Abstract
Neuroinflammation after traumatic brain injury (TBI) is related to chronic neurodegenerative diseases and is one of the causes of acute secondary injury after TBI. Therefore, it is particularly important to clarify the role of cellular mechanisms in the neuroinflammatory response after TBI. The objective of this article is to understand the involvement of cells during the TBI inflammatory response (for instance, astrocytes, microglia, and oligodendrocytes) and shed light on the recent progress in the stimulation and interaction of granulocytes and lymphocytes, to provide a novel approach for clinical research. We searched articles in PubMed published between 1950 and 2023, using the following keywords: TBI, neuroinflammation, inflammatory cells, neuroprotection, clinical. Articles for inclusion in this paper were finalized based on their novelty, representativeness, and relevance to the main arguments of this review. We found that the neuroinflammatory response after TBI includes the activation of glial cells, the release of inflammatory mediators in the brain, and the recruitment of peripheral immune cells. These inflammatory responses not only induce secondary brain damage, but also have a role in repairing the nervous system to some extent. However, not all of the mechanisms of cell-to-cell interactions have been well studied. After TBI, clinical treatment cannot simply suppress the inflammatory response, and the inflammatory phenotype of patients’ needs to be defined according to their specific conditions after injury. Clinical trials of personalized inflammation regulation therapy for specific patients should be carried out in order to improve the prognosis of patients.
Keywords: inflammatory cells, neuroinflammation, traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) is a functional or pathological change in the brain caused by external forces. It is a major public health problem around the world. Studies suggest that post-traumatic stress disorder, memory disorder, Parkinson’s disease, epilepsy, and chronic traumatic encephalopathy are all related to neuroinflammatory response.[1–5] Inflammation is associated with fighting, invading pathogens, and maintaining tissues’ physiological health. However, under pathological conditions such as trauma, inflammation can also aggravate tissue damage.[6–8] Initially, it was believed that TBI’s inflammatory response occurs via impaired blood–brain barrier (BBB) by peripheral immune mediators, however, it was then proved that it occurs through a complex interaction of central, peripheral, and soluble cells, and is controlled by a variety of factors. After TBI, microglia initiate the infiltration of peripheral neutrophils followed by lymphocytes and mononuclear macrophages, while various inflammatory cytokines and chemokines stimulate immune cells and accumulate in the lesion area.[9–12] Inflammatory response after TBI has advantages along with certain disadvantages, such as it promotes debris clearance, neuronal regeneration, glial cell differentiation, and angiogenesis and is harmful as it mediates neuronal death and neurodegenerative diseases.[5,10,12,13] Therefore, a comprehensive understanding of neuroinflammatory responses after TBI is particularly important. This review focuses on the latest research associated with cells related to neuroinflammatory response after TBI and their interactions concerning the 2 aforementioned aspects.
2. Inflammatory response and glial cells after TBI
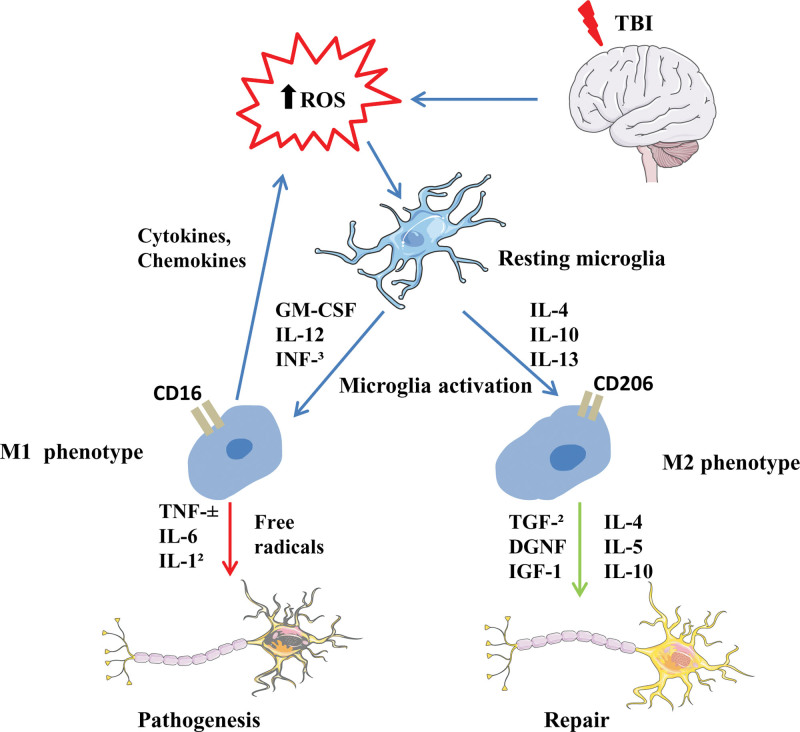
2.1. Inflammatory response and microglia initiation after TBI
The microglia cells have functions similar to that of peripheral macrophages and are easily affected by the surrounding microenvironment to polarize to different phenotypes. Polarized microglial cells are categorized into the M1 and M2 types, and the M2 is further divided into M2a, M2b, and M2c subtypes.[13] The M1-like phenotype is stimulated mainly by damage-associated molecular patterns (DAMPs), free radicals, or pro-inflammatory cytokines, and is manifested by increased chemokines, pro-inflammatory cell factors, reactive oxygen species production, and decreased cytophagocytosis activity. M1-like “pro-inflammatory” microglia cells are generally considered harmful, and their excess or prolonged responses can cause secondary brain damage. M2a-like microglia produce interleukins interleukin (IL)-4 and IL-13, which are linked with increased anti-inflammatory cytokines production and phagocytosis. M2c-like microglia may promote tissue repair and remodeling. M2b-like can be stimulated by toll-like receptor ligands and has both pro-inflammatory and anti-inflammatory effects (Fig. 1). However, the dominant polarization type of microglia changed with the time after TBI and the different TBI models, and most activated microglia were mixed with M1/M2-like microglia. Studies have shown that M2-type microglia can promote tissue repair and have anti-inflammatory effects, while the abnormal increase of M1-type microglia further advances pathological conditions.[14] Therefore, the early treatment strategy of TBI should not only inhibit the activation of microglia, but also pay more attention to increasing the proportion of M2-type microglia. Recent studies suggest that the following factors can improve the M2/M1 ratio: Atorvastatin, deoxycholic acid taurine, sinomenine, hydrogen inhalation, colony-stimulating factor-1, erythropoietin, TAK-242, Minocycline, Fingolimod, Peroxisome proliferator-activated receptors, Progranulin, Laquinimod, and Fingolimod, Fingormode, estrogen, and progesterone, omega-3 polyunsaturated fatty acids, Histone deacetylase inhibition, suppressors of cytokine signaling, complement components, IL-1 receptor antagonist, stem cells, hypothermia, and Maraviroc; all of these mediators have good anti-inflammatory effects at the level of animal models and play a certain role in neuroprotection after TBI.[15–22] Of course, large amounts of clinical data and further mechanistic studies are still needed in the future.
Figure 1.
Inflammatory response and microglia initiation after TBI. TBI = traumatic brain injury.
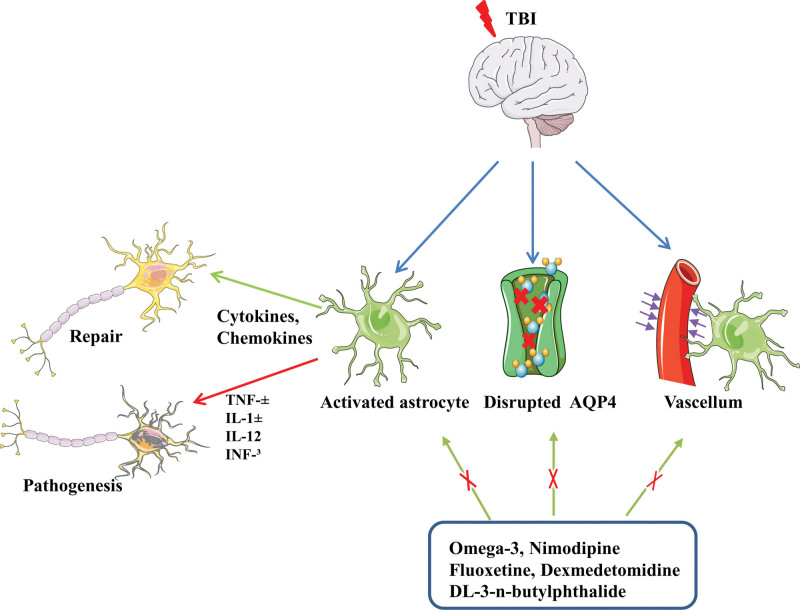
2.2. Inflammatory response and astrocyte initiation after TBI
Cell swelling is the main pathophysiological mechanism of increased intracranial pressure after TBI and astrocytoma-mediated nuclear factor kappa-B (NF-κB) signaling is responsible for this cell swelling. Therefore, inhibition of NF-κB signaling in astrocytes can reduce the inflammatory response after central nervous system (CNS) injury.[23–25] Small et al[26] found that after TBI, reactive astrocytes could increase the possibility of epilepsy after TBI by inducing JNK signal transduction. Additionally, after TBI, the stimulated high mobility group protein 1 (HMGB1) sends signals to microglia to induce IL-6 secretion and to reactive astrocytes to up-regulate the aquaporin-4 (AQP4) water channel involved in water uptake.[27] The negative side of reactive astrocytes is that they can directly increase intracranial pressure due to cytotoxic edema and produce harmful inflammatory mediators that aggravate brain injury. Although Walko et al[28] found that increasing the concentration of DAMPS, HMGB1 and mitochondrial deoxyribonucleic acid in cerebrospinal fluid (CSF) aggravates the condition of patients with TBI, astrocytic DAMPs can also cause them to interact with phagocytes, thus promoting the clearance of toxic debris.[29,30] Moreover, after the brain injury, the reactive astrocytes release signals such as HMGB1 that stimulate endothelial cells and their progenitors to boost BBB repair and neurovascular remodeling.[31,32] Rosa’s et al[33] study found that toll-like receptor 4-induced astrocyte activation could improve synaptic and cerebrovascular integrity after TBI. In addition, NF-κB signaling in astrocytes may be beneficial after brain injury as it stimulates the production and secretion of brain-derived neurotrophic factor, nerve and glia-protective growth factor, and nerve growth factor.[30] In addition, as a component of the glymphatic system, astrocytes participate in the clearance of metabolites in the brain, and the phalopodium of astrocytes intensively expresses AQP4 water channel, which helps cerebrospinal fluid flow into the brain parenchyma.[34] Ren et al[35] studied the expression and localization of AQP4 in mild and moderate TBI. They found that increased expression of this channel was associated with depolarization. These changes correlated with the proportion of reactive astrocyte hyperplasia. According to Iliff et al,[36] deletion of aquaporin 4 gene leads to increased Tau levels after TBI. This promotes intracellular aggregation of proteins, along with axonal degeneration, neuroinflammation, and worsening of cognitive impairment. Plog and Nedergaard[37] evaluated the clearance rates of TBI-specific markers such as S100b, GFAP and NSE through experiments. The results showed that the change of AQP4 channel reduced the clearance rate of TBI biomarkers. Therefore, targeting reactive gliosis may be a therapeutic strategy to normalize AQP4 expression and improve clearance of interstitial waste after TBI. Zhang et al[38] found that omega-3 intake may be a potential treatment for astrocyte alterations after TBI, as the administration of fish oil for 2 months prior to the determination of TBI significantly improved neuronal function in mouse models, preventing aßs from accumulating by partially restoring the expression and depolarization of TBI-damaged AQP4. In addition, drugs such as Nimodipine, Dexmedetomidine, Selective serotonin reuptake inhibitor: fluoxetine, DL-3-n-butylphthalide can improve the function of glymphatic system and play a neuroprotective role.[39] In summary, at different injury levels and their associated time points, astrocytes produce different inflammatory molecules, immunomodulatory molecules, cytokines, chemokines, and growth factors.[40–43] Therefore, during the investigation of the anti-inflammatory and proinflammatory effects of astrocytes produced by TBI, the influence of the degree and time associated with the injury should be considered (Fig. 2).
Figure 2.
Inflammatory response and astrocyte initiation after TBI. TBI = traumatic brain injury.
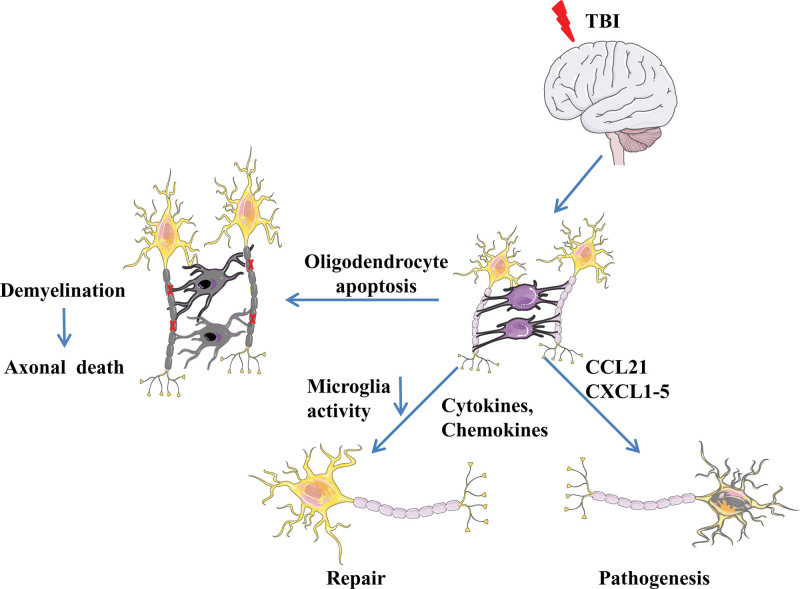
2.3. Inflammatory response and oligodendrocyte initiation after TBI
Axons and blood vessels are wrapped by mature oligodendrocytes to form a myelin sheath, which serves as nerve insulation and maintains normal neuronal functions. Mature oligodendrocytes are categorized into 3 types based on their distribution: intervascular, perineuronal, and perivascular oligodendrocytes. The intervascular subtype is mainly found along the nerve fibers and white matter and decreases rapidly during the myelin stage. The peri-neuronal subtype, also known as peri-neuronal satellite cells, can be generated from the oligodendrocyte progenitor cells (OPCs) after an injury or pathological condition. Activation of OPCs is considered to be a brain-protective response to regulate homeostasis and assist in post-injury repair.[43–46] After brain injury, oligodendrocytes can act as receptors of neuroinflammatory mediators, such as IL-6, IL-4, IL-7, and chemokine receptors 1, chemokine receptors 2, chemokine receptors 4. Chemokine receptors 4 can bind to CXCL12 secreted by astrocytes and microglia. Oligodendrocytes can communicate with microglia through their membrane glycoprotein CD200, which binds with its corresponding receptor on microglia and reduces its activity. Cd200-deficient microglia are spontaneously activated.[47–51] Furthermore, oligodendrocytes also secrete CXC chemokines, like CCL21 and CXCL1-5 in large quantities, which aggravate inflammation. After TBI, OPCs can also release matrix metallopeptidase (MMP)-9, which damages the BBB and promotes neutrophils and monocyte infiltration, thereby, further aggravating inflammation[52,53] (Fig. 3).
Figure 3.
Inflammatory response and oligodendrocyte initiation after TBI. TBI = traumatic brain injury.
3. Inflammatory response and neutrophils after TBI
Neuroinflammation is an inflammatory response in the CNS, involving both the brain and peripheral immune cells. Even though neuroinflammation is a response to shield the CNS from injury and infection, it is also a crucial secondary injury mechanism after TBI. TBI-associated neuroinflammation is manifested by stimulated glial cells, astrocytes, recruited white blood cells and up-regulated inflammatory cytokines in the brain center.[54,55] In the CNS, neutrophils are uncommon in the brain parenchyma due to the BBB.[56] Only a small quantity of neutrophils and other immune cells monitors specific compartments such as meninges, CSF, and pia meninges. However, during pathological conditions like infection, ischemia, trauma, and hemorrhage, the number of neutrophils entering the brain tissue increases significantly.[57,58] Zou et al[59] believe that neutrophils replace the role of macrophages after TBI, clearing apoptotic debris and performing post-traumatic repair. MMP9 and MMP13 stimulate neutrophils to rapidly and orderly migrate to the trauma site after TBI. Neutrophil derived cytokines are very complex, except for the common inflammatory cytokines (neutrophil elastase, tumor necrosis factor (TNF) family; Proinflammatory cytokines; CXC-chemokine; CC-chemokines), other anti-inflammatory cytokines, immunomodulatory cytokines, angiogenic factors and neurotrophic factors were also detected in neutrophils.[60–64] In addition to derived cytokines function, neutrophils can also damage the tight connection and permeability of BBB. Neutrophils can decompose the BBB for recruiting more immune cells in response to pathogens, causing tissue damage. Studies have shown that inhibition of activated neutrophils is beneficial to inflammatory regression and subsequent recovery.[65–71] Studies suggest that Maraviroc, TREM2 agonists COG1410, and cordycepin can all play a neuroprotective role by inhibiting neutrophil invasion after TBI.[22,69,72]
4. Inflammatory response and T lymphocytes after TBI
T cells have a great influence on microglia phenotype and function. After CNS injury, T cells infiltrate the injured tissues. Krämer et al[73] found that systemic reduction of immunosuppressive regulatory T cells (Tregs) causes enhanced T cell infiltration in the injured brain parenchyma through the TBI model of Controlled cortical impact. The presence of T cells in the brain is consistent with increased expression of the interferon-γ (IFN-γ) gene and hyperreactive astrocyte proliferation. Tregs cells depletion diminishes the brain’s acute immune responses. Tregs cells may critically regulate the TBI-associated pathophysiology. The study data of Caplan et al[74] showed that human Treg therapy changed the peripheral and central immune response after TBI in the rodent model and significantly reduced the chronic microglial hyperplasia in the damaged brain hemisphere. Moreover, Treg cells could effectively inhibit the pro-inflammatory immune response of rat spleen and microglial cells. Daglas et al[75] found that activation of CD8+ T cells after TBI in mice caused long-term nerve damage. Other studies revealed that IL-15 can stimulate neuronal apoptosis and enhance nerve damage by increasing the CD8 T cells’ function in TBI rat models.[76] Activation of IL-33/ST2 signals can regulate the function of T cells, reduce the size of brain injury, and alleviate functional defects after TBI.[77] Th17 cells, named for their production of IL-17 and other pro-inflammatory cytokines, are thought to be responsible for the demyelination of myelopathy in experimental autoimmune encephalomyelitis.[78] Th17 and other “type 17” T cells are linked with different autoimmune and inflammatory states. The type 17 response can promote and induce the recruitment of CXCL8 and neutrophils by cytokines such as IL-1β released after TBI, thereby exacerbating the inflammatory response.[79] Studies have indicated that umbilical cord mesenchymal stem cells and propofol can assist nerve repair after TBI by modulating Treg/Th17 homeostasis.[80,81] However, T cells are also a double-edged sword for TBI. Studies have found that T cell-deficient mice (due to RAG or MHCII gene defects) have a poor prognosis in CNS injury models, indicating a dominant neuroprotective effect of T cells.[82,83] The activation of mice’s autoimmune T cells is beneficial for the repair of CNS injury, and the underlying mechanism may involve neurotrophic factors produced by T cells acting on neurons and astrocytes to promote survival and repair.[83–89] T cells are necessary for normal CNS development because their deficiency in mice exhibits abnormal cognitive and behavioral development, suggesting they contribute to brain development and maintenance.[90] While regulating M1/M2-like homeostasis, T cells produced IL-4 protects neurons by enhancing neurotrophic signaling. IL-4-mediated T cell protection of damaged CNS tissues does not require T cells’ antigen-specific receptor initiation, and neurons can directly stimulate IL-4.[82,91] IL-33 also has a neuroprotective effect after CNS injury in mice.[92] It acts on IL-4-producing Th2 cells and therefore may be a link between CNS damage and the initiation of IL-4 production.[93–95] The function of T lymphocytes in TBI-mediated recovery and inflammation, and the process of antigen specificity and activation pathway, especially in chronic TBI models, requires further investigations.[96]
5. The interaction of various cells in the inflammatory response after TBI
5.1. Microglia initiation and astrocytes
In vitro investigations have shown that microglial and astrocytes’ interaction is mainly conducted by inflammatory regulators, and microglia-derived cytokines IL-1β, IL-6, TNF, IL-1α, and complement component C1q can inhibit the formation of astrocytes’ intercellular junction and restrict the intercellular traffic. In addition, it can cause loss of astrocytes function and increase astrocytes’ glucose uptake.[30,97–99] However, microglial and astrocytes’ gene expression in an in vitro environment changes rapidly, therefore, it is impossible to reconstruct the in vivo astrocytes and microglial interaction in a dish.[100–107] Recently, Liddelow et al[108] induced ischemia to cause acute CNS injury through injection of lipopolysaccharide or arterial occlusion respectively, and found that activated microglia-stimulated reactive astrocytes, which lost most of their normal cellular functions and produced new neurotoxic functions. They quickly killed neurons and matured differentiated oligodendrocytes. With the help of mice PD model, researchers found that glucagon-like peptide-1 receptor agonists inhibited microglia-mediated transformation of astrocytes into A1 neurotoxic phenotypes and thus played a neuroprotective role.[109] In a mouse experimental autoimmune encephalomyelitis model, microglia-derived transforming growth factor-α reduced the severity of the disease by directly signaling astrocytes.[110] Shinozaki et al[111] demonstrated in a rodent TBI model that inhibiting microglia notably damaged astrocyte scar formation and increased brain injury, indicating the presence of neuroprotective interaction of microglia and astrocyte after TBI.
5.2. Microglia initiation and neutrophils
Microglia are the brain’s innate immune cells, similar to macrophages in surrounding tissues. It removes debris, transmits inflammatory signals, and performs other activities. Microglia can be either resting or activated. Their resting state is stimulated by senescence, stroke, brain injury, and neurodegenerative diseases to become an activated state. Currently, no evidence suggests that neutrophils affect microglia subtypes (M1 and M2) in the TBI model. However, Moxon Emre and Schlichter revealed that neutrophils reduction could reduce the number of microglia/macrophages and their promoter marker CD68 after intracerebral hemorrhage, suggesting the involvement of neutrophils in the regulation of microglial status.[112] Kenne et al[113] also demonstrated in TBI mice models that neutrophil depletion decreased the activation of microglia, reduced edema, and loss of brain tissue. After brain injury, microglia stimulate endothelial cell activation and promote peripheral leukocyte recruitment to the CNS.[114] Activated microglia rapidly generate large quantities of inflammatory chemokines (IL-1β, TNF-α, IL-6, CXCL1-5, CXCL8-10) and cytokines. These strong inflammatory regulators recruit and activate neutrophils. Along with the aforementioned microglia-derived cytokines, neutrophils also induce other molecules to mutually initiate microglia, for instance, lipid carrier protein 2, reactive oxygen species, and MMP9.[13,115–118] Activated microglia are beneficial as well as harmful: on 1 hand, it stimulates neutrophils to secrete more pro-inflammatory cytokines, on the other hand, it reduces neuronal damage and the release of microglia neuroprotective factors.[119] However, whether neutrophils affect the M1/M2 polarization of microglia after TBI remains unclear.
5.3. Astrocytes and neutrophils
Astrocytes generally have a protective effect during neuroinflammatory processes.[120] Although, reactive astrocytes can form perivascular scarring during acute inflammatory responses, thereby limiting the diffusion of neutrophils from damaged tissue to healthy tissue.[121] in vitro experiments by Xie et al[122] revealed that neutrophils interact with astrocytes in both direct and indirect ways. During direct cell-cell interaction, astrocytes reduce apoptosis and neutrophils’ degranulation and improve the neutrophils’ phagocytosis and the expression of pro-inflammatory cytokines. Indirectly, astrocytes can reduce neutrophil apoptosis, enhance neutrophils’ necrosis and phagocytosis, and inhibit their granulation.[122] Neutrophils and astrocyte communication also affect astrocyte reactivity. Anti-Ly6G antibodies treatment in mice inhibited astrocyte proliferation and worsened the outcome of spinal cord injury.[123] Hooshmand and Nguyen in their in vitro investigation found that neutrophils can promote astrogenesis by producing C1q and C3a.[124] All the above investigations indicate that neutrophils and astrocytes are major sources of cytokines during neuroinflammation, stimulating each other to promote the inflammatory cascade. Neutrophils are important factors in promoting reactive astrocyte proliferation during brain injury. However, whether astrocyte proliferation is beneficial for brain injury is undetermined.
5.4. Oligodendrocytes and neutrophils
Oligodendrocytes not only interact with microglia and colloidal astrocytes to participate in immune regulation but also have a certain relationship with neutrophils. During acute brain injury, OPCs are the main component of MMP9-expressing cells. After TBI, OPCs release MMP-9, destroy the BBB, and promote neutrophil infiltration, thereby further aggravating the inflammatory response. Seo et al[53] treated brain-injured mice with MMP inhibitor GM6001 and found that after the second day of surgery, GM6001 reduced early BBB leakage and neutrophil infiltration.
5.5. Oligodendrocytes and T lymphocytes
Oligodendrocytes produce a variety of immunomodulators and express multiple receptors, for example, MHC class I molecules expressed by oligodendrocytes are recognized by CD8+ T lymphocytes,[125,126] and MHC class II molecules interact with CD4+ T lymphocytes. Atypical MHC class I molecules of human leukocyte antigen E were expressed in response to inflammatory cytokine invasion.[127,128] However, whether typical or atypical MHC molecules stimulate T-cell-mediated damage or initiate repair procedures remains to be further investigated. Kaya et al[129] found that oligodendrocytes and microglia cells induced by CD8+ T cells and responding to IFN are important ornaments of white matter aging. However, in the TBI model, the exact communication between CD8+ T cells and oligodendrocytes, their relation, and their association with IFN signal are still unknown.
6. Conclusions and future directions
Neuroinflammatory reactions after TBI include glial cell activation, the brain’s inflammatory regulator’s release, and peripheral immune cell recruitment. These inflammatory reactions not only induce secondary brain injury but also repair the nervous system to a certain extent. Therefore, the post-traumatic neuroinflammatory response has both positive and negative effects. Therefore, future research should focus on clarifying the specific roles of various inflammatory cells and factors and finding the corresponding inflammatory regulation strategies over time after TBI, to strategize the cure according to these different times and its associated factors, which play an important role in promoting nerve repair and regeneration while reducing the secondary damage of inflammatory response. Clinical treatment cannot simply involve the suppression of inflammatory response, the inflammatory phenotype of each patient based on their sex, genetic predisposition, age, presence or absence of secondary damage, CSF, serum, imaging, and biomarkers should also be defined. Clinical trials of patient-specific personalized inflammatory regulation therapy are expected to reduce secondary damage, enhance repair and improve patient outcomes.
Author contributions
Formal analysis: Jianhua Zhang.
Software: Hongru Li.
Writing – original draft: Huige Li.
Writing – review & editing: Qinghui Zhao, Fei Xie.
Abbreviations:
- AQP4
- aquaporin-4
- BBB
- blood–brain barrier
- CCL21
- chemokine(C-C motif) ligand 21
- CNS
- central nervous system
- CSF
- cerebrospinal fluid
- CXCL12
- chemokine (C-X-C motif) ligand 12
- CXCL1-5
- chemokine(C-X-C motif) ligand 1-5
- CXCL8-10
- chemokine(C-X-C motif) ligand 8-10
- DAMPs
- damage-associated molecular patterns
- HMGB1
- high mobility group protein 1
- IFN
- interferon
- IL
- interleukin
- MMP
- matrix metallopeptidase
- NF-KB
- nuclear factor kappa-B
- OPCs
- oligodendrocyte progenitor cells
- TBI
- traumatic brain injury
- TNF
- tumor necrosis factor
This work was supported by the Key Scientific Research Project in Colleges and Universities of Henan Province (grant number: 22A310002) and National Natural Science Foundation of China (grant number: 31500828).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Zhao Q, Li H, Li H, Xie F, Zhang J. Research progress of neuroinflammation-related cells in traumatic brain injury: A review. Medicine 2023;102:25(e34009).
Contributor Information
Qinghui Zhao, Email: qinghuizhao2046@163.com.
Huige Li, Email: hongru2046@163.com.
Hongru Li, Email: hongru2046@163.com.
Fei Xie, Email: xiefei990815@bjut.edu.cn.
References
- [1].Ruff RL, Riechers RG. Effective treatment of traumatic brain injury: learning from experience. JAMA. 2012;308:2032–3. [DOI] [PubMed] [Google Scholar]
- [2].Taylor CA, Bell JM, Breiding MJ, et al. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths-United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jiang JY, Gao GY, Feng JF, et al. Traumatic brain injury in Chinas. Lancet Neurol. 2019;18:286–95. [DOI] [PubMed] [Google Scholar]
- [4].Maas AIR, Menon DK, Manley GT, et al.; InTBIR Participants and Investigators. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022;21:1004–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hicks AJ, Ponsford JL, Spitz G, et al. β-Amyloid and Tau imaging in chronic traumatic brain injury: a cross-sectional study. Neurology. 2022;99:e1131–41. [DOI] [PubMed] [Google Scholar]
- [6].Webster KM, Sun M, Crack P, et al. Inflammation in epileptogenesis after traumatic brain injury. J Neuroinflammation. 2017;14:2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].DeKosky ST, Blennow K, Ikonomovic MD, et al. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat Rev Neurol. 2013;9:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shi K, Zhang J, Dong JF, et al. Dissemination of brain inflammation in traumatic brain injury. Cell Mol Immunol. 2019;16:523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jassam YN, Izzy S, Whalen M, et al. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95:1246–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hanscom M, Loane DJ, Shea-Donohue T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J Clin Invest. 2021;131:e143777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bolte AC, Lukens JR. Neuroimmune cleanup crews in brain injury. Trends Immunol. 2021;42:480–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Loane DJ, Kumar A. Microglia in the TBI brain: the good, the bad, and the dysregulated. Exp Neurol. 2016;275:316–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Simon DW, McGeachy M, Bayir H, et al. Neuroinflammation in the evolution of secondary injury, repair, and chronic neurodegeneration after traumatic brain injury. Nat Rev Neurol. 2017;13:171–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu X, Gao W, Cheng S, et al. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J Neuroinflammation. 2017;14:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao QH, Xie F, Guo DZ, et al. Hydrogen inhalation inhibits microglia activation and neuroinflammation in a rat model of traumatic brain injury. Brain Res. 2020;1748:147053. [DOI] [PubMed] [Google Scholar]
- [17].Li YF, Ren X, Zhang L, et al. Microglial polarization in TBI: signaling pathways and influencing pharmaceuticals. Front Aging Neurosci. 2022;14:901117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huang X, You W, Zhu Y, et al. Microglia: a potential drug target for traumatic axonal injury. Neural Plast. 2021;2021:5554824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhi YK, Li J, Yi L, et al. Sinomenine inhibits macrophage M1 polarization by downregulating α7nAChR via a feedback pathway of α7nAChR/ERK/Egr-1. Phytomedicine. 2022;100:154050. [DOI] [PubMed] [Google Scholar]
- [20].Sharma R, Kambhampati SP, Zhang Z, et al. Dendrimer mediated targeted delivery of sinomenine for the treatment of acute neuroinflammation in traumatic brain injury. J Control Release. 2020;323:361–75. [DOI] [PubMed] [Google Scholar]
- [21].Gronbeck KR, Rodrigues CM, Mahmoudi J, et al. Application of tauroursodeoxycholic acid for treatment of neurological and non-neurological diseases: is there a potential for treating traumatic brain injury? Neurocrit Care. 2016;25:153–66. [DOI] [PubMed] [Google Scholar]
- [22].Liu XL, Sun DD, Zheng MT, et al. Maraviroc promotes recovery from traumatic brain injury in mice by suppression of neuroinflammation and activation of neurotoxic reactive astrocytes. Neural Regen Res. 2023;18:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jayakumar AR, Tong XY, Ruiz-Cordero R, et al. Activation of NF-κB mediates astrocyte swelling and brain edema in traumatic brain injury. J Neurotrauma. 2014;31:1249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brambilla R, Hurtado A, Persaud T, et al. Transgenic inhibition of astroglial NF-kappa B leads to increased axonal sparing and sprouting following spinal cord injury. J Neurochem. 2009;110:765–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mettang M, Reichel SN, Lattke M, et al. IKK2/NF-κB signaling protects neurons after traumatic brain injury. FASEB J. 2018;32:1916–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Small C, Dagra A, Martinez M, et al. Examining the role of astrogliosis and JNK signaling in post-traumatic epilepsy. Egypt J Neurosurg. 2022;37:1.35035475 [Google Scholar]
- [27].Laird MD, Shields JS, Sukumari-Ramesh S, et al. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2014;62:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Walko TD, 3rd, Bola RA, Hong JD, et al. Cerebrospinal fluid mitochondrial DNA: a novel DAMP in pediatric traumatic brain injury. Shock. 2014;41:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp Neurol. 2016;275:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Greenhalgh AD, David S, Bennett FC. Immune cell regulation of glia during CNS injury and disease. Nat Rev Neurosci. 2020;21:139–52. [DOI] [PubMed] [Google Scholar]
- [31].Hayakawa K, Miyamoto N, Seo JH, et al. Highmobility group box 1 from reactive astrocytes enhances the accumulation of endothelial progenitor cells in damaged white matter. J Neurochem. 2012;125:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hayakawa K, Pham LD, Katusic ZS, et al. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci U S A. 2012;109:7505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rosa JM, Farré-Alins V, Ortega MC, et al. TLR4 pathway impairs synaptic number and cerebrovascular functions through astrocyte activation following traumatic brain injury. Br J Pharmacol. 2021;178:3395–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ren Z, Iliff JJ, Yang L, et al. “Hit & Run” model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab. 2013;33:834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Iliff JJ, Chen MJ, Plog BA, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol. 2018;13:379–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang E, Wan X, Yang L, et al. Omega-3 polyunsaturated fatty acids alleviate traumatic brain injury by regulating the glymphatic pathway in mice. Front Neurol. 2020;11:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Quintin S, Barpujari A, Mehkri Y, et al. The glymphatic system and subarachnoid hemorrhage: disruption and recovery. Explor Neuroprotective Ther. 2022;2:118–30.35756328 [Google Scholar]
- [40].Lucke-Wold BP, Turner RC, Logsdon AF, et al. Linking traumatic brain injury to chronic traumatic encephalopathy: identification of potential mechanisms leading to neurofibrillary tangle development. J Neurotrauma. 2014;31:1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2014;20:160–72. [DOI] [PubMed] [Google Scholar]
- [42].Michinaga S, Koyama Y. Pathophysiological responses and roles of astrocytes in traumatic brain injury. Int J Mol Sci. 2021;22:6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chancellor KB, Chancellor SE, Duke-Cohan JE, et al. Altered oligodendroglia and astroglia in chronic traumatic encephalopathy. Acta Neuropathol. 2021;142:295–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nishiyama A, Komitova M, Suzuki R, et al. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. [DOI] [PubMed] [Google Scholar]
- [45].Tanaka K, Nogawa S, Ito D, et al. Activation of NG2-positive oligodendrocyte progenitor cells during post-ischemic reperfusion in the rat brain. Neuroreport. 2001;12:2169–74. [DOI] [PubMed] [Google Scholar]
- [46].Song S, Hasan MN, Yu L, et al. Microglial-oligodendrocyte interactions in myelination and neurological function recovery after traumatic brain injury. J Neuroinflammation. 2022;19:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li Y, Tang G, Liu Y, et al. CXCL12 gene therapy ameliorates ischemia induced white matter injury in mouse brain. Stem Cells Transl Med. 2015;4:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rosenberger K, Dembny P, Derkow K, et al. Intrathecal heat shock protein 60 mediates neurodegeneration and demyelination in the CNSthrough a TLR4- and MyD88-dependent pathway. Mol Neurodegener. 2015;10:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pu H, Zheng X, Jiang X, et al. Interleukin-4 improves white matter integrity and functional recovery after murine traumatic brain injury via oligodendroglial PPARγ. J Cereb Blood Flow Metab. 2021;41:511–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Patel JR, McCandless EE, Dorsey D, et al. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci U S A. 2010;107:11062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Denieffe S, Kelly RJ, McDonald C, et al. Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav Immun. 2013;34:86–97. [DOI] [PubMed] [Google Scholar]
- [52].Watson AES, Goodkey K, Footz T, et al. Regulation of CNS precursor function by neuronal chemokines. Neurosci Lett. 2020;715:134533. [DOI] [PubMed] [Google Scholar]
- [53].Seo JH, Miyamoto N, Hayakawa K, et al. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest. 2013;123:782–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Postolache TT, Wadhawan A, Can A, et al. Inflammation in traumatic brain injury. J Alzheimers Dis. 2020;74:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kalra S, Malik R, Singh G, et al. Pathogenesis and management of traumatic brain injury (TBI): role of neuroinflammation and anti-inflammatory drugs. Inflammopharmacology. 2022;30:1153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mantovani A, Cassatella MA, Costantini C, et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. [DOI] [PubMed] [Google Scholar]
- [57].Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nasr IW, Chun Y, Kannan S. Neuroimmune responses in the developing brain following traumatic brain injury. Exp Neurol. 2019;320:112957. [DOI] [PubMed] [Google Scholar]
- [59].Zou D, Hu W, Qin J, et al. Rapid orderly migration of neutrophils after traumatic brain injury depends on MMP9/13. Biochem Biophys Res Commun. 2021;579:161–7. [DOI] [PubMed] [Google Scholar]
- [60].Johnson EA, Dao TL, Guignet MA, et al. Increased expression of the chemokines CXCL1 and MIP-1alpha by resident brain cells precedes neutrophil infiltration in the brain following prolonged soman-induced status epilepticus in rats. J Neuroinflammation. 2011;41:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fenn AM, Hall JC, Gensel JC, et al. IL-4 signaling drives a unique arginase(+)/IL-1β(+) microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Rα after traumatic spinal cord injury. J Neurosci. 2014;34:8904–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chen J, Zhang C, Jiang H, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Royo N, Conte V, Saatman KE, et al. Hippocampal vulnerability following traumatic brain injury: a potential role for neurotrophin-4/5 in pyramidal cell neuroprotection. Eur J Neurosci. 2006;23:1089–102. [DOI] [PubMed] [Google Scholar]
- [64].von Leden RE, Parker KN, Bates AA, et al. The emerging role of neutrophils as modifiers of recovery after traumatic injury to the developing brain. Exp Neurol. 2019;317:144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hayashi T, Kaneko Y, Yu S, et al. Quantitative analyses of matrix metalloproteinase activity after traumatic brain injury in adult rats. Brain Res. 2009;1280:172–7. [DOI] [PubMed] [Google Scholar]
- [66].Grossetete M, Phelps J, Arko L, et al. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009;65:702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chen XR, Besson VC, Palmier B, et al. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. Neurotrauma. 2007;24:1119–31. [DOI] [PubMed] [Google Scholar]
- [68].Mori T, Wang X, Aoki T, et al. Downregulation of matrix metalloproteinase-9 and attenuation of edema via inhibition of ERK mitogen activated protein kinase in traumatic brain injury. J Neurotrauma. 2002;19:1411–9. [DOI] [PubMed] [Google Scholar]
- [69].Yan J, Zhang Y, Wang L, et al. TREM2 activation alleviates neural damage via Akt/CREB/BDNF signalling after traumatic brain injury in mice. J Neuroinflammation. 2022;19:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gasche Y, Copin JC, Sugawara T, et al. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood–brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–400. [DOI] [PubMed] [Google Scholar]
- [71].Liu YW, Li S, Dai SS. Neutrophils in traumatic brain injury (TBI): friend or foe? J Neuroinflammation. 2018;15:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wei P, Wang K, Luo C, et al. Cordycepin confers long-term neuroprotection via inhibiting neutrophil infiltration and neuroinflammation after traumatic brain injury. J Neuroinflammation. 2021;18:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Krämer TJ, Hack N, Brühl TJ, et al. Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-γ gene expression in acute experimental traumatic brain injury. J Neuroinflammation. 2019;16:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Caplan HW, Prabhakara KS, Kumar A, et al. Human cord blood-derived regulatory T-cell therapy modulates the central and peripheral immune response after traumatic brain injury. Stem Cells Transl Med. 2020;9:903–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Daglas M, Draxler DF, Ho H, et al. Activated CD8+ T cells cause long-term neurological impairment after traumatic brain injury in mice. Cell Rep. 2019;29:1178–1191.e6. [DOI] [PubMed] [Google Scholar]
- [76].Wu L, Ji NN, Wang H, et al. Domino effect of interleukin-15 and CD8 T-cell-mediated neuronal apoptosis in experimental traumatic brain injury. J Neurotrauma. 2021;38:1450–63. [DOI] [PubMed] [Google Scholar]
- [77].Xie D, Miao W, Xu F, et al. IL-33/ST2 axis protects against traumatic brain injury through enhancing the function of regulatory T cells. Front Immunol. 2022;13:860772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fletcher JM, Lalor SJ, Sweeney CM, et al. T cells in multiple sclerosis andexperimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–50. [DOI] [PubMed] [Google Scholar]
- [80].Chen C, Hu N, Wang J, et al. Umbilical cord mesenchymal stem cells promote neurological repair after traumatic brain injury through regulating Treg/Th17 balance. Brain Res. 2022;1775:147711. [DOI] [PubMed] [Google Scholar]
- [81].Cui C, Zhang D, Sun K, et al. Propofol maintains Th17/Treg cell balance and reduces inflammation in rats with traumatic brain injury via the miR-145-3p/NFATc2/NF-κB axis. Int J Mol Med. 2021;48:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Walsh JT, Hendrix S, Boato F, et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125:699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kipnis J, Mizrahi T, Hauben E, et al. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002;99:15620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yoles E, Hauben E, Palgi O, et al. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci. 2001;21:3740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Moalem G, Yoles E, Leibowitz-Amit R, et al. Autoimmune T cells retard the loss of function in injured rat optic nerves. J Neuroimmunol. 2000;106:189–97. [DOI] [PubMed] [Google Scholar]
- [86].Hauben E, Nevo U, Yoles E, et al. Autoimmune T cells as potential neuroprotective therapy for spinal cord injury. Lancet. 2000;355:286–7. [DOI] [PubMed] [Google Scholar]
- [87].Hammarberg H, Lidman O, Lundberg C, et al. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci. 2000;20:5283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Linker R, Gold R, Luhder F. Function of neurotrophic factors beyond the nervous system: inflammation and autoimmune demyelination. Crit Rev Immunol. 2009;29:43–68. [DOI] [PubMed] [Google Scholar]
- [89].Bao W, Lin Y, Chen Z. The Peripheral Immune System and Traumatic Brain Injury: insight into the role of T-helper cells. Int J Med Sci. 2021;18:3644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Filiano AJ, Gadani SP, Kipnis J. Interactions of innate and adaptive immunity in brain development and function. Brain Res. 2015;1617:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Steinman L. Role reversal: infiltrating T cells protect the brain. J Clin Invest. 2015;125:493–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gadani SP, Walsh JT, Smirnov I, et al. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron. 2015;85:703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Burzyn D, Kuswanto W, Kolodin D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kuswanto W, Burzyn D, Panduro M, et al. Poor repair of skeletal muscle in aging mice reflects a defect in local,interleukin-33-dependent accumulation of regulatory T cells. Immunity. 2016;44:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Schiering C, Krausgruber T, Chomka A, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Javidi E, Magnus T. Autoimmunity after ischemic stroke and brain injury. Front Immunol. 2019;10:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Même W, Calvo C-F, Froger N, et al. Proinflammatory cytokines released from microglia inhibit gap junctions in astrocytes: potentiation by β-amyloid. FASEB J. 2006;20:494–6. [DOI] [PubMed] [Google Scholar]
- [98].Watanabe M, Masaki K, Yamasaki R, et al. Th1 cells downregulate connexin 43 gap junctions in astrocytes via microglial activation. Sci Rep. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Retamal MA, Froger N, Palacios-Prado N, et al. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bohlen CJ, Bennett FC, Tucker AF, et al. Diverse requirements for microglial survival, specification, and function revealed by defined medium cultures. Neuron. 2017;94:759–73.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Foo LC, Allen NJ, Bushong EA, et al. Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011;71:799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Butovsky O, Jedrychowski MP, Moore CS, et al. Identification of a unique TGF-β dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Greenhalgh AD, Zarruk JG, Healy LM, et al. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 2018;16:e2005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Gosselin D, Skola D, Coufal NG, et al. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356:eaal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Cronk JC, Filiano AJ, Louveau A, et al. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J Exp Med. 2018;215:1627–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bennett FC, Bennett ML, Yaqoob F, et al. A combination of ontogeny and CNS environment establishes microglial identity. Neuron. 2018;98:1170–1183.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Esmonde-White C, Yaqubi M, Bilodeau PA, et al. Distinct function-related molecular profile of adult human A2B5-positive pre-oligodendrocytes versus mature oligodendrocytes. J Neuropathol Exp Neurol. 2019;78:468–79. [DOI] [PubMed] [Google Scholar]
- [108].Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Yun SP, Kam T-I, Panicker N, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24:931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rothhammer V, Borucki DM, Tjon EC, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557:724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Shinozaki Y, Shibata K, Yoshida K, et al. Transformation of astrocytes to a neuroprotective phenotype by microglia via P2Y1 receptor downregulation. Cell Rep. 2017;19:1151–64. [DOI] [PubMed] [Google Scholar]
- [112].Moxon-Emre I, Schlichter LC. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropathol Exp Neurol. 2011;70:218–35. [DOI] [PubMed] [Google Scholar]
- [113].Kenne E, Erlandsson A, Lindbom L, et al. Neutrophil depletion reduces edema formation and tissue loss following traumatic brain injury in mice. J Neuroinflammation. 2012;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhou H, Lapointe BM, Clark SR, et al. A requirement for microglial TLR4 in leukocyte recruitment into brain in response to lipopolysaccharide. J Immunol. 2006;77:8103–10. [DOI] [PubMed] [Google Scholar]
- [115].Johnson NH, de Rivero Vaccari JP, Bramlett HM, et al. Inflammasome activation in traumatic brain injury and Alzheimer's disease. Transl Res. 2023;254:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Jang E, Lee S, Kim JH, et al. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J. 2013;27:1176–90. [DOI] [PubMed] [Google Scholar]
- [117].McPherson CA, Merrick BA, Harry GJ. In vivo molecular markers for proinflammatory cytokine M1 stage and resident microglia in trimethyltin induced hippocampal injury. Neurotox Res. 2014;25:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Needham EJ, Helmy A, Zanier ER, et al. The immunological response to traumatic brain injury. J Neuroimmunol. 2019;332:112–25. [DOI] [PubMed] [Google Scholar]
- [119].Liu YW, Li S, Dai SS. Neutrophils in traumatic brain injury (TBI): friend or foe? J Neuroinflammation. 2018;15:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hu S, Sheng WS, Ehrlich LC, et al. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation. 2000;7:153–9. [DOI] [PubMed] [Google Scholar]
- [121].Voskuhl RR, Peterson RS, Song B, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009;29:11511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Xie L, Poteet EC, Li W, et al. Modulation of polymorphonuclear neutrophil functions by astrocytes. J Neuroinflammation. 2010;53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Stirling DP, Liu S, Kubes P, et al. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci. 2009;29:753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Hooshmand MJ, Nguyen HX, Piltti KM, et al. Neutrophils induce astroglial differentiation and migration of human neural stem cells via C1q and C3a synthesis. J Immunol. 2017;199:1069–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ji Q, Castelli L, Goverman JM. MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8+ T cells. J Clin Invest. 2013;14:254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Kirby L, Jin J, Cardona JG, et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun. 2019;10:3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zaguia F, Saikali P, Ludwin S, et al. Cytotoxic NKG2C+ CD4 T cells target oligodendrocytes in multiple sclerosis. J Immunol. 2013;190:2510–8. [DOI] [PubMed] [Google Scholar]
- [128].Falcao AM, van Bruggen D, Marques S, et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat Med. 2018;24:1837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kaya T, Mattugini N, Liu L, et al. CD8+ T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging. Nat Neurosci. 2022;25:1446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]