Abstract
Background
Approximately 15‒20% of advanced gastric and gastroesophageal junction (GEJ) cancers overexpress HER2. In DESTINY-Gastric01, the HER2-targeted antibody-drug conjugate trastuzumab deruxtecan (T-DXd) improved response and overall survival (OS) versus chemotherapy in patients from Japan and Korea with locally advanced and/or metastatic HER2-positive gastric or GEJ cancer whose disease progressed after two lines of therapy including trastuzumab. Here, we report data from the single-arm, phase 2 DESTINY-Gastric02 trial, which examines T-DXd in Western patients.
Methods
DESTINY-Gastric02 is a multicentre, single-arm study in adult patients from the United States and Europe. Eligible patients were aged ≥18 years, had an Eastern Cooperative Oncology Group performance status of 0 or 1, had pathologically documented unresectable or metastatic gastric/GEJ cancer, had progressive disease on or after first-line therapy with a trastuzumab regimen, and had centrally confirmed HER2-positive disease on a postprogression biopsy. Patients received 6·4 mg/kg of T-DXd intravenously every 3 weeks until disease progression, withdrawal by patient, physician decision, or death. The primary endpoint was confirmed objective response rate by independent central review (ICR) which was assessed in the full analysis set. Safety was assessed in the safety analysis set. This ongoing trial is registered with ClinicalTrials.gov, NCT04014075.
Findings
Between November 26, 2019, and November 8, 2021, 79 patients were treated with T-DXd (57 male, 22 female), with a median follow-up of 10·2 months (IQR 5·6‒12·9 months). A confirmed objective response was reported in 33 patients (41·8% [95% CI 30·8‒53·4]), as assessed by ICR. The most common grade ≥3 treatment-emergent adverse events (TEAEs) occurring in ≥5% of patients were anaemia (n=11; 13·9%), nausea (n=6; 7·6%), neutrophil count decrease (n=6; 7·6%), and white blood cell decrease (n=5; 6·3%). Drug-related serious TEAEs occurred in 10 patients (12·7%). Drug-related TEAEs associated with the outcome of death occurred in 2 patients (2·5%) and were due to interstitial lung disease/pneumonitis. Eight patients (10·1%) had adjudicated drug-related interstitial lung disease/pneumonitis (two grade 1 [2·5%], four grade 2 [5·1%], and two grade 5 [2·5%]).
Interpretation
These clinically meaningful results, demonstrated by the robust ORR, prolonged PFS and OS, and tolerable safety profile, support the use of T-DXd as second-line therapy in patients with HER2-positive advanced gastric/GEJ cancer.
Funding
Daiichi Sankyo
Keywords: gastric cancer, gastroesophageal junction cancer, HER2-positive, antibody-drug conjugate, trastuzumab deruxtecan
Introduction
Approximately 15‒20% of advanced gastric and gastroesophageal junction (GEJ) cancers overexpress human epidermal growth factor receptor 2 (HER2). 1 Recommended first-line treatment for patients with HER2-positive gastric or GEJ cancer is chemotherapy in combination with the anti-HER2 antibody trastuzumab. 2 Results from the phase 3 KEYNOTE-811 study supported accelerated approval by the US Food and Drug Administration of pembrolizumab in combination with trastuzumab and chemotherapy for first-line treatment of locally advanced unresectable or metastatic HER2-positive gastric or GEJ cancer. 3,4 Key second-line trials of HER2-targeted therapy in patients with HER2-positive gastric or GEJ cancer have not met their primary endpoint, overall survival, 5,6 with evidence suggesting that a loss of HER2 expression after first-line trastuzumab therapy might be a mechanism contributing to resistance to subsequent lines of anti-HER2 treatment. 7
Trastuzumab deruxtecan (T-DXd) has been approved in multiple countries worldwide for use in the treatment of adult patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma who have previously received a trastuzumab-based regimen. 8, 9, 10 T-DXd is an antibody-drug conjugate composed of a humanised anti-HER2 monoclonal antibody linked to a topoisomerase I inhibitor payload through a tetrapeptide-based cleavable linker. 11, 12, 13 After being internalised by tumour cells, the linker is cleaved by lysosomal enzymes upregulated in those cells. The cytotoxic payload, which is cell membrane‒permeable post-cleavage, has a bystander antitumour effect that enables the killing of tumour cells proximal to HER2-expressing tumour cells. 11–13
T-DXd efficacy and safety in gastric or GEJ adenocarcinoma was established in the phase 2, open-label, multicentre, randomised DESTINY-Gastric01 trial of T-DXd in patients from Japan and the Republic of Korea with HER2-positive locally advanced or metastatic gastric or GEJ adenocarcinoma that had progressed after ≥2 prior lines of therapy, including a fluoropyrimidine agent, a platinum agent, and trastuzumab. 1,8–10 In DESTINY-Gastric01, T-DXd demonstrated statistically significant benefit versus physician’s choice standard chemotherapy in objective response rate (ORR) and overall survival (OS). 1 A range of factors, including diet, smoking status, and infection with Helicobacter pylori, may contribute to differences in outcomes for Asian and Western populations of patients with gastric cancer. 14,15 Gastric cancer is diagnosed earlier in many Asian countries because of robust screening guidelines in comparison to Western countries; this disparity results in poorer prognoses and lower 5-year survival rates for patients with gastric cancer in countries in Europe and the US. 14
Here we report results from the DESTINY-Gastric02 study. The objective of this study was to assess the activity and safety of T-DXd monotherapy in Western patients with metastatic and/or unresectable HER2-positive gastric or GEJ cancer whose disease had progressed on or after a trastuzumab-containing regimen and were confirmed to have retained tumour HER2 positivity. This first phase 2 trial of second-line T-DXd in Western patients with gastric or GEJ cancer was conducted to expand the results from DESTINY-Gastric01 from Asian patients to Western patients and to an earlier line of therapy.
Methods
Study design and participants
DESTINY-Gastric02 is an open-label, multicentre, single-arm, phase 2 study that recruited Western patients from 24 study sites in the United States, Belgium, Spain, Italy, and the United Kingdom. Eligible patients were aged ≥18 years and had pathologically documented unresectable or metastatic gastric or GEJ cancer, progressive disease on or after first-line therapy with a trastuzumab-containing regimen, centrally confirmed HER2-positive disease (defined as immunohistochemistry [IHC] 3+ or IHC 2+/in situ hybridisation [ISH]+ on a fresh post-trastuzumab progression biopsy), an Eastern Cooperative Oncology Group performance status of 0 or 1, a left ventricular ejection fraction of ≥50%, and adequate renal and hepatic function. The post-trastuzumab progression biopsy sample could be obtained from a primary or metastatic tumour site using standard biopsy per local practice or a surgical specimen. To account for possible heterogeneity of HER2 expression in tumour tissue, the study protocol specified that multiple core biopsies (at least 2) be taken. Needle biopsies of fluid or cells and biopsies derived from bone (metastatic) were considered not acceptable. Previous adjuvant therapy with a trastuzumab-containing regimen was counted as a line of therapy if progression occurred within 6 months of completing adjuvant therapy. Patients of reproductive/childbearing potential were required to use a highly effective form of contraception for at least 7 months for females and 4 months for males, after the last dose of the study drug. Patients were excluded if they had spinal cord compression or clinically active central nervous system metastases, uncontrolled or significant cardiovascular disease, clinically severe pulmonary compromise, pleural effusion, ascites, or pericardial effusion, or a history of noninfectious interstitial lung disease (ILD) or pneumonitis that required corticosteroid therapy, current ILD/pneumonitis, or were suspected to have ILD/pneumonitis that could not be ruled out by imaging at screening. In addition, patients were excluded if they used anticancer therapy after first-line treatment with a trastuzumab-containing regimen.
Patients must have had ≥1 measurable lesion as per Response Evaluation Criteria in Solid Tumours version 1·1 (RECIST v1·1). All lesions (target and non-target) were measured at screening according to RECIST v1·1. Tumour assessment was performed by computed tomography (CT) or magnetic resonance imaging (MRI) of the chest, abdomen, pelvis, and any other sites of disease. Baseline CT or MRI of the brain was required for all patients.
The study was performed in accordance with the principles outlined in the Declaration of Helsinki and the International Conference of Harmonisation Guidelines for Good Clinical Practice and the protocol was approved by the independent ethics committee or institutional review board at each site. Written informed consent was obtained from all patients before initiation of the study. The full study protocol is provided in the appendix.
Outcomes
Efficacy endpoints were based on tumour assessments performed at screening and every 6 weeks during the first year of treatment and every 12 weeks thereafter until objective disease progression, based on radiologic assessment, or the initiation of a new anticancer therapy. Tumour scans were assessed by independent central review (ICR). The primary endpoint was confirmed ORR, defined as the proportion of patients who achieved a best overall response of confirmed complete response (CR) or partial response (PR), by ICR using RECIST v1·1. The key secondary endpoint was progression-free survival (PFS), defined as the time interval from the date of first dose of study drug to the date of disease progression or death due to any cause, by ICR. Other secondary endpoints were OS (defined as the time interval from the date of first dose of study drug to the date of death due to any cause), PFS by investigator, ORR by investigator, duration of response (DOR; defined as the time from the date of first documentation of objective response to the date of the first documentation of disease progression) by ICR and investigator, and safety. The prespecified exploratory endpoints included disease control rate (DCR; defined as CR rate plus PR rate plus stable disease [SD] rate) by ICR and investigator, time to response by ICR and investigator, best percent change in sum of diameters of measurable tumours, and assessment of exploratory biomarkers, including serum extracellular domain of HER2 assessment, and biomarker analysis using cell-free DNA collected from blood, and their association with disease status and/or response to treatment.
Procedures
All patients received 6·4 mg/kg of T-DXd administered intravenously every 3 weeks until the occurrence of disease progression as per RECIST v 1·1; clinical progression; or withdrawal by subject, physician decision, or death. Each patient had a 40-day (+7 days) follow-up visit after the last study drug administration or before starting new anticancer treatment. Long-term/survival follow-up visits occurred every 12 weeks (±14 days) from the date of the 40-day (+7 days) follow-up visit, until death, withdrawal of consent, or loss to follow-up, whichever occurred first. Sex and/or gender and race and/or ethnicity were captured through the electronic data capture system.
Tumour assessments via CT or MRI scans were performed within 28 days of the first dose, every 6 weeks (±7 days) starting at cycle 1 day 1 for the first year of treatment, every 12 weeks (±7 days) after the first year until disease progression or start of a new anticancer treatment, and at the 40-day (+7 days) follow-up visit if tumour assessment was not performed at the end of treatment visit or if study treatment was discontinued for any reason other than disease progression. CT or MRI of the brain was performed for all patients within 28 days before recruitment and every 6 weeks (±7 days) from cycle 1 day 1; this was mandatory for all patients with stable brain metastases at baseline. The brain CT or MRI scan was performed as clinically indicated for all other patients. Laboratory monitoring was performed during screening (day −28 to day −1), within 3 days before the infusion for all cycles, at cycle 1 day 8 and cycle 1 day 15 (±1 days), at end of treatment (defined as the date the investigator decided to discontinue study treatment [+7 days]), and at the 40-day (+7 days) follow up visit. Blood samples for biomarker analysis were taken within 3 days before the administration of T-DXd on Cycle 1 Day 1, on Day 1 of every 3 cycles (Cycles 4, 7, etc), and end of treatment.
At each study visit, patients were evaluated for adverse events, which were directly observed, reported spontaneously by the patient, or determined through questioning of the patient. Treatment-emergent adverse events (TEAEs) were assessed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v5·0. Safety endpoints include serious adverse events, TEAEs, drug-related TEAEs, discontinuations due to adverse events, and adverse events of special interest. Suspected ILD/pneumonitis cases were adjudicated by an external independent adjudication committee.
Two dose reductions of T-DXd were allowed, from 6.4 mg/kg to 5.4 mg/kg and then 4.4 mg/kg; dose increases were not permitted. Patients with more than two dose reductions were withdrawn from the study treatment if further toxicity meeting the requirement for dose reductions occurred. A dose could be delayed for up to 28 days (49 days from the last infusion date) from the planned date of the administration. Patients that required a dose delay longer than 28 days were withdrawn from the study.
Statistical analysis
Based on a reported best ORR of ~27% in second-line treatment of Western patients with gastric or GEJ cancer, 16 a sample size of 72 patients would be needed to provide 90% power to achieve a lower limit of 95% confidence interval (CI) for the ORR exceeding 27% under the expected ORR of 45%. The study was planned to enroll an additional 10% of patients to account for potential withdrawals, for an estimated sample size of 80 patients.
Primary and secondary efficacy analyses were performed in the full analysis set, which included all subjects who were enrolled and received ≥1 dose of the study drug. Safety analyses were performed in the safety analysis set, which included all treated subjects. Other exploratory analyses were performed based on the full analysis set and the availability of assessment
The primary analysis of confirmed ORR by ICR was performed for the full analysis set when the last subject had ≥18 weeks of follow up. ORR estimate and its two-sided 95% exact CI were performed using the Clopper-Pearson method. For the PFS analysis, patients who were alive with no objective documentation of radiological disease progression as assessed by ICR at the time of data cutoff were censored at the date of their last evaluable tumour assessment. For the OS analysis, patients who were alive at the time of data analysis were censored at the last contact date at which the patient was known to be alive. For the DOR analysis, patients who were alive and progression-free at the time of the analyses were censored at the date of the last evaluable tumour assessment. Kaplan-Meier estimates and survival curves are presented. For PFS and DOR, 95% CI was calculated using the Brookmeyer and Crowley method. DOR was measured for responding patients (PR or CR) and was analysed based on tumour assessment by ICR. For time to response, median and IQR were based on Kaplan-Meier estimates.
TEAEs were defined as an adverse event occurring, which was absent before the first dose of T-DXd or had worsened in severity or seriousness after initiating T-DXd until 47 days after the last dose of T-DXd. TEAEs also included serious adverse events with an onset or worsening 48 days or more after last dose of the study drug, if related to the study drug.
All analyses were done with the use of SAS version 9.4. The study is registered with ClinicalTrials.gov, NCT04014075.
Role of the funding source
The study was designed and led by the funder, Daiichi Sankyo, who participated in data collection and analysis. In March 2019, AstraZeneca entered into a collaboration agreement with Daiichi Sankyo for further clinical development of T-DXd. Both Daiichi Sankyo and AstraZeneca were involved in study oversight and data collection; both funders also assisted in data interpretation, writing the report, reviewing the manuscript, and the decision to submit the paper for publication.
Results
Over the course of this study, which recruited from November 26, 2019, to December 2, 2020, 79 patients were enrolled and treated with T-DXd (figure 1). Ten patients failed screening after central HER2 confirmation for not satisfying inclusion/exclusion criteria and were not treated. Baseline demographics and disease characteristics of patients are presented in table 1. Patients had a median age of 60·7 years (IQR 52·0‒68·3), 57 patients (72·2%) were male, and 69 patients (87·3%) were White. Overall, 27 patients (34·2%) had gastric adenocarcinoma and 52 patients (65·8%) had GEJ adenocarcinoma. HER2 expression was IHC 3+ in 68 patients (86·1%), IHC 2+/ISH+ in 10 patients (12·7%), and not evaluable (NE) in 1 patient (1·3%; patient enrollment was based on local laboratory testing only). All patients had an adenocarcinoma histology.
Figure 1: Patient disposition.
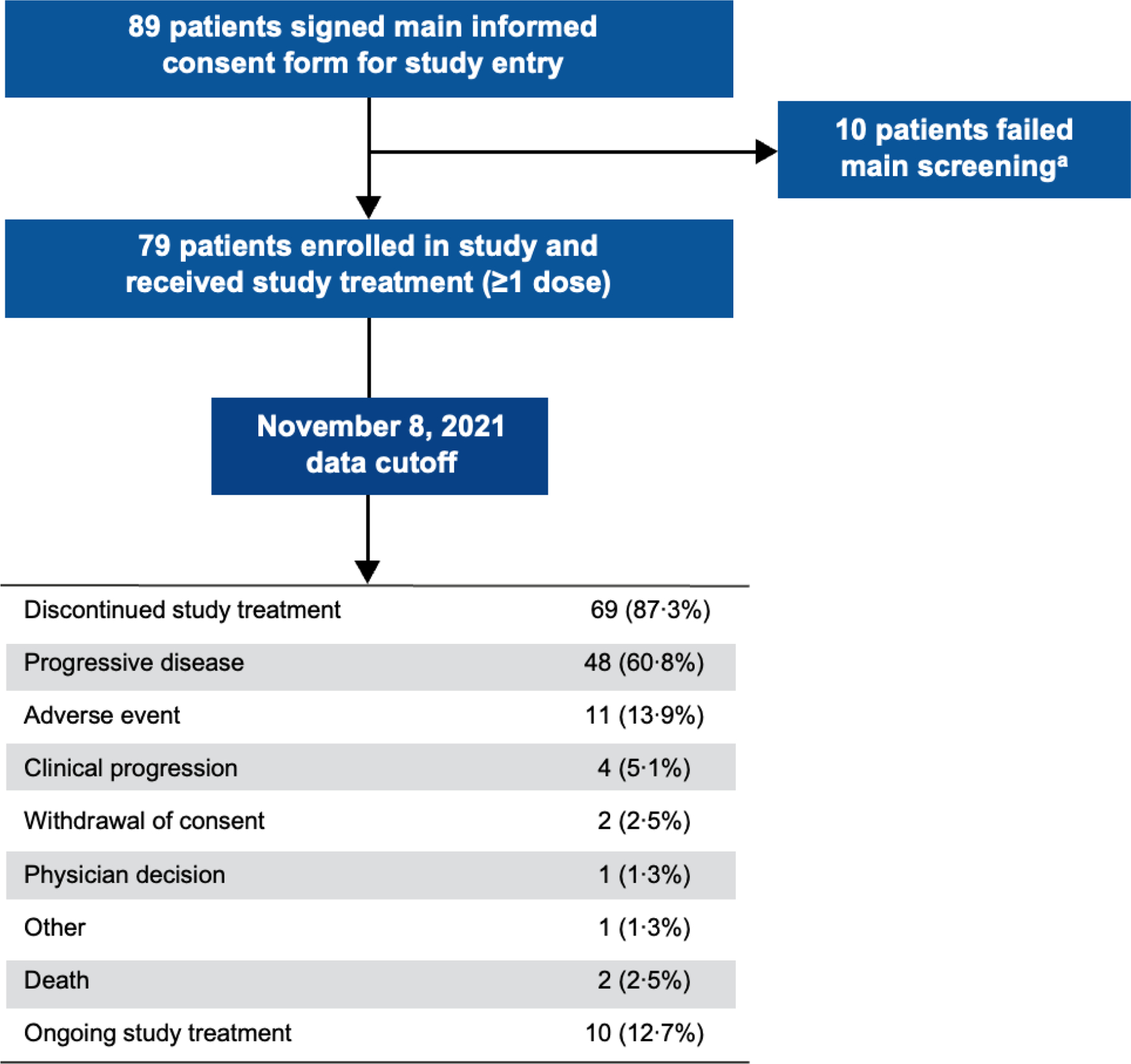
aThe 10 patients who failed main screening did not satisfy the inclusion/exclusion criteria of the study.
Table 1:
Patient baseline demographics and characteristics
| Baseline demographics and characteristics | Patients (N=79) |
|---|---|
| Age | |
| Median (IQR), years | 60·7 (52·0–68·3) |
| <65, n (%) | 46 (58·2) |
| ≥65, n (%) | 33 (41·8) |
| Sex, n (%) | |
| Male | 57 (72·2) |
| Female | 22 (27·8) |
| Race, n (%) | |
| White | 69 (87·3) |
| Black or African American | 1 (1·3) |
| Asian | 4 (5·1) |
| Native Hawaiian or Pacific Islander | 1 (1·3) |
| Other | 3 (3·8) |
| Missing | 1 (1·3) |
| ECOG PS, n (%) | |
| 0 | 29 (36·7) |
| 1 | 50 (63·3) |
| HER2 expression, n (%) | |
| IHC 3+ | 68 (86·1) |
| IHC 2+/ISH+ | 10 (12·7) |
| Not evaluable | 1 (1·3)a |
| Adenocarcinoma subtype, n (%) | 78 (98·7)b |
| Intestinal | 19 (24·1) |
| Diffuse | 1 (1·3) |
| Mixed | 1 (1·3) |
| Unknown | 57 (72·2) |
| Cancer type, n (%) | |
| Gastric | 27 (34·2) |
| GEJ | 52 (65·8) |
| Disease presentation at initial diagnosis, n (%) | |
| Metastatic disease or locally advanced | 69 (87·3) |
| Locoregional disease | 9 (11·4) |
| Unknown | 1 (1·3) |
| Number of metastatic sites, n (%) | |
| <2 | 5 (6·3) |
| ≥2 | 74 (93·7) |
| Previous total gastrectomy, n (%) | |
| Yes | 0 |
| No | 79 (100) |
| Liver metastasis at baseline, n (%) | 50 (63·3) |
| Time from diagnosis, median (IQR), months | 14·2 (10·2–25·9) |
| Lines of prior systemic therapy intended for locally advanced or metastatic cancer, n (%) | |
| 0 | 6 (7·6)c |
| 1 | 73 (92·4) |
| Prior platinum-based therapy, n (%) | 79 (100·0) |
| Prior trastuzumab therapy, n (%) | 79 (100·0) |
ECOG PS=Eastern Cooperative Oncology Group Performance Status; GEJ=gastroesophageal junction; IHC=immunohistochemistry; ISH=in situ hybridisation.
Demographics and baseline characteristics were not expected to change at the updated data cutoff and were not re-analysed. Data are reported based on the April 9, 2021, data cutoff.
Patient enrollment was based on local laboratory testing.
One patient had an intramucosal adenocarcinoma subtype and was not included in the 78 patients recorded as “adenocarcinoma.”
Patients were classified as having “unknown” adenocarcinoma subtype by the investigator as part of the case report form data entry.
These patients were included in this study as they had experienced progression after receiving adjuvant/neoadjuvant therapy.
A primary analysis was done with a data cutoff of April 9, 2021; results of the updated analysis that was requested as part of a health authority review are reported herein (data cutoff, November 8, 2021). As of the updated analysis, 10 (12·7%) patients remained on treatment.
Overall, 79 patients (100·0%) received prior treatment with a platinum-based therapy and trastuzumab. Seven patients (8·9%) received prior treatment with immune checkpoint inhibitors, with one patient (1·3%) previously receiving avelumab and six patients (7·6%) previously receiving pembrolizumab. The number of patients who had received prior immune checkpoint inhibitor therapy may have been greater, because seven patients (8·9%) had previously participated in trials in which they were randomised to double-blind treatment with either a checkpoint inhibitor or placebo. The post-trial anticancer treatments received are listed in appendix p 5.
A summary of antitumour efficacy outcomes is presented in table 2. As of the November 8, 2021, data cutoff (median follow-up of 10·2 months [IQR 5·6‒12·9 months]), 33 patients (41·8%) achieved a confirmed objective response (95% CI 30·8‒53·4), as assessed by ICR. In terms of best overall response, 4 patients (5·1%) had a CR, 29 patients (36·7%) had a PR, 31 patients (39·2%) had SD, and 13 (16·5%) had progressive disease. 64 patients (81·0%) experienced confirmed disease control (95% CI 70·6‒89·0), and median time to response was 1·4 months (95% CI 1·4‒2·7). Best percentage change of tumour size from baseline by ICR is shown in figure 2A. Patients with a HER2 score of IHC 3+ (n=68) had numerically greater ORR than those with IHC 2+/ISH+ (n=10) (47·1% [95% CI 34·8%‒59·6%] vs 10% [95% CI 0·3%‒44·5%]) (figure 2B). Patients with gastric tumours (n=27) had a numerically greater ORR than those with GEJ tumours (n=52) (55·6% [95% CI 35·3‒74·5] vs 34·6% [95% CI 22·0‒49·1]). Median DOR was 8·1 months (95% CI 5·9‒NE) (figure 3A); median PFS by ICR, a key secondary endpoint, was 5·6 months (95% CI 4·2‒8·3) (figure 3B); and median OS was 12·1 months (95% CI 9·4‒15·4) (figure 3C). The response assessed by the investigator was similar to the response assessed by ICR (appendix p 6).
Table 2:
Efficacy summary
| Response assessment by ICR | Patients (N=79)a |
|---|---|
| Confirmed ORR,b n (%) | 33 (41·8)[95% CI 30·8‒53·4] |
| Confirmed best overall response, n (%) | |
| CR | 4 (5·1) |
| PR | 29 (36·7) |
| SD | 31 (39·2) |
| PD | 13 (16·5) |
| Not evaluable | 2 (2·5) |
| Confirmed DCR, n (%) | 64 (81·0)[95% CI 70·6–89·0] |
| Median TTR, months | 1·4 (95% CI 1·4‒2·7) |
| Median DoR, months | 8·1 (95% CI 5·9‒NE)c |
| Median PFS, months | 5·6 (95% CI 4·2‒8·3)d |
| Patients with events, n (%) | 51 (64·6) |
| Progressive disease, n (%) | 44 (55·7) |
| Death, n (%) | 7 (8·9) |
| Median OS, months | 12·1 (95% CI 9·4‒15·4)e |
| Patients with events, n (%) | 26 (32·9) |
| Patients without events (censored), n (%) | 53 (67·1) |
| Alive, n (%) | 46 (58·2) |
| Lost to follow-up, n (%) | 7 (8·9) |
CI=confidence interval; CR=complete response; DCR=disease control rate; DoR=duration of response; ICR=independent central review; IQR=interquartile range; NE=not evaluable; ORR=objective response rate; OS=overall survival; PD=progressive disease; PFS=progression-free survival; PR=partial response; SD=stable disease; TTR=time to response.
Median follow up was 10·2 months (IQR, 5·6–12·9 months).
Primary endpoint.
Secondary endpoint analysis based on responders (n=33); 18 patients were censored (reasons: initiating new anticancer therapy, event after missing two consecutive assessments, ongoing without event, and adequate tumour assessment no longer available).
Secondary endpoint analysis conducted in the full analysis set based on 51 events.
Secondary endpoint analysis conducted in the full analysis set based on 46 events. OS is defined as the time from the date of the first dose to the date of death from any cause.
Figure 2: (A) Best percentage change of tumour size from baseline by independent central reviewa and (B) ORR across prespecified subgroups.
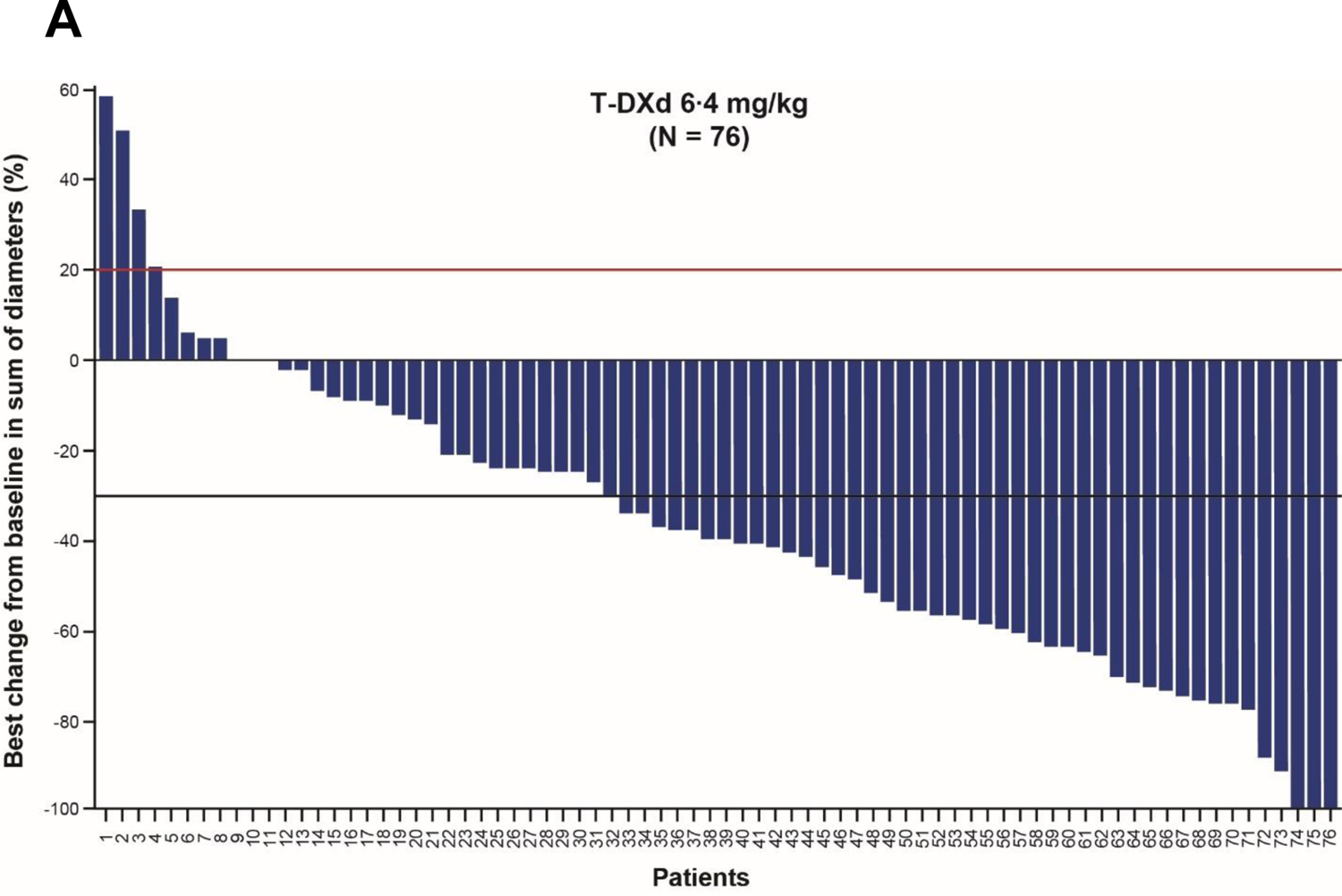
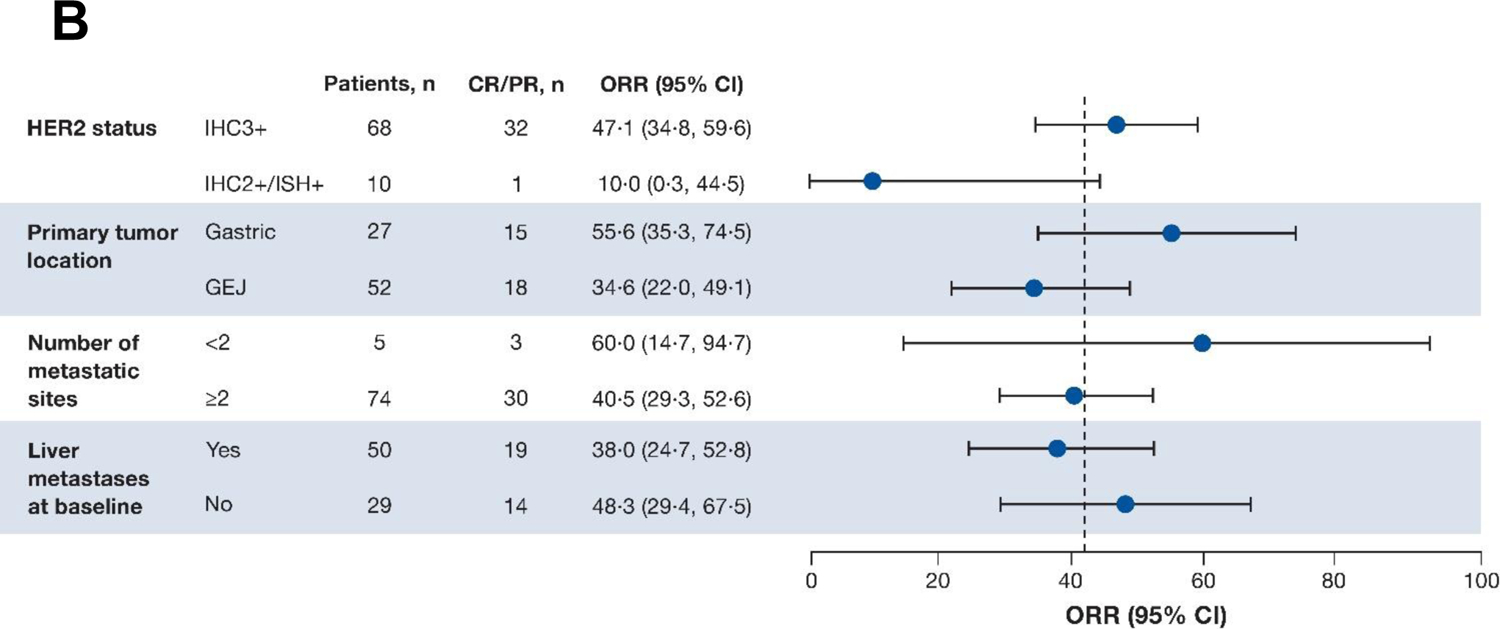
aThree patients were missing baseline or postbaseline target lesion assessment. Red line at 20% indicates progressive disease; black line at ‒30% indicates partial response. Analysis conducted in the full analysis set. Two-sided 95% CIs are based on the exact (Clopper-Pearson) binomial distribution. CI=confidence interval; CR=complete response; GEJ=gastroesophageal junction; HER2=human epidermal growth factor receptor 2; IHC=immunohistochemistry; ISH=in situ hybridisation; ORR=objective response rate; PR=partial response; T-DXd=trastuzumab deruxtecan.
Figure 3: (A) Duration of response by independent central review,a,b (B) progression-free survival by independent central review,c and (C) overall survivalc.
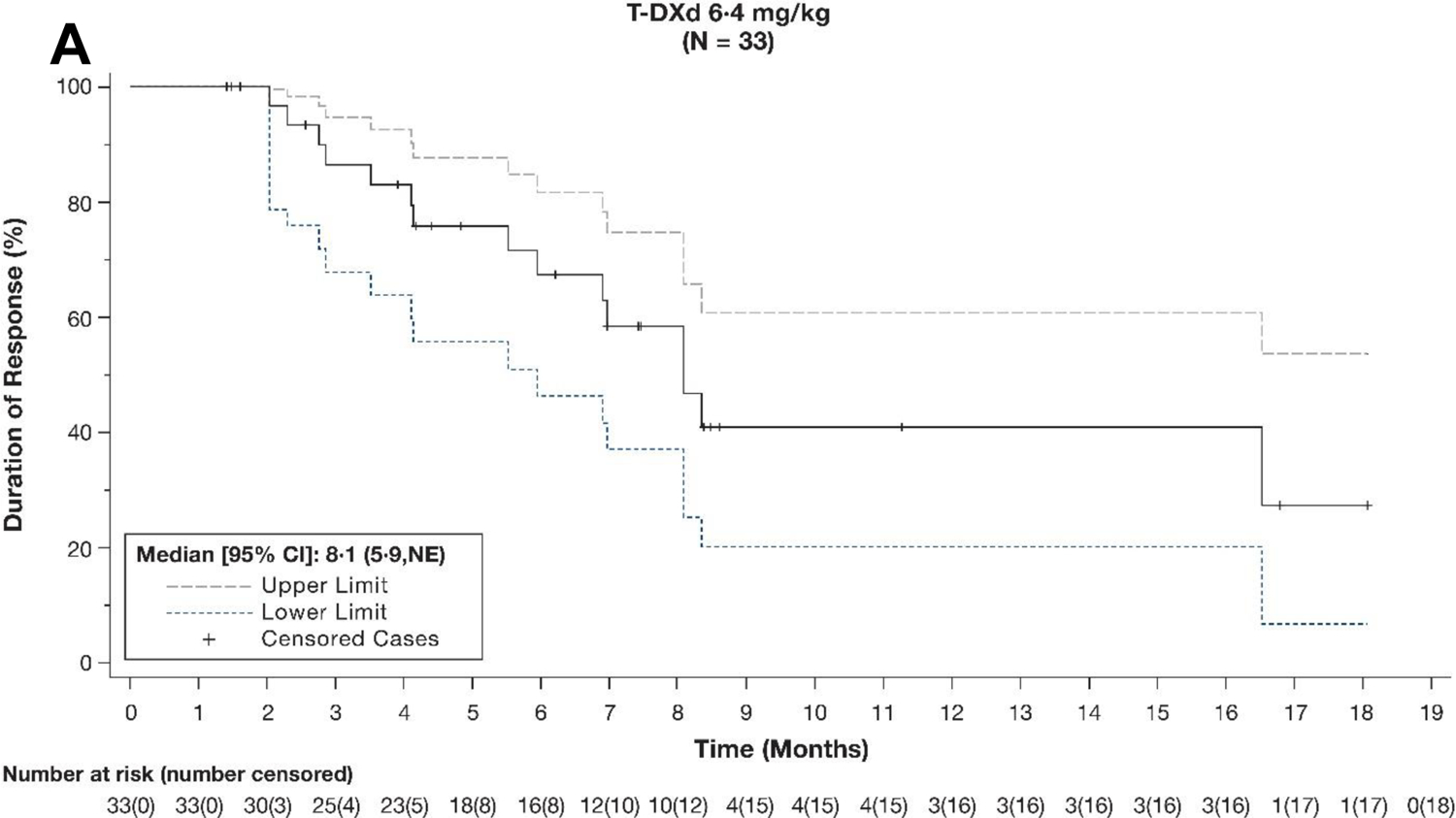
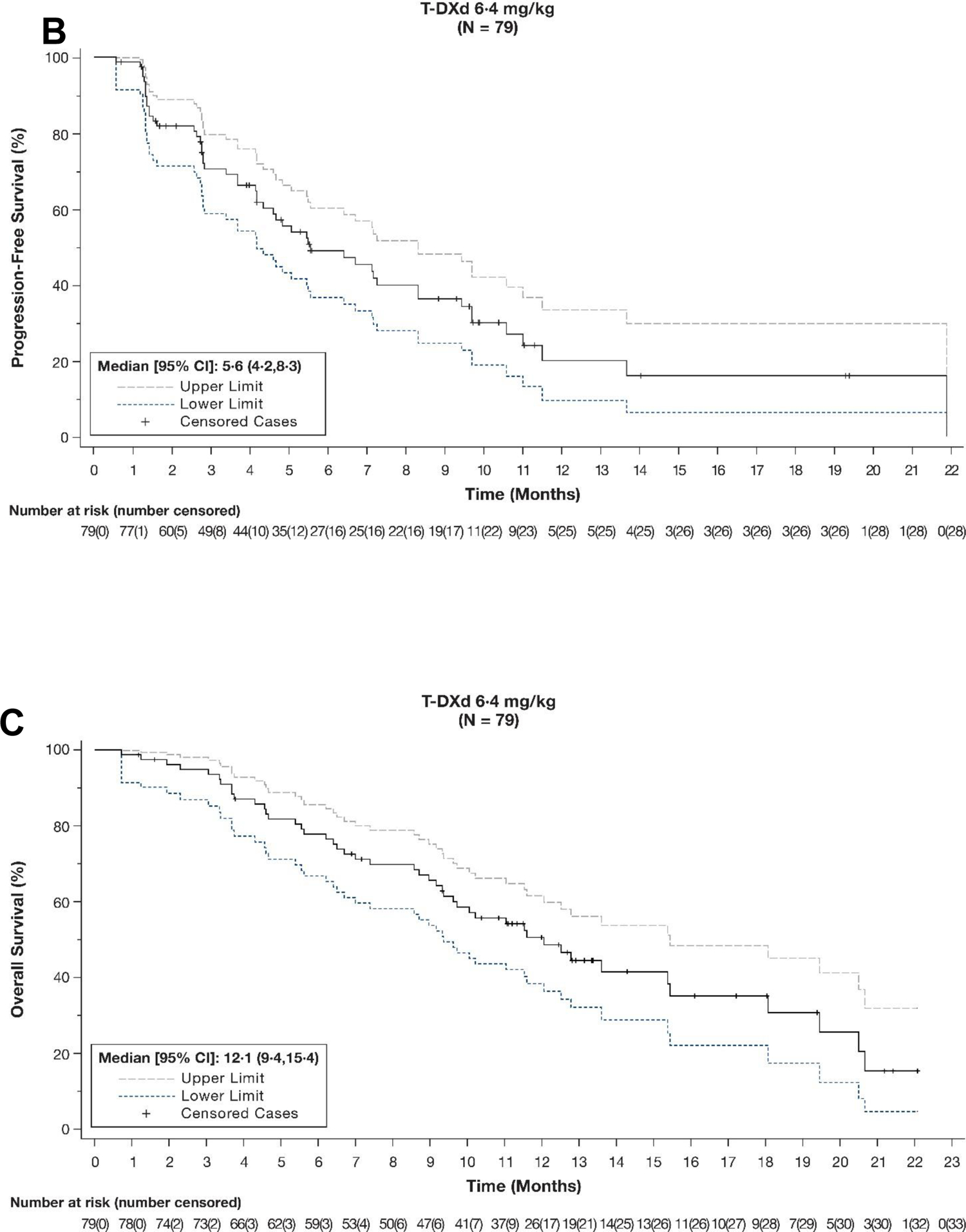
aAnalysis based on responders (n=33); 18 patients were censored (reasons: initiating new anticancer therapy, event after missing two consecutive assessments, ongoing without event, and adequate tumour assessment no longer available). bAnalysis conducted in the full analysis set based on 51 events (44 progressive disease, 7 deaths). cAnalysis conducted in the full analysis set. CI=confidence interval; NE=not evaluable; T-DXd=trastuzumab deruxtecan.
After a median treatment duration of 4·3 months (IQR 2·7‒10·1), 44 patients (55·7%) had experienced grade ≥3 TEAEs (appendix p 7). Drug-related TEAEs were reported in 75 patients (94·9%), with 24 (30·4%) being grade ≥3. The most common grade ≥3 TEAEs occurring in ≥5% of patients were anaemia (n=11, 13·9%), nausea (n=6, 7·6%), neutrophil count decrease (n=6, 7·6%), and white blood cell decrease (n=5, 6·3%) (table 3). TEAEs associated with study drug discontinuations, dose reductions, and dose interruptions occurred in 15 (19·0%), 17 (21·5%), and 23 (29·1%) patients, respectively, of which 10 (12·7%), 14 (17·7%), and 8 (10·1%) were drug-related (appendix p 7). Adjudicated ILD/pneumonitis was the most common drug-related TEAE associated with treatment discontinuation (eight patients [10·1%]). Nausea and neutrophil count decrease were the most common drug-related TEAEs associated with dose reduction (six [7·6%] and four patients [5·1%], respectively). Drug-related serious TEAEs were reported in 10 patients (12·7%); the most common were due to pneumonitis (n=3; 3·8%), ILD (n=2; 2·5%), and nausea (n=2; 2·5%).
Table 3:
TEAEs in ≥10% (grade 1–2) of patients by worst toxicity grade and all grade 3–5 events
| Patients (N = 79) | ||||
|---|---|---|---|---|
| TEAEs by Preferred Term, % (n) | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 |
| Nausea | 47 (59·5) | 6 (7·6) | 0 | 0 |
| Vomiting | 33 (41·8) | 2 (2·5) | 0 | 0 |
| Fatigue | 30 (38·0) | 3 (3·8) | 0 | 0 |
| Diarrhoea | 28 (35·4) | 1 (1·3) | 0 | 0 |
| Weight decreased | 25 (31·6) | 3 (3·8) | 0 | 0 |
| Constipation | 23 (29·1) | 0 | 0 | 0 |
| Decreased appetite | 22 (27·8) | 4 (5·1) | 0 | 0 |
| Anaemia | 19 (24·1) | 11 (13·9) | 0 | 0 |
| Alopecia | 19 (24·1) | 0 | 0 | 0 |
| Platelet count decreased | 12 (15·2) | 2 (2·5) | 0 | 0 |
| Aspartate aminotransferase increased | 12 (15·2) | 1 (1·3) | 0 | 0 |
| Hypokalaemia | 12 (15·2) | 0 | 1 (1·3) | 0 |
| Abdominal pain | 11 (13·9) | 2 (2·5) | 0 | 0 |
| Asthenia | 11 (13·9) | 1 (1·3) | 0 | 0 |
| Pyrexia | 9 (11·4) | 0 | 0 | 0 |
| Blood alkaline phosphatase increased | 8 (10·1) | 1 (1·3) | 0 | 0 |
| Cough | 8 (10·1) | 1 (1·3) | 0 | 0 |
| Epistaxis | 8 (10·1) | 0 | 0 | 0 |
| Gastroesophageal reflux disease | 8 (10·1) | 0 | 0 | 0 |
| Hypoalbuminemia | 8 (10·1) | 0 | 0 | 0 |
| Pneumonitis | 7 (8·9) | 0 | 0 | 1 (1·3) |
| Neutrophil count decreased | 7 (8·9) | 2 (2·5) | 4 (5·1) | 0 |
| Alanine aminotransferase increased | 7 (8·9) | 1 (1·3) | 0 | 0 |
| Dyspnoea | 6 (7·6) | 1 (1·3) | 0 | 0 |
| Hypotension | 5 (6·3) | 1 (1·3) | 0 | 0 |
| Neutropenia | 4 (5·1) | 4 (5·1) | 0 | 0 |
| Thrombocytopenia | 4 (5·1) | 1 (1·3) | 0 | 0 |
| White blood cell count decreased | 4 (5·1) | 5 (6·3) | 0 | 0 |
| Device-related infection | 3 (3·8) | 1 (1·3) | 0 | 0 |
| Dysphagia | 3 (3·8) | 3 (3·8) | 0 | 0 |
| Ascites | 3 (3·8) | 2 (2·5) | 0 | 0 |
| Blood bilirubin increased | 3 (3·8) | 2 (2·5) | 0 | 0 |
| Covid-19 | 2 (2·5) | 1 (1·3) | 0 | 2 (2·5) |
| Urinary tract infection | 2 (2·5) | 2 (2·5) | 0 | 0 |
| Hypophosphataemia | 2 (2·5) | 2 (2·5) | 0 | 0 |
| Hyperglycaemia | 1 (1·3) | 1 (1·3) | 0 | 0 |
| Hypertension | 2 (2·5) | 1 (1·3) | 0 | 0 |
| ILD | 2 (2·5) | 0 | 1 (1·3) | 1 (1·3) |
| Acute kidney injury | 2 (2·5) | 2 (2·5) | 0 | 0 |
| Cancer pain | 1 (1·3) | 1 (1·3) | 0 | 0 |
| Somnolence | 1 (1·3) | 1 (1·3) | 0 | 0 |
| Pleural effusion | 1 (1·3) | 1 (1·3) | 0 | 0 |
| Hepatotoxicity | 1 (1·3) | 1 (1·3) | 0 | 0 |
| Lymphocyte count decreased | 1 (1·3) | 2 (2·5) | 0 | 0 |
| Pneumonia | 0 | 0 | 2 (2·5) | 0 |
| Bacterial sepsis | 0 | 0 | 1 (1·3) | 0 |
| Catheter site infection | 0 | 1 (1·3) | 0 | 0 |
| Covid-19 pneumonia | 0 | 1 (1·3) | 0 | 0 |
| Staphylococcal infection | 0 | 1 (1·3) | 0 | 0 |
| Wound infection | 0 | 1 (1·3) | 0 | 0 |
| Malignant neoplasm progression | 0 | 0 | 0 | 2 (2·5) |
| Lymphangiosis carcinomatosa | 0 | 0 | 0 | 1 (1·3) |
| Tumour haemorrhage | 0 | 1 (1·3) | 0 | 0 |
| Febrile neutropenia | 0 | 1 (1·3) | 1 (1·3) | 0 |
| Lymphopenia | 0 | 1 (1·3) | 0 | 0 |
| Electrolyte imbalance | 0 | 1 (1·3) | 0 | 0 |
| Basal ganglia infarction | 0 | 1 (1·3) | 0 | 0 |
| Cerebrovascular accident | 0 | 0 | 0 | 1 (1·3) |
| Generalised tonic-clonic seizure | 0 | 1 (1·3) | 0 | 0 |
| Embolism | 0 | 2 (2·5) | 0 | 0 |
| Pulmonary embolism | 0 | 2 (2·5) | 0 | 0 |
| Anal fissure | 0 | 1 (1·3) | 0 | 0 |
| Anal fistula | 0 | 1 (1·3) | 0 | 0 |
| Colitis | 0 | 1 (1·3) | 0 | 0 |
| Enteritis | 0 | 1 (1·3) | 0 | 0 |
| Haematemesis | 0 | 1 (1·3) | 0 | 0 |
| Obstruction gastric | 0 | 1 (1·3) | 0 | 0 |
| Intestinal obstruction | 0 | 0 | 0 | 1 (1·3) |
| Oesophageal obstruction | 0 | 1 (1·3) | 0 | 0 |
| Bile duct stenosis | 0 | 1 (1·3) | 0 | 0 |
| Gallbladder obstruction | 0 | 1 (1·3) | 0 | 0 |
| Hydronephrosis | 0 | 1 (1·3) | 0 | 0 |
| Urinary tract obstruction | 0 | 1 (1·3) | 0 | 0 |
| Disease progression | 0 | 0 | 0 | 2 (2·5) |
| Oedema | 0 | 1 (1·3) | 0 | 0 |
| Animal bite | 0 | 1 (1·3) | 0 | 0 |
| Exposure to communicable disease | 0 | 1 (1·3) | 0 | 0 |
| Product issues | 0 | 1 (1·3) | 0 | 0 |
| Device occlusion | 0 | 1 (1·3) | 0 | 0 |
ILD=interstitial lung disease; TEAE=treatment-emergent adverse event.
As of the November 8, 2021, data cutoff, adjudicated ILD/pneumonitis related to T-DXd occurred in eight patients (10·1%). Two patients (2·5%) experienced grade 1 ILD/pneumonitis events, four patients (5·1%) experienced grade 2 events, and two patients (2·5%) experienced a grade 5 event. The median time to onset of adjudicated drug-related ILD/pneumonitis was 80·5 days (IQR 56·5‒148·0), with a median duration of 36·0 days (IQR 20·0‒56·0). Established ILD/pneumonitis management guidelines recommend to carefully monitor for signs or symptoms (cough, fever, dyspnea), discontinue or interrupt and/or dose reduce T-DXd, conduct imaging, and start corticosteroid therapy as soon as ILD/pneumonitis is suspected. 17
On the basis of laboratory values of the left ventricular ejection fraction (LVEF) collected during the study, grade 2 events (defined as a resting LVEF of ≥40 to <50%, or a 10% to <20% decrease from baseline) were observed in six patients (10·5%). No clinical heart failure events were reported.
TEAEs associated with the outcome of death were reported in 11 patients (13·9%), with 2 (2·5%) considered related to T-DXd (appendix p 7,8). Disease progression, neoplasm progression, and coronavirus disease 2019 (COVID-19) were reported in two patients each and were considered unrelated to T-DXd. Cerebrovascular accident, ILD, pneumonitis, intestinal obstruction, and lymphangiosis carcinomatosa were reported in one patient each. The two cases of ILD/pneumonitis were considered related to T-DXd by the investigator and by the ILD adjudication committee (appendix p 7,8).
Discussion
The DESTINY-Gastric02 study demonstrates clinically meaningful activity of T-DXd, with durable responses in Western patients with HER2-positive gastric or GEJ adenocarcinoma whose disease progressed on a trastuzumab-containing regimen. A high proportion of patients receiving T-DXd in this study achieved the primary endpoint of confirmed ORR by ICR (n=33, 41·8%; 95% CI 30·8‒53·4) compared with treatment groups in other second-line trials. 5,6,16,18 Furthermore, median PFS in this study was 5·6 months (95% CI 4·2‒8·3), and median OS was 12·1 months (95% CI 9·4‒15·4). The efficacy outcomes of second-line therapy with T-DXd in this study population are particularly promising when compared with historical data.
Efficacy data from DESTINY-Gastric01, conducted in Japan and South Korea in the third-line or later setting, were similar, with a confirmed ORR of 42·0% (95% CI 33·0‒51·4) with T-DXd versus 12·5% (95% CI 5·2‒24·1) with physician’s choice of irinotecan or paclitaxel (p=0·0001). Median PFS was 5·6 months with T-DXd versus 3·5 months with physician’s choice (hazard ratio (HR) 0·47 [95% CI 0·31‒0·71]; p=0·0003), and median OS was 12·5 months with T-DXd versus 8·9 months with physician’s choice (HR 0·60 [95% CI 0·42‒0·86]). 19
There have been limited numbers of studies in the second line in patients with HER2-positive gastric cancer, and the failure of previous trials for targeted therapies in this setting highlights the high unmet medical need. 5,6 Even though the historical 27% ORR in the second-line setting reported in this manuscript was not specific to patients with HER2-positive gastric cancer, similar ORRs have been reported in HER2-positive gastric cancer studies. In the randomised, open-label, phase 2/3 GATSBY study, 42/204 patients (20·6%) with previously treated HER2-positive advanced gastric cancer who received second-line trastuzumab emtansine 2.4 mg/kg weekly achieved an ORR, compared to 20/102 (19·6%) of those receiving taxane. 5 In the randomised, open-label, phase 3 TyTAN study, ORR was 27% in patients with HER2-positive advanced gastric cancer who received second-line treatment with lapatinib plus paclitaxel versus 9% in patients who received paclitaxel alone (OR, 3·85; 95% CI, 1·80 ‒8.87; p<0·001). 6 Lastly, in the randomised, open-label, phase 2 T-ACT study, second-line treatment with paclitaxel plus trastuzumab achieved a similar response rate (RR) (33%) to that with paclitaxel alone (32%) in patients with HER2-positive advanced gastric or GEJ cancer in Japan. 18
A more useful comparator for the results of the present DESTINY-Gastric02 study may be the RAINBOW trial of ramucirumab plus paclitaxel, which produced positive results in the second-line setting. 16 Although the patient populations differed somewhat, second-line therapy with ramucirumab plus paclitaxel in the RAINBOW trial was associated with consistently lower response rates and survival outcomes compared with second-line T-DXd in the DESTINY-Gastric02 trial. In the RAINBOW trial, ORR by investigator was 28% (95% CI 23‒33) for ramucirumab plus paclitaxel versus 16% (95% CI 13‒20) for placebo plus paclitaxel (p=0·0001). Median PFS with ramucirumab plus paclitaxel was 4·4 months (95% CI 4·2–5·3) versus 2·9 months (95% CI 2·8–3·0) for placebo plus paclitaxel (HR 0·635 [95% CI 0·536–0·752]; p<0·0001), and median OS with ramucirumab plus paclitaxel was 9·6 months (95% CI 8·5–10·8) versus 7·4 months (95% CI 6·3–8·4) for placebo plus paclitaxel (HR 0·807 [95% CI 0·678–0·962]; p=0·017). 16 In a subgroup analysis of Western patients in the RAINBOW trial, ORR by investigator (26·8%) and survival outcomes (median OS 8·6 months) with ramucirumab plus paclitaxel were also lower than in DESTINY-Gastric02. 20
Asian patients with gastric cancer participating in clinical trials typically have better survival outcomes than Western patients. 21,22 Explanations have been put forward to explain the differences in gastric cancer epidemiology and treatment outcome between these populations. Distal gastric cancer is more common in Asian patients, whereas GEJ cancer is more prevalent in Western patients. Evidence suggests that Asian patients tend to have less advanced or aggressive disease at initial diagnosis, thought to be in part due to gastric cancer screening programmes, and are more likely to receive systemic therapy; 21,22 those with gastric tumours tend to have better survival outcomes relative to GEJ tumours. 23 Risk factors for GEJ cancer include obesity, reflux disease, and smoking, which are more common in Western populations. 24 However, Asian patients may be at a higher risk of ILD/pneumonitis (mild/moderate in severity) associated with T-DXd compared with non-Asian patients, as suggested by a pooled analysis of nine clinical studies that compared rates of any-grade adjudicated drug-related ILD/pneumonitis in patients enrolled in Japan with those enrolled in other countries. 25 Of note, higher proportions of patients in the T-DXd treatment arm in DESTINY-Gastric02 had worse prognostic factors (such as ≥2 metastatic sites, presence of liver metastases, higher baseline tumour burden [as assessed by median sum of longest diameters], and an ECOG PS of 1) compared with those in the T-DXd arm of DESTINY-Gastric01. 1 The improved safety profile observed in Western patients in DESTINY-Gastric02 potentially can be attributable to the use of T-DXd in an earlier-line treatment setting than in DESTINY-Gastric01.
In DESTINY-Gastric02, patients were required to have biopsy-confirmed HER2-positive disease based on the trial inclusion criteria. After treatment with trastuzumab, some patients have been observed to lose HER2 expression, potentially making their disease refractory to subsequent HER2-directed treatment. 6,26–28 HER2 stability during disease progression has not been widely reported, but HER2 loss has been reported to occur in about a third of patients in the few studies that re-evaluated HER2 status.8,26 Studies to investigate whether HER2 expression is lost following treatment with T-DXd are ongoing.
The safety profile of T-DXd observed in DESTINY-Gastric02 was generally consistent with the established safety profile of T-DXd. The most common adverse events were gastrointestinal or hematological in nature, and the incidence rates of TEAEs, including those of any grade or grade ≥3, and adverse events of special interest such as ILD/pneumonitis, were lower than those reported in DESTINY-Gastric01. 19 It should be noted that DESTINY-Gastric02 enrolled and treated patients throughout the COVID-19 pandemic. COVID-19 infection led to six patients either discontinuing treatment or interrupting their dose, and was attributed as the cause of death of two patients (one of whom experienced dose discontinuation, while the other had neither discontinuation or interruption of dosing).
Limitations of the present study include the absence of a comparator or placebo group and a relatively small patient population. The small size of patient subgroups makes it difficult to draw more specific conclusions from the data. It is also worth noting that although a post-trastuzumab progression biopsy sample was needed for patients to be eligible for inclusion in this study, this may not always be feasible in clinical practice due to the difficulty in obtaining adequate biopsy material or considering the clinical condition of the patient. Nevertheless, such a practice should be encouraged, based on the potential loss of HER2 expression discussed above.
Although this study is limited by its single-arm design, there have been relatively few studies in the second line in patients with HER2-positive gastric cancer to date. DESTINY-Gastric02 provides data supporting clinically meaningful efficacy and a manageable safety profile and thus, compelling evidence that T-DXd is a valuable second-line HER2-targeted treatment option for Western patients, similar to what was observed in DESTINY-Gastric01 for Asian patients in the third-line and later settings. This has been validated by recent EU approval of T-DXd for patients with gastric/GEJ cancer who have received a prior trastuzumab-based regimen. 29 Future analyses of this study will evaluate circulating biomarkers including cell-free DNA, an emerging potential diagnostic biomarker. In addition, biomarker studies to evaluate the utility of liquid biopsies in gastric cancer and other solid tumours are ongoing. Results from DESTINY-Gastric02 also support the ongoing randomised, phase 3 DESTINY-Gastric04 (NCT04704934) trial, which is investigating the efficacy and safety of T-DXd compared with ramucirumab and paclitaxel in patients with gastric or GEJ cancer whose disease has progressed on or after a trastuzumab-containing regimen. 30
Supplementary Material
Research in Context.
Evidence before this study
We searched PubMed for clinical trials evaluating second-line treatments for human epidermal growth factor receptor 2 (HER2)‒positive gastric or gastroesophageal junction (GEJ) cancer published between July 2015 and April 2022. The search terms included were “HER2 positive” or “HER2 expressing” or “HER2 amplified” and “gastric cancer” or “gastroesophageal junction cancer”; the search was restricted to English language publications. The results of this search were used to frame the context of the DESTINY-Gastric02 study in terms of its objectives and findings. The HER2-targeted antibody-drug conjugate trastuzumab deruxtecan (T-DXd; DS-8201) is approved in several countries worldwide for the treatment of patients with locally advanced or metastatic gastric or GEJ adenocarcinoma who have previously received a trastuzumab-based regimen. Approvals were based on the phase 2, open-label, multicentre, randomised DESTINY-Gastric01 trial of T-DXd in patients from Japan and the Republic of Korea.
Added value of this study
This is the first phase 2 study of T-DXd in Western patients with unresectable or metastatic gastric or GEJ cancer who have had disease progression on or after a trastuzumab-containing regimen. T-DXd exhibited clinical efficacy in this patient population and a safety profile generally consistent with that observed in previous studies.
Implications of all the available evidence
These results support further clinical research into T-DXd, including the ongoing phase 3 DESTINY-Gastric04 trial, which will investigate the efficacy and safety of T-DXd compared with ramucirumab and paclitaxel in patients with gastric or GEJ cancer whose disease has progressed on or after a trastuzumab-containing regimen.
Acknowledgements
We thank the patients and their families for their participation and the study site staff for their contributions. This study was sponsored and designed by Daiichi Sankyo in collaboration with AstraZeneca. Medical writing support was provided by Laura Halvorson, PhD, Rachel Hood, PhD, and Greg Town, BSc (ApotheCom) and was funded by Daiichi Sankyo.
Funding:
Daiichi Sankyo and AstraZeneca
Footnotes
Declaration of interests
EvC has received research grants paid to his institution from Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Ipsen, Lilly, Merk Sharp & Dohme, Merck KGaA, Novartis, Roche, and Servier and has participated in advisory boards for AbbVie, Array, Astellas, AstraZeneca, Bayer, Beigene, Biocartis, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Halozyme, GSK, Helsinn, Incyte, Ipsen, Janssen Research, Lilly, Merck Sharp & Dohme, Merck KGaA, Mirati, Novartis, Pierre Fabre, Roche, Seagen, Servier, Sirtex, Terumo, Taiho, TRIGR, and Zymeworks.
MdB has nothing to disclose.
ES has received consulting fees from Amal Therapeutics, AstraZeneca, Bristol Myers Squibb, Beigene, Daiichi Sankyo, Merck, Novartis, Pfizer, Roche, Servier, Turning Point Therapeutics, and Zymeworks; speaker payments or honoraria from Amgen, Bristol Myers Squibb, Novartis, and Servier; support for meeting attendance/travel from Amgen, Bristol Myers Squibb, and Servier; participated on a data safety monitoring board or advisory board for Amgen, AstraZeneca, and Beigene; and held a leadership role (co-chair) of the EORTC GI Trials Group Gastric Cancer Task Force.
IC has received research funding from Eli Lilly and Janssen-Cilag, has participated in advisory boards for Eli Lilly, Bristol Myers Squibb, MSD, Roche, Merck-Serono, AstraZeneca, OncXerna, Pierre Fabre, Boehringer Ingelheim, Incyte, Astellas, GSK, Sotio, Eisai, Daiichi Sankyo, Taiho, Servier, Seagen, and Turning Point Therapeutics, and has received support for meeting attendance/travel from Bristol Myers Squibb.
HP has received grants or contracts from Adlai Nortye USA, Alpine Immune Sciences, Ambrx, Amgen, Aprea Therapeutics AB, Array BioPharma, Bayer, BeiGene, BJ Bioscience, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Elicio Therapeutics, EMD Serono, Exelixis, Genentech, Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Hoffman-LaRoche, Hutchison MediPharma, ImmuneOncia Therapeutics, Incyte, Jounce Therapeutics, Mabspace Biosciences, MacroGenics, Medimmune, Medivation, Merck, Millennium, Mirati Therapeutics, Novartis Pharmaceuticals, Oncologie, Pfizer, PsiOxus Therapeutics, Puma Biotechnology, Regeneron Pharmaceuticals, RePare Therapeutics, Seagen, Synermore Biologics, Taiho Pharmaceutical, TopAlliance Biosciences, Turning Point Therapeutics, Vedanta Biosciences, Xencor Inc, and participated on a data safety monitoring board or advisory board for Jacobio.
SS has participated in advisory boards for Agenus, AstraZeneca, Bayer, Bristol Myers Squibb, CheckmAb, Daiichi Sankyo, Guardant Health, Menarini, Merck, Novartis, Pierre Fabre, Roche-Genentech, and Seagen.
SL has received research funding paid to her institution from Daiichi Sankyo, Amgen, Merck Serono, Bayer, Roche, Lilly, AstraZeneca, and Bristol Myers Squibb; consulting fees from Amgen, Merck Serono, Lilly, AstraZeneca, Incyte, Daiichi Sankyo, Bristol Myers Squibb, Servier, and MSD; speaker payments or honoraria from Roche, Lilly, Bristol Myers Squibb, Servier, Merck Serono, Pierre Fabre, GSK, and Amgen.
ZAW has received consulting fees from Merck, Ibsen, Lilly, Five Prime, QED, Molecular Templates, Daiichi Sankyo, AstraZeneca, Bayer, and Novartis; support for meeting attendance/travel from Lilly, Merck, Novartis, and Daiichi Sankyo; and participated on a data safety monitoring board or advisory board for Array.
JA has nothing to disclose.
JC has received research funding paid to his institution from Daiichi Sankyo, Merck, and Brooklyn Immunotherapeutics; consulting fees from Lilly, Merck, AstraZeneca, Foundation Medicine, Daiichi Sankyo, Macrogenics, Amgen, Ono Pharmaceutical, Bristol Myers Squibb, Astellas, Turning Point Therapeutics, Silverback Therapeutics, Novartis, Coherus Biosciences, Geneos, and Roche; speaker payments or honoraria from Merck and Bristol Myers Squibb; and participated on a data safety monitoring board for Yiviva.
YJ has received grants or contracts from Bayer, Bristol Myers Squibb, Cycle for Survival, Department of Defense, Eli Lilly, Fred’s Team, Genentech/Roche, Merck, NCI, and RGENIX; speaker payments or honoraria from Amerisource Bergen, Arcus Biosciences, AstraZeneca, Basilea Pharmaceutical, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Geneos Therapeutics, GlaxoSmithKline, Imedex, Imugene, Lynx Health, Merck, Merck Serono, Michael J. Hennessy Associates, Paradigm Medical Communications, PeerView Institute, Pfizer, Research to Practice, RGENIX, Seagen, Silverback Therapeutics, and Zymeworks Inc; and stock options from RGENIX.
FB was an employee at the time of this study and held stocks or stock options in Daiichi Sankyo.
YK, AQ, and JS are employees of Daiichi Sankyo.
GK has received research funding paid to his institution from Arog, AstraZeneca, BMS, Daiichi Sankyo, Merck, Oncolys, Pieris, and Zymeworks; consulting fees from Apexigen, AstraZeneca, BMS, Merck, Pieris, and Zymeworks; support for meeting attendance/travel from BMS, Merck, and Pieris; and participated on a data safety monitoring board or advisory board for Zymeworks.
Contributor Information
Prof Eric Van Cutsem, University Hospitals Gasthuisberg, Leuven and University of Leuven (KUL), Leuven, Belgium.
Maria di Bartolomeo, Fondazione IRCCS Istituto Nazionale dei Tumori, Via G. Venezian, 20133 Milan, Italy.
Elizabeth Smyth, Cambridge University Hospitals NHS Foundation Trust, Hills Road, Cambridge CB2 0QQ, UK.
Ian Chau, The Royal Marsden Hospital, Downs Road, Sutton SM2 5PT, Surrey, UK.
Haeseong Park, Siteman Cancer Center, Washington University School of Medicine, 660 S. Euclid Avenue, St. Louis, MO, 63110, USA.
Prof Salvatore Siena, Università degli Studi di Milano and Grande Ospedale Metropolitano Niguarda, Piazza dell’Ospedale Maggiore, 3, 20162, Milan, Italy.
Sara Lonardi, Veneto Institute of Oncology IOV - IRCCS, Via Gattamelata, 64, 35128, Padova, Italy.
Zev A. Wainberg, Department of Medicine-Hematology/Oncology, University of California Los Angeles, 200 Medical Plaza Driveway, Los Angeles, CA, 90024, USA.
Prof Jaffer Ajani, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX, 77030, USA.
Joseph Chao, City of Hope Comprehensive Cancer Center, 1500 E Duarte Road, Duarte, CA, 91010, USA.
Yelena Janjigian, Memorial Sloan Kettering Cancer Center, 300 East 66th Street, New York, NY, 10065, USA.
Amy Qin, Daiichi Sankyo, 216 Mt. Airy Road, Basking Ridge, NJ, 07920, USA.
Jasmeet Singh, Daiichi Sankyo, 216 Mt. Airy Road, Basking Ridge, NJ, 07920, USA.
Ferdous Barlaskar, Daiichi Sankyo, 216 Mt. Airy Road, Basking Ridge, NJ, 07920, USA.
Yoshinori Kawaguchi, Daiichi Sankyo, 216 Mt. Airy Road, Basking Ridge, NJ, 07920, USA.
Geoffrey Ku, Memorial Sloan Kettering Cancer Center, 300 East 66th Street, New York, NY, 10065, USA.
Data sharing
Anonymised individual participant data (IPD) on completed studies and applicable supporting study documents may be available upon request at https://vivli.org/. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of company and our clinical study subjects. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi-sankyo/. For more information, see appendix (p 2).
References
- 1.Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 2020; 382: 2419–30. [DOI] [PubMed] [Google Scholar]
- 2.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–97. [DOI] [PubMed] [Google Scholar]
- 3.Janjigian YY, Kawazoe A, Yanez PE, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: Initial findings of the global phase 3 KEYNOTE-811 study. J Clin Oncol 2021; 39(15 suppl). Abstract 4013. [Google Scholar]
- 4.US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for HER2-positive gastric cancer. Published May 5, 2021. Accessed March 8, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-her2-positive-gastric-cancer
- 5.Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017; 18: 640–53. [DOI] [PubMed] [Google Scholar]
- 6.Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014; 32: 2039–49. [DOI] [PubMed] [Google Scholar]
- 7.Seo S, Ryu MH, Park YS, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer 2019; 22: 527–35. [DOI] [PubMed] [Google Scholar]
- 8.Enhertu® (fam-trastuzumab deruxtecan-nxki) for injection, for intravenous use. Prescribing information. Daiichi Sankyo, Inc.; 2022. [Google Scholar]
- 9.Enhertu® for intravenous drip infusion 100 mg. Prescribing information. Daiichi Sankyo Co., Ltd.; 2020. [Google Scholar]
- 10.Enhertu (fam-trastuzumab deruxtecan-nxki) for injection, for intravenous use. Prescribing information – Israel]. Daiichi Sankyo Europe GMBH; 2021. [Google Scholar]
- 11.Nakada T, Sugihara K, Jikoh T, Abe Y, Agatsuma T. The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem Pharm Bull 2019; 67: 173–85. [DOI] [PubMed] [Google Scholar]
- 12.Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 2016; 22: 5097–108. [DOI] [PubMed] [Google Scholar]
- 13.Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol 2017; 18: 1512–22. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda T, Saika K. The 5-year relative survival rate of stomach cancer in the USA, Europe and Japan. Jpn J Clin Oncol 2013; 43: 1157–8. [DOI] [PubMed] [Google Scholar]
- 15.Griniatsos J, Trafalis D. Differences in gastric cancer surgery outcome between East and West: diff erences in surgery or different diseases? J BUON 2018; 23: 1210–5. [PubMed] [Google Scholar]
- 16.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014; 15: 1224–35. [DOI] [PubMed] [Google Scholar]
- 17.Swain SM, Nishino M, Lancaster LH, et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-Focus on proactive monitoring, diagnosis, and management. Cancer Treat Rev 2022; 106: 102378. [DOI] [PubMed] [Google Scholar]
- 18.Makiyama A, Sukawa Y, Kashiwada T, et al. Randomized,pPhase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT Study). J Clin Oncol 2020; 38: 1919–27. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi k, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-positive advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: Final overall survival (OS) results from a randomized, multicenter, open-label, phase 2 study (DESTINY-Gastric01). J Clin Oncol 2021; 39(15 suppl). Abstract 4048. [Google Scholar]
- 20.Shitara K, Muro K, Shimada Y, et al. Subgroup analyses of the safety and efficacy of ramucirumab in Japanese and Western patients in RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer 2016; 19: 927–38. [DOI] [PubMed] [Google Scholar]
- 21.Theuer CP, Kurosaki T, Ziogas A, Butler J, Anton-Culver H. Asian patients with gastric carcinoma in the United States exhibit unique clinical features and superior overall and cancer specific survival rates. Cancer 2000; 89: 1883–92. [DOI] [PubMed] [Google Scholar]
- 22.Gill S, Shah A, Le N, Cook EF, Yoshida EM. Asian ethnicity-related differences in gastric cancer presentation and outcome among patients treated at a canadian cancer center. J Clin Oncol 2003; 21: 2070–6. [DOI] [PubMed] [Google Scholar]
- 23.Balakrishnan M, George R, Sharma A, Graham DY. Changing trends in stomach cancer throughout the world. Curr Gastroenterol Rep 2017; 19: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol 2013; 23: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell CA, Modi S, Iwata H, et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 2022; 7(4): 100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishimine Y, Goto A, Watanabe Y, et al. Loss of HER2 positivity after trastuzumab in HER2-positive gastric cancer: Is change in HER2 status significantly frequent? Case Rep Gastrointest Med 2015; 2015: 132030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saeki H, Oki E, Kashiwada T, et al. Re-evaluation of HER2 status in patients with HER2-positive advanced or recurrent gastric cancer refractory to trastuzumab (KSCC1604). Eur J Cancer 2018; 105: 41–9. [DOI] [PubMed] [Google Scholar]
- 28.Pietrantonio F, Caporale M, Morano F, et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int J Cancer 2016; 139: 2859–64. [DOI] [PubMed] [Google Scholar]
- 29.Enhertu® (fam-trastuzumab-nxki) for injection, for intravenous use. Prescribing information. Daiichi Sankyo Europe GmbH; 2023.. [Google Scholar]
- 30.US National Library of Medicine. Trastuzumab deruxtecan for subjects with HER2-positive gastric cancer or gastro-esophageal junction adenocarcinoma after progression on or after a trastuzumab-containing regimen (DESTINY-Gastric04). First posted January 12, 2021; Last updated February 22, 2023. Accessed March 8, 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised individual participant data (IPD) on completed studies and applicable supporting study documents may be available upon request at https://vivli.org/. In cases where clinical study data and supporting documents are provided pursuant to our company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of company and our clinical study subjects. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi-sankyo/. For more information, see appendix (p 2).


