Abstract
The nuclear envelope plays an essential role in organizing the genome inside of the nucleus. The inner nuclear membrane is coated with a meshwork of filamentous lamin proteins that provide a surface to organize a variety of cellular processes. A subset of nuclear lamina- and membrane-associated proteins functions as anchors to hold transcriptionally silent heterochromatin at the nuclear periphery. While most chromatin tethers are integral membrane proteins, a limited number are lamina-bound. One example is the mammalian PRR14 protein. PRR14 is a recently characterized protein with unique function that is different from other known chromatin tethers. Here, we review our current understanding of PRR14 structure and function in organizing heterochromatin at the nuclear periphery.
Keywords: PRR14, nuclear lamina, peripheral heterochromatin, HP1, LADs
Graphical Abstract

Interactions between heterochromatin and the nuclear lamina are mediated by structural proteins that function to tether heterochromatin to the nuclear periphery. Here, we present an overview of the PRR14 chromatin tether, focusing on PRR14 protein structure and the mechanisms underlying PRR14 interactions with heterochromatin and the nuclear lamina in interphase and mitosis.
Introduction
The nuclear envelope separates the genome inside of the nucleus from the rest of the eukaryotic cell. In addition to its barrier function, the nuclear envelope creates a zone at the nuclear periphery that serves as a hub for dozens of membrane proteins, nuclear lamins and lamina-binding proteins (1, 2). A significant proportion of the genome is localized to the nuclear periphery, largely in the form of transcriptionally silent chromatin, also known as the peripheral heterochromatin, which is packaged into lamina-associated domains (LADs) (3, 4). The peripheral heterochromatin, comprising around 40% of the genome in most mammalian cells studied to date (5–8), contributes to the silent state of genes that reside in LADs (9–12) and provides nongenetic functions such as forming a physical scaffold to support the nuclear envelope (13).
Interactions between heterochromatin and the nuclear lamina are mediated by structural proteins that function as tethers to connect heterochromatin to the nuclear periphery. To date, only a few such tethering proteins have been described in mammals (reviewed in (14)). One tethering protein, Proline Rich 14 (PRR14) has been recently discovered (15) and, like the histone H3K9me-tethering protein CEC-4 in C. elegans (8), PRR14 associates specifically with the nuclear lamina. This is in contrast to the many other tethering proteins that are embedded in the inner nuclear membrane (16).
Here, we present an overview of the current knowledge of the function of PRR14, focusing on PRR14 protein structure and the mechanisms underlying PRR14 interactions with heterochromatin and the nuclear lamina. We propose a model for the role of PRR14 in organizing the peripheral heterochromatin and its predicted function in reestablishing heterochromatin-nuclear lamina contacts during nuclear envelope reassembly at mitotic exit.
PRR14 protein domain structure and function
Human PRR14 is a ubiquitously expressed 585 amino acid nuclear protein (15). It is well conserved in mammals (15), and has orthologs in reptiles and amphibians (17). The protein name is derived from its unusual amino acid sequence, almost 18% of which is constituted by prolines. Most of the protein is thought to be disordered and only a limited number of functional motifs or small domains have been identified. Mapping studies of PRR14 domains have identified important functional elements of the protein that include heterochromatin- and nuclear lamina-binding motifs (15). These findings suggested a role for PRR14 as a tether that promotes heterochromatin association with the nuclear lamina.
PRR14 interacts with Heterochromatin Protein 1 (HP1) via the PxVxL pocket of the HP1 dimer (18) and detailed structure-function studies have demonstrated that the N-terminus of PRR14 contains two HP1-binding LxVxL motifs (LAVVL, 52–55aa and LVVML, 153–156aa) (19), a variation of the classic PxVxL HP1-binding motif (20) (Fig. 1). The first HP1-binding site plays a major role in heterochromatin binding, while the second site is required to stabilize the interaction with heterochromatin (19). Previous studies reported PRR14 association with the HP1α isoform (21–23), but more recent reports have shown that PRR14 is able to bind all three mammalian HP1 isoforms in vivo (19). An open question in the field is whether PRR14 interacts with two HP1 dimers simultaneously and thus stabilizes HP1 association with chromatin, as was demonstrated for the SENP7 protein (24). Alternatively, PRR14 may utilize two HP1 sites for stronger association with HP1-bound heterochromatin. No other elements of PRR14 that promote or regulate its interaction with HP1 have been identified; however, such elements might exist and remain to be discovered.
Figure 1. A schematic illustration of structural and functional elements of human PRR14 protein.
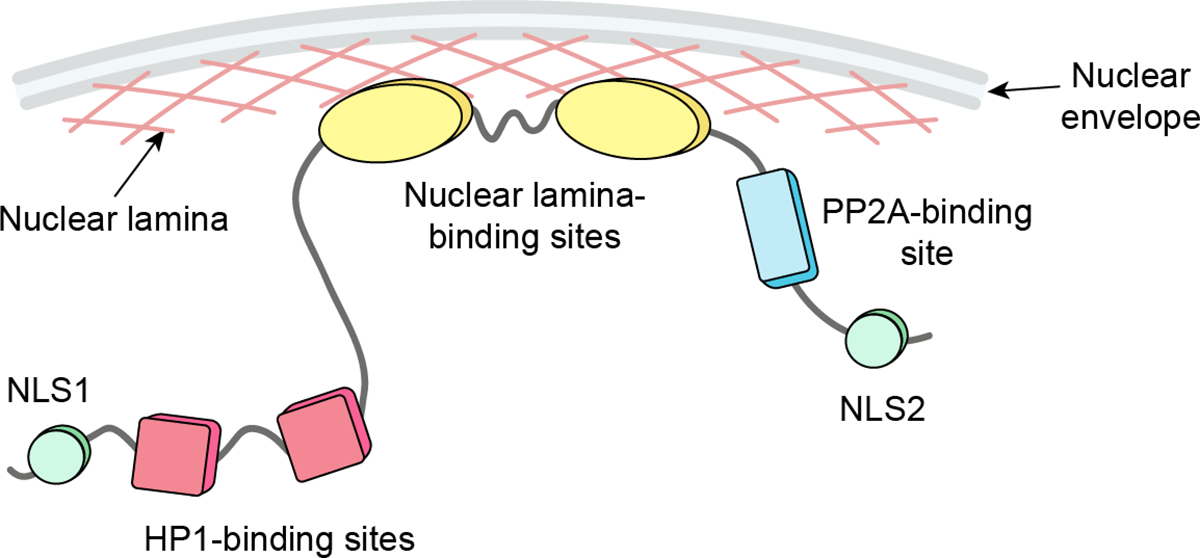
Among known functional motifs are two nuclear-localization sequences (NLS): NLS1 (30–36aa) and NLS2 (518–534aa); two HP1-binding motifs, LAVVL (52–56aa) and LVVML (153–157aa); two lamina-binding domains (LBD): LBD1 (231–282aa) and LBD2 (283–351aa); and a PP2A-binding site (495–500aa) that promotes interaction with PP2A B56α subunit.
The PRR14 association with the nuclear lamina is mediated by two lamina-binding domains (LBD) found in the central region of the protein (Fig. 1). Both domains (LBD1, 231–282aa and LBD2, 283–351aa) contain highly conserved but distinct functional motifs that promote nuclear lamina localization (17). For efficient association with the nuclear lamina, PRR14 requires the combination of both domains, but each domain is independently capable of nuclear lamina interaction (17). It has been demonstrated that PRR14 requires Lamin A/C proteins to localize to the nuclear periphery. Knockdown of Lamin A/C, but not Lamin B1 or B2 resulted in displacement of PRR14 from the nuclear periphery (15). Proteomics studies also confirmed PRR14 association with the nuclear lamina (25, 26).
Regulation of PRR14 is mediated through its C-terminus. This region contains a Protein Phosphatase 2 Subunit Alpha (PP2A) binding motif (FETIFE, 455–500aa) that is required for PP2A B56α subunit binding (17). Interaction with PP2A has been implicated in regulating phosphorylation of the PRR14 LBD and thus association of PRR14 with the nuclear lamina (discussed further below).
PRR14 organizes heterochromatin at the nuclear lamina
While the structural elements and individual functions of PRR14 support interaction with both heterochromatin and the nuclear lamina, the role of PRR14 as a tether that organizes heterochromatin at the nuclear periphery has also been demonstrated. Loss-of-function experiments revealed that knockdown of PPR14 resulted in detachment of H3K9me3-modified heterochromatin from the nuclear lamina and repositioning toward the nuclear interior (15, 19). In agreement with these observations, overexpression of PRR14 resulted in the relocalization of heterochromatin to the nuclear lamina and in an overall increase in the proportion of heterochromatin at the periphery (19). The spatial repositioning of H3K9me3-modified heterochromatin in the nucleus upon transient PRR14 knockdown or overexpression had no significant effect on the total amount of H3K9me3-modified heterochromatin (15, 19).
It is currently unclear if PRR14 has any preference for heterochromatin modified with specific post-translational modifications. The only known mechanism for PRR14 to tether chromatin is via its interaction with HP1. Mutation of HP1-binding sites of PRR14 results in no detectable PRR14-chromatin interaction (15, 19). As PRR14 can bind all HP1 isoforms, it is predicted that any preference for PRR14-chromatin tethering would be dictated by HP1-chromatin binding properties. HP1 has been shown to bind chromatin through a number of different interactions (reviewed in (27–30)). The most well-established interaction of HP1 is with the methylated tail of Histone H3 at Lys9 (H3K9me3 and H3K9me2). Pending further genome-wide occupancy studies, it is unclear which regions of the genome are organized by PRR14 and whether there is any specificity or preference for heterochromatin recognition and tethering by PRR14. The enrichment for H3K9me2/3 in the peripheral heterochromatin of LADs (7, 31, 32) and the strong correlation of HP1β and LADs (25) suggest that PRR14, as an HP1/H3K9me3-heterochromatin tether, plays an important role in the organization of LADs at the nuclear periphery.
Not every locus marked with H3K9me3 and bound by HP1 protein is positioned at the nuclear periphery. HP1/H3K9me3-marked heterochromatin is also found in other nuclear compartments, including chromocenters and the perinucleolar regions. This suggests that the heterochromatin-lamina tethering mechanism is likely regulated on many levels. It is also possible that PRR14 and other HP1 interactors compete for the same PxVxL-binding pocket on HP1 (18, 30).
Dynamic association of PRR14 with heterochromatin and the nuclear lamina
In contrast to chromatin tethers that are anchored in the nuclear membrane (e.g., LBR, LEM domain and SUN domain proteins (33–36)), PRR14 associates with the nuclear lamina. This could allow PRR14 to initiate complex formation with either heterochromatin or the nuclear lamina before linking them together. In vivo studies confirmed that PRR14 localization and interaction with heterochromatin and the nuclear lamina are dynamic (17, 19). Important characteristics of PRR14 dynamics have been obtained using fluorescent recovery after photobleaching (FRAP) experiments. These experiments showed that an average half-recovery time of PRR14 at the nuclear lamina is about 30 seconds (19), as compared to an average recovery time for lamina proteins at the nuclear periphery of around 10 minutes (37). Moreover, PRR14 recovery at the nuclear lamina is incomplete: approximately half of the proteins are not replaced at the periphery, suggesting an immobile fraction that has a tighter association with the nuclear lamina (19). The ability of PRR14 to bind heterochromatin has no impact on the strength of PRR14 association with the nuclear lamina, as was demonstrated using HP1-binding site mutants of PRR14 (19). In contrast to the full-length protein, an LBD-only fragment of PRR14 had no substantial immobile fraction. This suggests that yet unknown PRR14 motifs or perhaps interactions with other proteins might be involved in stabilizing the PRR14-nuclear lamina association. Conversely, PRR14 association with heterochromatin is very rapid. The half-recovery time of PRR14-heterochromatin association is around 5 seconds with a minimal immobile fraction (19). Such PRR14 dynamics are comparable to HP1-heterochromatin interaction which has been reported to be between 2.5 and 10 seconds (38–40). Together, these results demonstrate that PRR14 has a much stronger association with the nuclear lamina compared to more transient interaction with heterochromatin.
Super-resolution images of PRR14 localization in the nucleus showed that the majority of PRR14 proteins localized at the nuclear lamina and a much smaller fraction is observed in the nucleoplasm (19). At the nuclear periphery, PRR14 proteins create a seemingly uniform meshwork, similar to what is observed for nuclear lamins. In contrast, the nucleoplasmic PRR14 fraction has a scattered distribution without any discernable enrichment at heterochromatin loci or any other structures (19). It is currently unknown if nucleoplasmic PRR14 forms complexes with other proteins, for example with the nucleoplasmic fraction of Lamin A/C (41). Nucleoplasmic PRR14 might also represent a phosphorylated form of the protein that has lost association with the nuclear lamina (discussed below).
The observed behavior and properties of PRR14 displayed in interphase suggest a model where PRR14 proteins create a “sticky” surface overlaying the nuclear lamina meshwork that provides a platform for HP1-bound chromatin to attach to the nuclear periphery. While the interaction of individual HP1 and PRR14 molecules may be very rapid, large heterochromatin loci might have multiple transient HP1-PRR14 contacts across the surface to support heterochromatin-nuclear lamina association (Fig. 2).
Figure 2. A schematic model of PRR14 as a tether of HP1-bound heterochromatin to the nuclear lamina.
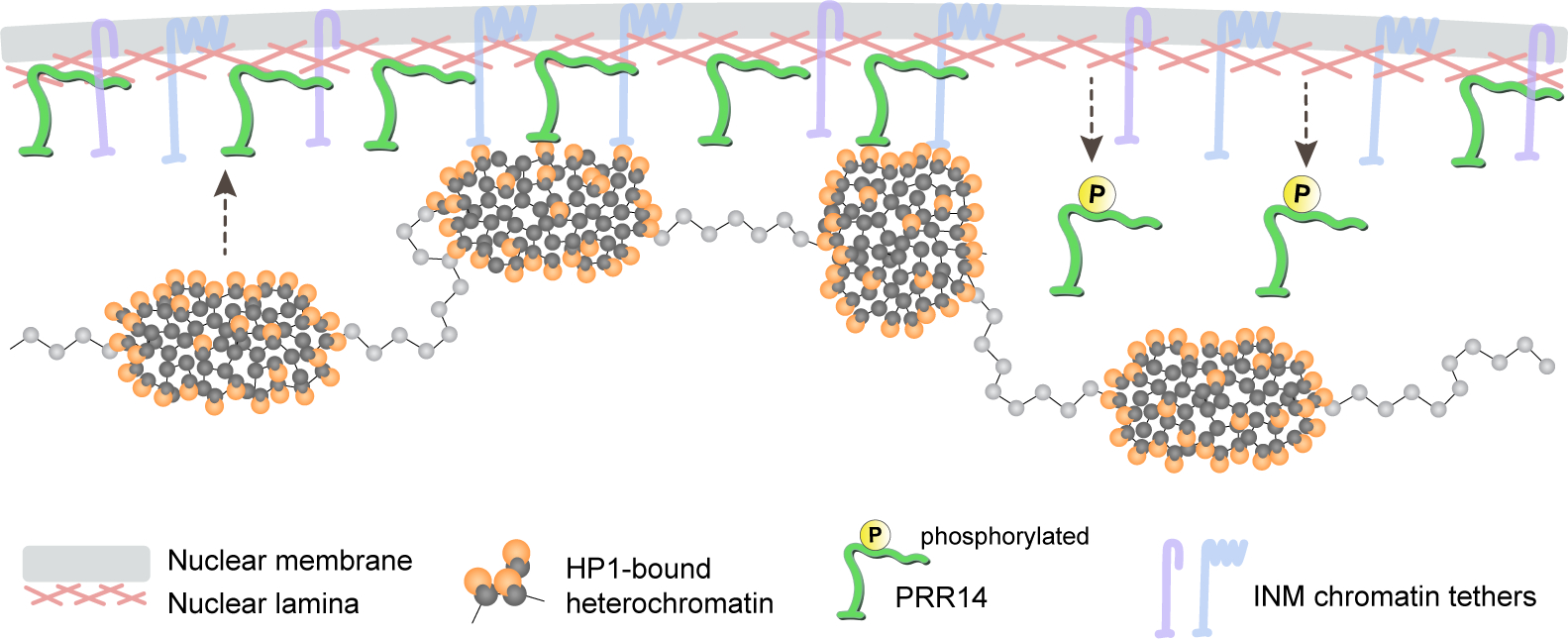
PRR14 is associated with the nuclear lamina, which distinguishes it from other mammalian inner-nuclear membrane (INM) tethers (e.g., LBR, LEM-domain proteins). PRR14 proteins are uniformly distributed at the nuclear periphery creating a “sticky” surface for HP1-bound heterochromatin. Association of PRR14 with the nuclear lamina is regulated via phosphorylation of PRR14 lamina-binding domain.
PRR14 function is regulated by phosphorylation
PRR14 association with the nuclear lamina is phosphoregulated (17). Four potential phosphorylation sites were identified within the Lamina-Binding Domain 1 (LBD1) of PRR14. Experiments with phospho-mimic versions of the PRR14 protein demonstrated that phosphorylation of these sites results in detachment of the PRR14 from the nuclear lamina. To function as a LAD tether, PRR14 must be able to bind both heterochromatin and the nuclear lamina. Thus, the prevention of PRR14 binding to the nuclear lamina via phosphorylation may abrogate its lamina binding and reduce the association of peripheral heterochromatin with the nuclear lamina (Fig. 2).
It is likely that PRR14 phosphorylation serves different functions during different stages of the cell cycle. We might speculate that, in interphase, the phosphoregulation of PRR14 via signaling pathways could be a mechanism to control heterochromatin localization at the nuclear periphery (Fig. 3). Such a mechanism might be utilized to release specific chromatin regions from the nuclear periphery in response to internal or external stimuli. PRR14 phosphorylation may also play an important role in mitosis where phosphorylation of PRR14 could trigger detachment of the peripheral heterochromatin from the nuclear lamina. Future studies are necessary to validate or refute these speculations.
Figure 3. A schematic model of PRR14 function during mitosis.

In prophase, multiple phosphorylation events occur, including phosphorylation of Ser10 on histone H3 tail (H3S10p) that displaces HP1 proteins from H3K9-methylated chromatin. At the same time, PRR14 is observed to detach from the nuclear lamina, potentially via phosphorylation of the PRR14 LBD. In anaphase, chromatin dephosphorylation triggers reassembly of the HP1/PRR14 complex of the mitotic chromosomes. During telophase progression and nuclear envelope reassembly, PRR14 is proposed as a platform for nuclear lamina formation on the surface of mitotic chromatin and re-establishment of heterochromatin-nuclear lamina contacts.
PRR14 function during mitosis
Mitosis in higher eukaryotes is a complex and highly coordinated process during which nuclear envelope proteins, including chromatin tethers, are critical to accomplish a complete disassembly of all nuclear structures and then reassembly in daughter cells (1, 42). Most chromatin tethers are integral membrane proteins and hence their localization and function are restricted to nuclear membranes/ER during mitosis.
Mitotic entry is accompanied by numerous phosphorylation events that trigger chromatin detachment from the nuclear periphery, nuclear lamina disassembly and nuclear envelope breakdown (43). Detachment of heterochromatin from the nuclear lamina requires disengagement of the tethering complex which is achieved through several steps: phosphorylation of Histone H3 Ser 10 (H3S10ph) displaces HP1 proteins from H3K9me3-modified heterochromatin (44) and, we propose, phosphorylation of PRR14 results in its detachment from the nuclear lamina (Fig. 3). It has not yet been confirmed that phosphorylation of the LBD of PRR14 is the precise cause of detachment of PRR14 from the nuclear lamina in prophase, but the observation that PRR14 separates from the nuclear lamina before the nuclear lamina disassembly (15) suggests that this may be the case.
Towards the later stages of mitosis, as the cell enters anaphase, rapid histone dephosphorylation results in HP1 and PRR14 rebinding to chromatin (45–47) (Fig. 3). As anaphase progresses, a majority of PRR14 protein rebinds chromatin (15). During this stage, PRR14 is predicted to remain phosphorylated to prevent PRR14-nuclear lamina interaction as the nuclear lamina remains disassembled.
Concordant with the cell entry into telophase, the nuclear envelope and nuclear lamina begin to reassemble on the surface of the mitotic chromatin. These processes are triggered by PP2A phosphatase activity, which is also required for dephosphorylation of PRR14 as well as another critical chromatin organizer, Barrier-to-Autointegration Factor (BAF) (48). BAF provides the chromatin-binding site for LEM domain proteins and is essential for nuclear membrane recruitment and nuclear envelope reassembly (49). Dephosphorylation of PRR14 exposes its lamina-binding domain for interaction with the nuclear lamina (specifically Lamin A/C). We note that such function of PRR14 on the surface of mitotic chromatin could potentially mimic BAF function but recruit Lamin A/C and promote nuclear lamina polymerization on the PRR14-coated chromatin (Fig. 3). We hypothesize that PRR14 is required for nuclear lamina assembly via its LBD domains which would serve as a platform on which to initiate nuclear lamina polymerization. Consistent with this hypothesis, knockdown experiments revealed that severe morphological defects in Lamin A/C occur in the absence of PRR14 (15).
It is intriguing that PRR14 potentially serves as a surface coating for both heterochromatin and the nuclear lamina. In interphase cells, PRR14 lines the nuclear lamina and allows for interaction of heterochromatin with the nuclear periphery, while in mitosis PRR14 coats mitotic chromatin and potentially promotes nuclear lamina recruitment (Fig. 4).
Figure 4. A comparison of PRR14 tethering function in interphase and mitotic cells.
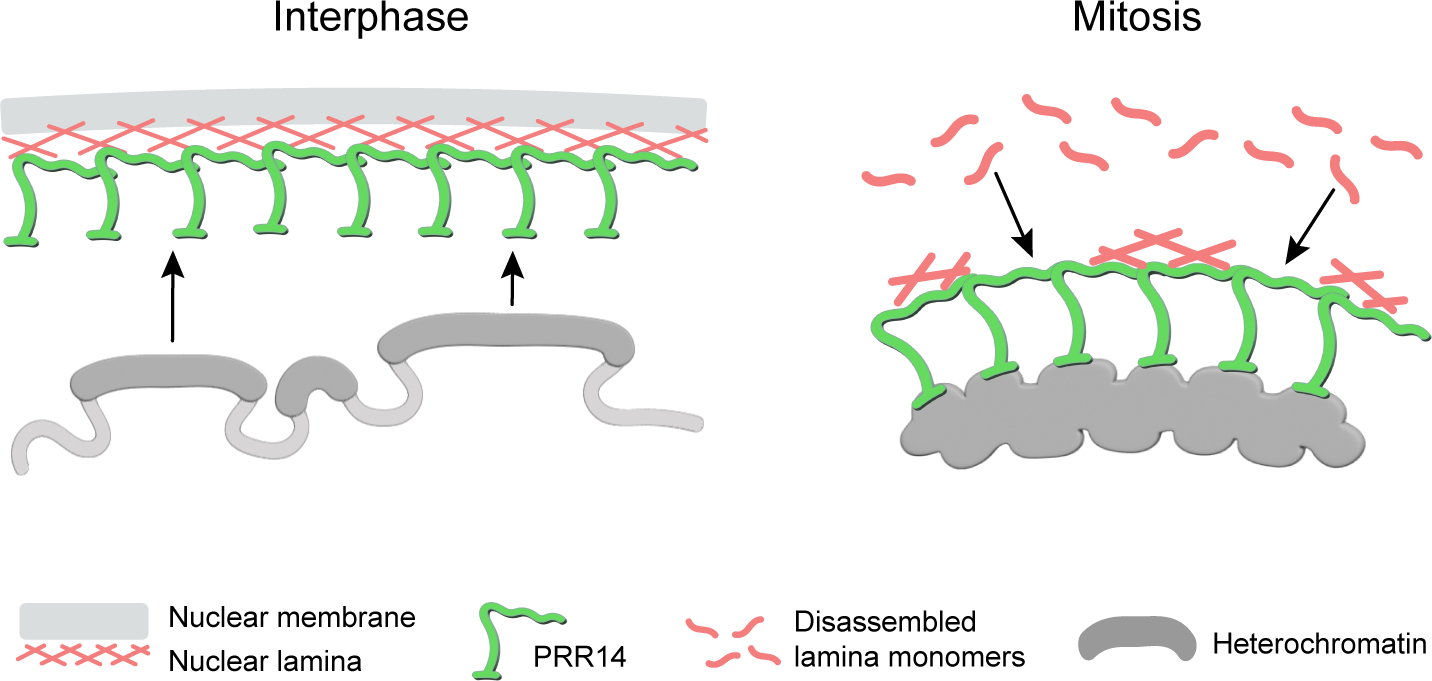
In interphase cells, PRR14 coats the nuclear lamina and provides a binding surface for heterochromatin at the nuclear periphery. In telophase, PRR14 coats mitotic chromatin and is predicted to promote nuclear lamina reassembly on the surface of mitotic chromatin.
A role for PRR14 in reestablishing the heterochromatin-lamina contacts
Upon nuclear lamina reassembly during telophase, the connections between PRR14 and the nuclear lamina are restored at the surface of the HP1/H3K9me3-modified heterochromatin. Such function of the tethering protein could be part of the cellular mechanism to bookmark the peripheral heterochromatin for reestablishment at the nuclear periphery after cell division. This may be part of the method by which the cell maintains cell type-specific nuclear organization in daughter cells.
Multiple studies have demonstrated that key features of the genome organization and function are bookmarked through mitosis to ensure correct re-establishment of the chromatin organization and gene expression programs (reviewed in (50–53)). These include pioneer transcription factors that remain associated with the mitotic chromatin to ensure early transcriptional activation of key lineage-specific genes (54, 55) and histone modifications that potentially mark different types of chromatin for specific nuclear localization (56). Here we propose that chromatin tethers function to recognize and position chromatin domains that are marked with a specific histone modification, namely H3K9me3.
The role of PRR14 in development and disease
It has been demonstrated that changes in PRR14 expression levels can significantly affect lamina-associated heterochromatin in the nucleus (19). It is well established that LADs play a critical role in epigenetic control of gene expression, lineage-specific gene programs (5, 7, 57–59), and further contribute to the restriction of alternative fate programs and maintenance of lineage-specific cell identity (8, 59–61). Multiple examples of abnormal cell differentiation and development have been reported following disruptions of peripheral heterochromatin and LADs (7, 8, 60). These aberrations are the result of mutations or loss of key members of tethering complexes, including nuclear lamin proteins, LEM-domain proteins, and others (15, 60, 62, 63). While the mechanism of heterochromatin tethering is likely redundant, loss of function of one of the components can result in small, but significant changes in genome organization, which may accumulate over time, and result in or exacerbate disease (60). These examples highlight the importance of appropriate, cell-type-specific spatial organization of chromatin in the nucleus.
We speculate that mutations in nuclear lamina proteins could affect the efficiency with which PRR14 binds to the nuclear lamina and thus reduce its tethering function and loosen the heterochromatin association with the nuclear periphery. Furthermore, PRR14 was originally identified as an epigenetic repressor (64). Knockdown of PRR14 not only affects localization of the heterochromatin but also results in activation of epigenetically silent genes (64). PRR14 mutations, including some at phosphorylation sites, have been reported in several cancers (17). This suggests that PRR14 might play a critical role in the maintenance of chromatin organization in the nucleus and likely impacts gene expression profiles.
Conclusions and Perspectives
Mounting evidence indicates that PRR14 as a chromatin tether is more than a simple, static anchor of heterochromatin at the nuclear periphery. PRR14 appears to play an important role in establishment, maintenance, and regulated organization of lamina-associated heterochromatin. While the function of nuclear lamina-heterochromatin tethers seems, on the surface, to be obvious and straightforward, the intricacies of peripheral heterochromatin organization turn out to be complex and dynamic. Recent findings show that tethering proteins, in addition to their primary function of anchoring heterochromatin to the nuclear lamina, can regulate the amount of heterochromatin at the nuclear periphery (19, 65), impact nuclear stiffness (63, 66, 67), genome compartmentalization (65, 68), and gene expression (64). They are also likely to be involved in the mitotic inheritance of spatial heterochromatin organization by re-establishing contacts between heterochromatin and the nuclear lamina upon mitotic exit. Further studies of chromatin tethers will deepen our understanding of the mechanisms and principles of peripheral heterochromatin organization, maintenance and regulation during cell differentiation and lineage maintenance.
Acknowledgments
We thank Cheryl L. Smith for the valuable insights on the discussion and editing of the manuscript. We also thank Jonathan A. Epstein, Richard A. Katz, and Rajan Jain for critical reading of the manuscript. Our research has been funded by the National Institutes of Health (R21AG081795, R35HL140018 and R35HL166663) and American Heart Association (827458).
Abbreviations
- BAF
Barrier-to-Autointegration Factor protein
- FRAP
fluorescent recovery after photobleaching
- HP1
Heterochromatin Protein 1
- INM
Inner-Nuclear Membrane
- LADs
lamina-associated domains
- LBD
lamina-binding domains
- LBR
Lamin B Receptor protein
- PRR14
Proline Rich 14 protein
- PP2A
Protein Phosphatase 2 Subunit Alpha
References
- 1.Guttinger S, Laurell E, Kutay U, Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol 10, 178–191 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Wong X, Luperchio TR, Reddy KL, NET gains and losses: the role of changing nuclear envelope proteomes in genome regulation. Curr Opin Cell Biol 28, 105–120 (2014). [DOI] [PubMed] [Google Scholar]
- 3.van Steensel B, Belmont AS, Lamina-associated domains: Links with chromosome architecture, heterochromatin, and gene repression. Cell 169, 780–791 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briand N, Collas P, Lamina-associated domains: peripheral matters and internal affairs. Genome Biol 21, 85 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peric-Hupkes D et al. , Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell 38, 603–613 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guelen L et al. , Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453, 948–951 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Poleshko A et al. , Genome-Nuclear Lamina Interactions Regulate Cardiac Stem Cell Lineage Restriction. Cell 171, 573–587 e514 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Sandoval A et al. , Perinuclear Anchoring of H3K9-Methylated Chromatin Stabilizes Induced Cell Fate in C. elegans Embryos. Cell 163, 1333–1347 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Kind J, van Steensel B, Genome-nuclear lamina interactions and gene regulation. Curr Opin Cell Biol 22, 320–325 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Buchwalter A, Kaneshiro JM, Hetzer MW, Coaching from the sidelines: the nuclear periphery in genome regulation. Nat Rev Genet 20, 39–50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerreiro I, Kind J, Spatial chromatin organization and gene regulation at the nuclear lamina. Curr Opin Genet Dev 55, 19–25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Towbin BD, Gonzalez-Sandoval A, Gasser SM, Mechanisms of heterochromatin subnuclear localization. Trends Biochem Sci 38, 356–363 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Bustin M, Misteli T, Nongenetic functions of the genome. Science 352, aad6933 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzo SG, Dauban L, van Steensel B, Lamina-associated domains: Tethers and looseners. Curr Opin Cell Biol 74, 80–87 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Poleshko A et al. , The human protein PRR14 tethers heterochromatin to the nuclear lamina during interphase and mitotic exit. Cell Rep. 5, 292–301 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czapiewski R, Robson MI, Schirmer EC, Anchoring a Leviathan: How the Nuclear Membrane Tethers the Genome. Front Genet 7, 82 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunlevy KL et al. , The PRR14 heterochromatin tether encodes modular domains that mediate and regulate nuclear lamina targeting. J Cell Sci 133, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiru A et al. , Structural basis of HP1/PXVXL motif peptide interactions and HP1 localisation to heterochromatin. EMBO J 23, 489–499 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiseleva AA, Cheng YC, Smith CL, Katz RA, Poleshko A, PRR14 organizes H3K9me3-modified heterochromatin at the nuclear lamina. Nucleus 14, 2165602 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechner MS, Schultz DC, Negorev D, Maul GG, Rauscher FJ 3rd, The mammalian heterochromatin protein 1 binds diverse nuclear proteins through a common motif that targets the chromoshadow domain. Biochem Biophys Res Commun 331, 929–937 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Rual JF et al. , Towards a proteome-scale map of the human protein-protein interaction network. Nature 437, 1173–1178 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Nozawa RS et al. , Human POGZ modulates dissociation of HP1alpha from mitotic chromosome arms through Aurora B activation. Nat Cell Biol 12, 719–727 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Yang M, Yuan ZM, A novel role of PRR14 in the regulation of skeletal myogenesis. Cell Death Dis 6, e1734 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romeo K et al. , The SENP7 SUMO-protease presents a module of two HP1 interaction motifs that locks HP1 protein at pericentric heterochromatin. Cell Rep. 10, 771–782 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Wong X et al. , Mapping the micro-proteome of the nuclear lamina and lamina-associated domains. Life Sci Alliance 4, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Go CD et al. , A proximity-dependent biotinylation map of a human cell. Nature 595, 120–124 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Hediger F, Gasser SM, Heterochromatin protein 1: don’t judge the book by its cover! Curr Opin Genet Dev 16, 143–150 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Canzio D, Larson A, Narlikar GJ, Mechanisms of functional promiscuity by HP1 proteins. Trends Cell Biol 24, 377–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sales-Gil R, Vagnarelli P, How HP1 Post-Translational Modifications Regulate Heterochromatin Formation and Maintenance. Cells 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A, Kono H, Heterochromatin protein 1 (HP1): interactions with itself and chromatin components. Biophys Rev 12, 387–400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah PP et al. , An atlas of lamina-associated chromatin across twelve human cell types reveals an intermediate chromatin subtype. Genome Biol 24, 16 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith CL, Lan Y, Jain R, Epstein JA, Poleshko A, Global chromatin relabeling accompanies spatial inversion of chromatin in rod photoreceptors. Sci Adv 7, eabj3035 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makatsori D et al. , The inner nuclear membrane protein lamin B receptor forms distinct microdomains and links epigenetically marked chromatin to the nuclear envelope. J Biol Chem 279, 25567–25573 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Barton LJ, Soshnev AA, Geyer PK, Networking in the nucleus: a spotlight on LEM-domain proteins. Curr Opin Cell Biol 34, 1–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horigome C, Okada T, Shimazu K, Gasser SM, Mizuta K, Ribosome biogenesis factors bind a nuclear envelope SUN domain protein to cluster yeast telomeres. EMBO J 30, 3799–3811 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pennarun G, Picotto J, Bertrand P, Close Ties between the Nuclear Envelope and Mammalian Telomeres: Give Me Shelter. Genes (Basel) 14, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP, Nuclear lamins: building blocks of nuclear architecture. Genes Dev 16, 533–547 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Cheutin T et al. , Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science 299, 721–725 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Festenstein R et al. , Modulation of heterochromatin protein 1 dynamics in primary Mammalian cells. Science 299, 719–721 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Romeo K et al. , The SENP7 SUMO-Protease Presents a Module of Two HP1 Interaction Motifs that Locks HP1 Protein at Pericentric Heterochromatin. Cell Rep 10, 771–782 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Naetar N, Ferraioli S, Foisner R, Lamins in the nuclear interior - life outside the lamina. J Cell Sci 130, 2087–2096 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Wandke C, Kutay U, Enclosing chromatin: reassembly of the nucleus after open mitosis. Cell 152, 1222–1225 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Dephoure N et al. , A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci U S A 105, 10762–10767 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirota T, Lipp JJ, Toh BH, Peters JM, Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature 438, 1176–1180 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Hendzel MJ et al. , Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348–360 (1997). [DOI] [PubMed] [Google Scholar]
- 46.McManus KJ, Hendzel MJ, The relationship between histone H3 phosphorylation and acetylation throughout the mammalian cell cycle. Biochem Cell Biol 84, 640–657 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Vagnarelli P et al. , Repo-Man coordinates chromosomal reorganization with nuclear envelope reassembly during mitotic exit. Dev Cell 21, 328–342 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asencio C et al. , Coordination of kinase and phosphatase activities by Lem4 enables nuclear envelope reassembly during mitosis. Cell 150, 122–135 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Haraguchi T et al. , Live cell imaging and electron microscopy reveal dynamic processes of BAF-directed nuclear envelope assembly. J Cell Sci 121, 2540–2554 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Kadauke S, Blobel GA, Mitotic bookmarking by transcription factors. Epigenetics Chromatin 6, 6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaidi SK, Lian JB, van Wijnen A, Stein JL, Stein GS, Mitotic Gene Bookmarking: An Epigenetic Mechanism for Coordination of Lineage Commitment, Cell Identity and Cell Growth. Adv Exp Med Biol 962, 95–102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Festuccia N, Gonzalez I, Owens N, Navarro P, Mitotic bookmarking in development and stem cells. Development 144, 3633–3645 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Ito K, Zaret KS, Maintaining Transcriptional Specificity Through Mitosis. Annu Rev Genomics Hum Genet 23, 53–71 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaret KS, Caravaca JM, Tulin A, Sekiya T, Nuclear mobility and mitotic chromosome binding: similarities between pioneer transcription factor FoxA and linker histone H1. Cold Spring Harb Symp Quant Biol 75, 219–226 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Caravaca JM et al. , Bookmarking by specific and nonspecific binding of FoxA1 pioneer factor to mitotic chromosomes. Genes Dev 27, 251–260 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poleshko A et al. , H3K9me2 orchestrates inheritance of spatial positioning of peripheral heterochromatin through mitosis. Elife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lochs SJA, Kefalopoulou S, Kind J, Lamina Associated Domains and Gene Regulation in Development and Cancer. Cells 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andrey G, Mundlos S, The three-dimensional genome: regulating gene expression during pluripotency and development. Development 144, 3646–3658 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Methot SP et al. , H3K9me selectively blocks transcription factor activity and ensures differentiated tissue integrity. Nat Cell Biol 23, 1163–1175 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah PP et al. , Pathogenic LMNA variants disrupt cardiac lamina-chromatin interactions and de-repress alternative fate genes. Cell Stem Cell 28, 938–954 e939 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harr JC et al. , Loss of an H3K9me anchor rescues laminopathy-linked changes in nuclear organization and muscle function in an Emery-Dreifuss muscular dystrophy model. Genes Dev 34, 560–579 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luke Y et al. , Nesprin-2 Giant (NUANCE) maintains nuclear envelope architecture and composition in skin. J Cell Sci 121, 1887–1898 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Lammerding J et al. , Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol 170, 781–791 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poleshko A et al. , Human factors and pathways essential for mediating epigenetic gene silencing. Epigenetics 9, 1280–1289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solovei I et al. , LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 152, 584–598 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Schreiner SM, Koo PK, Zhao Y, Mochrie SG, King MC, The tethering of chromatin to the nuclear envelope supports nuclear mechanics. Nat Commun 6, 7159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lionetti MC et al. , Chromatin and Cytoskeletal Tethering Determine Nuclear Morphology in Progerin-Expressing Cells. Biophys J 118, 2319–2332 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falk M et al. , Heterochromatin drives compartmentalization of inverted and conventional nuclei. Nature 570, 395–399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


