Abstract
Almost nothing is known about the diets of bathypelagic fishes, but functional morphology can provide useful tools to infer ecology. Here we quantify variation in jaw and tooth morphologies across anglerfishes (Lophiiformes), a clade spanning shallow and deep-sea habitats. Deep-sea ceratioid anglerfishes are considered dietary generalists due to the necessity of opportunistic feeding in the food-limited bathypelagic zone. We found unexpected diversity in the trophic morphologies of ceratioid anglerfishes. Ceratioid jaws span a functional continuum ranging from species with numerous stout teeth, a relatively slow but forceful bite, and high jaw protrusibility at one end (characteristics shared with benthic anglerfishes) to species with long fang-like teeth, a fast but weak bite and low jaw protrusibility at the other end (including a unique ‘wolftrap’ phenotype). Our finding of high morphological diversity seems to be at odds with ecological generality, reminiscent of Liem's paradox (morphological specialization allowing organisms to have broader niches). Another possible explanation is that diverse ceratioid functional morphologies may yield similar trophic success (many-to-one mapping of morphology to diet), allowing diversity to arise through neutral evolutionary processes. Our results highlight that there are many ways to be a successful predator in the deep sea.
Keywords: bathypelagic, anglerfish, Lophiiformes, jaw mechanics, Liem's paradox, teeth
1. Introduction
Studying the ecology of organisms that are rare, extinct or live in extreme habitats is very challenging. As an alternative to in situ observations, morphological characteristics can be used to infer functional abilities by comparison with more accessible species [1–3]. While functions such as prey acquisition correlate well with tooth shape or jaw mechanics in diverse taxa [4–8], our understanding of the correspondence of morphology to function or ecology is imperfect [9–16]. For example, species with specialized morphologies may still prefer to eat common food resources more often than their presumed specialized diet, a phenomenon called Liem's paradox [17–20]. This pattern is hypothesized to arise when periodic limitation of preferred food prompts organisms to use specialized morphologies to acquire alternative resources [21].
Liem's paradox has been related to freshwater [17,19], shallow marine [15,18] and terrestrial organisms [5]. However, these studies are from high-diversity, high-productivity systems. The bathypelagic zone of the deep sea (greater than 1000 m) represents the converse of these, being the largest and most food-limited habitat on Earth [22,23]. Little is known about the diets of bathypelagic fishes, but evidence suggests they must have generalized diets in order to meet their trophic demands [22,24–26]. Despite their dietary generalism, recent studies using phylogenetic comparative methods show that bathypelagic fishes have a greater diversity of body shapes than fishes from the continental shelf or slope [1,2,27]. It is unknown whether such morphological diversity corresponds to ecological diversity in deep-sea fishes.
Anglerfishes (order Lophiiformes) provide an opportunity to examine form and function with respect to habitat. Lophiiformes contain five suborders, four of which are benthic and found in shallow marine or continental slope settings. Frogfishes (Antennarioidei), one of the benthic suborders, are well recognized as an extreme among marine fishes regarding their feeding biomechanics. A functional trade-off is known among fishes between jaws with forceful bites but low protrusion, and highly protrusible jaws with low-force output [28–30]. Frogfishes are far on the latter end of this trade-off, with the largest buccal expansion and fastest prey capture speeds recorded for any fish [3,29,31–34]. The fifth suborder, Ceratioidei, is a bathypelagic radiation containing roughly half the species and family diversity of Lophiiformes [26]. Ceratioid anglerfishes are extremely difficult to observe owing to their low population density and inaccessibility [35]. Ceratioids have larger oral and opercular cavities than frogfishes, potentially indicating even greater buccal expansion and jaw-ram [26]. Some ceratioids have developed specialized morphologies and behaviours, such as the ‘wolftrap’ anglers which have a modified upper jaw for trapping prey [26].
Here we use high-resolution micro-computed tomography (µCT) scanning [36] of museum specimens to quantify jaw and tooth characteristics across Lophiiformes, including major lineages of ceratioids. Our results indicate that ceratioids span a wide range of trophic morphologies despite being putative dietary generalists.
2. Methods
(a) . Specimen imaging
We obtained µCT scans for 57 lophiiform species (n = 1 per species), including 42 female ceratioids (representing 73.5% of genera and 10 of 11 families in Ceratioidei) and 15 benthic species from three of four suborders and 50% of genera (electronic supplementary material, table S1). No batfish species (Ogcocephalidae) were included because their teeth were too small to quantify at our scanning resolution. Most µCT scans (48 of 57) were newly generated using a Bruker 1173 SkyScan at the Karel F. Liem Bio-Imaging Center at the University of Washington Friday Harbor Laboratories. We obtained nine scans from online repositories (electronic supplementary material, table S1).
(b) . Data collection
We took 13 measurements from the skull and teeth of each specimen (electronic supplementary material, table S2; figures S1 and S2). Measurements were taken from µCT scans using 3D Slicer version 4.11.20210226 [37] with the SlicerMorph extension [38]. Skull and jaw-related measurements were head length, head depth, premaxilla ascending process height (a predictor of jaw protrusion ability [3]), premaxilla length, lower jaw length, jaw-closing in-lever length, jaw opening in-lever length and horizontal gape (i.e. jaw width; a predictor of maximum prey size [39]). Given the wide gapes of many anglerfishes, we corrected the length of the lower jaw and premaxilla to anteroposterior lengths to ameliorate the effects of jaw curvature (electronic supplementary material, Extended Methods; electronic supplementary material, figure S2). Mechanical advantages of lower jaw opening and closing were formed from the ratios of the respective in-levers and corrected lower jaw length (electronic supplementary material, table S2). These lever ratios were proxies used to describe the continuous trade-off between more forceful jaw movements (high values) and faster jaw movements (low values) [14,28,40].
Tooth-related measurements were taken for upper and lower jaws. These were length of the tooth-bearing region, number of teeth, the mean height of the five tallest teeth, the mean width at the base of the five tallest teeth and the average spacing between the five tallest teeth. These features have been used to detect functional differences among piscivorous reef fishes [41] and predict feeding behaviour, water column use, and prey size [7]. Tall teeth are typically better suited for puncturing prey than stout ones; the same goes for teeth that are widely spaced, as tightly packed teeth may be better for gripping or processing prey than puncturing [41,42].
(c) . Phylogenetic inference
Existing phylogenies for Lophiiformes have poor sampling overlap with our µCT scan dataset. Phylogenetic trees were therefore newly estimated from publicly available cytochrome c oxidase (CO1) sequences for 43 of 57 scanned species (electronic supplementary material, table S3). We estimated two alternative trees using RAxML version 8.1.20 [43,44], enforcing backbone topological constraints based on phylogenomic studies by Hart et al. [45] and Miya et al. [46] (hereafter 'Hart' and 'Miya' trees). Trees were made ultrametric using non-parametric rate smoothing [47]. Details of phylogenetic inference are in the electronic supplementary material (Extended Methods).
(d) . Data analyses
All measurements were size-corrected by dividing by head length [48], then log-transformed. We tested for allometry following [41] (details in Extended Methods, electronic supplementary material).
To visualize morphological variation, we conducted principal component analyses (PCAs) without phylogenetic correction, including all species with µCT scan data (n = 57) using the ‘prcomp’ function in R. We used the first two components of this ordination to calculate the relative areas of morphospace occupied by benthic lophiiforms and ceratioid anglers, with and without accounting for sample size differences (Extended Methods, electronic supplementary material) [18]. We also conducted two phylogenetic PCAs (pPCA) using each alternative tree (n = 43 species). To perform pPCAs, we used the function phyl.pca from the package ‘phytools’ using a correlation matrix.
We fit three linear regressions comparing: (i) mean tooth length versus jaw-closing mechanical advantage, (ii) height of the ascending process versus jaw-closing mechanical advantage and (iii) mean tooth length versus mean tooth spacing. We chose these comparisons because, although jaw mechanics are well studied in frogfishes [3,29,31–33], it is unclear how these variables covary in other lophiiforms. Similarly, the relationship between tooth length and spacing was previously shown to distinguish functional groups in reef fishes [7,41]. Following [49], we compared the fit of three models: a non-phylogenetic ordinary least square model (OLS), phylogenetic generalized least squares with a Brownian motion model (PGLS-BM) and with an Ornstein–Uhlenbeck model (PGLS-OU). Upper and lower jaw tooth variables were averaged for these analyses. Regressions were performed using the ‘phylolm’ package version 2.6.2 [50]. We also performed ancestral state reconstructions for these four traits using the ‘fastAnc’ function in ‘phytools’.
3. Results
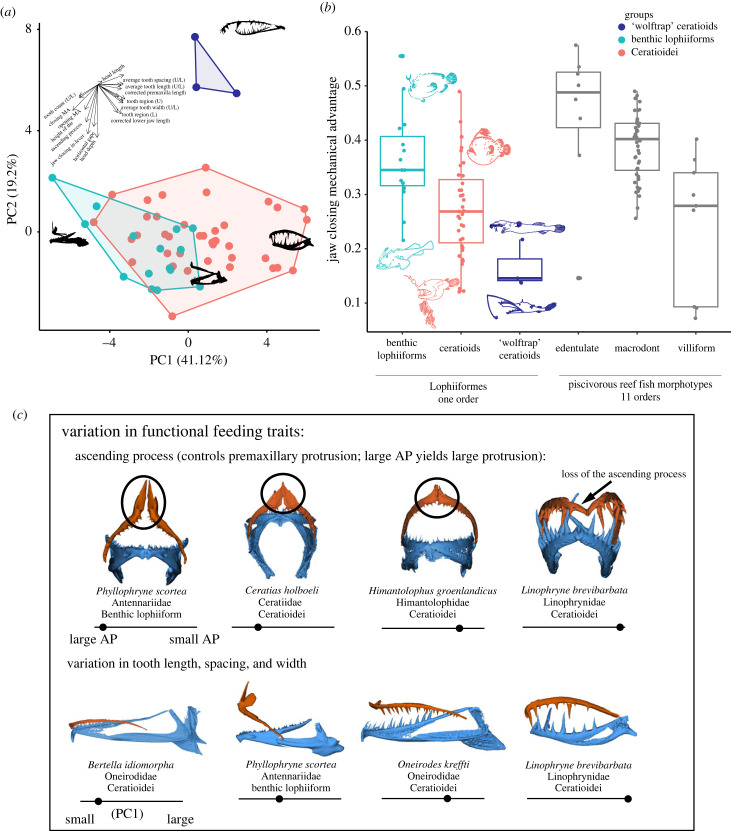
PCAs revealed that the morphospace occupied by the benthic lophiiforms was mostly subsumed within the larger ceratioid morphospace (figure 1). When accounting for sample size, Ceratioidei occupied a morphospace 2.1 (median) times larger than the benthic lophiiforms (electronic supplementary material, figure S3). The ‘wolftrap’ ceratioids (Thaumatichthys and Lasiognathus) form a distinct morphological group with high values on PC2 (figure 1; electronic supplementary material, figure S4). If these were excluded from the morphospace comparisons, the remaining ceratioids still occupied a morphospace 1.3 times larger than the benthic taxa after correcting for sample size (electronic supplementary material, figure S3).
Figure 1.
(a) PCA of jaw and tooth morphologies for 57 species of Lophiiformes. For pPCA, see electronic supplementary material, figure S5. (b) Mechanical advantage values measured for anglerfishes compared to morphotypes from [41]. Fish icons were digitized from FAO identification guides. (c) Examples of variation in trophic morphologies among Lophiiformes, where orange = upper jaw and blue = lower jaw. The bars below the jaws reflect the species' relative position with respect to ascending process height (top row; y-axis of figure 2b) or tooth length, spacing and width (bottom row; PC1 of (a)). Images not to scale.
Species were separated along PC1 (41.1% of var.) based on differences in jaw lengths, jaw-closing mechanical advantage and tooth characteristics (tooth lengths, widths, spacing and count). Benthic lophiiforms, with relatively low PC1 values, were characterized by forceful bites, high tooth counts and short, tightly packed teeth (figure 1a). PC2 (19.2% of var.) described differences in horizontal gape, the height of the ascending process and jaw-closing in-lever length. The ‘wolftrap’ ceratioids, with high PC2 scores, displayed shallow head depth, narrow gapes and a small or absent ascending process of the premaxilla. Results between PCAs with and without phylogenetic correction were similar (electronic supplementary material, figure S5).
Of lophiiform families, Oneirodidae and Linophrynidae have the greatest diversity (electronic supplementary material, figure S4). Both families have species with ‘average’ morphologies, near the PCA centroid and outliers with more extreme morphologies, such as the oneirodid wolftrap anglers Lasiognathus and the extreme macrodonts in Linophryne.
Regressions (figure 2) showed that several functional traits were correlated. Average tooth length was negatively related to jaw-closing mechanical advantage (p < 0.001; R2 = 0.45). The height of the ascending process was positively related to jaw-closing mechanical advantage, though this effect was not significant when accounting for phylogeny (p = 0.10; R2 = 0.06). Average tooth length was positively related to average tooth spacing (p < 0.001; R2 = 0.52). These results suggest a continuum of diversity in trophic morphologies across Lophiiformes, characterized on one end by species with many tightly packed and stout teeth, high jaw-closing mechanical advantage (i.e. slow but high-force bite), and high jaw protrusion. These traits characterize benthic lophiiforms. On the latter end of the continuum are species with large and spaced fang-like teeth, low jaw-closing mechanical advantage (i.e. fast but low-force bite) and low jaw protrusion. These traits characterize the wolftrap ceratioids at the most extreme. Ceratioids span this entire continuum (figure 2). Interestingly, we found that the (non-phylogenetic) OLS model had a better fit than BM or OU models in most cases (figure 2, table 1), likely because the branch lengths of ceratioids are so short that phylogenetic signal is minimal. Results were robust to the phylogeny used (table 1; electronic supplementary material, figures S5 and S6).
Figure 2.
Linear regressions and ancestral state reconstructions for the following traits: (a) average tooth length versus jaw-closing mechanical advantage, (b) height of the ascending process versus jaw-closing mechanical advantage and (c) average tooth length versus average distance between teeth. Analyses shown here use the Hart tree; for Miya tree, see electronic supplementary material, figure S6. See Table 1 for statistical results. For explanation of traits, see electronic supplementary material, figure S1.
Table 1.
Results of linear regressions of functional traits of interest using two alternative backbone topologies. If significant (alpha = 0.05), the best-fitting model is in italics.
| comparison | tree | p-value | R2 | AIC score, OLS | AIC score, BM | AIC score, OU | AICw, OLS | AICw, BM | AICw, OU |
|---|---|---|---|---|---|---|---|---|---|
| average tooth length versus jaw-closing mechanical advantage | Hart | <0.0001 | 0.453 | 42.08 | 64.53 | 43.82 | 0.705 | <0.001 | 0.295 |
| average tooth length versus jaw-closing mechanical advantage | Miya | <0.0001 | 0.453 | 42.08 | 60.89 | 44.12 | 0.735 | <0.001 | 0.265 |
| height of the ascending process versus jaw-closing mechanical advantage | Hart | 0.1000 | 0.065 | 127.52 | 83.07 | 88.09 | <0.001 | 0.925 | 0.075 |
| height of the ascending process versus jaw-closing mechanical advantage | Miya | 0.0531 | 0.088 | 127.52 | 78.54 | 84.50 | <0.001 | 0.952 | 0.048 |
| average tooth length versus average tooth spacing | Hart | <0.0001 | 0.516 | 36.78 | 68.02 | 39.30 | 0.779 | <0.001 | 0.221 |
| average tooth length versus average tooth spacing | Miya | <0.0001 | 0.516 | 36.78 | 53.34 | 38.82 | 0.735 | <0.001 | 0.265 |
4. Discussion
Our results suggest that morphological and functional diversity in ceratioids is much greater than would be predicted based on their generalist diets. Ceratioids span the gamut from short teeth, high jaw protrusion and slow-closing but high-force jaws on one end (similar to shallow-water lophiiforms [31]) and large teeth, low jaw protrusion and fast-closing but low-force jaws on the other, including the unique ‘wolftrap’ phenotype (figure 2). Note that ‘fast’ or ‘slow’ qualifications herein are relative to other lophiiforms, and all lophiiforms could be considered ‘fast’ when compared to force-adapted fishes like parrotfishes or piranhas [14]. As some examples of this diversity, Linophryne are extreme macrodonts with teeth as long as 25% of their head length (figure 2), and the lack of an ascending process implies poor jaw protrusion ability (figure 1c). Yet, other ceratioids have small teeth (e.g. Bertella), showing that the macrodont phenotype is far from universal in this deep-sea clade. Some ceratioids resemble shallow-water frogfishes (e.g. Cryptopsaras), which could imply functional similarities such as extreme buccal expansion [29,31,32]. Mechanical advantage values seen in ceratioids are comparable to reef fishes (figure 1b) and are not as extreme as those recorded in deep-sea dragonfishes, in which the applicability of this metric is questionable [51]. This suggests that established form–function relationships for shallow-water fishes should apply to ceratioids.
The two phenotypes described here are similar to the macrodont versus villiform functional groups previously described for piscivorous reef fishes [7,41]. The functional diversity of anglerfishes is striking. Ceratioids alone span most of the variation in mechanical advantage seen across 11 orders of reef fishes (figure 1b; data from [41]). Our results suggest that deep-sea ceratioids capture prey in varied ways. This is supported anecdotally by the few videos of ceratioids [26,35]. Existing behavioural inferences or direct observations are compiled in the electronic supplementary material, table S4 and are generally consistent with our results.
Yet, evidence suggests ceratioids have generalist diets [22,26]. Gut content observations are compiled in the electronic supplementary material, table S5; ceratioids eat a range of fishes and invertebrates with seemingly little connection to functional morphology (electronic supplementary material, table S4). For ceratioid feeding, the how seems to covary with morphology (prey capture), but the what does not (prey item). Why would this diversity arise? One possibility is that there are many ways of being successful predators in the deep sea (many-to-one mapping of functional morphology to diet [15,52]). If a broad range of phenotypes yields similar success at catching similar prey, morphological and functional diversity can accumulate through neutral evolution via relaxed selection [9,11]. Piscivory has been found to constrain evolution in shallow, visually dependent predators because of the functional constraints related to pursuing and capturing highly mobile prey [7,15,53]. Bathypelagic fishes live in darkness and are believed to use lures to attract rather than pursue prey [22]. These factors relax the typical demands on piscivores in high-light environments [1,54].
A second possibility is that ceratioid phenotypes are indeed adapted to capturing different types of prey. Liem's paradox suggests morphologically specialized species have the most generalized diets of all because they can access both common and unique food resources [17,18,20,21]. Unlike shallow organisms, ‘common’ and ‘unique’ items for a ceratioid may refer to prey size, not prey species. Perhaps some ceratioids play out Liem's paradox by maintaining the ability to eat any prey that comes along while simultaneously having morphologically specializations for capturing exceedingly large prey when available. This phenomenon might explain the success of ceratioids in the bathypelagic zone, the most food-limited habitat on Earth [22,23]. Future investigations of diet are necessary to distinguish between these alternatives and better understand evolution in the deep sea.
Acknowledgements
We are grateful to the following museums and personnel: Katherine Maslenikov and Luke Tornabene (UWFC), Alastair Graham (CSIRO), Amanda Hay (AM), Todd Clardy and Bill Ludt (LACM), Andrew Williston and Meaghan Sorce (MCZ), Ben Frable and Phil Hastings (SIO). We thank Adam Summers, Luke Tornabene, Karly Cohen, Edward Burress, Ted Pietsch and Ricardo Betancur-R for training and advice.
Data accessibility
Input data files and R scripts are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.qfttdz0n2 [55]. All CT scans were uploaded to Morphosource (see electronic supplementary material, table S1).
The data are provided in the electronic supplementary material [56].
Authors' contributions
Z.H.: data curation, formal analysis, investigation, methodology, visualization, writing—original draft and writing—review and editing; J.M.H.: conceptualization, data curation, investigation, methodology, resources, supervision, validation and writing—review and editing; A.P.M.M.: formal analysis, methodology and writing—review and editing; P.B.H.: methodology, supervision and writing—review and editing; C.H.R.G.: methodology, supervision and writing—review and editing; D.A.: methodology, supervision and writing—review and editing; E.C.M.: conceptualization, data curation, funding acquisition, project administration, resources, supervision, validation, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Z.H. was supported by an NSF REU internship (grant no. NSF DBI-1646666). Scanning was supported by the oVert TCN (grant no. NSF DBI-1701665). C.H.R.G. was supported by a Fulbright Future Scholarship Funded by the Kinghorn Foundation. E.C.M. was supported by an NSF PRFB (grant no. DBI-1906574). P.B.H. was supported by an NSF PRFB (grant no. DBI- 2109469). J.M.H. was supported by an NSF GRFP (grant no. DGE-1746914). D.A. was supported by NSF DEB-2144325 and NSF DEB-2015404.
References
- 1.Martinez CM, Friedman ST, Corn KA, Larouche O, Price SA, Wainwright PC. 2021. The deep sea is a hot spot of fish body shape evolution. Ecol. Lett. 24, 1788-1799. ( 10.1111/ele.13785) [DOI] [PubMed] [Google Scholar]
- 2.Myers EMV, Anderson MJ, Liggins L, Harvey ES, Roberts CD, Eme D. 2021. High functional diversity in deep-sea fish communities and increasing intraspecific trait variation with increasing latitude. Ecol. Evol. 11, 10 600-10 612. ( 10.1002/ece3.7871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellwood DR, Goatley CH, Bellwood O, Delbarre DJ, Friedman M. 2015. The rise of jaw protrusion in spiny-rayed fishes closes the gap on elusive prey. Curr. Biol. 25, 2696-2700. ( 10.1016/j.cub.2015.08.058) [DOI] [PubMed] [Google Scholar]
- 4.Huie JM, Thacker CE, Tornabene L. 2020. Co-evolution of cleaning and feeding morphology in western Atlantic and eastern Pacific gobies. Evolution 74, 419-433. ( 10.1111/evo.13904) [DOI] [PubMed] [Google Scholar]
- 5.Weinstein BG, Graham CH. 2017. Persistent bill and corolla matching despite shifting temporal resources in tropical hummingbird–plant interactions. Ecol. Lett. 20, 326-335. ( 10.1111/ele.12730) [DOI] [PubMed] [Google Scholar]
- 6.Hulsey CD, Cohen KE, Johanson Z, Karagic N, Meyer A, Miller CT, Sadier A, Summers AP, Fraser GJ. 2020. Grand challenges in comparative tooth biology. Integr. Comp. Biol. 60, 563-580. ( 10.1093/icb/icaa038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mihalitsis M, Bellwood DR. 2021. Functional groups in piscivorous fishes. Ecol. Evol. 11, 12 765-12 778. ( 10.1002/ece3.8020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segall M, Houssin C, Delapré A, Cornette R, Herrel A, Milgram J, Shahar R, Dumont M. 2023. Armed to the teeth: the underestimated diversity in tooth shape in snakes and its relationship to feeding behavior and diet. Ecol. Evol. 13, e10011. ( 10.1002/ece3.10011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wainwright PC. 2007. Functional versus morphological diversity in macroevolution. Ann. Rev. Ecol. Evol. Syst. 38, 381-401. ( 10.1146/annurev.ecolsys.38.091206.095706) [DOI] [Google Scholar]
- 10.Alfaro ME, Bolnick DI, Wainwright PC. 2004. Evolutionary dynamics of complex biomechanical systems: an example using the four-bar mechanism. Evolution 58, 495-503. [PubMed] [Google Scholar]
- 11.Alfaro ME, Bolnick DI, Wainwright PC. 2005. Evolutionary consequences of many-to-one mapping of jaw morphology to mechanics in labrid fishes. Am. Nat. 165, E140-E154. ( 10.1086/429564) [DOI] [PubMed] [Google Scholar]
- 12.Feilich KL, López-Fernández H. 2019. When does form reflect function? Acknowledging and supporting ecomorphological assumptions. Integr. Comp. Biol. 59, 358-370. ( 10.1093/icb/icz070) [DOI] [PubMed] [Google Scholar]
- 13.Kolmann MA, Huie JM, Evans K, Summers AP. 2018. Specialised specialists and the narrow niche fallacy: a tale of scale-feeding fishes. R. Soc. Open Sci. 5, 171581. ( 10.1098/rsos.171581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainwright PC, Bellwood DR, Westneat MW, Grubich JR, Hoey AS. 2004. A functional morphospace for the skull of labrid fishes: patterns of diversity in a complex biomechanical system. Biol. J. Linn. Soc. 82, 1-25. ( 10.1111/j.1095-8312.2004.00313.x) [DOI] [Google Scholar]
- 15.Bellwood DR, Wainwright PC, Fulton CJ, Hoey AS.. 2005. Functional versatility supports coral reef biodiversity. Proc. R. Soc. B 273, 101-107. ( 10.1098/rspb.2005.3276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wainwright PC. 2005. Many-to-one mapping of form to function: a general principle in organismal design? Integr. Comp. Biol. 45, 256-262. ( 10.1093/icb/45.2.256) [DOI] [PubMed] [Google Scholar]
- 17.Golcher-Benavides J, Wagner CE. 2019. Playing out Liem's paradox: opportunistic piscivory across Lake Tanganyikan cichlids. Am. Nat. 194, 260-267. ( 10.1086/704169) [DOI] [PubMed] [Google Scholar]
- 18.Brandl SJ, Bellwood DR. 2014. Individual-based analyses reveal limited functional overlap in a coral reef fish community. J. Anim. Ecol. 83, 661-670. ( 10.1111/1365-2656.12171) [DOI] [PubMed] [Google Scholar]
- 19.Binning SA, Chapman LJ, Cosandey-Godin A. 2009. Specialised morphology for a generalist diet: evidence for Liem's Paradox in a cichlid fish. J. Fish Biol. 75, 1683-1699. ( 10.1111/j.1095-8649.2009.02421.x) [DOI] [PubMed] [Google Scholar]
- 20.Liem KF. 1980. Adaptive significance of intra- and interspecific differences in the feeding repertoires of cichlid fishes. Am. Zool. 20, 295-314. ( 10.1093/icb/20.1.295) [DOI] [Google Scholar]
- 21.Robinson BW, Wilson DS. 1998. Optimal foraging, specialisation, and a solution to Liem's paradox. Am. Nat. 151, 223-235. ( 10.1086/286113) [DOI] [PubMed] [Google Scholar]
- 22.Drazen JC, Sutton TT. 2017. Dining in the deep: the feeding ecology of deep-sea fishes. Annu. Rev.Mar. Sci. 9, 337-366. ( 10.1146/annurev-marine-010816-060543) [DOI] [PubMed] [Google Scholar]
- 23.Sutton TT, Wiebe PH, Madin L, Bucklin A. 2010. Diversity and community structure of pelagic fishes to 5000m depth in the Sargasso Sea. Deep Sea Res. Part II 57, 2220-2233. ( 10.1016/j.dsr2.2010.09.024) [DOI] [Google Scholar]
- 24.Ebeling AW, Cailliet GM. 1974. Mouth size and predator strategy of midwater fishes. Deep Sea Res. Oceanogr. Abst. 21, 959-968. ( 10.1016/0011-7471(74)90028-X) [DOI] [Google Scholar]
- 25.Marshall NB. 1971. Explorations in the life of fishes. Cambridge, MA: Harvard University Press. [Google Scholar]
- 26.Pietsch TW. 2009. Oceanic anglerfishes: extraordinary diversity in the deep Sea. Berkeley, CA: University of California Press. [Google Scholar]
- 27.Maile AJ, May ZA, DeArmon ES, Martin RP, Davis MP. 2020. Marine habitat transitions and body-shape evolution in lizardfishes and their allies (Aulopiformes). Copeia 108, 820-832. ( 10.1643/CG-19-300) [DOI] [Google Scholar]
- 28.Burress ED, Muñoz MM. 2022. Functional trade-offs asymmetrically promote phenotypic evolution. Syst. Biol. 72, 150-160. ( 10.1093/sysbio/syac058) [DOI] [PubMed] [Google Scholar]
- 29.Corn KA, Martinez CM, Burress ED, Wainwright PC. 2021. A multifunction trade-off has contrasting effects on the evolution of form and function. Syst. Biol. 70, 681-693. ( 10.1093/sysbio/syaa091) [DOI] [PubMed] [Google Scholar]
- 30.Wainwright PC, McGee MD, Longo SJ, Patricia Hernandez L. 2015. Origins, innovations, and diversification of suction feeding in vertebrates. Integr. Comp. Biol. 55, 134-145. ( 10.1093/icb/icv026) [DOI] [PubMed] [Google Scholar]
- 31.Grobecker DB, Pietsch TW. 1979. High-speed cinematographic evidence for ultrafast feeding in antennariid anglerfishes. Science 205, 1161-1162. ( 10.1126/science.205.4411.1161) [DOI] [PubMed] [Google Scholar]
- 32.Olsson KH, Gurka R, Holzman R.. 2022. Trophic guilds of suction-feeding fishes are distinguished by their characteristic hydrodynamics of swimming and feeding. Proc. R. Soc. B 289, 20211968. ( 10.1098/rspb.2021.1968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pietsch TW, Arnold RJ. 2020. Frogfishes: biodiversity, zoogeography, and behavioral ecology. Baltimore, MA: Johns Hopkins University Press. [Google Scholar]
- 34.Longo SJ, McGee MD, Oufiero CE, Waltzek TB, Wainwright PC. 2015. Body ram, not suction, is the primary axis of suction feeding diversity in spiny-rayed fishes. J. Exp. Biol. 219, 119-128. [DOI] [PubMed] [Google Scholar]
- 35.Moore JA. 2002. Upside-down swimming behavior in a whipnose anglerfish (Teleostei: Ceratioidei: Gigantactinidae). Copeia 2002, 1144-1146. ( 10.1643/0045-8511(2002)002[1144:UDSBIA]2.0.CO;2) [DOI] [Google Scholar]
- 36.Buser TJ, Boyd OF, Cortés Á, Donatelli CM, Kolmann MA, Luparell JL, Pfeiffenberger JA, Sidlauskas BL, Summers AP. 2020. The natural historian's guide to the CT galaxy: step-by-step instructions for preparing and analysing computed tomographic (CT) data using cross-platform, open access software. Integr. Org. Biol. 2, obaa009. ( 10.1093/iob/obaa009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kikinis R, Pieper SD, Vosburgh KG. 2014. 3D slicer: a platform for subject-specific image analysis, visualization, and clinical support. In Intraoperative imaging and image-guided therapy (ed. Jolesz FA), pp. 277-289. New York, NY: Springer. [Google Scholar]
- 38.Rolfe S, et al. 2021. SlicerMorph: an open and extensible platform to retrieve, visualise and analyse 3D morphology. Methods Ecol. Evol. 12, 1816-1825. ( 10.1111/2041-210X.13669) [DOI] [Google Scholar]
- 39.Mihalitsis M, Bellwood DR. 2017. A morphological and functional basis for maximum prey size in piscivorous fishes. PLoS ONE 12, e0184679. ( 10.1371/journal.pone.0184679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westneat MW. 2004. Evolution of levers and linkages in the feeding mechanisms of fishes. Integr. Comp. Biol. 44, 378-389. ( 10.1093/icb/44.5.378) [DOI] [PubMed] [Google Scholar]
- 41.Mihalitsis M, Bellwood D. 2019. Functional implications of dentition-based morphotypes in piscivorous fishes. R. Soc. Open Sci. 6, 190040. ( 10.1098/rsos.190040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freeman PW, Lemen CA. 2007. The trade-off between tooth strength and tooth penetration: predicting optimal shape of canine teeth. J. Zool. 273, 273-280. ( 10.1111/j.1469-7998.2007.00325.x) [DOI] [Google Scholar]
- 43.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688-2690. ( 10.1093/bioinformatics/btl446) [DOI] [PubMed] [Google Scholar]
- 44.Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 57, 758-771. ( 10.1080/10635150802429642) [DOI] [PubMed] [Google Scholar]
- 45.Hart PB, Arnold RJ, Alda F, Kenaley CP, Pietsch TW, Hutchinson D, Chakrabarty P. 2022. Evolutionary relationships of anglerfishes (Lophiiformes) reconstructed using ultraconserved elements. Mol. Phylogenet. Evol. 171, 107459. ( 10.1016/j.ympev.2022.107459) [DOI] [PubMed] [Google Scholar]
- 46.Miya M, et al. 2010. Evolutionary history of anglerfishes (Teleostei: Lophiiformes): a mitogenomic perspective. BMC Evol. Biol. 10, 58. ( 10.1186/1471-2148-10-58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanderson M. 1997. A nonparametric approach to estimating divergence times in the absence of rate constancy. Mol. Biol. Evol. 14, 1218. ( 10.1093/oxfordjournals.molbev.a025731) [DOI] [Google Scholar]
- 48.Goatley CHR, Bellwood DR. 2009. Morphological structure in a reef fish assemblage. Coral Reefs 28, 449-457. ( 10.1007/s00338-009-0477-9) [DOI] [Google Scholar]
- 49.Medeiros APM, Santos BA, Betancur RR. 2022. Does genome size increase with water depth in marine fishes? Evolution 76, 1578-1589. ( 10.1111/evo.14510) [DOI] [PubMed] [Google Scholar]
- 50.Tung Ho LS, Ané C. 2014. A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63, 397-408. ( 10.1093/sysbio/syu005) [DOI] [PubMed] [Google Scholar]
- 51.Kenaley CP. 2012. Exploring feeding behaviour in deep-sea dragonfishes (Teleostei: Stomiidae): jaw biomechanics and functional significance of a loosejaw. Biol. J. Linn. Soc. 106, 224-240. ( 10.1111/j.1095-8312.2012.01854.x) [DOI] [Google Scholar]
- 52.Borstein SR, Fordyce JA, O'Meara BC, Wainwright PC, McGee MD. 2019. Reef fish functional traits evolve fastest at trophic extremes. Nat. Ecol. Evol. 3, 191-199. ( 10.1038/s41559-018-0725-x) [DOI] [PubMed] [Google Scholar]
- 53.Collar DC, O'Meara BC, Wainwright PC, Near TJ. 2009. Piscivory limits diversification of feeding morphology in centrarchid fishes. Evolution 63, 1557-1573. ( 10.1111/j.1558-5646.2009.00626.x) [DOI] [PubMed] [Google Scholar]
- 54.Childress JJ, Mickel T. 1985. Metabolic rates of animals from the hydrothermal vents and other deep-sea habitats. Bullet. Biol. Soc. Washington 6, 249-260. [Google Scholar]
- 55.Heiple Z, Huie JM, Medeiros APM, Hart PB, Goatley CHR, Arcila D, Miller EC. 2023. Data from: Many ways to build an angler: diversity of feeding morphologies in a deep-sea evolutionary radiation. Dryad Digital Repository. ( 10.5061/dryad.qfttdz0n2) [DOI] [PMC free article] [PubMed]
- 56.Heiple Z, Huie JM, Medeiros APM, Hart PB, Goatley CHR, Arcila D, Miller EC. 2023. Many ways to build an angler: diversity of feeding morphologies in a deep-sea evolutionary radiation. Figshare. ( 10.6084/m9.figshare.c.6700031) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Heiple Z, Huie JM, Medeiros APM, Hart PB, Goatley CHR, Arcila D, Miller EC. 2023. Data from: Many ways to build an angler: diversity of feeding morphologies in a deep-sea evolutionary radiation. Dryad Digital Repository. ( 10.5061/dryad.qfttdz0n2) [DOI] [PMC free article] [PubMed]
- Heiple Z, Huie JM, Medeiros APM, Hart PB, Goatley CHR, Arcila D, Miller EC. 2023. Many ways to build an angler: diversity of feeding morphologies in a deep-sea evolutionary radiation. Figshare. ( 10.6084/m9.figshare.c.6700031) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Input data files and R scripts are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.qfttdz0n2 [55]. All CT scans were uploaded to Morphosource (see electronic supplementary material, table S1).
The data are provided in the electronic supplementary material [56].