Abstract
BACKGROUND:
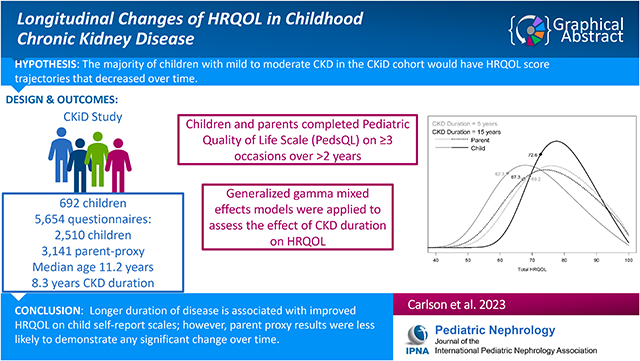
Few longitudinal studies have evaluated the impact of chronic kidney disease (CKD) duration on health-related quality of life (HRQOL). The study aim was to determine how HRQOL changes over time in childhood CKD.
METHODS:
Study participants were children in the Chronic Kidney Disease in Children (CKiD) cohort who completed the Pediatric Quality of Life Inventory (PedsQL) on three or more occasions over the course of two or more years. Generalized gamma (GG) mixed effects models were applied to assess the effect of CKD duration on HRQOL while controlling for selected covariates.
RESULTS:
692 children (median age=11.2) with a median of 8.3 years duration of CKD were evaluated. All subjects had a GFR greater than 15 ml/min/1.73m2. GG models with child self-report PedsQL data indicated that longer CKD duration was associated with improved Total HRQOL and the 4 domains of HRQOL. GG models with parent-proxy PedsQL data indicated that longer duration was associated with better Emotional but worse School HRQOL. Increasing trajectories of child self-report HRQOL were observed in the majority of subjects, while parents less frequently reported increasing trajectories of HRQOL. There was no significant relationship between Total HRQOL and time varying GFR.
CONCLUSIONS:
Longer duration of disease is associated with improved HRQOL on child self-report scales; however, parent proxy results were less likely to demonstrate any significant change over time. This divergence could be due to greater optimism and accommodation to CKD in children. Clinicians can use these data to better understand the needs of pediatric CKD patients.
Keywords: Quality of life, Pediatric Nephrology, chronic kidney disease, longitudinal, psychosocial, parent-child agreement
Graphical Abstract
Introduction
Pediatric onset kidney disease has been tied to numerous biological consequences such as deterioration in cardiovascular functioning, impairment of growth and development, and neurocognitive deficits [1–3]. Independent of biological consequences and medical complications, kidney disease progression has been associated with suboptimal long-term psychosocial outcomes, including lower or impaired perceived health-related quality of life (HRQOL) [4,5].
Health-related quality of life is a construct that represents the impact of disease on psychosocial outcomes. Publications examining HRQOL have primarily been cross-sectional and have suggested that children with chronic kidney disease (CKD) display suboptimal short-term and long-term HRQOL, especially in the areas of physical, social, educational and vocational functioning, as compared with children who do not have CKD [6,4,7–9]. In the majority of studies, longer duration of disease is associated with worse HRQOL [10,11] even though HRQOL may improve post-kidney transplant [12].
There are several limitations of the existing data. First, most published studies focus on CKD Stage 5, and there is less known about earlier stages of CKD. Since there are large numbers of patients with milder chronic kidney disease, it is important to better understand their HRQOL and how to create targeted interventions to improve HRQOL in this more common scenario [13,14,7]. Second, there is only one published study evaluating the longitudinal effects of CKD on HRQOL in children with mild to moderate CKD and this study has a number of limitations including absence of caregiver proxy ratings, a high rate of attrition, and 28% of eligible patients not participating and, consequently, not being included in the analysis [15]. The other longitudinal studies that do exist focus on patients with kidney failure, have small sample sizes, and often use analytical methods that fail to incorporate the richness of repeated measurements of HRQOL [10,12,14].
For more than sixteen years, the Chronic Kidney Disease in Children (CKiD) study has evaluated the HRQOL of a large cohort of children who have mild to moderate CKD at study enrollment. The objective of the present analysis was to evaluate longitudinally the impact of CKD duration on HRQOL using a robust epidemiological analytic method that evaluated trajectories of HRQOL. Our analysis aimed to create a better understanding of HRQOL in milder kidney disease and help clinicians better understand HRQOL trends in their patients and families. Based on the available literature, we hypothesized that the majority of children with mild to moderate CKD in the CKiD cohort would have HRQOL score trajectories that decreased over time. We predicted the parent proxy data would show a similar trend.
Methods
Study Population
Subjects were participants in the Chronic Kidney Disease in Children Study (CKiD), a longitudinal, observational cohort study of children with mild to moderate CKD being conducted at 58 pediatric nephrology centers in North America [16]. CKiD was initiated in 2003 with patient recruitment starting in 2005. Baseline estimated Glomerular Filtration Rate (eGFR) values for study participants were 30–90 ml/min/1.73m2 based on the U25 eGFR equation [17]. The CKiD study protocol was approved by the Institutional Review Boards at each participating center. All study participants or their parents provided informed consent.
Outcome Variable
Pediatric Quality of Life Inventory.
The 23-item PedsQL™ Generic Core Scales assess physical, emotional, social, and school functioning. In addition, a Total Score can be computed which reflects overall HRQOL. Respondents are asked to indicate the frequency with which each situation has been a problem in the past month using a 5-point Likert scale (never, almost never, sometimes, often, almost always). The reliability and validity of the PedsQL have been affirmed in studies of healthy children and children with other chronic medical conditions, including those with CKD [18–20]. Scores range from 0–100, with higher scores reflecting better HRQOL. There are child self-report (validated for ages 8–18 years old) and parent-proxy versions (validated for ages 2–18 years old). For this study, both self-report and parent proxy ratings were collected. The Pediatric Quality of Life Inventory (PedsQL) was administered to study participants and their parents at each annual CKiD study visit. The analysis included a subset of CKiD participants who had completed three or more PedsQL surveys.
Statistical Analysis
PedsQL data present a particular challenge for longitudinal analysis. Being bounded between 0 and 100 and highly skewed toward the upper boundary, particularly in certain subscales, renders conventional statistical methods based on normal (Gaussian) theory inadequate. However, the PedsQL outcomes can be rescaled and transformed into a form which can be appropriately described by the generalized gamma (GG) distribution [21]. This is the method used here, applying the transformation −log (HRQOL/100), with adjustment so that boundary values do not transform to 0 or infinity. Furthermore, to accommodate the repeated measurements of our longitudinal HRQOL outcomes, we chose to apply a mixed-effects GG model. We chose years with CKD as the primary independent variable as we considered this more directly relevant to HRQOL than years of age or years in the CKiD study. We included both random intercepts and slopes to allow for individual variability both at baseline and longitudinally.
Covariates
The variables included in the multivariate model were chosen based on published cross-sectional analyses by the CKiD research group and broad coverage of domains such as disease severity, cardiovascular, growth, socioeconomic status (SES), and medication use. These included eGFR [17]; urine protein/creatinine ratio (log2-transformed); height Z-score (corrected for age and sex); systolic blood pressure Z-score (corrected for age, sex and height); maternal education (high school or less as reference); anemia (defined as hemoglobin <5th percentile for age and sex based on CDC guidelines); glomerular vs non-glomerular; and ACE-inhibitor use. We also included an indicator for age ≥ 13 years, to account for potential differences in HRQOL reporting between adolescents and younger children [22,4]. Continuous variables (duration of disease, eGFR, and urine protein/creatinine) were centered at biologically meaningful values (10 years, 50 ml/min|1.73m2, and 1.0 mg/mg respectively). Models used records where data on all variables were collected.
The canonical summary statistic from GG models is the percentile (e.g., median), so that the effects of independent variables are characterized by contrasts in a given percentile (e.g., 25th percentile) for groups of individuals with different values in a variable of interest, while fixing other covariates at specified reference values. The hypothetical reference population has 10 years of CKD, non-glomerular disease, eGFR of 50 ml/min|1.73m2, urine protein/creatinine ratio of 1, age < 13, height and systolic BP at the mean for age, maternal education high school or less, no anemia, and no ACE-inhibitor use. For this analysis, we chose to present tabular results demonstrating effects at the 25th percentile, since the skewness of the distributions implies that the most noticeable shifts will occur in the lower tail. We also provide visual representations of the estimated distributions of HRQOL to enable observation of the impact of key predictors across the complete spectrum of PedsQL score outcomes.
To explore the effect of time in more detail, we performed an analysis using the empirical Bayes estimates of the random slopes to calculate individual adjusted trajectories of PedsQL for each subject. We calculated the percentage of children with positive trajectories (i.e., increasing HRQOL) and compared the proportions of increasers between normal-range and low baseline scores on the given PedsQL scale (≥1 SD below the mean based on normative data published by Varni et al., 2003) [23].
We also evaluated whether our analysis was subject to survivorship bias. This was accomplished by calculating whether children with negative HRQOL trajectories had a higher rate of kidney replacement therapy (KRT) than those with positive trajectories. A discernably higher percentage of KRT among subjects with negative trajectories would indicate survivorship bias, as children with decreasing HRQOL would tend to exit follow-up earlier and deprive our data of their low future PedsQL scores.
Analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC) using PROC NLMIXED for the GG models.
Results
Sample characteristics
The sample included 692 children and 5,651 PedsQL administrations: 2,510 from children and 3,141 from parent proxy. The parent and child surveys differed in quantity due to the limited age range of the PedsQL questionnaire. Children contributed a median of five years of HRQOL data (interquartile range [IQR] 4, 7). Table 1 displays demographic characteristics at the first visit with HRQOL available (typically 6 months after study entry). At this baseline, the median participant age was 11.2 years (IQR 7.5,14.6), median duration of CKD was 8.3 years (IQR 4.3, 12.0) and median eGFR was 51.2 ml/min/1.73m2 (IQR 38.6, 65.0). Twenty-two subjects were missing HRQOL parent ratings.
Table 1:
Demographic characteristics of 692 subjects with PedsQL data at three or more visits
| Characteristic | Median [IQR] or n (%) |
|---|---|
| Age, years | 11.2 [7.5, 14.6] |
| Years with CKD | 8.3 [4.3, 12.0] |
| Glomerular diagnosis | 182 (26%) |
| Male sex | 430 (62%) |
| African-American race | 144 (21%) |
| Number of study visits with PedsQL data | 5 [4, 7] |
| Estimated GFR, ml/min|1.73m2 | 51.2 [38.6, 65.0] |
| Annual change in eGFR | −2.7% [−7.2%, +0.0%] |
| Urine Protein/Creatinine | 0.31 [0.11, 0.84] |
| Height Z-score | −0.58 [−1.31, 0.25] |
| Systolic BP Z-score | 0.39 [−0.36, 1.13] |
| Maternal Education | |
| High school or less | 262 (39%) |
| Some college | 190 (28%) |
| College or more | 223 (33%) |
| Anemia | 154 (23%) |
| ACE-inhibitor use | 337 (49%) |
All variables measured at first visit with PedsQL data available except number of study visits.
PedsQL Findings from Child Self-Reported Ratings
Figure 1 displays the CKiD sample means in comparison to historical norms of healthy children and children with CKD Stage 5 [24] in order to provide context for our sample. On all PedsQL scales, the mean of the CKiD participant group based on the child self-report and parent-proxy ratings falls between the means of the comparison groups. PedsQL scores in all domains are generally lower than those seen in unaffected children and higher than other children who have kidney failure.
Figure 1:

Comparison of mean values of CKiD sample with historical norms of healthy children and children with ESRD. Healthy data from Varni et al.[24]
The effect of CKD duration on each HRQOL scale can be seen more fully in Figure 2. Each panel displays child self-report (solid lines) and parent-proxy (dashed lines) HRQOL for a given scale, at CKD durations of 5 years (gray) and 15 years (black), for the hypothetical reference population described above. The 25th percentiles shown in Figure 2 are indicated with diamonds on each distribution curve; these reflect the specific comparisons shown in Tables 2A and 2B. In each child self-report scale, the effect of longer CKD duration can be observed to pull the mass of the distribution toward the upper boundary of 100.
Figure 2:

Estimated distributions of HRQOL by duration of CKD for reference populations from generalized gamma mixed model.
Table 2A:
Significance of covariates from generalized gamma mixed effects models and effects on 25th percentile of HRQOL for selected reference values (child-rated scales) (576 subjects, 2510 observations).
| Estimated 25th percentiles and overall significance of child scales | |||||
|---|---|---|---|---|---|
| Total | Physical | Emotional | Social | School | |
| Duration of CKD 5 vs 15 years | 62.3 vs 72.6 | 68.4 vs 77.8 | 53.2 vs 65.1 | 66.1 vs 80.0 | 50.2 vs 56.9 |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.003 |
| Non-Glomerular vs Glomerular | 67.8 vs 76.8 | 73.4 vs 81.1 | 59.4 vs 67.2 | 73.8 vs 87.8 | 53.6 vs 60.0 |
| P value | <0.0001 | 0.0001 | 0.0008 | <0.0001 | 0.005 |
| Age < 13 vs ≥13 | 67.8 vs 69.5 | 73.4 vs 74.6 | 59.4 vs 65.2 | 73.8 vs 77.8 | 53.6 vs 54.1 |
| P value | 0.06 | 0.28 | <0.0001 | 0.007 | 0.71 |
| Estimated GFR 60 vs 30 ml/min | 67.2 vs 68.8 | 73.5 vs 73.4 | 58.4 vs 61.4 | 72.7 vs 75.8 | 53.5 vs 53.9 |
| P value | 0.07 | 0.91 | 0.01 | 0.04 | 0.78 |
| Urine P/C 0.5 vs 2 | 68.4 vs 67.1 | 74.1 vs 72.8 | 60.0 vs 58.8 | 74.1 vs 73.4 | 54.3 vs 53.0 |
| P value | 0.006 | 0.02 | 0.10 | 0.42 | 0.04 |
| Height Z 0 vs −2 | 67.8 vs 63.7 | 73.4 vs 68.1 | 59.4 vs 54.2 | 73.8 vs 69.2 | 53.6 vs 50.4 |
| P value | 0.0001 | <0.0001 | 0.0003 | 0.01 | 0.01 |
| SBP Z −1 vs +1 | 67.7 vs 67.8 | 73.6 vs 73.3 | 59.7 vs 59.0 | 74.2 vs 73.3 | 53.4 vs 53.9 |
| P value | 0.96 | 0.75 | 0.52 | 0.46 | 0.53 |
| M.E. Some coll vs HS or less | 67.4 vs 67.8 | 72.4 vs 73.4 | 60.3 vs 59.4 | 72.4 vs 73.8 | 53.5 vs 53.6 |
| P value | 0.81 | 0.55 | 0.62 | 0.60 | 0.96 |
| M.E. Coll or more vs HS or less | 73.9 vs 67.8 | 79.8 vs 73.4 | 64.3 vs 59.4 | 80.5 vs 73.8 | 61.9 vs 53.6 |
| P value | <0.0001 | <0.0001 | 0.006 | 0.002 | <0.0001 |
| Anemia no vs yes | 67.8 vs 67.2 | 73.4 vs 73.1 | 59.4 vs 59.2 | 73.8 vs 72.1 | 53.6 vs 53.2 |
| P value | 0.48 | 0.74 | 0.86 | 0.25 | 0.66 |
| ACEi no vs yes | 67.8 vs 67.0 | 73.4 vs 72.6 | 59.4 vs 58.5 | 73.8 vs 73.1 | 53.6 vs 52.1 |
| P value | 0.35 | 0.41 | 0.46 | 0.64 | 0.16 |
Table 2B:
Significance of covariates from generalized gamma mixed effects models and effects on 25th percentile of HRQOL for selected reference values (parent-rated scales) (660 subjects, 3372 observations).
| Estimated 25th percentiles and overall significance of parent proxy scales | |||||
|---|---|---|---|---|---|
| Total | Physical | Emotional | Social | School | |
| Duration of CKD 5 vs 15 years | 69.2 vs 67.3 | 72.1 vs 74.9 | 63.5 vs 67.6 | 74.3 vs 72.5 | 58.0 vs 48.0 |
| P value | 0.24 | 0.19 | 0.02 | 0.49 | <0.0001 |
| Non-Glomerular vs Glomerular | 68.3 vs 69.8 | 73.5 vs 74.6 | 65.6 vs 68.7 | 73.4 vs 78.8 | 53.1 vs 51.4 |
| P value | 0.45 | 0.69 | 0.11 | 0.054 | 0.48 |
| Age < 13 vs ≥13 | 68.3 vs 70.7 | 73.5 vs 72.7 | 65.6 vs 67.2 | 73.4 vs 78.0 | 53.1 vs 56.6 |
| P value | 0.009 | 0.58 | 0.18 | 0.001 | 0.005 |
| Estimated GFR 60 vs 30 ml/min | 68.4 vs 68.0 | 74.1 vs 72.4 | 65.7 vs 65.5 | 73.3 vs 73.7 | 53.2 vs 52.9 |
| P value | 0.70 | 0.21 | 0.85 | 0.77 | 0.82 |
| Urine P/C 0.5 vs 2 | 68.8 vs 67.7 | 74.6 vs 72.4 | 66.0 vs 65.2 | 74.2 vs 72.6 | 53.8 vs 52.4 |
| P value | 0.047 | 0.003 | 0.21 | 0.048 | 0.04 |
| Height Z 0 vs −2 | 68.3 vs 65.8 | 73.5 vs 69.5 | 65.6 vs 64.3 | 73.4 vs 68.2 | 53.1 vs 51.3 |
| P value | 0.03 | 0.01 | 0.28 | 0.004 | 0.17 |
| SBP Z −1 vs +1 | 68.5 vs 68.0 | 73.8 vs 73.3 | 65.6 vs 65.6 | 73.5 vs 73.3 | 53.3 vs 52.9 |
| P value | 0.53 | 0.63 | 0.99 | 0.86 | 0.67 |
| M.E. Some coll vs HS or less | 69.9 vs 68.3 | 77.5 vs 73.5 | 65.6 vs 65.6 | 74.4 vs 73.4 | 54.7 vs 53.1 |
| P value | 0.34 | 0.08 | >0.99 | 0.70 | 0.43 |
| M.E. Coll or more vs HS or less | 77.2 vs 68.3 | 83.9 vs 73.5 | 71.0 vs 65.6 | 82.5 vs 73.4 | 63.9 vs 53.1 |
| P value | <0.0001 | <0.0001 | 0.0008 | <0.0001 | <0.0001 |
| Anemia no vs yes | 68.3 vs 65.5 | 73.5 vs 69.2 | 65.6 vs 63.0 | 73.4 vs 70.1 | 53.1 vs 51.1 |
| P value | 0.002 | 0.002 | 0.02 | 0.02 | 0.07 |
| ACEi no vs yes | 68.3 vs 67.2 | 73.5 vs 72.5 | 65.6 vs 65.6 | 73.4 vs 71.9 | 53.1 vs 51.5 |
| P value | 0.23 | 0.45 | 0.99 | 0.29 | 0.15 |
Figure 3 similarly demonstrates the effect of short stature, comparing normal-height children to children whose height Z-score was 2 standard deviations or more below the mean for age. Approximately 11% of children had a height Z-score this low or lower in the current analysis. The effect of height Z-score is significant across all scales for parents and children (as shown in Tables 2A and 2B) and is most visually apparent in the Total and Physical PedsQL scales, where short stature clearly pulls the mass of the distribution away from 100.
Figure 3:

Estimated distributions of HRQOL by height Z-score level for reference populations from generalized gamma mixed model.
Table 2A summarizes the results of the five mixed effects GG models for child self-report scales. Each cell has both a comparison of the estimated 25th percentile of HRQOL on that scale for two given values of the covariate (generally structured as better vs. worse) holding all other covariates (including random effects) constant at their reference values, and a p-value reflecting the level of significance for the covariate’s effect on the overall distribution (i.e., all percentiles). It is important to understand that while these tables use estimated values for specific covariate levels in order to concretely illustrate the results, the significance and directionality of each effect are not dependent on the reference values.
According to the ratings by the child participants, longer duration of CKD did have a significant positive impact on Total HRQOL (p<.0001). Holding other covariates at the aforementioned reference categories, the 25th percentile of Total PedsQL would be 62.3 if they had 5 years of CKD, but 72.6 if they had 15 years of CKD. In addition, longer duration of disease is associated with significantly higher HRQOL values in the areas of physical (p.<0.0001), emotional (p<0.0001), social (p<0.0001), and school functioning (p=0.003).
With regards to hypothesized covariates of HRQOL, only glomerular disease, higher maternal education (college or more vs any other level) and taller stature were associated with improved HRQOL trajectories. These findings were observed across all of the PedsQL subscales and in the Total HRQOL score. Adolescence was associated with improved Emotional and Social HRQOL
PedsQL Findings from Parent Reports
As discussed above, Figure 2 shows the effect of CKD duration on HRQOL scale with parent-proxy results represented by the dashed lines. Compared to child ratings, the lines for parent ratings often overlap to the point of indistinguishability, although the negative effect of longer CKD duration for the School subscale can be seen.
In the GG models (Table 2B, structured identically to Table 2A), parent-proxy results differed from the children. In a hypothetical population as defined above, for parents, CKD duration did not affect the HRQOL trajectory except within the emotional and school domains, where longer CKD duration was associated with better emotional functioning but worse school functioning. Higher maternal education (college or more vs high school only) was associated with higher HRQOL scores across all of the PedsQL subscales and the Total PedsQL score. Taller stature was associated with higher HRQOL in the physical and social subscales as well as the Total PedsQL score. Adolescence was associated with higher parent-reported Social, School, and Total HRQOL.
Findings from the Trajectory Analysis for Child and Parent Ratings
Table 3 displays the percentage of participants with increasing model-estimated HRQOL trajectories in children with low and normal-range baseline PedsQL scores from the perspective of both the child and the parent. Children who started with low PedsQL scores had a higher percentage of increasing trajectories compared to children whose PedsQL scores were in the normal range at study entry, most noticeably in the physical and school subscales according to child self-report and in the social and school subscales according to parent report. The one exception is the parent-proxy scores on the emotional subscale for which the proportion of increasing trajectories was lower. The smallest percentage of increasing trajectories was found in the parent-proxy results for school functioning.
Table 3:
Percentage of subjects with estimated increasing HRQOL trajectories by baseline PedsQL, depression, and anxiety status.
| CHILD SCALES | |||||
|---|---|---|---|---|---|
| Total | Physical | Emotional | Social | School | |
| Low HRQOL at baseline | 168 (28%) | 157 (26%) | 121 (20%) | 134 (23%) | 241 (41%) |
| % with increasing trajectories | 88% | 85% | 98% | 88% | 81% |
| Normal HRQOL at baseline | 424 (72%) | 436 (74%) | 471 (80%) | 458 (77%) | 349 (59%) |
| % with increasing trajectories | 75% | 69% | 88% | 77% | 66% |
| PARENT SCALES | |||||
| Total | Physical | Emotional | Social | School | |
| Low HRQOL at baseline | 176 (26%) | 145 (21%) | 170 (25%) | 145 (21%) | 233 (35%) |
| % with increasing trajectories | 48% | 82% | 78% | 61% | 20% |
| Normal HRQOL at baseline | 501 (74%) | 533 (79%) | 508 (75%) | 531 (79%) | 437 (65%) |
| % with increasing trajectories | 44% | 79% | 84% | 50% | 9% |
Survivorship bias
The overall observed rate of kidney replacement therapy (KRT) was 32% in this sample. This rate was generally comparable between children with increasing and decreasing trajectories in each model. Where differences were noted, they generally indicated that subjects with increasing trajectories had a somewhat higher rate of KRT, indicating survivorship bias is not resulting in an inflation of positive trajectories in this sample.
Discussion
Physicians and parents share many concerns about children with complex chronic diseases. None is more important than the burden of disease on the maturing child and the degree to which the child’s perception of their quality of life is affected. Findings from this study suggest a relatively optimistic view from the perspective of the children with CKD versus a less confident assessment from their parents. We anticipated that results would illustrate a linear trend over time such that disease progression, or length of time with CKD, might reveal a worsening of both child and parent perceptions of HRQOL. However, this was not the case. Indeed, for the children and adolescents, we found the results to be the opposite of previously published trends in children diagnosed with CKD for a longer period of time [25,26]. Although our findings are unexpected, the demonstrated positive trajectories of child-reported HRQOL from our large cohort of participants are reassuring given that, at a minimum, they do not suggest deterioration of HRQOL over time.
Moreover, we found that children with CKD have HRQOL scores that remain well below the scores of unaffected children for all domains; however, children appear to adapt over time as their HRQOL trajectories were shown to change in a positive direction. Of note, although the magnitude of changes in our models varies depending on the percentile examined, observed effect sizes range from small to medium. Even among children we would consider at highest risk for poor quality of life, including those who endorsed lower self-report ratings of HRQOL at study entry, the majority described positive trajectories longitudinally. This finding was observed despite progression to kidney replacement therapy (KRT) in a substantial percentage of the sample. As others have shown, increased height z-scores are associated with improved child-reported HRQOL in all domains [27]. Additionally, the highest level of maternal education (college graduate or more) was associated with improved child-reported HRQOL in almost all domains which has also been shown previously [4]. Our analysis using GG models confirmed previously published results [27] that there is a significant effect of short stature on total quality of life from the perspectives of both children and parents. CKiD has also recently published data on the impact of anemia on quality of life and found height Z-scores had positive effects on HRQOL[22]. Overall, this finding reinforces the importance of physicians monitoring height closely and considering intervention (such as growth hormone) if clinically indicated. We also found adolescents had higher HRQOL than younger age groups which was seen in previous studies looking at the same CKD pediatric population [22,4].
Despite the relatively positive self-reports from children and adolescents, parents presented more concerns than their children. These findings are consistent with those of Park and colleagues [13] and Marciano and colleagues [28]. The wide diversity observed in the subscale distributions demonstrates why a flexible statistical method such as the GG was necessary for appropriate analysis. Overall, we found that parents report mostly increasing PedsQL trajectories for their children in the social, emotional and physical domains. These findings are consistent with the findings from Johnson and colleagues [29], who found improvement in parent ratings of emotional-behavioral functioning over time. However, there are differences in the total and school domains between parents and children. To what can we ascribe this divergence of experience? There are several possible explanations. Whereas children may have accommodated to their disease and its limitations in terms of their functional expectations, their parents may be unable to overcome the concerns they have about their child’s disease progression and the future challenges of kidney failure. Children might be focusing on the present while adults are worrying more about the future. Parents may not be able to fully appreciate or see the positive changes in their children’s lives and/or their perceptions of CKD. Further, parents could be more inclined to focus on issues of quality of life, particularly with respect to health-related factors as opposed to the myriad of other domains of functioning (e.g., social).
Our findings in no way invalidate the perceptions of the child or the parent. Indeed, since parents and children may view CKD differently over time, it is important for clinicians to recognize the validity of both perspectives to optimally address HRQOL. In addition to interventions that target aspects of HRQOL that either parents or children (or both) perceive as in need, there may be opportunities to highlight ways that children adapt and are resilient even when managing a significant chronic health condition. Identifying characteristics of individuals or situations that facilitate positive adaptation to disease may allow nephrologists, parents, and behavioral health professionals to support continued adaptation for individuals and foster positive supports to others. Marciano et al. [28] also showed the importance of coping strategies on parents’ perspective of their child’s HRQOL, which can be reviewed and strengthened with families. Parents might also need additional psychological, social and economic support to help them deal with a child with CKD, and future studies should examine whether these types of support affect parent ratings of their children. It is important to note, however, that children with CKD in the CKiD study presumably have better management of their disease over time due to being in the study, and this could be a likely factor leading to higher HRQOL than patients not in the study. Additionally, we did not look at changes in HRQOL when patients were transitioned to adult providers and do not know if the same trends would continue.
To the best of our knowledge, our study is the second published analysis that describes HRQOL longitudinally for children with mild to moderate CKD. Two study advantages are that the sample size is large (692 participants) and we include repeated HRQOL assessments of both parent proxy and self-report ratings. We also only had a small percentage of missing data (<5%). The study does have several limitations. First, even though the missing data was low, when we compared the patients that were missing assessments to the ones who completed surveys, we found that the patients with missing data were more likely to be older and have higher proteinuria. While this might suggest some mild potential bias in our sample, the overall strength of the findings likely would not change in a significant fashion given the small percentage of missing data. Additionally, due to the complexity of the modeling approach, we could not report coefficients in a traditional sense. Rather, we illustrated our findings by looking at differences in a hypothetical reference population controlling for all other variables, which may not capture the heterogeneity of effect magnitudes in real-world scenarios where subjects’ profiles differ in many characteristics simultaneously. Next, we chose years with CKD as the time scale in our model. Future studies could look at participant age or years in study as possible other time variants to explore. Also, we did not analyze age of onset since for over 70% of patients (those with non-glomerular disease) the age of onset was birth. In addition, we did not have HRQOL assessments after kidney replacement therapy, baseline data describing the parents’ characteristics, and any metric of parent emotional health; nonetheless, it is possible that status of parent emotional health may have influenced their ratings, and this should be explored in future studies. Finally, we only included maternal education as a marker for SES; additional studies could examine a more robust profile of SES since it is a powerful determinant of HRQOL.
Conclusion
In summary, our longitudinal analysis of CKiD PedsQL ratings among parents and children studied over 16 years indicates that children’s self-report trajectories support a more positive outlook when compared to previously published data. In contrast, parents appear to have a more pessimistic view of their children’s HRQOL over time. Clinicians should use this knowledge to support both groups and help achieve the best possible HRQOL for children with CKD and their families. This divergence of findings should guide clinicians to examine both child and parent HRQOL in determining the best treatment plan for a specific child or adolescent.
Funding source:
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri - Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD and Derek Ng, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U24-DK-082194, U24-DK-66116). The CKiD website is located at https://statepi.jhsph.edu/ckid.
Footnotes
Statements and declarations: None
Data availability:
Data from this manuscript will not be publicly released.
References
- 1.Gerson AC, Butler R, Moxey-Mims M, Wentz A, Shinnar S, Lande MB, Mendley SR, Warady BA, Furth SL, Hooper SR (2006) Neurocognitive outcomes in children with chronic kidney disease: Current findings and contemporary endeavors. Ment Retard Dev Disabil Res Rev 12 (3):208–215. doi: 10.1002/mrdd.20116 [DOI] [PubMed] [Google Scholar]
- 2.Rodig NM, McDermott KC, Schneider MF, Hotchkiss HM, Yadin O, Seikaly MG, Furth SL, Warady BA (2014) Growth in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children Study. Pediatr Nephrol 29 (10):1987–1995. doi: 10.1007/s00467-014-2812-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady TM, Roem J, Cox C, Schneider MF, Wilson AC, Furth SL, Warady BA, Mitsnefes M (2020) Adiposity, Sex, and Cardiovascular Disease Risk in Children With CKD: A Longitudinal Study of Youth Enrolled in the Chronic Kidney Disease in Children (CKiD) Study. Am J Kidney Dis 76 (2):166–173. doi: 10.1053/j.ajkd.2020.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerson AC, Wentz A, Abraham AG, Mendley SR, Hooper SR, Butler RW, Gipson DS, Lande MB, Shinnar S, Moxey-Mims MM, Warady BA, Furth SL (2010) Health-related quality of life of children with mild to moderate chronic kidney disease. Pediatrics 125 (2):e349–357. doi: 10.1542/peds.2009-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreira JM, Bouissou Morais Soares CM, Teixeira AL, Simoes ESAC, Kummer AM (2015) Anxiety, depression, resilience and quality of life in children and adolescents with pre-dialysis chronic kidney disease. Pediatr Nephrol 30 (12):2153–2162. doi: 10.1007/s00467-015-3159-6 [DOI] [PubMed] [Google Scholar]
- 6.Tong A, Wong G, McTaggart S, Henning P, Mackie F, Carroll RP, Howard K, Craig JC (2013) Quality of life of young adults and adolescents with chronic kidney disease. J Pediatr 163 (4):1179–1185 e1175. doi: 10.1016/j.jpeds.2013.04.066 [DOI] [PubMed] [Google Scholar]
- 7.Francis A, Didsbury MS, van Zwieten A, Chen K, James LJ, Kim S, Howard K, Williams G, Bahat Treidel O, McTaggart S, Walker A, Mackie F, Kara T, Nassar N, Teixeira-Pinto A, Tong A, Johnson D, Craig JC, Wong G (2019) Quality of life of children and adolescents with chronic kidney disease: a cross-sectional study. Arch Dis Child 104 (2):134–140. doi: 10.1136/archdischild-2018-314934 [DOI] [PubMed] [Google Scholar]
- 8.Baek HS, Kang HG, Choi HJ, Cheong HI, Ha IS, Han KH, Kim SH, Cho HY, Shin JI, Park YS, Lee JH, Lee J, Ahn C, Cho MH (2017) Health-related quality of life of children with pre-dialysis chronic kidney disease. Pediatr Nephrol 32 (11):2097–2105. doi: 10.1007/s00467-017-3721-5 [DOI] [PubMed] [Google Scholar]
- 9.Alhamed AA, Toly VB, Hooper SR, Dell KM (2022) The link between executive function, socio-emotional functioning and health-related quality of life in children and adolescents with mild to moderate chronic kidney disease. Child Care Health Dev 48 (3):455–464. doi: 10.1111/cch.12946 [DOI] [PubMed] [Google Scholar]
- 10.Neul SK, Minard CG, Currier H, Goldstein SL (2013) Health-related quality of life functioning over a 2-year period in children with end-stage renal disease. Pediatr Nephrol 28 (2):285–293. doi: 10.1007/s00467-012-2313-7 [DOI] [PubMed] [Google Scholar]
- 11.Selewski DT, Troost JP, Massengill SF, Gbadegesin RA, Greenbaum LA, Shatat IF, Cai Y, Kapur G, Hebert D, Somers MJ, Trachtman H, Pais P, Seifert ME, Goebel J, Sethna CB, Mahan JD, Gross HE, Herreshoff E, Liu Y, Song PX, Reeve BB, DeWalt DA, Gipson DS (2015) The impact of disease duration on quality of life in children with nephrotic syndrome: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol 30 (9):1467–1476. doi: 10.1007/s00467-015-3074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim HJ, Koo TY, Lee J, Huh KH, Park JB, Cho J, Lee S, Ro H, Han S, Park B, Park S, Chung W, Park SK, Kim C, Kim SJ, Kim YS, Ahn C, Yang J, Group K-KS (2016) Health-Related Quality of Life of Kidney Transplantation Patients: Results from the KoreaN Cohort Study for Outcome in Patients With Kidney Transplantation (KNOW-KT) Study. Transplant Proc 48 (3):844–847. doi: 10.1016/j.transproceed.2015.12.101 [DOI] [PubMed] [Google Scholar]
- 13.Park KS, Hwang YJ, Cho MH, Ko CW, Ha IS, Kang HG, Cheong HI, Park YS, Lee YJ, Lee JH, Cho HY (2012) Quality of life in children with end-stage renal disease based on a PedsQL ESRD module. Pediatr Nephrol 27 (12):2293–2300. doi: 10.1007/s00467-012-2262-1 [DOI] [PubMed] [Google Scholar]
- 14.Tjaden LA, Vogelzang J, Jager KJ, van Stralen KJ, Maurice-Stam H, Grootenhuis MA, Groothoff JW (2014) Long-term quality of life and social outcome of childhood end-stage renal disease. J Pediatr 165 (2):336–342 e331. doi: 10.1016/j.jpeds.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 15.Guha C, van Zwieten A, Khalid R, Kim S, Walker A, Francis A, Didsbury M, Teixeira-Pinto A, Barton B, Prestidge C, Lancsar E, Mackie F, Kwon J, Howard K, Mallitt KA, Howell M, Jaure A, Hayes A, Raghunandan R, Petrou S, Lah S, McTaggart S, Craig JC, Wong G (2023) Longitudinal assessment of the health-related quality of life of children and adolescents with chronic kidney disease. Kidney Int 103 (2):357–364. doi: 10.1016/j.kint.2022.09.026 [DOI] [PubMed] [Google Scholar]
- 16.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA (2006) Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1 (5):1006–1015. doi: 10.2215/CJN.01941205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng DK, Pierce CB (2021) Kidney Disease Progression in Children and Young Adults With Pediatric CKD: Epidemiologic Perspectives and Clinical Applications. Semin Nephrol 41 (5):405–415. doi: 10.1016/j.semnephrol.2021.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varni JW, Limbers CA, Burwinkle TM (2007) How young can children reliably and validly self-report their health-related quality of life?: an analysis of 8,591 children across age subgroups with the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 5:1. doi: 10.1186/1477-7525-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varni JW, Seid M, Kurtin PS (2001) PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care 39 (8):800–812. doi: 10.1097/00005650-200108000-00006 [DOI] [PubMed] [Google Scholar]
- 20.Varni JW, Limbers CA (2009) The pediatric quality of life inventory: measuring pediatric health-related quality of life from the perspective of children and their parents. Pediatr Clin North Am 56 (4):843–863. doi: 10.1016/j.pcl.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 21.Cox C, Chu H, Schneider MF, Munoz A (2007) Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med 26 (23):4352–4374. doi: 10.1002/sim.2836 [DOI] [PubMed] [Google Scholar]
- 22.Carlson J, Gerson AC, Matheson MB, Manne S, Warady BA, Hooper SR, Lande M, Harshman LA, Johnson RJ, Shinnar S, Kogon AJ, Furth S (2020) A longitudinal analysis of the effect of anemia on health-related quality of life in children with mild-to-moderate chronic kidney disease. Pediatr Nephrol 35 (9):1659–1667. doi: 10.1007/s00467-020-04569-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varni JW, Burwinkle TM, Seid M, Skarr D (2003) The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 3 (6):329–341. doi: [DOI] [PubMed] [Google Scholar]
- 24.Varni JW, Limbers CA, Burwinkle TM (2007) Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 5:43. doi: 10.1186/1477-7525-5-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein SL, Graham N, Warady BA, Seikaly M, McDonald R, Burwinkle TM, Limbers CA, Varni JW (2008) Measuring health-related quality of life in children with ESRD: performance of the generic and ESRD-specific instrument of the Pediatric Quality of Life Inventory (PedsQL). Am J Kidney Dis 51 (2):285–297. doi: 10.1053/j.ajkd.2007.09.021 [DOI] [PubMed] [Google Scholar]
- 26.Kogon AJ, Vander Stoep A, Weiss NS, Smith J, Flynn JT, McCauley E (2013) Depression and its associated factors in pediatric chronic kidney disease. Pediatr Nephrol 28 (9):1855–1861. doi: 10.1007/s00467-013-2497-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Uzri A, Matheson M, Gipson DS, Mendley SR, Hooper SR, Yadin O, Rozansky DJ, Moxey-Mims M, Furth SL, Warady BA, Gerson AC, Chronic Kidney Disease in Children Study G (2013) The impact of short stature on health-related quality of life in children with chronic kidney disease. J Pediatr 163 (3):736–741 e731. doi: 10.1016/j.jpeds.2013.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marciano RC, Soares CM, Diniz JS, Lima EM, Silva JM, Canhestro MR, Gazzinelli A, Melo CC, Dias CS, Simoes e Silva AC, Correa H, Oliveira EA (2011) Behavioral disorders and low quality of life in children and adolescents with chronic kidney disease. Pediatr Nephrol 26 (2):281–290. doi: 10.1007/s00467-010-1683-y [DOI] [PubMed] [Google Scholar]
- 29.Johnson RJ, Gerson AC, Harshman LA, Matheson MB, Shinnar S, Lande MB, Kogon A, Gipson DS, Warady BA, Furth SL, Hooper SR (2020) A longitudinal examination of parent-reported emotional-behavioral functioning of children with mild to moderate chronic kidney disease. Pediatr Nephrol. doi: 10.1007/s00467-020-04511-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this manuscript will not be publicly released.


