Abstract
Pro-inflammatory cytokines upregulate the expression of the H2O2-producing NADPH oxidase dual oxidase 2 (DUOX2)2 which, when elevated, adversely affects survival from pancreatic ductal adenocarcinoma (PDAC). Because the cGAS-STING pathway is known to initiate pro-inflammatory cytokine expression following uptake of exogenous DNA, we examined whether activation of cGAS-STING could play a role in the generation of reactive oxygen species by PDAC cells. Here, we found that a variety of exogenous DNA species markedly increased the production of cGAMP, the phosphorylation of TBK1 and IRF3, and the translocation of phosphorylated IRF3 into the nucleus, leading to a significant, IRF3-dependent enhancement of DUOX2 expression, and a significant flux of H2O2 in PDAC cells. However, unlike the canonical cGAS-STING pathway, DNA-related DUOX2 upregulation was not mediated by NF-κB. Although exogenous IFN-β significantly increased Stat1/2-associated DUOX2 expression, intracellular IFN-β signaling that followed cGAMP or DNA exposure did not itself increase DUOX2 levels. Finally, DUOX2 upregulation subsequent to cGAS-STING activation was accompanied by the enhanced, normoxic expression of HIF-1α and VEGF-A as well as DNA double strand cleavage, suggesting that cGAS-STING signaling may support the development of an oxidative, pro-angiogenic microenvironment that could contribute to the inflammation-related genetic instability of pancreatic cancer.
Keywords: cGAS, STING, dual oxidase 2, pancreatic cancer, reactive oxygen species
1. Introduction
Dual oxidase 2 (DUOX2) is an NADPH oxidase family member that plays an important role in mediating innate immunity at mucosal surfaces. Reactive oxygen species (ROS) produced by DUOX2 contribute to chronic inflammation-related tissue injury as well as angiogenesis and can support the growth of epithelial malignancies [1–3]. DUOX2 expression is significantly increased in patients with chronic pancreatitis; furthermore, patients with repetitive bouts of pancreatic inflammation are predisposed to develop pancreatic ductal adenocarcinoma (PDAC), suggesting that DUOX2-mediated ROS could play a role in pancreatic carcinogenesis [4, 5]. Our recent studies focusing on the control of DUOX2 expression have revealed that pro-inflammatory cytokines, including IFN-γ (interferon γ), IL-4 (interleukin 4), and IL-17A (interleukin 17A), upregulate DUOX2 expression in pancreatic cancer cells, producing oxidative DNA damage and DNA double strand breaks that could contribute to the pathogenesis of PDAC [4, 6, 7].
The cGAS-STING (cyclic GMP-AMP Synthase [cGAS]; Stimulator of Interferon Genes [STING]) signaling axis has been shown to play a vital role in innate immunity, protecting the host from viral infection. This signaling axis has also been demonstrated to promote cancer progression and oncogenesis, as well as enhance antitumor immunity [8–11]. Intratumoral injection of cyclic GMP-AMP (cGAMP) in murine cancer models produces an accumulation of macrophages in the microenvironment of various malignancies, such as breast cancer and melanoma, which subsequently leads to the recruitment of CD8+ T cells that secrete a variety of pro-inflammatory cytokines (TNF-α and IFN-β) [11]. However, activation of the cGAS-STING pathway has also been demonstrated to stimulate carcinogenesis, in part by supporting an immunosuppressive and pro-metastatic microenvironment [12] as well as suppressing DNA repair [9].
The cytosolic DNA sensor cGAS detects and binds double-stranded DNA (dsDNA) that is ~90 bp in length and longer [13] in a sequence-independent fashion. It then catalyzes the formation of cGAMP from GTP and ATP [14, 15]. cGAMP, in turn, binds to the ER-bound protein STING which translocates to the Golgi and recruits tank-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3). Subsequently, TBK1 phosphorylates itself as well as IRF3 [16]. Phosphorylated IRF3 can then translocate into the nucleus along with NF-κB to promote the transcription of Type I Interferons (IFN) [14, 17, 18].
cGAS-STING appears to be involved in both the initiation and progression phases of PDAC [19, 20]. Activation of STING signaling and enhancement of pancreatic inflammation have been demonstrated in a murine model of pancreatitis. Zhao and colleagues found that DNA released by necrotic pancreatic acinar cells was taken up by phagocytes in the microenvironment and activated STING signaling and production of type 1 IFN [21].
The importance of the pancreatic tumor microbiome in oncogenesis, cancer progression, and patient outcomes has recently been demonstrated [22–24]. Enrichment of certain microbes in the pancreatic tumor microbiome can contribute to pro-cancer phenotypes, depending on the type and genus of the microbes involved. In murine models, gram-negative bacteria can traverse the intestine to reside in the normal pancreas; and in certain patients with PDAC, translocation of gram-negative Proteobacteria from the gut to the pancreas drives immune suppression and disease progression [22].
The mechanisms by which microbes or DNA released from necrotic cells into the microenvironment affect pancreatic cancer cells at the molecular level remain elusive despite increasing knowledge about the influential role that the pancreatic tumor microbiome plays in the severity and outcome of PDAC [25]. In this study, we report that uptake of exogenous DNA into the cytosol induces cGAMP synthesis and significantly enhances DUOX2 expression at both the mRNA and protein levels in a panel of human PDAC cell lines following the activation of cGAS-STING signaling. Importantly, exposure to exogenous cGAMP as well as siRNA knockdown of cGAS confirmed the requirement for cGAS expression and enzymatic function in DNA-mediated enhancement of DUOX2 expression. Notably, cGAS-STING-mediated enhancement of DUOX2 expression was also associated with an increase in normoxic HIF-1α and VEGF-A expression, H2O2 formation, and the production of DNA double strand breaks in PDAC cells. Consistent with the known deregulation of STING signaling in colon cancer [26], DUOX2 expression was not enhanced by exogenous DNA in human colon cancer cell lines, suggesting that the crosstalk between cGAS-STING signaling and DUOX2 is context-dependent for tumors of the gastrointestinal tract.
In summary, these data suggest that extracellular DNA of mammalian or bacterial origin, by activating the cGAS-STING pathway, could support a DUOX2-induced, H2O2-mediated pro-inflammatory milieu that produces DNA double strand breaks which may contribute to the pathogenesis of PDAC.
2. Materials and methods
2.1. Cell culture, antibodies, plasmids, primers, and reagents
Human pancreatic cancer cell lines: BxPC-3 (CRL-1687), CFPAC-1 (CRL-1918), AsPC-1 (CRL-1682), and Hs 766T (HTB-134); human colon cancer cell lines: Ls513 (CRL-2134), HT-29 (HTB-38), and T84 (CCL-248) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cell lines were authenticated at the Johns Hopkins genetics resources core facility. BxPC-3, AsPC-1, and Ls513 cells were cultured in RPMI-1640 medium (HyClone SH30027.01) supplemented with 10% Fetal Bovine Serum (FBS, Gemini Bio-Products 100-106) and 1% 100 mM sodium pyruvate (Gibco) by volume. Hs 766T cells were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) (ATCC 30-2002) supplemented with 10% FBS. CFPAC-1 cells were cultured in Iscove’s Modified Dulbecco’s medium (ATCC 30-2005) supplemented with 10% FBS. HT-29 cells were cultured with McCoy’s 5a medium (ATCC 30-2007) supplemented with 10% FBS. T84 cells were cultured in DMEM:F-12 medium (ATCC 30-2006) supplemented with 5% FBS.
Antibodies against cGAS (15102), STING (3337), p-NF-κB p65S536 (3033), NF-κB p65 (8242), TBK1 (38066), p-TBK1 (5483), p-IRF3S396 (4947), IRF3 (4302), p-Stat2Y690 (88410), IRF9 (76684), HIF1β (3718), Stat1 (9175), p-Stat1Y701 (9167), γ-H2AXS139 (2577), Lamin A/C (2032), and β-actin (3700) were purchased from Cell Signaling Technologies (CST, Beverly, MA). HIF-1α (610959) and Stat2 (610187) antibodies were obtained from the BD Transduction Laboratory. The IRF1 (s497) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibody, which reacts with both human DUOX1 and DUOX2, was developed by Creative Biolabs (Port Jefferson Station, NY) and characterized by our laboratory [27].
The plasmids used in these studies included: pGL3-Basic Vector (pGL3-BV, E1751), bacterial strain JM109 for bacterial genomic DNA extraction (P9751) from Promega, pcDNA3.1-HA plasmid (pcDNA3,128034) from Addgene, and pReceiver-M08 plasmid (EX-NEG-M08) from GeneCopoeia. The STING agonist MSA-2 (HY-136927) was obtained from MedChem Express. Recombinant human cytokines Human IL-4 (204-IL-050), Human IL-17A (317-ILB-050), Human IFN-α (11100-1) and Human IFN-β (8499-IF-010) were from R & D Systems. 2’-3’ cGAMP (tlrl-nacga23-5) was obtained from InvivoGen,
The following primers used for Q-PCR in this study were purchased from Applied Biosystems: DUOX2 (Hs00204187_m1), DUOXA2 (Hs01595310_m1), DUOX1 (Hs00213694_m1), DUOXA1 (Hs00328806_m1), NOX1 (Hs00246589_m1), NOX4 (Hs00418356_m1), NOX5 (Hs00225846_m1), cGAS (Hs00403553_m1), STING (Hs00736955_g1), IRF3 ( Hs01547283_m1), IRF1 (Hs00971965_m1), β-actin (Hs01060665_g1), IFN-β (Hs01077958_s1), IRF-9 (Hs00196051_m1), STAT2 (Hs01013119_g1), RELA (Hs01042010_m1), VEGF-A (Hs00900055_m1), and STAT1 (Hs00234829_m1). IRF-1 siRNA-A: Silencer Select Human IRF-1 siRNA, was from Ambion (s7501); IRF-1 siRNA-B: On-Target plus Smart pool Human IRF1 was obtained from Dharmacon (011704-00-10). RELA siRNA, On-Target Plus SMART pool Human RELA (L-003533-00) was obtained from Dharmacon; RELA-A and B, RELA Silencer select siRNA, were obtained from Ambion (S11914 and S11915); On-Target Plus Human STAT1 siRNA, Smart pool, was purchased from Dharmacon (L-003543-00-0010). cGAS siRNA-2, On-Target Plus Human MB21D1 siRNA, SMART Pool, was from Dharmacon (L-015607-02-0020); cGAS siRNA-3, Silencer pre-designed siRNA, was from Ambion (s129126). On-Target plus Human STAT2 siRNA-A, Smart Pool(L-012064-00-0020); On-Target plus SMART siRNA Human STAT2-B (J-012064-08-0010); and On-Target plus SMART siRNA Human STAT2-C (J-012064-06-0010) were from Dharmacon. IRF3 siRNA-A and B, IRF3 Silencer Select siRNA (s7508 and s7509) were from Ambion; IRF3 siRNA-C: siGENOME human IRF3 siRNA SMART pool (M-006875-02-0020) was from Dharmacon.
2.2. Western analysis
Tumor cells (4 × 106) were plated in 100 mm dishes (Corning) and harvested after treatment or transfection. Cell lines were washed once with ice-cold PBS (1X), followed by scraping in PBS, collection into 1.7 ml microcentrifuge tubes, and centrifuged at 5,000 rpm for 2 min at 4 °C. Supernatant was aspirated, and cell pellets were frozen at −80 °C. Cell pellets were lysed and resuspended in 1X RIPA lysis buffer (Millipore Sigma) supplemented with 1X cOmplete Protease Inhibitor Cocktail (Roche) and PhosSTOP phosphatase inhibitors (Roche). Lysates were incubated on ice for 10 min and subsequently centrifuged at 14,000 × g for 10 min at 4 °C; the supernatant was evaluated for protein content using a Pierce BCA Protein Assay (ThermoFisher Scientific). In all experiments, 50 μg protein was loaded onto Novex™ 4-20% Tris-Glycine Mini Gels (ThermoFisher Scientific), transferred to nitrocellulose membranes using the iBlot™ 2 Gel Transfer Device (ThermoFisher Scientific), and probed with the specified antibodies overnight at 4 °C in 1 × TBS-Tween (Tris-buffered saline plus 0.02% Tween 20) containing 5% non-fat milk. Immunoblots were visualized using either a LICOR Odyssey Fc imaging instrument or by development with HyBlot CL Autoradiography Film (Thomas Scientific).
2.3. Quantitative real-time PCR (Q-PCR)
For real-time PCR experiments, total RNA was extracted from 1 x 106 cells using the QIAGEN RNeasy Mini Kit (74104) following the manufacturer’s instructions. Two micrograms of total RNA were used for cDNA synthesis in a 20 μl reaction. The cDNA synthesis steps consisted first of a 5 min incubation at 65 °C of the hexameric random primers, dNTP, and RNA, followed by cycles of 25 °C for 10 min, 42 °C for 50 min, and 75 °C for 10 min with the addition of 0.1 M DTT, 5X Reaction Buffer, Superscript III Reverse Transcriptase (18080-044), and RNaseOUT inhibitor (all from Life Technologies). The synthesized cDNA was diluted to 100 μl with molecular grade H2O, and quantitative PCR was conducted in 384-well plates in a 20 μl volume consisting of 2 μl diluted cDNA, 1 μl primers, 7 μl H2O, and 10 μl TaqMan Universal PCR Master Mix (4364340; Life Technologies). The PCR was performed using the default cycling conditions (50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 10 min) with the ABI QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems). Triplicate samples were used for the Q-PCR, and the mean values were calculated. The data in all figures represent three independent experiments. Relative gene expression was calculated as the ratio of the target gene expression to the expression of the internal reference gene (β-actin) based on the cycle threshold values.
2.4. Transfection and siRNA knockdown
Lonza Kit V (VCA-1003) was used for electroporation of BxPC-3 cells with the Lonza Nucleofector 2b device (Cat# AAB-1001). For this line, 1 × 106 cells were resuspended in the electroporation solution provided in the kit, according to the manufacturer’s recommendations, and 2 μg of DNA was added to the cell suspension before transferring to a clean electroporation cuvette. This ratio of cells to DNA was maintained when scaling up experiments for immunoblot analysis. After the electric charge was applied, the cells were transferred using a transfer pipette to culture dishes containing full-serum and media. A cell-type-specific program was used for the electroporation procedure. Lipofectamine RNAIMAX reagent (Thermo Fisher Scientific, Cat# 13778075) was used to transfect siRNA into CFPAC-1 cells following the manufacturer’s protocol. Lipofectamine 2000 (Thermo Fisher Scientific, Cat# 11668027) was used to transfect plasmid DNA into CFPAC-1, HT-29, and HTB134 cells. Purified E. coli genomic DNA fragments of approximately 1000 bp were transfected into both BxPC-3 and CFPAC-1 cells using Lipofectamine 2000 according to the manufacturer’s protocols.
Lonza Kit V (VCA-1003) was also used for co-electroporation of siRNA and DNA into BxPC-3 cells using the Lonza Nucleofector 2b device for 48 h. 20nM of scrambled siRNA or target gene siRNAs as indicated in the figures were used for co-transfection experiments when siRNA and plasmid DNA were simultaneously co-transfected.
2.5. ELISA
Intracellular cGAMP levels following exposure to transfected DNA were measured in BxPC-3 tumor cell extracts. Following transfection, medium was removed from adherent cells, and the cells were then washed with 1 × PBS. Mammalian protein extraction reagent (M-PER, Themo Scientific, Cat# 78501) lysis buffer was then added to the cells that were subsequently centrifuged for 10 min at 14,000 × g. The supernatant was used in an ELISA assay to quantitate intracellular cGAMP levels. The cGAMP ELISA kit was purchased from Cayman Chemical (cat# 501700), and the manufacturer’s protocol was followed. Absorbance was quantitated at 450 nm using a plate reader. Triplicate studies were performed for each experimental condition, and the mean values were determined for graphical presentation.
2.6. Amplex Red® assay to detect extracellular H2O2
The Amplex Red® Hydrogen Peroxide/Peroxidase Assay Kit (cat# A22188; Life Technologies) was used to detect extracellular H2O2 generation. BxPC-3 cells were either transfected with 2 μg of pGL3-BV plasmid or treated with solvent for 48 h and then washed twice with 1 X PBS, trypsinized, and counted. 2 x 104 live cells in 20 μl of 1X Krebs-Ringer phosphate glucose [KRPG] buffer was mixed with 100 μl of a solution containing 50 μM Amplex Red® and 0.1 U/ml horse radish peroxidase in KRPG buffer with or without 1 μM ionomycin and then incubated at 37 °C for the indicated times. The fluorescence of the oxidized 10-acetyl-3,7-dihydroxyphenoxazine was then measured at excitation and emission wavelengths of 530 nm and 590 nm, respectively, using a SpectraMax Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA, USA); the amount of extracellular H2O2 was calculated based on a standard curve using 0-2 μM H2O2. Each value in the figures represents the mean value of four samples.
2.7. Tumor cell proliferation
BxPC-3 cells (1 × 105 cells in 60 mm dishes) were treated in growth medium with either solvent (PBS for cGAMP; DMSO for MSA-2) or cGAMP (25 μg/ml)/MSA-2 (10 μM) for three days (N=4-5 for these experiments). Cell viability and growth rate were evaluated by manually counting cell numbers under the different treatment conditions. CFPAC cells (1 × 105 cells in 60 mm dishes) were treated with DMSO or MSA-2 for 5 days; cell numbers were determined at the end of the 5-day growth period; N=5 for these experiments.
2.8. Data mining and bioinformatic analyses of The Cancer Genome Atlas datasets
Level 3 normalized (RNASeq V2) gene expression data sets were generated by The Cancer Genome Atlas (TCGA) Research Network (http://cancergenome.nih.gov); the data sets were downloaded from the cBioPortal for Cancer Genomics (https://www.cbioportal.org/) for cGAS, STING1, and DUOX2. cGAS and STING1 gene expression levels were plotted according to tissue type, and tissues were sorted from highest to lowest mean expression. For the pancreatic cancer cohort (PDAC, n=177), the Pearson and Spearman-rank correlation and two-tailed P values between DUOX2 and cGAS and DUOX2 and STING1 were evaluated using GraphPad Prism. The same analysis was conducted for the colorectal adenocarcinoma cohort in TCGA (COADREAD, N=592 specimens).
2.9. Quantification and statistical analysis
Data are displayed as the mean ± SD from at least three independent experiments, unless otherwise specified. The normalized gene expression data is generally assumed to be normally distributed; therefore, we applied the unpaired two-tailed Student’s t-test for 2-group comparisons, and one-way or two-way analyses of variance (ANOVA) for comparisons among multiple groups, with Turkey’s post hoc test for evaluation of inter-group statistical significance. Statistical significance was determined as a P value < 0.05 and is shown with an asterisk (*); a P value < 0.01 is represented with two asterisks (**) and a P value < 0.001 with three asterisks (***).
3. Results
3.1. Exogenous DNA activates cGAS-STING signaling and enhances DUOX2 expression in human pancreatic cancer cells
Uptake of DNA into cell cytosol from necrotic cell debris, the formation of micronuclei due to DNA damage, or pathogens is common in areas of inflammatory tissue injury or abnormal tissue growth, such as in the tumor microenvironment [25]. Because recent studies from our laboratory have demonstrated the potential of pro-inflammatory cytokines to enhance oxidative stress in pancreatic ductal adenocarcinoma (PDAC) cells [7], we examined the effects of exogenous DNA on the NADPH oxidase family member that is prevalent in PDAC cells, DUOX2. We first evaluated the expression of STING and cGAS in PDAC cell lines that we have previously examined for their response to cytokines; we found that BxPC-3 and CFPAC-1 cells express both STING and cGAS protein in amounts that are easily demonstrable, whereas STING protein expression is limited in the AsPC-1 line (Fig. 1A). DUOX2 mRNA expression was significantly enhanced in BxPC-3, CFPAC-1, and HTB134 cells 24 h following transfection of exogenous DNA into cytosol (Figs. 1B, C, D). For the CFPAC-1 and HTB134 cell lines, the effect of transfected DNA plasmids was similar to the effects of exposure to the pro-inflammatory cytokines IL-17A and IL-4, respectively, for 24 h. As demonstrated in Fig. 1E, DNA plasmid increased the protein expression of DUOX 48 h after transfection; upregulation of DUOX occurred concomitantly with the phosphorylation of TBK1 and IRF3. The enhanced DUOX levels are also associated with increased expression of HIF-1α and the presence of DNA double strand scission in BxPC-3 cells as measured by the production of γH2AX. We found that total IRF3 and STING expression was diminished following plasmid transfection, an observation that is consistent with previous studies describing the degradation of IRF3 after sustained activation of cGAS-STING signaling [21, 28–30]. On the other hand, an increase in DUOX expression produced by exposure to IFN-γ for 24 h was, as expected, accompanied by a strong Stat1 phosphorylation signal without activation of TBK1 or IRF3.
Fig. 1. Introduction of exogenous DNA activates cGAS-STING signaling and enhances the expression of DUOX2 in human pancreatic cancer cells.
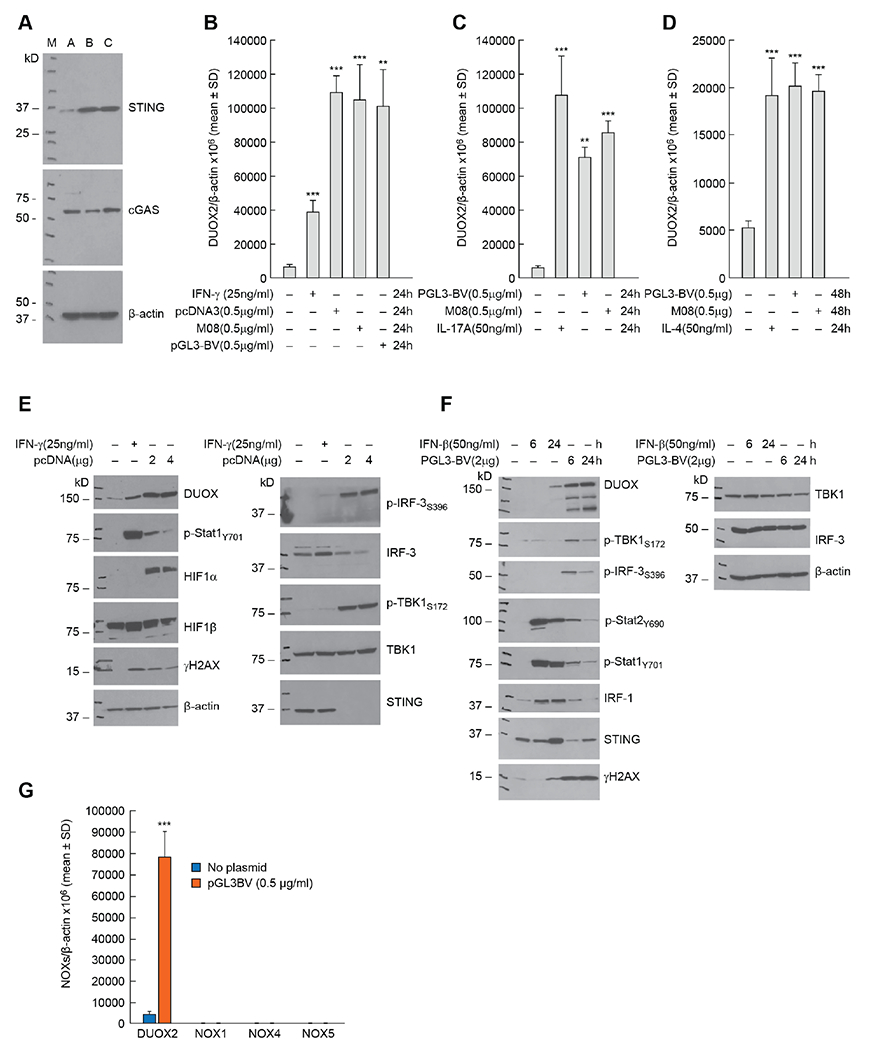
A, The protein expression levels of STING and cGAS were examined in three human pancreatic cancer cell lines: A: AsPC-1; B: BxPC-3; and C: CFPAC-1. B, The effects of three different DNA plasmids on DUOX2 mRNA expression in BxPC-3 cells 24 h following transfection were examined by RT-PCR and compared to the effect of IFN-γ on DUOX2 expression used here as a positive control [4]. ***p < 0.001; **P < 0.01. C, DUOX2 expression in CFPAC-1 cells was determined 24 h after transfection of two DNA plasmids and compared to the effect of exposing these cells to IL-17A for 24 h [7]. ***p < 0.001; **P < 0.01. D, DUOX2 expression was measured by RT-PCR in HTB134 human pancreatic cancer cells 48 h following plasmid transfection or after exposure to 50 ng/ml IL-4 for 24 h [41]. ***P < 0.001. E, DUOX expression, cell signaling, DNA damage, and cGAS-STING activation were examined in BxPC-3 cells by Western blot following exposure to pcDNA plasmid (48 h following transfection) compared to the same cell line treated for 24 h with 25 ng/ml of IFN-γ. F, CFPAC-1 cells were evaluated in these experiments to compare the time course of DUOX expression and activation of the cGAS-STING pathway following transfection of a DNA plasmid or exposure to IFN-β in complete media. G, Comparison of DUOX2, NOX1, NOX4, and NOX5 mRNA expression levels measured by RT-PCR in BxPC-3 cells 24 h following transfection with DNA plasmid. ***p < 0.001. All the experiments shown here were repeated at least in triplicate.
To confirm these results, we evaluated the time-dependent activation of cGAS-STING signaling in a second PDAC cell line, CFPAC-1 (Fig. 1F). In these experiments, plasmid-related activation of the cGAS-STING pathway was demonstrable as early as 6 h following transfection, as shown by phosphorylation of TBK1 and IRF3; DUOX expression was also increased 6 h following plasmid exposure and was accompanied by evidence of enhanced DNA double strand breakage. We also found that treatment with IFN-β for 24 h upregulated DUOX expression as well as phosphorylation of Stat1 and Stat2 and the expression of IRF1 and γH2AX.
To evaluate the specificity of our results for DUOX2 in human PDAC cells, we compared the expression of other NADPH oxidases in BxPC-3 cells following plasmid transfection. As shown in Fig. 1G, we found no evidence that exogenous DNA increased the expression levels of NOX1, NOX4, or NOX5 in these tumor cells. With respect to tumor histological context, we examined the effect of exogenous DNA on NOX expression in human colon cancer cell lines (Supplementary Fig. S1). Transfection of plasmid DNA had no significant effect on either NOX1 or DUOX2 expression in the Ls513 line, nor on DUOX2 mRNA expression in either T84 or HT-29 colon cancer cells. However, in the same cell lines, pro-inflammatory cytokine exposure significantly increased DUOX2 mRNA levels (Supplementary Fig. S1A, B, C). In a previous study, double stranded DNA was found to produce a limited effect on type I IFN production by the HT-29 cell line [26]. Taken together, these data suggest that the presence of intracellular DNA activates cGAS-STING signaling and induces DUOX2 expression primarily in pancreatic rather than colon cancer cells.
3.2. Concentration- and time-dependent enhancement of DUOX expression following DNA transfection is associated with increased H2O2 production by PDAC cells
Next, we examined the effect of DNA concentration and time following transfection on the expression of DUOX in PDAC cells. A plasmid level as low as 25 ng/ml DNA increased DUOX2 protein expression 48 h after transfection of BxPC-3 cells (middle panel, Fig. 2A). The same amount of DNA increased TBK1 phosphorylation (middle panel, Fig. 2A). DNA-dependent upregulation of DUOX2 mRNA expression was significantly increased 24 h following transfection in CFPAC-1 cells (P < 0.001, right panel, Fig. 2A). Solvent alone in the absence of DNA produced no effect on the expression of DUOX2 (Fig. 2A, middle panel). The upregulation of DUOX2 in BxPC-3 cells by DNA led to the expression of a fully functional NADPH oxidase as shown in Fig. 2B. Forty-eight hours following plasmid transfection, BxPC-3 cells in the presence of ionomycin (1 μM) produced significantly higher levels of extracellular H2O2 (compared to solvent-treated control cells) as measured by the Amplex Red® assay, P < 0.001.
Fig. 2. Concentration- and time-dependent enhancement of DUOX expression by plasmid DNA leads to significantly increased H2O2 production in PDAC cells while exogenous bacterial DNA is as effective as plasmid DNA in stimulating cGAS-STING signaling.
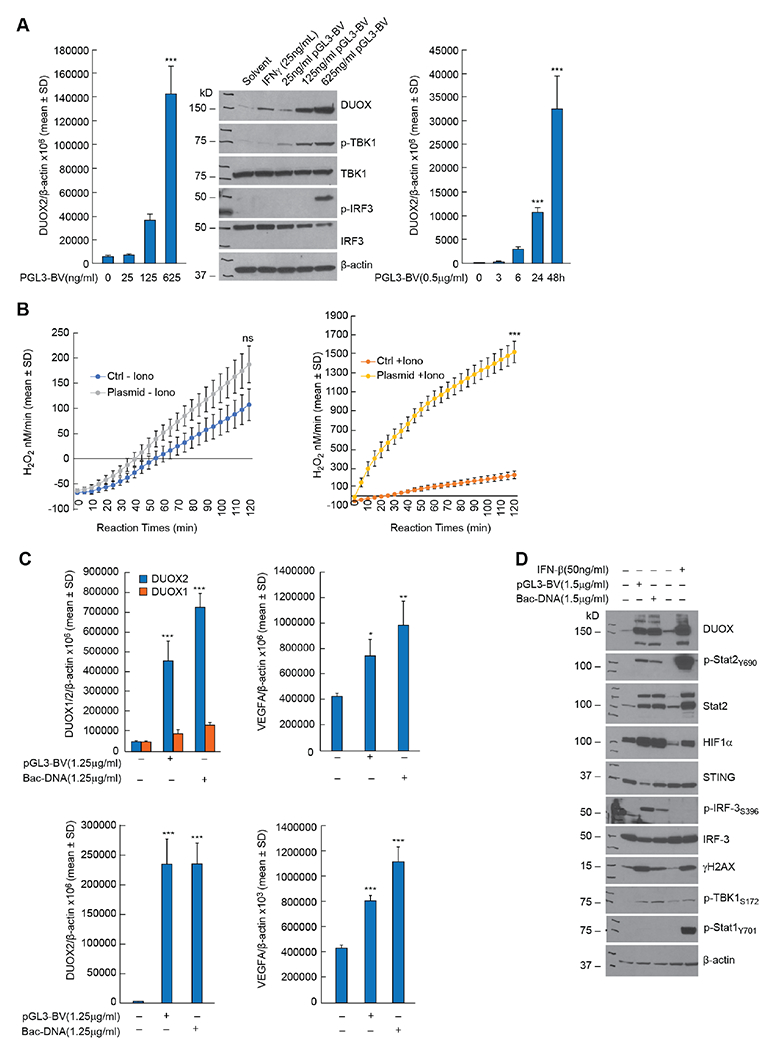
A, 48 h following transfection of PGL3-BV plasmid into BxPC-3 cells DUOX2 expression was markedly increased (middle panel); enhanced DUOX2 expression 48 h following plasmid transfection was accompanied by phosphorylation of TBK1 and IRF3 (middle panel). The time course for plasmid-enhanced DUOX2 expression in CFPAC-1 cells propagated in complete media is shown in the right panel. ***p < 0.001. B, Time-dependent H2O2 production by BxPC-3 cells was measured using the Amplex Red® assay 48 h following transfection of 2μg of pGL3-BV plasmid; the rate of H2O2 formation was compared in the absence (left panel) and presence (right panel) of ionomycin (1 μM) to that of solvent treated cells. NS = not significant; *** p < 0.001. C, In the top left panel, the expression levels of DUOX1 and DUOX2 were determined 48 h following transfection of a DNA plasmid or E. coli DNA (Bac-DNA) into BxPC-3 cells. ***P < 0.001. The top right panel demonstrates the expression of VEGF-A mRNA in BxPC-3 cells 48 h after DNA transfection. *P < 0.05; **P < 0.01. The bottom left panel demonstrates DUOX2 mRNA expression 48 h following transfection of either plasmid or E. coli DNA into CFPAC-1 cells. ***P < 0.001. The bottom right panel shows the expression of VEGF-A mRNA 48 h after transfection of DNA into CFPAC-1 cells. ***P < 0.001. D, For BxPC-3 cells, the upregulation of DUOX and the downstream effects of increased DUOX expression, including activation of HIF-1α and DNA double strand scission measured by γH2AX, were similar 48 h following transfection with either plasmid or E. coli DNA. The effects of a 24 h IFN-β exposure on DUOX expression, DNA damage, and interferon-related signaling pathways are also shown. Lane 1 is the control for lanes 2 and 3 with transfection reagents but without exogenous DNA; lane 4 is the control (solvent without cytokines) for lane 5. All experimental results shown are the result of at least three independent experiments.
Because of the recent demonstration that gram-negative bacteria are found in both human and murine pancreatic cancers [22], we compared the effect of plasmid DNA to that of E. coli for the ability to induce DUOX2 expression in PDAC cell lines (Fig. 2C). E. coli DNA significantly increased DUOX2 levels in BxPC-3 cells to the same degree as plasmid DNA, P < 0.001 (top left panel); DUOX1 mRNA expression increased but to a much smaller degree. Upregulation of DUOX2 expression by plasmid or E. coli DNA was accompanied by a significant increase in VEGF-A expression (top right panel). In CFPAC-1 cells, DUOX2 expression was enhanced similarly by both plasmid and E. coli DNA (bottom left panel) and was also associated with an increase in the expression of VEGF-A (bottom right panel), P < 0.001. The western blot shown in Fig. 2D confirms that transfection of bacterial DNA into BxPC-3 cells activates the cGAS-STING pathway and enhances the expression of DUOX, HIF-1α, and γH2AX.
3.3. Role of specific components of the cGAS-STING pathway in upregulation of DUOX2 expression by exogenous DNA
Using siRNAs against cGAS, we found that the enhanced expression of DUOX2 following plasmid transfection was significantly decreased in BxPC-3 cells when cGAS expression was diminished (Fig. 3A). cGAS siRNA blocked plasmid-stimulated DUOX protein expression, phosphorylation of TBK1 and IRF3, as well as expression of cGAS itself in these cells (Fig. 3B). Because cGAS binds to and is activated by double stranded DNA to produce cGAMP, we examined by ELISA whether pGL3-BV plasmid altered cGAMP levels in a concentration-dependent fashion 48 h following transfection. A positive linear relationship was demonstrated between intracellular cGAMP levels and plasmid DNA, with an R2 of 0.94 for the BxPC-3 line (Fig. 3C). We also found using 2 μg of pGL3-BV DNA that the time course of cGAMP production following plasmid transfection rose linearly to ≈ 125 pg/ml for the first 8 h and then began to plateau for the subsequent 40 h of observation (data not shown). Because of the effect of transfected DNA on intracellular cGAMP levels in BxPC-3 cells, we examined whether exposure to extracellular cGAMP, which can be imported by tumor cells [31, 32], altered DUOX2 mRNA expression. As shown in Fig. 3D, exposure of BxPC-3 cells for 24 h to extracellular cGAMP at a concentration of ≥ 1 μg/ml significantly increased DUOX2 mRNA expression, P < 0.001. This effect was time dependent, reaching significance following a 6 h exposure to 25 μg/ml cGAMP, left panel of Fig. 3E; cGAMP exposure also significantly increased IFN-β expression in BxPC-3 cells within 3 h, middle panel of Fig. 3E. The right panel of Fig. 3E demonstrates that cGAMP exposure was also associated with a significant increase in VEGF-A expression in the BxPC-3 line.
Fig. 3. Role of the cGAS-STING pathway in the enhancement of DUOX2 expression by extracellular DNA in BxPC-3 pancreatic cancer cells.
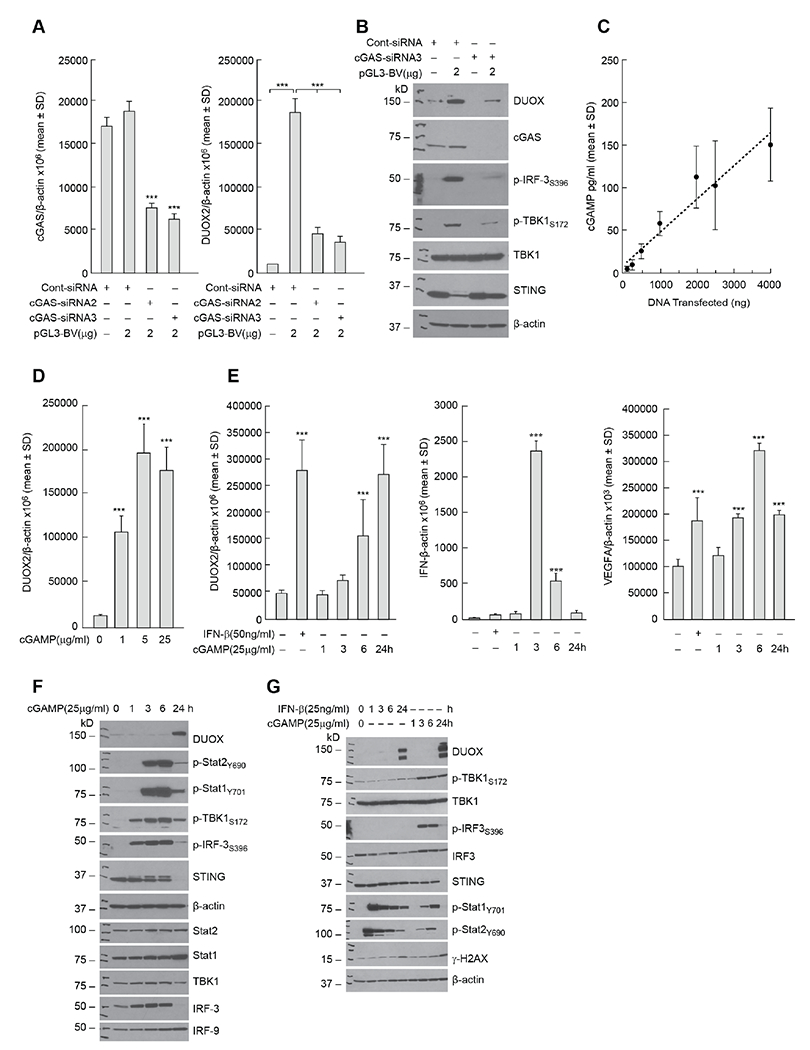
A, Effect of intracellular cGAS levels (left panel) on expression of DUOX2 (right panel) 48 h following transfection of pGL3-BV plasmid examined using cGAS siRNA in BxPC-3 cells. ***P < 0.001. B, Evaluation of the role of cGAS in plasmid-enhanced DUOX protein expression and cell signaling in the BxPC-3 cell line. Western analysis was performed 48 h following plasmid and siRNA transfection. C, DNA plasmid concentration-dependent increase in cellular cGAMP production by BxPC-3 cells. Tumor cells were transfected with increasing amounts of pGL3-BV DNA; 48 h following transfection, intracellular cGAMP was measured by ELISA. P < 0.05 for all DNA levels ≥ 500 ng. D, Concentration-dependent increase in DUOX2 expression following 24 h exposure to extracellular cGAMP in BxPC-3 cells. ***P < 0.001. E, Left panel; time-dependent increase in cGAMP-related DUOX2 expression in BxPC-3 cells compared to the effect of 24 h IFN-β treatment on DUOX2 mRNA levels. Middle panel; effect of cGAMP exposure time on expression of IFN-β mRNA. Right panel; effect of cGAMP exposure time on the expression of VEGF-A in BxPC-3 cells. ***P < 0.001. F, Time course for cGAMP-related DUOX protein expression and cGAS-STING signaling. G, Comparison of the time course of the effects of IFN-β and cGAMP on Stat and cGAS-STING signaling, DUOX expression, and DNA damage in BxPC-3 cells. All experiments shown in this figure were repeated a minimum of three times.
The time course of cGAMP-enhanced cGAS-STING signaling is shown in Fig. 3F; while increased DUOX protein expression was observed 24 h following cGAMP exposure, activation of TBK1 and IRF3 occurred as early as 1 h following the addition of cGAMP, and evidence of IFN-β-related signal transduction (phosphorylation of Stat1 and Stat2 and increased expression of IRF9) was demonstrated within 3 h. These experiments suggest that the cGAS-STING pathway, including upregulation of IFN-β-related signaling, is activated following the engagement/activation of cGAS by double stranded DNA in BxPC-3 cells. However, as shown in Fig. 3G, when examined concurrently, both the time course and the degree of IFN-β-related Stat phosphorylation differed when the effect of exogenous type I interferon was compared to cGAMP treatment; IFN-β activated Stat1/2 within 1 h of cytokine exposure, and activation lasted for at least 24 h; whereas treatment with cGAMP appeared to activate Stat signaling to a lesser degree and for a shorter duration.
3.4. Signal transduction downstream of activated cGAS-STING in PDAC cell lines
To examine cGAS-STING-dependent signaling in PDAC cells without DNA transfection, we evaluated the effects of the STING agonist MSA-2 [33] on the events downstream of cGAS that may contribute to enhanced DUOX expression. MSA-2 treatment, similar to plasmid DNA, increased the expression of DUOX in a time-dependent fashion in BxPC-3 cells; increased DUOX expression is preceded by phosphorylation of TBK1 and IRF3 beginning 1 h following drug exposure (Fig. 4A). DNA double strand breakage occurs in concert with increased DUOX expression, while phosphorylation of Stat1 and Stat2, suggestive of IFN-β signaling, is demonstrable 6 h following initiation of MSA-2 exposure. On the other hand, for AsPC-1 cells, which demonstrated a more modest baseline expression of STING compared to BxPC-3 cells (Fig. 1A and Fig. 4A), MSA-2 failed to activate IRF3 or Stat transcription factors and did not increase DUOX expression. Significant enhancement of DUOX2 expression by MSA-2 was concentration dependent in both BxPC-3 (Supplementary Fig. S2A) and CFPAC-1 (Supplementary Fig. S2B) cells; furthermore, the time course of MSA-2 activation of cGAS-STING signaling resembled that produced by exposure to cGAMP, differing only in the modest activation of Stat2 by the STING agonist (Supplementary Fig. S2C). Finally, because we previously demonstrated that dexamethasone co-treatment blunts pro-inflammatory cytokine-related upregulation of DUOX [34], we evaluated the effect of the glucocorticoid on signal transduction following cGAMP and MSA-2 exposure. Dexamethasone treatment partially blocked DUOX upregulation by either agent, as well as DNA double strand breakage, and cGAS-STING-mediated phosphorylation of IRF3 and TBK1 (Supplementary Fig. S2D).
Fig. 4. Activation of signaling pathways downstream of cGAS-STING in PDAC cell lines.
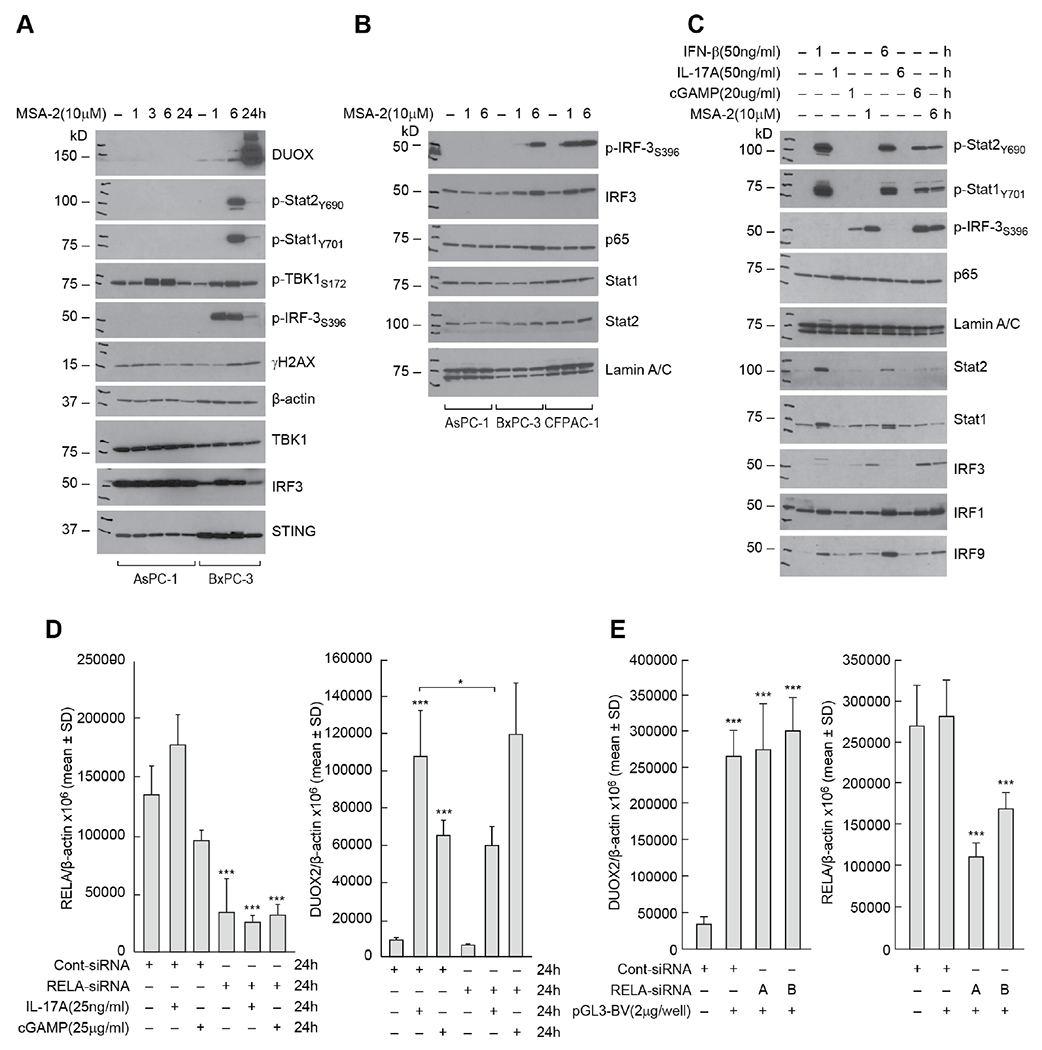
A, Comparison of cell signaling and DNA damage time course following exposure to the STING agonist MSA-2 (10 μM) in AsPC-1 and BxPC-3 cells. B, Time course for cGAS-STING and cytokine nuclear signaling following exposure to 10 μM MSA-2 in AsPC-1, BxP-3, and CFPAC-1 tumor cells. C, Comparison of cGAMP/MSA-2 induced cell signaling to that produced by IFN-β and IL-17A in BxPC-3 cells. Cells were untreated or exposed for 1 or 6 h to each of the compounds studied. D, Effect of NF-κB signaling on cGAMP-related DUOX2 expression. The role of RELA expression in cGAMP- and IL-17A-related DUOX2 (right panel) and RELA (left panel) expression was examined in BxPC-3 cells using siRNA. In these experiments, control siRNA and RELA siRNA, where indicated, were transfected into BxPC-3 cells; 24 h following transfection, cells were propagated in serum free medium alone or with the addition of either IL-17A or cGAMP for another 24 h. *P < 0.05; ***P < 0.001. E, Role of NF-κB signaling in plasmid-related upregulation of DUOX2 expression in BxPC-3 cells evaluated using two different RELA siRNAs; left panel demonstrates effects of RELA siRNAs on DUOX2 expression 48 h after plasmid transfection, and the right panel shows the effect of the siRNAs on RELA expression itself. ***p < 0.001. The results presented represent at least three independent experiments.
We next examined the nuclear translocation of IRF3, phospho-IRF3, p65, Stat1 and Stat2 following MSA-2 treatment in PDAC cell lines (Fig. 4B). Nuclear translocation of phosphorylated IRF3 by 6 h was clear for both BxPC-3 and CFPAC-1 cells, but not in AsPC-1 cells. However, translocation of the NF-κB component p65 (RELA) following MSA-2 exposure was not prominent in any PDAC cell line. To broaden our evaluation of nuclear signaling, we compared the effects of IFN-β, IL-17A, cGAMP, and MSA-2 on pathways downstream of cGAS-STING in BxPC-3 cells (Fig. 4C). As expected, IFN-β activated Stat1 and Stat2 and increased the expression of IRF9. Furthermore, exposure to both cGAMP and MSA-2 led to the nuclear translocation of phosphorylated IRF3. However, only IL-17A treatment modestly increases the expression of p65 in the nucleus.
To explore the role of NF-κB signaling further, the effect of RELA siRNA on DUOX expression was studied in BxPC-3 cells. Despite > 75% knockdown of RELA mRNA expression (Fig. 4D, left panel), siRNA treatment did not decrease cGAMP-mediated upregulation of DUOX2 (Fig. 4D, right panel). On the other hand, the increase in DUOX2 levels induced by IL-17A, which we have previously shown to be regulated, in part, by NF-κB [7], was significantly decreased by RELA siRNA, P < 0.001. RELA siRNAs did not diminish the significantly enhanced DUOX2 expression that occurred 48 h following plasmid transfection (Fig. 4E).
3.5. IRF3, but not Stat1 or Stat2, plays an important role in the cGAS-STING-mediated enhancement of DUOX2 expression
To further elucidate the mechanism by which exogenous DNA mediates increased DUOX2 expression following activation of cGAS-STING signaling, we employed an siRNA knockdown strategy. Knockdown of IRF3 expression in BxPC-3 cells by ~50% (Fig. 5A, right panel) significantly decreased DNA-mediated DUOX2 mRNA expression relative to the scrambled siRNA control (Fig. 5A, left panel). In contrast, siRNA knockdown of IRF1 did not alter DNA-mediated DUOX2 expression (Fig. 5A, left and middle panels; Supplementary Fig. S3G). These results were confirmed for MSA-2-mediated, as well as plasmid-mediated, DUOX2 expression using three different IRF3 siRNAs (Figs. 5B and 5C). Experiments evaluating CFPAC-1 cells exposed to cGAMP demonstrated similar results, confirming the role of IRF3 in cGAS-STING-mediated DUOX2 expression (data not shown). At the protein level, knockdown of IRF-3 with two different siRNAs markedly diminished plasmid-enhanced DUOX and HIF-1α expression in the BxPC-3 cell line (Fig. 5D).
Fig. 5. Role of IRF3 in the control of cGAS-STING-mediated enhancement of DUOX2 expression.
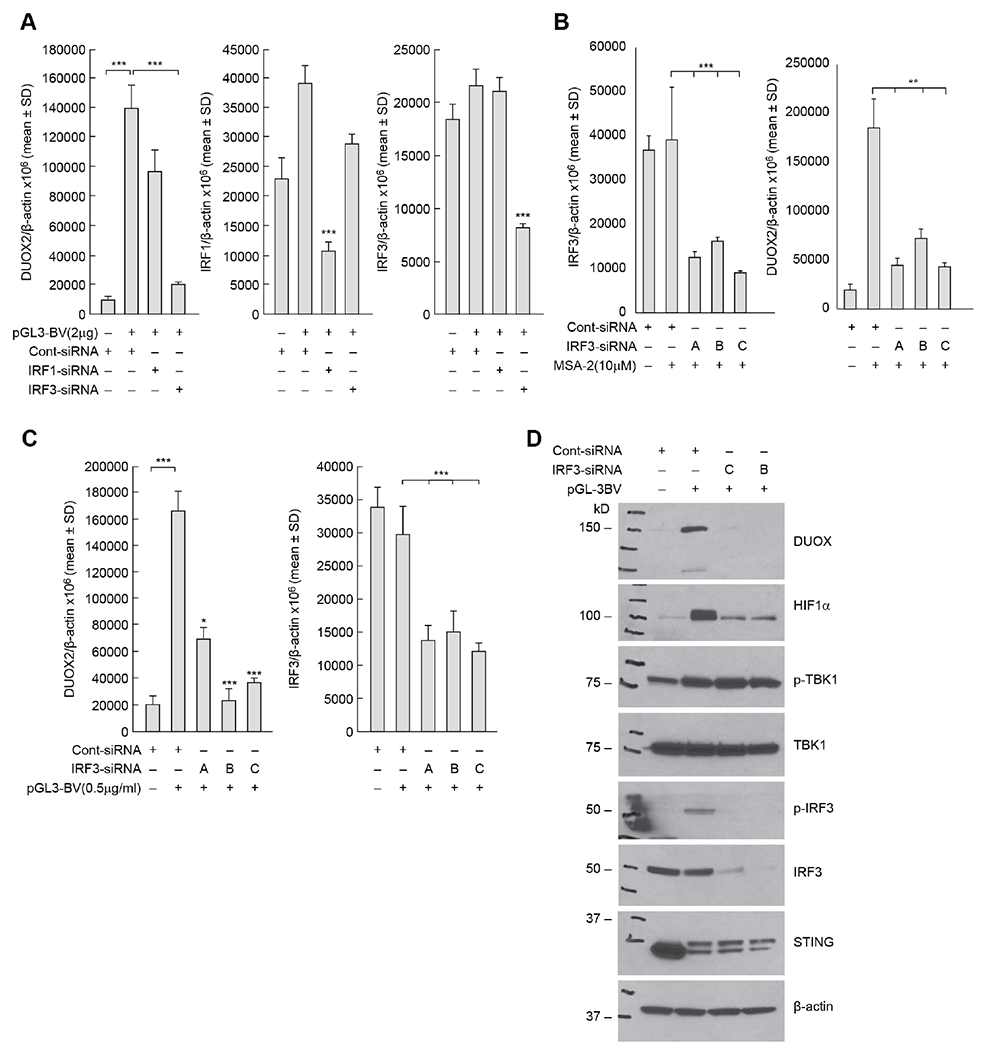
A, The contributions of IRF1 and IRF3 to plasmid-enhanced expression of DUOX2 measured using RT-PCR were examined in the BxPC-3 cell line using IRF1 or IRF3 siRNAs in the left panel, and on IRF1 or IRF3 expression levels in the middle and right panels, respectively. ***p < 0.001. B, In the left panel, downregulation of IRF3 by siRNAs was examined; in the right panel, the effect of IRF3 siRNAs on MSA-2-enhanced DUOX2 expression was determined for BxPC-3 cells. **P < 0.01; ***p < 0.001. C, IRF-3 siRNAs block the upregulation of DUOX2 mRNA expression following plasmid transfection (left panel) and baseline IRF3 mRNA after pGL3-BV transfection (right panel) in BxPC-3 cells. *P < 0.05; ***P < 0.001. D, At the protein level, IRF3 siRNA diminishes the enhanced expression of DUOX by the pGL3-BV plasmid in BxPC-3 pancreatic cancer cells. These results are representative of three independent experiments.
Because we had demonstrated in these studies that DUOX2 expression is significantly enhanced when PDAC cells are treated with IFN-β (Fig. 3E), and that Stat signaling pathways downstream of IFN-β are activated by cGAMP and MSA-2 (Fig.4C), we examined the role of Stat signaling in DNA-mediated upregulation of DUOX2 (Supplementary Fig. S3). As shown in Supplementary Fig. S3A and Supplementary Fig. S3B, transfection of plasmid DNA into BxPC-3 cells significantly increased DUOX2 expression; however, knockdown of either Stat1 or Stat2 with siRNA did not alter DNA-enhanced upregulation of DUOX2 mRNA expression. Furthermore, while Stat2 knockdown blocked IFN-β-stimulated DUOX2 expression, at least in part, it did not inhibit MSA-2-related upregulation of DUOX2 (Supplementary Fig. S3C, left panel). These results were confirmed for IFN-β by using multiple Stat2 siRNAs (Supplementary Fig. S3D). Finally, the ineffectiveness of Stat2 knockdown on MSA-2-related upregulation of DUOX2 expression was confirmed for plasmid DNA- and cGAMP-enhancement of DUOX2 expression using multiple siRNAs (Supplementary Fig. S3E and Supplementary Fig. S3F).
3.6. Effect of activation of the cGAS-STING pathway with subsequent enhancement of DUOX2 expression on tumor cell proliferation
To address the question of the role of activation of the cGAS-STING pathway and associated increases in DUOX2 expression on pancreatic cancer cell viability and growth in vitro, both BxPC-3 and CFPAC-1 cells were treated with cGAS-STING activating agents. As shown in Fig. 6, no significant differences were observed in the growth rate of either PDAC cell line under conditions wherein DUOX2 expression levels had previously been shown to be significantly increased.
Fig. 6. Effect of activating the cGAS-STING pathway on PDAC cell growth.
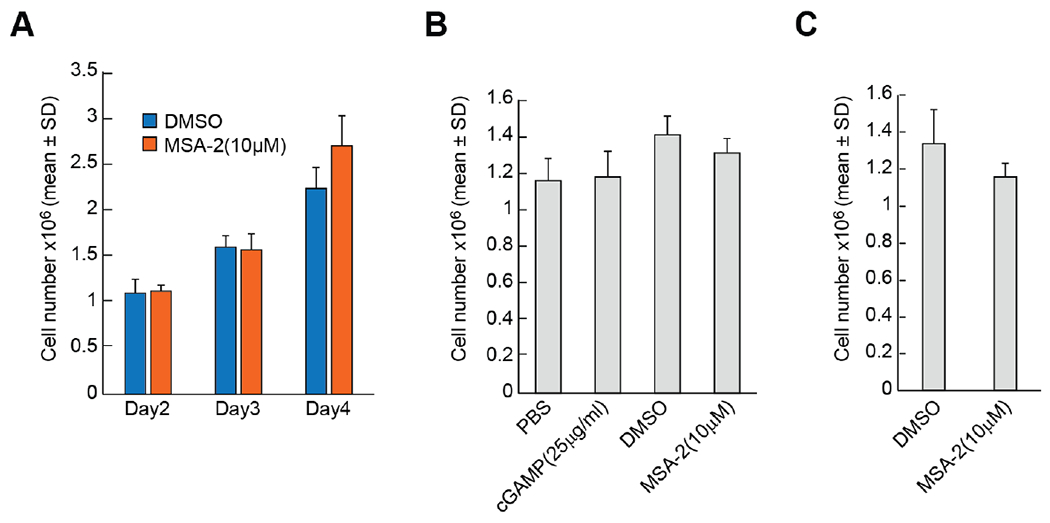
A, Effect of the STING agonist MSA-2 (10 μM) on the growth of BxPC-3 cells in complete growth medium over 4 days. B, Effect of cGAMP or MSA-2 on the growth of BxPC-3 cells for 3 days. C, Effect of MSA-2 in the proliferation of CFPAC-1 cells for 5 days.
3.7. Relationship of cGAS-STING and DUOX2 expression levels in human pancreatic ductal adenocarcinomas and human colon adenocarcinomas
We previously reported that DUOX2 expression in PDACs is the second highest (next to thyroid carcinomas) of all major human malignancies [7]. To assess the potential clinical relevance of the cGAS-STING pathway in primary pancreatic cancer in humans, we examined whether a relationship existed between expression levels of cGAS, STING, and DUOX2 in PDACs using The Cancer Genome Atlas (TCGA) database. As shown in Figs. 7A and B, the mean levels of cGAS, and particularly STING, mRNA in PDACs are relatively high compared to those in other tissue types. Furthermore, both cGAS and STING mRNA expression levels significantly correlated with DUOX2 expression in PDAC (Figs. 7C and D). In contrast, as shown in Supplementary Fig. S1D, cGAS mRNA levels are not significantly correlated with DUOX2 expression in colorectal tumor specimens from the TCGA.
Fig. 7. Expression of cGAS and STING in TCGA studies and relationship to DUOX2 expression levels in human pancreatic ductal adenocarcinomas.
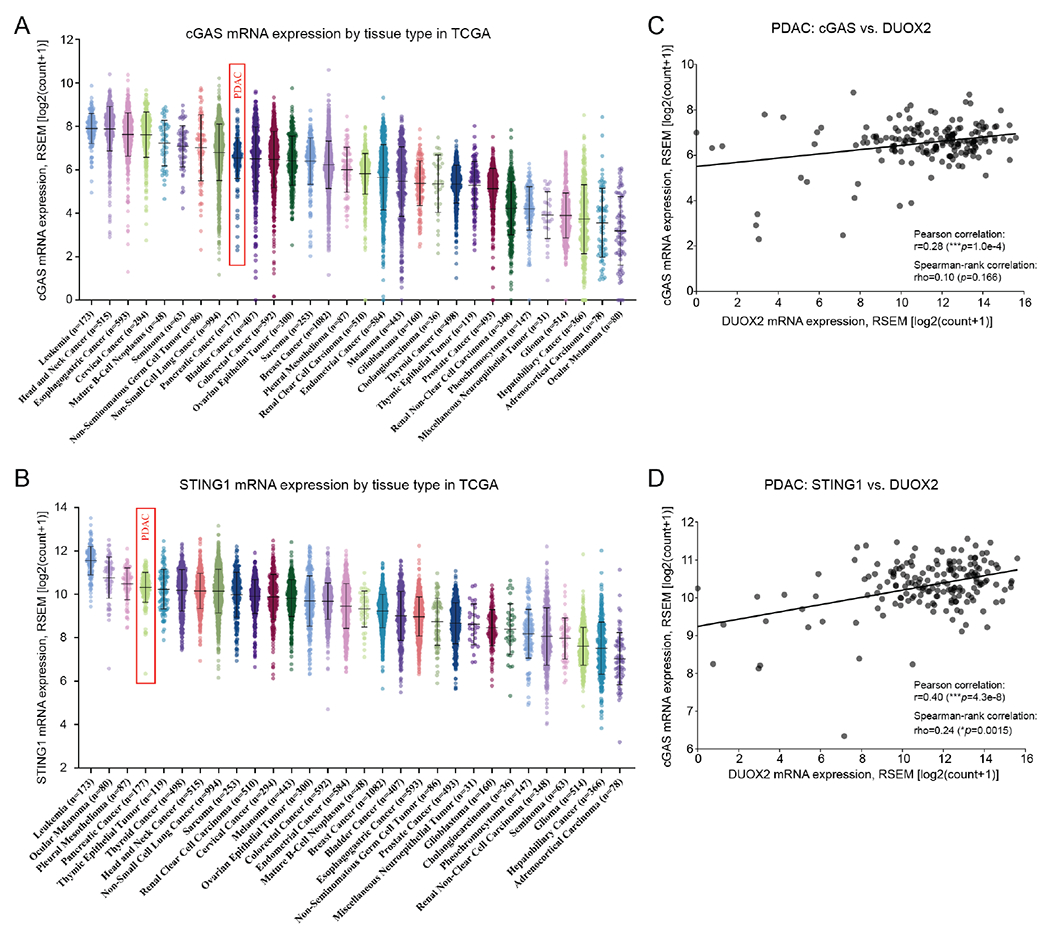
A, Mean cGAS mRNA levels in pancreatic ductal adenocarcinomas (PDAC, denoted by a red rectangle) are in the top third of common human malignancies as measured in The Cancer Genome Atlas (TCGA). B, STING mRNA levels in PDAC are the fourth highest reported in the TCGA (red rectangle). C, cGAS mRNA levels are significantly related to DUOX2 expression levels in PDACs. D, STING mRNA levels are significantly correlated with DUOX2 expression levels in PDACs.
4. Discussion
In a recent study, our laboratory demonstrated that high DUOX2 expression is adversely correlated with survival in patients with PDAC and that TH2 and TH17 cytokines synergistically induce expression of DUOX2 in PDAC cell lines [7]. These experiments broadened the known range of pro-inflammatory cytokines, beyond IFN-γ and LPS, capable of enhancing DUOX2 expression and DUOX2-mediated H2O2 production in pancreatic cancer cells [35]. Because the cGAS-STING pathway plays an important role in the regulation of pro-inflammatory cytokine expression [36] and is known to be highly expressed in both human PDACs and in murine pancreatic cancer models [37], we examined whether a relationship exists between cGAS-STING signaling and DUOX2 expression.
The demonstration that exogenous DNA from plasmids or bacteria significantly enhanced DUOX2 expression and function in a panel of PDAC cell lines in a concentration- and time-dependent manner initially suggested that the canonical cGAS-STING pathway was operating in our study (Fig. 8). Uptake of exogenous DNA led to the formation of cGAMP, the phosphorylation of TBK1 and IRF3, and the translocation of phosphorylated IRF3 to the nucleus of PDAC cells. These results were confirmed by exposure to exogenous cGAMP as well as by treatment with the STING agonist MSA-2 (Figs. 3D and 3E; Supplementary Fig. S2). Furthermore, cGAS siRNAs significantly diminished both cGAS expression and upregulation of DUOX2 by the DNA plasmid. We also found using multiple siRNAs that IRF3, but not IRF1, was necessary for DNA-mediated induction of DUOX2 expression (Fig. 5).
Fig. 8. cGAS-STING-mediated enhancement of DUOX2 expression in PDAC cells.
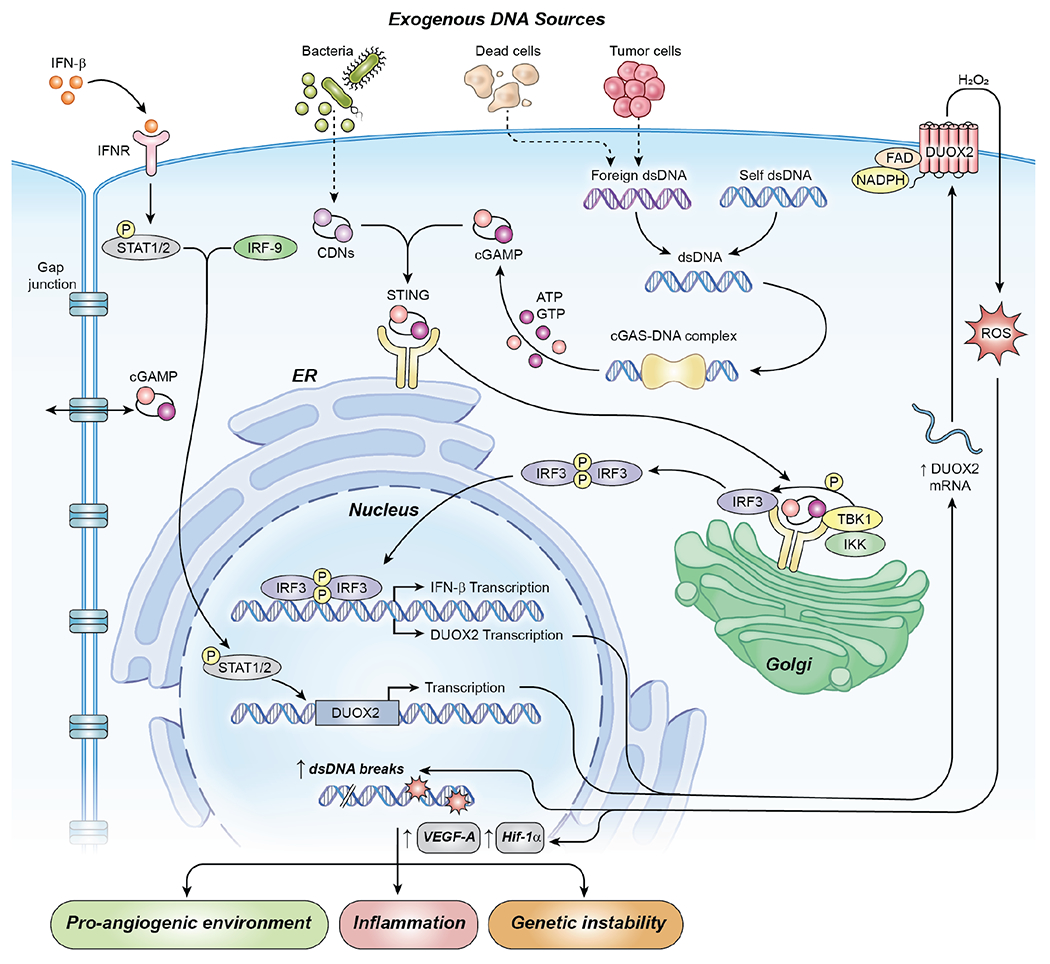
In this model, foreign DNA, from exogenous plasmids or from bacterial sources, when transferred into human PDAC cells activates cGAS-STING signaling. After binding DNA in the cytosol, cGAS catalyzes the formation of cGAMP from GTP and ATP. cGAMP in turn binds to the ER-bound protein STING which translocates to the Golgi and recruits Tank-binding kinase 1 (TBK1) and Interferon regulatory factor 3 (IRF3). The formation of this signaling complex allows TBK1 to phosphorylate IRF3 and auto-phosphorylate itself. For PDAC cells, in a non-canonical fashion, phosphorylated IRF3, but not NF-κB, appears to be responsible for enhancing the transcription of DUOX2 mRNA and the subsequent production of an enzymatically active oxidase that produces a flux of H2O2 capable of crossing cell membranes. Our experiments have also shown that extracellular cGAMP can be imported into PDAC cells, enhancing DUOX2 expression directly in the absence of extracellular DNA. Exogenous IFN-β signals downstream through Stat1/2 to increase DUOX2 protein expression. However, although exogenous IFN-β capably upregulates DUOX2, when the cytokine is generated intracellularly as a consequence of cGAS-STING signaling in PDAC cells, IFN-β signaling is limited and does not appear to contribute substantively to the expression of DUOX2. Reactive oxygen species generated by DUOX2 facilitate increased, normoxic expression of HIF-1α and VEGF-A, as well as DNA double strand cleavage that could sustain an oxidative, pro-inflammatory environment. Acutely, this might foster tumor immunity; however, chronic cGAS-STING-induced DUOX2 expression could promote DNA double strand breakage enhancing genetic instability. (Abbreviations used in the figure: DUOX2, dual oxidase 2; CDNs, cyclic dinucleotides; cGAS, cyclic GMP-AMP Synthase; STING, Stimulator of Interferon Genes; cGAMP, cyclic GMP-AMP; IFN-β, interferon beta; dsDNA, double stranded DNA; TBK1, tank-binding kinase 1; IRF3, interferon regulatory factor 3; IRF9, interferon regulatory factor 9; STAT1/2, signal transducer and activator of transcription 1 or 2; ROS, reactive oxygen species; HIF-1α, hypoxia-inducible factor 1; figure adapted from [42])
However, while exogenous DNA activated cGAS-STING signaling and generated cGAMP, activation of NF-κB with translocation to the nucleus was not observed following cGAMP exposure; furthermore, although RELA siRNA partially inhibited the upregulation of DUOX2 by IL-17A (consistent with our prior experiments demonstrating the NF-κB-dependence of this effect), multiple RELA siRNAs did not alter either plasmid- or cGAMP-mediated enhancement of DUOX2 expression (Figs. 4D and 4E). The observation that siRNA knockdown of NF-κB did not appear to have a significant effect on DNA-mediated DUOX2 expression suggests that the mechanism underlying the upregulation of DUOX2 by cGAS-STING diverges, in part, from the canonical pathway.
Moreover, we demonstrated that exposure to the pro-inflammatory cytokine IFN-β strongly upregulated DUOX2 expression in PDAC cells. DUOX2 upregulation by IFN-β was accompanied by increased expression of IRF-9 as well as phosphorylation of Stat1 and Stat2. This effect of IFN-β on DUOX2 expression may have been expected because of our previous demonstration that IFN-γ-mediated upregulation of DUOX2 is produced by Stat1 binding to the DUOX2 promoter [35]. Upregulation of DUOX2 by IFN-β has also been demonstrated in the airway in the context of an antiviral response [38]. However, despite the cGAMP-mediated increase in IFN-β expression that we observed (Fig. 3E), siRNAs against Stat1 and Stat2, although capable of blocking IFN-β-enhanced DUOX2 expression, did not inhibit MSA-2-or plasmid-mediated increases in DUOX2 levels (Supplementary Fig. S3). Thus, although the cGAS-STING pathway appears capable of regulating IFN-β transcription in PDAC cells, signaling by intrinsically-produced IFN-β does not appear to explain the enhanced DUOX2 expression following the uptake of exogenous DNA.
In conclusion, our findings have identified a novel crosstalk between the cGAS-STING immune sensing pathway and NADPH oxidase expression, both of which are implicated in mediating innate immunity at mucosal surfaces and in cancer progression. The clinical relevance of our investigations is supported by the finding that cGAS-STING and DUOX2 expression levels are significantly correlated in primary pancreatic ductal adenocarcinomas (Fig. 7). Since cGAS-STING-related enhancement of DUOX2 levels (and subsequent H2O2 formation) is associated with an increase in the normoxic expression of HIF-1α and VEGF-A, and promotes DNA double strand scission (as measured by γH2AX), without altering tumor cell proliferation in vitro (Fig. 6), our experiments suggest that peroxide-mediated DNA base oxidation and double strand breaks occurring downstream of DUOX2 [7] might provide a feedback mechanism that could sustain cGAS-STING activation [39, 40]. These results also suggest that the cGAS-STING-mediated activation of DUOX2 expression by extracellular DNA may contribute to oxidant-related pancreatic carcinogenesis by reinforcing a pro-angiogenic, oxidative pancreatic microenvironment rather than through intrinsic tumor cell proliferative mechanisms (Fig. 8).
Supplementary Material
Highlights.
Introduction of exogenous DNA by pancreatic cancer cells enhances the expression of DUOX2
DNA-induced DUOX2 activity results from activation of the cGAS-STING pathway
cGAS-STING-related DUOX2 expression leads to H2O2-related DNA double strand scission
Funding
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health (ZIA BC011076-05), and under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: DUOX2, dual oxidase 2; ROS, reactive oxygen species; PDAC, pancreatic ductal adenocarcinoma; NOX, NADPH oxidase; cyclic GMP-AMP synthase (cGAS); Stimulator of interferon genes (STING)
Data statement
Data generated in this study was both a) generated by the authors and is available upon reasonable request from the corresponding author and b) generated from The Cancer Genome Atlas public database.
Declaration of competing interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data for this article can be found online at:
References
- [1].Wu Y, Antony S, Meitzler JL, Doroshow JH, Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett 345 (2014) 164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aviello G, Knaus UG, NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol 11 (2018) 1011–23. [DOI] [PubMed] [Google Scholar]
- [3].De Deken X, Corvilain B, Dumont JE, Miot F, Roles of DUOX-mediated hydrogen peroxide in metabolism, host defense, and signaling. Antioxid Redox Signal 20 (2014) 2776–93. [DOI] [PubMed] [Google Scholar]
- [4].Wu Y, Lu J, Antony S, Juhasz A, Liu H, Jiang G et al. , Interferon-gamma-induced toll-like receptor 4 is required for the synergistic induction of dual oxidase 2 and dual oxidase A2 by lipopolysaccharide and interferon-gamma in human pancreatic cancer cell lines. J Immunol 190 (2013) 1859–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cheung EC, DeNicola GM, Nixon C, Blyth K, Labuschagne CF, Tuveson DA et al. , Dynamic ROS Control by TIGAR Regulates the Initiation and Progression of Pancreatic Cancer. Cancer Cell 37 (2020) 168–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu Y, Meitzler JL, Antony S, Juhasz A, Lu J, Jiang G et al. , Dual Oxidase2 and pancreatic adenocarcinoma: IFN-gamma-mediated dual oxidase2 overexpression results in H2O2-induced, ERK-associated up-regulation of HIF-1a and VEGF-A. Oncotarget 7 (2016)68412–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu Y, Konate MM, Lu J, Makhlouf H, Chuaqui R, Antony S et al. , IL-4 and IL-17A cooperatively promote hydrogen peroxide production, oxidative DNA damage, and upregulation of dual oxidase 2 in human colon and pancreatic cancer cells. J Immunol 203 (2019) 2532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN, Inflammation-driven carcinogenesis is mediated through STING. Nat Commun 5 (2014) 5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu H, Zhang H, Wu X, Ma D, Wu J, Wang L et al. , Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature 563 (2018) 131–6. [DOI] [PubMed] [Google Scholar]
- [10].Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE et al. , Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep 11 (2015) 1018–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ohkuri T, Kosaka A, Ishibashi K, Kumai T, Hirata Y, Ohara K et al. , Intratumoral administration of cGAMP transiently accumulates potent macrophages for anti-tumor immunity at a mouse tumor site. Cancer Immunol Immunother 66 (2017) 705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zheng Z, Jia S, Shao C, Shi Y, Irradiation induces cancer lung metastasis through activation of the cGAS-STING-CCL5 pathway in mesenchymal stromal cells. Cell Death Dis 11 (2020) 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Luecke S, Holleufer A, Christensen MH, Jonsson KL, Boni GA, Sorensen LK et al. , cGAS is activated by DNA in a length-dependent manner. EMBO Rep 18 (2017) 1707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sun L, Wu J, Du F, Chen X, Chen ZJ, Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339 (2013) 786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu J, Sun L, Chen X, Du F, Shi H, Chen C et al. , Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339 (2013) 826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tanaka Y, Chen ZJ, STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal 5 (2012) ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ishikawa H, Barber GN, STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455 (2008) 674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ishikawa H, Ma Z, Barber GN, STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461 (2009) 788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E et al. , STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med 7 (2015) 283ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang L, Shureiqi I, Stroehlein JR, Wei D, Novel and emerging innate immune therapeutic targets for pancreatic cancer. Expert Opin Ther Targets 22 (2018) 977–81. [DOI] [PubMed] [Google Scholar]
- [21].Zhao Q, Wei Y, Pandol SJ, Li L, Habtezion A, STING signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology 154 (2018) 1822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A et al. , The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov 8 (2018) 403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W et al. , Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178 (2019) 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI et al. , The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574 (2019) 264–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hoong BYD, Gan YH, Liu H, Chen ES, cGAS-STING pathway in oncogenesis and cancer therapeutics. Oncotarget 11 (2020) 2930–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xia T, Konno H, Ahn J, Barber GN, Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep 14 (2016) 282–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wu Y, Antony S, Hewitt SM, Jiang G, Yang S, Meitzler JL et al. , Functional activity and tumor-specific expression of Dual Oxidase 2 in pancreatic cancer cells and human malignancies characterized with a novel monoclonal antibody. Int J Oncol 42 (2013) 1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Higgs R, Ni GJ, Ben LN, Breen EP, Fitzgerald KA, Jefferies CA, The E3 ubiquitin ligase Ro52 negatively regulates IFN-beta production post-pathogen recognition by polyubiquitin-mediated degradation of IRF3. J Immunol 181 (2008) 1780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Long L, Deng Y, Yao F, Guan D, Feng Y, Jiang H et al. , Recruitment of phosphatase PP2A by RACK1 adaptor protein deactivates transcription factor IRF3 and limits type I interferon signaling. Immunity 40 (2014) 515–29. [DOI] [PubMed] [Google Scholar]
- [30].Prabakaran T, Bodda C, Krapp C, Zhang BC, Christensen MH, Sun C et al. , Attenuation of cGAS-STING signaling is mediated by a p62/SQSTM1-dependent autophagy pathway activated by TBK1. EMBO J 37 (2018) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ritchie C, Cordova AF, Hess GT, Bassik MC, Li L, SLC19A1 is an importer of the immunotransmitter cGAMP. Mol Cell 75 (2019) 372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Luteijn RD, Zaver SA, Gowen BG, Wyman SK, Garelis NE, Onia L et al. , SLC19A1 transports immunoreactive cyclic dinucleotides. Nature 573 (2019) 434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pan BS, Perera SA, Piesvaux JA, Presland JP, Schroeder GK, Cumming JN et al. , An orally available non-nucleotide STING agonist with antitumor activity. Science 369 (2020) . [DOI] [PubMed] [Google Scholar]
- [34].Wu Y, Antony S, Meitzler JL, Lu J, Juhasz A, Jiang G et al. Dexamethasone suppresses cytokine-induced dual oxidase 2 (Duox2) and VEGF-A expression in human pancreatic cancer cells in vitro and pancreatic cancer growth in xenografts. Cancer Res 76 (Suppl. 14), Abstract # 1456; page 371. 2016. [Google Scholar]
- [35].Wu Y, Antony S, Juhasz A, Lu J, Ge Y, Jiang G et al. , Up-regulation and sustained activation of Stat1 are essential for interferon-gamma (IFN-gamma)-induced dual oxidase 2 (Duox2) and dual oxidase A2 (DuoxA2) expression in human pancreatic cancer cell lines. J Biol Chem 286 (2011) 12245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ablasser A, Chen ZJ, cGAS in action: expanding roles in immunity and inflammation. Science 363 (2019) DOI: 10.1126/science.aat8657. [DOI] [PubMed] [Google Scholar]
- [37].Baird JR, Friedman D, Cottam B, Dubensky TW Jr., Kanne DB, Bambina S et al. , Radiotherapy combined with novel STING-targeting oligonucleotides results in regression of established tumors. Cancer Res 76 (2016) 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fink K, Martin L, Mukawera E, Chartier S, De D,X, Brochiero E. et al. , IFNbeta/TNFalpha synergism induces a non-canonical STAT2/IRF9-dependent pathway triggering a novel DUOX2 NADPH oxidase-mediated airway antiviral response. Cell Res 23 (2013) 673–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S et al. , Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 39 (2013) 482–95. [DOI] [PubMed] [Google Scholar]
- [40].Dai E, Han L, Liu J, Xie Y, Zeh HJ, Kang R et al. , Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat Commun 11 (2020) 6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu H, Moura-Alves P, Pei G, Mollenkopf HJ, Hurwitz R, Wu X et al. , cGAS facilitates sensing of extracellular cyclic dinucleotides to activate innate immunity. EMBO Rep 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zheng J, Mo J, Zhu T, Zhuo W, Yi Y, Hu S et al. , Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol Cancer 19 (2020)133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


