See Clinical Research on Page 1407
Acute kidney injury (AKI) is a syndrome characterized by a rapid decline of renal function in combination with renal tissue injury. The incidence of AKI in postcardiac surgery and septic shock patients is as high as 40% to 60% and dialysis-requiring AKI is associated with a very high mortality of approximately 50%.1 Although many promising therapeutics have been suggested by basic studies that have clarified possible mechanisms underlying AKI,2 no drug has been shown as effective for human AKI. This underscores the failure of translational research to drive the basic research results to a clinical setting. This failure is considered because of complex and heterogeneous disease characteristics of AKI, irrelevant animal models that insufficiently mimic human AKI, and inappropriate designs of clinical trials, such as delayed drug administration, canceled therapeutic benefit by adverse events, and inadequate sample size. It should be noted that identifying the right targets of a drug (i.e., responder) is crucial to demonstrate the benefit of therapeutic intervention in clinical trials.
Correction of renal hypoperfusion by fluid resuscitation will rapidly improve renal function in prerenal AKI. Including such prerenal AKI patients in clinical trials will interfere with detecting potential drug effects because most new drugs target acute tubular injury. However, determining prerenal AKI over renal AKI requires a fluid challenge and certain observation time. A longer observation time will decrease the chance of response to new AKI treatment in clinical trials. For early detection and differentiation of renal AKI, monitoring the tubular injury is expected to be a better strategy than monitoring the serum creatinine and urine output in AKI diagnosis based on the Kidney Disease–Improving Global Outcomes criteria. Several AKI biomarkers have been developed for early detection of renal tubular injury. Among them, the urinary tissue inhibitor of metalloproteinase 2 and insulin-like growth factor binding protein 7 ([TIMP-2] × [IGFBP7]), which are cell-cycle arrest proteins expressed in renal tubular cells during periods of cellular stress or injury, could predict the primary end point of AKI development to Kidney Disease–Improving Global Outcomes stage 2 or 3 within 12 hours in the Sapphire study.3 In 2014, the US Food and Drug Administration approved the marketing of a test of [TIMP-2] × [IGFBP7] to determine whether certain critically ill patients are at risk of developing moderate to severe AKI.
Because no specific drug that can currently prevent or treat AKI is clinically available, including biomarker measurement in AKI clinical trials may be a possible solution. In this case, patients are screened for eligibility of clinical trials by measuring biomarkers. Prevalence of renal tubular injury (i.e., high biomarker value) will increase the proportion of target patients at enrollment and decrease the sample size and trial cost. If drug A is demonstrated to be effective in patients with high biomarker B (responder), both drug A and biomarker B will be considered simultaneously. Several clinical trials have recently used biomarkers as the basis for patient enrollment. The PrevAKI study evaluated the impact of the Kidney Disease–Improving Global Outcomes guideline bundle implementation for preventing postcardiac surgery AKI in high-risk patients characterized by the urinary [TIMP-2] × [IGFBP7].4 In addition to a significant reduction in AKI occurrence in the intervention group, 495 of 882 screened patients were excluded because of low [TIMP-2] × [IGFBP7] values.
In this issue, Van Till et al.5 reported a randomized, double-blind, placebo-controlled, biomarker assignment-driven, multicenter study, in which efficacy and safety of a novel PPARδ modulator (ASP1128) were evaluated. Of note, this study enrolled only the adult patients at risk for postcardiac surgery AKI based on clinical characteristics and postoperative urinary [TIMP2] × [IGFBP7] measurement. The primary end point was AKI diagnosed by serum creatinine within 72 hours postsurgery and the secondary end point was the composite end point of major adverse kidney events (death, renal replacement therapy, and/or ≥25% reduction of estimated glomerular filtration rate) at days 30 and 90. The data monitoring committee recommended discontinuing the study because the result met the futility stop criteria. The subject disposition flow chart showed that a total number of 428 patients were screened, 77 were excluded, 151 patients were randomized to AP1128 (N = 70) or placebo (N = 81), and 200 patients were enrolled in the observational cohort because their urinary [TIMP-2] × [IGFBP7] values measured at 2 to 22 hours after cardiac surgery was smaller than the cutoff value of 0.3.
The results showed no difference in the rates of AKI diagnosed by serum creatinine within 72 hours postsurgery or moderate/severe AKI between the AP1128 and the placebo groups. There was also no difference in major adverse kidney events occurrence within 30 and 90 days. No safety issues were reported by ASP1128 administration, although postoperative atrial fibrillation was less frequent than in the placebo group. Although excluding the patients with low [TIMP-2] × [IGFBP7] values after the surgery was expected to enrich the AKI rate, overall AKI occurrence was lower in the randomized cohort (22.7%) than in the observational cohort (35.0%). In contrast, major adverse kidney events at day 30 and 90, especially sustained estimated glomerular filtration rate loss, were higher in the randomized cohort (12.0% and 12.7%) than in the observational cohort (7.0% and 6.5%). Complications of chronic kidney disease were similar between the randomized and the observational cohorts (18.0% vs. 18.5%). These observations suggested that urinary [TIMP-2] × [IGFBP7] values measured after cardiac surgery failed to identify the expected targets of high AKI risk patients; however, they might predict longer impact on kidneys caused by cardiac surgery.
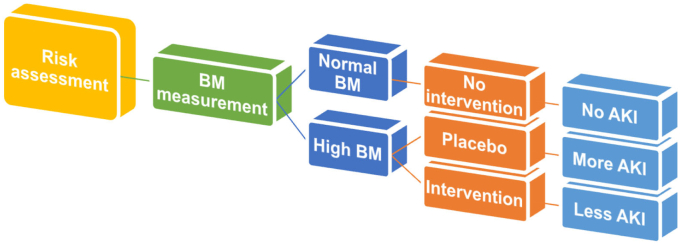
Biomarker assignment-driven patient enrollment will increase the AKI event rate and AKI severity (i.e., more renal AKI) (Figure 1); however, we need to be aware of the possible bias caused by biomarker measurement. As described above, the PrevAKI study reported a significant reduction of postcardiac surgery AKI occurrence not by any drug administration but by implementing the Kidney Disease–Improving Global Outcomes guideline bundle in high-risk patients identified by the urinary [TIMP-2] × [IGFBP7].4 Information of high biomarker values may influence physicians to select treatment strategies that are potentially renoprotective. The other issue we need to consider is the selection of biomarkers to identify the right target for a specific treatment. In this study, urinary [TIMP-2] × [IGFBP7] levels in the randomized cohorts were not changed after the surgery, whereas urinary kidney injury molecule-1 showed a step-up rise from just after the surgery to 24 hours after. This result indicates that a certain renal tubular injury had occurred in the patients of the randomized cohort, though no information regarding urinary kidney injury molecule-1 was shown in the patients of observational cohort. Obviously, different biomarkers will show different responses and clinical AKI is complex and heterogeneous, therefore we need to recognize that measuring 1 single biomarker cannot perfectly predict AKI development or other outcomes. Clinical studies applying machine learning to predict AKI have grown steadily over the past decade. Combinations of several biomarker measurements with machine learning-based prediction models that use many clinical parameters will enable the identification of the right targets of novel AKI drugs.
Figure 1.
Concept of biomarker assignment-driven clinical trial in acute kidney injury.
Disclosure
The author declared no competing interests.
References
- 1.Miyamoto Y., Iwagami M., Aso S., et al. Temporal change in characteristics and outcomes of acute kidney injury on renal replacement therapy in intensive care units: analysis of a nationwide administrative database in Japan, 2007–2016. Crit Care. 2019;23:172. doi: 10.1186/s13054-019-2468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jo S.K., Rosner M.H., Okusa M.D. Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 3.Kashani K., Al-Khafaji A., Ardiles T., et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meersch M., Schmidt C., Hoffmeier A., et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Till J.W.O., Nojima H., Kameoka C., et al. The effects of peroxisome proliferator-activated receptor delta modulator ASP1128 in patients at risk for acute kidney injury following cardiac surgery. Kidney Int Rep. 2023;8:1407–1416. doi: 10.1016/j.ekir.2023.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]