Abstract
Objective:
Pseudomonas aeruginosa is an opportunistic pathogen that can establish chronic infections and form biofilm in wounds. Because the wound environment is largely devoid of oxygen, P. aeruginosa may rely on anaerobic metabolism, such as nitrate respiration, to survive in wounds. While nitrate reductase (Nar) typically reduces nitrate to nitrite, it can also reduce chlorate to chlorite, which is a toxic oxidizing agent. Therefore, chlorate can act as a prodrug to specifically eradicate hypoxic/anoxic, nitrate-respiring P. aeruginosa populations, which are often tolerant to conventional antibiotic treatments.
Approach:
Using a diabetic mouse model for chronic wounds, we tested the role that anaerobic nitrate respiration plays in supporting chronic P. aeruginosa infections.
Results:
P. aeruginosa forms biofilm deep within the wound where the environment is anoxic. Daily treatment of P. aeruginosa-infected wounds with chlorate supported wound healing. Chlorate treatment was as effective as a treatment with ciprofloxacin (a conventional antibiotic that targets both oxic and hypoxic/anoxic P. aeruginosa populations). Chlorate-treated wounds showed markers of good-quality wound healing, including well-formed granulation tissue, reepithelialization and microvessel development. Loss- and gain-of-function experiments showed that P. aeruginosa requires nitrate respiration to establish a chronic wound infection and form biofilms.
Innovation:
We show that the small molecule chlorate, kills the opportunistic pathogen, P. aeruginosa, by targeting a form of anaerobic metabolism called nitrate respiration.
Conclusion:
Chlorate holds promise as a treatment to combat diverse bacterial infections where oxygen is limiting and/or where pathogens grow as biofilms because many other pathogens possess Nar and survive using anaerobic metabolism.
Keywords: Pseudomonas aeruginosa, chronic wounds, chlorate, antibiotics, anaerobic respiration, MiPACT-HCR

Manuela Martins-Green, PhD

Dianne K. Newman, PhD
INTRODUCTION
Most chronic wounds occur in patients with an underlying pathology or systemic disease, such as diabetes. The critical need for an effective treatment for diabetic chronic wounds is underlined by the continuous increase in type II diabetes, which accounts for 90–95% of all diabetic population.1,2 It has been reported that diabetes affects 387M people globally with 28M in the United States alone, and prediabetes affects 316M more globally and 86M in the United States.1 These statistics are daunting, considering that a significant number of these patients will develop lower limb ulcers. Current therapeutic approaches are frequently unable to achieve wound closure, leading to limb amputations in 12% of these individuals, who have a 5-year mortality rate of ∼50%.3
Diabetic wounds frequently become infected with microbes that form biofilms. Importantly, these infections and biofilms must be resolved before proper wound healing can proceed.4–7 Antibiotic treatments often fail to resolve wound infections.8–11 We posit that the limitation of oxygen in the wound environment is a key driver of treatment failure because many antibiotics are less toxic to bacteria under hypoxic/anoxic conditions.12–14
Bacteria experience oxygen deprivation in the wound in two ways. First, the wound environment is largely devoid of oxygen, likely due to the high metabolic activity of immune cells15 and the lack of arterial circulation.16 Direct measurements show that wounds are anoxic at 1–2 mm below the wound surface.17 Second, bacteria in biofilms within diabetic wounds generate oxygen gradients when bacterial cells in the biofilm periphery consume oxygen faster than oxygen diffuses to the interior, such that biofilms harbor antibiotic-susceptible peripheral populations and also oxygen-starved interior populations that survive antibiotic treatment.18,19 This is one of the reasons biofilms are notoriously recalcitrant to conventional antibiotic therapies.20
Biofilm formation is also considered a critical tipping point that pushes wounds into stalled healing states.21 Thus, there is an urgent need to identify therapeutics that effectively kill pathogens under oxygen-limited conditions when many conventional antibiotics are less effective.
Pseudomonas aeruginosa is an opportunistic bacterial pathogen that frequently infects and forms biofilms in human chronic wounds.22 As expected, P. aeruginosa biofilms are highly tolerant to conventional antibiotics,23–25 and studies show that attempts to eliminate P. aeruginosa biofilms through debridement usually result in biofilm resurgence within a few days of the procedure.26–29 Given that the bacteria will encounter a range of oxygen tensions within the wound environment, P. aeruginosa is predicted to employ a range of metabolic strategies. P. aeruginosa grows through aerobic respiration in the presence of oxygen but can also grow through anaerobic nitrate respiration when oxygen tensions are low.30
In nitrate respiration, nitrate is reduced to nitrite by nitrate reductase (Nar). Although P. aeruginosa encodes three distinct Nars, Nar is the only energy-conserving enzyme that directly couples nitrate reduction to proton translocation.31 Substantial nitrate concentrations (∼100–500 μM) are often found in environments with chronic inflammation, including chronic wounds, because it is generated as a byproduct of the inflammatory response.32–34 P. aeruginosa nar transcripts have been detected in human chronic wound samples.35
There is also evidence that Nar supports chronic P. aeruginosa infections within the airways of people with cystic fibrosis. P. aeruginosa nar transcripts,36,37 Nar antibodies,38 and nitrate respiration-derived metabolites39,40 have been detected in the sputa, sera, and breath of people with cystic fibrosis, respectively. Previously, we proposed nitrate respiration as a viable drug target for treating chronic infections given that it is conserved across many bacterial pathogens and predicted to contribute to survival in hypoxic/anoxic chronic infection contexts.19
It has been known for decades that, in addition to reducing nitrate to nitrite, Nar can also reduce chlorate to chlorite.41,42 While chlorate is a relatively nontoxic compound, chlorite is a toxic oxidizing agent.43–47 Recently, we used an in vitro biofilm system to demonstrate that chlorate treatment kills hypoxic/anoxic, antibiotic-tolerant P. aeruginosa populations by hijacking Nar activity to reduce nontoxic chlorate to toxic chlorite.19 Extensive research has shown that chlorate can be ingested by livestock at dosages that are sufficient to kill gut bacteria, while resulting in no health consequences for the animal (reviewed in48).
In this study, we use our diabetic mouse chronic wound model to test the role that nitrate respiration plays in supporting infections by P. aeruginosa that lead to biofilm formation as well as to assess the potential for chlorate to eradicate such infections. We chose to compare the efficacy of chlorate treatment to ciprofloxacin treatment. Unlike other antibiotics (e.g., tobramycin19), ciprofloxacin kills P. aeruginosa under both oxic and anoxic conditions, making ciprofloxacin a gold-standard therapeutic benchmark against which to evaluate chlorate's efficacy.
We demonstrate that using chlorate to target anoxic P. aeruginosa populations is as effective as ciprofloxacin at supporting chronic wound healing. This finding motivates future studies to test the potential of chlorate to accelerate chronic wound healing in humans.
CLINICAL PROBLEM ADDRESSED
Diabetic chronic wounds are frequently infected with a diverse community of microbes that form biofilms, hindering proper wound healing processes. Antibiotics are used to control bacterial infections; however, these treatments often fail to resolve such infections and these wounds persist.
A deeper understanding of how key microbes survive and colonize chronic wounds is crucial for developing more effective treatment strategies. Most antibiotics are effective when bacteria are in their planktonic forms, that is, when they are free floating and able to grow rapidly in the presence of abundant resources, including oxygen. In contrast, slow-growing bacteria, such as those found in oxygen-limited environments and biofilms, are highly tolerant to many antibiotics. Furthermore, extracellular polymeric substances that form the biofilm matrix can limit diffusion of drugs, exposing bacteria in the biofilm to subeffective concentrations. Bacteria that reside within the interiors of biofilms are typically limited for oxygen and therefore rely on anaerobic metabolisms for survival.
Studying the efficacy of small, nontoxic anions that target conserved anaerobic bacterial metabolisms may help resolve biofilm infections and thereby promote wound healing.
MATERIALS AND METHODS
Bacterial strains and mutant strain construction
All experiments used Luria-Bertani (LB; Difco) as the growth medium, supplemented with KNO3 as specified. Aerobic liquid cultures were incubated at 37°C with shaking at 250 RPM. Anaerobic work was conducted in an anaerobic glove box with a 95% N2–5% H2 atmosphere, and anaerobic cultures were incubated at 37°C.
For our studies, we used a P. aeruginosa strain referred to as RPA (Riverside PA) that was previously isolated from our chronic wound mouse model. Additionally, for these studies we constructed an RPA strain lacking genes for the Nar enzyme (ΔnarGHJI, hereby referred to as Δnar) using methods described previously,19 and mutants were confirmed through PCR fragment size and their inability to grow anaerobically with nitrate.
We also constructed a complement strain referred to as RPA Δnar+att::mTnnar by genomically inserting the nar operon into the att site, a neutral cloning location in the RPA genome, through miniTn7 integration.49 To construct the complement strain, narK1K2GHJI genes and promoter were amplified from RPA genomic DNA using the following forward primer: 5'-aagctaattcgatcatgcatgagctgtgaaaagccttttaaaacagtg-3'; and reverse primer: 5'-ggcctgcaaggccttcgcgaggtactcaggcaggacgtttctg-3'. The amplicon was ligated into restriction-digested (KpnI and SacI) plasmid pJM220 by Gibson assembly and transformed into electrocompetent Escherichia coli (EC) DH5a to generate pJM220_nar.
Plasmids were isolated through mini-prep, sequence verified, and transformed into EC SM10 λpir. Conjugation of pJM220_nar into RPA Δnar was achieved through triparental mating by mixing equal volumes of overnight cultures of RPA Δnar, EC SM10 λpir containing pJM220_nar, and EC SM10 λpir containing pTNS1, and incubating a 100 μL concentrated spot on LB agar at 37°C overnight.50 The following day, RPA conjugates were selected by streaking for single colonies on LB agar containing 20 μg/mL gentamicin and 10 μg/mL chloramphenicol. Complementation was verified by screening colonies for restored anaerobic growth with nitrate.
In vitro drug treatment assays
For in vitro planktonic drug treatment studies, overnight RPA cultures were grown aerobically in LB broth supplemented with 40 mM KNO3. Overnight cultures were pelleted, pellets were washed once with LB, and then resuspended to OD500 = 2 in either LB, LB with 10 μg/mL ciprofloxacin, LB with 10 mM sodium chlorate, or LB with 10 μg/mL ciprofloxacin and 10 mM sodium chlorate. Cell resuspensions were added to 96-well plates (150 μL per well), and one 96-well plate was incubated statically (not shaking) at 37°C at ambient oxygen, while a duplicate 96-well plate was incubated statically at 37°C under anoxia (anaerobic glove box).
Drug treatments were incubated for 24 h, after which cultures were serially diluted for viable cell counts. Viable cell counts were performed by serially diluting samples in phosphate-buffered saline (PBS). Dilutions spanning 7 orders of magnitude were plated on LB agar plates as 10 μL drips. After incubation at 37°C, colonies were counted to quantify colony-forming units (CFU) per milliliter for each culture.
Percent survival was calculated for each condition by dividing CFU/mL values from a drug-treated sample by the average CFU/mL value from control (no drug) samples and multiplying by 100. All viable cell counts were carried out under oxic conditions.
For in vitro biofilm drug studies, we used the agar block biofilm assay (ABBA).19 Briefly, to set up ABBA, overnight RPA cultures were diluted to OD500 = 0.001 into cooled molten agar (44°C) composed of LB with 5mM KNO3 and 1% agar. Before the agar solidified, 500 μL of the cell–agar suspension was added to 2-mL Eppendorf tubes, after which samples were allowed to solidify.
Eppendorf tubes were incubated at 37°C for 24 h to allow cells to grow as aggregate biofilms suspended within the agar medium. After this growth incubation, ABBA samples were washed twice with 200 μL of PBS to remove cell growth that was not suspended within the agar. ABBA samples were then treated with 100 μL of PBS (control), 100 μL of 60 μg/mL ciprofloxacin (final concentration = 10 μg/mL), 100 μL of 60 mM sodium chlorate (final concentration = 10 mM), or 100 μL of a 60 μg/mL ciprofloxacin plus 60 mM sodium chlorate solution (final concentrations = 10 μg/mL ciprofloxacin and 10 mM sodium chlorate).
Drug treatments were incubated for 24 h at 37°C. To determine viable cell counts, ABBA samples were first homogenized by adding 300 μL of PBS to each sample and homogenizing on speed 3 with a Bio-Gen PRO200 Homogenizer (PRO Scientific). Viable cell counts and percent survival were calculated for ABBA samples as described above.
Biofilm visualization through MiPACT-HCR
Mice were sacrificed on day 10 and wound tissue was collected and fixed by placing directly into 4% paraformaldehyde (PFA) buffered in PBS at pH 7.4. Wound tissue was embedded in 1% agarose and agarose-embedded wound samples were incubated at 4° C in a 4% PFA solution for 2 days and then transferred to PBS-only for 5 days.
Wound samples were then sectioned into 1.5 mm slices using a Leica VT 1000 S Vibratome. Postslicing, samples were processed for MiPACT-HCR by adapting a published protocol.51 In brief, samples were further stabilized and fixed by placing them in 4% bisacrylamide (29:1 bisacrylamide/acrylamide) with 1% PFA and VA-044 for 1 day before clearing with 8% SDS at pH 8.0, followed by treatment with 2mg/ml Proteinase K (Sigma-Aldrich) in 10 mM Tris (pH 7.5) for 3 h at 37°C shaking to clarify the epidermis. Samples took about 10 days to fully clear. Cleared samples were hybridized with 30 nM each of even and odd DNA probes for P. aeruginosa 16S rRNA containing the B3 initiator sequence51–53 at 46°C with shaking for 1 day, followed by a wash in 84 mM NaCl for 3 h at 51°C.
Amplification was performed with 120 nM of hairpin conjugated with fluorophore pairs in amplification buffer for 1 day with shaking at RT.51 Samples were then washed in 337.5 mM NaCl wash buffer for 1 h at 42°C before staining with DAPI (10 ug/mL, shaking, RT for 24 h) and then stored in RIMS at 4°C before imaging. P. aeruginosa 16S rRNA was labeled with the B3 initiator/hairpin system, with B3 hairpins conjugated to fluorophore AlexaFluor 549.51–54 Hairpins conjugated to fluorophores were purchased from Molecular Instruments. Imaging was performed on a Zeiss LSM 800 confocal microscope and image reconstructions were made in the FIJI distribution of ImageJ.55
Chronic wound model
All experiments were completed in accordance and compliance with federal regulations and the University of California policy and procedures approved by the University of California, Riverside Institutional Animal Care and Use Committee (IACUC).
The description of how to obtain chronic wounds in db/db−/− mice have been published in detail by us previously.56 Briefly, db/db−/− mice are bred in our conventional vivarium from B6.BKS(D)-Leprdb/J heterozygotes obtained from the Jackson Laboratories (Stock no. 000697). Only db/db−/− that are 5 months old are used to create chronic wounds because at this age they are fully diabetic and obese. The mice must weigh at least 50 g to be able to withstand the burden of the wound. The back of the mouse was shaved, naired, and then allowed to recover from the irritation of the Nair for 24 h. The hairless skin of the mouse was wiped and disinfected with iodine and 70% ethanol immediately before surgery to remove the natural skin microbiota.57
As soon as the wounds were made, they were sealed with sterile Tegaderm, which provides a barrier to external contaminants and environmental bacteria from the cage and bedding.
To create chronic wounds, a 7 mm full-thickness skin excision wound was made under isoflurane anesthesia and the mouse was treated with specific inhibitors for catalase and glutathione peroxidase, 3-amino-1, 2, 4-triazole (ATZ) (TCI Chemicals, Tokyo, Japan), and mercaptosuccinic acid (MSA) (Sigma-Aldrich, St. Louis, MO), respectively. ATZ was injected intraperitoneally at 0.75 g ATZ/kg of mouse weight in sterile PBS ∼20 min before surgery. MSA was administered topically onto the wound site under the Tegaderm at 150 mg MSA/kg of mouse weight in sterile PBS within 10 min after surgery. All mice were treated with buprenorphine, a pain reliever, injected intraperitoneally at 0.05 mg buprenorphine/kg of mouse weight in sterile PBS before surgery and 6 h after surgery.
Wound area and healing
The area of each wound was traced and measured over time with ImageJ.55 Briefly, the scale of the wound was set using the straight segment tool along a ruler. Using the freehand selection, the outline of the open wound was traced. The outline was analyzed to obtain the area. Wound healing was evaluated by examining parameters related to granulation tissue formation such as collagen types and deposition, reepithelialization, and the number of microvessels in the granulation tissue.
Wound drug treatments
Wounds were infected with 104 or 106 CFU, as specified in the figure legends, of either RPA, RPA Δnar, or RPA Δnar+att::mTnnar at 24 h postwounding. Daily wound treatments began 10 days after surgery with one of the following treatments: 100 μL of 200 μg/mL ciprofloxacin (i.e., 20 μg total/wound), 10 mM sodium chlorate, or 200 μg/mL ciprofloxacin plus 10 mM sodium chlorate. Treatment was injected under the Tegaderm into the wound bed with an insulin syringe. All drugs were dissolved in filter-sterilized water. Control wounds were treated with 100 μL of sterile water. The Tegaderm was changed when sealing to the skin began to be breached.
Antibodies
The following primary antibodies were used: anti-collagen IV (col IV) ab6586 and anti-alpha smooth muscle actin antibody [1A4] (ab7817) from Abcam (Cambridge, United Kingdom) and anti-keratin 14/16 AF0736 antibody from Affinity Bioscience (Cincinnati, OH). The following secondary antibodies were used: goat anti-rabbit antibody conjugated with AlexaFluor 594 A11012, and goat anti-rat antibody conjugated with Fluorescein A10527 from Invitrogen (Carlsbad, CA).
Histological staining
Wound tissues were collected and fixed in 4% PFA in PBS for 4–6 h at room temperature. The tissues were washed three times in PBS for 15 min to remove excess PFA. Noncrosslinked PFA was quenched by incubation in 0.1 M glycine in PBS for 30 min. The tissues were washed with PBS and then incubated first in 15% sucrose in PBS for 4–6 h at room temperature, and then 30% sucrose in PBS overnight at 4°C.
The tissues were then embedded in optimal cutting temperature compound (OCT), sectioned (8–10 μm thick) in a cryostat and stained with Hematoxylin & Eosin (H&E) and Masson's Trichrome (MT) as we have previously described.57,58 Staining with Picrosirius Red (PSR) (Polysciences, Inc.) was performed as previously described.59 Briefly, the sectioned tissue was fixed in 4% PFA for 10 min and then rinsed with DI water before staining with PSR (Solution B) for 90 min, rinsed two times with 0.1 N HCl at pH 4.0 (Solution C) for 1 min before rinsing again in DI water. The sections were dehydrated in 70% ethanol for 30 s and allowed to dry before mounting in Krystalon. Stained sections were visualized using a Nikon Microphot-FXA microscope (Nikon Instruments, Inc., Melville, NY) and photographed between cross-polarizers.
Immunofluorescent labeling
The OCT was removed by incubating the section with PBS for 5 min. The sections were fixed in 4% PFA in PBS for 20 min. After washing with PBS, excess PFA was quenched with 0.1 M glycine in PBS for 20 min. Tissue sections were incubated in 0.3% Triton X-100 for 30 min when probing for intracellular proteins. Sections were blocked with Power Block for 4 min and then incubated with the primary antibodies in 1% BSA/PBS for 4 h at room temperature, washed three times with 0.1% BSA in PBS, and then incubated with secondary antibodies for 1 h at room temperature. After washing with PBS, slides were mounted in VECTASHIELD containing DAPI (4′,6-diamidino-2-phenylindole) (Vector Laboratories, Newark, CA). Immunofluorescence was visualized and imaged using a Nikon Microphot-FXA fluorescence microscope with a Nikon DS-Fi1 digital camera and NIS-Elements software (Nikon Instruments, Inc., Melville, NY).
Statistics
One-way analysis of variance (ANOVA) was used to calculate the significance of the number of blood vessels between the different treatments. The number of blood vessels were counted in five frames (area = 0.02 mm2) of the granulation tissue. The calculation of the significance was followed by the Dunnett test to determine significant differences between treatment groups to untreated control. The Student's t-test was also used to calculate the significance between different treatments of the wound area. An electronic laboratory notebook was not used.
RESULTS
RPA requires Nar for chlorate sensitivity
Chlorate is activated by and specifically kills Nar-containing bacterial cells (Fig. 1A), while bacterial cells lacking Nar, are spared. The P. aeruginosa UCBPP-PA14 strain is highly aggressive and causes severe biofilms in our mouse model, which frequently results in the death of the animal (data not shown). Thus, for these studies we used a P. aeruginosa strain isolated directly from biofilms of our chronic wound mouse model, referred to as RPA (Riverside P. aeruginosa).
Figure 1.
RPA requires Nar for chlorate susceptibility. (A) Nar is a hypoxically induced enzyme that reduces chlorate to toxic chlorite. (B) Survival of WT and Δnar RPA after treatment with ciprofloxacin, chlorate, or both drugs under oxic and anoxic planktonic conditions and grown as biofilms. For each treatment, n ≥ 3 replicates and bars show mean ± standard error of the mean. RPA, Riverside Pseudomonas aeruginosa; Nar, nitrate respiration.
First, we tested whether RPA responds to chlorate in vitro in similar ways as P. aeruginosa UCBPP-PA14.19,60 Alongside chlorate, we also tested RPA susceptibility to the antibiotic, ciprofloxacin, which serves as a therapeutic gold standard/control treatment because it is capable of killing bacterial cells under both oxic and anoxic conditions. As expected, we found that oxic planktonic cultures of RPA are highly susceptible to ciprofloxacin and highly tolerant to chlorate, whereas anoxic planktonic cultures of RPA are highly susceptible to both chlorate and ciprofloxacin (Fig. 1B).
Our results also show that the combined ciprofloxacin and chlorate treatment is more effective in killing planktonic cultures than either drug alone; cell survival is below our detection limit (> 6-log killing) for combined treatment under both oxic and anoxic conditions.
We performed similar experiments growing RPA as aggregate biofilms (through the agar block biofilm assay, ABBA) and found that RPA biofilms are susceptible to ciprofloxacin but show greater survival than planktonic cells. Combined ciprofloxacin and chlorate treatment also resulted in more killing of bacterial cells in biofilms than either drug alone, although combined treatment did not reduce survival to below our detection limit. Finally, we constructed an RPA Δnar strain to test whether chlorate sensitivity is dependent on Nar. As expected, RPA Δnar cultures are highly resistant to chlorate treatment, and the combined ciprofloxacin and chlorate treatment resembles ciprofloxacin-only treatment in the RPA Δnar strain (Fig. 1B). Based on these findings, we conclude that chlorate toxicity requires the hypoxically induced Nar enzyme in RPA, as previously shown for UCBPP-PA14.19
Treatment of RPA biofilms in db/db−/− chronic wounds with chlorate, ciprofloxacin, or both, leads to healing of chronic wounds
To test whether our in vitro results would extend to the in vivo context, we used our diabetic mouse model of chronic wounds that mimics chronic wounds in humans.57,61 First, we confirmed that mouse wounds contained RPA biofilms at day 10 postwounding using the tissue clearing and microbial visualization method, MiPACT-HCR51 (Fig. 2A). Fluorescence microscopy revealed confluent biofilm growth to thicknesses >50 μm at the surface of the wound. Given that the rate of oxygen consumption by P. aeruginosa outpaces its diffusion, it is well established that 10–20 μm below the surface of such densely packed biofilms, interior cells experience steep oxygen gradients.39,62 We also observed RPA growth deep within the wound tissue (∼1 mm), where direct measurements have shown the environment is hypoxic/anoxic.17
Figure 2.
Ciprofloxacin and chlorate treatment support healing of RPA-infected wounds. Wounds were infected with RPA (106 CFU) 24 h after injury and daily treatment began 10 days after infection. Wounds were treated daily with either vehicle (n = 5), ciprofloxacin (cip, n = 7), chlorate (chlor, n = 8), or both ciprofloxacin and chlorate (cip/chlor, n = 9). (A) Visualization of RPA biofilms using MiPACT-HCR on a section of chronic wound tissue collected at 10 days postwounding and before treatment (wound surface is on the top). Yellow fluorescence shows RPA, as detected by 16S rRNA amplification, and DAPI (blue) was used to visualize the nuclei of the cells in the mouse tissue. (B) Representative images of untreated and treated RPA-infected wounds over 40 days. Untreated RPA-infected wounds did not undergo wound closure and have robust biofilm formation at day 40, whereas treated wounds had decreased amount of biofilm and were smaller in size. (C) Quantification of wound areas over time for untreated and treated RPA-infected wounds. The student t-test was used to determine significant differences between treatment groups compared with the untreated control. *p-value <0.05 is between RPA+cip/chlor and RPA. (D) Individual data points for control and treatments groups are shown for RPA-infected wounds at day 40. Student's t-test was used to determine significant differences between treatment groups compared with the untreated control. *p-value <0.05. CFU, colony-forming unit; MiPACT-HCR, Microbial identification after Passive Clarity Technique–Hybridization Chain Reaction; ns, non significant.
Having confirmed biofilm growth and RPA's likely oxygen limitation within the wound, wound treatments began at 10 days postwounding when biofilms are already present,57,61 and included 100 μL of either ciprofloxacin alone (200 μg/mL, i.e., 20 μg total/wound), chlorate alone (10 mM), or combined ciprofloxacin and chlorate treatment.
Wounds were treated daily between 10 and 40 days postwounding. Untreated RPA-infected wounds remained opened and contained biofilms until day 40. All treated wounds showed a visual decrease in biofilm formation compared with untreated wounds (Fig. 2B and Supplementary Fig. S1). By measuring wound area over time, we observed that the wound area increased during the first 15 days after injury for all mice (both untreated and treated) (Fig. 2C). However, by day 40 postinjury, untreated wounds maintained their maximum surface area, whereas all treated wounds had significantly decreased in size (Fig. 2C, D). Therefore, we conclude that chlorate treatment is as effective as ciprofloxacin at promoting wound healing, and that combined ciprofloxacin and chlorate treatment is as effective as either drug alone.
Treatment with ciprofloxacin, chlorate, or both promote healing of chronic wounds
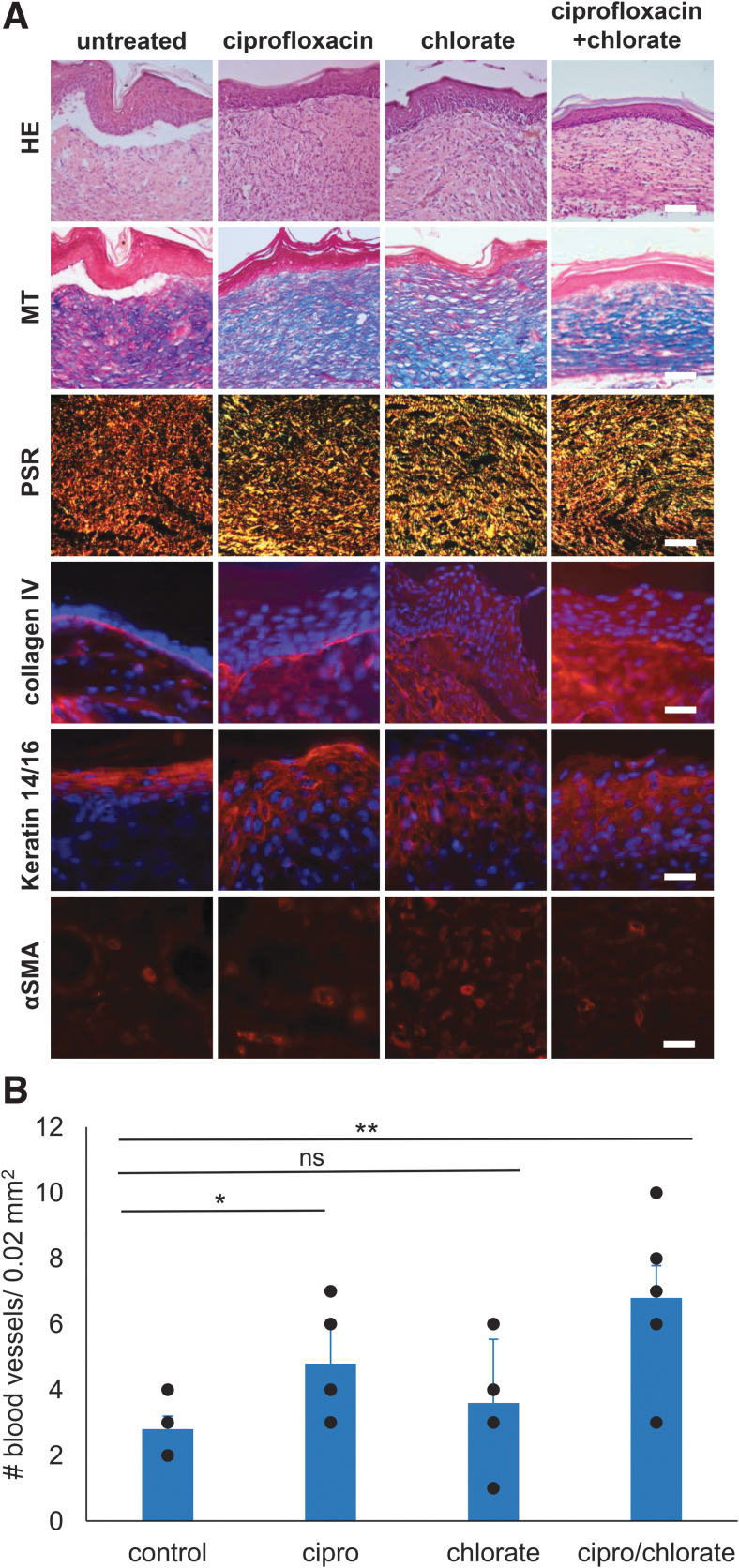
Because a subset of treated wounds had healed by day 40, we sought to examine the quality of wound healing in these mice by performing histological and immunofluorescence analyses on day 40 wounds (Fig. 3A). HE staining showed that untreated RPA-infected wounds fail to properly undergo reepithelialization or granulation tissue formation. MT staining showed that no collagen formed in the granulation tissue of untreated wounds. By contrast, HE staining of all healed treated wound tissues showed strong reepithelialization with granulation tissue formation beneath the newly formed epidermis. MT staining showed that collagen fibers were present in the granulation tissue of all treated wounds, suggesting that the quality of the wound tissue is significantly improved when wounds are treated with ciprofloxacin, chlorate, or both (Fig. 3A).
Figure 3.
Ciprofloxacin and chlorate treatment support wound healing. (A) Wound tissue was collected at day 40 for treated wounds that had healed and also for untreated wounds to perform histology and immunofluorescence staining. Cryosections of the skin were taken from wound tissue for HE, MT, and PSR. Scale bar = 100 μm. Collagen III is shown in green, collagen I is shown in red, and the colocalization of collagen III and collagen I is shown in yellow. Wound tissues were immunolabeled with collagen IV (red), αSMA (red), keratin 14/16 (red), and all samples were labeled with DAPI to stain the DNA in the nuclei of the mouse cells (blue). Scale bar = 20 μm. (B) The number of blood vessels were counted in five frames (area = 0.02 mm2) of the granulation tissue. Blood vessel of RPA-infected wounds were compared using one-way ANOVA: ***p-value <0.001. H&E, Hematoxylin & Eosin; MT, Masson's Trichrome; ns, non significant; PSR, Picrosirius Red.
To distinguish between and visualize the distribution of collagen I and collagen III in the granulation tissue, the wounds were also stained with PSR. PSR staining of untreated wounds showed thick bandings of collagen I (Fig. 3A, stained red) in the granulation tissue, indicating that the wounds did not close and heal. Healed wounds treated with ciprofloxacin, chlorate, or both showed staining of both collagen I and collagen III (Fig. 3A, stained red and green, respectively—overlap appears as yellow) in the granulation tissue, indicative of healing wounds.
Collagen IV immunolabeling was used to show whether basal lamina under the epidermis was present, an important indicator of proper connectivity of the epithelium with the underlying dermis. Collagen IV acts as a physical, chemical, and functional structure between the epidermis and the dermis. Immunofluorescence labeling for collagen IV showed that untreated RPA-infected wounds, which were not reepithelialized, did not show collagen IV-containing basement membrane even at the margins of the wound (Fig. 3A). Healed wounds treated with either chlorate, ciprofloxacin, or both formed a basement membrane under the epithelium as is the case in normal healing.
Keratin 14/16 is a cytokeratin found in proliferating keratinocytes of the epidermis and is observed in the cytoplasm surrounding the nucleus. Keratin 14/16 was absent in the untreated wounds but present in all treated wounds. The quality of the keratinocytes of the epidermis was observably better in wounds treated with both ciprofloxacin and chlorate (Fig. 3A).
Blood vessel development during wound healing is another indicator of proper healing. We quantified the number of microvessels present in the granulation tissue by immunolabeling the wound tissue with α-smooth muscle actin (αSMA), a protein that is present in the cells surrounding blood vessels. Untreated RPA-infected wounds showed no αSMA labeling, indicating that these wounds contained few or no blood vessels and confirming that wound healing did not occur properly in these wounds. In contrast, wounds treated with ciprofloxacin, chlorate, or both showed microvessels in the granulation tissue (Fig. 3A). Compared with untreated controls, wounds treated with ciprofloxacin, chlorate, or both, all had significantly higher number of microvessels in the granulation tissue, although there was no statistically significant difference between the treatments (Fig. 3B).
RPA requires nitrate respiration to establish a chronic infection
To understand the role that anaerobic nitrate respiration plays in the pathogenesis of RPA, we infected our diabetic mouse model of chronic wounds with a Δnar RPA strain. Similar to WT RPA-infected wounds, wounds infected with Δnar RPA produced visible biofilms over the first 10–15 days of the infection (Fig. 4A). However, Δnar RPA-infected wounds did not develop as robust biofilms as WT RPA, and Δnar RPA biofilms began to detach from the wound bed after day 20. More strikingly, all Δnar RPA-infected wounds healed without the need for treatment (Fig. 4A and Supplementary Fig. S2). The Δnar RPA-infected wounds were similar in size to WT RPA-infected wounds until day 10–15, after which Δnar RPA-infected wounds began to decrease in size and were smaller than those infected with WT RPA, with wound closure occurring for all Δnar RPA-infected wounds at day 35–40 after injury (Figs. 4B, C).
Figure 4.
RPA requires Nar to establish a chronic wound infection. Wounds were infected with Δnar RPA (106 CFU) 24 h after injury and daily treatment began 10 days after infection. Wounds were treated daily with either vehicle (n = 6), ciprofloxacin (cip, n = 6), chlorate (chlor, n = 6), or both ciprofloxacin and chlorate (cip/chlor, n = 6). (A) Representative images of untreated and treated Δnar RPA-infected wounds over 40 days. (B) Quantification of wound areas over time. All untreated and treated Δnar RPA-infected wounds underwent wound closure. The Student's t-test was used to determine significant differences between treatment groups compared with the untreated control group. No significant differences in wound areas were observed. (C) Individual data points for control and treatment groups are shown for Δnar RPA-infected wounds at day 40. The Student's t-test was used to determine significant differences between treatment groups compared with the untreated control group. No significant (ns) differences in wound areas were observed. Nar, nitrate respiration; ns, non significant.
As excepted, Δnar RPA-infected wounds did not close faster when treated with chlorate. They also did not close faster when treated with ciprofloxacin (or both drugs), suggesting that killing oxic RPA populations does not make a significant contribution to wound healing of Δnar RPA-infected wounds.
These findings were also supported by HE staining, which showed the presence of well-developed epithelium and granulation tissue in Δnar RPA-infected wounds (Fig. 5A). However, we found that some untreated Δnar RPA-infected wounds did not have a proper connection between the epithelium and the granulation tissue. MT staining showed that despite the presence of granulation tissue, the collagen bundles were not properly organized. This suggests that while Δnar RPA-infected wounds healed, the quality of interstitial collagen in the granulation tissue was not good. As seen with PSR staining, untreated wounds contained collagen fibers primarily of collagen I. Collagen IV immunolabeling showed the presence of basement membrane components attached to the granulation tissue, even though from HE and MT staining we can see that the connection with the epithelium was visibly not secured.
Figure 5.
Ciprofloxacin and chlorate treatment enhance healing of Δnar RPA-infected wounds. Wound tissue was collected at day 40 for untreated and treated Δnar RPA-infected wounds to perform histology and immunofluorescence staining. (A) Cryosections of the skin were taken from wound tissue for HE, MT, and PSR. Collagen III is shown in green, collagen I is shown in red, and the colocalization of collagen III and collagen I is shown in yellow. Scale bar = 100 μm. Wounds tissues were immunolabeled with collagen IV (red), αSMA (red), keratin 14/16 (red), and all samples were labeled with DAPI to stain the DNA in the nuclei of the mouse cells (blue) (scale bar = 20 μm). (B) The number of blood vessels were counted in six frames (area = 0.02 mm2) of the granulation tissue. Blood vessel of Δnar RPA-infected wounds were compared using one-way ANOVA: *p-value <0.05, **p-value <0.01; ns, non significant.
When Δnar RPA-infected wounds were treated with ciprofloxacin, chlorate, or with both drugs, the quality of wound healing was improved compared with untreated wounds. In all treated wounds, HE staining showed reepithelialization and the presence of granulation tissue. Across all drug treatments, MT staining showed collagen bundles were present and more organized. PSR staining supports that all treated wounds showed colocalization of collagen I and collagen III (shown in yellow), whereas untreated Δnar RPA-infected wounds lacked collagen III despite wound closure. Immunolabeling for collagen IV showed that the basement membrane was present in all Δnar RPA-infected wounds (Fig. 5A).
Keratin 14/16 staining of untreated Δnar RPA-infected wounds showed localization in the most superficial layers of the epithelium, whereas there was staining throughout the epithelium of treated wounds. Labeling for αSMA showed blood vessel development, but on average, the density of blood vessels was lower in untreated wounds compared with treated wounds, although there was no statistical difference between untreated and chlorate-treated wounds (Fig. 5B). Altogether, these findings suggest that the quality of healing was better in treated compared with untreated Δnar RPA-infected wounds.
Finally, to further confirm that RPA requires nitrate respiration to establish a chronic infection, we generated a complement strain where the nar genes were reintroduced at a neutral site in the genome of the Δnar RPA background (complement strain Δnar+att::mTnnar). Wounds infected with the complement strain looked similar to those infected with WT RPA, forming robust biofilms that persist in the wound bed for the duration of the experiment (Fig. 6A). These wounds increased in size over time and remained large, similar to WT RPA-infected wounds (Fig. 6B, C). WT RPA and Δnar+att::mTnnar-infected wounds were significantly larger at days 30 and 40 compared with Δnar RPA-infected wounds (Fig. 6C). Interestingly, the complement strain was more aggressive than WT RPA, such that a lower starting inoculum of 104 CFU was used when infecting wounds with Δnar+att::mTnnar RPA to prevent mice from dying.
Figure 6.
Mice infected with the Δnar-complement strain (Δnar+att::mTnnar) develop chronic wounds. (A) Representative images of wounds infected with either WT (106 CFU), Δnar (106 CFU), or Δnar+att::mTnnar (104 CFU) RPA 24 h after injury and left undisturbed for 40 days. Wounds infected with either WT or Δnar+att::mTnnar RPA displayed biofilm formation throughout the 40-day experiment, whereas Δnar RPA-infected wounds did not display biofilm at day 40. (B) Quantification of wound areas. Both WT and Δnar+att::mTnnar RPA-infected wounds were open 40 days after injury, whereas Δnar RPA-infected wounds had closed. Note: the data shown for WT and Δnar RPA-infected wounds are the same as the data shown in Figs. 2C and 4B, respectively, and have been added for ease of comparison to the Δnar+att::mTnnar RPA strain. (C) Individual data points for control and treatment groups are shown for wounds infected with either RPA, Δnar RPA, or Δnar+att::mTnnar at day 30 and 40. The Student's t-test was used to determine significant differences in wound areas between mutant strains compared with RPA. **p-value <0.01, ***p-value <0.001. ns, non significant.
Our experiments with the complement strain demonstrate that Δnar RPA phenotypes are a direct result of nar mutation, as opposed to off-target genetic effects, strengthening our conclusion that P. aeruginosa requires anaerobic nitrate respiration to establish chronic wound infections.
DISCUSSION
Chronic wound infections and biofilms must be resolved before proper healing can occur, yet our current antibiotic treatments often fail to clear either.4–11 Identifying drugs that target energetic processes in bacteria is a promising strategy for combatting recalcitrant infections.63
First, while opportunistic pathogens are diverse, they possess highly conserved energy-conserving metabolic pathways that are not found in mammalian systems (e.g., the nitrate reductase, Nar). For this reason, drugs that target anaerobic metabolism might be less likely to crossreact with host cells. Second, energy-conserving processes are directly linked to bacterial growth and survival, so drugs that disrupt them might prove particularly lethal. This approach has shown promising results for treating Mycobacterium tuberculosis, the causative agent of the chronic infection, tuberculosis. Several drug inhibitors of M. tuberculosis respiratory chain enzymes are either being studied, undergoing clinical trials, or are in use.64
In our study, we demonstrate that (1) Nar is an excellent drug target for treating chronic P. aeruginosa wound infections because the pathogen requires this metabolism to establish a chronic infection, and (2) Using chlorate to target P. aeruginosa-infected chronic wounds effectively contributes to healing of these wounds.
Our finding that P. aeruginosa requires anaerobic nitrate respiration is in line with prior work showing that the wound environment is largely anoxic.17 However, it is interesting to note that although Δnar RPA-infected wounds ultimately healed, these wounds still increased in size during the first ∼10 days postwounding. At around day 10–15, Δnar RPA-infected wounds appear to change course and become smaller in size compared with WT RPA-infected wounds. This suggests that nitrate respiration does not initially contribute to P. aeruginosa establishing itself in the wound, and only later becomes an essential metabolic process for chronically inhabiting the wound. Our imaging studies reveal that thick P. aeruginosa biofilms form by day 10, suggesting that oxygen availability becomes limiting for P. aeruginosa by this time.
Our previous work analyzing the transcriptomic landscape of the wound microenvironment shows that the wound endures hypoxia and an inflammatory assault during the development of chronicity.65,66 Additional studies are needed to better understand oxygen dynamics of the wound environment over time and the establishment of bacterial biofilms.
In prior work, we used a genetic screen to demonstrate that the primary mechanism of chlorate resistance in P. aeruginosa is acquiring mutations that decrease or abolish Nar activity.60 In other words, the best way to become chlorate resistant is to avoid reducing chlorate to chlorite, since the cell appears to have limited mechanisms for fighting chlorite toxicity.60
Our current findings demonstrate that P. aeruginosa requires Nar to establish a chronic wound infection so acquiring chlorate resistance would be expected to hinder pathogen fitness in chronic wounds. Because Nar is an essential enzyme for pathogenesis, the accumulation of mutations that render P. aeruginosa resistant to chlorate would be unlikely to arise. In addition, there are also examples of bacteria that have evolved to grow through chlorate respiration.67 Some of these bacteria also use Nar for nitrate respiration, however, these organisms take extra precautions to prevent these metabolisms from co-occurring. Some species use mobile genetic elements to disrupt the nar operon during times of chlorate respiration,67 suggesting that nature has not successfully evolved a Nar enzyme that can distinguish between nitrate and chlorate.
Thus, in the case of chlorate treatment, P. aeruginosa might face a lose–lose scenario: chlorate susceptible (Nar+) populations die during chlorate treatment, or chlorate-resistant (Nar-) populations cannot thrive in the chronic wound environment over time.
Compared with untreated wounds, all treated wounds (ciprofloxacin, chlorate, combination) were smaller in size, on average, and began decreasing in size starting ∼day 30 postwounding. None of the untreated RPA-infected wounds healed, whereas treated wounds that healed displayed markers of good-quality healing. It is interesting that, although all Δnar RPA-infected wounds had healed, chlorate treatment (which presumably kills populations using Nar) does not result in the same striking outcome. Although we do not know why this is the case, it is possible that the chlorate dosage used in these studies was insufficient to kill the entire Nar-utilizing P. aeruginosa population.
We also observed that the combined ciprofloxacin and chlorate treatment resulted in similar healing outcomes compared with ciprofloxacin or chlorate-only treatment. Although ciprofloxacin can kill both oxic and anoxic populations of P. aeruginosa, it does not have better outcomes than a drug that only kills anoxic populations.
Given that Δnar RPA-infected wounds (which would consist of predominantly oxygen-respiring P. aeruginosa population) cannot establish chronic infections, our data indicate that anoxic/Nar-utilizing populations of P. aeruginosa are important for sustaining long-term chronicity. Except for ciprofloxacin, many other commonly used antibiotics selectively kill oxic but not anoxic pathogen populations.18,19 Because our data indicate anoxic populations sustain chronic wound infections, our finding that treatment with a simple anion-like chlorate selectively targets these populations has significant implications for how chronic wound infections should be managed.
We also observed that ciprofloxacin-only and combined ciprofloxacin and chlorate-treated Δnar RPA-infected wounds showed higher quality healing outcomes compared with their untreated counterparts. Thus, while anoxic/Nar-utilizing populations promote stalled healing, the presence of oxic P. aeruginosa populations might still prevent healing processes such as collagen deposition as seen by the lack of collagen III. Chlorate-treated Δnar RPA-infected wounds also showed higher quality healing for some metrics compared with their untreated counterparts. Given that chlorate treatment should have no impact on the fully chlorate-resistant Δnar RPA strain, understanding the effects of chlorate treatment on host tissue remains an important goal.
While this study focused on the common wound pathogen, P. aeruginosa, chronic wounds are colonized by polymicrobial communities.21 Many other wound-relevant pathogens encode Nar and might be susceptible to chlorate under hypoxic/anoxic conditions, including Staphylococcus aureus, E. coli, Enterobacter spp., and Corynebacterium spp. Accordingly, we propose that nitrate respiration may function beyond P. aeruginosa as a keystone metabolism that supports microbial community recalcitrance and thus stalled healing of chronic wounds. Future studies should explore the potential role that nitrate respiration plays in defining wound community structure and in stabilizing wound communities, since community stabilization is associated with longer-lasting wounds.68
Chlorate treatment has a range of potential advantages in comparison with other antimicrobial treatments. Compared with antiseptic compounds, chlorate toxicity is more targeted because it requires a bacterial enzyme to become activated to chlorite. Chlorite is generated inside the bacterial cytoplasm and we have been unable to detect chlorite in P. aeruginosa cultures,19 suggesting that it does not escape to harm neighboring cells.
Chlorate is also an inexpensive and shelf-stable salt, which might lend itself to ease of use in a range of clinical settings. Importantly, chlorate treatment is unlikely to select for resistant pathogens, given that mutations in the nar gene are the primary drivers of chlorate resistance,60 but mutants lacking a functional nar gene product cannot survive long term in the wound environment. In light of challenges like antibiotic tolerance and resistance, our finding that chlorate is as effective at supporting chronic wound healing as ciprofloxacin is highly significant. Ciprofloxacin itself has been widely used for decades, although there is growing concern regarding the rise in ciprofloxacin resistance.69
Although most drugs are screened for their toxicity against fast-growing pathogens under oxic conditions, our findings indicate that such populations do not perpetuate chronic wound infections. In our work, we use chlorate to leverage the metabolic shifts that pathogens make in hypoxic/anoxic environments to improve chronic wound outcomes.
INNOVATION
Many conventional antibiotics are less effective at killing bacteria in oxygen-limited environments, such as those found in chronic wounds. We demonstrate the therapeutic potential of the small molecule, chlorate, which kills the opportunistic pathogen, Pseudomonas aeruginosa, by targeting a form of anaerobic metabolism called nitrate respiration. We showed that P. aeruginosa requires anaerobic nitrate respiration to sustain chronic wound infections, and that chlorate treatment supports chronic wound healing. These studies were performed in a unique diabetic mouse model for chronic wounds wherein infecting bacteria produce robust biofilms in wounds that stay open for weeks to months as the mouse survives.
KEY FINDINGS
Anaerobic P. aeruginosa populations play a dominant role in preventing healing of chronic wounds.
P. aeruginosa lacking nitrate reductase, Nar, an enzyme important for its survival under anaerobic conditions, was unable to effectively colonize wounds and form biofilms in our diabetic, chronic wound mouse model system.
Wounds treated with chlorate (which targets bacterial cells using Nar) healed effectively. Chlorate holds promise as a treatment to fight diverse infections where oxygen is limiting and/or where pathogens grow as biofilms, because many other opportunistic pathogens have Nars and survive using anaerobic metabolism.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Aaron Chavez for technical support with the mouse experiments and the specimen preparation for histology.
ABBREVIATIONS AND ACRONYMS
- αSMA
α-smooth muscle actin
- ABBA
agar block biofilm assay
- ATZ
3-amino-1, 2, 4-triazole
- CFU
colony-forming unit
- col I
collagen I
- col III
collagen III
- col IV
collagen IV
- DAPI 4′
6-diamidino-2-phenylindole
- H&E
Hematoxylin & Eosin
- LB
Luria-Bertani
- MiPACT-HCR
Microbial identification after Passive Clarity Technique–Hybridization Chain Reaction
- MSA
mercaptosuccinic acid
- MT
Masson's Trichrome
- Nar
nitrate reductase
- OCT
optimal cutting temperature compound
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- RPA
Riverside Pseudomonas aeruginosa
- PSR
Picrosirius Red
AUTHORs' CONTRIBUTIONS
J.H.K. performed all in vivo experiments and immunofluorescence labeling. M.A.S. generated the Δnar strain, tested in vitro drug susceptibilities against P. aeruginosa planktonic cultures, and assisted with writing. E.G.L. measured wound areas. Z.R.L. generated the Δnar complement strain and tested in vitro drug susceptibilities against P. aeruginosa biofilms. I.B.T. performed MiPACT and imaging on wound tissue. D.K.N. and M.M.G. contributed to the experimental design, analysis of the data, and writing of the article.
AUTHOR DISCLOSURE AND GHOSTWRITING
The authors state no competing financial interests. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
ABOUT THE AUTHORS
Jane H. Kim, PhD is a postdoctoral scholar at the University of California, Riverside. Her research focuses on understanding cellular and molecular mechanisms of chronic wound initiation. Melanie A. Spero, PhD was a postdoctoral scholar at the California Institute of Technology and is now an Assistant Professor of Biology in the Institute of Molecular Biology at the University of Oregon. Her research focuses how bacterial pathogens grow and survive within host environments during chronic infections. Elyson Gavin Lebig, BS was a biochemistry undergraduate student at the University of California, Riverside. Zachery R. Lonergan, PhD is a postdoctoral scholar at the California Institute of Technology. Inês B. Trindade, PhD is a postdoctoral scholar at the California Institute of Technology.
Dianne K. Newman, PhD is the Gordon M. Binder/Amgen Professor of Biology and Geobiology at California Institute of Technology and Executive Officer for Biology and Biological Engineering. Manuela Martins-Green, PhD is Professor of Cell Biology & Neuroscience at the University of California, Riverside.
FUNDING INFORMATION
This work was supported by a fellowship from the Jane Coffin Childs Memorial Fund for Medical Research to Z.R.L., a postdoctoral fellowship from the Cystic Fibrosis Foundation (SPERO19F0) to M.A.S., a gift from the Doren Family Foundation to D.K.N., and by NIH grant 1R21AI146987-01 to M.M.G. and D.K.N.
SUPPLEMENTARY MATERIAL
REFERENCES
- 1. Galaviz KI, Narayan KMV, Lobelo F, et al. Lifestyle and the prevention of type 2 diabetes: A status report. Am J Lifestyle Med 2018;12:4–20; doi: 10.1177/1559827615619159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 2011;8:228–236; doi: 10.1038/nrendo.2011.183 [DOI] [PubMed] [Google Scholar]
- 3. Edmonds M, Manu C, Vas P. The current burden of diabetic foot disease. J Clin Orthop Trauma 2021;17:88–93; doi: 10.1016/j.jcot.2021.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gardiner M, Vicaretti M, Sparks J, et al. A longitudinal study of the diabetic skin and wound microbiome. PeerJ 2017;5:e3543; doi: 10.7717/peerj.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith K, Collier A, Townsend EM, et al. One step closer to understanding the role of bacteria in diabetic foot ulcers: Characterising the microbiome of ulcers. BMC Microbiol 2016;16:54; doi: 10.1186/s12866-016-0665-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spichler A, Hurwitz BL, Armstrong DG, et al. Microbiology of diabetic foot infections: From Louis Pasteur to ‘crime scene investigation’. BMC Med 2015;13:2; doi: 10.1186/s12916-014-0232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pang M, Zhu M, Lei X, et al. Changes in Foot Skin Microbiome of Patients with Diabetes Mellitus Using High-Throughput 16S rRNA Gene Sequencing: A Case Control Study from a Single Center. Med Sci Monit 2020;26:e921440; doi: 10.12659/MSM.921440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schaper NC, Kujath P, Bruch HP, et al. Efficacy and safety of IV/PO moxifloxacin and IV piperacillin/tazobactam followed by PO amoxicillin/clavulanic acid in the treatment of diabetic foot infections: Results of the RELIEF study. Infection 2013;41:175–186; doi: 10.1007/s15010-012-0367-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lipsky BA, Itani K, Norden C; Linezolid Diabetic Foot Infections Study Group. Treating foot infections in diabetic patients: A randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin Infect Dis 2004;38:17–24; doi: 10.1086/380449 [DOI] [PubMed] [Google Scholar]
- 10. Lipsky BA, Stoutenburgh U. Daptomycin for treating infected diabetic foot ulcers: Evidence from a randomized, controlled trial comparing daptomycin with vancomycin or semi-synthetic penicillins for complicated skin and skin-structure infections. J Antimicrob Chemother 2005;55:240–245; doi: 10.1093/jac/dkh531 [DOI] [PubMed] [Google Scholar]
- 11. Siami G, Christou N, Eiseman I, et al. Clinafloxacin versus piperacillin-tazobactam in treatment of patients with severe skin and soft tissue infections. Antimicrob Agents Chemother 2001;45:525–531; doi: 10.1128/AAC.45.2.525-531.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narten M, Rosin N, Schobert M, et al. Susceptibility of Pseudomonas aeruginosa urinary tract isolates and influence of urinary tract conditions on antibiotic tolerance. Curr Microbiol 2012;64:7–16; doi: 10.1007/s00284-011-0026-y [DOI] [PubMed] [Google Scholar]
- 13. Hamad MA, Austin CR, Stewart AL, et al. Adaptation and antibiotic tolerance of anaerobic Burkholderia pseudomallei. Antimicrob Agents Chemother 2011;55:3313–3323; doi: 10.1128/AAC.00953-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gutierrez A, Jain S, Bhargava P, et al. Understanding and sensitizing density-dependent persistence to quinolone antibiotics. Mol Cell 2017;68:1147–1154 e1143; doi: 10.1016/j.molcel.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 15. Kragh KN, Alhede M, Jensen PQ, et al. Polymorphonuclear leukocytes restrict growth of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Infect Immun 2014;82:4477–4486; doi: 10.1128/IAI.01969-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast Reconstr Surg 2006;117:35S–41S; doi: 10.1097/01.prs.0000225431.63010.1b [DOI] [PubMed] [Google Scholar]
- 17. James GA, Ge Zhao A, Usui M, et al. Microsensor and transcriptomic signatures of oxygen depletion in biofilms associated with chronic wounds. Wound Repair Regen 2016;24:373–383; doi: 10.1111/wrr.12401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borriello G, Werner E, Roe F, et al. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother 2004;48:2659–2664; doi: 10.1128/AAC.48.7.2659-2664.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Spero MA, Newman DK. Chlorate specifically targets oxidant-starved, antibiotic-tolerant populations of Pseudomonas aeruginosa biofilms. mBio 2018;9:e01400-18; doi: 10.1128/mBio.01400-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis 2015;34:877–886; doi: 10.1007/s10096-015-2323-z [DOI] [PubMed] [Google Scholar]
- 21. Kalan LR, Brennan MB. The role of the microbiome in nonhealing diabetic wounds. Ann N Y Acad Sci 2019;1435:79–92; doi: 10.1111/nyas.13926 [DOI] [PubMed] [Google Scholar]
- 22. Bodey GP, Bolivar R, Fainstein V, et al. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis 1983;5:279–313. [DOI] [PubMed] [Google Scholar]
- 23. Taylor PK, Yeung AT, Hancock RE. Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies. J Biotechnol 2014;191:121–130; doi: 10.1016/j.jbiotec.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 24. Mah TF, Pitts B, Pellock B, et al. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 2003;426:306–310; doi: 10.1038/nature02122 [DOI] [PubMed] [Google Scholar]
- 25. Drenkard, E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect 2003;5:213–1219; doi: 10.1016/j.micinf.2003.08.009 [DOI] [PubMed] [Google Scholar]
- 26. Wolcott RDD, Kennedy JPP, Dowd SEE. Regular debridement is the main tool for maintaining a healthy wound bed in most chronic wounds. J Wound Care 2009;18:54–56. [DOI] [PubMed] [Google Scholar]
- 27. Salamone JC, Salamone AB, Swindle-Reilly K, et al. Grand challenge in Biomaterials-wound healing. Regen Biomater 2016;3:127–128; doi: 10.1093/rb/rbw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verbanic S, Shen Y, Lee J, et al. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiomes 2020;6:21; doi: 10.1038/s41522-020-0130-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalan L, Zhou M, Labbie M, et al. Measuring the microbiome of chronic wounds with use of a topical antimicrobial dressing - A feasibility study. PLoS One 2017;12:e0187728; doi: 10.1371/journal.pone.0187728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer KL, Brown S, Whiteley M. Membrane-bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J Bacteriol 2007;189:4449–4455; doi: 10.1128/JB.00162-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stolz JF, Basu P. Evolution of nitrate reductase: Molecular and structural variations on a common function. Chembiochem 2002;3:198–206; doi: [DOI] [PubMed] [Google Scholar]
- 32. Winter SE, Winter MG, Xavier MN, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2013;339:708–711; doi: 10.1126/science.1232467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernatchez SF, Menon V, Stoffel J, et al. Nitric oxide levels in wound fluid may reflect the healing trajectory. Wound Repair Regen 2013;21:410–417; doi: 10.1111/wrr.12048 [DOI] [PubMed] [Google Scholar]
- 34. Line L, Alhede M, Kolpen M, et al. Physiological levels of nitrate support anoxic growth by denitrification of Pseudomonas aeruginosa at growth rates reported in cystic fibrosis lungs and sputum. Front Microbiol 2014;5:554; doi: 10.3389/Fmicb.2014.00554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cornforth DM, Dees JL, Ibberson CB, et al. Pseudomonas aeruginosa transcriptome during human infection. Proc Natl Acad Sci U S A 2018;115:E5125–E5134; doi: 10.1073/pnas.1717525115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Son MS, Matthews WJ, Kang Y, et al. In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun 2007;75:5313–5324; doi: 10.1128/IAI.01807-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quinn RA, Lim YW, Maughan H, et al. Biogeochemical forces shape the composition and physiology of polymicrobial communities in the cystic fibrosis lung. mBio 2014;5:e00956-13; doi: 10.1128/mBio.00956-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beckmann C, Brittnacher M, Ernst R, et al. Use of phage display to identify potential Pseudomonas aeruginosa gene products relevant to early cystic fibrosis airway infections. Infect Immun 2005;73:444–452; doi: 10.1128/IAI.73.1.444-452.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cowley E, Kopf S, LaRiviere A, et al. Pediatric cystic fibrosis sputum can be chemically dynamic, anoxic, and extremely reduced due to hydrogen sulfide formation. mBio 2015;6:e00767; doi: 10.1128/mBio.00767-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kolpen M, Kühl M, Bjarnsholt T, et al. Nitrous oxide production in sputum from cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. PLoS One 2014;9:e84353; doi: 10.1371/journal.pone.0084353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Puig J, Azoulay E, Pichinoty F, et al. Genetic mapping of the chl C gene of the nitrate reductase A system in Escherichia coli K12. Biochem Biophys Res Commun 1969;35:659–662. [DOI] [PubMed] [Google Scholar]
- 42. Hochstein LI, Tomlinson GA. The enzymes associated with denitrification. Annu Rev Microbiol 1988;42:231–261; doi: 10.1146/annurev.mi.42.100188.001311 [DOI] [PubMed] [Google Scholar]
- 43. Canada H. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Chlorite and Chlorate. Ottawa, Ontario, 2008. [Google Scholar]
- 44. van Wijk DJ, Kroon SG, Garttener-Arends IC. Toxicity of chlorate and chlorite to selected species of algae, bacteria, and fungi. Ecotoxicol Environ Saf 1998;40:206–211; doi: 10.1006/eesa.1998.1685 [DOI] [PubMed] [Google Scholar]
- 45. Jackson RC, Elder WJ, McDonnell, H. Sodium-chlorate poisoning complicated by acute renal failure. Lancet 1961;2:1381–1383. [DOI] [PubMed] [Google Scholar]
- 46. Helliwell M, Nunn J. Mortality in sodium chlorate poisoning. Br Med J 1979;1:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zou XH, Foong WC, Cao T, et al. Chondroitin sulfate in palatal wound healing. J Dent Res 2004;83:880–885; doi: 10.1177/154405910408301111 [DOI] [PubMed] [Google Scholar]
- 48. Smith DJ, Oliver CE, Taylor JB, et al. Invited review: Efficacy, metabolism, and toxic responses to chlorate salts in food and laboratory animals. J Anim Sci 2012;90:4098–4117; doi: 10.2527/jas.2011-4997 [DOI] [PubMed] [Google Scholar]
- 49. Choi KH, Schweizer HP. mini-Tn7 insertion in bacteria with single attTn7 sites: Example Pseudomonas aeruginosa. Nat Protoc 2006;1:153–161; doi: 10.1038/nprot.2006.24 [DOI] [PubMed] [Google Scholar]
- 50. Basta DW, Bergkessel M, Newman DK. Identification of fitness determinants during energy-limited growth arrest in Pseudomonas aeruginosa. mBio 2017;8:e01170-17; doi: 10.1128/mBio.01170-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. DePas WH, Starwalt-Lee R, Van Sambeek L, et al. Exposing the three-dimensional biogeography and metabolic states of pathogens in cystic fibrosis sputum via hydrogel embedding, clearing, and rRNA labeling. mBio 2016;7:e00796-16; doi: 10.1128/mBio.00796-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jorth P, Spero MA, Livingston J, et al. Quantitative visualization of gene expression in mucoid and nonmucoid Pseudomonas aeruginosa aggregates reveals localized peak expression of alginate in the hypoxic zone. mBio 2019;10:e02622-19; doi: 10.1128/mBio.02622-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Livingston J, Spero MA, Lonergan ZR, et al. Visualization of mRNA expression in Pseudomonas aeruginosa aggregates reveals spatial patterns of fermentative and denitrifying metabolism. Appl Environ Microbiol 2022;88:e0043922; doi: 10.1128/aem.00439-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Choi HM, Beck VA, Pierce NA. Next-generation in situ hybridization chain reaction: Higher gain, lower cost, greater durability. ACS Nano 2014;8:4284–4294; doi: 10.1021/nn405717p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int 2004;11:36–42. [Google Scholar]
- 56. Kim JH, Martins-Green M. Protocol to create chronic wounds in diabetic mice. J Vis Exp 2019; doi: 10.3791/57656 [DOI] [PubMed] [Google Scholar]
- 57. Kim JH, Yang B, Tedesco A, et al. High levels of oxidative stress and skin microbiome are critical for initiation and development of chronic wounds in diabetic mice. Sci Rep 2019;9:19318; doi: 10.1038/s41598-019-55644-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dhall S, Do DC, Garcia M, et al. Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J Diabetes Res 2014;2014:562625; doi: 10.1155/2014/562625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Petreaca ML, Do D, Dhall S, et al. Deletion of a tumor necrosis superfamily gene in mice leads to impaired healing that mimics chronic wounds in humans. Wound Repair Regen 2012;20:353–366; doi: 10.1111/j.1524-475X.2012.00785.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spero MA, Jones J, Lomenick B, et al. Mechanisms of chlorate toxicity and resistance in Pseudomonas aeruginosa. Mol Microbiol 2022;118:321–335; doi: 10.1111/mmi.14972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim JH, Ruegger PR, Lebig EG, et al. High levels of oxidative stress create a microenvironment that significantly decreases the diversity of the microbiota in diabetic chronic wounds and promotes biofilm formation. Front Cell Infect Microbiol 2020;10:259; doi: 10.3389/fcimb.2020.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stewart PS. Diffusion in biofilms. J Bacteriol 2003;185:1485–1491; doi: 10.1128/JB.185.5.1485-1491.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cook GM, Greening C, Hards K, et al. Energetics of pathogenic bacteria and opportunities for drug development. Adv Microb Physiol 2014;65:1–62; doi: 10.1016/bs.ampbs.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 64. Hasenoehrl EJ, Wiggins TJ, Berney M. Bioenergetic inhibitors: Antibiotic efficacy and mechanisms of action in Mycobacterium tuberculosis. Front Cell Infect Microbiol 2020;10:611683; doi: 10.3389/fcimb.2020.611683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Basu P, Kim JH, Saeed S, et al. Using systems biology approaches to identify signalling pathways activated during chronic wound initiation. Wound Repair Regen 2021;29:881–898; doi: 10.1111/wrr.12963 [DOI] [PubMed] [Google Scholar]
- 66. Basu P, Martins-Green M. Signaling pathways associated with chronic wound progression: A systems biology approach. Antioxid Basel 2022;11:1506; doi: 10.3390/antiox11081506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Youngblut MD, Wang O, Barnum TP, et al. (Per)chlorate in biology on Earth and beyond. Annu Rev Microbiol 2016;70:435–457; doi: 10.1146/annurev-micro-102215-095406 [DOI] [PubMed] [Google Scholar]
- 68. Loesche M, Gardner SE, Kalan L, et al. Temporal stability in chronic wound microbiota is associated with poor healing. J Invest Dermatol 2017;137:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mintz I, Chowers M, Obolski U. Prediction of ciprofloxacin resistance in hospitalized patients using machine learning. Commun Med (Lond) 2023;3:43; doi: 10.1038/s43856-023-00275-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.