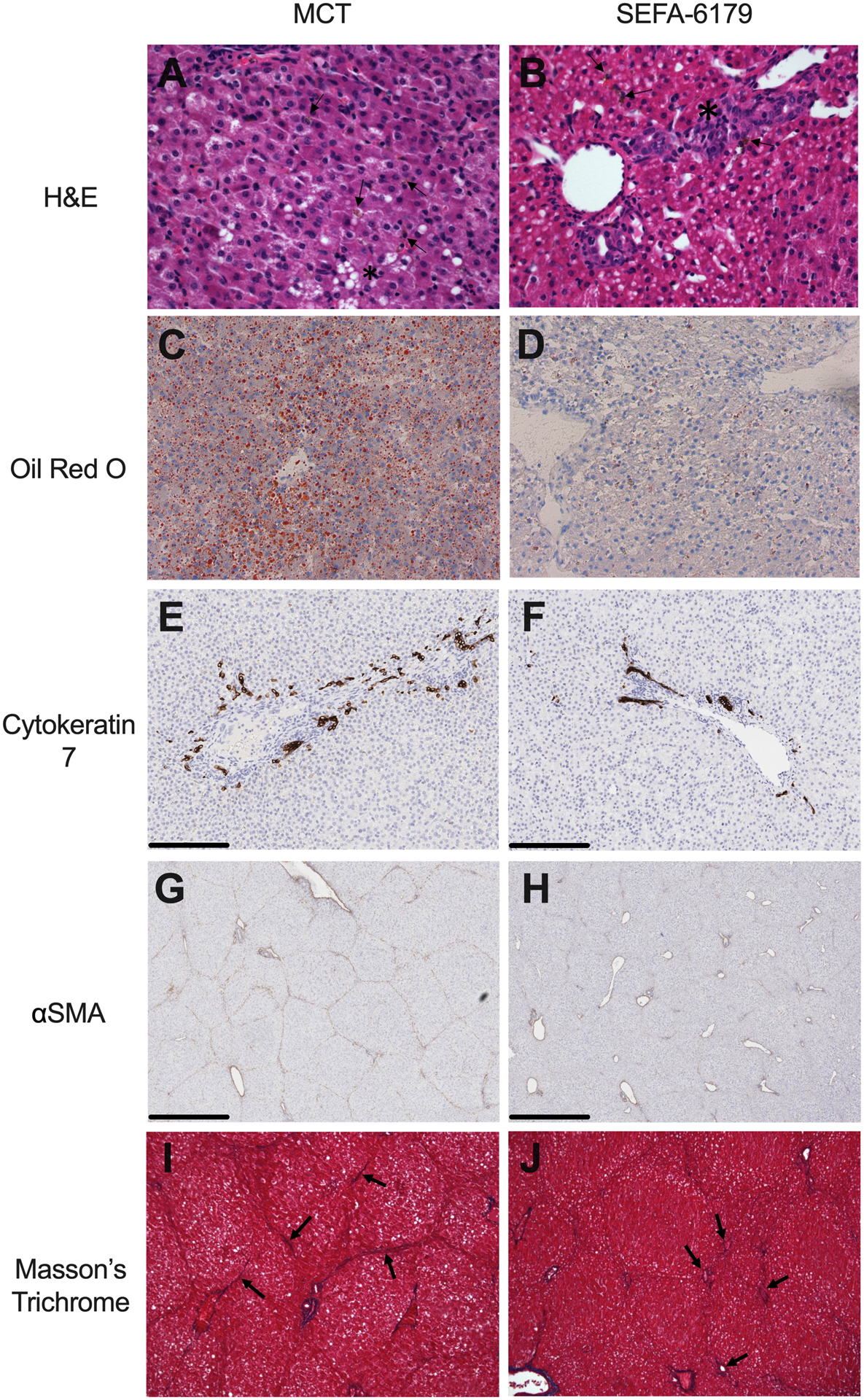
Formalin-fixed paraffin-embedded liver sections were stained with hematoxylin & eosin (H&E, A-B). Frozen liver sections were stained with oil red O (C-D). Immunohistochemical staining (DAB, brown) of formalin-fixed paraffin-embedded liver sections was performed for cytokeratin 7 (E-F) and αSMA (G-H). Formalin-fixed paraffin-embedded liver sections were stained with Masson’s trichrome (I-J). MCT piglets demonstrate steatosis (A, asterisks; C), extensive bile pigment (A, arrows), bile duct proliferation (E), extensive αSMA staining (G), and fibrosis indicated by bridging portal-portal and portal-central vein fibrosis (I, arrows). SEFA-6179 piglets demonstrate no steatosis (D), mild bile pigment (B, arrows), mild bile duct proliferation (B, asterisks; F), reduced αSMA staining (H), and minimal fibrotic portal expansion (J, arrows). Magnification: A– 400X; B – 200x; C – 200nm reference bars. D – 1mm reference bars. E – 100X.
Fig. 6. SEFA-6179 reduces liver steatosis, bile duct proliferation, hepatic stellate cell activation, and Ishak fibrosis score.

SEFA-6179 piglets, compared to MCT piglets, demonstrated decreased oil red O staining (A) and liver triglyceride content (B) consistent with minimal hepatosteatosis, decreased cytokeratin 7 staining (C) consistent with minimal bile duct proliferation, decreased αSMA staining (D) consistent with decreased hepatic stellate cell activation, and decreased Ishak fibrosis score (E) assessed by a masked pathologist. Boxplots with range. Comparisons (A-D) made with exact Wilcoxon rank-sum tests. The mean Ishak score is presented as a diamond. Ishak fibrosis scores were compared with Fisher’s exact test. * P < 0.05 ** P < 0.01.
Hepatic stellate cell activation–a key step in fibrogenesis–was assessed using immunohistochemical staining for α smooth muscle actin (αSMA). MCT piglets demonstrated increased αSMA staining compared to SEFA-6179 piglets, consistent with increased hepatic stellate cell activation (Fig. 5G–H, Fig. 6D; 3.0 [2.8–5.2] vs. 1.8 [1.6–2.8] % area stained, P = 0.004). A masked veterinary pathologist assessed liver fibrosis on formalin-fixed paraffin embedded liver tissue stained with Masson’s Trichrome (Fig. 5I–J, Fig. 6E). MCT piglets had more severe fibrosis than SEFA-6179 piglets with a median Ishak fibrosis score of 3 (fibrous expansion of most portal areas with occasional portal-portal bridging) versus median score of 1 (fibrous expansion of some portal areas ± short fibrous septa) (P = 0.007).
Key functional pathways activated by SEFA-6179 include increased fatty acid β-oxidation and retinol/RXR-mediated signaling, while inflammatory pathways are inhibited
We next focused on differences in gene expression between the two groups to identify key pathways mediating the hepatoprotective effects of SEFA-6179 demonstrated in this model. In vitro, SEFA-6179 is a GPR84/PPARα/PPARγ agonist, a receptor profile that would be expected to have broad inhibitory and stimulatory effects on inflammatory and metabolic pathways, respectively. Serum adiponectin, a sensitive and specific marker of PPARγ activation,25 was elevated in the SEFA-6179 piglets at DOL 15 compared to the MCT piglets (Fig. 4E, 234.1 [80.5–348.6] vs. 12.5 [6.5–53.1] ng/ml, P = 0.004) (Fig. 4E).
mRNA-Seq was performed on liver tissue from all MCT and SEFA-6179 piglets. One-hundred and forty-seven differentially expressed genes (52 up, 95 down) were identified (Fig. 7A). Functional analysis was performed using Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Ingenuity Pathway Analysis (IPA) (Fig. 7B–D). Commonly enriched pathways across multiple functional analyses included fatty acid oxidation and metabolic processes (GO, KEGG, and IPA), retinol metabolism/retinoid X receptor (RXR) function (KEGG and IPA), and inflammation/cellular adhesion (GO and IPA).
Fig. 7. RNA-Seq demonstrates enrichment of fatty acid oxidation, retinal metabolism, and PPAR signaling from SEFA-6179 treatment.

RNA-Seq was performed on the Illumina NovaSeq 6000 platform and differentially expressed genes were identified (A). Gene ontogeny analysis (B) demonstrated biological process (BP), cellular component (CC), and molecular function (MF) categories of commonly enriched genes, including fatty acid oxidation, lipid oxidation, and cellular adhesion. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis (C) demonstrated key enriched pathways including retinol metabolism and PPAR signaling. Key canonical pathways determined using Ingenuity Pathway Analysis (D) include increased oxidative phosphorylation and fatty acid β-oxidation, with downregulated granzyme A signaling and LPS/IL-1 Mediated Inhibition of RXR Function.
GO enrichment analysis demonstrated that the most enriched biological processes were fatty acid oxidation and lipid oxidation, while the most enriched cellular components were involved in cell adhesion (Fig. 7B). KEGG analysis found the most enriched pathways involved retinol metabolism, PPAR signaling, and fatty acid degradation (Fig. 7C). IPA demonstrated the key upregulated canonical pathways from SEFA-6179 treatment included pathways involved in oxidative phosphorylation, eukaryotic initiation factor 2 signaling, fatty acid β-oxidation I, and glutathione redox reactions I (Fig. 7D). Both eukaryotic initiation factor 2 phosphorylation and glutathione redox reactions are critical in mediating the antioxidant capacity in the liver and responding to oxidative stress.26 Key downregulated canonical pathways from SEFA-6179 treatment included the Sirtuin signaling pathway, Granzyme A signaling, and lipopolysaccharide/interleukin-1 (LPS/IL-1) mediated inhibition of RXR function.
Specific canonical pathways of interest were also analyzed in IPA based upon observed differentially expressed genes. In the SEFA-6179 treated piglets, PPARα/RXR activation was predicted with increased fatty acid oxidation, fatty acid uptake, glucose homeostasis, lipoprotein metabolism, β-oxidation, and anti-inflammation (Fig. S2). Consistent with this activation, investigation of the inhibited LPS/IL-1 Mediated Inhibition of RXR Function pathway (Fig. S3) demonstrates predicted activation of retinoid X receptor-mediated pathways including master regulators liver X receptor, farsenoid X receptor, retinoic acid receptor, pregnane X receptor, and PPARs. Notably, this includes genes involved in bile acid and organic ion transport, in addition to fatty acid metabolism. Nuclear factor κ B (NF-κB) signaling (which is downregulated by both PPARγ and PPARα agonism) was predicted to be inhibited (Fig. S4). Finally, hepatic fibrosis signaling was investigated (Fig. S5). Fibrosis pathways mediated by transforming growth factor β (TGF-β), SMAD 2/3/4, c-Jun N-terminal kinase (JNK), and signal transducer and activator of transcription 3 (STAT3) were predicted to be inhibited with downstream decreased hepatic stellate cell activation and liver fibrosis.
Predicted upstream regulators of differentially expressed genes/pathways are consistent with GPR84, PPARγ and PPARα agonism
Upstream regulator analysis by IPA identified candidate upstream regulators consistent with observed downstream pathway enrichment from SEFA-6179 treatment (Table S3). The activation z-score provides the degree of downstream activation or inhibition, with upstream regulators with a z-score > |2| considered significant. Identification of PPARγ co-activator 1α (PPARGC1A) as one of the greatest activated upstream regulators (z-score 3.234) is consistent with PPARγ agonism (as it is a transcriptional coactivator for PPARγ). Similarly, hepatocyte nuclear factor 4 alpha (HNF4A) interacts with PPARα to drive fatty acid metabolism, consistent with activation of pathways downstream of PPARα. Inhibited upstream regulators included proinflammatory signaling molecules such as myeloid differentiation primary response 88 and tumor necrosis factor (TNF)–consistent with anti-inflammatory effects. Multiple transcription regulators were identified as possible activated upstream regulators that all inhibit transcription of collagen genes COL1A2 and COL3A1, consistent with downstream anti-fibrotic activity. Eukaryotic initiation factor 6 inhibition is also associated with reduction in nonalcoholic fatty liver disease through downregulation of de novo lipogenesis genes and collagenencoding genes, resulting in decreased steatosis and fibrosis.27 Predicted upstream regulators are consistent with GPR80, PPARα, and PPARγ agonism as the key mechanisms mediating the metabolic, anti-inflammatory, and anti-fibrotic effects of SEFA-6179 in this model.
DISCUSSION
Here, we demonstrate that SEFA-6179 prevents IFALD in a cholestatic preterm piglet model. The swine gastrointestinal system is highly similar to that of humans with respect to intestinal and biliary physiology, intestinal absorption, hormonal regulation, and nutritional requirements.28 The neonatal piglet model of IFALD closely reproduces the pathophysiology of human IFALD. Administration of parenteral nutrition to neonatal piglets–especially with preterm delivery, a key risk factor in human IFALD–results in cholestatic liver disease with both biochemical and histologic biomarkers that parallel human disease.15,16,29,30 In this study, piglets receiving MCT vehicle developed active cholestatic disease, marked by elevated plasma biomarkers (direct bilirubin, total bilirubin, gamma glutamyl transferase, bile acids) and histologic liver injury with early progression to steatosis, bile duct proliferation, and fibrosis. SEFA-6179 prevented cholestasis and steatosis, and reduced bile duct proliferation and fibrosis. Transcriptome analysis demonstrated that SEFA-6179 activated anti-inflammatory pathways, fatty acid β-oxidation, and anti-fibrotic pathways consistent with GPR84, PPARα and PPARγ activation.
Advances in modern management of intestinal failure have allowed patients to survive years or even decades on parenteral nutrition. Fish oil lipid emulsion and hepatoprotective management has transformed IFALD into a chronic liver disease, but few therapeutic options exist to interrupt further disease progression. Current estimates suggest that 20–30% of children with intestinal failure will develop overt IFALD, although this may be an underestimate of the true disease burden.2 Recent literature has identified a new poorly understood entity of non-cholestatic IFALD marked by subclinical histologic progression with long-term parenteral nutrition, despite an absence of overt biochemical cholestasis. A recently published cohort from our center found 79% of pediatric intestinal failure patients dependent on long-term PN have (typically mild-to-moderate) elevations in alanine aminotransferase.8 IFALD is classified histologically into ‘active’ (cholestasis and inflammation) or ‘chronic’ (steatosis and fibrosis) stages.2 On routine liver biopsy of a modern cohort of pediatric intestinal failure patients, two-thirds demonstrated histologic liver disease: 48% had active IFALD and 21% had chronic IFALD.7 On subsequent biopsy of patients with active IFALD (median time between biopsies was 2.5 years), two-thirds demonstrated either persistent active IFALD or transition to chronic IFALD. Even after weaning from parenteral nutrition, most pediatric intestinal failure patients will have persistent fibrosis and half will have steatosis on biopsy.31 A major need exists for therapeutics to both prevent the development of IFALD and interrupt the progression of established disease. Our results suggest that SEFA-6179 may prevent development of IFALD; further research is necessary to investigate whether SEFA-6179 can interrupt the progression of established disease.
IFALD in premature neonates is characterized by an inflammatory and cholestatic phenotype. The immature liver is poorly able to handle the numerous inflammatory insults in this population including pro-inflammatory lipids and phytosterols, translocation of bacterial products across a disrupted intestinal epithelium into the portal circulation, and sepsis from necrotizing enterocolitis or central line-associated blood stream infections.2 Even with newer generation mixed oil lipid emulsions with a decreased (more anti-inflammatory) ω6: ω3 fatty acid ratio and reduced phytosterols, IFALD still occurs at a high incidence in susceptible patient populations.32 GPR84 activation has recently been identified as a hepatoprotective MCFA receptor, exhibiting both antiinflammatory and anti-fibrotic activity in response to lipotoxicity via downregulation of macrophage activation.12 Although MCFAs are established endogenous ligands, GPR84 remains an orphan receptor as its downstream signaling has not been fully elucidated.14 Importantly, SEFA-6179 has a similar potency towards GPR84 as naturally occurring MCFAs (low micromolar range), a potential differentiating factor from highly potent synthetic GPR84 agonists that display pro-inflammatory effects.12
A key proposed mechanism of IFALD is an inflammatory response to bacterial products either from recurrent sepsis (e.g., from central line-associated bloodstream infection) or from absorption into the portal circulation.2 Several lines of evidence support the role of enteric bacterial products in development of IFALD: patients with short bowel syndrome have increased intestinal permeability, small bowel bacterial overgrowth/dysbiosis is associated with gram-negative bloodstream infections and IFALD progression, and flagellin and LPS (along with increased anti-flagellin/anti-LPS immunoglobulins) have been directly detected in serum samples from short bowel syndrome patients.33,34 Both PPARα and PPARγ have anti-inflammatory effects mediated by a variety of mechanisms that may interrupt the inflammatory liver response to bacterial products. Liver-specific PPARα agonism prevents the systemic inflammatory response induced by LPS-toll like receptor 4 (TLR4) signaling.35 In TLR4 knockout mouse models of IFALD and nonalcoholic steatohepatitis, TLR4−/− mice are protected from liver disease.36,37 PPARα agonism also suppresses the interleukin-6-induced acute phase response via down-regulation of interleukin 6 receptor components gp80 and gp130 (reducing downstream STAT3 and JNK signal transduction) and decreases expression of CCAAT enhancer-binding proteins (transcription factors for acute phase response genes).38 Both PPARα and PPARγ inhibit the NF-κB pathway.39,40 Furthermore, macrophage polarization to a M2 anti-inflammatory phenotype is driven by PPARγ.41 On transcriptomic analysis of SEFA-6179 treatment, inhibition of the canonical pathways NF-κB and LPS/IL-1 Mediated Inhibition of RXR Function, as well as predicted inhibition of transcription downstream from myeloid differentiation primary response 88 and TNF, was consistent with the anti-inflammatory effects of both PPARα and PPARγ agonism.
In adults with intestinal failure, IFALD is characterized by a steatosis-predominant phenotype. Minimizing parenteral lipids may drive de novo lipogenesis; alternatively, parenteral administration of lipids bypasses the normal physiologic trafficking of micelles with resultant first-pass metabolism occurring in the liver which contributes to increased hepatic lipid deposition.42 PPARα is a key ligand-activated transcription factor that regulates lipid transport and fatty acid oxidation in the liver, controlling the rate-limiting enzymes of peroxisomal β-oxidation and mitochondrial β-oxidation.43 SEFA-6179 effectively prevented hepatosteatosis in this study. In nonalcoholic fatty liver disease, PPARα agonists including clofibrate and fenofibrate have little effect on biochemical and histologic disease, while multiple randomized controlled trials demonstrate that PPARγ agonists (rosiglitazone and pioglitazone) decrease liver enzymes, steatosis, and inflammation, but minimally affect fibrosis.44 These findings suggest that dual PPARα and PPARγ agonism, in addition to GPR84 activation, may play an additive and/or synergistic role in the observed anti-inflammatory, anti-steatotic, and anti-fibrotic effects.
Multiple pathways may mediate the anti-fibrotic effects of SEFA-6179. Anti-inflammatory effects mediated by GPR84, PPARα and PPARγ may interrupt macrophage and hepatic stellate cell activation, resulting in decreased extracellular matrix deposition. In addition to downregulation of inflammation, PPARγ inhibits hepatic stellate cell activation through modulation of TGF-β signaling pathways.45 Decreased αSMA staining in the SEFA-6179 piglets is consistent with prevention of hepatic stellate cell activation. PPARγ agonists are effective in reducing fibrosis in models of pulmonary fibrosis, renal fibrosis, liver fibrosis, cardiac fibrosis.46–49 A recent phase 2b double-blind randomized placebo-controlled trial of the pan-PPAR agonist lanifibranor in nonalcoholic steatohepatitis found substantial resolution of nonalcoholic steatohepatitis and an improvement in fibrosis stage.50
There are several important limitations to this work. The model used recapitulates the disease of the preterm neonate with cholestatic IFALD, but it may not be broadly applicable to older children and adults who less frequently demonstrate a cholestasis-predominant phenotype. In addition, while most long-term parenteral nutrition patients have short bowel syndrome (from either a congenital or acquired cause), the piglets retained the entirety of their bowel. For an enterally-administered therapy, this may be an important limitation and careful pharmacokinetic investigation will be critical in translation to human populations. Due to the tenuous clinical status of premature piglets, all piglets required parenteral nutrition for survival and a ‘normal’ control without parenteral nutrition was not possible. Finally, in this study, SEFA-6179 was evaluated as a preventative treatment, and further investigations of its efficacy in treating established IFALD are warranted.
Despite advances in IFALD management with hepatoprotective strategies, many patients with intestinal failure have progressive chronic liver disease and fibrosis. IFALD remains a key life-limiting complication of intestinal failure. In a preterm piglet model of IFALD that is highly analogous to the preterm neonate with cholestatic IFALD, SEFA-6179 prevented cholestasis and steatosis, and reduced bile duct proliferation and fibrosis. Transcriptomic analysis revealed that these effects are consistent with the demonstrated in vitro GPR84/PPARα/PPARγ agonism, with increased fatty acid metabolism, inhibition of key inflammatory pathways (NF-κB, IL-1/LPS), and inhibition of fibrosis via TGF-β, SMAD 2/3/4, and JNK pathways. Further investigation will be critical to evaluate the efficacy of SEFA-6179 in chronic non-cholestatic IFALD. SEFA6179 has recently completed a Phase I clinical trial and will soon begin a Phase II clinical trial for the treatment of IFALD. The unique mechanism of action of SEFA-6179 (GPR84/PPARα/PPARγ agonism) may also provide a new avenue of investigation for other cholestatic liver diseases with limited therapeutic options.
Materials and Methods
Preterm Piglet Delivery, Resuscitation, and Central Venous Catheter Placement
A pregnant Yorkshire sow (Sus scrofa domesticus) were obtained (Parson’s Farm, Hadley, MA) and housed in our facility five days prior to caesarean section. The sow was induced with atropine (0.04 mg/kg), tiletamine/zolazepam (2.2 mg/kg), xylazine (1.1 mg/kg), and propofol (1 mg/kg). General anesthesia was maintained with inhaled isoflurane (1–5%) via mask inhalation. Five-hundred ml of whole blood was drawn from the left femoral vein into a collection bag containing citrate phosphate double dextrose (CP2D) solution (Haemonetics, Braintree, MA). Following this, a lower midline incision was made. One horn of the uterus was exteriorized and incised with electrocautery over the back of a piglet. The piglet was delivered from the uterus, oropharynx suctioned, umbilical cord ligated and divided, and handed off the surgical field for resuscitation. Subsequent piglets were delivered in this fashion. After all piglets were delivered, the sow was euthanized using sodium pentobarbital (110 mg/kg).
After delivery, piglets were dried, vigorously stimulated, warmed, and administered doxepram (4 mg), atropine (0.11 mg), and vitamin B12 (500 μg) via intramuscular injection. If necessary, ventilation and supplemental oxygen was provided via bag-valve mask. Epinephrine was administered if piglets developed bradycardia and additional doxepram was given for hypoventilation. Piglets were then placed in a humified incubator at 37°C. After adequate resuscitation, piglets were induced and intubated. Single-lumen (3Fr × 8cm) central venous catheters (Cook Medical, Bloomington, IN) were placed preferentially in the right external jugular vein. Intravenous cefazolin (20mg/kg), banamine (1 mg/kg), buprenorphine (0.01 mg/kg), cerenia (1 mg/kg), and intramuscular iron (75 mg/kg) were administered. The piglets were returned to incubators and cohoused per animal welfare recommendations (two piglets per incubator bay).
Total Parenteral Nutrition
Total parenteral nutrition (TPN) was initiated on DOL 1, advanced to goal over 5 days, and continued until sacrifice on DOL 15. The goal daily volume was 162 ml/kg/d, dextrose 16 g/kg/d, amino acids 9 g/kg/d, fat 5.7 g/kg/d, and caloric content 143 kcal/kg/g. The parenteral nutrition composition was based upon the previously published work of Vlaardingerbroek at al, with the volume decreased after the pilot cohort demonstrated intolerance of a higher dextrose infusion rate (see Fig. S1). TPN was compounded daily in a class II biosafety cabinet with sterile technique using a premixed parenteral nutrition solution (Clinimix e 8/14, Baxter, Deerfield, IL), soybean oil lipid emulsion (Nutrilipid, B. Braun, Bethlehem, PA), intravenous multivitamins (Infuvite, Baxter, Deerfield, IL), and sterile water for injection (Baxter, Deerfield, IL). TPN was advanced at the following daily rate as a % of goal: Day 1, 43%; Day 2, 69%; Day 3, 82%; Day 4, 95%; Day 5, 100%. Piglets were monitored for signs of TPN intolerance including hyperglycemia on ear stick blood glucose, and evidence of dehydration such as lethargy. If there was concern for TPN intolerance, the rate of TPN was decreased to the previous rate, supplemental isotonic intravenous fluids were provided, and TPN was readvanced following normalization of glucose or activity.
Immunohistochemistry
Formalin-fixed paraffin embedded liver sections were stained using DAB immunohistochemistry by HistoWiz Inc. (Brooklyn, NY, USA) using a fully automated workflow on a Bond Rx autostainer (Leica Biosystems, Wetzlar, Germany) for αSMA (Bond epitope retrieval solution 2, 20 minutes, Leica Biosystems; Primary antibody: Abcam ab5694 1:1000, Cambridge, UK) and cytokeratin 7 (Bond epitope retrieval solution 1, 40 minutes, Leica Biosystems. Primary antibody: Abcam ab181598 1:8000, Cambridge, UK). DAB staining was performed using the IHC Polymer Detection Kit (Leica Biosystems, Wetzlar, Germany). Whole slide scanning (40x) was performed on an Aperio AT2 (Leica Biosystems, Wetzlar, Germany).
mRNA-Seq and Transcriptomic Analysis
Liver tissue was collected at necropsy, flash frozen in liquid nitrogen, and stored at −80° C until analysis. mRNA-Seq was performed by Novogene Co. (Sacramento, CA, USA). RNA integrity was assessed with the RNA Nano 6000 Assay kit (Agilent Technologies, CA, USA). All samples had RIN> 4 and passed the established quality thresholds. mRNA was purified using poly-T oligo-attached magnetic beads and 150 bp paired-end reads were generated using the NovaSeq 6000 platform (Illumina, San Diego, CA). Raw data were cleaned with removal of low quality reads and then mapped to the Sscrofa11.1 pig genome using Hisat2 v2.0.5 (1,2). Mapped reads were assembled using StringTie v1.3.3b (3). Read numbers for each mapped gene were calculated with featureCounts v1.5.0-p3 (4). Differentially expressed genes were determined using the DESeq2 R package 1.20.0 (5). Adjusted P value < 0.05 using the Benjamini and Hochberg approach for multiple testing were used for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis using clusterProfiler. Further functional pathway analysis and investigation of upstream regulators was performed using Ingenuity Pathway Analysis (Qiagen Inc.) using differentially expressed genes with adjusted P < 0.1 and log2foldchange > |0.5|. RNA-Seq data are filed in the National Center for Biotechnology Information Gene Expression Omnibus with the accession number GSE223347. Detailed methods are provided in the Supporting Information.
GPR84 Activation Assay
The activity of SEFA-6179 towards GPR84 was determined in vitro using a Hit Hunter® cAMP assay (Eurofins DiscoverX, CA, US) with embelin acting as a positive control. cAMP Hunter cell lines were expanded from freezer stocks according to standard procedures; cells were seeded in a total volume of 20 μl into white walled, 384-well microplates and incubated at 37°C/5% CO2 overnight. Following overnight incubation, cells were then treated with either SEFA-6179 or Embelin at 37°C/5% CO2 or room temperature for 60 minutes. A 10-point concentration curve from 0.001 to 100 μM was constructed. Compound activity was determined via chemiluminescence using β-galactosidase (β-Gal) as the functional reporter. Percentage activity was calculated using the following formula: % Activity=100% × (mean relative light units [RLU] of test sample - mean RLU of vehicle control) / (mean MAX control ligand - mean RLU of vehicle control).
PPARα and PPARγ activation assays
The activity of SEFA-6179 towards human PPARα and PPARγ was determined in vitro using PathHunter® nuclear hormone receptor (NHR) cell lines (Eurofins DiscoverX, CA, US) with GW7647 or troglitazone acting as respective positive controls. NHR cell lines were expanded from freezer stocks according to standard procedures; cells were seeded in a total volume of 20 μl into white walled, 384-well microplates and incubated at 37°C/5% CO2 overnight. Following overnight incubation, cells were then treated with SEFA-6179, GW7647, or Troglitazone at 37°C/5% CO2 or room temperature for 3–16 hours. A 10-point concentration curve from 0.001 to 100 μM was constructed. Compound activity was determined via chemiluminescence after the addition of PathHunter Detection reagent cocktail. Microplates were read following signal generation with a PerkinElmer EnvisionTM instrument for chemiluminescent signal detection. Percentage activity was calculated using the following formula: % Activity=100% × (mean RLU of test sample - mean RLU of vehicle control) / (mean MAX control ligand - mean RLU of vehicle control).
Supplementary Material
BACKGROUND AND CONTEXT.
Long-term parenteral nutrition in patients with intestinal failure often results in intestinal failureassociated liver disease (IFALD). SEFA-6179 is a medium chain fatty acid analogue under development for IFALD.
NEW FINDINGS.
In a preterm piglet model of IFALD, SEFA-6179 treatment prevented biochemical cholestasis and hepatosteatosis, while reducing liver fibrosis. RNA-Seq demonstrated that SEFA-6179 broadly impacted inflammatory, metabolic, and fibrotic pathways.
LIMITATIONS.
The preterm piglet model is highly analogous to the preterm human infant with cholestatic IFALD, but older children and adults often have non-cholestatic liver disease.
CLINICAL RESEARCH RELEVENCE.
IFALD is a common complication of long-term parenteral nutrition in intestinal failure patients, for which there are few therapeutic options. SEFA-6179 is a promising first-in-class therapy for the prevention and treatment of IFALD that will be investigated in an upcoming phase II clinical trial.
BASIC RESEARCH RELEVANCE.
SEFA-6179 is a first-in-class GPR84/PPARα/PPARγ agonist that targets inflammatory, metabolic, and fibrotic pathways. The unique mechanism of action of SEFA-6179 may provide a new avenue of investigation for other cholestatic liver diseases with limited therapeutic options.
Acknowledgements:
Assistance with the study: The authors would like to thank the certified veterinary technicians Robin Grammer and Brittany Pattison for their excellent care of the study animals.
Grant Support:
This study was funded by NorthSea Therapeutics under a sponsored research agreement, National Institutes of Health grants 5T32HL007734 (SCF, TIH) and 2T32DK007754-22 (STT), the Beth Israel Deaconess Medical Center Richard and Sandra Cummings Research Fellowship (SCF), the Boston Children’s Hospital Vascular Biology Program, and Boston Children’s Hospital Surgical Foundation.
Conflicts of Interest:
This study was primarily funded via a sponsored research agreement with NorthSea Therapeutics. Dr. Fraser is the Chief Scientific Officer of NorthSea Therapeutics and was responsible for the in vitro analysis. Drs. Puder and Gura are external consultants for Northsea Therapeutics. The study was designed in consultation with NorthSea Therapeutics, but final study design decisions were made by Drs. Puder and Fligor. Other than the in vitro analysis, NorthSea Therapeutics and Dr. Fraser were not involved in the collection, analysis, or interpretation of data.
List of Abbreviations:
- αSMA
α smooth muscle actin
- JNK
c-Jun N-terminal kinase
- DOL
Day of life
- GPR
G protein-coupled receptor
- GPR84
G protein-coupled receptor 84
- GO
Gene Ontology
- HNF4A
Hepatocyte nuclear factor 4 alpha
- IPA
Ingenuity Pathway Analysis
- IL-1
Interleukin 1
- IL-6
Interleukin-6
- IFALD
Intestinal failure-associated liver disease
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LPS
Lipopolysaccharide
- MCT
Medium chain triglycerides
- MCFAs
Medium chain fatty acids
- NF-κB
Nuclear factor κ B
- PPARα
Peroxisome proliferator-activated receptor α
- PPARγ
Peroxisome proliferator-activated receptor γ
- PPARGC1A
Peroxisome proliferator-activated receptor γ co-activator 1α
- RXR
Retinoid X receptor
- STAT3
Signal transducer and activator of transcription 3
- TLR4
Toll like receptor 4
- TPN
Total parenteral nutrition
- TGF-β
Transforming growth factor β
Footnotes
Transcript Profiling: RNA-Seq data are available from the National Center for Biotechnology Information Gene Expression Omnibus, accession GSE223347.
Data Transparency Statement: Original data are available as a downloadable excel file.
Presentation: Preliminary data were presented at Digestive Disease Week (May 2022, San Diego, USA) as a late-breaking basic science plenary and at the International Pediatric Intestinal Failure and Rehabilitation Symposium as a top abstract (October, 2022; Toronto, Canada).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lauriti G, Zani A, Aufieri R, et al. Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J Parenter Enteral Nutr 2014;38:70–85. [DOI] [PubMed] [Google Scholar]
- 2.Khalaf RT, Sokol RJ. New Insights Into Intestinal Failure-Associated Liver Disease in Children. Hepatology 2020;71:1486–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen RD, Henry E, Wiedmeier SE, et al. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J Perinatol 2007;27:284–290. [DOI] [PubMed] [Google Scholar]
- 4.Lee WS, Chew KS, Ng RT, et al. Intestinal failure-associated liver disease (IFALD): insights into pathogenesis and advances in management. Hepatol Int 2020;14:305–316. [DOI] [PubMed] [Google Scholar]
- 5.Gura KM, Premkumar MH, Calkins KL, et al. Fish Oil Emulsion Reduces Liver Injury and Liver Transplantation in Children with Intestinal Failure-Associated Liver Disease: A Multicenter Integrated Study. J Pediatr 2021;230:46–54.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secor JD, Yu L, Tsikis S, et al. Current strategies for managing intestinal failure-associated liver disease. Expert Opin Drug Saf 2021;20:307–320. [DOI] [PubMed] [Google Scholar]
- 7.Mutanen A, Lohi J, Merras-Salmio L, et al. Prediction, identification and progression of histopathological liver disease activity in children with intestinal failure. J Hepatol 2021;74:593–602. [DOI] [PubMed] [Google Scholar]
- 8.Keefe G, Culbreath K, Knell J, et al. Long-term assessment of bilirubin and transaminase trends in pediatric intestinal failure patients during the era of hepatoprotective parenteral nutrition. J Pediatr Surg 2022;57:122–126. [DOI] [PubMed] [Google Scholar]
- 9.Huysentruyt K, Belza C, Wong-Sterling S, et al. Use of a combined transient elastography and biochemical strategy to determine liver fibrosis in pediatric intestinal failure. Clin Nutr 2023;42:136–142. [DOI] [PubMed] [Google Scholar]
- 10.Greenberger NJ, Rodgers JB, Isselbacher KJ. Absorption of medium and long chain triglycerides: factors influencing their hydrolysis and transport. J Clin Invest 1966;45:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fougerat A, Montagner A, Loiseau N, et al. Peroxisome Proliferator-Activated Receptors and Their Novel Ligands as Candidates for the Treatment of Non-Alcoholic Fatty Liver Disease. Cells 2020;9:1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohue-Kitano R, Nonaka H, Nishida A, et al. Medium-chain fatty acids suppress lipotoxicity-induced hepatic fibrosis via the immunomodulating receptor GPR84. JCI Insight 2023;8:e165469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. New England Journal of Medicine 2010;362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luscombe VB, Lucy D, Bataille CJR, et al. 20 Years an Orphan: Is GPR84 a Plausible Medium-Chain Fatty Acid-Sensing Receptor? DNA and Cell Biology 2020;39:1926–1937. [DOI] [PubMed] [Google Scholar]
- 15.Vlaardingerbroek H, Ng K, Stoll B, et al. New generation lipid emulsions prevent PNALD in chronic parenterally fed preterm pigs. J Lipid Res 2014;55:466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng K, Stoll B, Chacko S, et al. Vitamin E in New-Generation Lipid Emulsions Protects Against Parenteral Nutrition-Associated Liver Disease in Parenteral Nutrition-Fed Preterm Pigs. JPEN J Parenter Enteral Nutr 2016;40:656–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, D.C.: National Academies Press; 2011:12910. Available at: http://www.nap.edu/catalog/12910 [Accessed October 31, 2022]. [Google Scholar]
- 18.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shu J, Qiu G, Mohammad I. A Semi-automatic Image Analysis Tool for Biomarker Detection in Immunohistochemistry Analysis. In: 2013 Seventh International Conference on Image and Graphics.; 2013:937–942. [Google Scholar]
- 20.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu G, Wang L-G, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012;16:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krämer A, Green J, Pollard J, et al. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014;30:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.[dataset] Fligor SC, Tsikis ST, Puder M. Transcriptomic analysis of SEFA-6179 treatment in preterm piglets receiving parenteral nutrition. National Center for Biotechnology Information Gene Expression Omnibus. Available at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?&acc=GSM6945905 [Accessed February 16, 2023]. [Google Scholar]
- 24.Onofrio FQ, Hirschfield GM. The Pathophysiology of Cholestasis and Its Relevance to Clinical Practice. Clinical Liver Disease 2020;15:110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakota K, Wei J, Carns M, et al. Levels of adiponectin, a marker for PPAR-gamma activity, correlate with skin fibrosis in systemic sclerosis: potential utility as biomarker? Arthritis Res Ther 2012;14:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi W-G, Han J, Kim J-H, et al. eIF2α phosphorylation is required to prevent hepatocyte death and liver fibrosis in mice challenged with a high fructose diet. Nutr Metab (Lond) 2017;14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scagliola A, Miluzio A, Ventura G, et al. Targeting of eIF6-driven translation induces a metabolic rewiring that reduces NAFLD and the consequent evolution to hepatocellular carcinoma. Nat Commun 2021;12:4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez LM, Moeser AJ, Blikslager AT. Porcine models of digestive disease: the future of large animal translational research. Transl Res 2015;166:12–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guthrie G, Stoll B, Chacko S, et al. Depletion and enrichment of phytosterols in soybean oil lipid emulsions directly associate with serum markers of cholestasis in preterm parenteral nutrition-fed pigs. JPEN J Parenter Enteral Nutr 2022;46:160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isaac DM, Alzaben AS, Mazurak VC, et al. Mixed Lipid, Fish Oil, and Soybean Oil Parenteral Lipids Impact Cholestasis, Hepatic Phytosterol, and Lipid Composition. J Pediatr Gastroenterol Nutr 2019;68:861–867. [DOI] [PubMed] [Google Scholar]
- 31.Mutanen A, Lohi J, Heikkilä P, et al. Persistent abnormal liver fibrosis after weaning off parenteral nutrition in pediatric intestinal failure. Hepatology 2013;58:729–738. [DOI] [PubMed] [Google Scholar]
- 32.Yu LJ, Anez-Bustillos L, Mitchell PD, et al. Incidence and development of cholestasis in surgical neonates receiving an intravenous mixed-oil lipid emulsion. JPEN J Parenter Enteral Nutr 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole CR, Frem JC, Schmotzer B, et al. The rate of bloodstream infection is high in infants with short bowel syndrome: relationship with small bowel bacterial overgrowth, enteral feeding, and inflammatory and immune responses. J Pediatr 2010;156:941–947.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziegler TR, Luo M, Estívariz CF, et al. Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. Am J Physiol Regul Integr Comp Physiol 2008;294:R402–R410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansouri RM, Baugé E, Staels B, et al. Systemic and distal repercussions of liver-specific peroxisome proliferator-activated receptor-alpha control of the acute-phase response. Endocrinology 2008;149:3215–3223. [DOI] [PubMed] [Google Scholar]
- 36.Barron LK, Bao JW, Aladegbami BG, et al. Toll-like receptor 4 is critical for the development of resection-associated hepatic steatosis. Journal of Pediatric Surgery 2017;52:1014–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutter AG, Palanisamy AP, Lench JH, et al. Dietary Saturated Fat Promotes Development of Hepatic Inflammation Through Toll-Like Receptor 4 in Mice. J Cell Biochem 2016;117:1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gervois P, Kleemann R, Pilon A, et al. Global suppression of IL-6-induced acute phase response gene expression after chronic in vivo treatment with the peroxisome proliferator-activated receptor-alpha activator fenofibrate. J Biol Chem 2004;279:16154–16160. [DOI] [PubMed] [Google Scholar]
- 39.Kleemann R, Gervois PP, Verschuren L, et al. Fibrates down-regulate IL-1-stimulated C-reactive protein gene expression in hepatocytes by reducing nuclear p50-NFkappa B-C/EBP-beta complex formation. Blood 2003;101:545–551. [DOI] [PubMed] [Google Scholar]
- 40.Scirpo R, Fiorotto R, Villani A, et al. Stimulation of nuclear receptor PPAR-γ limits NF-kB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 2015;62:1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao Q, Liu J, Zhang Z, et al. Peroxisome proliferator–activated receptor γ (PPARγ) induces the gene expression of integrin αVβ5 to promote macrophage M2 polarization. Journal of Biological Chemistry 2018;293:16572–16582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guthrie G Parenteral Nutrition Associated Hepatic Steatosis and NAFLD Intersect at AMPK. Cellular and Molecular Gastroenterology and Hepatology 2022;14:724–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. Journal of Hepatology 2015;62:720–733. [DOI] [PubMed] [Google Scholar]
- 44.Liss KHH, Finck BN. PPARs and Nonalcoholic Fatty Liver Disease. Biochimie 2017;136:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kon K, Ikejima K, Hirose M, et al. Pioglitazone prevents early-phase hepatic fibrogenesis caused by carbon tetrachloride. Biochem Biophys Res Commun 2002;291:55–61. [DOI] [PubMed] [Google Scholar]
- 46.Avouac J, Konstantinova I, Guignabert C, et al. Pan-PPAR agonist IVA337 is effective in experimental lung fibrosis and pulmonary hypertension. Annals of the Rheumatic Diseases 2017;76:1931–1940. [DOI] [PubMed] [Google Scholar]
- 47.Németh Á, Mózes MM, Calvier L, et al. The PPARγ agonist pioglitazone prevents TGF-β induced renal fibrosis by repressing EGR-1 and STAT3. BMC Nephrology 2019;20:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyer-Diaz Z, Aristu-Zabalza P, Andrés-Rozas M, et al. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. Journal of Hepatology 2021;74:1188–1199. [DOI] [PubMed] [Google Scholar]
- 49.Ogata T, Miyauchi T, Sakai S, et al. Stimulation of peroxisome-proliferator-activated receptor alpha (PPAR alpha) attenuates cardiac fibrosis and endothelin-1 production in pressure-overloaded rat hearts. Clin Sci (Lond) 2002;103 Suppl 48:284S–288S. [DOI] [PubMed] [Google Scholar]
- 50.Francque SM, Bedossa P, Ratziu V, et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. New England Journal of Medicine 2021;385:1547–1558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


