Abstract
Objective
This study aimed to establish and validate nomograms to predict the probability of intravesical recurrence (IVR) after radical nephroureterectomy (RNU) for upper urinary tract epithelial carcinoma (UTUC).
Methods
Clinical data of 528 patients with UTUC after RNU were collected from two medical centers between 2009 and 2020. We used the least absolute shrinkage and selection operator (LASSO) regression to select variables for multivariable Cox regression analysis in the training cohort and included independent risk factors into nomogram models predicting IVR-free survival (IVRFS). Another center was applied as the external cohort to validate the predictive accuracy and discriminative ability of the nomogram by performing area under the receiver operating curve (AUC), consistency index (C-index), and calibration curve.
Results
History of bladder cancer, tumor size, preoperative urine cytology, postoperative instillation, Ki-67, and platelet-to-lymphocyte ratio (PLR) were identified as independent risk factors for IVR. The prognosis model including these predictors demonstrated excellent discriminatory performance in both the training cohort (C-index, 0.814) and external validation cohort (C-index, 0.748). The calibration plots of the nomogram revealed good consistency in both cohorts. Finally, patients could be classified into two risk groups based on scores obtained from the nomogram, with significant differences in IVRFS.
Conclusion
Our study provided a reliable nomogram for predicting the probability of IVR in patients with UTUC after RNU. Risk stratification based on this model may assist urologists make optimal clinical decisions on the management of UTUC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-023-05016-2.
Keywords: Upper tract urothelial carcinoma, Intravesical recurrence, Radical nephroureterectomy, Nomogram
Introduction
Upper tract urothelial carcinoma (UTUC) is a malignant tumor that occurs in the urothelial cells of the renal pelvis or ureter. Its incidence rate is far lower than that of bladder cancer, accounting for 5–10% of all urothelial carcinomas (Rouprêt et al. 2021; Siegel et al. 2022). Compared with other urological tumors, UTUC has a more rapid clinical progression and a worse prognosis. Radical nephroureterectomy (RNU) with bladder sleeve resection is the standard surgical treatment for UTUC, especially for high-risk patients (Ito et al. 2013). However, it has been reported that intravesical recurrence (IVR) occurs in 15–50% of all patients with UTUC who underwent RNU, and with the majority occurring within 1 year after surgery (Raman and Park 2017). Once IVR occurs, patients have to undergo additional adjuvant treatment, such as transurethral bladder tumor resection surgery and intravesical instillation chemotherapy (Doeveren et al. 2018; Zhou et al. 2019). Therefore, IVR could cause a worse survival prognosis and significantly increase the psychological stress and financial burden of patients, severely affecting their quality of life (Zamboni et al. 2019).
Although the history of bladder cancer and tumor multifocality are the most commonly reported risk factors, factors that reliably predict IVR have not been identified (Azémar et al. 2011). Thus, how to leverage patient stratification tools to better identify patients who may have IVR at an early stage has become a timely issue to be addressed (Loizzo et al. 2022). Xylinas et al. constructed prediction models based on a larger population from Western countries; however, the discriminatory power of these models was not satisfactory in external validation and was only applicable to specific populations (Xylinas et al. 2014). For the Chinese population, there are significant differences in race, dietary habits, lifestyle choices, and exposure to common carcinogenic factors, especially aristolochic acid, compared with patients in Western countries (Chen et al. 2013; Singla et al. 2017). In this study, risk factors for IVR in patients with UTUC were identified. We developed a nomogram and conducted external validation, and hope to assist the urologist to optimize clinical decision-making.
Materials and methods
Patients selection
This retrospective study was approved by the Institutional Research Ethics Committee of China–Japan Friendship Hospital and Beijing Chaoyang Hospital. Informed consent was obtained from all eligible participants in advance. This work has been reported in line with the STROCSS guideline (Agha et al. 2019). The information of patients diagnosed with UTUC who received RNU treatment in the two hospitals from January 2009 to December 2020 were collected retrospectively, and all patient details have been de-identified. We included the patients who met the following criteria: (1) patients with UTUC confirmed pathologically; (2) patients with primary disease; (3) patients with unilateral onset; and (4) patients subject to RNU combined with cystic sleeve resection. We excluded patients according to the following criteria: (1) patients with bilateral UTUC; (2) patients subject to no RNU combined with cystectomy; and (3) patients with metastatic uroepithelial carcinoma.
Data collection
We obtained the following clinicopathological features of patients for analysis: sex, age at the first diagnosis, body mass index (BMI), tumor laterality, pathologic tumor stage (pT), lymph node status (pN0, pNx or pN+), tumor grade, tumor size, tumor multifocality, margin positivity, and lymphovascular invasion (LVI). Patients with previous history of hypertension, diabetes, and bladder cancer, expression of Ki-67, preoperative urine cytology, presence of hydronephrosis on the affected side, requiring postoperative instillation, and high serum creatinine level were also enrolled. We specially collected some blood inflammation biomarkers including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR), which were obtained from routine blood examination performed 1 week before surgery. All resected tumor specimens were sent for pathological examination by senior pathologists. The pathologic tumor stage and grade were evaluated according to the 2009 International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) TNM classification system (Edge and Compton 2010) and the 2016 World Health Organization (WHO) grading system (Humphrey et al. 2016).
Follow-up
We monitored patients every 3 months during the first 2 years after surgery and every 6 months thereafter until 5 years. Follow-up data included blood tests, cystoscopic examination, urinary system ultrasound, chest and abdomen CT, urine exfoliated cytology, and urography. Selective bone scan, PET/CT or magnetic resonance (MRI) examination was performed if clinically indicated. Overall survival (OS) was defined as the time from the date of RNU to death from any cause. Intravesical recurrence-free survival (IVRFS) was defined as the time from the date of RNU to the date of the first IVR according to cystoscopic examination.
Nomogram development and validation
Patients of Beijing Chaoyang Hospital were enrolled in the training cohort and validated in the cohort of China–Japan Friendship Hospital. The least absolute shrinkage selection operator (LASSO) regression was utilized to screen variables and prevent overfitting. Multivariate Cox regression analyses were performed in the training cohort to determine the potential independent risk factors. Based on the prognostic model, we developed a new nomogram to predict IVR at 1-, 3-, and 5-year intervals in patients with UTUC who underwent RNU. Receiver operating characteristic (ROC) analyses were conducted in both cohorts, and the area under the ROC curve (AUC) and concordance index (C-index) were used to evaluate discrimination ability. To validate the accuracy of the model, calibration curves were drawn from 1000 bootstrap samples from the training and validation cohorts.
Statistical analysis
The categorical variables were expressed as the frequency (percentage). Continuous variables were presented as median values with ranges. We utilized the X-tile software (version 3.6.1) to determine the optimal cutoff for these biomarkers classified as low and high level (Camp et al. 2004). The cutoff values of NLR, PLR, and LMR were 5.0, 166.0, and 4.2. Pearson’s Chi-square test and non-parametric U test were used to compare the two groups. Kaplan–Meier survival curves were plotted and the log-rank test conducted to demonstrate the difference in different risk groups. Prognostic risk factors related to IVR were assessed by univariate and multivariate Cox regression analyses models, and the results were shown as hazard ratio (HR) with 95% confidence interval (CI). All p values were obtained from two-sided tests, and p < 0.05 indicated that the difference was statistically significant. R software (Version 4.1.2) and IBM SPSS Statistics (Version 24) were utilized to complete all statistical analyses and figures.
Results
Patient characteristics
A total of 528 patients matched the inclusion and exclusion criteria and included, with 308 patients in the training cohort and 220 patients in the external validation cohort. The demographic and clinicopathological variables are summarized in Table 1. 61 patients (19.8%) in the training cohort and 58 patients (26.4%) in the validation cohort developed IVR after RNU. There were no significant differences between the two cohorts except for the tumor location, pT stage, grade, tumor size, multifocality, postoperative instillation, PLR, and LMR. The median follow-up of the training cohort was 41 months (range 1–131 months), which was similar to the validation cohort (42 months, 2–143 months).
Table 1.
Characteristics of patients from two hospitals
| Characteristic | Training cohort | Validation cohort | p value |
|---|---|---|---|
| N = 308 | N = 220 | ||
| Age (years) | 67 (33–88) | 68 (38–86) | 0.896 |
| Sex | 0.221 | ||
| Male | 155 (50.3%) | 98 (44.5%) | |
| Female | 153 (49.7%) | 122 (55.5%) | |
| BMI | 24.77 (14.54–34.69) | 24.69 (16.44–33.13) | 0.391 |
| History of hypertension | 0.988 | ||
| Yes | 154 (50.0%) | 109 (49.5%) | |
| No | 154 (50.0%) | 111 (50.5%) | |
| History of diabetes | 0.386 | ||
| Yes | 77 (25.0%) | 47 (21.4%) | |
| No | 231 (75.0%) | 173 (78.6%) | |
| History of bladder cancer | 0.743 | ||
| Yes | 15 (4.9%) | 13 (5.9%) | |
| No | 293 (95.1%) | 207 (94.1%) | |
| Tumor location | 0.004 | ||
| Ureter | 121 (39.3%) | 110 (50.0%) | |
| Renal pelvis | 147 (47.7%) | 98 (44.5%) | |
| Both | 40 (13.0%) | 12 (5.5%) | |
| Laterality | 0.401 | ||
| Left | 154 (50.0%) | 119 (54.1%) | |
| Right | 154 (50.0%) | 101 (45.9%) | |
| Pathologic stage | < 0.001 | ||
| pT2 or less | 183 (59.4%) | 166 (75.5%) | |
| pT3 or greater | 125 (40.6%) | 54 (24.5%) | |
| Lymph node status | 1.000 | ||
| pN0/pNx | 289 (93.8%) | 207 (94.0%) | |
| pN+ | 19 (6.2%) | 13 (6.0%) | |
| Tumor grade | < 0.001 | ||
| Low | 120 (39.0%) | 22 (10.0%) | |
| High | 188 (61.0%) | 198 (90.0%) | |
| Tumor size | 0.009 | ||
| < 3 cm | 154 (50.0%) | 136 (61.8%) | |
| ≥ 3 cm | 154 (50.0%) | 84 (38.2%) | |
| Tumor multifocality | 0.001 | ||
| Present | 110 (35.7%) | 49 (22.3%) | |
| Absent | 198 (64.3%) | 171 (77.7%) | |
| Hydronephrosis | 0.258 | ||
| Present | 99 (32.1%) | 82 (37.3%) | |
| Absent | 209 (67.9%) | 138 (62.7%) | |
| LVI | 0.396 | ||
| Present | 62 (20.1%) | 37 (16.8%) | |
| Absent | 246 (79.9%) | 183 (83.2%) | |
| Margin positive | 0.540 | ||
| Negative | 289 (93.8%) | 210 (95.5%) | |
| Positive | 19 (6.2%) | 10 (4.5%) | |
| Urine cytology | 0.712 | ||
| Normal | 166 (53.9%) | 123 (55.9%) | |
| Abnormal | 142 (46.1%) | 97 (44.1%) | |
| Postoperative instillation | 0.001 | ||
| Yes | 105 (34.1%) | 107 (48.6%) | |
| No | 203 (65.9%) | 113 (51.4%) | |
| Ki-67 | 0.276 | ||
| < 20% | 152 (49.4%) | 120 (54.5%) | |
| ≥ 20% | 156 (50.6%) | 100 (45.5%) | |
| Creatinine (mmol/L) | 85.5 (34.9–1077.2) | 90.9 (32.6–836.8) | 0.090 |
| NLR | 2.49 (0.75–16.61) | 2.29 (0.67–16.74) | 0.600 |
| PLR | 133.35 (27.56–379.58) | 100.57 (35.99–310.54) | < 0.001 |
| LMR | 4.04 (0.58–11.14) | 4.03 (0.76–9.79) | 0.015 |
| Follow-up (months) | 41 (1–131) | 42 (2–143) | 0.411 |
BMI body mass index, LVI lymphovascular invasion, NLR neutrophil-to-lymphocyte ratio, PLR platelet-to-lymphocyte ratio, LMR lymphocyte-monocyte ratio
Identification of risk factors for IVR
LASSO regression and multivariate analysis were applied to identify the prognostic risk factors related to IVR. In LASSO regression analysis, variables with non-zero coefficients at λmin were screened out in the training cohort (Fig. S1). Further multivariate Cox regression analysis revealed history of bladder cancer, tumor size, preoperative urine cytology, postoperative instillation, Ki-67, and PLR to be independent prognostic factors for IVR (Table S1).
Development and external validation of the nomogram for IVR
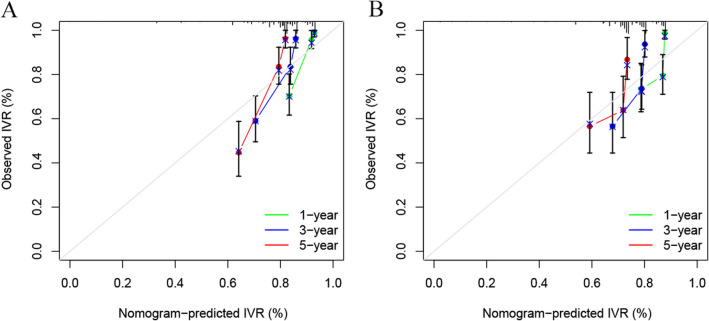
We constructed the nomogram based on the independent risk factors identified by multivariate analysis. The nomogram for predicting IVR is shown in Fig. 1. At the top of the nomogram, a scale provides a score for each prognostic variable. According to the sum of all scores axis at the bottom of the nomogram, probabilities of 1-, 3-, and 5-year IVRFS were able to be estimated. The median risk score of patients in the training cohort was determined as the cutoff score. All patients with UTUC were divided into two risk subgroups by the cutoff score: low risk (scores ≤ 83) and high risk (scores > 83). Kaplan–Meier curves showed shorter IVRFS in the high-risk group than in the low-risk group in both the training and validation cohorts (Fig. 2). To validate the accuracy and predictive ability of the nomograms, we used time-dependent ROC analysis and C-index. In the training group, the AUC values for 1-, 3-, and 5-year IVRFS were 0.820, 0.776, and 0.823, respectively, while in the validation group, they were 0.748, 0.766, and 0.732 (Fig. 3). In addition, the C-index of nomograms for IVR was 0.814 in the training group and 0.748 in the validation group. These results indicated that the nomogram provides a good discriminatory performance. Finally, the calibration curves for the training and validation group revealed that the IVR predicted by the nomogram was consistent with the actual observation results in Fig. 4.
Fig. 1.
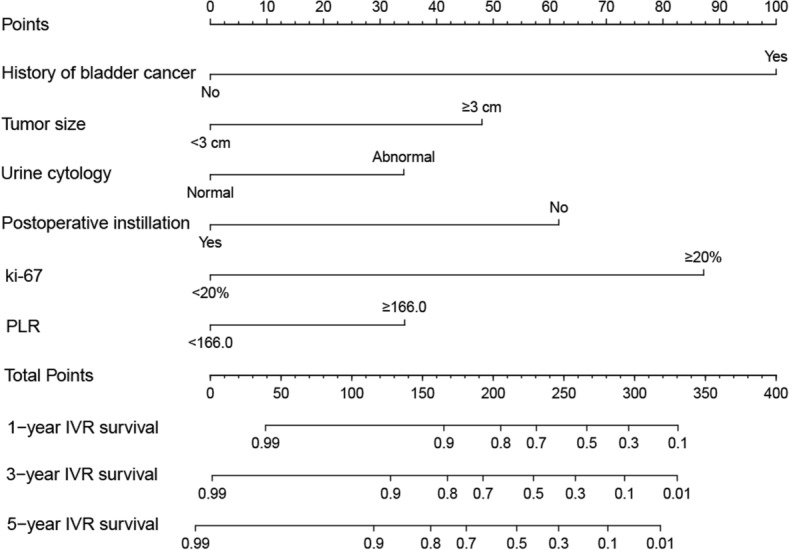
Nomogram for predicting 1-,3-, and 5-year IVRFS
Fig. 2.
The Kaplan–Meier curves of nomogram in the training cohort (A) and validation cohort (B)
Fig. 3.
Nomogram ROC curves to predict 1-,3-, and 5-year IVRFS in the training cohort (A) and validation cohort (B)
Fig. 4.
The calibration curves of nomogram at 1,3, and 5 years in the training cohort (A) and validation cohort (B)
Discussion
The high incidence of IVR after RNU is a characteristic of UTUC, which has a significant impact on the survival outcome of patients (Rouprêt et al. 2021; Jiang et al. 2020). The modern management of UTUC patients should aim at customized methods to minimize the risk of tumor recurrence. Ishioka et al. built a risk stratification model for IVR based on patients with UTUC at 13 institutions in Japan (Ishioka et al. 2015). Higher tumor stage (≥ pT2), papillary tumor architecture, male sex, and LVI were identified as the risk factors and included in the model. According to the number of risk factors, patients were classified into different risk groups. Although the model is simple and practical, the discriminatory performance is poor with a C-index of 0.600. Some scholars thought it is not sufficiently effective for clinical decision-making (Laguna 2015). Besides, Xylinas et al. constructed nomograms to calculate the probabilities of IVR after RNU based on a larger population with 1839 patients (Xylinas et al. 2014). However, some predictors such as surgical access, the distal ureter management technique, age, and carcinoma in situ are not significantly associated with IVR in external validation, which reduced the calibration of nomograms. In this study, in addition to a history of bladder, cytology, chemotherapy, and tumor size, which have been reported to be risk factors for IVR (Ito et al. 2013; Azémar et al. 2011; Tanaka et al. 2014; Pieras et al. 2010), we also identified Ki-67 and PLR as independent predictors for IVR and added them to the nomogram, which acquired ideal discriminatory ability in both cohorts.
The pathophysiological mechanism of IVR is still unclear. There are currently two mainstream academic views, namely, ‘tumor seeding theory’ and ‘field-cancerization hypothesis’ (Habuchi et al. 1993; Li et al. 2020). Due to the complex pathogenesis of IVR, many scholars argued that the early IVR after surgery could be attributed to the dissemination and implantation of primary cancer cells, which is a true progression of disease recurrence. The late IVR may be a novel uroepithelial carcinoma induced by multiple carcinogens, unrelated to the primary tumor (Fang et al. 2014). The predictors in our nomogram echoed the two viewpoints. The positive urinary exfoliative cytology indicates a high risk of dissemination and implantation of tumor cells in the bladder (Raman et al. 2007). The history of bladder cancer could reflect the IVR is not caused by the primary tumor, but by independent gene alterations in different parts of the urothelium (Jones et al. 2005). Certainly, more research is required to reveal the detailed mechanism.
As an immunostaining marker of nuclear cell proliferation, Ki-67 was found to be frequently expressed in several malignancies such as breast, colon, and ovarian cancers (Krabbe et al. 2015; Wu et al. 2015). High expression rates of Ki-67 represents more aggressive and active proliferation of cancer cells, suggesting that primary tumors are more likely to progress and metastasize distantly (Yang et al. 2018). Krabbe et al. reported that the overexpression of Ki-67 was associated with poor prognosis in UTUC patients treated with RNU (Krabbe et al. 2015). However, the exact mechanism of Ki-67 in IVR remains controversial. Long et al. mentioned patients with low Ki-67 levels were more sensitive to intravesical instillations for IVR prevention after RNU (Long et al. 2016). By contrast, Lai et al. proposed that low-level Ki-67 expression was an independent predictor of IVR (Lai et al. 2021). In our study, we defined the Ki-67 overexpression as 20% after referring to relevant literature (Tsai et al. 2021; Fan et al. 2016). The results confirmed that the Ki-67 overexpression was closely related to IVR. We speculated that tumors with higher rate of Ki-67 overexpression are more prone to produce exfoliative cancer cell, thus increasing the risk of bladder implantation. However, the specific pathophysiological mechanisms remain to be further explored.
Recent research has demonstrated that inflammatory effects in the tumor microenvironment play an important role in the development, progression, and metastasis of human cancer (Mantovani et al. 2008; Hanahan and Weinberg 2011). The body’s immune response to malignancy may lead to changes in the levels of lymphocytes, neutrophils, monocytes, or platelets in peripheral blood. Our study showed that higher NLR is also associated with a higher risk of IVR. Platelets are generally considered to be mainly involved in the process of physiological hemostasis. However, during tumor cell migration, platelets can play a role in chemotactic integration, causing the immune escape of tumor cells and promoting the spread of cancer cells (Gresele et al. 2017). In addition, platelets can secrete protein aggregation factors and increase the adhesion between tumor cells and vascular endothelial cells, thus promoting the metastasis of tumor cells (Denis et al. 2005). In contrast, the level of lymphocytes reflects the cell-mediated immune response (Massagué and Obenauf 2016). Tumor-infiltrating lymphocytes play an important anti-tumor immune role in the tumor microenvironment. The study of Gooden et al. showed that a high level of tumor lymphocyte infiltration affects the prognosis of cancer patients (Gooden et al. 2011). The reduction of lymphocyte count in peripheral blood weakens the body's immune response ability, leading to immune tolerance and decreased host immune surveillance, which in turn lead to poor survival of tumor patients. Therefore, elevated PLR not only represents a stronger inflammatory response in patients with malignancy, but also reflects the impairment of cell-mediated immune function, thus providing a theoretical basis for PLR to predict the prognosis of tumor patients. The cutoff values in the present study were determined by X-tile software and are consistent with the ranges reported by previous studies (Shao et al. 2020). However, the results should be interpreted with more caution due to the differences in cutoff values.
The present study had several strengths as follows: first, the nomogram performed well in external validation, which increased credibility; in addition, the follow-up was relatively long and the sample size was relative large compared to previous studies; third, the PLR can be obtained before surgery compared to other risk factors, and thus guide further treatment reasonably. However, the current study had several limitations. First of all, an inherent bias was inevitable due to the design of the retrospective study. Moreover, due to the relatively long time span, the criteria related to blood tests, urine exfoliation cytology, and pathological examinations may have changed during this period, further affecting the reliability of the collected data. Additionally, this study involved two hospitals and the difference in surgical techniques, medical equipment, and the postoperative follow-up of patients should be considered. Finally, this nomogram was constructed based on the Chinese population and additional medical centers in other countries are needed to validate the generalizability of the model.
Conclusion
In summary, we developed a reliable nomogram based on clinical and pathological variables to predict the probability of IVR in patients with UTUC after RNN. Risk stratification based on the model may be an effective tool to assist clinicians in assessing individualized IVR rates and making optimal treatment decisions. Further prospective multicenter trials in different countries are required to validate our nomogram.
Supplementary Information
Below is the link to the electronic supplementary material.
Figure S1. LASSO coefficient profiles of all variables predicting IVR (A). 10-fold cross-validation for tuning parameter selection in the least LASSO model related to IVR (B) (TIF 4243 KB)
Acknowledgements
We gratefully acknowledge Ruihuan Shen and Ziqi Pan for their professional statistical help.
Author contributions
Study design: ZD, GZ. Data collection: TH, HZ, BJ. Data analysis and interpretation: WH, YB, ZL. Manuscript writing: ZL, BJ.
Funding
No funding was received.
Data availability statement
All the data were presented in the manuscript. No additional data are available.
Declarations
Conflict of interest
The authors declare that this study has received no financial support and there was no conflict of interest.
Statement of ethics
The studies involving human participants were reviewed and approved by the China–Japan Friendship Hospital and Beijing Chaoyang Hospital. The patients/participants provided their written informed consent to participate in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenkai Luo and Binbin Jiao contributed to the work equally and should be regarded as co-first authors.
Contributor Information
Zhenshan Ding, Email: dzsfighting@126.com.
Guan Zhang, Email: gzhang2016@sina.com.
References
- Agha R, Abdall-Razak A, Crossley E, Dowlut N, Iosifidis C, Mathew G et al (2019) STROCSS 2019 Guideline: strengthening the reporting of cohort studies in surgery. Int J Surg 72:156–165 [DOI] [PubMed] [Google Scholar]
- Azémar MD, Comperat E, Richard F, Cussenot O, Rouprêt M (2011) Bladder recurrence after surgery for upper urinary tract urothelial cell carcinoma: frequency, risk factors, and surveillance. Urol Oncol 29(2):130–136 [DOI] [PubMed] [Google Scholar]
- Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10(21):7252–7259 [DOI] [PubMed] [Google Scholar]
- Chen CH, Dickman KG, Huang CY, Moriya M, Shun CT, Tai HC et al (2013) Aristolochic acid-induced upper tract urothelial carcinoma in Taiwan: clinical characteristics and outcomes. Int J Cancer 133(1):14–20 [DOI] [PubMed] [Google Scholar]
- Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S et al (2005) Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell 122(3):379–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474 [DOI] [PubMed] [Google Scholar]
- Fan B, Zhang H, Jin H, Gai Y, Wang H, Zong H et al (2016) Is overexpression of Ki-67 a prognostic biomarker of upper tract urinary carcinoma? A retrospective cohort study and meta-analysis. Cell Physiol Biochem 40(6):1613–1625 [DOI] [PubMed] [Google Scholar]
- Fang D, Xiong GY, Li XS, Chen XP, Zhang L, Yao L et al (2014) Pattern and risk factors of intravesical recurrence after nephroureterectomy for upper tract urothelial carcinoma: a large Chinese center experience. J Formos Med Assoc 113(11):820–827 [DOI] [PubMed] [Google Scholar]
- Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW (2011) The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 105(1):93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresele P, Momi S, Malvestiti M, Sebastiano M (2017) Platelet-targeted pharmacologic treatments as anti-cancer therapy. Cancer Metastasis Rev 36(2):331–355 [DOI] [PubMed] [Google Scholar]
- Habuchi T, Takahashi R, Yamada H, Kakehi Y, Sugiyama T, Yoshida O (1993) Metachronous multifocal development of urothelial cancers by intraluminal seeding. Lancet 342(8879):1087–1088 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674 [DOI] [PubMed] [Google Scholar]
- Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol 70(1):106–119 [DOI] [PubMed] [Google Scholar]
- Ishioka J, Saito K, Kijima T, Nakanishi Y, Yoshida S, Yokoyama M et al (2015) Risk stratification for bladder recurrence of upper urinary tract urothelial carcinoma after radical nephroureterectomy. BJU Int 115(5):705–712 [DOI] [PubMed] [Google Scholar]
- Ito A, Shintaku I, Satoh M, Ioritani N, Aizawa M, Tochigi T et al (2013) Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP Monotherapy Study Group Trial. J Clin Oncol 31(11):1422–1427 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Yao Z, Zhu X, Wu B, Bai S (2020) Risk factors and oncological outcome for intravesical recurrence in organ-confined upper urinary tract urothelial carcinoma patients after radical nephroureterectomy: a propensity score-matched case control study. Int J Surg 76:28–34 [DOI] [PubMed] [Google Scholar]
- Jones TD, Wang M, Eble JN, MacLennan GT, Lopez-Beltran A, Zhang S et al (2005) Molecular evidence supporting field effect in urothelial carcinogenesis. Clin Cancer Res 11(18):6512–6519 [DOI] [PubMed] [Google Scholar]
- Krabbe LM, Bagrodia A, Haddad AQ, Kapur P, Khalil D, Hynan LS et al (2015) Multi-institutional validation of the predictive value of Ki-67 in patients with high grade urothelial carcinoma of the upper urinary tract. J Urol 193(5):1486–1493 [DOI] [PubMed] [Google Scholar]
- Laguna MP (2015) Re: Risk stratification for bladder recurrence of upper urinary tract urothelial carcinoma after radical nephroureterectomy. J Urol 194(6):1582–1583 [DOI] [PubMed] [Google Scholar]
- Lai S, Long X, Wu P, Liu J, Seery S, Hou H et al (2021) Developing a nomogram for predicting intravesical recurrence after radical nephroureterectomy: a retrospective cohort study of mainland Chinese patients. Jpn J Clin Oncol 51(7):1132–1141 [DOI] [PubMed] [Google Scholar]
- Li R, Du Y, Chen Z, Xu D, Lin T, Jin S et al (2020) Macroscopic somatic clonal expansion in morphologically normal human urothelium. Science 370(6512):82–89 [DOI] [PubMed] [Google Scholar]
- Loizzo D, Pandolfo SD, Del Giudice F, Cerrato C, Chung BI, Wu Z et al (2022) Ureteroscopy and tailored treatment of upper tract urothelial cancer: recent advances and unmet needs. BJU Int 130(1):35–37 [DOI] [PubMed] [Google Scholar]
- Long X, Zu X, Li Y, He W, Hu X, Tong S et al (2016) Epidermal growth factor receptor and Ki-67 as predictive biomarkers identify patients who will be more sensitive to intravesical instillations for the prevention of bladder cancer recurrence after radical nephroureterectomy. PLoS ONE 11(11):e0166884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444 [DOI] [PubMed] [Google Scholar]
- Massagué J, Obenauf AC (2016) Metastatic colonization by circulating tumour cells. Nature 529(7586):298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieras E, Frontera G, Ruiz X, Vicens A, Ozonas M, Pizá P (2010) Concomitant carcinoma in situ and tumour size are prognostic factors for bladder recurrence after nephroureterectomy for upper tract transitional cell carcinoma. BJU Int 106(9):1319–1323 [DOI] [PubMed] [Google Scholar]
- Raman JD, Park R (2017) Endoscopic management of upper-tract urothelial carcinoma. Expert Rev Anticancer Ther 17(6):545–554 [DOI] [PubMed] [Google Scholar]
- Raman JD, Sosa RE, Vaughan ED Jr, Scherr DS (2007) Pathologic features of bladder tumors after nephroureterectomy or segmental ureterectomy for upper urinary tract transitional cell carcinoma. Urology 69(2):251–254 [DOI] [PubMed] [Google Scholar]
- Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM et al (2021) European Association of Urology Guidelines on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol 79(1):62–79 [DOI] [PubMed] [Google Scholar]
- Shao Y, Li W, Wang D, Wu B (2020) Prognostic value of preoperative lymphocyte-related systemic inflammatory biomarkers in upper tract urothelial carcinoma patients treated with radical nephroureterectomy: a systematic review and meta-analysis. World J Surg Oncol 18(1):273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33 [DOI] [PubMed] [Google Scholar]
- Singla N, Fang D, Su X, Bao Z, Cao Z, Jafri SM et al (2017) A multi-institutional comparison of clinicopathological characteristics and oncologic outcomes of upper tract urothelial carcinoma in China and the United States. J Urol 197(5):1208–1213 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Kikuchi E, Kanao K, Matsumoto K, Shirotake S, Kobayashi H et al (2014) The predictive value of positive urine cytology for outcomes following radical nephroureterectomy in patients with primary upper tract urothelial carcinoma: a multi-institutional study. Urol Oncol 32(1):48.e19–26 [DOI] [PubMed] [Google Scholar]
- Tsai MY, Chiang PC, Chen CH, Sung MT, Huang SC, Suen JL et al (2021) Comparison of the prognostic value of Ki-67 and programmed cell death ligand-1 in patients with upper tract urothelial carcinoma. J Clin Med 10(16):3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doeveren T, van Leeuwen PJ, Aben KKH, van der Aa M, Barendrecht M, Boevé ER et al (2018) Reduce bladder cancer recurrence in patients treated for upper urinary tract urothelial carcinoma: the REBACARE-trial. Contemp Clin Trials Commun 9:121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Liu S, Zhang W, Zhang Y, Zhu G, Wei D et al (2015) Low-level Ki-67 expression as an independent predictor of bladder tumour recurrence in patients with primary upper tract urothelial carcinoma after radical nephroureterectomy. Jpn J Clin Oncol 45(12):1175–1181 [DOI] [PubMed] [Google Scholar]
- Xylinas E, Kluth L, Passoni N, Trinh QD, Rieken M, Lee RK et al (2014) Prediction of intravesical recurrence after radical nephroureterectomy: development of a clinical decision-making tool. Eur Urol 65(3):650–658 [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang J, Ding M, Xu K, Li L, Mao L et al (2018) Ki67 targeted strategies for cancer therapy. Clin Transl Oncol 20(5):570–575 [DOI] [PubMed] [Google Scholar]
- Zamboni S, Baumeister P, Mattei A, Mordasini L, Antonelli A, Simeone C et al (2019) Single postoperative instillation for non-muscle invasive bladder cancer: are there still any indication? Transl Androl Urol 8(1):76–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhao S, Lu Y, Wu J, Li Y, Gao Z et al (2019) Meta-analysis of efficacy and safety of continuous saline bladder irrigation compared with intravesical chemotherapy after transurethral resection of bladder tumors. World J Urol 37(6):1075–1084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. LASSO coefficient profiles of all variables predicting IVR (A). 10-fold cross-validation for tuning parameter selection in the least LASSO model related to IVR (B) (TIF 4243 KB)
Data Availability Statement
All the data were presented in the manuscript. No additional data are available.