Key Points
Question
Is early hysterectomy before natural menopause in women associated with an increased risk of cardiovascular disease (CVD)?
Findings
In this Korean nationwide cohort study including 135 575 women, early surgical hysterectomy before age 50 years was independently associated with an increased risk of CVD, especially stroke. Even after excluding women who underwent oophorectomy, the hysterectomy group had higher risks of CVD than the nonhysterectomy group.
Meaning
This study noted an association between early hysterectomy and an increased risk of CVD; because the incidence of CVD was not high, a change in clinical practice may not be needed.
Abstract
Importance
Women who undergo surgical hysterectomy before natural menopause may have an earlier increase in hematocrit and storage iron levels than those who continue menstruation, thereby increasing the risk of cardiovascular disease (CVD) at ages younger than usually seen. Examining this issue may provide important implications for women’s cardiovascular health to both physicians and patients.
Objective
To evaluate the association of hysterectomy with the risk of incident CVD among women before age 50 years.
Design, Setting, and Participants
In this Korean population-based cohort study, 135 575 women aged 40 to 49 years were evaluated from January 1, 2011, to December 31, 2014. After propensity score matching in covariates including age, socioeconomic status, region, Charlson Comorbidity Index, hypertension, diabetes, dyslipidemia, menopause, menopausal hormone therapy, and adnexal surgery before inclusion, 55 539 pairs were included in the hysterectomy and nonhysterectomy groups. Participants were followed up until December 31, 2020. Data analysis was conducted from December 20, 2021, to February 17, 2022.
Main Outcomes and Measures
The primary outcome was an incidental CVD, a composite of myocardial infarction, coronary artery revascularization, and stroke. The individual components of the primary outcome were also evaluated.
Results
A total of 55 539 pairs were included; median age in the combined groups was 45 (IQR, 42-47) years. During median follow-up periods in the hysterectomy group of 7.9 (IQR, 6.8-8.9) years and nonhysterectomy group of 7.9 (IQR, 6.8-8.8) years, the incidence of CVD was 115 per 100 000 person-years for the hysterectomy group and 96 per 100 000 person-years for the nonhysterectomy group. After adjusting for confounding factors, the hysterectomy group had an increased risk of CVD compared with the nonhysterectomy group (hazard ratio [HR], 1.25; 95% CI, 1.09-1.44). The incidences of myocardial infarction and coronary artery revascularization were comparable between the groups, whereas the risk of stroke was significantly higher in the hysterectomy group (HR, 1.31; 95% CI, 1.12-1.53). Even after excluding women who underwent oophorectomy, the hysterectomy group had higher risks of CVD (HR, 1.24; 95% CI, 1.06-1.44).
Conclusions and Relevance
The findings of this cohort study suggest early menopause owing to hysterectomy was associated with increased risks for a composite of CVD, particularly stroke.
This cohort study examines the incidence of cardiovascular disease in Korean women who undergo hysterectomy when younger than 50 years.
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in women and affects up to 36% of all women worldwide.1,2 The risk of CVD rapidly increases after menopause, and early menopause is associated with an increased risk of coronary artery disease and stroke.3,4,5 The increasing incidence of CVD in postmenopausal women may be due to lower female sex hormone levels, which have cardiovascular protective effects.6 However, hemorheologic changes that occur after menopause may also affect the occurrence of CVD. Women’s hematocrit levels increase substantially as women enter into the fifth decade of life, which is the average age of menopause.7 Elevated hematocrit levels increase whole blood viscosity, which can lead to endothelial injury, rupture of plaques by increasing shear stress on the vessel wall, and thrombus formation by red cell aggregation.8,9,10 An increase in hematocrit levels is also associated with an increase in iron and ferritin levels, which are prooxidant cofactors linked to the production of hydroxy radicals and progression of atherosclerosis.11 Women who undergo hysterectomy before natural menopause may have an earlier increase in hematocrit and storage iron levels than those who continue menstruation, thereby increasing the risk of early CVD.12
Examining the use of hysterectomy at a younger age and women’s risk of CVD is important since it is a commonly performed gynecologic procedure with well-documented benefits in relieving symptoms and improving quality of life.13 Thus, this nationwide, population-based cohort study conducted in Korea to evaluate the risk of incident CVD between women with and without hysterectomy before age 50 years after propensity score matching.
Methods
Because the Korean National Health Insurance Service (NHIS) is a universal health coverage system in South Korea and is necessarily linked to all Korean medical facilities, the NHIS database contains medical information (age, sex, diagnosis code, prescription drug information, surgical procedure code, type of medical insurance, hospitalization, and outpatient records) of the entire Korean population (approximately 51 million individuals).14 The Korean Health Insurance Review and Assessment Service (HIRA) is an institution that screens whether medical expenses claimed by medical institutions are clinically valid and shares a significant part of NHIS data. The HIRA data are publicly available and can be requested from the HIRA data site.15 This population-based, retrospective cohort study comprised health insurance data provided by the HIRA from January 1, 2007, to December 31, 2020. The study protocol conformed with the ethical guidelines of the 1975 Declaration of Helsinki16and was approved by the institutional review board of Sanggye Paik Hospital. All personal information was deidentified, and the analysis was possible only using a virtual server within the HIRA. Therefore, the requirement for patient informed consent was waived according to the Bioethics and Safety Act of South Korea. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Selection of Participants
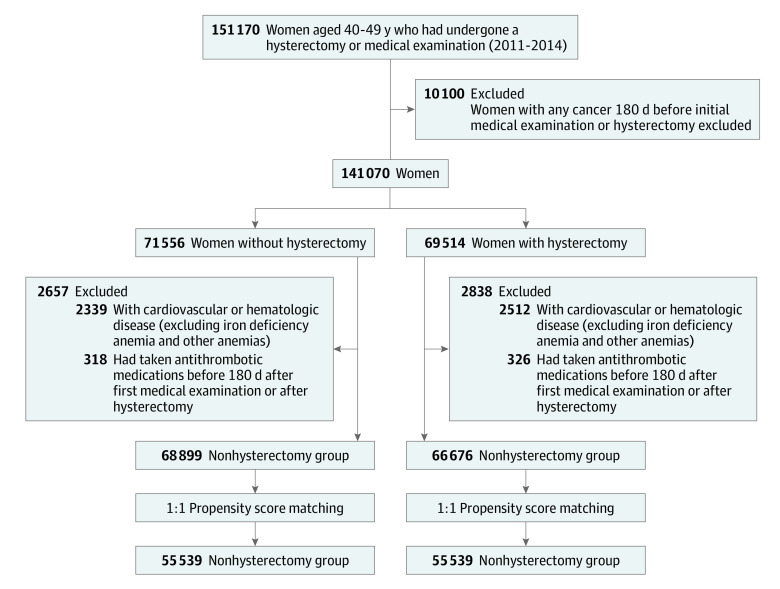
We used diagnostic codes included in the claims that were classified as per the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), the surgical and treatment codes from the Health Insurance Medical Care Expenses (2016 and 2019 version) for selection of participants, extraction of variables, and assessment of outcomes. Participant flow and reasons for exclusion are provided in Figure 1. In this study, for the case and control groups, women aged 40 to 50 years in 2011 to 2014 were screened. The hysterectomy group included patients who underwent hysterectomy owing to uterine myoma or adenomyosis from January 1, 2011, to December 31, 2014. The date of hysterectomy was considered the inclusion day for these patients. The control group included women who visited a medical institute for health check-ups during the period. Among this population, patients with a history of hysterectomy after 2014 were excluded. The first visit date to a medical institute for health examination was considered the inclusion day of the control group. Patients with an ICD-10 code of cancer (any Cxx codes), CVD (codes I21-23, I60-64), and hematologic disease (codes D50-89) except for iron deficiency anemia (code D50), anemia after acute bleeding (code D62), anemia with chronic disease (code D63), and other anemia (code D64) before 180 days of inclusion were excluded from both groups. In the selected hysterectomy and control groups, 1:1 propensity matching was conducted for age at 5-year intervals, year at inclusion, socioeconomic status, region, Charlson Comorbidity Index (CCI) score, hypertension, diabetes, dyslipidemia, menopause before inclusion, menopausal hormone therapy (MHT) before inclusion, and adnexal surgery before inclusion. The accuracy of coding of menopause has not been well validated; however, a previous study reported that it is not overestimated compared with other studies.17 The CCI assigns constant weights from 1 to 6 for 19 diseases defined through medical record investigations, and then corrects the sum of these weights.18 Women were followed up from the inclusion day to death from any cause or to the end of the study (December 31, 2020).
Figure 1. Selection of Participants From the Korean Health Insurance Review and Assessment Service Data.
Age per 5-year interval, year at inclusion, socioeconomic status, region, Charlson Comorbidity Index, hypertension, diabetes, dyslipidemia, menopause before inclusion, menopausal hormone therapy before inclusion, and adnexal surgery before inclusion were used in propensity matching.
Cardiovascular Outcomes
The primary outcome was incidental CVD, which was defined as the first hospitalization or death for myocardial infarction (MI), coronary artery revascularization, or stroke. The individual components of the primary outcome were also evaluated. Myocardial infarction was defined as records of ICD-10 codes I21 to 23 during an inpatient visit. Coronary artery revascularization was defined as a record of receiving percutaneous coronary intervention (transluminal coronary angioplasty, transcatheter placement of intracoronary stent, transluminal coronary atherectomy, transluminal angioplasty, intravascular installation of metallic stent, and thrombus removal) during an inpatient visit. To identify the participants’ incident stroke events, we investigated cerebral bleeding and infarction (ICD-10 code I60–64) after excluding transient ischemic attacks (code G45) or other kinds of thromboembolisms. We only considered hospitalization or deaths with the above-listed diagnoses as primary diagnoses of the hospitalization or the primary causes of death.
Variables
The receipt of medical aid as medical insurance served as a proxy for socioeconomic status. The region was classified as an urban area if the administrative district was metropolitan city level or larger and a rural area if the administrative district was nonmetropolitan. The CCI score was obtained using diagnostic codes from the date of inclusion to the previous 1 year.19 Hypertension (code I10-15), diabetes (code E10-14), dyslipidemia (code E78), and menopause (codes N95, N80.0, M81.0, and E28.3) were defined as visiting medical institutes more than 2 times with the corresponding codes before the study inclusion day. A history of adnexal surgery was defined if any surgery of extirpation of adnexal tumor (unilateral or bilateral salpingoophorectomy, unilateral or bilateral oophorectomy, unilateral or bilateral salpingectomy, and unilateral or bilateral ovarian cystectomy), ovarian wedge resection, incision and drainage of ovarian cyst, or adnexectomy for adhesion was performed more than once. Receiving MHT (tibolone, estradiol valerate, estradiol hemihydrate, dydrogesterone, norethisterone acetate, medroxyprogesterone acetate, drospirenone, or cyproterone) more than 180 days before inclusion was considered as MHT before inclusion. When the first MHT was used after inclusion, it was considered as MHT after inclusion.
Statistical Analysis
Data analysis was conducted from December 20, 2021, to February 17, 2022. Continuous variables are reported as medians (IQRs), and categorical variables are expressed as numbers and frequencies. Continuous variables were analyzed using the Wilcoxon signed rank test, and categorical variables were compared using the Cochran-Mantel-Haenszel test. Standardized difference was used to evaluate matching variables. An absolute standardized difference less than 0.1 for the matching variables indicated an appropriate balance between the groups.20 Stratified Cox proportional hazards regression analysis was performed to analyze the association between hysterectomy and the clinical end points by confounding factors. Time-to-event data according to hysterectomy status were presented using Kaplan-Meier curves. For proportional hazards assumptions, the Schoenfeld residual test was used. There were no missing data regarding the baseline characteristics of the cohort that we can select since HIRA has national data linked to the resident registration number given by the state to all citizens. For the sensitivity test, a stratified Cox regression analysis was performed to compare the risk of CVD between women who underwent only laparoscopic hysterectomy in the hysterectomy group and the nonhysterectomy group. All tests were 2-sided, and the results were considered statistically significant at P < .05. These analyses were conducted using SAS Enterprise Guide, version 6.1 (SAS Institute Inc) and R Statistical software, version 3.5.3 (R Foundation for Statistical Computing).
Results
Baseline Characteristics
Overall, 135 575 women were included in this analysis (66 676 in the hysterectomy group and 68 899 in the nonhysterectomy group) (Figure 1). The baseline characteristics of the study population after propensity score matching are reported in Table 1. After propensity score matching, among the 55 539 matched pairs, the absolute standardized value between the hysterectomy and nonhysterectomy groups was less than 0.1. The median age of the matched hysterectomy and nonhysterectomy groups was 45 (IQR, 42-47) years, and 52 550 women (47.3%) were younger than 45 years. In the cohort, 8.3% of women had menopause before inclusion, and 1.1% of women were receiving MHT. A total of 1.7% of women underwent adnexal surgery before inclusion.
Table 1. Baseline Characteristics of Participants Based on Whether Hysterectomy Was Performed.
| Characteristic | Participants, No. (%) | Standardized difference | ||
|---|---|---|---|---|
| Total (N = 111 078) | Nonhysterectomy (n = 55 539) | Hysterectomy (n = 55 539) | ||
| Age, median (IQR), y | 45 (42-47) | 45 (42-47) | 45 (43-47) | .09 |
| Age at inclusion, y | ||||
| 40-44 | 52 550 (47.3) | 26 615 (47.9) | 25 935 (46.7) | .03 |
| 45-49 | 58 528 (52.7) | 28 924 (52.1) | 29 604 (53.3) | |
| Year at inclusion | ||||
| 2011 | 26 332 (23.7) | 13 559 (24.4) | 12 773 (23.0) | .05 |
| 2012 | 27 397 (24.7) | 13 291 (23.9) | 14 106 (25.4) | |
| 2013 | 28 952 (26.1) | 14 819 (26.7) | 14 133 (25.4) | |
| 2014 | 28 397 (25.6) | 13 870 (25.0) | 14 527 (26.2) | |
| Socioeconomic status | ||||
| Receipt of medical aid as medical insurance | 3420 (3.1) | 1758 (3.2) | 1662 (3.0) | .01 |
| Region | ||||
| Urban area | 62 555 (56.3) | 30 995 (55.8) | 31 560 (56.8) | .02 |
| Rural area | 48 523 (43.7) | 24 544 (44.2) | 23 979 (43.2) | |
| CCI score | ||||
| 0 | 85 071 (76.6) | 42 501 (76.5) | 42 570 (76.6) | .02 |
| 1 | 15 582 (14) | 7960 (14.3) | 7622 (13.7) | |
| ≥2 | 10 425 (9.4) | 5078 (9.1) | 5347 (9.6) | |
| Hypertension | 12 302 (11.1) | 6065 (10.9) | 6237 (11.2) | .01 |
| Diabetes | 7503 (6.8) | 3756 (6.8) | 3747 (6.7) | <.001 |
| Dyslipidemia | 18 490 (16.6) | 9268 (16.7) | 9222 (16.6) | .002 |
| Menopause before inclusion | 9215 (8.3) | 4672 (8.4) | 4543 (8.2) | .008 |
| MHT before inclusion | 1187 (1.1) | 569 (1) | 618 (1.1) | .009 |
| Adnexal surgery before inclusion | 1899 (1.7) | 952 (1.7) | 947 (1.7) | <.001 |
| First MHT after inclusion | 11 146 (10) | 3856 (6.9) | 7290 (13.1) | <.001 |
Abbreviations: CCI, Charlson Comorbidity Index; MHT, menopausal hormone therapy.
Cardiovascular Outcomes
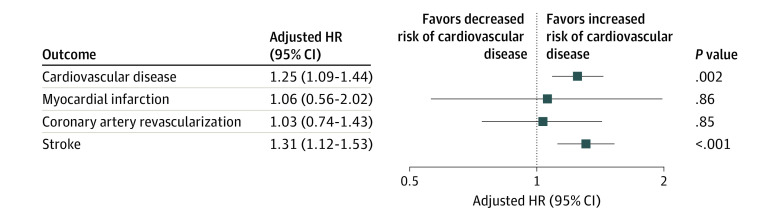
The incidence and risk of cardiovascular outcomes between the groups after propensity score matching are presented in Table 2 and eTable 1 in Supplement 1. During the follow-up period (hysterectomy group: median, 7.9 [IQR, 6.8-8.9] years; nonhysterectomy group: median, 7.9 [IQR, 6.8-8.8] years), the incidence of CVD was 115 per 100 000 person-years for the hysterectomy group and 96 per 100 000 person-years for the nonhysterectomy group (eTable 1 in Supplement 1). The hysterectomy group had an increased risk of CVD compared with the nonhysterectomy group (hazard ratio [HR], 1.25; 95% CI, 1.09-1.44; P = .002) (Table 2 and Figure 2). For individual outcomes, the incidence of MI and coronary revascularization were comparable between the groups, whereas the risk of stroke was significantly higher in the hysterectomy group than in the nonhysterectomy group (HR, 1.31; 95% CI, 1.12-1.53; P < .001).
Table 2. Incidence and Risk of Cardiovascular Outcomes in the Hysterectomy and Nonhysterectomy Groups.
| Cardiovascular outcome | No. (%) of events | Unadjusted | Adjusteda | |||||
|---|---|---|---|---|---|---|---|---|
| Nonhysterectomy (n = 55 539) | Hysterectomy (n = 55 539) | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Cardiovascular disease | 422 (0.8) | 507 (0.9) | 1.17 (1.03-1.34) | .02 | 1.25 (1.09-1.44) | .002 | ||
| Myocardial infarction | 26 (0) | 25 (0) | 0.96 (0.54-1.70) | .88 | 1.06 (0.56-2.02) | .86 | ||
| Coronary artery revascularization | 110 (0.2) | 112 (0.2) | 0.97 (0.74-1.27) | .84 | 1.03 (0.74-1.43) | .85 | ||
| Stroke | 322 (0.6) | 406 (0.7) | 1.24 (1.07-1.44) | .004 | 1.31 (1.12-1.53) | <.001 | ||
Abbreviation: HR, hazard ratio.
Adjusted for age, socioeconomic status, region, Charlson Comorbidity Index, hypertension, diabetes, dyslipidemia, menopause before inclusion, menopausal hormone therapy before inclusion, adnexal surgery before inclusion, and menopausal hormone therapy after inclusion.
Figure 2. Hazard Ratios (HRs) for Risk of Adverse Cardiovascular Events.
Adjustments made for age, socioeconomic status, region, Charlson Comorbidity Index, hypertension, diabetes, dyslipidemia, menopause before inclusion, menopausal hormone therapy before inclusion, adnexal surgery before inclusion, and menopausal hormone therapy after inclusion.
Sensitivity Analysis
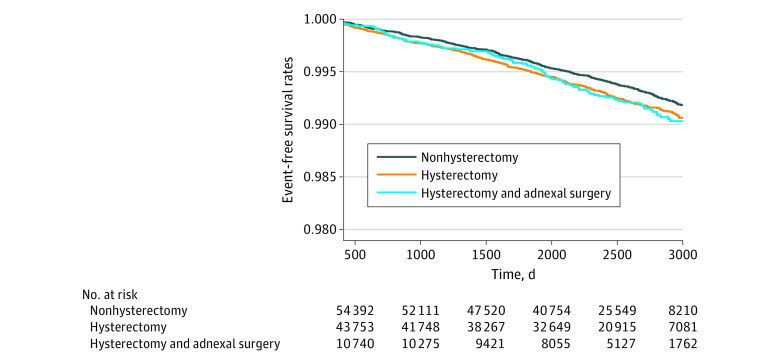
To minimize the possible effect of female sex hormones, subgroup analysis was performed based on whether participants underwent adnexal surgery simultaneously with hysterectomy. Regardless of adnexal surgery, the results tended to be similar. Women who underwent hysterectomy without adnexal surgery had a higher risk of CVD than those who did not undergo hysterectomy (HR, 1.24; 95% CI, 1.06-1.44; P = .008) (eTable 2 in Supplement 1). This result was noted mainly with stroke (HR, 1.28; 95% CI, 1.08-1.52; P = .005). Kaplan-Meier curves showed that the CVD events gradually started to differ between hysterectomy and nonhysterectomy groups over time (Figure 3). Kaplan-Meier curves regarding MI or stroke are shown in the eFigure in Supplement 1. In addition, the outcomes of laparoscopic hysterectomy were evaluated. The characteristics of the women who underwent laparoscopic hysterectomy are presented in eTable 3 in Supplement 1. The risk of stroke in the laparoscopic hysterectomy group was significantly higher than that in the nonhysterectomy group (HR, 1.32; 95% CI 1.01-1.72; P = .04) (eTable 4 in Supplement 1). However, the difference of MI or coronary revascularization was not detected (eTable 4 in Supplement 1).
Figure 3. Cardiovascular Disease Events Over Time.
Discussion
The present analysis from a nationwide, population-based, cohort study noted that women who underwent hysterectomy when younger than 50 years had an increased risk of incidental CVD, noted mainly with stroke, compared with those without hysterectomy. This risk increase was consistent even after adjusting for confounders and after excluding patients who underwent adnexal surgery simultaneously with hysterectomy.
Previous studies have provided evidence of the association between hysterectomy and CVD; however, the results are contradictory.21,22,23,24,25 Although a Swedish population study reported the association of hysterectomy in women younger than 50 years with an elevated risk of CVD, the result was limited because traditional cardiovascular risk factors were not adjusted in the Cox proportional hazards regression model.21 Another nested cohort study noted that hysterectomy did not increase the risk of death from CVD; however, that study reported only mortality as the end point and did not specify the status of oophorectomy in women with hysterectomy.22 Population-based studies in the Olmsted County, Minnesota, region23 and Australia24 noted that hysterectomy without oophorectomy performed in women younger than 35 years was associated with an increased risk of coronary heart disease23 and cardiovascular mortality.24 In the Olmsted County cohort study, the risk of stroke was not significantly different between the hysterectomy and nonhysterectomy groups.23 However, there was no consideration of the use of MHT that could affect cardiovascular outcomes in that study. On the contrary, a nationwide, population-based study from Taiwan reported that women who underwent hysterectomy with ovarian conservation when younger than 45 years had an increased risk of stroke, while there was no significant difference of CVD risk after age 45 years.25 That result was analyzed after excluding women who had undergone menopause or those receiving MHT. As with the above study, we noted that, in women younger than 50 years, hysterectomy was independently associated with an increased risk of CVD, especially stroke. Our study adjusted for the use of MHT before and after inclusion. The result was consistent even after excluding patients who received oophorectomy. These findings suggest that the uterus may have a cardiovascular protective effect in women, independent of female sex hormones.
The pathobiologic mechanism by which hysterectomy increases the risk of CVD in women before the age of menopause remains uncertain. It has been previously proposed that one of the possible mechanisms is disruption of ovarian blood flow from ovarian ligaments during hysterectomy, which may result in premature ovarian failure.21 Decreased ovarian blood flow and low ovarian sex steroid levels have been noted after hysterectomy.26,27,28 However, there is controversy about whether ovarian function changes after hysterectomy; some studies have shown unchanged ovarian function after hysterectomy and salpingectomy.29,30
Another possible mechanism is that the loss of menstruation after hysterectomy may result in a hemorheologic deleterious effect. After menopause, an increase in hematocrit levels occurs. Elevated hematocrit levels are associated with increased blood viscosity, leading to endothelial injury, rupture of plaques by increasing shear stress on the vessel wall, and thrombus formation by red blood cell aggregation,8,9,10 thereby increasing the risk of adverse cardiovascular events. Several epidemiologic studies reported that a high hematocrit level might be related to greater risks for MI, stroke, and cardiovascular mortality in the general population.31,32,33 In addition, an increased hematocrit level can lead to excessive iron and ferritin levels, which is associated with increased hydroxyl radical production and progression of atherosclerosis.11 Furthermore, frequent phlebotomy via voluntary blood donation in healthy men can reduce their heme iron and ferritin levels by 44% and lead to reduction in CVD risk.34,35,36 Before menopause, women have substantially lower hematocrit, iron, and ferritin levels than men. Serum ferritin levels increase in postmenopausal women and this upward curve almost coincides with the curve of increasing CVD incidence after menopause.37,38 These observational studies suggest that early hysterectomy may cause hematocrit and ferritin level increases and thereby increase the risk of early CVD.
Using data from the HIRA, we noted that women who underwent hysterectomy before the average age of menopause had a higher risk of CVD compared with women with an intact uterus. Since the data regarding hematocrit or serum ferritin levels in this cohort population were limited, it could not be verified whether hemorheologic changes after hysterectomy affect the CVD risk. Further studies are needed to clarify the association between hysterectomy and hematocrit, iron, and ferritin levels and its possible effect on cardiovascular outcomes. Furthermore, it is difficult to explain why the risk of stroke appears to be significantly increased, while the risk of coronary artery disease and MI are not significantly different. Further mechanistic studies are required to clarify the noted association.
Limitations
This study has several limitations. First, this was a retrospective, observational study. Second, since the study population comprised a homogeneous group of women of Korean ethnicity, the findings may not be generalizable. Third, studies using administrative databases are prone to errors caused by inaccurate coding.39 To minimize this problem, we applied definitions that were already validated in previous studies using the Korean NHIS database.40 Fourth, the inability to determine the severity of the underlying disease or treatment status that can affect the primary outcome may bias the study results. In addition, there was no consideration for time-varying covariates, such as MHT duration or treatment for CVD risk factors after inclusion, which could act as a residual confounder. Fifth, although propensity score matching was performed by categorizing the age groups as 40 to 44 and 45 to 49 years to reduce the confounding variable according to the wide age range, stratification of age groups into tighter bands would have reduced the age-dependent confounding effect. Sixth, some factors, such as body mass index and family history, could not be corrected since the corresponding data were limited. Seventh, the association between increased blood viscosity or ferritin levels with early hysterectomy and their outcomes could not be evaluated because data regarding hematocrit or ferritin levels were omitted. Eighth, because data on female sex hormone levels were absent, it was difficult to note whether hysterectomy is associated with the risk of CVD independently of female sex hormones. However, the subgroup analysis that excluded women who underwent salpingo-oophorectomy showed results that are consistent with those of our main analysis. Further research is needed on the mechanisms for the effects of the uterus on women’s cardiovascular health.
Conclusions
In this cohort study of Korean women, we noted that hysterectomy in women younger than 50 years was independently associated with an increased risk of stroke. A difference of the risk of MI or coronary artery revascularization was not detected. Although we found that widely performed hysterectomy with a broad indication for benign diseases at premenopausal ages slightly increases the risk of CVD, the incidence is not high, so a change in clinical practice may not be needed.
eTable 1. Case/Person-Years of Cardiovascular Disease According to Hysterectomy
eTable 2. Subgroup Analysis for the Risk of Cardiovascular Outcomes According to Whether Adnexal Surgery Was Performed Simultaneously With Hysterectomy
eTable 3. Characteristics of Participants According to Whether or not Hysterectomy Was Performed (Non-Hysterectomy Versus Laparoscopic Hysterectomy) (Sensitivity Test)
eTable 4. Hazard Ratios for Risk of Cardiovascular Disease (Laparoscopic Hysterectomy vs Non-Hysterectomy)
eFigure. Kaplan-Meier Curves of Individual Outcomes
Data Sharing Statement
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. ; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38-e360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603. doi: 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roeters van Lennep JE, Heida KY, Bots ML, Hoek A; collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders . Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23(2):178-186. doi: 10.1177/2047487314556004 [DOI] [PubMed] [Google Scholar]
- 4.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2012;19(10):1081-1087. doi: 10.1097/gme.0b013e3182517bd0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley SH, Li Y, Tobias DK, et al. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc. 2017;6(11):e006713. doi: 10.1161/JAHA.117.006713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Khoudary SR, Thurston RC. Cardiovascular implications of the menopause transition: endogenous sex hormones and vasomotor symptoms. Obstet Gynecol Clin North Am. 2018;45(4):641-661. doi: 10.1016/j.ogc.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 7.Cruickshank JM. Some variations in the normal haemoglobin concentration. Br J Haematol. 1970;18(5):523-529. doi: 10.1111/j.1365-2141.1970.tb00773.x [DOI] [PubMed] [Google Scholar]
- 8.Eshtehardi P, Brown AJ, Bhargava A, et al. High wall shear stress and high-risk plaque: an emerging concept. Int J Cardiovasc Imaging. 2017;33(7):1089-1099. doi: 10.1007/s10554-016-1055-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BG, Kim H, Hong SJ, et al. Relation of preprocedural hemoglobin level to outcomes after percutaneous coronary intervention. Am J Cardiol. 2019;124(9):1319-1326. doi: 10.1016/j.amjcard.2019.07.056 [DOI] [PubMed] [Google Scholar]
- 10.Cho SW, Kim BG, Kim BO, et al. Hemorheological and glycemic parameters and HDL cholesterol for the prediction of cardiovascular events. Arq Bras Cardiol. 2016;106(1):56-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araujo JA, Romano EL, Brito BE, et al. Iron overload augments the development of atherosclerotic lesions in rabbits. Arterioscler Thromb Vasc Biol. 1995;15(8):1172-1180. doi: 10.1161/01.ATV.15.8.1172 [DOI] [PubMed] [Google Scholar]
- 12.Peuranpää P, Heliövaara-Peippo S, Fraser I, Paavonen J, Hurskainen R. Effects of anemia and iron deficiency on quality of life in women with heavy menstrual bleeding. Acta Obstet Gynecol Scand. 2014;93(7):654-660. doi: 10.1111/aogs.12394 [DOI] [PubMed] [Google Scholar]
- 13.Lycke KD, Kahlert J, Damgaard R, Mogensen O, Hammer A. Trends in hysterectomy incidence rates during 2000-2015 in Denmark: shifting from abdominal to minimally invasive surgical procedures. Clin Epidemiol. 2021;13:407-416. doi: 10.2147/CLEP.S300394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim L, Kim JA, Kim S. A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiol Health. 2014;36:e2014008. doi: 10.4178/epih/e2014008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Healthcare Bigdata Hub . Accessed January 26, 2022. http://opendata.hira.or.kr
- 16.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.Yuk JS, Kim M. Incidence and prevalence of primary ovarian insufficiency in South Korea: a population-based study. Arch Gynecol Obstet. 2021;304(3):823-831. doi: 10.1007/s00404-021-05962-7 [DOI] [PubMed] [Google Scholar]
- 18.D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med. 1993;32(5):382-387. doi: 10.1055/s-0038-1634956 [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676-682. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 20.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387-398. doi: 10.1016/S0895-4356(00)00321-8 [DOI] [PubMed] [Google Scholar]
- 21.Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745-750. doi: 10.1093/eurheartj/ehq477 [DOI] [PubMed] [Google Scholar]
- 22.Iversen L, Hannaford PC, Elliott AM, Lee AJ. Long term effects of hysterectomy on mortality: nested cohort study. BMJ. 2005;330(7506):1482. doi: 10.1136/bmj.38483.669178.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laughlin-Tommaso SK, Khan Z, Weaver AL, Smith CY, Rocca WA, Stewart EA. Cardiovascular and metabolic morbidity after hysterectomy with ovarian conservation: a cohort study. Menopause. 2018;25(5):483-492. doi: 10.1097/GME.0000000000001043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuesley KM, Protani MM, Webb PM, et al. Hysterectomy with and without oophorectomy and all-cause and cause-specific mortality. Am J Obstet Gynecol. 2020;223(5):723.e1-723.e16. doi: 10.1016/j.ajog.2020.04.037 [DOI] [PubMed] [Google Scholar]
- 25.Yeh JS, Cheng HM, Hsu PF, et al. Hysterectomy in young women associates with higher risk of stroke: a nationwide cohort study. Int J Cardiol. 2013;168(3):2616-2621. doi: 10.1016/j.ijcard.2013.03.042 [DOI] [PubMed] [Google Scholar]
- 26.Xiangying H, Lili H, Yifu S. The effect of hysterectomy on ovarian blood supply and endocrine function. Climacteric. 2006;9(4):283-289. doi: 10.1080/13697130600865774 [DOI] [PubMed] [Google Scholar]
- 27.Hehenkamp WJ, Volkers NA, Broekmans FJ, et al. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod. 2007;22(7):1996-2005. doi: 10.1093/humrep/dem105 [DOI] [PubMed] [Google Scholar]
- 28.Yuan Z, Cao D, Bi X, Yu M, Yang J, Shen K. The effects of hysterectomy with bilateral salpingectomy on ovarian reserve. Int J Gynaecol Obstet. 2019;145(2):233-238. doi: 10.1002/ijgo.12798 [DOI] [PubMed] [Google Scholar]
- 29.Findley AD, Siedhoff MT, Hobbs KA, et al. Short-term effects of salpingectomy during laparoscopic hysterectomy on ovarian reserve: a pilot randomized controlled trial. Fertil Steril. 2013;100(6):1704-1708. doi: 10.1016/j.fertnstert.2013.07.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venturella R, Lico D, Borelli M, et al. 3 To 5 years later: long-term effects of prophylactic bilateral salpingectomy on ovarian function. J Minim Invasive Gynecol. 2017;24(1):145-150. doi: 10.1016/j.jmig.2016.08.833 [DOI] [PubMed] [Google Scholar]
- 31.Toss F, Nordström A, Nordström P. Association between hematocrit in late adolescence and subsequent myocardial infarction in Swedish men. Int J Cardiol. 2013;168(4):3588-3593. doi: 10.1016/j.ijcard.2013.05.065 [DOI] [PubMed] [Google Scholar]
- 32.Gotoh S, Hata J, Ninomiya T, et al. Hematocrit and the risk of cardiovascular disease in a Japanese community: the Hisayama Study. Atherosclerosis. 2015;242(1):199-204. doi: 10.1016/j.atherosclerosis.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 33.Kunnas T, Solakivi T, Huuskonen K, Kalela A, Renko J, Nikkari ST. Hematocrit and the risk of coronary heart disease mortality in the TAMRISK study, a 28-year follow-up. Prev Med. 2009;49(1):45-47. doi: 10.1016/j.ypmed.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 34.Tuomainen TP, Salonen R, Nyyssönen K, Salonen JT. Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland. BMJ. 1997;314(7083):793-794. doi: 10.1136/bmj.314.7083.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyers DG, Strickland D, Maloley PA, Seburg JK, Wilson JE, McManus BF. Possible association of a reduction in cardiovascular events with blood donation. Heart. 1997;78(2):188-193. doi: 10.1136/hrt.78.2.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy K V, Shastry S, Raturi M, Baliga B P. Impact of regular whole-blood donation on body iron stores. Transfus Med Hemother. 2020;47(1):75-79. doi: 10.1159/000499768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook JD, Finch CA, Smith NJ. Evaluation of the iron status of a population. Blood. 1976;48(3):449-455. doi: 10.1182/blood.V48.3.449.449 [DOI] [PubMed] [Google Scholar]
- 38.Kim C, Nan B, Kong S, Harlow S. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21(8):872-877. doi: 10.1089/jwh.2012.3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burns EM, Rigby E, Mamidanna R, et al. Systematic review of discharge coding accuracy. J Public Health (Oxf). 2012;34(1):138-148. doi: 10.1093/pubmed/fdr054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service–National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46(2):e15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Case/Person-Years of Cardiovascular Disease According to Hysterectomy
eTable 2. Subgroup Analysis for the Risk of Cardiovascular Outcomes According to Whether Adnexal Surgery Was Performed Simultaneously With Hysterectomy
eTable 3. Characteristics of Participants According to Whether or not Hysterectomy Was Performed (Non-Hysterectomy Versus Laparoscopic Hysterectomy) (Sensitivity Test)
eTable 4. Hazard Ratios for Risk of Cardiovascular Disease (Laparoscopic Hysterectomy vs Non-Hysterectomy)
eFigure. Kaplan-Meier Curves of Individual Outcomes
Data Sharing Statement