Abstract
Background
This meta-analysis was conducted given the contradictory findings from studies on the influence of diabetes duration or age at onset on mortality in patients with insulin-dependent diabetes mellitus (IDDM).
Methods
Electronic databases (PubMed, Embase, Cochrane, Web of Knowledge, Scopus, and CINHAL) were comprehensively searched to identify relevant studies until October 31, 2022. All of the selected articles contained statistics on hazard ratios, relative risks (RRs), or odds ratios, or data for estimating the association between diabetes duration or age at onset and total mortality in IDDM patients. Regardless the heterogeneity assessed by the I2 statistic, pooled RRs and 95% confidence intervals (CI) for total mortality were acquired via random effect meta-analysis with inverse variance weighting.
Results
This meta-analysis finally included 19 studies involving 122, 842 individuals. Both age at onset and diabetes duration were positively associated with an increased mortality rate in IDDM patients. Specifically, the pooled RRs for age at onset and diabetes duration were 1.89 (95%CI 1.43–2.50) and 1.89 (95%CI 1.16–3.09) respectively. Subgroup analyses revealed that only prepubertal onset was associated with a greater survival advantage than pubertal or postpubertal onset.
Conclusions
The findings of this meta-analysis and systematic review suggest that a later age at onset or longer diabetes duration is associated with increased risk of total mortality in IDDM patients. However, this conclusion shall be interpreted with caution due to the possibility of residual confounding and be confirmed in the future by well-designed studies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-023-01113-x.
Keywords: Diabetes duration, Age at onset, IDDM, Mortality, Risk, Systematic review, Meta-analysis
Background
Diabetes mellitus (DM) is a serious public global health concern. More than half a billion people (536.6 million) aged 20–79 years were expected to develop DM in 2021, with about 90% having noninsulin-dependent diabetes mellitus (NIDDM) [1]. In recent decades, diabetes-related health and economic burdens have increased globally, especially in low- and middle-income countries [2]. Most studies estimating diabetes burdens have focused on NIDDM, but paid scant attention to insulin-dependent diabetes mellitus (IDDM) [3]. As a significant chronic autoimmune disease, IDDM affects adolescents and children frequently [4], and becomes more prevalent worldwide in the last decade [5]. Since 1989, the global incidence of IDDM in children under 14 has increased by 3% annually [6], and about 8.4 million people worldwide would have IDDM in 2021 [7]. Misdiagnosis, underdiagnosis, a considerable chance of complications, and premature mortality are obstacles [8, 9].
Evidence from the past confirms that age at onset is closely related to premature mortality among IDDM patients [10]. According to a Swedish study, it discovered that patients who developed IDDM before the age of 10 years, and 26—30 years had a threefold, and less than twofold increase in mortality respectively compared to controls [11]. A Finnish study determined that the standardised mortality ratio for the early onset (0–14 years) cohort was 3.6 and that for the late onset (15–29 years) cohort was 2.80 [12]. Likewise, a recent narrative review found a correlation between the onset age of IDDM and total mortality [13]. This review had numerous limitations, including the absence of subgroup analysis and failure to recognize heterogeneity. Specifically, the omission of certain confounders (e.g., study design, early-onset criteria, and model adjustment ignorance) may result in bias. In addition, a previous analysis of 13 population-based EURODIAB registers from 12 countries found inconsistent results and no significant difference in the standardised mortality ratios by age at diagnosis [14].
In terms of diabetes duration, a longer duration implies an earlier onset, and a lower mortality risk shall be anticipated. This is not the situation, however. No systematic review or meta-analysis of the relationship between diabetes duration and mortality in diabetic populations has been conducted to date. In light of these considerations, our objective is to determine if diabetes duration or age at onset influences the total mortality of diabetic patients.
Methods
Search strategy and inclusion criteria
PubMed EMBASE, Wiley Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, Scopus, and CINAHL were exhaustively searched until October 31, 2022 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [15]. The search terms included "diagnostic age" or "diagnosis age" or "age of diagnosis" or "age on diagnosis" or "age at diagnosis" or "age at onset" or "onset age" or "age of onset" or "childhood onset" or "adolescent onset" or "puberty onset" or "prepuberty onset" or "duration of diabetes" or "time of diabetes" or "period of diabetes" (search strategy shown in Additional file 1). The references in pertinent articles were manually looked through to identify potential articles. WD and YZ searched independently, and any disagreement between them was coped by team consensus. The authors were contacted to obtain required information. If a site-specific dataset had been previously published, the most recent publication was chosen.
The inclusion criteria were: (i) observational study (cohort or case–control); (ii) reporting the relationship of diabetes duration or age at onset (age at diagnosis) with total mortality in IDDM patients; (iii) reporting effect estimates: standardized mortality or incidence ratio (SMR or SIR), or hazard ratio (HR), relative risk (RR) or odds ratios (OR), and relevant raw data for re-calculation. The exclusion criteria were: (I) case report, quasiexperiment (non-random subject allocation), editorial, remark, review, or unpublished study; (II) only published as abstract or conference proceeding. Studies that did not report such estimates for IDDM but also included T2D were excluded.
Data extraction and quality evaluation
Information regarding the study, participants (mean attained and current age, and gender), analysis strategy (statistical models, adjustment factors), and effect magnitude (e.g., SMR or IRR, or HRs, RRs or ORs) was gathered along with relevant raw data for recalculation. Specifically, the study information included design, name of first author, title, publication year, country/region, calendar time period of study, follow-up time, endpoints, sample size, adjustment level, measure of association, numbers of observed and expected events. The study quality was evaluated separately by WD and YZ using the 9-star Newcastle–Ottawa Scale (NOS). A rating greater than six stars indicates high quality [16]. Disagreements were handled by discussion.
Statistical analysis
The principal result measure was total mortality. As a control for the age at onset, the earliest age at onset or prepubertal onset was used. The associations between age groups and total mortality in the same study, relative to the controls, were merged before being combined with measurements from other studies. In terms of diabetes duration, only the shortest duration was used as a control, and the associations of different age groups, relative to the controls, with total mortality from the same study were combined before being combined with measurements from other studies.
The percent of between-study variability attributable to between-study heterogeneity was estimated by the I2 statistic [17] and categorized as high, modest or low with I2 ≥ 50%, < 50% and < 25%, respectively. The iterative non-central Chi-2 method [18] was used to identify a CI for I2.
Regarding the a-priori discrepancy of OSs, we performed subgroup analyses for the association between age at onset and total mortality according to sample size (< 1000, and ≥ 1000), control group (prepubertal, and others), age at onset/age at diagnosis, and type of effect measure (reported and calculated). Identifiable sources of dissimilarity were clarified by removing the articles one by one in a sensitivity test.
When 5 or more studies were available for analysis, Begg’s and Egger’s tests were used to test publication bias, and the dissymmetry of funnel plots of estimated effects versus standard errors was visually inspected [19]. Duval & Tweedie’s trim-and-fill method was used to correct for any publication bias (P < 0.10). All other analyses were performed on STATA 12.0 (US) at the significance level of P < 0.05.
Results
Study identification and quality evaluation
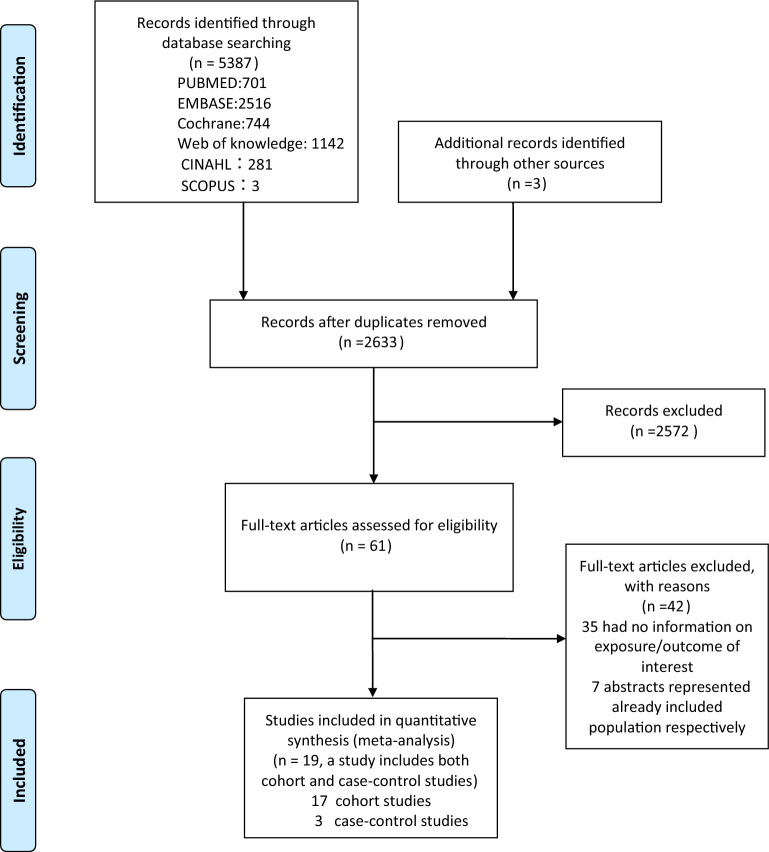
The systematic search yielded 5,390 publications, of which 61 were chosen for additional review (Fig. 1). Two articles [20, 21] from the same data source presented contradictory findings, and thus were both included. Two studies [22, 23] from the same team or institution with different results were included for analysis. Two studies [24, 25] from the same team or institution both reported an independent variable with age at onset and age at diagnosis and thus were both included. Finally, 20 papers provided information on the link between diabetes duration or age at onset and total death (Table 1). The 20 studies included two case–control studies [10, 26], 17 cohort studies [11, 20–23, 25, 27–37], and one study [24] that combined both case–control and cohort studies. The sample sizes of these studies ranged from 103 to 27,195 patients, and the follow-up periods ranged from 3.0 to 33.0 years. The share increased from 40.05% to 72.22%, while diabetes duration increased from 3.5 to 31.9 years.
Fig.1.
Flow-diagram of study selection
Table 1.
Detailed characteristics of studies included in the meta-analysis
| Study | Data source/Country/Region | Study design | Calendar time period of study | Age at onset/Age at diagnosis/diabetes duration | Sample size | Mean attained age/Mean current age (years) | Female (%total) | Mean diabetes duration(Years) | Mean follow-up time(Years) | Effect estimate of all-cause mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| Kostraba et al.(1991) | Children's Hospital of Pittsburgh Insulindependent Diabetes Mellitus Registry of/USA | Case–control | 1950–1981 | Age at onset | 924 | 28.32 | 87.88 | 19.1 | 20.0 | RR |
| Modan et al.(1991) | the DERI study group activitie/Israel | Cohort | 1965–1979 | Age at diagnosis | 614 | NR | NR | NR | 11.5 | OR(calc) |
| COLLADO-MESA et al.(1997) | Havana City Province data from the National Registry of IDDM/Cuba | Cohort | 1965–1980 | Age at diagnosis | 504 | NR | 48.6 | 25.0 | 17.5 | HR(extracting from Kapla-Meier curves) |
| NlSHIMURA et al.(1998) | two nationwide IDDM surveys/Japan |
Case–control Cohort |
1965–1979 | Age at onset | 1,286 | 23.28 | 40.05 | NR | 11.55 |
Case–control: RR Cohort: OR(calc) |
|
Mühlhause et al. (2000) |
the diabetes centre of the Düsseldorfuniversity Hospital/Germany | Cohort | 1978–1994 | Diabetes duration | 3,570 | 27.5 | 50.3 | 10.6 | 10.3 | HR |
| ASAO et al.(2003) | Nationwide surveys/Japan; the National Social Insurance Institution /Finland | Cohort | 1965–1979 | Age at diagnosis |
Japan:1,408 Finland:5,126 |
NR |
Japan:59.80 Finland:45.04 |
Japan:3.5 Finland: 4.2 |
Japan: 16.3 Finland:17.8 |
Japan: aHR Finland: aHR |
| Barceló et al.(2007) | Havana City Province data from the National Registry of IDDM/Cuba | Cohort |
AC:1965–1979 HA:1965–1980 |
Age at onset | 504 | NR | 48.61 | 16.5 | NR | OR(calc) |
| Rendas-Baum et al. (2006) | the New Jersey 725 study/USA | Case–control | 1993–1998 | Diabetes duration | 725 | 29.00 | 58.34 | 9.50 | 3.0 | HR |
| Dawson et al.(2008) | the Canterbury Diabetes Registry/ New Zealand | Cohort | 1984–2004 | Age at onset | 989 | 52.36 | 50.66 | 16.80 | 13.60 | OR(calc) |
| SECREST et al.(2010) | The Allegheny County Type 1 Diabetes Registry cohort/ USA | Cohort | 1965–1979 | Age at onset | 1,075 | 42.8 | 48.0 | 31.9 | 33.0 | OR(calc) |
| Washington et al.(2014) | the USVI Childhood (< 19 years old) Diabetes Registry/USA | Cohort | 1979–2005 | Age at onset | 103 | 28.2 | 52.4 | 16.8 | 16.8 | OR(calc) |
| Gagnum et al.(2015) | the nationwide, population based Norwegian Childhood Diabetes Registry/Norway | Cohort | 1973–1982; 1989–2012 | Age at diagnosis | 7,884 | NR | 46.0 | 16.8 | 16.8 | aHR |
| Marshall et al.(2016) |
Rwanda Life For a Child (LFAC) program/USA、 Australia、Rwanda |
Cohort | 2004–2012 | Age at diagnosis | 488 | 20.95 | 57.38 | 4.5 | NR | aHR |
| Cheung et al.(2017) | YRDCYP/United Kingdom | Cohort | 1978–2013 | Age at diagnosis | 5,498 | NR | 45.96 | NR | 17.42 | aHR |
| Gomes et al. (2017) | BNHCS /Brazil | Cohort | 2014–2015 | Age at diagnosis/diabetes duration | 986 | 28.5 | 54.4 | 15.9 | 16.1 | OR(calc) |
| Rawshani et al. (2018) | the Swedish National Diabetes Register/Sweden | Cohort | 1998–2012 | Age at onset | 27,195 | NR | 44.2 | 13.0 | 10.0 | OR(calc) |
| Conway et al. (2018) | the Southern Community Cohort Study/USA | Cohort | 2002–2009 | Age at diagnosis | 475 | 49.87 | 66.32 | 29.06 | 9.5 | OR(calc) |
| Groop et al. (2018) | FinnDiane/Finland | Cohort | 1980–2005 | Age at onset | 10,737 | 35.51 | 45.83 | 16.2 | 14.0 | OR(calc) |
| Majaliwa et al. (2022) | CYLDM/ Tanzania | Cohort |
1991–2004; 2005–2010; 2011–2019 |
Age at diagnosis | 3,235 | NR | 49.2 | 5.0 | NR | OR(calc) |
BNHCS the Brazilian National Health Care System, DERI Diabetes Epidemiology Research International, YRDCYP The Yorkshire Register of Diabetes in Children and Young People, AC Allegheny County, HA Havana, USVI U.S. Virgin Islands, FinnDiane the Finnish Diabetic Nephropathy Study, CYLDM Children and Youth Living with Diabetes Mellitus, IDDM Insulin dependent diabetes mellitus, aHR Adjusted Hazard Ratio, RR Relative Risk/Risk Ratio, OR Odds Ratio, calc Calculate, NR not reported
Aside from the multinational origins in one study [33], other origins included developed countries or regions in 15 articles [10, 11, 22–32, 35, 36], and developing countries in only four articles [20, 21, 34, 37]. Three articles [22, 26, 28] and sixteen studies [10, 11, 20, 21, 23–25, 27, 29–33, 35–37] presented data comparing diabetes duration and age at onset, respectively, with the risk of total mortality, and one study [34] presented data comparing either diabetes duration or age at onset with total mortality.
With ≥ 7 NOS scores (mean = 7.5; Additional file 2: Table S2), all 20 OSs were of high methodological quality.
Impact of diabetes duration on total mortality in IDDM patients
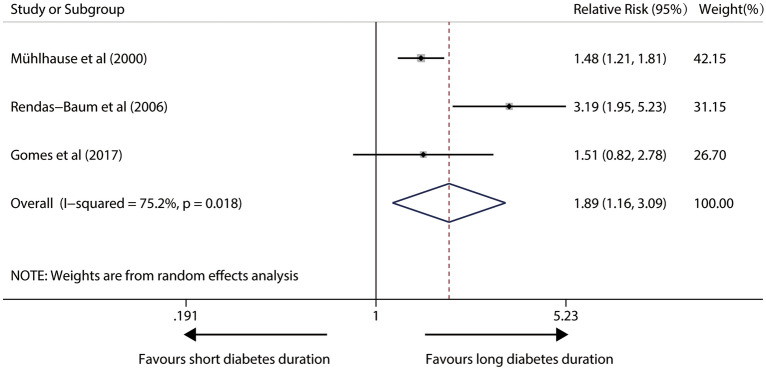
In a random-effects model with significant heterogeneity (I2 = 75.2%; P = 0.018) from four relevant studies, patients with longer diabetes duration had a 89% higher risk (pooled RR, 1.89; 95%CI 1.16–3.09; P = 0.011) in susceptibility to any death. Figure 2 illustrates forest plots of the meta-analysis.
Fig. 2.
Forest plot for the association between diabetes duration and risk of total mortality in IDDM patients (The X-axis represents the log scale; the solid square represents the relative risk; and the horizontal lines represent the 95% CIs. The same in other figures)
Impact of age at onset on total mortality in IDDM patients
Seventeen relevant studies were included in the analysis of age at onset/age at diagnosis with the danger of total mortality. Overall, the pooled RR showed a 89% greater risk of total death in late-onset TID patients compared to early-onset IDDM patients (RR,1.89; 95%CI 1.43–2.50; p < 0.001), however, there was evident heterogeneity among trials (I2 = 92.3%, p < 0.001) (Fig. 3). After removing single studies, sensitivity analysis showed that heterogeneity did not disappear. Neither Egger's test (P = 0.183) nor visual check revealed any substantial publication bias (Additional file 3: Fig.S1).
Fig. 3.
Forest plot for the association between age at onset and risk of total mortality in IDDM patients
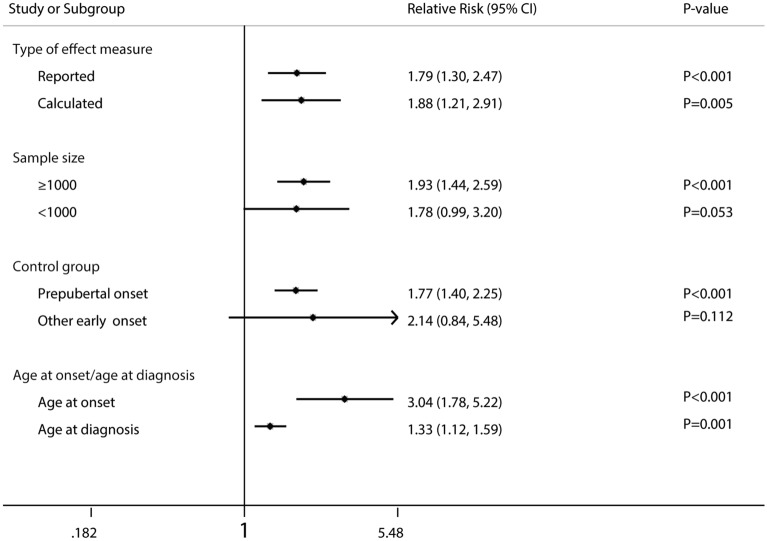
The pooled RRs were broadly consistent across the large sample size (≥ 1000 patients, P < 0.001), age at onset/age at diagnosis (both P ≤ 0.001), prepubertal onset as control group (P < 0.001), and type of effect measure (reported, P < 0.001; calculated, P = 0.005) (Fig. 4).
Fig. 4.
Forest plot for the association between age at onset and risk of total mortality in IDDM patients according to some clinically important variables. IDDM insulin-dependent diabetes mellitus
Discussion
We are the first to comprehensively examine and meta-analyze the discrepancy between early-late onset or diabetes duration and total mortality in IDDM patients. Our findings reveal that IDDM people have a greater risk of mortality over longer time period with diabetes. In regard to early-late onset, we searched all current studies involving 69,031 people and discovered a significantly higher risk of total mortality for those with a later onset than those with an earlier onset. Only prepubertal onset offered a survival advantage according to further subgroup analyses. Furthermore, age at onset and age at diagnosis were both positively connected with the risk of total mortality, with age at onset being more evenly distributed. Although the underlying biological mechanisms directly linking prepubertal onset or diabetes duration to death have yet to be determined, there are three accepted hypotheses. (1) As reported [38], diabetes duration has been linked to the prevalence and prognosis of diabetic complications, and the impact of age at onset on mortality may be attributed to a differential effect of puberty on duration in the etiology of microvascular complications [10]. It is further proposed that the age at onset is marked significantly by duration and not by age, because the persistence of diabetes after puberty will predict mortality, regardless of age. (2) The etiology of IDDM is heterogeneous, with a more benign disease diagnosed before the age of 12 years, and the diabetic complications progress due to a different pathogenesis [10, 39–41] and worse glycemic control. This is so due to the impaired insulin action [42] caused by increased secretion of various hormones during puberty [43–45], by psychosocial issues [46], and differences in self-management education and experience, additional to differences in shifting health services from pediatric to adult populations [35]. (3) In the peripubertal group, the duration from onset to diagnosis may be longer than in the younger population, contributing to a longer period of potential organ damage [20].
Notably, Araz Rawshani et al. [11] discovered that patients who developed IDDM between the age of 0 and 10 had hazard ratios for all-cause mortality of 4.11 in contrast to the general population, after adjusting for duration. They believed that diabetes duration was significant since total correction was unattainable. Diabetes duration is a component of total glycemic load. Glycaemic load is defined as the cumulative exposure of the vasculature to glucose and is affected by diabetes duration and glycaemic variability. A larger glycemic burden and hence the damage lead to longer duration of diabetes (similar to the area under the LDL cholesterol exposure curve) [47, 48]. Obviously, coronary arteries are particularly sensitive to hyperglycemia, and possibly even more when hyperglycemia first manifests during the first 10 to 15 years of life. Another possible explanation for their findings is that individuals with a younger age of onset have a more severe and rapid loss of β-cells due to a distinct type of insulitis [49, 50], which contributes to elevated glycemia. Furthermore, after 10 years of disease duration, children and teenagers with IDDM begin to suffer subclinical cardiovascular disease abnormalities, as exemplified by numerous methodologies [51–53]. Given these contradicting reports, more research is needed to determine whether such disparities among age-at-onset groups are connected to a different etiology of disease based on age at onset.
Using raw data from Araz Rawshani's et al. study, we discovered that prepubertal onset was preferable to later onset. The explanation is unknown, but can be due to an ambiguous achieved age, as there is considerable debate about whether diabetes duration or age of onset is the key predictor of increased relative mortality. This was because the pubertal group had diabetes for a shorter time period than the prepubertal group at a given attained age. A given attained age simultaneously represents background risks. Studies [25, 32, 54, 55] found that attained age, rather than diabetes duration or age at diagnosis, was the most crucial predictor of outcome. Because attained age is the sum of age at onset and duration of diabetes, it cannot be assessed in a single multivariate analysis model. Reportedly, diabetes duration had a strong but variable impact on relative mortality, and age at diagnosis, in conjunction with diabetes duration, was the most crucial predictor [55]. None of the parameters influenced the relative mortality of patients with a transient diabetes history. Age at initiation and diabetes duration at admission were the primary indicators of higher relative mortality in diabetic individuals with median duration. However, after 40 years, almost all of the factors had lost their significance. One study [23] also revealed that duration of diabetes did not impact diabetic complications as severely as previously thought.
The detailed retrieval plan utilizing Cochrane protocols and the relatively large sample size are two significant strengths of this meta-analysis. This study has some limitations. First, the avoidance of unpublished reports may have skewed our results. Second, the onset age and diagnosis of IDDM cannot be discriminated precisely, and the misclassification may obscure a significant link. Third, significant heterogeneity was discovered due to the lack of uniformity in the case elucidation method, study design and period, endpoint classification, and the adjusted level of between-study confounding. These results need be confirmed by other research because we cannot take into account the vast majority of between-study heterogeneity, despite the sensitivity testing. Fourth, no information was available on the treatment or other clinical factors (e.g., HbA1c level). In consequence, it is challenging to identify the factors that influence how mortality risk changes. Fifth, studies with smaller sample sizes (N < 1000) were included in the meta-analysis, but these studies very probably lacked the statistical power to identify the true association.
Conclusion
In summary, our study provides further evidence for the association between age at onset or diabetes duration, and mortality risk in IDDM patients. However, this conclusion must be understood carefully given the potential remaining confounding factors and be further validated by well-planned prospective studies.
Supplementary Information
Additional file 1: Table S1. Search stragely.
Additional file 2: Table S2. Quality assessment of observational studies included in the meta-analysis by NOS. NOS Newcastle–Ottawa scale.
Additional file 3: Fig. S1. Funnel plot for the association between age at onset and risk of total mortality in IDDM patients. IDDM insulin-dependent diabetes mellitus.
Acknowledgements
None.
Author contributions
X-MW and F-YZG conceptualized and designed the study, conducted the literature search, data extraction, data analyses, and the drafting and review of the final manuscript. S-PZ and G-FL interpreted the analyzed data, critically reviewed the manuscript, and helped to draft the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and analyzed in this investigation are accessible from the corresponding author in response to a legitimate request.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
None of the authors have any relevant conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan M, Hashim MJ, King JK, Govender RD, Mustafa H, Al KJ. Epidemiology of Type 2 diabetes—global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maahs DM, Daniels SR, de Ferranti SD, Dichek HL, Flynn J, Goldstein BI, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American heart association. Circulation. 2014;130:1532–1558. doi: 10.1161/CIR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 5.Tao Z, Shi A, Zhao J. Epidemiological perspectives of diabetes. Cell Biochem Biophys. 2015;73:181–185. doi: 10.1007/s12013-015-0598-4. [DOI] [PubMed] [Google Scholar]
- 6.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 7.Gregory GA, Robinson T, Linklater SE, Wang F, Colagiuri S, de Beaufort C, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 2022;10:741–760. doi: 10.1016/S2213-8587(22)00218-2. [DOI] [PubMed] [Google Scholar]
- 8.Thomas NJ, Lynam AL, Hill AV, Weedon MN, Shields BM, Oram RA, et al. Type 1 diabetes defined by severe insulin deficiency occurs after 30 years of age and is commonly treated as type 2 diabetes. Diabetologia. 2019;62:1167–1172. doi: 10.1007/s00125-019-4863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo SJ, Shao H. Growing global burden of type 1 diabetes needs multitiered precision public health interventions. Lancet Diabetes Endocrinol. 2022;10:688–689. doi: 10.1016/S2213-8587(22)00257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kostraba JN, Dorman JS, LaPorte RE, Kuller LH, Orchard TJ, Becker DJ, et al. The investigation of age at onset as a risk factor for mortality in persons with insulin-dependent diabetes mellitus using Cox proportional hazards models. Am J Epidemiol. 1991;133:67–72. doi: 10.1093/oxfordjournals.aje.a115804. [DOI] [PubMed] [Google Scholar]
- 11.Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson AM, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392:477–486. doi: 10.1016/S0140-6736(18)31506-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harjutsalo V, Forsblom C, Groop PH. Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. BMJ. 2011;343:d5364. doi: 10.1136/bmj.d5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ang GY. Age of onset of diabetes and all-cause mortality. World J Diabetes. 2020;11:95–99. doi: 10.4239/wjd.v11.i4.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson CC, Dahlquist G, Harjutsalo V, Joner G, Feltbower RG, Svensson J, et al. Early mortality in EURODIAB population-based cohorts of type 1 diabetes diagnosed in childhood since 1989. Diabetologia. 2007;50:2439–2442. doi: 10.1007/s00125-007-0824-8. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Zhang Y, Wu H, Zhu P, Mo X, Ma X, et al. Intake of polyunsaturated fatty acids and risk of preclinical and clinical type 1 diabetes in children-a systematic review and meta-analysis. Eur J Clin Nutr. 2018;73:1–8. doi: 10.1038/s41430-018-0185-z. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Hedges LV, Pigott TD. The power of statistical tests in meta-analysis. Psychol Methods. 2001;6:203–217. doi: 10.1037/1082-989X.6.3.203. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collado-Mesa F, Díaz-Díaz O, Melián-Torres R, Suárez-Pérez R, Vera-González M, Aldana-Padilla D. Mortality of childhood-onset IDDM patients A cohort study in Havana City Province Cuba. Diabetes Care. 1997;20:1237–1241. doi: 10.2337/diacare.20.8.1237. [DOI] [PubMed] [Google Scholar]
- 21.Barceló A, Bosnyak Z, Orchard T. A cohort analysis of type 1 diabetes mortality in Havana and Allegheny County, Pittsburgh PA. Diabetes Res Clin Pract. 2007;75:214–219. doi: 10.1016/j.diabres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Conway BN, May ME, Signorello LB, Blot WJ. Mortality experience of a low-income population with young-onset diabetes. Diabetes Care. 2012;35:542–548. doi: 10.2337/dc11-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conway BN, Lopes-Virella MF, Blot WJ. Late adulthood mortality among African-American and white American people with type 1 diabetes according to age at diabetes diagnosis. Diabet Med. 2018;35:729–736. doi: 10.1111/dme.13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura R, Tajima N, Matsushima M, LaPorte RE. Puberty, IDDM, and death in Japan. diabetes epidemiology research international study group. Diabetes Care. 1998;21:1674–1679. doi: 10.2337/diacare.21.10.1674. [DOI] [PubMed] [Google Scholar]
- 25.Asao K, Sarti C, Forsen T, Hyttinen V, Nishimura R, Matsushima M, et al. Long-term mortality in nationwide cohorts of childhood-onset type 1 diabetes in Japan and Finland. Diabetes Care. 2003;26:2037–2042. doi: 10.2337/diacare.26.7.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy M, Rendas-Baum R, Skurnick J. Mortality in African-Americans with Type 1 diabetes: The New Jersey 725. Diabet Med. 2006;23:698–706. doi: 10.1111/j.1464-5491.2006.01901.x. [DOI] [PubMed] [Google Scholar]
- 27.Modan M, Karp M, Bauman B, Gordon O, Danon YL, Laron Z. Mortality in Israeli Jewish patients with type 1 (insulin-dependent) diabetes mellitus diagnosed prior to 18 years of age: a population based study. Diabetologia. 1991;34:515–520. doi: 10.1007/BF00403289. [DOI] [PubMed] [Google Scholar]
- 28.Mühlhauser I, Overmann H, Bender R, Jörgens V, Berger M. Predictors of mortality and end-stage diabetic complications in patients with Type 1 diabetes mellitus on intensified insulin therapy. Diabet Med. 2000;17:727–734. doi: 10.1046/j.1464-5491.2000.00372.x. [DOI] [PubMed] [Google Scholar]
- 29.Dawson SI, Willis J, Florkowski CM, Scott RS. All-cause mortality in insulin-treated diabetic patients: a 20-year follow-up. Diabetes Res Clin Pract. 2008;80:e6–9. doi: 10.1016/j.diabres.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 30.Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County type 1 diabetes registry. Diabetes Care. 2010;33:2573–2579. doi: 10.2337/dc10-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Washington RE, Orchard TJ, Arena VC, LaPorte RE, Secrest AM, Tull ES. All-cause mortality in a population-based type 1 diabetes cohort in the U.S. Virgin Islands. Diabetes Res Clin Pract. 2014;103:504–509. doi: 10.1016/j.diabres.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagnum V, Stene LC, Sandvik L, Fagerland MW, Njølstad PR, Joner G, et al. All-cause mortality in a nationwide cohort of childhood-onset diabetes in Norway 1973–2013. Diabetologia. 2015;58:1779–1786. doi: 10.1007/s00125-015-3623-7. [DOI] [PubMed] [Google Scholar]
- 33.Marshall SL, Edidin DV, Arena VC, Becker DJ, Orchard TJ. Mortality and natural progression of type 1 diabetes patients enrolled in the Rwanda LFAC program from 2004 to 2012. Int J Diabetes Dev Ctries. 2016;37:1–9. [Google Scholar]
- 34.Gomes MB, Almeida AP, et al. Cause-specific mortality in a cohort of Brazilian patients with type 1 diabetes. Acta Diabetol. 2017;54:535–542. doi: 10.1007/s00592-017-0975-0. [DOI] [PubMed] [Google Scholar]
- 35.Evans-Cheung TC, Bodansky HJ, Parslow RC, Feltbower RG. Mortality and acute complications in children and young adults diagnosed with Type 1 diabetes in Yorkshire, UK: a cohort study. Diabet Med. 2018;35:112–120. doi: 10.1111/dme.13544. [DOI] [PubMed] [Google Scholar]
- 36.Groop PH, Thomas M, Feodoroff M, Forsblom C, Harjutsalo V, FinnDiane Study Group Excess mortality in patients with type 1 diabetes without albuminuria-separating the contribution of early and late risks. Diabetes Care. 2018;41:748–754. doi: 10.2337/dc17-1618. [DOI] [PubMed] [Google Scholar]
- 37.Majaliwa ES, Minja L, Ndayongeje J, Ramaiya K, Mfinanga SG, Mmbaga BT. Survival of children and youth with type 1 diabetes mellitus in Tanzania. Pediatr Diabetes. 2022;23:1560–1566. doi: 10.1111/pedi.13425. [DOI] [PubMed] [Google Scholar]
- 38.Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 39.Arslanian SA, Becker DJ, Rabin B, Atchison R, Eberhardt M, Cavender D, et al. Correlates of insulin antibodies in newly diagnosed children with insulin-dependent diabetes before insulin therapy. Diabetes. 1985;34:926–930. doi: 10.2337/diab.34.9.926. [DOI] [PubMed] [Google Scholar]
- 40.Vandewalle CL, Falorni A, Svanholm S, Lernmark A, Pipeleers DG, Gorus FK. High diagnostic sensitivity of glutamate decarboxylase autoantibodies in insulin-dependent diabetes mellitus with clinical onset between age 20 and 40 years. the belgian diabetes registry. J Clin Endocrinol Metab. 1995;80:846–851. doi: 10.1210/jcem.80.3.7883841. [DOI] [PubMed] [Google Scholar]
- 41.Yokota I, Shirakawa N, Shima K, Matsuda J, Naito E, Ito M, et al. Relationship between GAD antibody and residual beta-cell function in children after overt onset of IDDM. Diabetes Care. 1996;19:74–75. doi: 10.2337/diacare.19.1.74. [DOI] [PubMed] [Google Scholar]
- 42.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. a contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–219. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 43.Merimee TJ, Zapf J, Froesch ER. Insulin-like growth factors. studies in diabetics with and without retinopathy. N Engl J Med. 1983;309:527–530. doi: 10.1056/NEJM198309013090904. [DOI] [PubMed] [Google Scholar]
- 44.Williamson JR, Rowold E, Chang K, Marvel J, Tomlinson M, Sherman WR, et al. Sex steroid dependency of diabetes-induced changes in polyol metabolism, vascular permeability, and collagen cross-linking. Diabetes. 1986;35:20–27. doi: 10.2337/diab.35.1.20. [DOI] [PubMed] [Google Scholar]
- 45.Alzaid AA, Dinneen SF, Melton LJ, 3rd, Rizza RA. The role of growth hormone in the development of diabetic retinopathy. Diabetes Care. 1994;17:531–534. doi: 10.2337/diacare.17.6.531. [DOI] [PubMed] [Google Scholar]
- 46.Moberg E, Kollind M, Lins PE, Adamson U. Acute mental stress impairs insulin sensitivity in IDDM patients. Diabetologia. 1994;37:247–251. doi: 10.1007/BF00398050. [DOI] [PubMed] [Google Scholar]
- 47.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. evidence from genetic, epidemiologic, and clinical studies. a consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arif S, Leete P, Nguyen V, Marks K, Nor NM, Estorninho M, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes. 2014;63:3835–3845. doi: 10.2337/db14-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leete P, Willcox A, Krogvold L, Dahl-Jørgensen K, Foulis AK, Richardson SJ, et al. Differential Insulitic profiles determine the extent of β-Cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65:1362–1369. doi: 10.2337/db15-1615. [DOI] [PubMed] [Google Scholar]
- 51.Järvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, et al. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109:1750–1755. doi: 10.1161/01.CIR.0000124725.46165.2C. [DOI] [PubMed] [Google Scholar]
- 52.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 53.Margeirsdottir HD, Stensaeth KH, Larsen JR, Brunborg C, Dahl-Jørgensen K. Early signs of atherosclerosis in diabetic children on intensive insulin treatment: a population-based study. Diabetes Care. 2010;33:2043–2048. doi: 10.2337/dc10-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorman JS, Laporte RE, Kuller LH, Cruickshanks KJ, Orchard TJ, Wagener DK, et al. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study mortality results. Diabetes. 1984;33:271–276. doi: 10.2337/diab.33.3.271. [DOI] [PubMed] [Google Scholar]
- 55.Borch-Johnsen K, Kreiner S, Deckert T. Mortality of type 1 (insulin-dependent) diabetes mellitus in Denmark: a study of relative mortality in 2930 Danish type 1 diabetic patients diagnosed from 1933 to 1972. Diabetologia. 1986;29:767–772. doi: 10.1007/BF00873214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Search stragely.
Additional file 2: Table S2. Quality assessment of observational studies included in the meta-analysis by NOS. NOS Newcastle–Ottawa scale.
Additional file 3: Fig. S1. Funnel plot for the association between age at onset and risk of total mortality in IDDM patients. IDDM insulin-dependent diabetes mellitus.
Data Availability Statement
The datasets used and analyzed in this investigation are accessible from the corresponding author in response to a legitimate request.