Abstract
Outcome selection to evaluate interventions to support a successful transition from hospital to home of children with medical complexity (CMC) may be difficult due to the variety in available outcomes. To support researchers in outcome selection, this systematic review aimed to summarize and categorize outcomes currently reported in publications evaluating the effectiveness of hospital-to-home transitional care interventions for CMC. We searched the following databases: Medline, Embase, Cochrane library, CINAHL, PsychInfo, and Web of Science for studies published between 1 January 2010 and 15 March 2023. Two reviewers independently screened the articles and extracted the data with a focus on the outcomes. Our research group extensively discussed the outcome list to identify those with similar definitions, wording or meaning. Consensus meetings were organized to discuss disagreements, and to summarize and categorize the data. We identified 50 studies that reported in total 172 outcomes. Consensus was reached on 25 unique outcomes that were assigned to six outcome domains: mortality and survival, physical health, life impact (the impact on functioning, quality of life, delivery of care and personal circumstances), resource use, adverse events, and others. Most frequently studied outcomes reflected life impact and resource use. Apart from the heterogeneity in outcomes, we also found heterogeneity in designs, data sources, and measurement tools used to evaluate the outcomes.
Conclusion: This systematic review provides a categorized overview of outcomes that may be used to evaluate interventions to improve hospital-to-home transition for CMC. The results can be used in the development of a core outcome set transitional care for CMC.
|
What is Known: • Studies on the effectiveness of interventions to support the hospital-to-home transition of CMC are numerous. •Heterogeneity in outcomes hamper comparisons across studies and therewith the ability to move research forward. | |
|
What is New: •This systematic review summarizes and categorizes outcomes reported in publications that evaluated interventions to improve the hospital-to-home transition for CMC. •In total 172 reported outcomes were summarized to 25 unique outcomes that were assigned to six outcome domains. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-023-05050-9.
Keywords: Transitional care - children with medical complexity, Core outcome set, Systematic review, Reported outcomes
Introduction
Hospital-to-home care for children with medical complexity (CMC) and their families is an expanding research area. This is motivated by the substantial increase in numbers of CMC, resulting from medical developments and consequently in the improved life expectancy of children with previously considered untreatable diseases [1, 2]. CMC are defined as children with concurrent chronic conditions, family-identified service needs, functional limitations and high healthcare use [3]. CMC consists of a diverse group of children (e.g. children with severe cerebral palsy or metabolic diseases), and are characterized by frequent emergency department visits, and lengthy and complicated (re)hospitalizations that create pressure on the healthcare system [4, 5]. However, the length of hospital stay of CMC is decreasing [6], and the complex care, such as tracheostomies, enteral feeding tubes, intravenous infusions, dialysis, and complex medication regimens is often provided by families at home [7]. Despite the benefits of being home, caring for CMC is challenging for families, and experiences of parents reveal emotional, social and financial hardships [8–10].
The transition from hospital to home of CMC should only take place when parents feel ready, and when the continuity of care is optimally organized. However, this is not always successful [11], and parents do not always feel supported and adequately prepared [12, 13]. Publications on the effectiveness of interventions to support a successful transition from hospital to home of CMC are numerous, with coordination of care, collaboration between families and the multidisciplinary team, and communication as key elements [14, 15]. However, the heterogeneity in outcomes hamper comparisons across trials and therewith the ability to move research forward in this field [16–18]. An overview of available outcomes may support researchers and program evaluators in outcome selection. The aim of this systematic review was to summarize and categorize outcomes currently reported in publications evaluating the effectiveness of hospital-to-home transitional care interventions for CMC.
Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews [19], and the recommendations for a Core Outcome Set development and reporting [20, 21].
We considered this literature review as the first step in developing a Core Outcome Set (COS), an agreed method to overcome problems of variability in outcome selection, measurement and synthesis [22]. The full protocol, describing this systematic review and the next steps (a Delphi study and focus groups), has been registered in the Core Outcome Measures in Effectiveness Trials (COMET) Initiative database [22].
Study eligibility
Search strategy
We searched Medline, EMBASE, Cochrane library, CINAHL, PsychInfo and Web of Science with help of a clinical librarian. The search was limited to articles published in English between 1 January 2010 and 15 March 2023. The search was executed on 15 November 2020, and updated on 18 March 2023. This period was considered adequate for the aim of our study as the reported outcomes may change over time depending on e.g. societal perspective on health and healthcare, and population characteristics. The key terms referring to the patient group included “children with medical complexity” or related terms, such as “children with complex chronic conditions”. The key terms referring to the intervention included “transitional care”, “follow-up” and “discharge”. Comparison interventions and outcomes were not specified in the search. The detailed search strategies are presented in Appendix A. Duplicates were removed electronically; the selection was carried out using the web application Rayyan (http://rayyan.qcri.org).
Inclusion criteria
We included comparative studies evaluating the effectiveness of an intervention to improve the hospital-to-home transition for CMC, e.g. randomized controlled trials, controlled clinical trials, cohort studies, and before-after designs. Studies had to report on quantitative outcomes to be eligible for inclusion. We focused on studies with participants described as children with medical complexity. Studies based in all inpatient settings that provided transitional care were included, as well as all types of interventions or types of care that aimed to improve the hospital-to-home transitional care. We only included studies if full text publications were available.
Exclusion criteria
Publications were excluded if it concerned qualitative research, reviews, guidelines, case studies, editorials and abstracts.
Study selection
Two reviewers independently screened all titles and abstracts to identify potentially relevant studies based on the inclusion criteria. If they could not assess a publication for relevance based on title and abstract, full text was obtained. Subsequently, the two reviewers independently studied the full texts for final inclusion. We used snowball sampling by hand-searched the reference lists of all included articles and relevant (systematic) reviews to identify additional publications. After each step, the reviewers discussed their findings to reach consensus. When disagreement needed to be solved, a third reviewer was consulted.
Data extraction
Data were extracted by one reviewer and verified by a second reviewer. A self-designed extraction form was used. This form was pilot tested by two reviewers by comparing the data extraction results of the first 10 included studies. The following data were systematically collected: first author, publication year, country or origin, design of the study, setting, sample size, medical complexity of the child, age of the child, hospital-to-home intervention studied, outcomes, outcome measures, sources of the outcomes, and the time-frame of the outcome measures. Any outcomes that were described in the studies were included, and no distinction was made between primary and secondary outcomes.
Analysis
A narrative synthesis was undertaken to summarize the outcomes. We anticipated that outcomes would vary in terminology and in measurement tools used. We merged the outcomes with similar definitions and/or concepts. Therefore, two researchers independently reviewed the list of outcomes as reported in the studies to identify similarities. Their findings were discussed in consensus meetings with the multidisciplinary hospital-to-home research group that included pediatricians, a pediatric intensivist, a pediatric rehabilitation specialist, (pediatric) nurses, and a clinical epidemiologist. Two researchers checked the merged outcomes by re-reading the publications.
After comparing several frameworks to classify the outcomes, we decided to use the taxonomy of Dodd et al. [23]. Based on this taxonomy, we categorized the outcomes into the following domains: (a) mortality and survival, (b) physical health, (c) life impact, (d) resource use, and (e) adverse events [23]. We created a domain (f) others to report on those outcomes that would not fit in well, but we considered important to include in this review. The domain mortality and survival includes all-cause mortality and cause-specific mortality. Physical health refers to measures of physiological function, signs and symptoms related to a body system, or general physiological outcomes, such as weight, fatigue and pain. Life impact refers to the impact of a disease or condition on functioning (e.g. social, emotional, and cognitive), quality of life, delivery of care (e.g. compliance), and personal circumstances (e.g. finances, work). Resource use includes outcomes related to healthcare utilization and costs. The domain adverse events includes any unintended consequence of the intervention. Two reviewers categorized the outcomes into one of the six domains. A third reviewer resolved uncertainties.
Results
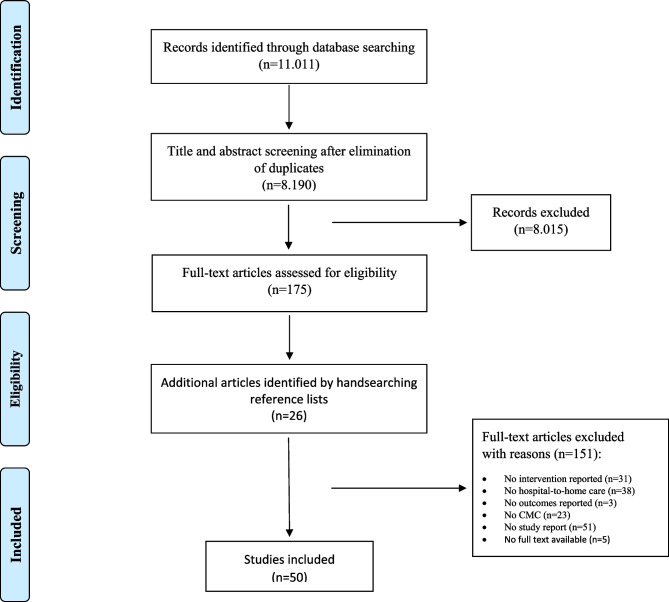
The database searches resulted in 11.011 records and after elimination of duplicates 8.190 records remained. A total of 8.015 papers were excluded based on title and abstract. After reading the remaining 175 publications full texts, 48 studies were deemed eligible according the pre-defined inclusion and exclusion criteria. Two additional publications were identified from hand searching the reference lists, resulting in a total of 50 studies included in this systematic review [24–73]. Figure 1 shows the selection process.
Fig. 1.
Study selection process
Study characteristics
Of the 50 studies, 43 (86%) were performed in pediatric hospitals in the United States of America [24–28, 32–59, 61, 62, 64–70, 72], three studies in Canada (6%) [30, 31, 60], two in Italy (4%) [63, 73], one in Australia (2%) [29], and one in Turkey (2%) [71]. Fourty one studies (82%) were published between 2016 and 2021 [24–29, 32–38, 40, 42–47, 49–55, 59, 61–63, 65–70, 72, 73,57—]. In total, 29 studies (58%) characterized their study population as CMC, labeled with criteria, but no specific diagnosis [24, 25, 27–33, 35, 36, 38–42, 44, 46, 47, 49–52, 56, 59, 61, 63, 68, 73]. Other study populations were based on specific diagnoses: (preterm) neonates (six studies, 12%) [43, 48, 54, 66, 67, 70], neurological conditions or epilepsy (three studies, 6%) [34, 58, 60], and childhood cancer (one study, 2%) [71]. Some publications defined their population based on technology assistance: mechanical ventilation (two studies, 4%) [37, 53], tracheostomy (five studies, 10%) [45, 55, 57, 65, 72], or both (four studies, 8%) [26, 62, 64, 69].
The following designs were described in the publications: cohort studies (22 studies, 44%) [25, 27–29, 33, 35–37, 40, 41, 43, 53–56, 58, 59, 63, 66, 67, 70, 73], quality improvement projects with a pre-post design (13 studies, 26%) [24, 26, 38, 42, 45, 50, 51, 61, 62, 64, 65, 68, 72], randomized controlled trials (six studies, 12%) [32, 34, 46, 47, 49, 60], quasi-experimental studies (five studies, 10%) [39, 44, 48, 52, 71], mixed method studies (two studies, 4%) [30, 31], or other (two studies, 4%) [57, 69].
The hospital-to-home interventions studied were mainly multi-faceted including a wide variety of activities, e.g. care coordination, parental education programs, home visits, and telehealth applications. The study characteristics are summarized in Table 1.
Table 1.
Characteristics of included studies
| Author (year) | Country | Study type | Setting | Sample size | Medical condition | Age of children | Intervention | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Antolick et al. (2020) [24] | USA | Quality improvement project with a pre-post design | Children's hospital | 40 | High risk children (criteria described) | Median 12 years | Post Hospitalization Action Grid (PHAG) and a standardized discharge handoff process. |
- Provider and staff perceptions of integrated care - Provider and staff intentions to use the PHAG |
| Appachi et al. (2017) [25] | USA | Retrospective medical record review with a pre-post design | Tertiary Care Center for children with medical complexity | 113 | Children with complex medical needs, specifically with aerodigestive disorders (definition described) | Range 0 to 20 years | A multidisciplinary aerodigestive clinic providing a comprehensive and coordinated care program (inpatient and outpatient). |
- Number of hospital admissions - Number of hospital days for all admissions per year - Number of admissions in the aerodigestive clinic - Costs |
| Baker et al. (2016) [26] | USA | Quality improvement project with a pre-post design | Tertiary care children's hospital | 48 | Children requiring chronic mechanical ventilation via tracheostomy | Neonates | An interdisciplinairy Ventilator Care Program including: education materials, a chronic ventilation road map and instructional videos for caregivers, assessment of discharge readiness, the involvement of an advanced practice nurse and rehabilitation respiratory therapist, and case management. |
- Mortality - Number of unplanned hospital readmissions - Hospital length of stay - Number of Emergency Department visits - Costs |
| Barreda et al. (2021) [27] | USA | Propensity-matched retrospective cohort design | Children’s Hospital | 566 | Children with medical complexity (criteria described) | Median 5 years (IQR 4-6) | Pediatric Complex Care Program including: comprehensive care planning and care coordination, support by the complex care team for urgent visits, monthly phone calls. |
- Number of hospitalisation per year, related to device complications - Number of Emergency Department visits per year, related to device complications |
| Braun et al. (2021) [28] | USA | Retrospective matched cohort design | Children’s Hospital | 64 | Children with medical complexity (criteria described) | Mean 5.6 years (SD 7.2) and 5.5 (SD 7.7) | Family Integrated Healthcare Transitions (FLIGHT) team providing care coordination and complex care by a predominantly telemedicine-mediated format. |
- Number of hospital admissions per year - Hospital days per year and per admission - Number of subspeciality appointments per year - Missed appointments per year |
| Breen et al. (2018) [29] | Australia | Pre-post implementation cohort evaluation | Children’s Hospital Network (two tertiary pediatric hospitals) | 534 | Children with medical complexity (criteria described) | Median 5 years (range 7-19) | Care coordination service: access to a care coordinator, shared-care plan, linkage with local general practitioners, and acces to a 24-hours hotline. |
- School absences child - Prevented family travel costs - Number of hospital encounters and admissions (overnight and day-only) - Number of Emergency Department visits - Costs |
| Cohen et al. (2010) [30] | Canada | Mixed method descriptive study with a pre-post design | Tertiary care hospital | 28 | Children with medical complexity (criteria described) | Median 1.5 years (range 30 days to 14 years) | Nurse practitioner/pediatrician-run complex care clinic in a tertiary care hospital. |
- Quality of life of parents - Parental perceptions of care - Healthcare utilization (inpatient days, Emergency Department visits, visits to hospital and community based practitioners) - Healthcare providers perceptions of care |
| Cohen et al. (2012) [31] | Canada | Mixed method, intervention study with a pre-post design | A tertiary care children's hospital and two community hospitals | 81 | Children with medical complexity (criteria described) | Mean 5.8 years (SD 4.7) | Community-based complex care clinics integrated with a tertiary care center. |
- Quality of life child - Quality of life of parents - Parental perception of family centered care - Health care utilization (inpatient days, Emergency Department visits, visits to hospital and community based practitioners) - Costs (healthcare system costs and parents reported out of pocket expenses) |
| Coller et al. (2018) [32] | USA | Randomized Controlled Trial | Tertiary medical center | 147 | Children with medical complexity | < 18 years | Plans for Action and Care Transition (PACT): written action plans and care transition coaching during the period of hospital discharge. |
- Mortality - Number of readmissions - Number of hospitalizations - Costs |
| Donnelly et al. (2020) [33] | USA | Retrospective cohort study with a pre-post design | Tertiary children's hospital | 31 | Children with medical complexity (criteria described) | Mean 5.2 years (SD 3.5) | The Advanced Practice Nurse and Care Coordination Assistent model medical care coordination program. |
- Quality of life child - Self-efficacy regarding managing child's healthcare needs - Quality of Life of parents - Satisfaction of parents with healthcare |
| Duffy and Vessey (2016) [34] | USA | Randomized Controlled Trial | Pediatric teaching hospital | 46 | Children with chronic neurological conditions | 2 to 6 years | Creating Opportunities for Parent Empowerment (COPE) program: an intervention that teaches parents what behaviors they can expect in their child as normal response to illness, and how to help their child to cope with illness experiences. |
- Number of behavior problems of the child - Parental belief in their caregiving skills - Anxiety of parents - Depression of parents |
| Gay et al. (2016) [35] | USA | Retrospective, matched cohort study | Home Health Care Nursing Care services across 19 states | 10144 | Children with medical complexity | 0 to 18 years | Home Health service: intermittent skilled nursing visits or private duty nursing. |
- Number of readmissions - Number of hospitalizations - Number of hospital days - Costs |
| Gillen and Morris (2019) [36] | USA | Cohort study with a pre-post design | Urban PICU | 50 | Children with medical complexity | < 18 years | Information and materials to improve the ability of caregivers to care for their children in case of a prolonged home power failure. | - Families' disaster preparedness |
| Graham et al. (2018) [37] | USA | Prospective cohort study | Tertiary care center | 346 | Children with chronic respiratory failure | Median 6 years (IQR 1 to 16) | Critical Care, Anesthesia, Perioperative Extension Program (CAPE): an individual tailored and comprehensive longitudinal service and home ventilator program. |
- Number of hospitalizations - Number of Emergency Department visits - Costs |
| Hogan et al. (2022) [38] | USA | Pre-post design | Pediatric health system with inpatient and outpatient services | 105 | Children with medical complexity (criteria described) | 90% of the children were < 10 years | The Compass Care Program, a consultative complex care program across inpatient and outpatient settings. |
- Caregiver satisfaction (communication and access to care) - Number of hospital (re)admissions - Number of hospital readmissions within 7 days. - Number of hospital days - Length of stay per inpatient admission - Number of Emergency Department visits - Cost |
| Holland (2015) [39] | USA | Prospective quasi-experimental, non-equivalent comparison group design | Tertriary children's hospital | 300 | Pediatric patients hospitalized in the acute care setting | Mean 8.7 years (SD 5.9) | Early Screen for Discharge Planning Child Version (ESDP-C): a screening tool to identify children with medical complexity that would benefit from early engagement of a discharge planner. |
- Time from admission to discharge planner involvement - Number of readmissions - Length of hospital stay |
| Howard et al. (2017) [40] | USA | Retrospective cohort study | A Pediatric Hospital and other urban hospitals | 183 | Children with medical complexity, including cerebral palsy | Mean 12.4 years (SD 5.8) and 6.7 (SD 4.4) | Care beyond The Bedside Model: increasing the preparedness and comfort level of children and their caregivers to manage ongoing health care needs independently. |
- Number of readmissions - Number of inpatient days - Number of Emergency Department visits - Costs |
| Knight et al. (2013) [41] | USA | Observational survey-based study | Quarternary, academic pediatric hospital | 117 | High risk children (criteria described) | 0 to 18 years | Cardiopulmonary resuscitation (CPR) discharge training. |
- Parental knowledge of the core skills of CPR - Parents comfort levels in performing CPR - Frequency of video review and practising core skills after discharge by parents - Disemmination of the kit to other family and friends - Nurses' impression of the program and suggestions for improvement |
| Lerret et al. (2020) [42] | USA | Quality improvement project with a pre-post two group design | Academic medical center | 395 | Children with and without a chronic condition | 2 to 21 years | The engaging Parents in Education for Discharge (ePed): a tool that explores parents experiences with discharge teaching and care coordination. |
- Quality of discharge teaching - Quality of care coordination - Number of readmissions |
| Liu et al. (2018) [43] | USA | Cohort study with propensity score weighting | Tertiary Women and Infant hospital | 686 | High risk neonates (criteria are described) | 0 to 1 year | Transition to Home Plus (THP) program: enhanced support services before and after discharge. |
- Number of unplanned readmissions - Number of Emergency Department visits - Costs |
| Ming et al. (2022) [44] | USA | Non-randomized pilot study | Tertiary children’s hospital | 48 | Children with medical complexity (criteria described) | Mean 8.5 years (SD 5.6) and 10.1 years (SD 5.1) | Post-hospitalization telemedicine video visits during hospital-to-home transitions. |
- Acceptability of the intervention (parents) - Saved travel costs - Self-efficacy - Number of hospital admissions - Length of hospital stay - Length of ICU stay - Number of Emergency Department visits |
| Moreno and Peck (2020) [45] | USA | Quality improvement project with a pre-post intervention design | Freestanding pediatric hospital | 2 | Children with a newly placed tracheostomy | Not reported | A hospital-based discharge protocol and telehealth follow-up care. |
- Caregiver knowledge, competence, self-efficacy and satisfaction - Number of hospitalizations - Number of Emergency Room visits - Number of tracheostomy-associated complications |
| Mosquera et al. (2021a) [46] | USA | Randomized Quality Improvement Trial | High-Risk Children’s Clinic | 422 | Children with medical complexity (criteria described) | Mean 6.2 years (SD 5.4) and 5.7 years (SD 4.5) | Care coordination with telemedicine. |
- Mortality - Serious illness - Hospital (re)admissions - Number of PICU admission - Number of hospital days - Hospital length of stay - PICU length of stay - Number of Emergency Department visits - Number of office visits - Well-child checks - Costs |
| Mosquera et al. (2021b) [47] | USA | Randomized Quality Improvement Trial | High-Risk Children’s Clinic | 342 | Children with medical complexity (criteria described) | Mean 5.8 years (SD 4.2) and 6.3 years (SD 4.7) | A hospital consultation service for CMC from their outpatient comprehensive care clinicians |
- Mortality - Serious illness - Parental satisfaction healthcare - Hospital (re)admissions - Number of PICU admission - Number of hospital days - Hospital length of stay - PICU length of stay - Number of Emergency Department visits - Number of telephone conversations after discharge - Costs |
| Moyer et al. (2014) [48] | USA | Concurrent cohort study | Children's hospital | 229 | Preterm children (criteria described) | Preterm infants | Care Transitions Intervention: a care transition coach to assist families, and an enhanced personal health record to improve the quality of information available to parents and community professionals. |
- Mortality - Number of sick visits - Number of unplanned readmissions - Number of Emergency Department visits - Caregiver perception of perceived quality of transitional care - Non-compliance with follow-up appointments |
| Nguyen et al. (2018) [50] | USA | Prospective cohort study | Freestanding pediatric hospital | 311 | Children with medical complexity (criteria described) | Range 0 to 17 years | Pharmacy discharge services additional to an existing nurse-led discharge service. |
- Number and type of pharmacist interventions during discharge and telephone encounters - Costs |
| Nkoy et al. (2021) [49] | USA | Randomized controlled trial | Tertiary children’s hospital | 50 | Children with medical complexity (criteria described) | Mean 9.65 years (95% CI 7.40 to 11.91) and 7.29 years (95% CI 4.90 to 9.69) | A home monitoring application (mobile app) additional to the usual care coordination program. |
- Health deteriorations - Quality of life child - Satisfaction with healthcare - Hospital admissions - Hospital days - Number of Emergency Department visits |
| Noritz et al. (2017) [51] | USA | Quality improvement project with a pre-post design | Freestanding quaternary children's hospital and the affiliated accountable care organizations | 1070 | Children with medical complexity (criteria described) | Range 1 to 18 years | Program with 3 interventions: standardizing percutaneous feeding tube management, improving family education, and care coordination. |
- Number of children with a weight between 5th and 95th percentile on standard growth curve - Length of stay - Costs |
| Osorio et al. (2021) [52] | USA | Factorial design of a natural experiment | Four children’s hospitals | 7725 | Multiple or complex chronic conditions, including technology-supported patients | Median 1.9, 2.4, 0.7 and 3.6 years | Pediatric Care Transition (PACT) bundel: (a) a transition readiness checklist, (b) predischarge teach-back education, (c) written handoff to primary care professional, and (d) postdischarge phone call. | - Hospital readmission rate |
| Parker et al. (2020) [53] | USA | Cohort study with a comparison group | University-affiliated tertiary care children's hospital | 126 | Children with chronic respiratory insufficiency requiring technological support | Median 7 years (IQR 3 to 12) | The Center for Children with Complex and Chronic Conditions (C5) program: inpatient and outpatient care coordination. |
- Number of readmissions - Number of Emergency Department visits - Costs |
| Patel et al. (2017) [54] | USA | Prospective observational cohort study | Regional NICU | 241 | High risk neonates (criteria described) | born < 32 weeks gestational age | BRIDGE: home visits by pediatric nurse practitioners. |
- Number of adverse events: homecare and healthcare utilization errors, such as errors with medication, feeding and equiment use, - Non-compliance with follow-up appointments |
| Petitgout (2018) [55] | USA | Retrospective cohort study with a pre-post design | Tertiary children's hospital | 158 | Children with a tracheostomy | ≤ 21 years | Hospital-based care coordination program: interdisciplinary family centered care, continuity of care, psychosocial support, assessment of recources and services, communication, avoidance of duplication of services and improving overall health of the child. |
- Number of unplanned hospital readmissions - Length of stay - Costs |
| Postier et al. (2014) [56] | USA | Retrospective cohort study with a pre-post design | Pediatric hospital | 425 | Children with life-limiting and life-threatening illnesses | 1 to 21 years | Pediatric Palliative Care program (PCC): a home-based pediatric hospice program. |
- Number of hospital admissions - Length of stay - Costs |
| Prickett et al. (2019) [57] | USA | Evaluation study | Pediatric hospital | 39 | Children with a newly placed tracheostomy | Not reported | Tracheostomy simulation-based education program for caregivers: classroom learning, one-on-one teaching, bedside teaching and caregivers skills demonstrations. |
- Parental self confidence with tracheostomy emergency management - Utility of tracheostomy emergency management |
| Roundy et al. (2016) [58] | USA | Cohort study with historical controls | Tertiary children's hospital. | 120 | Children with epilepsy | 0 to 18 years | Seizure Action Plan (SAP): information that might be forgotten and difficult to remember, and that would be helpful in a situation of breakthrough seizures or for determining timing of follow-up. |
- Number of readmissions - Length of stay - Number of Emergency Department visits - Number of neurology follow-up clinic visits - Number of telephone calls to pediatric neurology offices |
| Sarik et al. (2018) [59] | USA | Retrospective review | Pediatric acute care setting in a Magnet designated hospital | 398 | Children with medical complexity (criteria described) | 0 to 20 years | Patient navigation program: assessment of the readiness for discharge, planning of the sequential order of appointments, implementing stategies to help families succesfully care for their child. |
- Number of readmissions - Rate of no-show at scheduled follow-up appointments |
| Sigalet et al. (2014) [60] | Canada | Randomized Controlled pilot Trial | Children's hospital | 61 | Children with acute seizure disorders | < 18 years | Simulation-based seizure management teachingprogram. |
- Caregivers self-efficacy - Caregivers performance assessed by the trainer |
| Statile et al. (2016) [61] | USA | Quality improvement project | Freestanding pediatric hospital | 227 | Children with neurologic medical complexity (categories described) | Median 5.3 years (IQR 2.2 to 15.6) | Quality improvement interventions, such as defining medical discharge goals, admission order sets, care coordination rounds, needs assessment tool, and medication pathway. |
- Number of children discharged within 2 hours of meeting medical discharge goals - Number of readmissions - Length of hospital stay |
| Thrasher et al (2018) [62] | USA | Quality improvement project | University-affiliated tertiary care children's hospital and regional referral centers | 87 | Children with a tracheostomy requiring long-term mechanical ventilation | Median 11.5 months (range 2 to 410) | Simulation training incorporated into a multimodal discharge preparedness training with instructional videos, printed handouts, scenario training, and video based debriefing. | - Number of readmissions |
| Tiozzo et al. (2022) [63] | Italy | Prospective cohort study | Pediatric hospital | 310 | Children with chronic diseases and complex therapeutic plans | 0 to 18 years | A Medication Safety at Home cell-phone app. | - Number and features of out-of-hospital medication errors |
| Tofil (2013) | USA | Quality improvement project | Department of pediatric pulmonology | 7 | Children with a trachestomy and home ventilation | Range 15 months to 15 years | Home ventilator program with simulation training. | - Parents perceived preparedness and confidence to provide care to their child |
| Tolomeo et al (2017) [65] | USA | Quality improvement project | Pediatric Respiratory Care Unit | 30 | Children with a trachestomy | < 1 year | Standardizing the care and skills proficiency training for parents of infants with trachestomy tubes: welcome binder and educational material. |
- Number of developmental interventions for infants with tracheostomy tubes - Length of stay |
| Vohr et al. (2017) [66] | USA | Prospective cohort study | NICU (level 3-4) | 804 | High risk / very low birth weight preterm infants | Born ≤ 37 weeks gestational age | Comprehensive transition home services: predischarged interventions (e.g. education, support to assess community resources) and postdischarge interventions (e.g. contact with primary care provider). | - Number of rehospitalization |
| Vohr et al. (2018) [67] | USA | Prospective cohort study | NICU (level 3-4) | 804 | High risk / very low birth weight preterm infants | Early, moderate and late preterm infants. | NICU transition support services: predischarged interventions (e.g. education, support to assess community resources) and postdischarge interventions (e.g. contact with primary care provider and visits to follow-up clinic). | - Number of Emergency Department visits |
| Wells et al. (2017) [68] | USA | Prospective cohort study | Children's hospital | 38 | Children with medical complexity (criteria described) | Median 6 years (IQR 2 to 18) | Postdischarge home visits. |
- Unresolved health issues - Parental satisfaction with home visits - Postdischarge problems (number and type) |
| Whalen et al. (2020) [69] | USA | Descriptive quantitative design | Children's hospital | 8 | Childeren with a tracheostomy, some requiring mechanical ventilation | ≤ 18 years | Parental Airway Assessment with Simulation program. | - Parent tracheostomy skills |
| Willard et al. (2018) [70] | USA | Prospective cohort study | NICU (quaternary level 4) | 93 | Children with medical surgical complexity | Infants | Postdischarge telemedicine visits, as additional to the existing discharge process (education and home care coordination). |
- Caregivers knowledge and practice gaps uncovered - Caregiver perceived satisfaction |
| Yilmaz and Ozsoy (2010) [71] | Turkey | A quasi-experimental study | Pediatric oncology unit | 49 | Children with newly diagnosed cancer |
Mean 8.7 years (SD 5.9) and 10.7 years (SD 4.2) |
A discharge planning program, including discharge planning, discharge teaching, home visits and postdischarge telephone consultation. |
- Physical care needs of the children (number and characteristics) - Number of readmissions Number of clinic visits |
| Yuen et al. (2021) [72] | USA | Pilot study with a pre-post design | Children’s hospital within a tertiary academic medical center | 25 | Children with tracheostomy | < 21 years | Simulation-based Discharge Program |
- Caregivers’ comfort and confidence to perform care at home - Caregivers’ skills to provide care |
| Zanello et al. (2017) [73] | Italy | Prospective cohort study | Hospitals participating in the Special Needs Kids Research Project | 61 | Children with medical complexity (criteria described) | Mean 5.8 months (SD 11.8) | Family Pediatrician: care coordination by pediatricians in primary care. |
- Patient's needs requiring care coordination - Activities by the Family Pediatricians (number and type) - Prevented healthcare utilization |
USA United States of America, IQR Inter Quartile Range, SD Standard Deviation, 95% CI 95% Confidence Interval
Outcomes
We identified 172 outcomes among the 50 included studies. Our research group extensively reviewed and discussed the outcome list to identify those with similar definitions, wording or meaning. Finally, consensus was reached on a list of 25 unique outcomes that were assigned to the six outcome domains. We present the outcomes per study (Table 1) and per domain (Table 2).
Table 2.
Outcomes per domain
| Outcome | Data sources | References |
|---|---|---|
| Domain 1: mortality and survival (1 outcome) | ||
| Mortality (five studies, 10%) | Data from institutional databases or electronic medical records | Baker et al. [26], Coller et al. [32], Mosquera et al. [46, 47], Moyer et al. [48] |
| Domain 2: physical health (1 outcome) | ||
| Disease management, in terms of e.g. serious illness, health deteriorations, or physical care needs (seven studies, 14%) |
Electronic medical records, e.g. growth curve, development assessments, unresolved health issues Mobile app for monitoring child’s vital signs and symptom Children's Physical Care Needs (CPCN) measurement tool |
Mosquera [46, 47], Nkoy et al [49], Noritz et al. [51], Tolomeo et al. [65], Wells et al. [68], Yilmaz and Ozsoy [71] |
| Domain 3: life impact (13 outcomes) | ||
| Outcomes reflecting the impact on the life of the child | ||
| Quality of Life (three studies, 6%) |
Measurement tools: Child Health-Related Quality of Life (PedsQL) Caregiver Priorities and Child Health Index of Life with Disabilities (CPCHILD) A self-structured questionnaire based on existing validated surveys: Medical Home Family Index and the Client Perception of Coordination Questionnaire (CPCQ) Adapted survey based on Parent Perceptions of quality of life and healthcare satisfaction for CMC |
Cohen et al. [31], Donnelly et al. [33], Nkoy et al. [49] |
| Behavioral problems (one study, 2%) |
Measurement tool: Behavior Assessment System for Children - Parent Report Scale (BASC2-PRS) |
Duffy and Vessey [34] |
| School absences among children agedover 5 years (one study, 2%) | Data collected by care coordinators on prevented hospital visits | Breen et al. [29] |
| Outcomes reflecting the impact on the life of the parents | ||
| Self-efficacy (nine studies, 18%) |
Measurement tools: Parental Beliefs Scale A self-structured questionnaire based on existing validated surveys: Medical Home Family Index and the Client Perception of Coordination Questionnaire (CPCQ) KidSIM-ASPIRE Parent Seizure Self-efficacy Questionnaire Care Transitions Measure Survey (CTMS) CPR Skill Competence Checklist 10-point rating scale Caregiving self-efficacy (CSE) Family self-management (FSM) self-constructed survey/assessment tool |
Donnelly et al. [33], Duffy and Vessey [34], Knight et al. [41], Ming et al. [44], Moreno and Peck [45], Prickett et al. [57], Sigalet et al. [60], Tofil et al. [64], Yuen et al. [72] |
|
The competency of parents to provide care for their child (eight studies, 16%) |
Measurement tools: Parents Airway Assessment with Simulation skill checklists Caregiver Knowledge Checklist (CKC) the KidSIM-ASPIRE Emergent Seizure Management Checklist CPR Skill Competence Checklist self-constructed survey / assessment tool |
Gillen and Morris [36], Knight et al. [41], Moreno and Peck [45], Prickett et al. [57], Sigalet et al. [60], Whalen et al. [69], Willard et al. [70], Yuen et al. [72] |
| Satisfaction with hospital-to-home transitional care (eight studies, 16%) |
Measurement tools: Self-structured questionnaire based on existing validated surveys: Medical Home Family Index and the Client Perception of Coordination Questionnaire (CPCQ) Telehealth Satisfaction Survey (TSS) Care Transition Measure (CTM) Care Transition Measure neo (CTM Neo) delivery subscale of the Quality of Discharge Teaching Scale (QDTS-D) Self-constructed survey / assessment tool Telehealth Usability Questionnaire Survey adapted from: (a) the Consumer Assessment of Healthcare Providers and Systems Patient-Centered Medical Home survey and b) Medicare Coordinated Care Demonstration survey. |
Donnelly et al. [33], Hogan et al. [38], Lerret et al. [42], Ming et al. [44], Moreno and Peck [45], Moyer et al. [48], Wells et al. [68], Willard et al. [70] |
| Compliance, in term of missed appointments to an outpatient department/clinic/subspecialist (four studies, 8%) | Data from institutional databases or electronic medical records | Braun et al. [28], Moyer et al. [48], Patel et al. [54], Sarik et al. [59] |
| Quality of Life (three studies, 6%) |
Measurement tools: Parental Health-Related Quality of Life (SF-36) A self-structured questionnaire based on existing validated surveys: Medical Home Family Index and the Client Perception of Coordination Questionnaire (CPCQ) |
Cohen et al. [30], Cohen et al. [31], Donnelly et al. [33] |
| Satisfaction with healthcare in general (three studies, 6%) |
Measurement tool: Larsen's Client Satisfaction Questionnaire (LCSQ) Consumer Assessment of Healthcare Providers and Systems (CAHPS) Adapted survey based on the Client Satisfaction Questionnaire |
Cohen et al. [30], Mosquera et al. [47], Nkoy et al. [49] |
| Out-of-pocket expenses (three study, 6%) |
Measurement tool: Health and Social Service Utilization Questionnaire for expenditures: parents reported out of pocket expenses Travel costs savings estimated from prevented hospital visits |
Breen et al. [29], Cohen et al. [31], Ming et al. [44] |
| Satisfaction with family centered care (two studies, 4%) |
Measurement tool: Measures of Processes of Care (MPOC) |
Cohen et al. [30], Cohen et al. [31] |
| Anxiety (one study, 2%) |
Measurement tool: State-Trait Anxiety Inventory (STAI-Y) |
Duffy and Vessey [34] |
| Depression (one study, 2%) |
Measurement tool: Beck Depression Inventory II (BDI-11) |
Duffy and Vessey [34] |
| Domain 4: Resource use (8 outcomes) | ||
| Hospital (re)admissions (30 studies, 60%) |
Measurement tool: Adapted Care Coordination Measurement Tool (CCMT) Data from institutional databases or electronic medical records |
Appachi et al. [25], Baker et al. [26], Barreda et al. [27], Braun et al. [28], Breen et al. [29], Coller et al. [32], Gay et al. [35], Graham et al. [37], Hogan et al. [38], Holland et al. [39], Howard et al. [40], Lerret et al. [42], Liu et al. [43], Ming et al. [44], Moreno and Peck [45], Mosquera et al. [46, 47], Moyer et al. [48], Nkoy et al. [49], Osorio et al. [52], Parker et al. [53], Petitgout [55], Postier et al. [56], Roundy et al. [58], Sarik et al. [59], Statile et al. [61], Thrasher et al. [62], Vohr et al. [70],Yilmaz et al. [71], Zanello et al. [73] |
| Length of hospital stay (19 studies, 38%) | Data from institutional databases or electronic medical records | Appachi et al. [25], Baker et al. [26], Braun et al. [28], Cohen et al. [30], Cohen et al. [31], Gay et al. [35], Hogan et al. [38], Holland et al. [39], Howard et al. [40], Ming et al. [44], Mosquera et al. [46, 47], Nkoy et al. [49], Noritz et al. [51], Petitgout [55], Postier et al. [56], Roundy et al. [58], Statile et al. [61], Tolomeo et al. [65] |
| Number of visits Emergency Department (19 studies, 38%) | Data from institutional databases or electronic medical records | Baker et al. [26], Barreda et al. [27], Breen et al. [29], Cohen et al. [30], Cohen et al. [31], Graham et al. [37], Hogan et al. [38], Howard et al. [40], Liu et al. [43], Ming et al. [44], Moreno and Peck [45], Mosquera et al. [46, 47], Moyer et al. [48], Nkoy et al. [49], Parker et al. [53], Roundy et al. [58], Vohr et al. [66], Zanello et al. [73] |
| Costs (17 studies, 34%) | Data from institutional databases or insurance databases | Appachi et al. [25], Baker et al. [26], Breen et al. [29], Cohen et al. [31], Coller et al. [32], Gay et al. [35], Graham et al. [37], Hogan et al. [38], Howard [40], Liu et al. [43], Mosquera et al. [46, 50], Noritz et al. [51], Parker et al. [53], Petitgout [55], Postier et al. [56] |
| Number of contacts to an outpatient department/clinic/ subspecialist (nine studies, 18%) | Data from institutional databases or electronic medical records | Braun et al. [28], Breen et al. [29], Cohen et al. [31], Mosquera et al. [46, 47], Roundy et al. [58], Yilmaz and Ozsoy [71], Zanello et al. [73] |
| Number of primary care consultations or visits to a community based clinic (two studies, 4%) | Data from institutional databases or electronic medical records | Cohen et al. [30], Cohen et al. [31] |
| Services carried out by a pharmacist (one study, 2%) | Electronic medical records and a self-constructed tool | Nguyen et al. [50] |
|
Number of activities performed by primary care professionals, e.g. laboratory tests, examinations, coordination services (one study, 2%) |
Measurement tool: Special Needs Kids-Family Pediatrician (SpeNK-FP) |
Zanello et al. [73] |
| Domain 5: adverse events (1 outcome) | ||
| Identification, number and features of out-of-hospital medication and equipment errors (four studies, 8%) |
Data collected by care coordinators on adverse events at home during home visits. Home cell-phone app |
Moreno and Peck [45], Patel et al. [54], Tiozzo et al. [63], Wells et al. [68] |
| Other: Staff perceptions (1 outcome) | ||
| Staff perception about the transitional care, in term of feasibility, usability and satisfaction (three studies, 6%) |
Provider and Staff Perceptions of Integrated Care Survey Self-constructed survey |
Antolick et al. [24], Cohen et al. [31], Knight et al. [41] |
Mortality and survival
Mortality was considered as an outcome in five studies (10%) [26, 32, 46–48]. Mortality was reported differently: 1-year mortality [26], number of children deceased during the study period [32, 46, 47], and 30-days mortality [48].
Physical Health
In seven studies (14%) the outcomes referred to disease management [46, 47, 49, 51, 65, 68, 71]. Studies reported on different outcomes: serious illness [46, 47], health deterioration [49], weight on standard growth curve [51], physical development assessments [65], unresolved health issues [68], and physiological care needs, e.g. bowel control and pain [71].
Life impact
This domain was evaluated in 24 studies (48%) [28–31, 33, 34, 36, 38, 41, 42, 44, 45, 47–49, 54, 57, 59, 60, 64, 47–70, 72] and we differentiated 13 outcomes. Five studies reported on outcomes reflecting the impact on the life of the child: quality of life (three studies, 6%) [31, 33, 49], behavioral problems (one study, 2%) [34], and school absences (one study, 2%) [29]. Ten outcomes concerned the impact on the lives of the parents: self-efficacy (nine studies, 18%) [33, 34, 41, 44, 45, 57, 60, 64, 72], the competency of parents to provide care for their child (eight studies, 16%) [36, 41, 45, 57, 60, 69, 70, 72], satisfaction with hospital-to-home transitional care (eight studies, 16%) [33, 38, 42, 44, 45, 48, 68, 70], compliance in terms of missed appointments were explored in four studies (8%) [28, 48, 54, 59], quality of life (three studies, 6%) [30, 31, 33], satisfaction with healthcare in general (three studies, 6%) [30, 47, 49], out-of-pocket expenses (three studies, 6%) [29, 30, 44], satisfaction with family centered care (two studies, 4%) [30, 31], anxiety (one study, 2%) [34], and depression (one study, 2%) [34].
Resource use
The majority of the studies (36 studies, 72%) had chosen outcomes in the domain resource use [25–32, 35, 37–40, 42–53, 55, 56, 58, 59, 61, 62, 65–67, 71, 73]. Hospital (re)admission was the most frequently reported outcome (30 studies, 60%) [25–29, 32, 35, 37–40, 42–49, 52, 53, 55, 56, 58, 59, 61, 62, 66, 71, 73], followed by length of stay in the hospital (19 studies, 38%) [25, 26, 28, 30, 31, 35, 38–40, 44, 46, 47, 49, 51, 55, 56, 58, 61, 65], the number of visits to an Emergency Department (19 studies, 38%) [26, 27, 29–31, 37, 38, 40, 43–49, 53, 58, 67, 73], and costs (17 studies, 34%) [25, 26, 29, 31, 32, 35, 37, 38, 40, 43, 46, 47, 50, 51, 53, 55, 56]. Other reported outcomes in this domain were: the number of contacts to an outpatient department/clinic/subspecialist (nine studies, 18%) [28, 31, 46, 47, 58, 71, 73], the number of primary care consultations or visits to a community based clinic (two studies, 4%) [30, 31], services carried out by a pharmacist (one study, 2%) [50], and the number of activities performed by primary care professionals (one study, 2%) [73].
Adverse events
Adverse events in terms of numbers and features of medication and equipment errors at home were assessed in four studies (8%) [45, 54, 63, 68].
Other
Staff perception about the transitional care, in term of feasibility, usability and satisfaction was evaluated in three studies (6%) [24, 30, 41].
Sources of outcome data and outcome measurements
Different data sources and tools were used to evaluate the outcomes. All studies reporting on mortality and healthcare use collected their data from institutional databases, insurance databases, and electronic medical records. Physical health data came from electronic medical records, a telehealth application and a measurement tool. Outcomes on physical health, life impact, adverse events, and staff perception were measured by a big variety of questionnaires or assessment tools. For example, in the studies reporting on life impact, we found 35 different measurement tools, of which several were modified or self-structured. See Table 2.
Period of outcome measurements
The total duration of the study periods varied from three months [24, 45] to 10 years or more [25, 35, 55, 56]. We found great variation in the frequencies and intervals of the outcome measurements. Some studies reported a single observation, while other studies collected outcomes biweekly, monthly, quarterly, six-monthly, or yearly. Some studies included the measurements over time in the analyses [30, 34, 36, 43, 51, 59, 67, 71] and/or gave visual insight in the trends, e.g. with run charts [26, 29, 31, 49, 50, 54, 59, 61]. In general, studies were unclear in reporting their timelines.
Discussion
In this systematic review, we identified outcomes currently reported in publications evaluating the effectiveness of hospital-to-home transitional care interventions for CMC. Despite a substantial degree of heterogeneity in the definitions and descriptions of the outcomes, we agreed on 25 unique outcomes. These outcomes were assigned to six main outcome domains: mortality and survival, physical health, life impact, resource use, adverse events, and others. This overview of outcomes shows the outcomes researchers have prioritized to evaluate hospital-to-home interventions.
We are aware of important previous work by Looman et al. [17] and Barnert et al. [74] that also reports on outcome measures in publications concerning CMC and their families. Looman et al. aim to identify patterns and gaps in classification systems, data, and outcomes in studies of CMC [17]. Barnert et al. aim to contribute to the development of summary measures to describe the health of CMC [74]. Our systematic review is of additional value due to its focus on outcomes used in evaluations of hospital-to-home interventions. In addition, we specifically aim to use this systematic review in the development of a COS in order to standardize and prioritize meaningful outcomes in studies that aim to improve hospital-to-home transitional care interventions for CMC and their families.
Most studies in our review had chosen resource use outcomes, such as visits to an ED, number of hospital admissions, length of hospital stay, and costs, which is congruent with the two aforementioned reviews [17, 74]. The focus on resource use outcomes might reflect the perceived importance by different stakeholders, e.g. policy makers, insurance companies, and healthcare professionals. The importance of resource use outcomes is obvious, as CMC account substantially in healthcare resource use, such as 20% of ED visits at children’s hospitals, and up to 33% of all children’s healthcare costs [4, 75, 76]. Furthermore, resource use outcomes might also be chosen as indicators of physical health, and the extent to which the medical complexity impacts on the child, parents and families.
Many studies in our systematic review had chosen outcomes reflecting the life impact. Within the domain life impact a variety of different outcomes were collected, of which most reflected the impact on the life of parents. The impact on the life of the children was less represented with six studies focusing on the child. We did not find studies reporting on the specific impact on siblings, other members of the family (e.g. grandparents), or family’s interactions. An explanation could be that those themes are more often explored in qualitative studies. To create a comprehensive view of hospital-to-home transitional care another review of qualitative studies should be of additional value. Thomson et al. pointed out that having a CMC may have a major negative impact on the financial situation of a family [8]. This is supported by Barnert et al. who underlined that a comprehensive and continuous health insurance is considered an important contribution to the health of CMC [77]. Apart from three study that reported out-of-pockets expenses [29, 31, 44], no other study in our review reported the financial impact as an outcome. This is in line with a recent review showing that out of 27 studies only three reported on costs of CMC from the family perspective [78].
Outcomes in the domains mortality and survival, and physical health were less represented in our results. It can be reasoned that the outcomes on physical health are associated with resource use, and therefore less chosen. For example, it is likely that disease exacerbations (physical health) result in more ED visits and hospitalizations (resource use outcomes), making resource use outcomes surrogate outcomes for physical health. We identified two studies that explicitly described the seriousness of the disease in terms of death and resource use [46, 47].
Adverse events outcomes were reported in only a few studies. This might be explained by the challenges in identification and reporting. The identification of errors at home depends mainly on self-reporting by parents. Lack of awareness, parents’ perceived value and decision-making of reporting, and non-transparent reporting processes might hamper data in this outcome domain [79]. However, minimizing medical errors was defined as an important outcome for a healthy life for CMC [77].
Population
Although abundant literature exists on CMC, a uniform definition of CMC is lacking [80], and studies might not have been indexed clearly in the literature databases. Therefore, we also included studies that described the participants as children with complex chronic conditions. As a result, the studies included in this review represent a great variety of medical conditions and diseases, and over-inclusion in our study cannot be ruled out. As the aim of this SR was to identify all outcomes relevant for the hospital-to-home transition, we consider the broad inclusion as a strength of this study. On the other hand, also under-inclusion might have occurred as we excluded studies that did not provide a definition or clear description of the medical complexity of the participants. It is remarkable that most included studies are conducted in the USA. As outcome priorities may be influenced by national policy and the organization of care, this may have resulted in missing outcomes considered relevant in other countries.
Period of outcome measurements
Obviously, the timing of outcome measurement in the course of disease is crucial and dependent on the aim of a study. Publication guidelines stress out to publish the rationale for the frequency, time between measurements and duration of the follow-up in studies. Especially in complex care, outcomes should not be considered final endpoints, but rather ongoing indicators of the well-being of patients and their families, and healthcare needs [81, 82]. It can be expected that the outcomes change over time influenced by the dynamic and unpredictable course of the condition of CMC, and the context. Longitudinal measurement should be encouraged, as it provides the opportunity to use results in feedback loops evaluating the impact and connecting the results with treatment, care, and support activities [82, 83].
Limitations
We acknowledge some limitations of this systematic review. Firstly, we did not execute a quality assessment of the studies included in this systematic review. Because the aim of this systematic review was to summarize and categorize the reported outcomes regardless the quality of the study, we believe a quality assessment was not appropriate to address the research question of interest. However, future review of the existing literature is needed to quantify the effects of the interventions, including an assessment of the quality of the evidence, as this will inform the trustworthiness of the reported effectivity of the hospital-to-home interventions. Secondly, the final set of unique outcomes was established by consensus among the researchers. Despite the expertise of the researchers and the conscientious and careful process, subjective interpretations might have been of influence. In future research, validation by an independent group of experts might increase the trustworthiness of the results.
Conclusion
This systematic review found a big variety of outcomes used in studies evaluating interventions to improve hospital-to-home transition for CMC. However, it was possible to summarize them in a short-list with 25 unique outcomes that reflect mortality, physical health, impact on the life of the child and the parents, resource use, adverse events and staff perceptions. This short-list may support researchers and program evaluators in outcome selection, and can be used in the development of a core outcome set transitional care for CMC in future.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CMC
Children with Medical Complexity
- COS
Core Outcome Set
- COMET
Core Outcome Measures in Effectiveness Trials
- ED
Emergency Department
- PICU
Pediatric Intensive Care Unit
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Author contribution
Annemieke de Lange, Clara van Karnebeek, Jeroen van Woensel en Jolanda Maaskant contributed to the study conception and design. Material preparation, data collection and analysis were performed by Annemieke de Lange, Heleen Haspels, Mattijs van Alsem, Faridi van Etten-Jamaludin and Jolanda Maaskant. The first draft of the manuscript was written by Annemieke de Lange and all authors commented on previous versions of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This project is made possible by the Foundation “Steun Emma Kinderziekenhuis” and The Netherlands Organisation for Health Research and Development (ZonMw; 845008701). Both funders had no role in the design and conduct of the study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
The Institutional Review Board of the Amsterdam UMC location AMC waived the need for ethical approval (W20_220 #20.007).
Consent to participate
Not applicable to this manuscript.
Consent to publish
Not applicable to this manuscript.
Competing interest
The authors have no conflict of interest relevant to this article to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yamada H, Ohno K, Shiota M, Togawa M, Utsunomiya Y, Akaboshi S, Tsuchie H, Okada T, Oguri M, Higami S, Noma H, Maegaki Y. Prevalence and clinical characteristics of children with medical complexity in Tottori Prefecture, Japan: A population-based longitudinal study. Brain Dev. 2020;42(10):747–755. doi: 10.1016/j.braindev.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Fraser LK, Gibson-Smith D, Jarvis S, Norman P, Parslow RC. Estimating the current and future prevalence of life-limiting conditions in children in England. Palliat Med. 2021;35(9):1641–1651. doi: 10.1177/0269216320975308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen E, Kuo DZ, Agrawal R, Berry JG, Bhagat SK, Simon TD, Srivastava R. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127(3):529–538. doi: 10.1542/peds.2010-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo DZ, Melguizo-Castro M, Goudie A, Nick TG, Robbins JM, Casey PH. Variation in child health care utilization by medical complexity. Matern Child Health J. 2015;19(1):40–48. doi: 10.1007/s10995-014-1493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leyenaar JK, Schaefer AP, Freyleue SD, Austin AM, Simon TD, Van Cleave J, Moen EL, O'Malley AJ, Goodman DC (2022) Prevalence of children with medical complexity and associations with health care utilization and in-hospital mortality. JAMA pediatrics 176(6):e220687. 10.1001/jamapediatrics.2022.0687 [DOI] [PMC free article] [PubMed]
- 6.Brown CM, Williams DJ, Hall M, Freundlich KL, Johnson DP, Lind C, Rehm K, Frost PA, Doupnik SK, Ibrahim D, Patrick S, Howard LM, Gay JC. Trends in Length of Stay and Readmissions in Children's Hospitals. Hosp Pediatr. 2021;11(6):554–562. doi: 10.1542/hpeds.2020-004044. [DOI] [PubMed] [Google Scholar]
- 7.Elias ER, Murphy NA, Council on Children with Disabilities (2012) Home care of children and youth with complex health care needs and technology dependencies. Pediatrics 129(5):996–1005. 10.1542/peds.2012-0606 [DOI] [PubMed]
- 8.Thomson J, Shah SS, Simmons JM, Sauers-Ford HS, Brunswick S, Hall D, Kahn RS, Beck AF. Financial and Social Hardships in Families of Children with Medical Complexity. J Pediatr. 2016;172:187–193.e1. doi: 10.1016/j.jpeds.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kish AM, Newcombe PA, Haslam DM. Working and caring for a child with chronic illness: A review of current literature. Child Care Health Dev. 2018;44(3):343–354. doi: 10.1111/cch.12546. [DOI] [PubMed] [Google Scholar]
- 10.Bayer ND, Wang H, Yu JA, Kuo DZ, Halterman JS, Li Y (2021) A national mental health profile of parents of children with medical complexity. Pediatrics 148(2):e2020023358. 10.1542/peds.2020-023358 [DOI] [PubMed]
- 11.Nageswaran S, Sebesta MR, Golden SL. Transitioning Children With Medical Complexity From Hospital to Home Health Care: Implications for Hospital-Based Clinicians. Hosp Pediatr. 2020;10(8):657–662. doi: 10.1542/hpeds.2020-0068. [DOI] [PubMed] [Google Scholar]
- 12.Ronan S, Brown M, Marsh L. Parents' experiences of transition from hospital to home of a child with complex health needs: A systematic literature review. J Clin Nurs. 2020;29(17–18):3222–3235. doi: 10.1111/jocn.15396. [DOI] [PubMed] [Google Scholar]
- 13.Seppänen AV, Sauvegrain P, Draper ES, Toome L, El Rafei R, Petrou S, Barros H, Zimmermann LJ, Cuttini M, Zeitlin J, SHIPS Research Group Parents' ratings of post-discharge healthcare for their children born very preterm and their suggestions for improvement: a European cohort study. Pediatric research. 2021;89(4):1004–1012. doi: 10.1038/s41390-020-01120-y. [DOI] [PubMed] [Google Scholar]
- 14.Conkol KJ, Martinez-Strengel A, Coller RJ, Bergman DA, Whelan EM. Pediatric Hospitalists' Lessons Learned From an Innovation Award to Improve Care for Children With Medical Complexity. Hosp Pediatr. 2020;10(8):694–701. doi: 10.1542/hpeds.2020-0069. [DOI] [PubMed] [Google Scholar]
- 15.Williams LJ, Waller K, Chenoweth RP, Ersig AL (2021) Stakeholder perspectives: Communication, care coordination, and transitions in care for children with medical complexity. J Spec Pediatr Nurs 26(1):e12314. 10.1111/jspn.12314 [DOI] [PMC free article] [PubMed]
- 16.Breneol S, Belliveau J, Cassidy C, Curran JA. Strategies to support transitions from hospital to home for children with medical complexity: A scoping review. Int J Nurs Stud. 2017;72:91–104. doi: 10.1016/j.ijnurstu.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Looman WS, Park YS, Gallagher TT, Weinfurter EV. Outcomes research on children with medical complexity: A scoping review of gaps and opportunities. Child Care Health Dev. 2020;46(1):121–131. doi: 10.1111/cch.12725. [DOI] [PubMed] [Google Scholar]
- 18.Mantler T, Jackson KT, Baer J, White J, Ache B, Shillington K, Ncube N. Changes in care- a systematic scoping review of transitions for children with medical complexities. Curr Pediatr Rev. 2020;16(3):165–175. doi: 10.2174/1573396316666191218102734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (Clinical research ed.) 372:n160. 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed]
- 20.Kirkham JJ, Gorst S, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, Moher D, Schmitt J, Tugwell P, Tunis S, Williamson PR (2016) Core Outcome Set-STAndards for Reporting: The COS-STAR Statement. PLoS medicine 13(10):e1002148. 10.1371/journal.pmed.1002148 [DOI] [PMC free article] [PubMed]
- 21.Kirkham JJ, Davis K, Altman DG, Blazeby JM, Clarke M, Tunis S, Williamson PR (2017) Core outcome set-standards for development: the cos-stad recommendations. PLoS Med 14(11): e1002447. 10.1371/journal.pmed.1002447 [DOI] [PMC free article] [PubMed]
- 22.Core Outcome Measures in Effectiveness trials. Available: www.comet-initiave.org, Assessed February 2023.
- 23.Dodd S, Clarke M, Becker L, Mavergames C, Fish R, Williamson PR. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol. 2018;96:84–92. doi: 10.1016/j.jclinepi.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antolick MM, Looman WS, Cady RG, Kubiatowicz K. Identifying and Communicating Postdischarge Goals for Hospitalized Children With Medical Complexity: A Process Improvement Pilot in a Specialty Pediatric Setting. J Pediatr Health Care. 2020;34(2):90–98. doi: 10.1016/j.pedhc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Appachi S, Banas A, Feinberg L, Henry D, Kenny D, Kraynack N, Rosneck A, Carl J, Krakovitz P. JAMA Otolaryngol Head Neck Surg. 2017;143(11):1117–1121. doi: 10.1001/jamaoto.2017.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker CD, Martin S, Thrasher J, Moore HM, Baker J, Abman SH, Gien J (2016) A Standardized discharge process decreases length of stay for ventilator-dependent children. Pediatrics 137(4):e20150637. 10.1542/peds.2015-0637 [DOI] [PMC free article] [PubMed]
- 27.Barreda CB, Ehlenbach ML, Nackers A, Kelly MM, Shadman KA, Sklansky DJ, Edmonson MB, Zhao Q, Warner G, Coller RJ (2021) Complex care program enrollment and change in ed and hospital visits from medical device complications. Pediatr Qual Saf 6(5):e450. 10.1097/pq9.0000000000000450 [DOI] [PMC free article] [PubMed]
- 28.Braun L, Steurer M, Henry D (2021) Healthcare utilization of complex chronically ill children managed by a telehealth-based team. Front Pediatr 16(9):689572. 10.3389/fped.2021.689572 [DOI] [PMC free article] [PubMed]
- 29.Breen C, Altman L, Ging J, Deverell M, Woolfenden S, Zurynski Y. Significant reductions in tertiary hospital encounters and less travel for families after implementation of Paediatric Care Coordination in Australia. BMC Health Serv Res. 2018;18(1):751. doi: 10.1186/s12913-018-3553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen E, Friedman JN, Mahant S, Adams S, Jovcevska V, Rosenbaum P. The impact of a complex care clinic in a childrens’s hospital. Child Care Health Dev. 2010;36(4):574–582. doi: 10.1111/j.1365-2214.2009.01069.x. [DOI] [PubMed] [Google Scholar]
- 31.Cohen E, Lacombe-Duncan A, Spalding K, MacInnis J, Nicholas D, Narayanan UG, Gordon M, Margolis I, Friedman JN. Integrated complex care coordination for children with medical complexity: a mixed-methods evaluation of tertiary care-community collaboration. BMC health services research. 2012;12:66. doi: 10.1186/1472-6963-12-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coller RJ, Klitzner TS, Lerner CF, Nelson BB, Thompson LR, Zhao Q, Saenz AA, Ia S, Flores-Vazquez J, Chung PJ (2018) Complex care hospital use and postdischarge coaching: a randomized controlled trial. Pediatrics 142(2):e20174278. 10.1542/peds.2017-4278 [DOI] [PMC free article] [PubMed]
- 33.Donnelly S, Shaw E, Timoney P, Foca M, Hametz P. Parents' Assessment of an Advanced-Practice Nurse and Care Coordination Assistant Model Medical Care Coordination Program for Children With Medical Complexity. J Pediatr Health Care. 2020;34(4):325–332. doi: 10.1016/j.pedhc.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Duffy LV, Vessey JA. A Randomized Controlled Trial Testing the Efficacy of the Creating Opportunities for Parent Empowerment Program for Parents of Children With Epilepsy and Other Chronic Neurological Conditions. J Neurosci Nurs. 2016;48(3):166–174. doi: 10.1097/JNN.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gay JC, Thurm CW, Hall M, Fassino MJ, Fowler L, Palusci JV, Berry JG (2016) Home health nursing care and hospital use for medically complex children. Pediatrics 138(5):e20160530. 10.1542/peds.2016-0530 [DOI] [PubMed]
- 36.Gillen JK, Morris MC. Preparing Families of Technology-Dependent Children for Emergencies. Hosp Pediatr. 2019;9(11):874–879. doi: 10.1542/hpeds.2019-0091. [DOI] [PubMed] [Google Scholar]
- 37.Graham RJ, McManus ML, Rodday AM, Weidner RA, Parsons SK. Pediatric Specialty Care Model for Management of Chronic Respiratory Failure: Cost and Savings Implications and Misalignment With Payment Models. Pediatr Crit Care Med. 2018;19(5):412–420. doi: 10.1097/PCC.0000000000001472. [DOI] [PubMed] [Google Scholar]
- 38.Hogan AK, Galligan MM, Stack NJ, Leach KF, Aredas BL, English R, Dye M, Rubin D. A Tertiary Care-based Complex Care Program: Improving Care for Children With Medical Complexity. Med Care. 2022;58(11):958–962. doi: 10.1097/MLR.0000000000001388. [DOI] [PubMed] [Google Scholar]
- 39.Holland DE, Conlon PM, Rohlik GM, Gillard KL, Messner PK, Mundy LM. Identifying hospitalized pediatric patients for early discharge planning: a feasibility study. J Pediatr Nurs. 2015;30(3):454–62. doi: 10.1016/j.pedn.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Howard SW, Zhang Z, Buchanan P, Armbrecht E, Williams C, Wilson G, Hutchinson J, Pearson L, Ellsworth S, Byler CM, et al. The Effect of a Comprehensive Care Transition Model on Cost and Utilization for Medically Complex Children With Cerebral Palsy. J Pediatr Health Care. 2017;31(6):634–647. doi: 10.1016/j.pedhc.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Knight LJ, Wintch S, Nichols A, Arnolde V, Schroeder AR. Saving a life after discharge: CPR training for parents of high-risk children. J Healthc Qual. 2013;35(1):9–17. doi: 10.1111/j.1945-1474.2012.00221.x. [DOI] [PubMed] [Google Scholar]
- 42.Lerret SM, Johnson NL, Polfuss M, Weiss M, Gralton K, Klingbeil CG, Gibson C, Garnier-Villarreal M, Ahamed SI, Adib R, et al. Using the Engaging Parents in Education for Discharge (ePED) iPad Application to Improve Parent Discharge Experience. J Pediatr Nurs. 2020;52:41–48. doi: 10.1016/j.pedn.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, McGowan E, Tucker R, Glasgow L, Kluckman M, Vohr B. Transition Home Plus Program Reduces Medicaid Spending and Health Care Use for High-Risk Infants Admitted to the Neonatal Intensive Care Unit for 5 or More Days. J Pediatr. 2018;200:91–97.e3. doi: 10.1016/j.jpeds.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 44.Ming DY, Li T, Ross MH, Frush J, He J, Goldstein BA, Jarrett V, Krohl N, Docherty SL, Turley CB, Bosworth HB. Feasibility of Post-hospitalization Telemedicine Video Visits for Children With Medical Complexity. J Pediatr Health Care. 2022;36(2):e22–e35. doi: 10.1016/j.pedhc.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreno L, Peck JL. Nurse Practitioner-Led Telehealth to Improve Outpatient Pediatric Tracheostomy Management in South Texas. J Pediatr Health Care. 2020;34(3):246–255. doi: 10.1016/j.pedhc.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Mosquera RA, Avritscher EBC, Pedroza C, Lee KH, Ramanathan S, Harris TS, Eapen JC, Yadav A, Caldas-Vasquez M, Poe M et al (2021a) Telemedicine for children with medical complexity: a randomized clinical trial. Pediatrics 148(3):e2021050400. 10.1542/peds.2021-050400 [DOI] [PubMed]
- 47.Mosquera RA, Avritscher EBC, Pedroza C, Bell CS, Samuels CL, Harris TS, Eapen JC, Yadav A, Poe M, Parlar-Chun RL et al (2021b) Hospital consultation from outpatient clinicians for medically complex children: a randomized clinical trial. JAMA Pediatr 175(1):e205026. 10.1001/jamapediatrics.2020.5026 [DOI] [PMC free article] [PubMed]
- 48.Moyer VA, Papile LA, Eichenwald E, Giardino AP, Khan MM, Singh H (2014) An intervention to improve transitions from NICU to ambulatory care: quasi-experimental study. BMJ Qual Saf 23(12):e3. 10.1136/bmjqs-2012-001726 [DOI] [PubMed]
- 49.Nkoy F, Stone B, Hofmann M, Fassl B, Zhu A, Mahtta N, Murphy N. Home-Monitoring Application for Children With Medical Complexity: A Feasibility Trial. Hosp Pediatr. 2021;11(5):492–502. doi: 10.1542/hpeds.2020-002097. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen V, Sarik DA, Dejos MC, Hilmas E. Development of an Interprofessional Pharmacist-Nurse Navigation Pediatric Discharge Program. J Pediatr Pharmacol Ther. 2018;23(4):320–328. doi: 10.5863/1551-6776-23.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noritz G, Madden M, Roldan D, Wheeler TA, Conkol K, Brilli RJ, Barnard J, Gleeson S (2017) A population intervention to improve outcomes in children with medical complexity. Pediatrics 139(1):e20153076. 10.1542/peds.2015-3076 [DOI] [PubMed]
- 52.Osorio SN, Gage S, Mallory L, Soung P, Satty A, Abramson EL, Provost L, Cooperberg D, IMPACT STUDY GROUP (2021) Factorial analysis quantifies the effects of pediatric discharge bundle on hospital readmission. Pediatrics 148(4):e2021049926. 10.1542/peds.2021-049926 [DOI] [PubMed]
- 53.Parker CL, Wall B, Tumin D, Stanley R, Warren L, Deal K, Stroud T, Crickmore K, Ledoux M. Care coordination program for children with complex chronic conditions discharged from a rural tertiary-care academic medical center. Hosp Pediatr. 2020;10(8):687–693. doi: 10.1542/hpeds.2019-0323. [DOI] [PubMed] [Google Scholar]
- 54.Patel R, Nudelman M, Olarewaju A, Pooley SW, Jegatheesan P, Song D, Govindaswami B. Homecare and healthcare utilization errors post-neonatal intensive care unit discharge. Adv Neonatal Care. 2017;17(4):258–264. doi: 10.1097/ANC.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petitgout JM. The Financial Impact of a Hospital-Based Care Coordination Program for Children With Special Health Care Needs. J Pediatr Health Care. 2018;32(1):3–9. doi: 10.1016/j.pedhc.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Postier A, Chrastek J, Nugent S, Osenga K, Friedrichsdorf J. Exposure to home-based pediatric palliative and hospice care and its impact on hospital and emergency care charges at a single institution. J Palliat Med. 2014;17(2):183–8. doi: 10.1089/jpm.2013.0287. [DOI] [PubMed] [Google Scholar]
- 57.Prickett K, Deshpande A, Paschal H, Simon D, Hebbar KB. Simulation-based education to improve emergency management skills in caregivers of tracheostomy patients. Int J Pediatr Otorhinolaryngol. 2019;120:157–161. doi: 10.1016/j.ijporl.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 58.Roundy LM, Filloux FM, Kerr L, Rimer A, Bonkowsky JL. Seizure Action Plans Do Not Reduce Health Care Utilization in Pediatric Epilepsy Patients. J Child Neurol. 2016;31(4):433–8. doi: 10.1177/0883073815597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarik D, Winterhalter M, Calamaro C. Improving the Transition from Hospital to Home for Clinically Complex Children. Pediatr Nurs. 2018;44(6):281–287. [Google Scholar]
- 60.Sigalet E, Cheng A, Donnon T, Koot D, Chatfield J, Robinson T, Catena H, Grant VJ. A simulation-based intervention teaching seizure management to caregivers: A randomized controlled pilot study. Paediatr Child Health. 2014;19(7):373–378. doi: 10.1093/pch/19.7.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Statile AM, Schondelmeyer AC, Thomson JE, Brower LH, Davis B, Redel J, Hausfeld J, Tucker K, White DL, White CM (2016) Improving discharge efficiency in medically complex pediatric patients. Pediatrics 138(2):e20153832. 10.1542/peds.2015-3832 [DOI] [PubMed]
- 62.Thrasher J, Baker J, Ventre KM, Martin SE, Dawson J, Cox R, Moore HM, Brethouwer S, Sables-Baus S, Baker CD. Hospital to home: a quality improvement initiative to implement high-fidelity simulation training for caregivers of children requiring long-term mechanical ventilation. J Pediatr Nurs. 2018;38:114–121. doi: 10.1016/j.pedn.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tiozzo E, Rosati P, Brancaccio M, Biagioli V, Ricci R, d'Inzeo V, Scarselletta G, Piga S, Vanzi V, Dall'Oglio I, Gawronski O et al (2022) A Cell-phone medication error ehealth app for managing safety in chronically ill young patients at home: a prospective Study. Telemed J E Health Epub Sep 7. 10.1089/tmj.2022.0042 [DOI] [PubMed]
- 64.Tofil NM, Rutledge C, Zinkan JL, Youngblood AQ, Stone J, Peterson DT, Slayton D, Makris C, Magruder T, White ML. Ventilator caregiver education through the use of high-fidelity pediatric simulators: a pilot study. Clin Pediatr. 2013;52(11):1038–1043. doi: 10.1177/0009922813505901. [DOI] [PubMed] [Google Scholar]
- 65.Tolomeo CT, Major NE, Szondy MV, Bazzy-Asaad A. Standardizing Care and Parental Training to Improve Training Duration, Referral Frequency, and Length of Stay: Our Quality Improvement Project Experience. J Pediatr Nurs. 2017;32:72–79. doi: 10.1016/j.pedn.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Vohr B, McGowan E, Keszler L, Alksninis B, O'Donnell M, Hawes K, Tucker R. Impact of a transition home program on rehospitalization rates of preterm infants. J Pediatr. 2017;181:86–92.e1. doi: 10.1016/j.jpeds.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 67.Vohr B, McGowan E, Keszler L, O’Donnell M, Hawes K, Tucker R. Effects of a transition home program on preterm infant emergency room visits within 90 days of discharge. J Perinatol. 2018;38(2):185–190. doi: 10.1038/jp.2017.136. [DOI] [PubMed] [Google Scholar]
- 68.Wells S, O'Neill M, Rogers J, Blaine K, Hoffman A, McBride S, Tschudy MM, Shumskiy I, Mauskar S, Berry JG. Nursing-led home visits post-hospitalization for children with medical complexity. J Pediatr Nurs. 2017;34:10–16. doi: 10.1016/j.pedn.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Whalen M, Aebersold ML, Nelson K, Rooney DM. The feasibility and use of simulation to assess parent learning. Clin Simul Nurs. 2020;38:23–26. doi: 10.1016/j.ecns.2019.10.003. [DOI] [Google Scholar]
- 70.Willard A, Brown E, Masten M, Brant M, Pouppirt N, Moran K, Lioy J, Chuo J. Complex surgical infants benefit from postdischarge telemedicine visits. Adv Neonatal Care. 2018;18(1):22–30. doi: 10.1097/ANC.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 71.Yilmaz MC, Ozsoy SA. Effectiveness of a discharge-planning program and home visits for meeting the physical care needs of children with cancer. Support Care Cancer. 2010;18(2):243–53. doi: 10.1007/s00520-009-0650-2. [DOI] [PubMed] [Google Scholar]
- 72.Yuen A, Rodriguez N, Osorio SN, Nataraj C, Ward MJ, Clapper TC, Abramson E, Ching K. Simulation-Based Discharge Education Program for Caregivers of Children With Tracheostomies. Hosp Pediatr. 2021;11(6):571–578. doi: 10.1542/hpeds.2020-000984. [DOI] [PubMed] [Google Scholar]
- 73.Zanello E, Calugi S, Sanders LM, Lenzi J, Faldella G, Rucci P, Fantini MP. Care coordination for children with special health care needs: a cohort study. Ital J Pediatr. 2017;43(1):18. doi: 10.1186/s13052-017-0342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barnert ES, Coller RJ, Nelson BB, Thompson LR, Tran J, Chan V, Padilla C, Klitzner TS, Szilagyi M, Chung PJ. Key population health outcomes for children with medical complexity: a systematic review. Matern Child Health J. 2019;23(9):1167–1176. doi: 10.1007/s10995-019-02752-1. [DOI] [PubMed] [Google Scholar]
- 75.Agrawal R, Hall M, Cohen E, Goodman D., Kuo DZ, Neff JM, O'Neill M, Thomson J, Berry JG (2016) Trends in health care spending for children in medicaid with high resource use. Pediatrics 138(4):e20160682. 10.1542/peds.2016-0682 [DOI] [PubMed]
- 76.O'Mahony L, O'Mahony DS, Simon TD, Neff J, Klein EJ, Quan L. Medical complexity and pediatric emergency department and inpatient utilization. Pediatrics. 2013;131(2):e559–e565. doi: 10.1542/peds.2012-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barnert ES, Coller R, Nelson BB, Thompson LR, Klitzner TS, Szilagyi M, Breck AM, Chung PJ (2018) A healthy life for a child with medical complexity: 10 domains for conceptualizing health. Pediatrics 142(3):e20180779. 10.1542/peds.2018-0779 [DOI] [PubMed]
- 78.Sidra M, Sebastianski M, Ohinmaa A, Rahman S (2022) Reported costs of children with medical complexity-a systematic review. J Child Health Care 24:13674935221109683. 10.1177/13674935221109683 [DOI] [PubMed]
- 79.Khan A, Baird J, Kelly MM, Blaine K, Chieco D, Haskell H, Lopez K, Ngo T, Mercer A, Quiñones-Pérez B et al (2022) Family safety reporting in medically complex children: parent, staff, and leader perspectives. Pediatrics 149(6):e2021053913. 10.1542/peds.2021-053913 [DOI] [PMC free article] [PubMed]
- 80.Oliveira PV, Enes CC, Nucci LB (2022) How are children with medical complexity being identified in epidemiological studies? A systematic review. World J Pediatr 27. 10.1007/s12519-022-00672-9 [DOI] [PubMed]
- 81.Giovannetti ER, Dy S, Leff B, Weston C, Adams K, Valuck TB, Pittman AT, Blaum CS, McCann BA, Boyd CM. Performance measurement for people with multiple chronic conditions: conceptual model. Am J Manag Care. 2013;19(10):e359–e366. [PMC free article] [PubMed] [Google Scholar]
- 82.Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012;65(10):1041–1051. doi: 10.1016/j.jclinepi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 83.Zullig LL, Whitson HE, Hastings SN, Beadles C, Kravchenko J, Akushevich I, Maciejewski ML. A Systematic Review of Conceptual Frameworks of Medical Complexity and New Model Development. J Gen Intern Med. 2016;31(3):329–337. doi: 10.1007/s11606-015-3512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.