Abstract
There is growing interest in reducing the number of synthetic products or additives and replacing them with natural ones. The pharmaceutical, cosmetic, and food industries are especially focused on natural and bioactive chemicals isolated from plants or microorganisms. The main challenge here is to develop efficient and ecological methods for their isolation. According to the strategies and rules of sustainable development and green chemistry, green solvents and environmentally friendly technologies must be used. The application of deep eutectic solvents as efficient and biodegradable solvents seems to be a promising alternative to traditional methods. They are classified as being green and ecological but, most importantly, very efficient extraction media compared to organic solvents. The aim of this review is to present the recent findings on green extraction, as well as the biological activities and the possible applications of natural plant ingredients, namely, phenolics, flavonoids, terpenes, saponins, and some others. This paper thoroughly reviews modern, ecological, and efficient extraction methods with the use of deep eutectic solvents (DESs). The newest findings, as well as the factors influencing the efficiency of extraction, such as water content, and hydrogen bond donor and acceptor types, as well as the extraction systems, are also discussed. New solutions to the major problem of separating DESs from the extract and for solvent recycling are also presented.
Keywords: bioactive compounds from plants, bioactive ingredients in cosmetics, natural pharmaceuticals, natural food additives, deep eutectic solvents, green solvents
1. Introduction
Nowadays, reducing global pollution and introducing sustainable development in all areas of life should be everyone’s priority. A sustainable development strategy to save the planet and the environment consists of many goals, and one of the most important is responsible production and consumption. A growing amount of interest and demand for natural components with promising properties such as antiaging, anti-inflammatory, anticancer, and antimicrobial has been observed in the pharmaceutical, cosmetic, and food industries. The most interesting groups of valuable, plant-originated compounds include phenolics, flavonoids, terpenoids, and saponins. The major concern involves efficient and ecological isolation of these compounds from natural sources. The technologies applied should meet the requirements of green chemistry, which means that the use of many organic solvents is restricted. There are still many attempts to find green, efficient, and inexpensive methods for the isolation of natural plant ingredients, both hydrophilic and hydrophobic. The new trend, widely explored in many fields of industry, is to apply deep eutectic solvents (DESs), composed of naturally occurring ingredients. These have been proven to be efficient, biodegradable, and environmentally friendly media, useful in many branches of industry (Figure 1).1−16
Figure 1.
DESs were first introduced in 2003 by Abbott et al., and since then, huge development and interest in these solvents have been observed.17 They have been considered as potential replacements for ionic liquids (ILs), after aspects of the possible toxicity of some ILs to living organisms have emerged. Initially, the research focused mostly on hydrophilic DESs, and the first information on hydrophobic ones appeared in 2015. The structures of the most popular DESs are based on noncovalent interactions. Depending on the character of the hydrogen bond acceptor (HBA) and the hydrogen bond donor (HBD), interactions such as hydrogen bonding, van der Waals interactions, or π–π stacking occur. Recently, novel eutectic systems, namely, eutectic molecular liquids (EMLs), were defined, and several other noncovalent interactions were identified, including not only hydrogen bonding and π–π stacking but also σ-hole (halogen, pnicogen, and tetrel bonds), π-hole, κ-hole, and μ-hole bonding interactions.18,19 There is a huge interest in composing DESs with new and interesting properties as well as in explaining the structure–activity relationship and the interactions occurring between different HBAs and HBDs.20−22 The most valuable feature of DESs seems to be the possibility of obtaining almost unlimited numbers of tailor-made solvents by selecting appropriate HBDs and HBAs. Additionally, many DESs (also named natural deep eutectic solvents, NADESs) are bioderived, green, biocompatible, biodegradable, and nontoxic chemicals, unlike classic solvents (including solvents belonging to volatile organic compounds, VOCs) or some ILs. However, there are a few negative aspects for DES applications. First is the high viscosity of many DESs. Also, due to the high hygroscopicity of some DESs (especially those with choline chloride), they can absorb moisture from the air, which may cause problems with handling and storage. Additionally, even though the majority of DESs are considered thermostable, some can decompose at high temperatures and some can be corrosive.23 The major challenge in using DESs as extraction media is to efficiently separate them from the extract and to recycle them for reuse. The main advantages and disadvantages of DESs as extraction media are presented in Figure 2. It is clear that the advantages far outweigh the disadvantages, so DESs are much better and more preferable substitutes for organic solvents.
Figure 2.
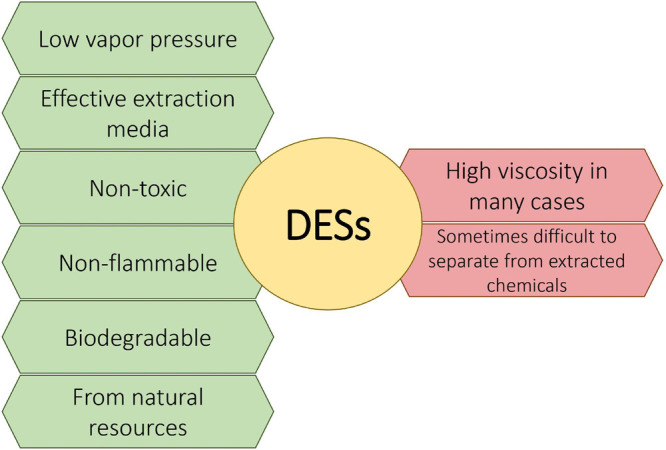
Advantages (green) and disadvantages (red) of deep eutectic solvents applied as extraction media.
Many papers discussing the extractions of plant ingredients with DESs have been published in recent times. These focus on several important aspects of efficient extraction, including the types and properties of the solvents, the time of extraction, and the water content and temperature.24,25 Different extraction techniques, e.g., microwave- and ultrasound-assisted extraction and microextraction, have been reviewed.26−28 The analytical methods applied to the characteristics of the extracted substances, as well as the problems with the viscosity and recovery of the extracted compounds, are also widely discussed.29,30 Special attention is paid to the extraction of phenolic compounds, flavonoids, proteins, biopolymers, and carbohydrates from waste biomass or algae.24,31,32 Among all of the types of DESs used in modern extraction, the potential for hydrophobic DESs was underlined in many recent papers, and thoroughly reviewed by Sportiello et al.33 These DESs can be characterized by the presence of compounds with long alkyl chain or cycloalkyl groups. They are composed of, e.g., lauric acid, decanoic acid, thymol, menthol, tetraheptylammonium chloride, or tetraoctylammonium chloride as HBAs and long chain carboxylic acids, levulinic acid, lidocaine, or camphor as HBDs. Efficient extraction of food contaminants, chelating properties, and also extraction of bioactive compounds such as artemisinin, carotenoids, or tocols are widely reported.33 The importance of the extraction of natural substances with DESs is also confirmed by the number of patent applications filed in recent years. According to the Espacenet database, in only 2022–2023, out of 91 documents claiming extractions using DESs, ∼67 patent applications were filed for the extraction of natural compounds (https://worldwide.espacenet.com/; query: deep eutectic solvent AND extraction; selected by earliest publication date; accessed 2023-10-05). The newest inventions, apart from the classic DESs, focus on extractions with NADESs, hydrophobic DESs, binary solvents (e.g., composed of ILs and DESs), or deep eutectic solvent microemulsion extraction systems, as well as the use of ultrasound- or microwave-assisted processes.
This review provides a compilation of the newest information on the significance of the most important groups of bioactive compounds from plants, namely, phenolics, flavonoids, terpenes, saponins, polysaccharides, and some others. It focuses on the novel findings of their biological activities, the new potential applications of the extracted chemicals, and ecologically sound and efficient extraction methods with the use of DESs. The advantage of applying DESs, as well as the influence of their structures on the efficiency of extraction, is thoroughly discussed. Additionally, technological aspects, including the influence of additives, the extraction system, and conditions, are discussed. The newest solutions of the crucial problem concerning solute recovery after extraction and DES recycling are also presented. Further perspectives, taking into account the strengths and weaknesses of extraction using DESs, are also discussed.
2. The Most Interesting Bioactive Compounds Extracted with DESs from Plant Material
The most interesting, widely investigated plant ingredients belong to one of the following groups: phenolics, flavonoids, terpenoids, saponins, polysaccharides, or alkaloids. Each group contains many bioactive compounds with interesting properties, which makes them promising chemicals for the pharmaceutical, cosmetic, and food industries. In this section, the properties and potencies of the compounds most frequently mentioned in the recent literature are presented.
2.1. Phenolic Compounds
Phenolic compounds can be successfully extracted from plants occurring in all habitats. It is also worth mentioning that the majority of them are already known for their interesting properties that are useful in medicine, foods, or cosmetics. They are edible, e.g., found in tomatoes, olives, grapes, common purslane, and common knotgrass; or they are part of the group of herbs, e.g., mint, lavender, and Plantago asiatica.34,35 The antimicrobial activities and perspectives of phenolic applications as food preservatives or biocides are widely discussed.36 Phenolics such as chlorogenic acid, gallic acid, caffeic acid, and rosmarinic acid are secondary metabolites in plants.37 Because of their antioxidant and anti-inflammatory properties, they can be used as active ingredients in the cosmetic, food, and pharmaceutical industries.38−40Table 1 presents the newest findings in the bioactivities and possible future applications of the chosen phenolic compounds extracted from plants.41−86 Based on the information presented in Table 1, it is clear that current research focuses mostly on the medical application of phenolics in anticancer preparations. It is worth mentioning that phenolics have shown activities in the treatment of other civilization and neurodegenerative diseases.
Table 1. Chemical Structures, Properties, and Application of Phenolic Compounds Present in Plants.
The extraction of phenolic compounds has been widely discussed in the literature. Scientists are attempting to replace classic methods with more ecologically sound methods. An overview of the newest findings in the extraction of these valuable compounds from different types of plant materials using DESs is presented in Table 2. Several factors (the molar ratio of the HBD and HBA in DES, the method of extraction, the temperature, water addition, and the time of extraction) influencing the efficiency of the process are included.87−108
Table 2. Extraction of Phenolic Compounds Using Deep Eutectic Solventsa.
| biomass (part) | DES | molar ratio | method of extraction | temp [°C] | water content [%] | time [min] | extracted compd | yield (calcd on 1 g of dry biomass) | ref |
|---|---|---|---|---|---|---|---|---|---|
| Vitis rotundifolia (fruit skins) | ChCl:lactic acid | 1:2 | UAE | 60 | 20 | 30 | ellagic acid | 21300 μg/g | (87) |
| ChCl:oxalic acid | 1:1 | UAE | 60 | 30 | 30 | ellagic acid | 16000 μg/g | ||
| ChCl:lactic acid | 1:2 | UAE | 60 | 20 | 30 | gallic acid | 10300 μg/g | ||
| ChCl:oxalic acid | 1:1 | UAE | 60 | 30 | 30 | gallic acid | 10400 μg/g | ||
| Olea europaea L. (leaves) | ChCl:acetic acid | 1:1 | SAE | 50 | 20 | 180 | total phenolic content | 470 μg GA/gb | (88) |
| ChCl:malic acid | 1:1 | SAE | 50 | 20 | 180 | total phenolic content | 260 μg GA/gb | ||
| ChCl:malonic acid | 1:1 | SAE | 50 | 20 | 180 | total phenolic content | 250 μg GA/gb | ||
| ChCl:citric acid | 2:1 | SAE | 50 | 20 | 180 | total phenolic content | 240 μg GA/gb | ||
| Solanum lycopersicum (fruit peels) | ChCl:1,2-propanediol | 1:2 | UAE | 65 | 10 | 60 | caffeic acid | 2 μg/g | (89) |
| ChCl:1,2-propanediol | 1:2 | UAE | 65 | 10 | 60 | chlorogenic acid | 37 μg/g | ||
| ChCl:1,2-propanediol | 1:2 | UAE | 65 | 10 | 60 | gallic acid | 10 μg/g | ||
| ChCl:lactic acid | 1:2 | UAE | 65 | 10 | 60 | caffeic acid | 5 μg/g | ||
| ChCl:lactic acid | 1:2 | UAE | 65 | 10 | 60 | chlorogenic acid | 52 μg/g | ||
| ChCl:lactic acid | 1:2 | UAE | 65 | 10 | 60 | gallic acid | 14 μg/g | ||
| Apocynum ventem L. (leaves) | ChCl:levulinic acid | 1:2 | SAE | 50 | 45 | 30 | gallic acid | 1009 μg/g | (90) |
| ChCl:levulinic acid | 1:2 | SAE | 50 | 45 | 30 | protocatechuic acid | 418 μg/g | ||
| ChCl:levulinic acid | 1:2 | MAE | – | 45 | 0.5 | gallic acid | 924 μg/g | ||
| ChCl:levulinic acid | 1:2 | MAE | – | 45 | 0.5 | protocatechuic acid | 575 μg/g | ||
| Brewer’s spent grain | ChCl:glycerol | 1:2 | MAE | – | 25 | 20 | total phenolic content | 3780 μg GA/gb | (91) |
| ChCl:ethylene glycol | 1:2 | MAE | – | 25 | 20 | total phenolic content | 2150 μg GA/gb | ||
| Leptospermum scoparium (leaves) | ChCl:ethylene glycol | 1:2 | SAE | RT | 0 | 60 | total phenolic content | 56870 μg GA/gb | (92) |
| ChCl:1,3-propanediol | 1:3 | SAE | RT | 0 | 60 | total phenolic content | 50670 μg GA/gb | ||
| ChCl:lactic acid | 1:2 | SAE | RT | 0 | 60 | total phenolic content | 52520 μg GA/gb | ||
| ChCl:acetic acid | 1:2 | SAE | RT | 0 | 60 | total phenolic content | 32490 μg GA/gb | ||
| Lavandula angustifolia (flowers) | ChCl:glycerol | 1:2 | UAE | 40 | 30 | 30 | total phenolic content | 44650 μg GA/gb | (93) |
| ChCl:glycerol | 1:3 | UAE | 40 | 30 | 30 | total phenolic content | 41460 μg GA/gb | ||
| ChCl:glycerol | 1:4 | UAE | 40 | 30 | 30 | total phenolic content | 40750 μg GA/gb | ||
| Lilium lancifolium Thunb. (bulbs) | ChCl:ethylene glycol | 1:2 | SAE | 50 | 20 | 40 | regaloside C | 310 μg/g | (94) |
| ChCl:ethylene glycol | 1:2 | SAE | 50 | 20 | 40 | regaloside E | 290 μg/g | ||
| ChCl:ethylene glycol | 1:2 | SAE | 50 | 20 | 40 | regaloside B | 3040 μg/g | ||
| Plantago asiatica L. (aerial part) | ChCl:lactic acid:ethylene glycol | 1:4:2 | UAE | 60 | 50 | 35 | plantamajoside | 3830 μg/g | (95) |
| ChCl:lactic acid:ethylene glycol | 1:4:2 | UAE | 60 | 50 | 35 | acteoside | 4230 μg/g | ||
| Sanghuangporus baumii (fruiting body) | ChCl:malic acid | 1:2 | UAE | 50 | 40 | 30 | total phenolic content | 6370 μg GA/gb | (96) |
| Polygonum aviculare (leaves) | ChCl:levulinic acid | 1:2 | UAE | 70 | 38 | 60 | gallic acid | 39800 μg/g | (97) |
| ChCl:levulinic acid | 1:2 | UAE | 70 | 38 | 60 | 5-caffeoylquinic acid | 11830 μg/g | ||
| ChCl:levulinic acid | 1:2 | UAE | 70 | 38 | 60 | chlorogenic acid | 7160 μg/g | ||
| Eucommia ulmoides (leaves) | ChCl:1,4-butanediol:ascorbic acid | 1:1:0.1 | MAE | – | 20 | 20 | chlorogenic acid | 3659 μg/g | (98) |
| Achillea millefolium L. (aerial parts) | ChCl:lactic acid | 1:2 | UAE | 50 | 25 | 30 | chlorogenic acid | 2970 μg/g | (99) |
| ChCl:urea | 2:1 | 2900 μg/g | |||||||
| Mentha piperita (leaves) | ChCl:glycerol | 1:1 | SAE | 60 | 30 | 30 | chlorogenic acid | 160 μg/g | (100) |
| ChCl:glycerol | 1:1 | SAE | 60 | 30 | 30 | rosmarinic acid | 3233 μg/g | ||
| ChCl:citric acid | 1:1 | SAE | 60 | 30 | 30 | chlorogenic acid | 206 μg/g | ||
| ChCl:citric acid | 1:1 | SAE | 60 | 30 | 30 | rosmarinic acid | 12005 μg/g | ||
| Rhus coriaria L. (fruits) | ChCl:lactic acid | 1:2 | UAE | 25 | 20 | 30 | total phenolic content | 124960 μg GA/gb | (101) |
| ChCl:acetic acid | 1:2 | UAE | 25 | 20 | 30 | total phenolic content | 119570 μg GA/gb | ||
| Ginkgo biloba (leaves) | ChCl:ascorbic acid | 1:1 | UAE | 70 | 30 | 30 | ginkgolic acid | 10434 μg/g | (102) |
| ChCl:fructose | 1:1 | UAE | 70 | 30 | 30 | ginkgolic acid | 8155 μg/g | ||
| Malus domestica (fruit pomace) | ChCl:glycerol | 1:2 | UAE | 30 | 30 | 30 | total phenolic content | 5600 μg GA/gb | (103) |
| ChCl:citric acid | 1:1 | UAE | 30 | 30 | 30 | total phenolic content | 4600 μg GA/gb | ||
| Mentha piperita (leaves) | ChCl:glycerol | 1:2 | SAE | 70 | 20 | 180 | rosmarinic acid | 5090 μg/g | (104) |
| Curcuma longa L. (rhizomes) | ChCl:citric acid | 1:1 | MAE | 30 | 30 | 6 | curcuminoids | 89870 μg/g | (105) |
| Phoenix dactylifera L. (seeds) | ChCl:lactic acid | 1:2 | UAE | 40 | 30 | 15 | total phenolic content | 145540 μg GA/gb | (106) |
| Cosmos sulphureus (seeds) | ChCl:lactic acid | 1:2 | UAE | 47.5 | 32.6 | 40 | total phenolic content | 35300 μg GA/gb | (107) |
| Curcuma longa L. (rhizomes) | ChCl:malonic acid | 1:1 | UAE | 90 | 50 | 60 | curcuminoids | 165510 μg/g | (108) |
| ChCl:propionic acid | 1:2 | UAE | 90 | 50 | 60 | curcuminoids | 120920 μg/g | ||
| ChCl:oxalic acid | 1:1 | UAE | 90 | 50 | 60 | curcuminoids | 112210 μg/g | ||
| ChCl:ethylene glycol | 1:2 | UAE | 90 | 50 | 60 | curcuminoids | 50870 μg/g |
RT, room temperature; GA, gallic acid; UAE, ultrasound-assisted extraction; MAE, microwave-assisted extraction; SAE, stirring-assisted extraction.
Mass of all phenolic compounds, calculated as mass of the gallic acid.
The yields of extracted chemicals depend on a few factors. The major factor is the type of plant and the contents of the active compounds. However, some trends in the influences of other factors can also be observed. When the type of HBD in the DES is considered, the application of an acidic HBD, rather than polyalcohols and sugars, is more favorable. For instance, in an extraction from Solanum lycopersicum, higher yields of gallic acid, chlorogenic acid, and caffeic acid were obtained while using DES ChCl:lactic acid, compared to ChCl:1,2-propanediol (for gallic acid, the difference was 4 μg/g; for chlorogenic acid, 15 μg/g; and for caffeic acid, 3 μg/g).89 The same phenomenon was observed in the isolation of rosmarinic acid from Mentha piperita. The DES ChCl:citric acid was much more efficient than ChCl:glycerol (the yield was 8800 μg/g higher).99 Similar results were observed by Alrugaibah et al.87 The extraction of ellagic acid from Vitis rotundifolia was much more efficient with DES ChCl:lactic acid (21.3 mg/g of dry biomass) and ChCl:oxalic acid (16.0 mg/g), compared to ChCl:1,2-propanediol (8.4 mg/g).
The other important issue in the extraction of phenolics with DESs is the chosen technique. As can be seen in Table 2, ultrasound- or microwave-assisted processes allow for a reduced time of extraction and a high yield of the desired chemical to be obtained. For example, in the microwave extraction of protocatechuic acid from Apocynum ventem L. with the DES ChCl:levulinic acid, a yield of 575 μg/g was obtained in 30 s, while traditional stirring extraction required 30 min to obtain 418 μg/g.90
2.2. Flavonoids
Flavonoids can also be considered as phenolic compounds, but because of their distinctive chemical structures, based on 2-phenyl-chromone, they are presented separately.109 Similar to phenolics, flavonoids can be found in plants from all over the world, especially in edible ones. The richest sources are fruits from Prunus (e.g., cherry and plum) and Vaccinium (e.g., cranberry and blueberry) species. There have also been many attempts to utilize byproducts or wastes from the forestry and food industries. An interesting example is the utilization of bark from Larix decidua (European larch), which is a byproduct of timber production. Studies revealed that it can be a promising and cheap source of flavonoids.110,111 Flavonoids have great potential as anticancer and anti-inflammatory pharmaceuticals, as well as antiaging agents in cosmetics. Examples of these compounds, extracted from plants with DESs, are presented in Table 3.46,112−181
Table 3. Chemical Structures, Properties, and Application of Flavonoids Present in Plants.
Flavonoids are commonly presented as glycosides, such as cyanidin-3-O-glucoside and quercetin 3-rutinoside, because of their increased efficiencies as pharmaceuticals. After glycosylation their water solubilities and stabilities are enhanced, which improves their bioavailability.182
Natural flavonoids have also been studied as additives/preservatives in the food industry. They have very good antioxidative and antimicrobial properties, which makes them promising natural preservatives. Examples of flavonoid extraction with DESs from plant material are presented in Table 4.89,90,95,97,98,100,104,183−196
Table 4. Extraction of Flavonoids Using Deep Eutectic Solvents.
| biomass | DES | molar ratio | method of extractiona | temp [°C] | water content [%] | time [min] | extracted compd | yield (calcd on 1 g of dry biomass) | ref |
|---|---|---|---|---|---|---|---|---|---|
| Polygonatum odoratum (rhizomes) | ChCl:malic acid | 1:1 | UAE | 50 | 0 | 20 | total flavonoid content | 4760 μg/g | (183) |
| ChCl:citric acid | 1:1 | UAE | 50 | 0 | 20 | total flavonoid content | 6830 μg/g | ||
| ChCl:lactic acid | 1:1 | UAE | 50 | 0 | 20 | total flavonoid content | 7320 μg/g | ||
| Larix decidua (bark) | ChCl:urea | 1:2 | SAE | 58 | 75 | 94 | total flavonoid content | 159000 μg/g | (184) |
| ChCl:urea | 1:2 | SAE | 58 | 50 | 94 | total flavonoid content | 305000 μg/g | ||
| ChCl:urea | 1:2 | SAE | 58 | 25 | 94 | total flavonoid content | 275000 μg/g | ||
| ChCl:1,4-butanediol | 1:2 | SAE | 58 | 75 | 94 | total flavonoid content | 376000 μg/g | ||
| ChCl:1,4-butanediol | 1:2 | SAE | 58 | 50 | 94 | total flavonoid content | 376000 μg/g | ||
| ChCl:1,4-butanediol | 1:2 | SAE | 58 | 25 | 94 | total flavonoid content | 383000 μg/g | ||
| Polygonatum sibiricum (whole plant) | ChCl:acetic acid | 1:4 | UAE | 50 | 30 | 30 | total flavonoid content | 3000 μg/g | (185) |
| Solanum lycopersicum (fruit peels) | ChCl:1,2-propanediol | 1:2 | UAE | 65 | 10 | 60 | quercetin | 1.02 μg/g | (89) |
| ChCl:lactic acid | 1:2 | UAE | 65 | 10 | 60 | quercetin | 1.47 μg/g | ||
| Apocynum ventem L. (leaves) | ChCl:levulinic acid | 1:2 | SAE | 50 | 45 | 30 | catechin | 4338 μg/g | (90) |
| ChCl:levulinic acid | 1:2 | SAE | 50 | 45 | 30 | rutin | 169 μg/g | ||
| ChCl:levulinic acid | 1:2 | SAE | 50 | 45 | 30 | isoquercetin | 2031 μg/g | ||
| ChCl:levulinic acid | 1:2 | MAE | – | 45 | 0.5 | catechin | 5025 μg/g | ||
| ChCl:levulinic acid | 1:2 | MAE | – | 45 | 0.5 | rutin | 255 μg/g | ||
| ChCl:levulinic acid | 1:2 | MAE | – | 45 | 0.5 | isoquercetin | 3411 μg/g | ||
| Plantago asiatica L. (aerial part) | ChCl:lactic acid:ethylene glycol | 1:4:2 | UAE | 60 | 50 | 35 | quercetin | 560 μg/g | (95) |
| ChCl:lactic acid:ethylene glycol | 1:4:2 | UAE | 60 | 50 | 35 | kaempferol | 190 μg/g | ||
| Syzygium cumini (fruits) | ChCl:citric acid | 1:1 | MAE | – | 40 | 2.5 | total anthocyanine content | 7864 μg/g | (186) |
| ChCl:citric acid | 1:1 | UAE | 70 | 40 | 150 | total anthocyanine content | 8525 μg/g | ||
| Polygonum aviculare (leaves) | ChCl:levulinic acid | 1:2 | UAE | 70 | 38 | 60 | myricitrin | 10470 μg/g | (97) |
| ChCl:levulinic acid | 1:2 | UAE | 70 | 38 | 60 | 3″-O-galloylmyricitrin | 7920 μg/g | ||
| ChCl:levulinic acid | 1:2 | UAE | 70 | 38 | 60 | quercitrin | 4260 μg/g | ||
| ChCl:levulinic acid | 1:2 | UAE | 70 | 38 | 60 | avicularin | 3020 μg/g | ||
| Chrysanthemum indicum L. (flowers) | ChCl:ethylene glycol | 1:2 | UAE | RT | 30 | 32 | linarin | 14230 μg/g | (187) |
| Fagopyrum esculentum (sprouts) | ChCl:urea | 1:2 | UAE | 40 | 20 | 40 | isoorientin | 5110 μg/g | (188) |
| ChCl:triethylene glycol | 1:4 | UAE | 40 | 20 | 40 | isoorientin | 6660 μg/g | ||
| ChCl:urea | 1:2 | UAE | 40 | 20 | 40 | rutin | 1840 μg/g | ||
| ChCl:triethylene glycol | 1:4 | UAE | 40 | 20 | 40 | rutin | 2220 μg/g | ||
| pollen typhae (pollen) | ChCl:1,2-propanediol | 1:4 | UAE | RT | 30 | 35 | quercetin | 383 μg/g | (189) |
| ChCl:1,2-propanediol | 1:4 | UAE | RT | 30 | 35 | naringenin | 48 μg/g | ||
| ChCl:1,2-propanediol | 1:4 | UAE | RT | 30 | 35 | kaempferol | 391 μg/g | ||
| ChCl:1,2-propanediol | 1:4 | UAE | RT | 30 | 35 | isorhamnetin | 3149 μg/g | ||
| cranberry pomace | ChCl:lactic acid | 1:2 | UAE | 60 | 20 | 30 | cyanidin-3-galactoside | 180 μg/g | (190) |
| ChCl:lactic acid | 1:2 | UAE | 60 | 20 | 30 | peonidin 3-arabinoside | 320 μg/g | ||
| ChCl:lactic acid | 1:2 | UAE | 60 | 50 | 30 | cyanidin-3-galactoside | 300 μg/g | ||
| ChCl:lactic acid | 1:2 | UAE | 60 | 50 | 30 | peonidin 3-arabinoside | 600 μg/g | ||
| Eucommia ulmoides (leaves) | ChCl:1,4-butanediol:ascorbic acid | 1:1:0.2 | MAE | – | 20 | 20 | rutin | 1143 μg/g | (98) |
| ChCl:1,4-butanediol:ascorbic acid | 1:1:0.2 | MAE | – | 20 | 20 | isoquercetin | 1087 μg/g | ||
| Jaboticaba processing byproduct | ChCl:propylene glycol | 1:2 | pressurized liquid extraction | 90 | 53 | 12 | cyanidin-3-O-glucoside | 29400 μg/g | (191) |
| ChCl:malic acid | 1:2 | pressurized liquid extraction | 90 | 53 | 12 | cyanidin-3-O-glucoside | 25780 μg/g | ||
| Buddleja globosa (leaves) | ChCl:1,2-propanediol | 1:3 | SAE | 60 | 20 | 20 | luteolin-7-O-glucoside | 4387 μg/g | (192) |
| Mentha piperita (leaves) | ChCl:glycerol | 1:1 | SAE | 60 | 30 | 30 | luteolin-7-O-glucoside | 5856 μg/g | (100) |
| ChCl:glycerol | 1:1 | SAE | 60 | 30 | 30 | apigenin | 361 μg/g | ||
| ChCl:citric acid | 1:1 | SAE | 60 | 30 | 30 | luteolin 7-O-glucoside | 7998 μg/g | ||
| ChCl:citric acid | 1:1 | SAE | 60 | 30 | 30 | apigenin | 384 μg/g | ||
| Polygonum maritimum L. (leaves and stems) | ChCl:sucrose | 1:2 | UAE | RT | 30 | 30 | rhamnetin-3-galactoside | 1470 μg/g | (193) |
| sour cherry pomace | ChCl:malic acid | 1:1 | SAE | 40 | 20 | 30 | quercetin-3-rutinoside | 172 μg/g | (194) |
| ChCl:malic acid | 1:1 | UAE | 40 | 20 | 30 | quercetin-3-rutinoside | 119 μg/g | ||
| ChCl:malic acid | 1:1 | MAE | – | 20 | 0.25 | quercetin-3-rutinoside | 138 μg/g | ||
| Potentilla Fruticose L. (leaves and stems) | ChCl:citric acid | 1:1 | UAE | 28 | 30 | 30 | catechin-7-O-glucoside | 12200 μg/g | (195) |
| Mentha piperita (leaves) | ChCl:glycerol | 1:2 | SAE | 70 | 20 | 180 | hesperidin | 3220 μg/g | (104) |
| Dendrobium officinale (stems) | ChCl:lactic acid | 1:4 | UAE | 50 | 30 | 40 | total flavonoid content | 35240 μg/g | (196) |
UAE, ultrasound-assisted extraction; MAE, microwave-assisted extraction; SAE, stirring-assisted extraction.
As can be seen from Table 4, there is no clear tendency for the influence of DES composition on the yield of flavonoids. For example, in the extraction of quercetin from tomatoes, the yield was similar for DES with acidic and diol HBDs (1.02 μg/g for DES ChCl:1,2-propanediol and 1,47 μg/g for ChCl:lactic acid).89 In some cases, better results were obtained in an extraction with DES containing diols compared to other HBDs (e.g., the yield of rutin extracted from Fagopyrum esculentum with ChCl:triethylene glycol was higher than that extracted with ChCl:urea; the difference was 380 μg/g).188
2.3. Terpenes, Terpenoids, and Saponins
Another class of compounds with great importance is terpenes and their derivatives—terpenoids are considered to be one of the most diverse groups of plant metabolites.168 They are present in many plants and in some marine organisms. A unique feature of all terpenes is a chemical structure based on isoprene molecules. Many of them have pharmacological activities—anticancer, antimicrobial, and anti-inflammatory.169,170 Because of their diversity and interesting properties, they have become important ingredients in food, cosmetics, and pharmaceuticals. Their chemical synthesis is usually unprofitable or unsustainable, so efficient extraction from natural sources seems to be a very important direction in research and industrial development.197−199Table 5 presents a few examples of terpenes and terpenoids, with their activities and possible applications.133,200−225
Table 5. Chemical Structures, Properties, and Application of Terpenes, Terpenoids, and Saponins Present in Plants.
The extraction of these groups of compounds from the plant material can be effectively carried out with DESs based on choline chloride as the HBA, summarized in Table 6.102,226−233
Table 6. Extraction of Terpenes, Terpenoids, and Saponins Using Deep Eutectic Solvents.
| biomass | DES | molar ratio | method of extractiona | temp [°C] | water content [%] | time [min] | extracted compd | yield (calcd on 1 g of dry biomass) | ref |
|---|---|---|---|---|---|---|---|---|---|
| Glycyrrhiza glabra (roots) | ChCl:glycerol | 1:1 | SAE | 30 | 15 | 60 | glycyrrhizic acid | 23250 μg/g | (226) |
| ChCl:malic acid | 1:1 | SAE | 30 | 15 | 60 | glycyrrhizic acid | 36700 μg/g | ||
| ChCl:oxalic acid | 1:1 | SAE | 30 | 15 | 60 | glycyrrhizic acid | 39600 μg/g | ||
| ChCl:lactic acid | 1:1 | SAE | 30 | 15 | 60 | glycyrrhizic acid | 42820 μg/g | ||
| ChCl:succinic acid | 1:1 | SAE | 30 | 15 | 60 | glycyrrhizic acid | 43650 μg/g | ||
| ChCl:citric acid | 1:1 | SAE | 30 | 15 | 60 | glycyrrhizic acid | 30670 μg/g | ||
| Glycyrrhiza glabra (roots) | ChCl:lactic acid | 1:1 | SAE | 40 | 30 | 30 | glycyrrhizic acid | 53720 μg/g | (227) |
| Betula pendula (bark) | ChCl:lactic acid | 1:1 | SAE | 60 | 5.4 | 150 | betulinic acid | 141 μg/g | (228) |
| ChCl:lactic acid | 1:1 | SAE | 80 | 5.4 | 150 | betulinic acid | 263 μg/g | ||
| ChCl:lactic acid | 1:1 | SAE | 60 | 5.4 | 75 | betulin | 1217 μg/g | ||
| ChCl:lactic acid | 1:1 | SAE | 60 | 5.4 | 105 | betulin | 1015 μg/g | ||
| ChCl:lactic acid | 1:1 | SAE | 60 | 5.4 | 150 | betulin | 878 μg/g | ||
| ChCl:lactic acid | 1:1 | SAE | 80 | 5.4 | 75 | betulin | 1332 μg/g | ||
| ChCl:lactic acid | 1:1 | SAE | 80 | 5.4 | 105 | betulin | 1788 μg/g | ||
| ChCl:lactic acid | 1:1 | SAE | 80 | 5.4 | 150 | betulin | 1329 μg/g | ||
| Dioscorea nipponica (rhizomes) | ChCl:malonic acid | 1:1 | UAE | RT | 30 | 20 | pseudoprotodioscin | 9710 μg/g | (229) |
| ChCl:malonic acid | 1:1 | UAE | RT | 30 | 20 | protodioscin | 29300 μg/g | ||
| ChCl:malonic acid | 1:1 | UAE | RT | 30 | 20 | protogracillin | 15860 μg/g | ||
| ChCl:malonic acid | 1:1 | UAE | RT | 30 | 20 | pseudoprotogracillin | 3660 μg/g | ||
| Salvia officinalis L. (leaves) | ChCl:lactic acid | 1:2 | SAE | 50 | 10 | 90 | carnosic acid | 14.43 μg/g | (230) |
| ChCl:lactic acid | 1:2 | SAE | 30 | 10 | 60 | carnosic acid | 10.68 μg/g | ||
| ChCl:lactic acid | 1:2 | SAE | 70 | 10 | 60 | carnosic acid | 10.16 μg/g | ||
| Ginkgo biloba(leaves) | ChCl:ascorbic acid | 1:1 | UAE | 70 | 30 | 30 | bilobalide | 7518 μg/g | (102) |
| ChCl:fructose | 1:1 | UAE | 70 | 30 | 30 | bilobalide | 6552 μg/g | ||
| Xanthoceras sorbifolia Bunge. (husks) | ChCl:glycolic acid | 1:2 | SAE | 60 | 30 | 30 | total saponin content | 60720 μg/g | (231) |
| Panax quinquefolius L. (roots) | ChCl:ethylene glycol | 1:2 | SAE | 60 | 20 | 30 | ginsenosides | 71590 μg/g | (232) |
| Trillium govanianum (rhizomes) | ChCl:lactic acid | 1:1 | UAE | 50 | 0 | 60 | total saponin content | 114400 μg/g | (233) |
UAE, ultrasound-assisted extraction; MAE, microwave-assisted extraction; SAE, stirring-assisted extraction.
Licorice (Glycyrrhiza glabra) is one of the most commonly used plants, because of its high content of glycyrrhizic acid. In the isolation of terpenoids and saponins, the impact of HBDs in applied DESs is similar to the case of phenolic compound extraction: higher yields of products are achieved with DESs when acidic HBDs are used. For example, in a process of bilobalide extraction from Ginkgo biloba, extraction with the DES ChCl:ascorbic acid yielded 7518 μg/g product while in the presence of ChCl:fructose 6552 μg/g was obtained.102 Similar results were reported by Lanjekar and Rathod—the yield of glycyrrhizic acid obtained from licorice was nearly twice as high when succinic acid was the HBD, compared to DES with glycerol.226
2.4. Other Chemicals Extracted from Plants with DESs
There are many other interesting bioactive molecules that can be isolated from plants, apart from the mentioned phenolics, flavonoids, terpenoids, and saponins. Many of them present biological activities that are required by industries, especially the pharmaceutical industry. For instance, polysaccharides extracted from Zizyphus jujube fruits show prebiotic activity, as tested on bacteria.234 Similar properties were demonstrated for polysaccharides from Lithocarpus litseifolius leaves. In addition, these compounds could be applied in diabetes treatment.235 Polysaccharides extracted from lotus leaves were examined in terms of their antioxidant capacities. Through extraction with DESs, the antioxidant properties of compounds were more intense compared to the extracts isolated with water.236 Other interesting natural chemicals are alkaloids with potential anti-inflammatory bioactivities. This group of compounds has many possible applications, such as the improvement of immunological defense and the treatment of cardiovascular and liver diseases.237−239 Carotenoids are chemicals that are commonly present in edible plants, but due to their hydrophobic nature, the recent literature focuses on applying hydrophobic DESs in their extraction.240Table 7 presents a brief review on other chemicals extracted using DESs.234,236,241−244
Table 7. Extraction of Other Chemicals Using Deep Eutectic Solvents.
| biomass | DES | molar ratio | method of extraction | temp [°C] | water content [%] | time [min] | extracted compd | yield (calcd on 1 g of dry biomass) | ref |
|---|---|---|---|---|---|---|---|---|---|
| Ziziphus jujuba Mill. (fruits) | ChCl:ethylene glycol | 1:3.75 | SAE | 90 | 53 | 120 | polysaccharides | 63600 μg/g | (234) |
| Nelumbo nucifera Gaertn. (leaves) | ChCl:ethylene glycol | 1:3 | SAE | 90 | 30 | 180 | polysaccharides | 778900 μg/g | (236) |
| ChCl:ethylene glycol | 1:3 | MAE | – | 0 | 6.5 | polysaccharides | 832000 μg/g | ||
| Nelumbo nucifera Gaertn. (leaves) | ChCl:propylene glycol | 1:4 | UAE | RT | 30 | 30 | O-nornuciferine | 690 μg/g | (241) |
| ChCl:propylene glycol | 1:4 | UAE | RT | 30 | 30 | nuciferine | 3340 μg/g | ||
| Peumus boldus Mol. (leaves) | ChCl:levulinic acid | 1:1 | SAE | 60 | 20 | 50 | boldine | 427 μg/g | (242) |
| Phellodendri amurensis (cortex) | ChCl:citric acid | 1:2 | UAE | 25 | 30 | 30 | berberine | 10820 μg/g | (243) |
| ChCl:citric acid | 1:2 | UAE | 25 | 30 | 30 | palmatine | 4740 μg/g | ||
| apricot pulp | ChCl:tartaric acid | 2:1 | UAE | 30 | 20 (MeOH) | 10 | β-carotene | 413 μg/g | (244) |
3. Effect of Type of DES and Process Conditions for Extraction Efficiency
3.1. Comparison of DESs and Organic Solvents in the Extraction of Bioactive Plant Ingredients
Traditional methods of extraction include Soxhlet extraction with solvents such as ethanol, methanol, and acetone245 and extraction with the aid of stirring and heating, commonly used together with the assistance of ultrasound or microwaves.246−249 The application of organic solvents in the extraction processes comes with some disadvantages, such as high flammability, high vapor pressure, and, in some cases, toxicity.250,251 Additionally, some extracted compounds, e.g., anthocyanins, are prone to degradation at high temperatures. Thus, it is even more difficult to efficiently extract them with organic solvents. Because of that, there is a need for a simple and ecological extraction method under mild conditions.252 The application of DESs can be an option for solving the above-mentioned problems.253 In many cases, they are more efficient as extraction media, compared with traditional organic solvents.30,254 It was shown that higher antioxidant activity was retained by plant extracts obtained using DESs as the extraction media when compared to organic solvents.255 Apart from a higher extraction efficiency, DESs can be more selective than organic solvents. By changing the HBA and HBD, it is possible to adjust the polarity of a DES to that of the target compound.256 Additionally, extraction with a DES requires less solvent.30 A comparison of extraction efficiencies using DESs and organic solvents is presented in Table 8.88,90,100,102,257,258
Table 8. Advantage of Using DESs over Organic Solvents in Extraction of Bioactive Compounds from Plant Material.
| biomass | extracted compd | organic solvent | yield [μg/g] (calcd on 1 g of dry biomass) | DES | yield [μg/g] (calcd on 1 g of dry biomass) | ref |
|---|---|---|---|---|---|---|
| Mentha piperita (leaves) | chlorogenic acid | ethanol | 175.94 | ChCl:malonic acid | 194.01 | (100) |
| Apocynum ventem L. (leaves) | protocatechuic acid | methanol | 284.00 | ChCl:levulinic acid | 418.00 | (90) |
| Ginkgo biloba L. (leaves) | bilobalide | ethanol | 5100.00 | ChCl:ascorbic acid | 7520.00 | (102) |
| Lavandula pedunculata subsp. Lusitanica (aerial parts) | caffeic acid | methanol | 81.50 | ChCl:urea | 248.30 | (257) |
| Olea europaea L. (leaves) | total phenolic content | ethanol | 16030.00 | ChCl:acetic acid | 34610.00 | (88) |
| Carthamus tinctorius L. (flowers) | hydroxysafflor yellow A | methanol | 15540.00 | ChCl:urea | 22810.00 | (258) |
The biggest challenge in extraction processes with DESs is the recovery of the extracted chemicals from the solvent. The low vapor pressure of the DES is an advantage from an ecological point of view, but this makes it impossible to separate the solvent from the extract via distillation, which is a common method in organic solvent extraction. In the latest reports, macroporous resins or solid-phase extraction were applied during the separation of extracted compounds from DESs.259
3.2. Impact of HBA and HBD in DES
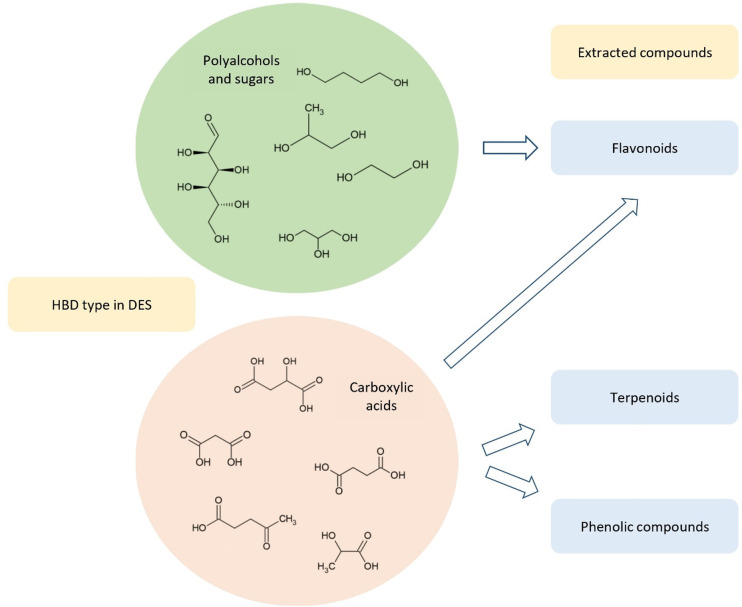
As has been mentioned in previous sections, the impact of the HBD on the extraction efficiency depends on the type of the extracted compound. The extraction of flavonoids can be efficiently performed with DESs that possess HBDs such as polyalcohols and organic acids. In the case of phenolic compounds and terpenoids, higher yields of products are obtained in the presence of DESs composed of organic acids as HBDs102,108,226 (Figure 3).
Figure 3.
Dependence of HBD in DES and efficiency of extracted metabolites.
This phenomenon is connected with the polarities of DESs and extracted compounds.260 The interaction of solute and solvent is indicated by the polarity, which in turn can be studied using solvatochromic data. The ETN parameter (normalized polarity) and Kamlet–Taft parameters, namely, α (hydrogen bond donating ability), β (hydrogen bond accepting ability), and π* (dipolarity/polarizability), give information about intermolecular interactions. It has been shown that, based on ETN values of DESs having the same HBD (levulinic acid), a hydrophilic HBA makes the solvent more polar. This has been explained by the stability of the hydrogen bond (chloride anion in hydrophilic choline chloride HBA forms a more stable hydrogen bond with HBD than hydrophobic methanol). It can be assumed that, in DESs based on the same HBD, the character of the HBA controls their dipolarity/polarizability as well as the β parameter. Additionally, DESs with carboxylic acids displayed high α values. It was also shown that the addition of water to DESs based on choline chloride and polyalcohols decreases the β parameter and increases π*.261 The extraction efficiency can be partially predicted by measuring the αβ value, which represents the strength of hydrogen bonding in the solvent. The efficiency of isolation should increase with higher αβ values.262 Many phenolic compounds (e.g., gallic acid and glycyrrhizic acid) are polar, so DESs with higher polarities should be more appropriate for extraction.263,264 A study presented by Jurić et al. confirmed that DESs containing organic acids with higher polarities compared to polyalcohols and sugars are better extraction solvents for polar metabolites in the plant material.265 In some cases, extraction with polyalcohol-based DESs is more efficient. For instance, the extraction of phenolic compounds from Leptospermum scoparium with DES ChCl:ethylene glycol allowed for better yields of phenolics to be obtained (56.87 mg/g of dry biomass, calculated as equivalents of gallic acid) than those with ChCl:lactic acid (52.51 mg/g of dry biomass) or ChCl:acetic acid (32.49 mg/g).92 This could be explained by lower viscosities of some DESs with diols as HBDs than with organic acids, which facilitates mass transport during extraction (e.g., the viscosity of ChCl:lactic acid at 25 °C is equal 301.902 mPa·s and that of ChCl:ethylene glycol is equal to 32.045 mPa·s).92,192 Some hydrophobic DESs, based on fatty acids, also show low viscosities, which improves extraction efficiency.15 The extraction efficiency also depends on the pKa of the HBD and the extracted compound. Metabolites with pKa values that are lower than or similar to that of the HBD can form stronger hydrogen bond based interactions with the chloride anion in the HBA, compared to the HBD. This results in a higher extraction efficiency.226,266 Furthermore, if the extracted compound has more donor groups (carboxylic acid or hydroxyl groups), it can interact with HBAs even more strongly. The formation of hydrogen bonds is connected with solvation enthalpy, which is preferably exothermic. The isolation effectiveness is also driven by the extraction entropy change, which is an effect of breaking the DES structure via the solute. In the solvent structure, a cavity needs to be created to accommodate the solubilized compound. This fact is an explanation for the low extraction efficiencies of larger compounds—more energy is needed to create a cavity. It could be concluded that the isolation effectiveness depends mostly on the hydrogen bond creation between the compound and the chloride atom in the HBA, which is the most significant in extraction of polar chemicals.266
The same general rules and exceptions apply to flavonoids.267 Additionally, many flavonoids are soluble in alkaline solutions, so an extraction with acidic solvents is not preferable.268
3.3. Impact of the Extraction Method and Temperature
Natural compounds can be extracted using DESs through various methods, including assistance by ultrasound or microwaves or subcritical extraction techniques. These techniques are considered to be more effective than traditional stirring with heating because the plant cell walls can be more easily disrupted, which translates into a more efficient release of bioactive compounds.269,270 It also decreases the time needed for extraction.74,153,190 Unfortunately, microwave-assisted extraction can sometimes cause the degradation of sensitive metabolites.194,271,272
Considering the influence of the temperature of extraction, most of the processes are performed in the range 40–70 °C. A further increase in the temperature is risky, because some of the compounds, especially phenolics, tend to decompose at high temperatures, which affects their antioxidant properties.88,194,273 DESs can also react with extracted chemicals under such conditions, forming undesirable polymeric side products.274 On the other hand, the higher the temperature, the lower the viscosities of DESs and thus the better the mass transfer. Higher temperatures also enhance the cleavage of bonds between extracted compounds and the plant matrix.275
3.4. Impact of Water Content
Water content in the extraction media is another crucial element in the isolation of secondary metabolites from plants. Generally, DESs based on choline chloride have high viscosities, which have a negative effect on the extraction yield due to poor mass transfer. This problem can be solved by increasing the temperature of extraction (as was discussed in previous sections) or via the addition of water. In some cases, an addition of 25% (v/v) water to a DES can decrease the viscosity to the level of the water viscosity.276 Water can also increase the DES polarity, which can facilitate the extraction of some polar compounds, which creates a possibility for enhancing the selectivity of the extraction.277−279 Because of the high water concentration in the reaction system, the isolation of compounds with weak polarities was reduced, which was shown by Liu et al.: by increasing the water content to beyond 15%, the yield of curcuminoids extracted from Curcuma longa L. significantly decreased.280 On the other hand, too high a water percentage in the solvent can break the hydrogen bond structure of the DES, which leads to the deterioration of its properties and its abilities to extract bioactive compounds from the plant biomass.273,276,281,282 Apart from hydrogen bond breaking, there is also another possible mechanism that explains this phenomenon. An increase in the water content for the DES ChCl:ethylene glycol to above 30% (v/v) water creates aggregates in which the DES particles are suspended. The hydrogen bonds between the HBA and HBD compounds are preserved, but the eutectic properties of the solvent are significantly lower.283 A brief comparison of the extraction efficiencies, dependent on the water content, is presented in Table 9.103,184,188,226
Table 9. Yields of Extracted Compounds Using DESs, Dependent on Water Content.
| biomass | DES | extracted compd | water content [%] | yield [mg/g on mass of dry biomass] | ref |
|---|---|---|---|---|---|
| Glycyrrhiza glabra (roots) | ChCl:lactic acid | glycyrrhizic acid | 0 | 28.00 | (226) |
| ChCl:lactic acid | glycyrrhizic acid | 30 | 50.00 | ||
| Fagopyrum esculentum (sprouts) | ChCl:oxalic acid | orientin | 0 | 0.44 | (188) |
| ChCl:oxalic acid | orientin | 20 | 2.26 | ||
| Larix decidua (bark) | ChCl:urea | total flavonoid content | 25 | 159.00 | (184) |
| ChCl:urea | total flavonoid content | 50 | 305.00 | ||
| ChCl:urea | total flavonoid content | 75 | 275.00 | ||
| Malus domestica (fruit pomace) | ChCl:lactic acid | total phenolic content | 10 | 3.90 | (103) |
| ChCl:lactic acid | total phenolic content | 30 | 5.20 | ||
| ChCl:lactic acid | total phenolic content | 50 | 1.20 |
4. Recovery of Isolated Chemicals from DESs
The major challenge in the extraction of bioactive chemicals is their recovery from DESs. Because of the negligible vapor pressures of these solvents, it is impossible to separate them from isolated compounds by distillation. There are a few developed methods, which were presented in recent literature, for example, macroporous resin adsorption, solid phase extraction, antisolvent method, and back extraction. The most applied method is adsorption on a macroporous resin, carried out in a packed column or by batch extraction. The recovery rate depends mainly on the type of the resin and its polarity, which should be similar to the polarity of the adsorbate. The most used resins are nonpolar (D-101, HP-20, X-5), middle polar (AB-8), and polar (HPD-600, XAD-7HP). Apart from the resin type, it is important to select the proper eluent, with an optimal desorption capacity.284,285 Solid phase extraction (SPE) is also one of the most popular methods of bioactive compounds recovery. It requires application of cartridges filled with sorbent, mainly reversed phase or hydrophilic–lipophilic balanced, which interacts noncovalently with the eluted analyte. It is one of the most effective methods, which allows recovery of analytes at a level above 90%.188 Even though solid phase extraction is considered an efficient method at the laboratory scale, recent findings in sorbent types (e.g., hierarchically porous silica monoliths providing a durable, high surface area that can be further functionalized with ligands) can open the way to industrial application of that technique.286 Another recovery method is adding antisolvent to the obtained DES extract, which breaks bonds between the HBA and the HBD. This causes the loss of DES properties and precipitation of the extracted chemicals as the antisolvent water is mostly applied, but also buffers.287 However, this method is not suitable for all isolated compounds.288 Back extraction is also a simple method for recovery of chemicals from DESs. It involves re-extraction of analytes with solvents which are immiscible with the DES, but the isolated compounds are soluble in them. The selection of the proper solvent increases the selectivity of re-extraction.288 Ethyl acetate, n-butanol, hexane, and acetone are the solvents most applied; however, they are not considered green chemicals, which makes this method not fully ecological. The economic issue, including the costs of additional solvent, its recovery, and recycling should also be considered. Table 10 presents a brief review on recovery of bioactive compounds from DESs.
Table 10. Recovery of Isolated Bioactive Compounds from DESs after Extraction.
| biomass | DES | extracted compds | recovery method | recovery rate [%] | ref |
|---|---|---|---|---|---|
| Acanthopanax senticosus | lactic acid:glycerol | flavonoids | resin absorption (D101) | 55.76 | (284) |
| lactic acid:glycerol | flavonoids | resin absorption (AB-8) | 77.10 | ||
| Carthamus tinctorius L. | ChCl:sucrose | carthamin | resin absorption (Diaion HP-20) | 90.00 | (285) |
| citrus fruit peels | ChCl:levulinic acid:N-methyl urea | polymethoxylated flavones | back extraction (ethyl acetate) | 95.87 | (288) |
| ChCl:levulinic acid:N-methyl urea | hesperidin | back extraction (n-butanol) | 86.32 | ||
| Fagopyrum esculentum Möench | ChCl:triethylene glycol | orientin | SPE (C18 cartridges) | 97.81 | (188) |
| ChCl:triethylene glycol | rutin | SPE (C18 cartridges) | 98.47 | ||
| Ampelopsis grossedentata | ChCl:glycerol | flavonoids | antisolvent (phosphate buffered saline) | 86.75 | (287) |
| Ipomoea batatas L. | ChCl:malic acid | flavonoids | resin absorption (AB-8) | 85.46 | (289) |
| Citri Reticulatae Pericarpium Viride | l-proline:urea | narirutin | resin absorption (SP825) | 88.00 | (290) |
| l-proline:urea | hesperidin | resin absorption (SP825) | 86.00 |
5. Future Prospects
DESs are considered the solvents of the future that may be used in many fields. They can be applied not only as extraction media but also as drug carriers and adsorbents for harmful metals and organic molecules, H2S, and SO2, which are common byproducts in various processes. DESs may also be used as medium or catalysts in organic syntheses.291 There is an impressive number of publications and recently patented processes of extraction of natural products, with different types of DESs and systems containing DESs.292 The dramatic increase in the number of publications and patents in recent months (67 patent applications within 2022–2023, based on Espacenet) emphasizes the importance of the discussed topic. DESs have been demonstrated to have better extraction properties than many organic solvents, and they seem to be a promising alternative to them, due to the higher yields of extracted bioactive chemicals, preservation of their bioactivity, the mild extraction conditions, and their biodegradability. The proper selection of HBAs and HBDs in DESs allows for a natural, “tailor-made” solvent to be made for the best possible efficiency in the isolation of active compounds. According to the examples presented, the most frequently used solvents are based on organic acids of natural origin, especially lactic acid, which is one of the most suitable HBDs for obtaining high yields for many chemicals. In order to maximize the yields of isolated chemicals, extraction with the assistance of ultrasound or microwaves is recommended. A combination of these techniques with green solvents makes the extraction process more sustainable and consistent with green chemistry rules. Undeniably, studies regarding extractions with DESs are constantly being developed.
This review presented the recent results achieved with DESs, especially solvents based on choline chloride as the HBA. However, there is a growing trend to modify DESs and their properties. Many researchers focus on hydrophobic DESs, which have a great potential for the extraction of less polar compounds, e.g., carotenoids, sulforaphane, and ginkgolic acids.240,293,294 The other tendency is to prepare ternary DESs and NADESs, composed usually of choline chloride, diol or polyalcohol, and acid, e.g., choline chloride/lactic acid/ethylene glycol or choline chloride/butanediol/citric acid, where the acidity can be tuned by using different amounts of acids.95,98 In addition, the development of switchable systems is receiving more attention. With the use of such a system, an efficient and selective separation of compounds with different polarities is possible. For instance, rosmarinic acid and carnosic acid were extracted from rosemary, using a thermo-switchable system composed of a DES and an IL, namely ChCl:levulinic acid and [BMIM]PF6. Due to the different polarities of the solvents, the selective extraction of compounds was possible: the IL phase was rich with less polar carnosic acid and, in the DES phase, polar rosmarinic acid was present.295 Thermo- and pH-switchable DESs are also promising solvents in green extraction. For example, by increasing the pH value, it is possible to create a homogeneous hydrophobic DES–water mixture, and addition of acid after the extraction separates the DES and aqueous phases.296,297 This is a promising approach to the problem of DES separation after extraction. This problem can also be solved by applying magnetic DESs containing metal oxides or salts, e.g., Fe3O4, TiO2, FeCl3, NiCl2, and ZnCl2. These solvents can be separated from the aqueous solution by using a magnetic field. Furthermore, there is evidence from the literature that magnetic DESs are more effective extraction media than traditional DESs.298,299 From the separation of the solute after extraction point of view, deep eutectic solvent molecularly imprinted polymers (DES-MMIPs) have great potential. For example, MMIPs with choline choride/caffeic acid/formic acid used as functional monomers were obtained and successfully used in the extraction and determination of hormones from lotion.300 When new deep eutectic systems are designed for extraction, their price must be taken into account. Classic DESs are valued for their properties but also for the simple way of obtaining them and their relatively low price. Modern systems require more work and are more expensive. In the final calculation, however, the entire extraction process, the entire economy, the amount of waste, and, above all, the yield of the isolated, pure product must be taken into account.
The most important difficulties in the application of DESs in industrial extraction processes are the high viscosities of many of them, as well as the separation of the solute after extraction. Design of DESs with lower viscosities by selecting new, biodegradable, natural, and cheap HBAs and HBDs, and mixing them in appropriate ratios, seems to be one of the challenges on the way to the industrial use of these solvents. The viscosity problem can also be solved by the addition of water to the extraction system or an increase of temperature. The amount of added water should be thoroughly examined, in order to maintain the properties of DESs. According to the literature, usually addition of up to 30% of water is safe, but there are also publications where even 50% of water was used, especially in ternary systems.95 Another issue is an efficient separation of the extracted compounds from the solvents, which is crucial in the application of extracts in pharmaceuticals, cosmetics, or foods. As previously mentioned, application of switchable systems, ternary systems, or magnetic DESs can be a solution for that problem. However, a simple, more economical and efficient method of solute recovery and solvent recycling should be elaborated. Finally, even though most DESs are considered biocompatible, nontoxic, and environmentally friendly, their application in the extraction of cosmetic, food, and pharmaceutical ingredients needs an individual, thorough safety examination.
The authors declare no competing financial interest.
References
- Wen Q.; Chen J. X.; Tang Y. L.; Wang J.; Yang Z. Assessing the Toxicity and Biodegradability of Deep Eutectic Solvents. Chemosphere 2015, 132, 63–69. 10.1016/j.chemosphere.2015.02.061. [DOI] [PubMed] [Google Scholar]
- Radošević K.; Cvjetko Bubalo M.; Gaurina Srček V.; Grgas D.; Landeka Dragičević T.; Radojčić Redovniković I. Evaluation of Toxicity and Biodegradability of Choline Chloride Based Deep Eutectic Solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. 10.1016/j.ecoenv.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Juneidi I.; Hayyan M.; Hashim M. A. Evaluation of Toxicity and Biodegradability for Cholinium-Based Deep Eutectic Solvents. RSC Adv. 2015, 5 (102), 83636–83647. 10.1039/C5RA12425E. [DOI] [Google Scholar]
- Cicco L.; Dilauro G.; Perna F. M.; Vitale P.; Capriati V. Advances in Deep Eutectic Solvents and Water: Applications in Metal- And Biocatalyzed Processes, in the Synthesis of APIs, and Other Biologically Active Compounds. Org. Biomol. Chem. 2021, 19, 2558–2577. 10.1039/D0OB02491K. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Li S.; Li T.; Hu T.; Ge X. Deep Eutectic Solvents (DESs) for Green Recycling of Wasted Lithium-Ion Batteries (LIBs): Progress on Pushing the Overall Efficiency. Min. Metall. Explor. 2022, 39, 2149–2165. 10.1007/s42461-022-00660-7. [DOI] [Google Scholar]
- Smith E. L.; Abbott A. P.; Ryder K. S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- Mišan A.; Nađpal J.; Stupar A.; Pojić M.; Mandić A.; Verpoorte R.; Choi Y. H. The Perspectives of Natural Deep Eutectic Solvents in Agri-Food Sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. 10.1080/10408398.2019.1650717. [DOI] [PubMed] [Google Scholar]
- Svigelj R.; Dossi N.; Grazioli C.; Toniolo R. Deep Eutectic Solvents (Dess) and Their Application in Biosensor Development. Sensors. 2021, 21 (13), 4263. 10.3390/s21134263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płotka-Wasylka J.; Rutkowska M.; de la Guardia M. Are Deep Eutectic Solvents Useful in Chromatography? A Short Review. J. Chromatogr. A 2021, 1639, 461918. 10.1016/j.chroma.2021.461918. [DOI] [PubMed] [Google Scholar]
- Tran M. K.; Rodrigues M. T. F.; Kato K.; Babu G.; Ajayan P. M. Deep Eutectic Solvents for Cathode Recycling of Li-Ion Batteries. Nat. Energy 2019, 4 (4), 339–345. 10.1038/s41560-019-0368-4. [DOI] [Google Scholar]
- Abo-Hamad A.; Hayyan M.; AlSaadi M. A. H.; Hashim M. A. Potential Applications of Deep Eutectic Solvents in Nanotechnology. Chem. Eng. J. 2015, 273, 551–567. 10.1016/j.cej.2015.03.091. [DOI] [Google Scholar]
- García G.; Aparicio S.; Ullah R.; Atilhan M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29 (4), 2616–2644. 10.1021/ef5028873. [DOI] [Google Scholar]
- Troter D. Z.; Todorović Z. B.; Dokić-Stojanović D. R.; Stamenković O. S.; Veljković V. B. Application of Ionic Liquids and Deep Eutectic Solvents in Biodiesel Production: A Review. Renew. Sust. Energy Rev. 2016, 61, 473–500. 10.1016/j.rser.2016.04.011. [DOI] [Google Scholar]
- Cai T.; Qiu H. Application of Deep Eutectic Solvents in Chromatography: A Review. TrAC - Trends Anal. Chem. 2019, 120, 115623. 10.1016/j.trac.2019.115623. [DOI] [Google Scholar]
- Florindo C.; Romero L.; Rintoul I.; Branco L.; Marrucho I. M. From Phase Change Materials to Green Solvents: Hydrophobic Low Viscous Fatty Acid-based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2018, 6 (3), 3888–3895. 10.1021/acssuschemeng.7b04235. [DOI] [Google Scholar]
- Jiang Z.-M.; Liu W.-J.; Li Y.; Liu J.; Wang H.-Y.; Li P.; Liu E.-H. Eco-friendly Deep Eutectic Solvents Contribute to Improving the Separation of Isoquinoline Alkaloids in Supercritical Fluid Chromatography. ACS Sustain. Chem. Eng. 2020, 8 (36), 13777–13783. 10.1021/acssuschemeng.0c04743. [DOI] [Google Scholar]
- Abbott A. P.; Capper G.; Davies D. L.; Rasheed R. K.; Tambyrajah V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 70–71. 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- Yu D.; Mou H.; Zhao X.; Wang Y.; Mu T. Eutectic Molecular Liquids Based on Hydrogen Bonding and π-π Interaction for Exfoliating Two-dimensional Materials and Recycling Polymers. Chem.—Asian J. 2019, 14 (19), 3350–3356. 10.1002/asia.201900990. [DOI] [PubMed] [Google Scholar]
- Yu D.; Mu T. Strategy to Form Eutectic Molecular Liquids Based on Noncovalent Interactions. J. Phys. Chem. B 2019, 123, 4958–4966. 10.1021/acs.jpcb.9b02891. [DOI] [PubMed] [Google Scholar]
- Kaur S.; Kumari M.; Kashyap H. K. Microstructure of Deep Eutectic Solvents: Current Understanding and Challenges. J. Phys. Chem. B 2020, 124, 10601–10616. 10.1021/acs.jpcb.0c07934. [DOI] [PubMed] [Google Scholar]
- Yu D.; Mou H.; Fu H.; Lan X.; Wang Y.; Mu T. “Inverted” Deep Eutectic Solvents Based on Host-Guest Interactions. Chem.—Asian J. 2019, 14 (23), 4183–4188. 10.1002/asia.201901365. [DOI] [PubMed] [Google Scholar]
- Shi R.; Yu D.; Zhou F.; Yu J.; Mu T. An Emerging Deep Eutectic Solvent Based on Halogen-Bonds. Chem. Commun. 2022, 58 (29), 4607–4610. 10.1039/D2CC00528J. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Mu T. Revisiting Greenness of Ionic Liquids and Deep Eutectic Solvents. Green Chem. Eng. 2021, 2 (2), 174–186. 10.1016/j.gce.2021.01.004. [DOI] [Google Scholar]
- Saini A.; Kumar A.; Panesar P. S.; Thakur A. Potential of Deep Eutectic Solvents in the Extraction of Value-added Compounds from Agro-industrial By-products. Appl. Food Res. 2022, 2 (2), 100211. 10.1016/j.afres.2022.100211. [DOI] [Google Scholar]
- Lu W.; Liu S. Choline Chloride-Based Deep Eutectic Solvents (Ch-DESs) as Promising Green Solvents for Phenolic Compounds Extraction from Bioresources: State-of-the-Art, Prospects, and Challenges. Biomass Conv. Bioref. 2022, 12, 2949–2962. 10.1007/s13399-020-00753-7. [DOI] [Google Scholar]
- Ali Redha A. Review on Extraction of Phenolic Compounds from Natural Sources Using Green Deep Eutectic Solvents. J. Agric. Food. Chem. 2021, 69 (3), 878–912. 10.1021/acs.jafc.0c06641. [DOI] [PubMed] [Google Scholar]
- Nakhle L.; Kfoury M.; Mallard I.; Landy D.; Greige-Gerges H. Microextraction of Bioactive Compounds Using Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19, 3747–3759. 10.1007/s10311-021-01255-2. [DOI] [Google Scholar]
- Gil-Martín E.; Forbes-Hernández T.; Romero A.; Cianciosi D.; Giampieri F.; Battino M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. 10.1016/j.foodchem.2021.131918. [DOI] [PubMed] [Google Scholar]
- Kalyniukova A.; Holuša J.; Musiolek D.; Sedlakova-Kadukova J.; Płotka-Wasylka J.; Andruch V. Application of Deep Eutectic Solvents for Separation and Determination of Bioactive Compounds in Medicinal Plants. Ind. Crops Prod. 2021, 172, 114047. 10.1016/j.indcrop.2021.114047. [DOI] [Google Scholar]
- Alam M. A.; Muhammad G.; Khan M. N.; Mofijur M.; Lv Y.; Xiong W.; Xu J. Choline Chloride-Based Deep Eutectic Solvents as Green Extractants for the Isolation of Phenolic Compounds from Biomass. J. Clean. Prod. 2021, 309, 127445. 10.1016/j.jclepro.2021.127445. [DOI] [Google Scholar]
- Mehariya S.; Fratini F.; Lavecchia R.; Zuorro A. Green Extraction of Value-Added Compounds Form Microalgae: A Short Review on Natural Deep Eutectic Solvents (NaDES) and Related Pre-Treatments. J. Environ. Chem. Eng. 2021, 9 (5), 105989. 10.1016/j.jece.2021.105989. [DOI] [Google Scholar]
- Boateng I. D. Evaluating the Status Quo of Deep Eutectic Solvent in Food Chemistry. Potentials and Limitations. Food Chem. 2023, 406, 135079. 10.1016/j.foodchem.2022.135079. [DOI] [PubMed] [Google Scholar]
- Sportiello L.; Favati F.; Condelli N.; di Cairano M.; Caruso M. C.; Simonato B.; Tolve R.; Galgano F. Hydrophobic Deep Eutectic Solvents in the Food Sector: Focus on Their Use for the Extraction of Bioactive Compounds. Food Chem. 2023, 405A, 134703. 10.1016/j.foodchem.2022.134703. [DOI] [PubMed] [Google Scholar]
- Mahnashi M. H.; Alyami B. A.; Alqahtani Y. S.; Alqarni A. O.; Jan M. S.; Hussain F.; Zafar R.; Rashid U.; Abbas M.; Tariq M.; Sadiq A. Antioxidant Molecules Isolated from Edible Prostrate Knotweed: Rational Derivatization to Produce More Potent Molecules. Oxid. Med. Cell. Longev. 2022, 2022, 3127480. 10.1155/2022/3127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukalova Fukalova T.; García-Martínez M. D.; Raigón M. D. Nutritional Composition, Bioactive Compounds, and Volatiles Profile Characterization of Two Edible Undervalued Plants: Portulaca Oleracea L. and Porophyllum Ruderale (Jacq.) Cass. Plants 2022, 11 (3), 377. 10.3390/plants11030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulahal N.; Degraeve P. Phenolic-Rich Plant Extracts With Antimicrobial Activity: An Alternative to Food Preservatives and Biocides?. Front. Microbiol. 2022, 12, 753518. 10.3389/fmicb.2021.753518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzella L. Natural Phenolic Compounds for Health, Food and Cosmetic Applications. Antioxidants. 2020, 9 (5), 427. 10.3390/antiox9050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.; Witkamp G. J.; Verpoorte R.; Choi Y. H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. 10.1016/j.foodchem.2015.03.123. [DOI] [PubMed] [Google Scholar]
- Vernès L.; Vian M.; Chemat F.. Ultrasound and Microwave as Green Tools for Solid-Liquid Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, Netherlands, 2020; pp 355–374. 10.1016/B978-0-12-816911-7.00012-8. [DOI] [Google Scholar]
- Xiang J.; Apea-Bah F. B.; Ndolo V. U.; Katundu M. C.; Beta T. Profile of Phenolic Compounds and Antioxidant Activity of Finger Millet Varieties. Food Chem. 2019, 275, 361–368. 10.1016/j.foodchem.2018.09.120. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Tang G.; Zhang C.; Wang N.; Feng Y. Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress. Molecules. 2021, 26 (23), 7115. 10.3390/molecules26237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y.-P.; Hung P.-F.; Ku W.-Y.; Chang C.-Y.; Wu B.-H.; Wu M.-H.; Yao J.-Y.; Yang J.-R.; Lee C.-H. The Inhibitory Activity of Gallic Acid against DNA Methylation: Application of Gallic Acid on Epigenetic Therapy of Human Cancers. Oncotarget 2018, 9, 361–374. 10.18632/oncotarget.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S.; Al-Harrasi A.; Al-Azri M. S.; Ullah S.; Makeen H. A.; Meraya A. M.; Albratty M.; Najmi A.; Anwer M. K. Gallic Acid Crosslinked Gelatin and Casein Based Composite Films for Food Packaging Applications. Polymers. 2022, 14 (19), 4065. 10.3390/polym14194065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velderrain-Rodríguez G. R.; Torres-Moreno H.; Villegas-Ochoa M. A.; Ayala-Zavala J. F.; Robles-Zepeda R. E.; Wall-Medrano A.; González-Aguilar G. A. Gallic Acid Content and an Antioxidant Mechanism Are Responsible for the Antiproliferative Activity of “Ataulfo” Mango Peel on LS180 Cells. Molecules 2018, 23 (3), 695. 10.3390/molecules23030695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badhani B.; Sharma N.; Kakkar R. Gallic Acid: A Versatile Antioxidant with Promising Therapeutic and Industrial Applications. RSC Adv. 2015, 5 (35), 27540–27557. 10.1039/C5RA01911G. [DOI] [Google Scholar]
- Luzi F.; Pannucci E.; Santi L.; Kenny J. M.; Torre L.; Bernini R.; Puglia D. Gallic Acid and Quercetin as Intelligent and Active Ingredients in Poly(Vinyl Alcohol) Films for Food Packaging. Polymers. 2019, 11 (12), 1999. 10.3390/polym11121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K.; Lin L.; Xu H. Research on Antioxidant Performance of Diglucosyl Gallic Acid and Its Application in Emulsion Cosmetics. Int. J. Cosmet. Sci. 2022, 44, 177–188. 10.1111/ics.12766. [DOI] [PubMed] [Google Scholar]
- Ceci C.; Lacal P. M.; Tentori L.; de Martino M. G.; Miano R.; Graziani G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrients. 2018, 10 (11), 1756. 10.3390/nu10111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos J. L.; Giner R. M.; Marín M.; Recio M. C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. 10.1055/a-0633-9492. [DOI] [PubMed] [Google Scholar]
- Gupta A.; Singh A. K.; Kumar R.; Jamieson S.; Pandey A. K.; Bishayee A. Neuroprotective Potential of Ellagic Acid: A Critical Review. Adv. Nutr. 2021, 12, 1211–1238. 10.1093/advances/nmab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moccia F.; Liberti D.; Giovando S.; Caddeo C.; Monti D. M.; Panzella L.; Napolitano A. Chestnut Wood Mud as a Source of Ellagic Acid for Dermo-Cosmetic Applications. Antioxidants 2022, 11 (9), 1681. 10.3390/antiox11091681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X.; Liu Z.; Liu H.; Guo J.; Long S. Hybrid Molecules Based on Caffeic Acid as Potential Therapeutics: A Focused Review. Eur. J. Med. Chem. 2022, 243, 114745. 10.1016/j.ejmech.2022.114745. [DOI] [PubMed] [Google Scholar]
- Alam M.; Ahmed S.; Elasbali A. M.; Adnan M.; Alam S.; Hassan M. I.; Pasupuleti V. R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. 10.3389/fonc.2022.860508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F.; Bamunuarachchi N. I.; Tabassum N.; Kim Y. M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. 10.1021/acs.jafc.0c07579. [DOI] [PubMed] [Google Scholar]
- Maity S.; Kinra M.; Nampoothiri M.; Arora D.; Pai K. S. R.; Mudgal J. Caffeic Acid, a Dietary Polyphenol, as a Promising Candidate for Combination Therapy. Chem. Pap. 2022, 76, 1271–1283. 10.1007/s11696-021-01947-7. [DOI] [Google Scholar]
- Thongchai K.; Chuysinuan P.; Thanyacharoen T.; Techasakul S.; Ummartyotin S. Characterization, Release, and Antioxidant Activity of Caffeic Acid-Loaded Collagen and Chitosan Hydrogel Composites. J. Mater. Res. Technol. 2020, 9 (3), 6512–6520. 10.1016/j.jmrt.2020.04.036. [DOI] [Google Scholar]
- Sun Y.; Lan W.; Liu S.; Guan Y.; Zhu S.; Xie J. Preparation of Chitosan Grafted Caffeic Acid Coating and Its Effect on Pompano (Trachinotus Ovatus) Preservation. J. Sci. Food. Agric. 2022, 102 (7), 2835–2845. 10.1002/jsfa.11624. [DOI] [PubMed] [Google Scholar]
- Topal M.; Gulcin I. Evaluation of the in Vitro Antioxidant, Antidiabetic and Anticholinergic Properties of Rosmarinic Acid from Rosemary (Rosmarinus Officinalis L.). Biocatal. Agric. Biotechnol. 2022, 43, 102417. 10.1016/j.bcab.2022.102417. [DOI] [Google Scholar]
- Cândido T. M.; Ariede M. B.; Pinto C. A. S. d. O.; Lima F. V.; Magalhães W. V.; Pedro N. M. E.; Padovani G.; Sufi B. d. S.; Rijo P.; Velasco M. V. R.; Rosado C.; Baby A. R. Rosmarinic Acid Multifunctional Sunscreen: Comet Assay and In Vivo Establishment of Cutaneous Attributes. Cosmetics 2022, 9 (6), 141. 10.3390/cosmetics9060141. [DOI] [Google Scholar]
- Matwiejczuk N.; Galicka A.; Zaręba I.; Brzóska M. M. The Protective Effect of Rosmarinic Acid Against Unfavorable Influence of Methylparaben and Propylparaben on Collagen in Human Skin Fibroblasts. Nutrients 2020, 12 (5), 1282. 10.3390/nu12051282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchev A. S.; Vasileva L. v.; Amirova K. M.; Savova M. S.; Koycheva I. K.; Balcheva-Sivenova Z. P.; Vasileva S. M.; Georgiev M. I. Rosmarinic Acid - From Bench to Valuable Applications in Food Industry. Trends Food Sci. Technol. 2021, 117, 182–193. 10.1016/j.tifs.2021.03.015. [DOI] [Google Scholar]
- Nwafor E. O.; Lu P.; Zhang Y.; Liu R.; Peng H.; Xing B.; Liu Y.; Li Z.; Zhang K.; Zhang Y.; Liu Z. Chlorogenic Acid: Potential Source of Natural Drugs for the Therapeutics of Fibrosis and Cancer. Transl. Oncol. 2022, 15 (1), 101294. 10.1016/j.tranon.2021.101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.; Atanasov A. G.; Li Y.; Kumar N.; Bishayee A. Chlorogenic Acid for Cancer Prevention and Therapy: Current Status on Efficacy and Mechanisms of Action. Pharmacol. Res. 2022, 186, 106505. 10.1016/j.phrs.2022.106505. [DOI] [PubMed] [Google Scholar]
- Xue N.; Liu Y.; Jin J.; Ji M.; Chen X. Chlorogenic Acid Prevents UVA-Induced Skin Photoaging through Regulating Collagen Metabolism and Apoptosis in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2022, 23 (13), 6941. 10.3390/ijms23136941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.-H.; Do H.-K.; Kim D.-Y.; Kim W. Impact of Chlorogenic acid on Modulation of Significant Genes in Dermal Fibroblasts and Epidermal Keratinocytes. Biochem. Biophys. Res. Commun. 2021, 583, 22–28. 10.1016/j.bbrc.2021.10.057. [DOI] [PubMed] [Google Scholar]
- He D.; Xing Y.; Wang Y.; Zeng W.; Gao W.; Su N.; Zhang C.; Chen H.; Xing X. H. Improved Functional Properties of Wheat Gluten Hydrolysate by Covalent Conjugation with Chlorogenic Acid. Int. J. Food. Sci. Technol. 2023, 58 (1), 454–462. 10.1111/ijfs.16025. [DOI] [Google Scholar]
- Acquaviva R.; Tomasello B.; Di Giacomo C.; Santangelo R.; La Mantia A.; Naletova I.; Sarpietro M. G.; Castelli F.; Malfa G. A. Protocatechuic Acid, a Simple Plant Secondary Metabolite, Induced Apoptosis by Promoting Oxidative Stress through HO-1 Downregulation and P21 Upregulation in Colon Cancer Cells. Biomolecules 2021, 11 (10), 1485. 10.3390/biom11101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Gai Z.; Gui T.; Chen J.; Chen Q.; Li Y. Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid.-Based Complement. Altern. Med. 2021, 2021, 6139308. 10.1155/2021/6139308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.; He Y.; Luo C.; Feng B.; Ran F.; Xu H.; Ci Z.; Xu R.; Han L.; Zhang D. New Progress in the Pharmacology of Protocatechuic Acid: A Compound Ingested in Daily Foods and Herbs Frequently and Heavily. Pharmacol. Res. 2020, 161, 105109. 10.1016/j.phrs.2020.105109. [DOI] [PubMed] [Google Scholar]
- Shin S.; Cho S. H.; Park D.; Jung E. Anti-skin Aging Properties of Protocatechuic Acid In Vitro and In Vivo. J. Cosmet. Dermatol. 2020, 19, 977–984. 10.1111/jocd.13086. [DOI] [PubMed] [Google Scholar]
- Zhong C.; Hou P.-F.; Li Y.-X.; Yang W.-Y.; Shu M.; Wu G.-P. Characterization, Antioxidant and Antibacterial Activities of Gelatin Film Incorporated with Protocatechuic Acid and Its Application on Beef Preservation. LWT 2021, 151, 112154. 10.1016/j.lwt.2021.112154. [DOI] [Google Scholar]
- Wu M.; Tian L.; Fu J.; Liao S.; Li H.; Gai Z.; Gong G. Antibacterial Mechanism of Protocatechuic Acid Against Yersinia enterocolitica and Its Application in Pork. Food Control 2022, 133A, 108573. 10.1016/j.foodcont.2021.108573. [DOI] [Google Scholar]
- Thi N. N.; Song H. S.; Oh E. J.; Lee Y. G.; Ko J. H.; Kwon J. E.; Kang S. C.; Lee D. Y.; Jung I. H.; Baek N. I. Phenylpropanoids from Lilium Asiatic Hybrid Flowers and Their Anti-Inflammatory Activities. Appl. Biol. Chem. 2017, 60 (5), 527–533. 10.1007/s13765-017-0307-7. [DOI] [Google Scholar]
- Goyal A.; Singh Kushwah P.; Agrawal N. Therapeutic Potential of Plantamajoside. Rev. Bras. Farmacogn. 2022, 32, 355–364. 10.1007/s43450-022-00252-y. [DOI] [Google Scholar]
- Luo S.; Jiang X.; Yin G.; Liu Y.; Liu Z.; Meng L.; Wu J.; Wu H. The Herbal Agent Plantamajoside, Exerts a Potential Inhibitory Effect on the Development of Hepatocellular Carcinoma. Exp. Ther. Med. 2021, 21 (6), 573. 10.3892/etm.2021.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.; Zhao G.; Jiang K.; Chen X.; Zhu Z.; Qiu C.; Li C.; Deng G. Plantamajoside Ameliorates Lipopolysaccharide-Induced Acute Lung Injury via Suppressing NF-ΚB and MAPK Activation. Int. Immunopharmacol. 2016, 35, 315–322. 10.1016/j.intimp.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Zuo X.; Li L.; Sun L. Plantamajoside Inhibits Hypoxia-Induced Migration and Invasion of Human Cervical Cancer Cells through the NF-ΚB and PI3K/Akt Pathways. J. Recep. Signal Transduc. 2021, 41 (4), 339–348. 10.1080/10799893.2020.1808679. [DOI] [PubMed] [Google Scholar]
- Lin S.; Lu J.; Chen Q.; Jiang H.; Lou C.; Lin C.; Wang W.; Lin J.; Pan X.; Xue X. Plantamajoside Suppresses the Activation of NF-κB and MAPK and Ameliorates the Development of Osteoarthritis. Int. Immunopharmacol. 2023, 115, 109582. 10.1016/j.intimp.2022.109582. [DOI] [PubMed] [Google Scholar]
- Yu N.; Li Y.; Wang Y.; Xu H.; Ye F.; Fu Q. Healing Effect of Carboxymethyl Chitosan-Plantamajoside Hydrogel on Burn Wound Skin. Burns 2022, 48, 902–914. 10.1016/j.burns.2022.01.019. [DOI] [PubMed] [Google Scholar]
- Firat F.; Türkoğlu C.; Ozdal Kurt F.; Vatansever H. S. Does Acteoside Have Effects on Colon Cancer Stem Cells Via Inflamation or Apoptosis?. Genel Tıp Derg. 2022, 32 (4), 372–379. 10.54005/geneltip.1053439. [DOI] [Google Scholar]
- Xiao Y.; Ren Q.; Wu L. The Pharmacokinetic Property and Pharmacological Activity of Acteoside: A Review. Biomed. Pharmacother. 2022, 153, 113296. 10.1016/j.biopha.2022.113296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Yan Y.; Liu H.; Wang J.; Hu J. Protective Effects of Acteoside Against X-ray-Induced Damage in Human Skin Fibroblasts. Mol. Med. Rep. 2015, 12 (2), 2301–2306. 10.3892/mmr.2015.3630. [DOI] [PubMed] [Google Scholar]
- Gao W.; Zheng S.; Hwang E.; Yi T.-H.; Wang Y.-S. Effects of Phenylethanol Glycosides from Orobanche cernua Loefling on UVB-Induced Skin Photodamage: A Comparative Study. Photochem. Photobiol. Sci. 2021, 20 (5), 599–614. 10.1007/s43630-021-00038-6. [DOI] [PubMed] [Google Scholar]
- Ding Y.; Ding Z.; Xu J.; Li Y.; Chen M. Pharmacological Activities of Ginkgolic Acids in Relation to Autophagy. Pharmaceuticals 2022, 15 (12), 1469. 10.3390/ph15121469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boateng I. D. A Critical Review of Ginkgolic Acids in Ginkgo Biloba Leaf Extract (Egb): Toxicity and Technologies to Remove Ginkgolic Acids and Their Promising Bioactivities. Food Funct. 2022, 13, 9226–9242. 10.1039/D2FO01827F. [DOI] [PubMed] [Google Scholar]
- Lee J.-H.; Kim Y.-G.; Ryu S. Y.; Cho M. H.; Lee J. Ginkgolic Acids and Ginkgo Biloba Extract Inhibit Escherichia Coli O157:H7 and Staphylococcus Aureus Biofilm Formation. Int. J. Food Microbiol. 2014, 174, 47–55. 10.1016/j.ijfoodmicro.2013.12.030. [DOI] [PubMed] [Google Scholar]
- Alrugaibah M.; Washington T. L.; Yagiz Y.; Gu L. Ultrasound-Assisted Extraction of Phenolic Acids, Flavonols, and Flavan-3-Ols from Muscadine Grape Skins and Seeds Using Natural Deep Eutectic Solvents and Predictive Modelling by Artificial Neural Networking. Ultrason. Sonochem. 2021, 79, 105773. 10.1016/j.ultsonch.2021.105773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Pontes P. V.; Ayumi Shiwaku I.; Maximo G. J.; Caldas Batista E. A. Choline Chloride-Based Deep Eutectic Solvents as Potential Solvent for Extraction of Phenolic Compounds from Olive Leaves: Extraction Optimization and Solvent Characterization. Food Chem. 2021, 352, 129346. 10.1016/j.foodchem.2021.129346. [DOI] [PubMed] [Google Scholar]
- Vorobyova V.; Skiba M.; Vasyliev G. Extraction of Phenolic Compounds from Tomato Pomace Using Choline Chloride-Based Deep Eutectic Solvents. J. Food Meas. Charact. 2022, 16 (2), 1087–1104. 10.1007/s11694-021-01238-5. [DOI] [Google Scholar]
- Wang R.; Zhang W.; He R.; Li W.; Wang L. Customized Deep Eutectic Solvents as Green Extractants for Ultrasonic-Assisted Enhanced Extraction of Phenolic Antioxidants from Dogbane Leaf-Tea. Foods 2021, 10 (11), 2527. 10.3390/foods10112527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Linares J. C.; Campillo V.; Coca M.; Lucas S.; García-Cubero M. T. Microwave-Assisted Deep Eutectic Solvent Extraction of Phenolic Compounds from Brewer’s Spent Grain. J. Chem. Technol. Biotechnol. 2021, 96 (2), 481–490. 10.1002/jctb.6565. [DOI] [Google Scholar]
- Alsaud N.; Shahbaz K.; Farid M. Application of Deep Eutectic Solvents in the Extraction of Polyphenolic Antioxidants from New Zealand Manuka Leaves (Leptospermum Scoparium): Optimization and Antioxidant Activity. J. Mol. Liq. 2021, 337, 116385. 10.1016/j.molliq.2021.116385. [DOI] [Google Scholar]
- Alasalvar H.; Yildirim Z. Ultrasound-Assisted Extraction of Antioxidant Phenolic Compounds from Lavandula Angustifolia Flowers Using Natural Deep Eutectic Solvents: An Experimental Design Approach. Sustain. Chem. Pharm. 2021, 22, 100492. 10.1016/j.scp.2021.100492. [DOI] [Google Scholar]
- Chen L.; Yang Y. Y.; Zhou R. R.; Fang L. Z.; Zhao D.; Cai P.; Yu R.; Zhang S. H.; Huang J. H. The Extraction of Phenolic Acids and Polysaccharides from: Lilium Lancifolium Thunb. Using a Deep Eutectic Solvent. Anal. Methods 2021, 13 (10), 1226–1231. 10.1039/D0AY02352C. [DOI] [PubMed] [Google Scholar]
- Guo H.; Feng C.; Hu L.; Zhao X.; Tang X.; Huang Y.; Luo J.; Xu M.; Xie W. Exploration of a Ternary Deep Eutectic Solvent for the Efficient Extraction of Plantamajoside, Acteoside, Quercetin and Kaempferol from Plantago Asiatica L. Phytochem. Anal. 2022, 33 (1), 94–104. 10.1002/pca.3071. [DOI] [PubMed] [Google Scholar]
- Zheng N.; Ming Y.; Chu J.; Yang S.; Wu G.; Li W.; Zhang R.; Cheng X. Optimization of Extraction Process and the Antioxidant Activity of Phenolics from Sanghuangporus Baumii. Molecules 2021, 26 (13), 3850. 10.3390/molecules26133850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Chen Z.; Li S.; Wang L.; Zhang J. Eco-Friendly and High-Efficient Extraction of Natural Antioxidants from Polygonum Aviculare Leaves Using Tailor-Made Deep Eutectic Solvents as Extractants. Sep. Purif. Technol. 2021, 262, 118339. 10.1016/j.seppur.2021.118339. [DOI] [Google Scholar]
- Yu L.; Cao L.; Chang Y. H.; Duan C. J.; Liu C.; Zhao X. L.; Yue G. L.; Wang X. Q.; Fu Y. J. Enhanced Extraction Performance of Iridoids, Phenolic Acids from Eucommia Ulmoides Leaves by Tailor-Made Ternary Deep Eutectic Solvent. Microchem. J. 2021, 161, 105788. 10.1016/j.microc.2020.105788. [DOI] [Google Scholar]
- Ivanović M.; Grujić D.; Cerar J.; Islamčevic Razboršek M. I.; Topalić-Trivunović L.; Savić A.; Kočar D.; Kolar M. Extraction of Bioactive Metabolites from Achillea Millefolium L. with Choline Chloride Based Natural Deep Eutectic Solvents: A Study of the Antioxidant and Antimicrobial Activity. Antioxidants 2022, 11 (4), 724. 10.3390/antiox11040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurić T.; Mićić N.; Potkonjak A.; Milanov D.; Dodić J.; Trivunović Z.; Popović B. M. The Evaluation of Phenolic Content, in Vitro Antioxidant and Antibacterial Activity of Mentha Piperita Extracts Obtained by Natural Deep Eutectic Solvents. Food Chem. 2021, 362, 130226. 10.1016/j.foodchem.2021.130226. [DOI] [PubMed] [Google Scholar]
- Zannou O.; Pashazadeh H.; Galanakis C. M.; Alamri A. S.; Koca I. Carboxylic Acid-Based Deep Eutectic Solvents Combined with Innovative Extraction Techniques for Greener Extraction of Phenolic Compounds from Sumac (Rhus Coriaria L.). J. Appl. Res. Med. Aromat. Plants. 2022, 30, 100380. 10.1016/j.jarmap.2022.100380. [DOI] [Google Scholar]
- Abouheif S. A.; Sallam S. M.; el Sohafy S. M.; Kassem F. F.; Shawky E. Optimization of Terpene Lactones and Ginkgolic Acids Extraction from Ginkgo Biloba L. Leaves by Natural Deep Eutectic Solvents Using Experimental Design and HPTLC-MS Analysis. Microchem. J. 2022, 176, 107246. 10.1016/j.microc.2022.107246. [DOI] [Google Scholar]
- Rashid R.; Mohd Wani S.; Manzoor S.; Masoodi F. A.; Masarat Dar M. Green Extraction of Bioactive Compounds from Apple Pomace by Ultrasound Assisted Natural Deep Eutectic Solvent Extraction: Optimisation, Comparison and Bioactivity. Food Chem. 2023, 398, 133871. 10.1016/j.foodchem.2022.133871. [DOI] [PubMed] [Google Scholar]
- Athanasiadis V.; Palaiogiannis D.; Grigorakis S.; Bozinou E.; Lalas S. I.; Makris D. P. Food-Grade Deep Eutectic Solvent Extraction of Antioxidant Polyphenols from Peppermint (Mentha × Piperita L.): Screening, Optimization and Metabolite Profile. J. Appl. Res. Med. Aromat. Plants. 2023, 33, 100456. 10.1016/j.jarmap.2022.100456. [DOI] [Google Scholar]
- Patil S. S.; Rathod V. K. Extraction and Purification of Curcuminoids from Curcuma Longa Using Microwave Assisted Deep Eutectic Solvent Based System and Cost Estimation. Process Biochem. 2023, 126, 61–71. 10.1016/j.procbio.2022.11.010. [DOI] [Google Scholar]
- Osamede Airouyuwa J.; Mostafa H.; Riaz A.; Maqsood S. Utilization of Natural Deep Eutectic Solvents and Ultrasound-Assisted Extraction as Green Extraction Technique for the Recovery of Bioactive Compounds from Date Palm (Phoenix Dactylifera L.) Seeds: An Investigation into Optimization of Process Parameters. Ultrason. Sonochem. 2022, 91, 106233. 10.1016/j.ultsonch.2022.106233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.-Y.; Ou H.; Gregersen H.; Zuo J. Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction of Polyphenols from Cosmos Sulphureus. J. Appl. Res. Med. Aromat. Plants. 2023, 32, 100444. 10.1016/j.jarmap.2022.100444. [DOI] [Google Scholar]
- Shekaari H.; Zafarani-Moattar M. T.; Mokhtarpour M. Effective Ultrasonic-Assisted Extraction and Solubilization of Curcuminoids from Turmeric by Using Natural Deep Eutectic Solvents and Imidazolium-Based Ionic Liquids. J. Mol. Liq. 2022, 360, 119351. 10.1016/j.molliq.2022.119351. [DOI] [Google Scholar]
- Wang T. Y.; Li Q.; Bi K. S. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J.Pharm. Sci. 2018, 13, 12–23. 10.1016/j.ajps.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggian M.; Bernabè G.; Ferrari S.; Francescato S.; Baratto G.; Castagliuolo I.; Dall’acqua S.; Peron G. Polyphenol-Rich Larix Decidua Bark Extract with Antimicrobial Activity against Respiratory-Tract Pathogens: A Novel Bioactive Ingredient with Potential Pharmaceutical and Nutraceutical Applications. Antibiotics 2021, 10 (7), 789. 10.3390/antibiotics10070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldan V.; Sut S.; Faggian M.; Dalla Gassa E.; Ferrari S.; de Nadai G.; Francescato S.; Baratto G.; Dall’Acqua S. Larix Decidua Bark as a Source of Phytoconstituents: An LC-MS Study. Molecules 2017, 22 (11), 1974. 10.3390/molecules22111974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Petrillo A.; Orrù G.; Fais A.; Fantini M. C. Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review. Phytother. Res. 2022, 36, 266–278. 10.1002/ptr.7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. L. A.; Bhattacharya D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27 (8), 2494. 10.3390/molecules27082494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki Dana P.; Sadoughi F.; Asemi Z.; Yousefi B. Anti-Cancer Properties of Quercetin in Osteosarcoma. Cancer Cell Int. 2021, 21, 349. 10.1186/s12935-021-02067-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorisio S.; Argentieri M. P.; Avato P.; Caderni G.; Chioccioli S.; Cirmi S.; Delfino D. V.; Greco G.; Hrelia P.; Iriti M.; Lenzi M.; Lombardo G. E.; Luceri C.; Maugeri A.; Montopoli M.; Muscari I.; Nani M. F.; Navarra M.; Gasperini S.; Turrini E.; Fimognari C. The Molecular Basis of the Anticancer Properties of Quercetin. Pharmadvances. 2021, 3 (3), 496. 10.36118/pharmadvances.2021.10. [DOI] [Google Scholar]
- Alzaabi M. M.; Hamdy R.; Ashmawy N. S.; Hamoda A. M.; Alkhayat F.; Khademi N. N.; al Joud S. M. A.; El-Keblawy A. A.; Soliman S. S. M. Flavonoids Are Promising Safe Therapy against COVID-19. Phytochem. Rev. 2022, 21, 291–312. 10.1007/s11101-021-09759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. C.; Benati R. B.; Massaro T. N. C.; Pereira K. C.; Gaspar L. R.; Marcato P. D. Antioxidant and Anti-tyrosinase Activities of Quercetin-loaded Olive Oil Nanoemulsion as Potential Formulation for Skin Hyperpigmentation. J. Dispers. Sci. Technol. 2022, 10.1080/01932691.2022.2116715. [DOI] [Google Scholar]
- Yong H.; Bai R.; Bi F.; Liu J.; Qin Y.; Liu J. Synthesis, Characterization, Antioxidant and Antimicrobial Activities of Starch Aldehyde-Quercetin Conjugate. Int. J. Biol. Macromol. 2020, 156, 462–470. 10.1016/j.ijbiomac.2020.04.035. [DOI] [PubMed] [Google Scholar]
- Łopusiewicz L.; Zdanowicz M.; Macieja S.; Kowalczyk K.; Bartkowiak A. Development and Characterization of Bioactive Poly(Butylene-Succinate) Films Modified with Quercetin for Food Packaging Applications. Polymers 2021, 13 (11), 1798. 10.3390/polym13111798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho W.-K.; Lee M.-M.; Ma J. Y. Antiviral Effect of Isoquercitrin against Influenza A Viral Infection via Modulating Hemagglutinin and Neuraminidase. Int. J. Mol. Sci. 2022, 23 (21), 13112. 10.3390/ijms232113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zymone K.; Benetis R.; Trumbeckas D.; Baseviciene I.; Trumbeckaite S. Different Effects of Quercetin Glycosides and Quercetin on Kidney Mitochondrial Function—Uncoupling, Cytochrome C Reducing and Antioxidant Activity. Molecules 2022, 27 (19), 6377. 10.3390/molecules27196377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G.; Hu J.; Huang J.; Li Y.; Deng Q.; Jiang W.; Huang G.; Wang Q. The Effect of Isoquercitrin on Cell Apoptosis and Cycle for HepG2 Cells. Sch. Acad. J. Pharm. 2022, 11 (10), 182–186. 10.36347/sajp.2022.v11i10.002. [DOI] [Google Scholar]
- Whangsomnuek N.; Mungmai L.; Mengamphan K.; Amornlerdpison D. Bioactive Compounds of Aqueous Extracts of Flower and Leaf of Etlingera elatior (JACk) R.M.Sm. for Cosmetic Application. Maejo Int. J. Sci. Technol. 2019, 13, 196–208. [Google Scholar]
- Cadoná F. C.; Dantas R. F.; de Mello G. H.; Silva-Jr F. P. Natural Products Targeting into Cancer Hallmarks: An Update on Caffeine, Theobromine, and (+)-Catechin. Crit. Rev. Food Sci. Nutr. 2022, 62, 7222–7241. 10.1080/10408398.2021.1913091. [DOI] [PubMed] [Google Scholar]
- Amiri S.; Rezazad Bari L.; Malekzadeh S.; Amiri S.; Mostashari P.; Ahmadi Gheshlagh P. Effect of Aloe Vera Gel-Based Active Coating Incorporated with Catechin Nanoemulsion and Calcium Chloride on Postharvest Quality of Fresh Strawberry Fruit. J. Food Process. Preserv. 2022, 46 (10), e15960 10.1111/jfpp.15960. [DOI] [Google Scholar]
- Bae J.; Kim N.; Shin Y.; Kim S.-Y.; Kim Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. 10.1186/s41702-020-0057-8. [DOI] [Google Scholar]
- Aljuffali I. A.; Lin C.-H.; Yang S.-C.; Alalaiwe A.; Fang J.-Y. Nanoencapsulation of Tea Catechins for Enhancing Skin Absorption and Therapeutic Efficacy. AAPS PharmSciTechnol. 2022, 23 (6), 187. 10.1208/s12249-022-02344-3. [DOI] [PubMed] [Google Scholar]
- Felice M. R.; Maugeri A.; de Sarro G.; Navarra M.; Barreca D. Molecular Pathways Involved in the Anti-Cancer Activity of Flavonols: A Focus on Myricetin and Kaempferol. Int. J. Mol. Sci. 2022, 23 (8), 4411. 10.3390/ijms23084411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangar S. P.; Chaudhary V.; Sharma N.; Bansal V.; Ozogul F.; Lorenzo J. M. Kaempferol: A Flavonoid with Wider Biological Activities and Its Applications. Crit. Rev. Food Sci. Nutr. 2022, 2022, 1–25. 10.1080/10408398.2022.2067121. [DOI] [PubMed] [Google Scholar]
- Silva dos Santos J.; Gonçalves Cirino J. P.; de Oliveira Carvalho P.; Ortega M. M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. 10.3389/fphar.2020.565700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-J.; Kim D.-W.; Lim S.-R.; Sung J.; Kim T. H.; Min I. S.; Choi C.-H.; Lee S.-J. Kaempferol Blocks the Skin Fibroblastic Interleukin 1β Expression and Cytotoxicity Induced by 12-O-tetradecanoylphorbol-13-acetate by Suppressing c-Jun N-terminal Kinase. Nutrients 2021, 13 (9), 3079. 10.3390/nu13093079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Kim H.-S.; Choi C.-H.; Choi J.; Cho S. Y.; Kim S.-H.; Baek H.-S.; Yoon K. D.; Son S. W.; Son E. D.; Hong Y.-D.; Ko J.; Cho S.-Y.; Park W.-S. Kaempferol Tetrasaccharides Restore Skin Atrophy via PDK1 Inhibition in Human Skin Cells and Tissues: Bench and Clinical Studies. Biomed. Pharmacother. 2022, 156, 113864. 10.1016/j.biopha.2022.113864. [DOI] [PubMed] [Google Scholar]
- Özkütük A. S. Antimicrobial Effects of Carnosic Acid, Kaempferol and Luteolin on Biogenic Amine Production by Spoilage and Food-Borne Pathogenic Bacteria. Food Biosci. 2022, 46, 101588. 10.1016/j.fbio.2022.101588. [DOI] [Google Scholar]
- Farha A. K.; Gan R. Y.; Li H.; Wu D. T.; Atanasov A. G.; Gul K.; Zhang J. R.; Yang Q. Q.; Corke H. The Anticancer Potential of the Dietary Polyphenol Rutin: Current Status, Challenges, and Perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. 10.1080/10408398.2020.1829541. [DOI] [PubMed] [Google Scholar]
- Enogieru A. B.; Haylett W.; Hiss D. C.; Bardien S.; Ekpo O. E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. 10.1155/2018/6241017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullón B.; Lú-Chau T. A.; Moreira M. T.; Lema J. M.; Eibes G. Rutin: A Review on Extraction, Identification and Purification Methods, Biological Activities and Approaches to Enhance Its Bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. 10.1016/j.tifs.2017.07.008. [DOI] [Google Scholar]
- Çelik H.; Kandemir F. M.; Caglayan C.; Özdemir S.; Çomaklı S.; Kucukler S.; Yardım A. Neuroprotective Effect of Rutin Against Colistin-induced Oxidative Stress, Inflammation and Apoptosis in Rat Brain Associated with the CREB/BDNF Expressions. Mol. Biol. Rep. 2020, 47, 2023–2034. 10.1007/s11033-020-05302-z. [DOI] [PubMed] [Google Scholar]
- Choi J. K.; Kim S.-H. Rutin Suppresses Atopic Dermatitis and Allergic Contact Dermatitis. Soc. Exp. Biol. Med. 2013, 238 (4), 410–417. 10.1177/1535370213477975. [DOI] [PubMed] [Google Scholar]
- Gong J.; Luo S.; Zhao S.; Yin S.; Li X.; Mou T. Myricitrin Attenuates Memory Impairment in a Rat Model of Sepsis-Associated Encephalopathy via the NLRP3/Bax/Bcl Pathway. Folia Neuropathol. 2019, 57 (4), 327–334. 10.5114/fn.2019.89856. [DOI] [PubMed] [Google Scholar]
- Weng W.; Wang Q.; Wei C.; Man N.; Zhang K.; Wei Q.; Adu-Frimpong M.; Toreniyazov E.; Ji H.; Yu J.; Xu X. Preparation, Characterization, Pharmacokinetics and Anti-hyperuricemia Activity Studies of Myricitrin-loaded Proliposomes. Int. J. Pharm. 2019, 572, 118735. 10.1016/j.ijpharm.2019.118735. [DOI] [PubMed] [Google Scholar]
- Jo S.; Kim S.; Shin D. H.; Kim M.-S. Inhibition of African Swine Fever Virus Protease by Myricetin and Myricitrin. J. Enzyme Inhib. Med. Chem. 2020, 35 (1), 1045–1049. 10.1080/14756366.2020.1754813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samant N. P.; Gupta G. L. Avicularin Attenuates Memory Impairment in Rats with Amyloid Beta-Induced Alzheimer’s Disease. Neurotox. Res. 2022, 40 (1), 140–153. 10.1007/s12640-021-00467-2. [DOI] [PubMed] [Google Scholar]
- Sá F. A. d. S.; Silva T. C.; Andrade W. M.; de Ávila R. I.; Valadares M. C.; Costa C. R.; Santos A. S.; Feitas V. A. Q.; de Paula J. R.; Rodrigues Silva M. d. R. Antifungal Activity of the Ethanolic Extract and Flavonoid Avicularin from Myrcia tomentosa (Aubl.) DC. on Virulence Factors of Candida Species. J. Herb. Med. 2023, 38, 100643. 10.1016/j.hermed.2023.100643. [DOI] [Google Scholar]
- Keerthanaa T.; Boobalan S.; Kamalanathan D.; Karunakaran G.; Sudha K. G.; Aarthi M.; Prasanna Rajeshkumar M. Elicitation of Apigenin in Green Leafy Vegetable Plants and Its Molecular Docking Evaluation for Effective Anticancer Applications. Plant Cell Tiss. Organ Cult. 2022, 150 (2), 459–478. 10.1007/s11240-022-02297-2. [DOI] [Google Scholar]
- Rahmani A. H.; Alsahli M. A.; Almatroudi A.; Almogbel M. A.; Khan A. A.; Anwar S.; Almatroodi S. A. The Potential Role of Apigenin in Cancer Prevention and Treatment. Molecules. 2022, 27 (18), 6051. 10.3390/molecules27186051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B.; Venditti A.; Sharifi-Rad M.; Kręgiel D.; Sharifi-Rad J.; Durazzo A.; Lucarini M.; Santini A.; Souto E. B.; Novellino E.; Antolak H.; Azzini E.; Setzer W. N.; Martins N. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20 (6), 1305. 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap P.; Shikha D.; Thakur M.; Aneja A. Functionality of Apigenin as a Potent Antioxidant with Emphasis on Bioavailability, Metabolism, Action Mechanism and in Vitro and in Vivo Studies: A Review. J. Food Biochem. 2022, 46 (4), e13950 10.1111/jfbc.13950. [DOI] [PubMed] [Google Scholar]
- Zare M. F. R.; Rakhshan K.; Aboutaleb N.; Nikbakht F.; Naderi N.; Bakhshesh M.; Azizi Y. Apigenin Attenuates Doxorubicin Induced Cardiotoxicity via Reducing Oxidative Stress and Apoptosis in Male Rats. Life Sci. 2019, 232, 116623. 10.1016/j.lfs.2019.116623. [DOI] [PubMed] [Google Scholar]
- Majma Sanaye P.; Mojaveri M. R.; Ahmadian R.; Sabet Jahromi M.; Bahramsoltani R. Apigenin and Its Dermatological Applications: A Comprehensive Review. Phytochemistry 2022, 203, 113390. 10.1016/j.phytochem.2022.113390. [DOI] [PubMed] [Google Scholar]
- Serim E.; Ceylan B.; Tekkeli S. E. K. Determination of Apigenin in Cosmetics Containing Chamomile by High-Performance Liquid Chromatography with Ultraviolet Detection (HPLC-UV). Anal. Lett. 2023, 56, 2113–2122. 10.1080/00032719.2022.2155180. [DOI] [Google Scholar]
- Li M.; Kong J.; Chen Y.; Li Y.; Xuan H.; Liu M.; Zhang Q.; Liu J. Comparative Interaction Study of Soy Protein Isolate and Three Flavonoids (Chrysin, Apigenin and Luteolin) and Their Potential as Natural Preservatives. Food Chem. 2023, 414, 135738. 10.1016/j.foodchem.2023.135738. [DOI] [PubMed] [Google Scholar]
- Lee H.; Krishnan M.; Kim M.; Yoon Y. K.; Kim Y. Rhamnetin, a Natural Flavonoid, Ameliorates Organ Damage in a Mouse Model of Carbapenem-Resistant Acinetobacter baumannii-Induced Sepsis. Int. J. Mol. Sci. 2022, 23 (21), 12895. 10.3390/ijms232112895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L.; Wang Y.; Pan Z.; Wang B.; Yue Z.; Jiang Z.; Li L.; Wang C.; Tang H. Rhamnetin Induces Apoptosis in Human Breast Cancer Cells via the miR-34a/Notch-1 Signaling Pathway. Oncol. Lett. 2019, 17, 676–682. 10.3892/ol.2018.9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.-K. Natural Products in Cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. 10.1007/s13659-022-00363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshit G.; Dagur P.; Satpathy S.; Patra A.; Jain A.; Ghosh M. Flavonoids as Potential Therapeutics against Novel Coronavirus Disease-2019 (NCOVID-19). J. Biomol. Struct. Dyn. 2022, 40, 6989–7001. 10.1080/07391102.2021.1892529. [DOI] [PubMed] [Google Scholar]
- Russo M.; Moccia S.; Spagnuolo C.; Tedesco I.; Russo G. L. Roles of Flavonoids against Coronavirus Infection. Chem.-Biol. Interact. 2020, 328, 109211. 10.1016/j.cbi.2020.109211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Stefano A.; Caporali S.; di Daniele N.; Rovella V.; Cardillo C.; Schinzari F.; Minieri M.; Pieri M.; Candi E.; Bernardini S.; Tesauro M.; Terrinoni A. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci. 2021, 22, 1321. 10.3390/ijms22031321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeujo F. M.; Ragazzi E.; Urettini M.; Sauro B.; Cichero E.; Tonelli M.; Froldi G. Magnolol and Luteolin Inhibition of α-Glucosidase Activity: Kinetics and Type of Interaction Detected by In Vitro and In Silico Studies. Pharmaceuticals 2022, 15 (2), 205. 10.3390/ph15020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J. Y.; Seok J. K.; Suh H.-J.; Choi Y.-H.; Hong S. S.; Kim D. S.; Boo Y. C. Antimelanogenic Effects of Luteolin 7-sulfate Isolated from Phyllospadix iwatensis Makino. Br. J. Dermatol. 2016, 175 (3), 501–511. 10.1111/bjd.14496. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Cheng R.; Liu J.; Fang J.; Wang X.; Cui Y.; Zhang P.; Du B. Linarin Protects against Cadmium-Induced Osteoporosis Via Reducing Oxidative Stress and Inflammation and Altering RANK/RANKL/OPG Pathway. Biol. Trace Elem. Res. 2022, 200, 3688–3700. 10.1007/s12011-021-02967-w. [DOI] [PubMed] [Google Scholar]
- Ye Y.; Chen Z.; Wu Y.; Gao M.; Zhu A.; Kuai X.; Luo D.; Chen Y.; Li K. Purification Process and In Vitro and In Vivo Bioactivity Evaluation of Pectolinarin and Linarin from Cirsium Japonicum. Molecules 2022, 27 (24), 8695. 10.3390/molecules27248695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Bai Z.; Yan J.; Liu T.; Li Y.; Xu J.; Meng X.; Bi Y. Anti-Diabetic Effects of Linarin from Chrysanthemi Indici Flos via AMPK Activation. Chin. Herb. Med. 2022, 14, 97–103. 10.1016/j.chmed.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández S.; Wasowski C.; Paladini A. C.; Marder M. Sedative and Sleep-Enhancing Properties of Linarin, a Flavonoid-Isolated from Valeriana Officinalis. Pharmacol., Biochem. Behav. 2004, 77 (2), 399–404. 10.1016/j.pbb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Zeng J.; Hu W.; Li H.; Liu J.; Zhang P.; Gu X.; Yu Y.; Wang W.; Wei Y. Purification of Linarin and Hesperidin from Mentha haplocalyx by Aqueous Two-phase Flotation Coupled with Preparative HPLC and Evaluation of the Neuroprotective Effect of Linarin. J. Sep. Sci. 2021, 44, 2496–2503. 10.1002/jssc.202001243. [DOI] [PubMed] [Google Scholar]
- Oh S.-Y.; Kang J. K.; Hyun C.-G. Linarin Enhances Melanogenesis in B16F10 Cells via MAPK and PI3K/AKT Signaling Pathways. J. Appl. Biol. Chem. 2021, 64 (4), 447–451. 10.3839/jabc.2021.060. [DOI] [Google Scholar]
- Cao Z.; Liu W.; Bi B.; Wu H.; Cheng G.; Zhao Z. Isoorientin Ameliorates Osteoporosis and Oxidative Stress in Postmenopausal Rats. Pharm. Biol. 2022, 60, 2219–2228. 10.1080/13880209.2022.2142614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Xiu Y. H.; Xue H.; Li Y. N.; Cao J. L.; Hou W. S.; Liu J.; Cui Y. H.; Xu T.; Wang Y.; Jin C. H. A Mechanism of Isoorientin-Induced Apoptosis and Migration Inhibition in Gastric Cancer AGS Cells. Pharmaceuticals 2022, 15 (12), 1541. 10.3390/ph15121541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefahal M.; Makhloufi E.-H.; Boussetla A.; Ayad R.; Rayane S. A.; Akkal S. Isoorientin Isolated from the Algerian Halophyte Limonium Thouinii (Viv.) Kuntze as a Multifunctional Cosmetic Ingredient: Antioxidant and Photoprotective Effects Evaluation. Proc. Natl. Acad. Sci., India Sect. B: Biol. Sci. 2022, 92, 889–896. 10.1007/s40011-022-01380-0. [DOI] [Google Scholar]
- Stabrauskiene J.; Kopustinskiene D. M.; Lazauskas R.; Bernatoniene J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines. 2022, 10 (7), 1686. 10.3390/biomedicines10071686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motallebi M.; Bhia M.; Rajani H. F.; Bhia I.; Tabarraei H.; Mohammadkhani N.; Pereira-Silva M.; Kasaii M. S.; Nouri-Majd S.; Mueller A. L.; Veiga F. J. B.; Paiva-Santos A. C.; Shakibaei M. Naringenin: A Potential Flavonoid Phytochemical for Cancer Therapy. Life Sci. 2022, 305, 120752. 10.1016/j.lfs.2022.120752. [DOI] [PubMed] [Google Scholar]
- Wen Q. H.; Wang R.; Zhao S. Q.; Chen B. R.; Zeng X. A. Inhibition of Biofilm Formation of Foodborne Staphylococcus Aureus by the Citrus Flavonoid Naringenin. Foods 2021, 10 (11), 2614. 10.3390/foods10112614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.; Li L.; Wang C.; Wang L.; Lu D.; Shen D.; Wang J.; Jiang C.; Cheng L.; Pan X.; Yang A.; Wang Y.; Zhu X.; Li B.; Li Y.; Zhang F. Naringenin Confers Defence against Phytophthora Nicotianae through Antimicrobial Activity and Induction of Pathogen Resistance in Tobacco. Mol. Plant Pathol. 2022, 23 (12), 1737–1750. 10.1111/mpp.13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Arceo M.; Gomez-Lopez I.; Carr-Ugarte H.; Eseberri I.; González M.; Cano M. P.; Portillo M. P.; Gómez-Zorita S. Anti-Obesity Effects of Isorhamnetin and Isorhamnetin Conjugates. Int. J. Mol. Sci. 2023, 24 (1), 299. 10.3390/ijms24010299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalai F. Z.; Boulaaba M.; Ferdousi F.; Isoda H. Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways. Int. J. Mol. Sci. 2022, 23 (2), 704. 10.3390/ijms23020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D K.; V A. Antimelanogenic and Anticancer Activity of Isorhamnetin isolated from Acalypha indica Linn. on A375 cell line. Curr. Trends Biotechnol. Pharm. 2022, 16 (4), 445–455. 10.5530/ctbp.2022.4.78. [DOI] [Google Scholar]
- Zhu Z.; Yang L.; Li Z.; Liu Q. Cyanidin-3-O-Glucoside, Cyanidin, and Oxidation Products of Cyanidin Protect Neuronal Function through Alleviating Inflammation and Oxidative Damage. J. Food Sci. 2022, 87, 2159–2172. 10.1111/1750-3841.16125. [DOI] [PubMed] [Google Scholar]
- Gao N.; Tian J.; Shu C.; Tan H.; Jiao X.; Lang Y.; Zang Z.; Cui H.; Li B. Protective Effects and Mechanism of Amino Acids as Chokeberry Cyanidin and Its Glycoside Protectant under the Condition of Vitamin C Coexistence. Food Chem. 2022, 397, 133783. 10.1016/j.foodchem.2022.133783. [DOI] [PubMed] [Google Scholar]
- Bai X.; Jiang M.; Wang J.; Yang S.; Liu Z.; Zhang H.; Zhu X. Cyanidin Attenuates the Apoptosis of Rat Nucleus Pulposus Cells and the Degeneration of Intervertebral Disc via the JAK2/STAT3 Signal Pathway in Vitro and in Vivo. Pharm. Biol. 2022, 60, 427–436. 10.1080/13880209.2022.2035773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira H.; Correia P.; Bessa L. J.; Guimarães M.; Gameiro P.; Freitas V. d.; Mateus N.; Cruz L.; Fernandes I. Cyanidin-3-glucoside Lipophilic Conjugates for Topical Application: Tuning the Antimicrobial Activities with Fatty Acid Chain Length. Processes 2021, 9 (2), 340. 10.3390/pr9020340. [DOI] [Google Scholar]
- Lin Y.; Li C.; Shao P.; Jiang L.; Chen B.; Farag M. A. Enzymatic Acylation of Cyanidin-3-O-glucoside in Raspberry Anthocyanins for Intelligent Packaging: Improvement of Stability, Lipophilicity and Functional Properties. Curr. Res. Food Sci. 2022, 5, 2219–2227. 10.1016/j.crfs.2022.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.; Zhang P.; Zhu Y.; Lou Q.; He S. Antioxidant and Prebiotic Activity of Five Peonidin-Based Anthocyanins Extracted from Purple Sweet Potato (Ipomoea Batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. 10.1038/s41598-018-23397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slámová K.; Kapešová J.; Valentová K. Sweet Flavonoids”: Glycosidase-Catalyzed Modifications. Int. J. Mol. Sci. 2018, 19 (7), 2126. 10.3390/ijms19072126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G. H.; Li X. H.; Jiang Y. Deep Eutectic Solvents as Green Media for Flavonoids Extraction from the Rhizomes of Polygonatum Odoratum. Alex. Eng. J. 2021, 60, 1991–2000. 10.1016/j.aej.2020.12.008. [DOI] [Google Scholar]
- Sillero L.; Prado R.; Welton T.; Labidi J. Extraction of Flavonoid Compounds from Bark Using Sustainable Deep Eutectic Solvents. Sustain. Chem. Pharm. 2021, 24, 100544. 10.1016/j.scp.2021.100544. [DOI] [Google Scholar]
- Zhang H.; Hao F.; Yao Z.; Zhu J.; Jing X.; Wang X. Efficient Extraction of Flavonoids from Polygonatum Sibiricum Using a Deep Eutectic Solvent as a Green Extraction Solvent. Microchem. J. 2022, 175, 107168. 10.1016/j.microc.2021.107168. [DOI] [Google Scholar]
- Sharma M.; Dash K. K. Microwave and Ultrasound Assisted Extraction of Phytocompounds from Black Jamun Pulp: Kinetic and Thermodynamics Characteristics. Innov. Food Sci. Emerg. Technol. 2022, 75, 102913. 10.1016/j.ifset.2021.102913. [DOI] [Google Scholar]
- Guo N.; Zou Y. P.; Li H. K.; Kou P.; Liu Z. M.; Fu Y. J. Effective Extraction and Recovery of Linarin from Chrysanthemum Indicum L. Flower Using Deep Eutectic Solvents. Microchem. J. 2020, 159, 105586. 10.1016/j.microc.2020.105586. [DOI] [Google Scholar]
- Mansur A. R.; Song N. E.; Jang H. W.; Lim T. G.; Yoo M.; Nam T. G. Optimizing the Ultrasound-Assisted Deep Eutectic Solvent Extraction of Flavonoids in Common Buckwheat Sprouts. Food Chem. 2019, 293, 438–445. 10.1016/j.foodchem.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Meng Z.; Zhao J.; Duan H.; Guan Y.; Zhao L. Green and Efficient Extraction of Four Bioactive Flavonoids from Pollen Typhae by Ultrasound-Assisted Deep Eutectic Solvents Extraction. J. Pharm. Biomed. Anal. 2018, 161, 246–253. 10.1016/j.jpba.2018.08.048. [DOI] [PubMed] [Google Scholar]
- Alrugaibah M.; Yagiz Y.; Gu L. Use Natural Deep Eutectic Solvents as Efficient Green Reagents to Extract Procyanidins and Anthocyanins from Cranberry Pomace and Predictive Modeling by RSM and Artificial Neural Networking. Sep. Purif. Technol. 2021, 255, 117720. 10.1016/j.seppur.2020.117720. [DOI] [Google Scholar]
- Benvenutti L.; Zielinski A. A. F.; Ferreira S. R. S. Pressurized Aqueous Solutions of Deep Eutectic Solvent (DES): A Green Emergent Extraction of Anthocyanins from a Brazilian Berry Processing by-Product. Food Chem. X. 2022, 13, 100236. 10.1016/j.fochx.2022.100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Vega J.; Gómez-Alonso S.; Pérez-Navarro J.; Alarcón-Enos J.; Pastene-Navarrete E. Polyphenolic Compounds Extracted and Purified from Buddleja Globosa Hope (Buddlejaceae) Leaves Using Natural Deep Eutectic Solvents and Centrifugal Partition Chromatography. Molecules. 2021, 26 (8), 2192. 10.3390/molecules26082192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukavina I.; Rodrigues M. J.; Pereira C. G.; Mansinhos I.; Romano A.; Ślusarczyk S.; Matkowski A.; Custódio L. Greener Is Better: First Approach for the Use of Natural Deep Eutectic Solvents (Nades) to Extract Antioxidants from the Medicinal Halophyte Polygonum Maritimum l. Molecules. 2021, 26 (20), 6136. 10.3390/molecules26206136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic B. M.; Micic N.; Potkonjak A.; Blagojevic B.; Pavlovic K.; Milanov D.; Juric T. Novel Extraction of Polyphenols from Sour Cherry Pomace Using Natural Deep Eutectic Solvents - Ultrafast Microwave-Assisted NADES Preparation and Extraction. Food Chem. 2022, 366, 130562. 10.1016/j.foodchem.2021.130562. [DOI] [PubMed] [Google Scholar]
- Xue H.; Li J.; Wang G.; Zuo W.; Zeng Y.; Liu L. Ultrasound-Assisted Extraction of Flavonoids from Potentilla Fruticosa L. Using Natural Deep Eutectic Solvents. Molecules. 2022, 27 (18), 5794. 10.3390/molecules27185794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui M.; Feng S.; Liu G.; Chen B.; Li Z.; Shao P. Deep Eutectic Solvent on Extraction of Flavonoid Glycosides from Dendrobium Officinale and Rapid Identification with UPLC-Triple-TOF/MS. Food Chem. 2023, 401, 134054. 10.1016/j.foodchem.2022.134054. [DOI] [PubMed] [Google Scholar]
- Isah M. B.; Tajuddeen N.; Umar M. I.; Alhafiz Z. A.; Mohammed A.; Ibrahim M. A.. Terpenoids as Emerging Therapeutic Agents: Cellular Targets and Mechanisms of Action against Protozoan Parasites. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, Netherlands, 2018; Vol. 59, pp 227–250. 10.1016/B978-0-444-64179-3.00007-4. [DOI] [Google Scholar]
- Arnesen J. A.; Borodina I. Engineering of Yarrowia Lipolytica for Terpenoid Production. Metab. Eng. Commun. 2022, 15, e00213 10.1016/j.mec.2022.e00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus O.; Hilgers F.; Nakielski A.; Hasenklever D.; Jaeger K. E.; Axmann I. M.; Drepper T. Engineering Phototrophic Bacteria for the Production of Terpenoids. Curr. Opin. Biotechnol. 2022, 77, 102764. 10.1016/j.copbio.2022.102764. [DOI] [PubMed] [Google Scholar]
- Sun Z.; He G.; Huang N.; Thilakavathy K.; Lim J. C. W.; Kumar S. S.; Xiong C. Glycyrrhizic Acid: A Natural Plant Ingredient as a Drug Candidate to Treat COVID-19. Front. Pharmacol. 2021, 12, 707205. 10.3389/fphar.2021.707205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed E. A. H.; Peng Y.; Wang Z.; Qiang X.; Zhao Q. Synthesis, Antiviral, and Antibacterial Activity of the Glycyrrhizic Acid and Glycyrrhetinic Acid Derivatives. Russ. J. Bioorg. Chem. 2022, 48, 906–918. 10.1134/S1068162022050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z.; He L.; Duan X.; Peng Z.; Han J. Glycyrrhizic Acid Exhibits Strong Anticancer Activity in Colorectal Cancer Cells via SIRT3 Inhibition. Bioengineered. 2022, 13, 2720–2731. 10.1080/21655979.2021.2001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; Wan Z.; Yang X. Glycyrrhizic Acid: Self-assembly and Applications in Multiphase Food Systems. Curr. Opin. Food Sci. 2022, 43, 107–113. 10.1016/j.cofs.2021.11.008. [DOI] [Google Scholar]
- Rocha V.; Quadros H.; Meira C.; Silva L.; Carvalho D.; Hodel K.; Moreira D.; Soares M. Potential of Triterpenic Natural Compound Betulinic Acid for Neglected Tropical Diseases New Treatments. Biomedicines. 2022, 10 (4), 831. 10.3390/biomedicines10040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H.; Li H.; Zhang S.; Lu H.; Chen Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules. 2021, 26 (18), 5583. 10.3390/molecules26185583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbubine C. O.; Adeyemi M. M.; Habila J. D. Isolation and Characterization of Betulinic Acid from the Stem Bark of Feretia Canthioides Hiern and Its Antimalarial Potential. Bull. Natl. Res. Cent. 2020, 44, 49. 10.1186/s42269-020-00302-2. [DOI] [Google Scholar]
- Chen X.; Lei Z.; Cao F.; Guo Q.; Wang J. Extraction, Purification of Saponins Components from Xanthoceras Sorbifolium Bunge Leaves: Potential Additives in the Food Industry. J. Food Meas. Charact. 2023, 17, 916–932. 10.1007/s11694-022-01669-8. [DOI] [Google Scholar]
- Kaur P.; Arora S.; Singh R. Isolation, Characterization and Biological Activities of Betulin from Acacia Nilotica Bark. Sci. Rep. 2022, 12, 9370. 10.1038/s41598-022-13338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordyjewska A.; Ostapiuk A.; Horecka A.; Kurzepa J. Betulin and Betulinic Acid: Triterpenoids Derivatives with a Powerful Biological Potential. Phytochem. Rev. 2019, 18, 929–951. 10.1007/s11101-019-09623-1. [DOI] [Google Scholar]
- Scheffler A. The Wound Healing Properties of Betulin from Birch Bark from Bench to Bedside. Planta Med. 2019, 85, 524–527. 10.1055/a-0850-0224. [DOI] [PubMed] [Google Scholar]
- Gai Y.; Li Y.; Xu Z.; Chen J. Pseudoprotodioscin Inhibits SREBPs and MicroRNA 33a/b Levels and Reduces the Gene Expression Regarding the Synthesis of Cholesterol and Triglycerides. Fitoterapia 2019, 139, 104393. 10.1016/j.fitote.2019.104393. [DOI] [PubMed] [Google Scholar]
- Chen Y. R.; Wang S. C.; Huang S. P.; Su C. C.; Liu P. L.; Cheng W. C.; Chuu C. P.; Chen J. K.; Bao B. Y.; Lee C. H.; Ke C. C.; Wu H. E.; Chang H. H.; Yeh H. C.; Li C. Y. Protodioscin Inhibits Bladder Cancer Cell Migration and Growth, and Promotes Apoptosis through Activating JNK and P38 Signaling Pathways. Biomed. Pharmacother. 2022, 156, 113929. 10.1016/j.biopha.2022.113929. [DOI] [PubMed] [Google Scholar]
- Ma Y. L.; Zhang Y. S.; Zhang F.; Zhang Y. Y.; Thakur K.; Zhang J. G.; Wei Z. J. Methyl Protodioscin from Polygonatum Sibiricum Inhibits Cervical Cancer through Cell Cycle Arrest and Apoptosis Induction. Food Chem. Toxicol. 2019, 132, 110655. 10.1016/j.fct.2019.110655. [DOI] [PubMed] [Google Scholar]
- Figueiredo C. C. M.; da Costa Gomes A.; Zibordi L. C.; Granero F. O.; Ximenes V. F.; Pavan N. M.; Silva L. P.; Sonvesso C. d. S. M.; Job A. E.; Nicolau-Junior N.; Gonçalves da Silva R. M. Biosynthesis of Silver Nanoparticles of Tribulus terrestris Food Supplement and Evaluated Antioxidant Activity and Collagenase, Elastase and Tyrosinase Enzyme Inhibition: In Vitro and In Silico Approaches. Food Bioprod. Process. 2023, 138, 150–161. 10.1016/j.fbp.2023.01.010. [DOI] [Google Scholar]
- Yang G.; Liu P.; Shi H.; Fan W.; Feng X.; Chen J.; Jing S.; Wang L.; Zheng Y.; Zhang D.; Guo L. Identification of Anti-Inflammatory Components in Dioscorea Nipponica Makino Based on HPLC-MS/MS, Quantitative Analysis of Multiple Components by Single Marker and Chemometric Methods. J. Chromatogr. B 2022, 1213, 123531. 10.1016/j.jchromb.2022.123531. [DOI] [PubMed] [Google Scholar]
- Pavić V.; Jakovljević M.; Molnar M.; Jokić S. Extraction of Carnosic Acid and Carnosol from Sage (Salvia Officinalis l.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants 2019, 8 (1), 16. 10.3390/plants8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elebeedy D.; Elkhatib W. F.; Kandeil A.; Ghanem A.; Kutkat O.; Alnajjar R.; Saleh M. A.; Abd El Maksoud A. I.; Badawy I.; Al-Karmalawy A. A. Anti-SARS-CoV-2 Activities of Tanshinone IIA, Carnosic Acid, Rosmarinic Acid, Salvianolic Acid, Baicalein, and Glycyrrhetinic Acid between Computational And In Vitro insights. RSC Adv. 2021, 11, 29267–29286. 10.1039/D1RA05268C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Minero F. J.; Bravo-Díaz L.; Ayala-Gómez A. Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics. Cosmetics 2020, 7 (4), 77. 10.3390/cosmetics7040077. [DOI] [Google Scholar]
- Park J.; Rho S. J.; Kim Y. R. Enhancing Antioxidant and Antimicrobial Activity of Carnosic Acid in Rosemary (Rosmarinus Officinalis L.) Extract by Complexation with Cyclic Glucans. Food Chem. 2019, 299, 125119. 10.1016/j.foodchem.2019.125119. [DOI] [PubMed] [Google Scholar]
- Feng Z.; Sun Q.; Chen W.; Bai Y.; Hu D.; Xie X. The Neuroprotective Mechanisms of Ginkgolides and Bilobalide in Cerebral Ischemic Injury: A Literature Review. Mol. Med. 2019, 25, 57. 10.1186/s10020-019-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.; Xie L.; Liu K.; Zhang X.; Wang X.; Dai X.; Liang Y.; Cao Y.; Li X. Bilobalide: A Review of Its Pharmacology, Pharmacokinetics, Toxicity, and Safety. Phytother. Res. 2021, 35, 6114–6130. 10.1002/ptr.7220. [DOI] [PubMed] [Google Scholar]
- Wang F.; Huang J.; Li J.; Chen K.; Zhang X.; Zhang Y.; Zhu Y. Bilobalide, a Bioactive Compound on Sepsis-Induced Acute Lung Injury through Its Anti-Inflammatory and Antioxidative Activity. Pharmacogn. Mag. 2021, 17, 163–169. 10.4103/pm.pm_448_20. [DOI] [Google Scholar]
- Wang X.; Gong X.; Zhang H.; Zhu W.; Jiang Z.; Shi Y.; Li L. In Vitro Anti-aging Activities of Ginkgo biloba Leaf Extract and Its Chemical Constituents. Food Sci. Technol. 2020, 40 (2), 476–482. 10.1590/fst.02219. [DOI] [Google Scholar]
- Sun H.; Zhang L.; Sui B.; Li Y.; Yan J.; Wang P.; Wang Y.; Liu S. The Effect of Terpenoid Natural Chinese Medicine Molecular Compound on Lung Cancer Treatment. Evid.-Based Complement. Altern. Med. 2021, 2021, 3730963. 10.1155/2021/3730963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H.; Baatar D.; Hwang S. G. Anticancer Activities of Ginsenosides, the Main Active Components of Ginseng. Evid.-based Complement. Altern. Med. 2021, 2021, 8858006. 10.1155/2021/8858006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanjekar K. J.; Rathod V. K. Green Extraction of Glycyrrhizic Acid from Glycyrrhiza Glabra Using Choline Chloride Based Natural Deep Eutectic Solvents (NADESs). Process Biochem. 2021, 102, 22–32. 10.1016/j.procbio.2020.11.023. [DOI] [Google Scholar]
- Lanjekar K. J.; Rathod V. K. Application of Ultrasound and Natural Deep Eutectic Solvent for the Extraction of Glycyrrhizic Acid from Glycyrrhiza Glabra: Optimization and Kinetic Evaluation. Ind. Eng. Chem. Res. 2021, 60, 9532–9538. 10.1021/acs.iecr.1c00862. [DOI] [Google Scholar]
- Jablonský M.; Šima J.; Strizincová P.; Hroboňová K.; Majová V.; Ház A. Valorization of Birch Bark Using a Low Transition Temperature Mixture Composed of Choline Chloride and Lactic Acid. Green Process. Synth. 2021, 10, 902–911. 10.1515/gps-2021-0083. [DOI] [Google Scholar]
- Yang G. Y.; Song J. N.; Chang Y. Q.; Wang L.; Zheng Y. G.; Zhang D.; Guo L. Natural Deep Eutectic Solvents for the Extraction of Bioactive Steroidal Saponins from Dioscoreae Nipponicae Rhizoma. Molecules. 2021, 26 (7), 2079. 10.3390/molecules26072079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovljević M.; Jokić S.; Molnar M.; Jerković I. Application of Deep Eutectic Solvents for the Extraction of Carnosic Acid and Carnosol from Sage (Salvia Officinalis l.) with Response Surface Methodology Optimization. Plants. 2021, 10 (1), 80. 10.3390/plants10010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J.; Wu G.; Wang L.; Cao F.; Jiang Y.; Zhao L. Oriented Deep Eutectic Solvents as Efficient Approach for Selective Extraction of Bioactive Saponins from Husks of Xanthoceras Sorbifolia Bunge. Antioxidants. 2022, 11 (4), 736. 10.3390/antiox11040736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R. R.; Huang J. H.; He D.; Yi Z. Y.; Zhao D.; Liu Z.; Zhang S. H.; Huang L. Q. Green and Efficient Extraction of Polysaccharide and Ginsenoside from American Ginseng (Panax Quinquefolius L.) by Deep Eutectic Solvent Extraction and Aqueous Two-Phase System. Molecules. 2022, 27 (10), 3132. 10.3390/molecules27103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh P. S.; Singh P. P.; Anmol; Kapoor S.; Padwad Y. S.; Sharma U. Lactic Acid-Based Deep Eutectic Solvent: An Efficient Green Media for the Selective Extraction of Steroidal Saponins from Trillium Govanianum. Sep. Purif. Technol. 2022, 294, 121105. 10.1016/j.seppur.2022.121105. [DOI] [Google Scholar]
- Zou X.; Xiao J.; Chi J.; Zhang M.; Zhang R.; Jia X.; Mei D.; Dong L.; Yi Y.; Huang F. Physicochemical Properties and Prebiotic Activities of Polysaccharides from Ziziyphus jujube Based on Different Extraction Techniques. Int. J. Biol. Macromol. 2022, 223, 663–672. 10.1016/j.ijbiomac.2022.11.057. [DOI] [PubMed] [Google Scholar]
- Wu D.-T.; Fu M.-X.; Guo H.; Hu Y.-C.; Zheng X.-Q.; Gan R.-Y.; Zou L. Microwave-Assisted Deep Eutectic Solvent Extraction, Structural Characteristics, and Biological Functions of Polysacchcarides from Sweet Tea (Lithocarpus litseifolius) Leaves. Antioxidants 2022, 11 (8), 1578. 10.3390/antiox11081578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K.-L.; Huang L.; Wu D.-T.; Li F.; Gan R.-Y.; Qin W.; Zou L. Physicochemical Properties and In Vitro Bioactivities of Polysaccharides from Lotus Leaves Extracted by Different Techniques and Solvents. J. Food Meas. Charact. 2022, 16, 1583–1594. 10.1007/s11694-021-01256-3. [DOI] [Google Scholar]
- Jiang Z.-M.; Wang F.-F.; Liu J.; Huang T.-Q.; Zhang H.; Liu E.-H. Deep Eutectic Solvents in the Efficient Detoxification and Extraction of Alkaloids of Aconiti Lateralis Radix Praeparata. ACS Sustain. Chem. Eng. 2022, 10, 5674–5682. 10.1021/acssuschemeng.2c00754. [DOI] [Google Scholar]
- Duan H.; Er-bu A.; Dongzhi Z.; Xie H.; Ye B.; He J. Alkaloids from Dendrobium and Their Biosynthetic Pathway, Biological Activity and Total Synthesis. Phytomedicine 2022, 102, 154132. 10.1016/j.phymed.2022.154132. [DOI] [PubMed] [Google Scholar]
- Song M.; Ying Z.; Ying X.; Jia L.; Yang G. Three Novel Alkaloids from Portulaca oleracea L. and Their Anti-inflammatory Bioactivities. Fitoterapia 2022, 156, 105087. 10.1016/j.fitote.2021.105087. [DOI] [PubMed] [Google Scholar]
- Viñas-Ospino A.; Panić M.; Radojčić-Redovniković J.; Blesa J.; Esteve M. J. Using Novel Hydrophobic Deep Eutectic Solvents to Improve a Sustainable Carotenoid Extraction from Orange Peels. Food Biosci. 2023, 53, 102570. 10.1016/j.fbio.2023.102570. [DOI] [Google Scholar]
- Liu Y.; chen Q.; Zhang S.; Zhang H.; Xu W. Evaluation of Green and Efficient Deep Eutectic Solvents as Media for Extracting Alkaloids from Lotus Leaf. Biomed. Chromatogr. 2022, 36 (3), e5293 10.1002/bmc.5293. [DOI] [PubMed] [Google Scholar]
- Torres-Vega J.; Gómez-Alonso S.; Pérez-Navarro J.; Pastene-Navarrete E. Green Extraction of Alkaloids and Polyphenols from Peumus boldus Leaves with Natural Deep Eutectic Solvents and Profiling by HPLC-PDA-IT-MS/MS and HPLC-QTOF-MS/MS. Plants 2020, 9 (2), 242. 10.3390/plants9020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Pan Z.; Wang B.; Yu W.; Song S.; Feng H.; Zhao W.; Zhang J. Ultrasound-assisted Extraction of Bioactive Alkaloids from Phellodendri amurensis Cortex Using Deep Eutectic Solvent Aqueous Solutions. New J. Chem. 2020, 44, 9172–9178. 10.1039/D0NJ00877J. [DOI] [Google Scholar]
- Koutsoukos S.; Tsiaka T.; Tzani A.; Zoumpoulakis P.; Detsi A. Choline Chloride and Tartaric Acid, a Natural Deep Eutectic Solvent for the Efficient Extraction of Phenolic and Carotenoid Compounds. J. Clean. Prod. 2019, 241, 118394. 10.1016/j.jclepro.2019.118384. [DOI] [Google Scholar]
- Alara O. R.; Abdurahman N. H.; Ukaegbu C. I. Soxhlet Extraction of Phenolic Compounds from Vernonia Cinerea Leaves and Its Antioxidant Activity. J. Appl. Res. Med. Aromat. Plants. 2018, 11, 12–17. 10.1016/j.jarmap.2018.07.003. [DOI] [Google Scholar]
- Irakli M.; Chatzopoulou P.; Ekateriniadou L. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds: Oleuropein, Phenolic Acids, Phenolic Alcohols and Flavonoids from Olive Leaves and Evaluation of Its Antioxidant Activities. Ind. Crops. Prod. 2018, 124, 382–388. 10.1016/j.indcrop.2018.07.070. [DOI] [Google Scholar]
- Caldas T. W.; Mazza K. E. L.; Teles A. S. C.; Mattos G. N.; Brígida A. I. S.; Conte-Junior C. A.; Borguini R. G.; Godoy R. L. O.; Cabral L. M. C.; Tonon R. v. Phenolic Compounds Recovery from Grape Skin Using Conventional and Non-Conventional Extraction Methods. Ind. Crops. Prod. 2018, 111, 86–91. 10.1016/j.indcrop.2017.10.012. [DOI] [Google Scholar]
- Rodsamran P.; Sothornvit R. Extraction of Phenolic Compounds from Lime Peel Waste Using Ultrasonic-Assisted and Microwave-Assisted Extractions. Food Biosci. 2019, 28, 66–73. 10.1016/j.fbio.2019.01.017. [DOI] [Google Scholar]
- Saifullah M.; McCullum R.; McCluskey A.; Vuong Q. Comparison of Conventional Extraction Technique with Ultrasound Assisted Extraction on Recovery of Phenolic Compounds from Lemon Scented Tea Tree (Leptospermum Petersonii) Leaves. Heliyon 2020, 6 (4), e03666 10.1016/j.heliyon.2020.e03666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nian B.; Li X. Can Deep Eutectic Solvents Be the Best Alternatives to Ionic Liquids and Organic Solvents: A Perspective in Enzyme Catalytic Reactions. Int. J. Biol. Macromol. 2022, 217, 255–269. 10.1016/j.ijbiomac.2022.07.044. [DOI] [PubMed] [Google Scholar]
- Dong J. N.; Wu G. D.; Dong Z. Q.; Yang D.; Bo Y. K.; An M.; Zhao L. S. Natural Deep Eutectic Solvents as Tailored and Sustainable Media for the Extraction of Five Compounds from Compound Liquorice Tablets and Their Comparison with Conventional Organic Solvents. RSC Adv. 2021, 11, 37649–37660. 10.1039/D1RA06338C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.; Rozema E.; Verpoorte R.; Choi Y. H. Application of Natural Deep Eutectic Solvents to the Extraction of Anthocyanins from Catharanthus Roseus with High Extractability and Stability Replacing Conventional Organic Solvents. J. Chromatogr. A 2016, 1434, 50–56. 10.1016/j.chroma.2016.01.037. [DOI] [PubMed] [Google Scholar]
- Huang H. J.; Yuan X. Z. Recent Progress in the Direct Liquefaction of Typical Biomass. Prog. Energy Combust. Sci. 2015, 49, 59–80. 10.1016/j.pecs.2015.01.003. [DOI] [Google Scholar]
- Tang W.; An Y.; Row K. H. Emerging Applications of (Micro) Extraction Phase from Hydrophilic to Hydrophobic Deep Eutectic Solvents: Opportunities and Trends. TrAC - Trends Anal. Chem. 2021, 136, 116187. 10.1016/j.trac.2021.116187. [DOI] [Google Scholar]
- Boli E.; Prinos N.; Louli V.; Pappa G.; Stamatis H.; Magoulas K.; Voutsas E. Recovery of Bioactive Extracts from Olive Leaves Using Conventional and Microwave-Assisted Extraction with Classical and Deep Eutectic Solvents. Separations 2022, 9 (9), 255. 10.3390/separations9090255. [DOI] [Google Scholar]
- Saar-Reismaa P.; Koel M.; Tarto R.; Vaher M. Extraction of Bioactive Compounds from Dipsacus Fullonum Leaves Using Deep Eutectic Solvents. J. Chromatogr. A 2022, 1677, 463330. 10.1016/j.chroma.2022.463330. [DOI] [PubMed] [Google Scholar]
- Mansinhos I.; Gonçalves S.; Rodríguez-Solana R.; Ordóñez-Díaz J. L.; Moreno-Rojas J. M.; Romano A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula Pedunculata Subsp. Lusitanica (Chaytor) Franco. Antioxidants 2021, 10 (4), 582. 10.3390/antiox10040582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X.; Yang J.; Zhao Y.; Wan H.; He Y.; Zhang L.; Wan H.; Li C. Greener Extraction Process and Enhanced in Vivo Bioavailability of Bioactive Components from Carthamus Tinctorius L. by Natural Deep Eutectic Solvents. Food Chem. 2021, 348, 129090. 10.1016/j.foodchem.2021.129090. [DOI] [PubMed] [Google Scholar]
- della Posta S.; Gallo V.; Gentili A.; Fanali C. Strategies for the Recovery of Bioactive Molecules from Deep Eutectic Solvents Extracts. TrAC Trends Anal. Chem. 2022, 157, 116798. 10.1016/j.trac.2022.116798. [DOI] [Google Scholar]
- Xu M.; Ran L.; Chen N.; Fan X.; Ren D.; Yi L. Polarity-Dependent Extraction of Flavonoids from Citrus Peel Waste Using a Tailor-Made Deep Eutectic Solvent. Food Chem. 2019, 297, 124970. 10.1016/j.foodchem.2019.124970. [DOI] [PubMed] [Google Scholar]
- Florindo C.; McIntosh A. J. S.; Welton T.; Branco L. C.; Marrucho I. M. A Closer Look Into Deep Eutectic Solvents: Exploring Intermolecular Interactions Using Solvatochromic Probes. Phys. Chem. Chem. Phys. 2018, 20 (1), 206–213. 10.1039/C7CP06471C. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Wu H.; Cai D.; Yang T.; Yang L. Effective Extraction of Harmine by Menthol/Anise Alcohol-Based Natural Deep Eutectic Solvents. Sep. Purif. Technol. 2020, 250, 117211. 10.1016/j.seppur.2020.117211. [DOI] [Google Scholar]
- Radzali S. A.; Markom M.; Saleh N. M. Co-Solvent Selection for Supercritical Fluid Extraction (SFE) of Phenolic Compounds from Labisia Pumila. Molecules. 2020, 25 (24), 5859. 10.3390/molecules25245859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L.; Dou L.-L.; Guo L.; Li P.; Liu E.-H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. 10.1021/acssuschemeng.6b00091. [DOI] [Google Scholar]
- Jurić T.; Uka D.; Holló B. B.; Jović B.; Kordić B.; Popović B. M. Comprehensive Physicochemical Evaluation of Choline Chloride-Based Natural Deep Eutectic Solvents. J. Mol. Liq. 2021, 343, 116968. 10.1016/j.molliq.2021.116968. [DOI] [Google Scholar]
- Alshammari O. A. O.; Almulgabsagher G. A. A.; Ryder K. S.; Abbott A. P. Effect of Solute Polarity on Extraction Efficiency Using Deep Eutectic Solvents. Green Chem. 2021, 23, 5097–5105. 10.1039/D1GC01747K. [DOI] [Google Scholar]
- Lei J.; Wang Y.; Li W.; Fu S.; Zhou J.; Lu D.; Wang C.; Sheng X.; Zhang M.; Xiao S.; Sun C.; Wang G. Natural Green Deep Eutectic Solvents-Based Eco-Friendly and Efficient Extraction of Flavonoids from Selaginella Moellendorffii: Process Optimization, Composition Identification and Biological Activity. Sep. Purif. Technol. 2022, 283, 120203. 10.1016/j.seppur.2021.120203. [DOI] [Google Scholar]
- Zhao B. Y.; Xu P.; Yang F. X.; Wu H.; Zong M. H.; Lou W. Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora Japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. 10.1021/acssuschemeng.5b00619. [DOI] [Google Scholar]
- Phaisan S.; Makkliang F.; Putalun W.; Sakamoto S.; Yusakul G. Development of a Colorless Centella Asiatica (L.) Urb. Extract Using a Natural Deep Eutectic Solvent (NADES) and Microwave-Assisted Extraction (MAE) Optimized by Response Surface Methodology. RSC Adv. 2021, 11, 8741–8750. 10.1039/D0RA09934A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J.; Zhang Y.; Tang C.; Hou Y.; Ai X.; Chen X.; Zhang Y.; Wang X.; Meng X. Gallic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Inflammation-Related Diseases. Biomed. Pharmacother. 2021, 133, 110985. 10.1016/j.biopha.2020.110985. [DOI] [PubMed] [Google Scholar]
- Pan C.; Zhao L.; Zhao D. Microwave-Assisted Green Extraction of Antioxidant Components from Osmanthus Fragrans (Lour) Flower Using Natural Deep Eutectic Solvents. J. Appl. Res. Med. Aromat. Plants. 2021, 20, 100285. 10.1016/j.jarmap.2020.100285. [DOI] [Google Scholar]
- Zhao Y.; Wang P.; Zheng W.; Yu G.; Li Z.; She Y.; Lee M. Three-Stage Microwave Extraction of Cumin (Cuminum Cyminum L.) Seed Essential Oil with Natural Deep Eutectic Solvents. Ind. Crops. Prod. 2019, 140, 111660. 10.1016/j.indcrop.2019.111660. [DOI] [Google Scholar]
- Shang X.; Dou Y.; Zhang Y.; Tan J. N.; Liu X.; Zhang Z. Tailor-Made Natural Deep Eutectic Solvents for Green Extraction of Isoflavones from Chickpea (Cicer Arietinum L.) Sprouts. Ind. Crops. Prod. 2019, 140, 111724. 10.1016/j.indcrop.2019.111724. [DOI] [Google Scholar]
- Shang X. C.; Chu D.; Zhang J. X.; Zheng Y. F.; Li Y. Microwave-Assisted Extraction, Partial Purification and Biological Activity in Vitro of Polysaccharides from Bladder-Wrack (Fucus Vesiculosus) by Using Deep Eutectic Solvents. Sep. Purif. Technol. 2021, 259, 118169. 10.1016/j.seppur.2020.118169. [DOI] [Google Scholar]
- Loukri A.; Sarafera C.; Goula A. M.; Gardikis K.; Mourtzinos I. Green Extraction of Caffeine from Coffee Pulp Using a Deep Eutectic Solvent (DES). Appl. Food Res. 2022, 2 (2), 100176. 10.1016/j.afres.2022.100176. [DOI] [Google Scholar]
- Gabriele F.; Chiarini M.; Germani R.; Tiecco M.; Spreti N. Effect of Water Addition on Choline Chloride/Glycol Deep Eutectic Solvents: Characterization of Their Structural and Physicochemical Properties. J. Mol. Liq. 2019, 291, 111301. 10.1016/j.molliq.2019.111301. [DOI] [Google Scholar]
- Oomen W. W.; Begines P.; Mustafa N. R.; Wilson E. G.; Verpoorte R.; Choi Y. H. Natural Deep Eutectic Solvent Extraction of Flavonoids of Scutellaria Baicalensis as a Replacement for Conventional Organic Solvents. Molecules. 2020, 25 (3), 617. 10.3390/molecules25030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk B.; Esteban J.; Gonzalez-Miquel M. Deterpenation of Citrus Essential Oils Using Glycerol-Based Deep Eutectic Solvents. J. Chem. Eng. Data 2018, 63, 2384–2393. 10.1021/acs.jced.7b00944. [DOI] [Google Scholar]
- Guo N.; Ping-Kou; Jiang Y. W.; Wang L. T.; Niu L. J.; Liu Z. M.; Fu Y. J. Natural Deep Eutectic Solvents Couple with Integrative Extraction Technique as an Effective Approach for Mulberry Anthocyanin Extraction. Food Chem. 2019, 296, 78–85. 10.1016/j.foodchem.2019.05.196. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Li J.; Fu R.; Zhang L.; Wang D.; Wang S. Enhanced Extraction of Natural Pigments from Curcuma Longa L. Using Natural Deep Eutectic Solvents. Ind. Crops. Prod. 2019, 140, 111620. 10.1016/j.indcrop.2019.111620. [DOI] [Google Scholar]
- Shikov A. N.; Kosman V. M.; Flissyuk E. v.; Smekhova I. E.; Elameen A.; Pozharitskaya O. N. Natural Deep Eutectic Solvents for the Extraction of Phenyletanes and Phenylpropanoids of Rhodiola Rosea L. Molecules. 2020, 25 (8), 1826. 10.3390/molecules25081826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh Y. H.; Li Y.; Pan Z.; Chen Z.; Lu J.; Yuan J.; Zhu Z.; Zhang J. Ultrasonication-Assisted Synthesis of Alcohol-Based Deep Eutectic Solvents for Extraction of Active Compounds from Ginger. Ultrason. Sonochem. 2020, 63, 104915. 10.1016/j.ultsonch.2019.104915. [DOI] [PubMed] [Google Scholar]
- Rozas S.; Benito C.; Alcalde R.; Atilhan M.; Aparicio S. Insights on the Water Effect on Deep Eutectic Solvents Properties and Structuring: The Archetypical Case of Choline Chloride + Ethylene Glycol. J. Mol. Liq. 2021, 344, 117717. 10.1016/j.molliq.2021.117717. [DOI] [Google Scholar]
- Zhang X.; Su J.; Chu X.; Wang X. A Green Method of Extracting and Recovering Flavonoids from Acanthopanax senticosus Using Deep Eutectic Solvents. Molecules 2022, 27 (3), 923. 10.3390/molecules27030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.; Witkamp G.-J.; Verpoorte R.; Choi Y. H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. 10.1021/ac400432p. [DOI] [PubMed] [Google Scholar]
- Brewer A.; Florek J.; Kleitz F. A Perspective on Developing Solid-phase Extraction Technologies for Industrial-scale Critical Materials Recovery. Green Chem. 2022, 24, 2752–2765. 10.1039/D2GC00347C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen S.; Chen S.; Geng S.; Zhang H.; Chen Y.; Liu B. Ultrasound-Assisted Natural Deep Eutectic Solvent Extraction and Bioactivities of Flavonoids in Ampelopsis grossedentata Leaves. Foods 2022, 11 (5), 668. 10.3390/foods11050668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M.; Ran L.; Chen N.; Fan X.; Ren D.; Yi L. Polarity-dependent Extraction of Flavonoids from Citrus Peel Waste Using a Tailor-made Deep Eutectic Solvent. Food Chem. 2019, 297, 124970. 10.1016/j.foodchem.2019.124970. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Bian S.; Hu J.; Liu G.; Peng S.; Chen H.; Jiang Z.; Wang T.; Ye Q.; Zhu H. Natural Deep Eutectic Solvent-Based Microwave-Assisted Extraction of Total Flavonoid Compounds from Spent Sweet Potato (Ipomoea batatas L.) Leaves: Optimization and Antioxidant and Bacteriostatic Activity. Molecules 2022, 27 (18), 5985. 10.3390/molecules27185985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.; Lei J.; Li S.; Jiang Y.; Zhang F.; Song C.; Xiao S.; Fu S.; Zhou J.; Wu F.; Wang G. Extraction of Flavonoids from Citri Reticulatae Pericarpium Viride Using a Deep Eutectic Solvent. RSC Adv. 2022, 12, 26975–26988. 10.1039/D2RA04276B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannu A.; Blangetti M.; Baldino S.; Prandi C. Promising Technological and Industrial Applications of Deep Eutectic Systems. Materials 2021, 14 (10), 2494. 10.3390/ma14102494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rente D.; Cvjetko Bubalo M. C.; Panić M.; Paiva A.; Caprin B.; Radojčic Redovniković I. R.; Duarte A. R. C. Review of Deep Eutectic Systems from Laboratory to Industry, Taking the Application in the Cosmetics Industry as an Example. J. Clean. Prod. 2022, 380 (2), 135147. 10.1016/j.jclepro.2022.135147. [DOI] [Google Scholar]
- Deng W.-w.; Mei X.-p.; Cheng Z.-j.; gan T.-x.; Tian X.; Hu J-n.; Zang C.-r.; Sun B.; Wu J.; Deng Y.; Ghiladi R. A.; Lorimer G. H.; Keceli G.; Wang J. Extraction of Weak Hydrophobic Sulforaphane From Broccoli by Salting-out Assisted Hydrophobic Deep Eutectic Solvent Extraction. Food Chem. 2023, 405, 134817. 10.1016/j.foodchem.2022.134817. [DOI] [PubMed] [Google Scholar]
- Dong Q.; Wang H.; Wu R.; Cao J.; Cao F.; Su E. A Highly Efficient Liquid-liquid Microextraction Pretreatment Method for Determination of Ginkgolic Acids Based on Hydrophobic Deep Eutectic Solvent. J. Mol. Liq. 2023, 376, 121425. 10.1016/j.molliq.2023.121425. [DOI] [Google Scholar]
- Wang T.; Guo Q.; Li P.; Yang H. Deep-eutectic Solvents/Ionic Liquids/Water Mixture as a Novel Type of Green Thermo-switchable Solvent System for Selective Extraction and Separation of Natural Products from Rosmarinus officialis Leaves. Food Chem. 2022, 390, 133225. 10.1016/j.foodchem.2022.133225. [DOI] [PubMed] [Google Scholar]
- Chen X.; Wang R.; Tan Z. Extraction and Purification of Grape Seed Polysaccharides Using pH-switchable Deep Eutectic Solvents-based Three-phase Partitioning. Food Chem. 2023, 412, 135557. 10.1016/j.foodchem.2023.135557. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Zhao W.; Bai T.; Fu L.; Chen Z.; Jing X.; Wang X. Sustainable Extraction of Polyphenols from Millet Using Switchable Deep Eutectic Solvents. LWT - Food Sci. Technol. 2022, 170, 114082. 10.1016/j.lwt.2022.114082. [DOI] [Google Scholar]
- Aguirre M. A.; Canals A. Magnetic Deep Eutectic Solvents in Microextraction Techniques. Trends Anal. Chem. 2022, 146, 116500. 10.1016/j.trac.2021.116500. [DOI] [Google Scholar]
- Makoś-Chełstowska P.; Kaykhaii M.; Płotka-Wasylka J.; de la Guardia M. Magnetic Deep Eutectic Solvents - Fundamentals and Applications. J. Mol. Liq. 2022, 365, 120158. 10.1016/j.molliq.2022.120158. [DOI] [Google Scholar]
- Hang Q.; Yin H.; Yuan Y.; Jiang X.; Zhao L.; Xiong Z. Magnetic Molecularly Imprinted Polymers Based on Eco-friendly Deep Eutectic Solvent for Recognition and Extraction of Three Glucocorticoids in Lotion. Microchem. J. 2022, 183, 107975. 10.1016/j.microc.2022.107975. [DOI] [Google Scholar]