Abstract
The vaginal contraceptive ring is very effective and user dependent. In this article, we will discuss the different types of vaginal contraceptive rings, namely, the etonogestrel/ethinyl estradiol (ENG/EE) ring (NuvaRing, Merck, Rahway, NJ, USA) and the segesterone acetate (SA)/EE (Annovera, Mayne Pharma, Raleigh, NC, USA) ring. The details of dosing and administration, indications, advantages, disadvantages, and cost-effectiveness are presented. This literature review was conducted using PubMed and Google Scholar. The search terms included ‘vaginal contraceptive ring’, ‘etonogestrel/ethinyl estradiol ring’, and ‘segesterone acetate/ethinyl estradiol ring’. The search was then sorted by year from 2000 until present, and the most recent articles were reviewed. The purpose of this article is to provide a comprehensive reference on the two vaginal contraceptive rings widely used in the United States for clinicians to guide management. Both vaginal contraceptive rings are combination of hormonal contraceptives that suppress ovulation and create physiologic conditions unfavorable for pregnancy. The ENG/EE ring is designed to be replaced monthly, while the SA/EE ring is a single device used over the course of 1 year. Common side effects of both devices include headaches, nausea, vomiting, and vaginitis. Serious adverse reactions can occur with the vaginal contraceptive rings including venous thromboembolism, psychiatric events, and hypersensitivity. Both devices are contraindicated in patients at high risk for arterial or venous thrombotic events, patients with a history of breast cancer or other estrogen/progesterone cancers, and patients with severe liver disease. Overall, the vaginal contraceptive ring is well tolerated and liked by patients. Patients should be well counseled on known severe adverse reactions. The vaginal contraceptive ring is more expensive than other forms of contraception and this should be an important point of discussion with patients.
Keywords: combined hormonal contraception, contraception, ethinyl estradiol, etonogestrel, segesterone acetate, vaginal contraceptive ring
Introduction
The vaginal contraceptive ring is considered a ‘second-tier’ contraceptive method, meaning that it is considered very effective; however, it is user dependent, which decreases its efficacy. 1 The very first Food and Drug Administration (FDA)-approved vaginal contraceptive ring was released in 2001. Since that time, numerous studies have been performed examining the efficacy of the vaginal contraceptive ring, and different versions have been released. There are limited resources that have synthesized this information into a single document. In this article, we will discuss the different types of vaginal contraceptive rings: the etonogestrel/ethinyl estradiol (ENG/EE) ring and the segesterone acetate (SA)/EE ring. The details of dosing and administration, indications, advantages, disadvantages, usage, adverse reactions, efficacy, cost-effectiveness, and newest research available will be reviewed for each method. The purpose of this article is to provide a comprehensive reference on the two vaginal contraceptive rings widely used in the United States for clinicians to guide management.
Methods
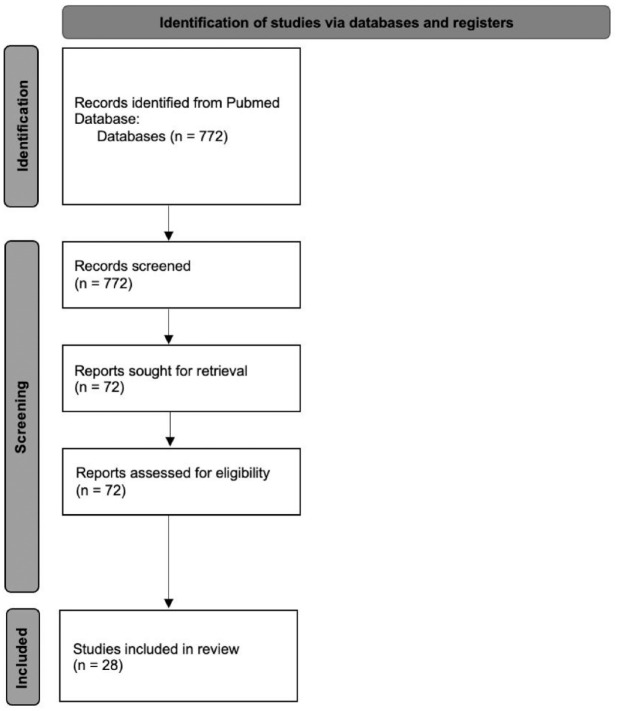
This literature review was conducted using PubMed. The search was conducted in June 2022. The search terms included ‘vaginal contraceptive ring’, ‘etonogestrel/ethinyl estradiol ring’, and ‘segesterone acetate/ethinyl estradiol ring’. The search was then sorted by year from 2000 till present in both databases. The search yielded 774 articles. Articles were screened by the first and second authors for relevance. The remaining approximately 72 articles were reviewed by the first two authors. Articles were selected by author’s perceived relevance to clinical practice and understanding of these medical devices (Figure 1). The FDA labels of the devices discussed were also reviewed. Additional websites were reviewed to view company data and recent customer perspectives.
Figure 1.
Flow diagram for database review.
ENG/EE ring
Introduction
In the United States, the intravaginal hormonal contraceptive containing ENG/EE is called the NuvaRing. ENG is a progestin (13-ethyl-17-hydroxy-11-methylene-18,19-dinor-17-a-pregn-4-en-20-yn-3-one) and EE is an estrogen (19-nor-17a-pregna-1,3,5(10)-trien-20-yne-3,17-diol). The ENG/EE ring is a flexible polymer ring with a diameter measuring 54 mm from the outer diameter, and 50 mm from the inner diameter. The ring itself is a nonbiodegradable, flexible, and colorless device. When the vaginal ring is inserted, it sits in the vaginal canal proximal and ideally around the cervix. When it is inserted, it releases a daily dose of 15 µg EE and 120 µg of ENG. The total dose in each ring when it is made is 11.7 mg of ENG and 2.7 mg EE. 1 It was approved for use by FDA in 2001 and is still used today. 2 The only FDA-approved indication for the NuvaRing is to prevent pregnancy. 2
Pharmacology
ENG/EE are rapidly absorbed by the body from the device. According to a study examining serum hormone levels cited in the FDA label, the bioavailability of ENG and EE is 100% and 56%, respectively. 2 This is comparable to oral administration of these hormones. When ENG is distributed in the blood, about 32% is bound to sex hormone-binding globulin (SHBG) and about 66% is bound to albumin. Likewise, about 98.5% of EE is bound to serum albumin and induces an increase in serum concentrations of SHBG. Both ENG and EE are metabolized in liver microsomes by the cytochrome P450 3A4 isoenzyme. EE, specifically, is primarily metabolized by aromatic hydroxylation, forming a variety of hydroxylated and methylated metabolites, which are present as free metabolites and as sulfate and glucuronide conjugates. 2 When these hormones are administered vaginally, gastrointestinal absorption and the hepatic first-pass metabolism are avoided. Despite this, concentrations of EE and ENG levels in the uterus are not elevated compared to combined oral contraceptives (COCs). 3 Indications for the ENG/EE ring include prevention of pregnancy, reduction in menorrhagia, and dysmenorrhea. 4
Mechanism of action
The ENG/EE ring is a combination of hormonal contraceptive. These contraceptives work by suppressing hypothalamic gonadotropin-releasing hormone. This, in turn, prevents pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone. Follicle-stimulating hormone is suppressed by estrogen, which stabilizes the endometrium. Therefore, when the ENG/EE ring is removed, the endometrium sheds, causing withdrawal bleeding. 1 LH is suppressed by progesterone, which prevents ovulation. When this occurs, the cervical mucus thickens to restrict the entry of sperm into the uterus. Suppression of LH also causes the endometrium to become unfavorable for implantation. 1
Usage
The ENG/EE ring is inserted inside the vagina and remains in place continuously for 3 weeks. The ring is then removed for 1 week to allow for withdrawal bleeding, then a new ring is placed. This withdrawal bleeding usually begins 2–3 days after the removal of the ring. The bleeding may not have stopped by the time a new ring should be placed; however, a new ring should be placed exactly 7 days after removal to remain effective. 2
To insert the ring, the patient can stand with one leg up, squatting, or lying down. The ring is compressed and inserted. The ring is removed by hooking the index finger under the forward rim, or by grasping the ring between two fingers and pulling it out. 2 The placement of the ring does not have to be perfect to be efficacious. In an in vivo study with magnetic resonance imaging, the anatomic location of the ring was identified to be superior to the urogenital diaphragm and surrounding the cervix. 5
The ENG/EE ring may be removed from the vagina and not lose contraceptive efficacy if it is reinserted within 3 h. To initiate the use of the ENG/EE ring, it should be inserted on the first day of menstrual bleeding. 2
Off-label continuous use is recommended for those who have issues with abnormal bleeding patterns. It is recommended for those who do not desire to take pills. Continuous use is achieved by immediately inserting a new contraceptive ring after 3 weeks, instead of waiting 7 days for withdrawal bleeding. The ring was found to be effective and tolerable with continuous use; however, women were more likely to experience breakthrough bleeding. 6
Efficacy and advantages
A widely accepted way to statistically report contraceptive efficacy is called the ‘Pearl Index’. The Pearl Index is defined as the expected number of pregnancies per 100 woman-years of exposure. This index can be calculated for the intent-to-treat (ITT) population and the per protocol (PP) population. The ITT population is defined as all patients who used a certain treatment. The PP population is defined as all patients who used a treatment without any protocol violations. 7 The Pearl Index demonstrates the effectiveness of the contraceptive method in terms of how well it can prevent pregnancy. The lower the index, the better the contraceptive is at preventing pregnancy. 8
In a study performed by Roumen et al., 1145 women used the ENG/EE ring for 12,109 cycles and the ITT Pearl Index was 0.65 with a 95% confidence interval (CI) of 0.24–1.41. 7 In a study performed by Dieben et al., the ITT Pearl Index was 1.18 with a 95% CI of 0.73–1.80. The PP Pearl Index was 0.77 with a 95% CI of 0.37–1.40. 9 Both studies demonstrate favorable Pearl Indices and provided evidence of patient satisfaction. When asked, 85% of patients were satisfied with the ring and 90% of patients would recommend it to others. In addition, individuals stated that the ring was easy to insert and remove and did not interfere with intercourse. 9
Another appealing factor of the ENG/EE ring is the favorable bleeding profile. In a 1-year, multicenter study in which 1156 patients were exposed to the vaginal ring for 12,109 cycles, irregular bleeding occurred in only 1.6–6.4% of the cycles, which mostly consisted of spotting. 7 Breakthrough bleeding was reported in only 0.4–1.1% of the cycles. Withdrawal bleeding occurred in 97.9–99.4% of cycles. 7 In a study by Dieben et al., 2322 women were followed for 23,298 cycles; similar findings were observed. 9 The incidence of irregular bleeding was 5.5% and was restricted to spotting. The incidence of withdrawal bleeding was 98.5%. 9
There have been various studies to examine the effects of concomitant vaginal products on the efficacy of the ENG/EE ring.4,9–12 The use of spermicide (specifically nonoxynol-9) with the ENG/EE ring was studied. Furthermore, it was demonstrated that the spermicide had no effect on the absorption, serum levels, and efficacy of the vaginal contraceptive device. 10 The use of tampons with the ENG/EE ring was also found to have no effect on hormone levels, and is therefore not expected to compromise its efficacy. 11
Adverse reactions
The most serious adverse reactions observed with the NuvaRing include venous thromboembolism (VTE), anxiety, cholelithiasis, and vomiting. In a study by Selvan et al., the incidence of stroke with NuvaRing was 2.5 times more likely than those without and occurred within the first year of use. 13 In a case series examining the incidence of stroke with NuvaRing use, it was suggested that approximately half of the strokes were venous, half were arterial, and that stroke typically occurred within the first year of use, as early as 2 weeks after initiation. 14 In a paper cited in the NuvaRing FDA label studying 2501 women, the three most common adverse reactions were vaginitis (13.8%), headache/migraine (11.2%), and mood changes (6.4%). 2 The mood changes observed with the ENG/EE ring include depression, mood swings, altered or depressed mood, and affect lability. Other common adverse reactions are device-related events (including expulsion, discomfort, coital problems, and foreign body sensation), nausea/vomiting, vaginal discharge, increased weight, vaginal discomfort, breast pain, dysmenorrhea, abdominal pain, acne, and decreased libido. 2
The most common adverse reaction to lead to discontinuation of the ENG/EE ring was device-related events. This was cited by approximately 2.7% of patients. 2 Other common causes of discontinuation include mood changes, headache/migraine, and vaginal symptoms. 2 Close follow-up is recommended for those affected by vaginal irritation, toxic shock syndrome, constipation, and uterovaginal prolapse. 4
A study was performed by Etminan et al. to determine the risk of pseudotumor cerebri syndrome (PTCS) with eight different types of hormonal contraceptives compared with oral levonorgestrel. An elevated risk for PTCS was found in patients who used the NuvaRing and Medroxyprogesterone suspension when compared with oral levonorgestrel (LNG). The relative risk was 4.45 compared to LNG, which was 2.2. 15
Patients should be counseled regarding the risk of VTE. A large prospective observational study called the Transatlantic Active Surveillance on Cardiovascular Safety of NuvaRing (TASC) compared the risk of VTE between the NuvaRing and COCs. The results were similar, with an incidence of 8.3 per 10,000 women-years for the NuvaRing and 9.2 per 10,000 women-years for COCs. 2 There have also been case reports describing the development of life-threatening ailments including portal venous thrombosis and cerebral sinus venous thrombosis.16,17
In a study conducted by Roumen et al., the cervical cytology of patients using NuvaRing was studied. Out of 1145 subjects, only 21 individuals with normal cervical cytology at screening experienced a shift to abnormal. 12
Contraindications
Absolute contraindications of the ENG/EE ring include history of cardiovascular disease, venous thromboembolism, hypertension, diabetes, liver disease, headaches with neurological symptoms, and smokers older than 35. It should also not be used in those with known or suspected breast, endometrial, vaginal, cervical cancer, and undiagnosed abnormal vaginal bleeding. Patients should also be counseled against the use of ENG/EE ring if they have a family history of benign breast disease, breast cancer, hyperlipidemia, liver disease, heart disease, hypertension, and migraines. 4 In addition, the ENG/EE ring should not be initiated within 30 days of planned surgery, as the estrogen component may lead to increased risk of thrombosis. 4 Some relative contraindications of the ENG/EE ring include uncontrolled dyslipidemia and non-migraine headaches. 18
Cost-effectiveness
Crespi et al. performed a model assessing the budgetary impact of eight hormonal contraceptives including branded or generic oral contraceptives, quarterly intramuscular depot medroxy-progesterone, ENG/EE vaginal ring, ENG implant, levonorgestrel intrauterine device (IUD), norelgestromin/EE transdermal contraceptive, and EE/levonorgestrel extended cycle oral contraceptives. The overall cost was calculated based on drug costs, typical use failure rates, discontinuation rates, and pregnancy costs for 1000 women initiating each type of hormonal contraceptive. The cost of the NuvaRing over 3 years was $4,082,093. This was the most expensive form of contraception, second only to the transdermal patch, which had a cost of $4,545,717 over 3 years. 19
SA/EE ring
Introduction
Another common vaginal contraceptive ring newly released in the United States is called Annovera. Like the NuvaRing, this form of contraception is completely under a patient’s control; however, it provides a full year’s worth of protection against unintended pregnancy rather than 1 month. 20 Annovera contains SA (16-methylene-17a-acetoxy-19-nor-pregn-4-ene-3,20-dione), which has high affinity to progesterone receptors and has an agonistic effect, and EE (19-Nor-17a-pregna-1,3,5(10)-trien-20-yne-3,17-diol). The device is toroidal-shaped, flexible, nonbiodegradable and opaque white. Its diameter is 56 mm and its cross-sectional diameter of 8.4 mm. The device contains 103 mg of SA and 17.4 mg EE. The daily dose of SA released is 0.15 mg per day and the dose of EE released is 0.013 mg per day. The only FDA-approved indication for this product is prevention of pregnancy. Annovera has not been adequately studied in individuals with a body mass index (BMI) >29 kg/m2. 21
Pharmacology
In vitro data have shown that the release of the hormones from the SA/EE ring varies over time. Initially, the rate of release is higher, especially during the first 24–48 h, then a lower steady state is reached during the rest of the cycle. 21 Once the device is inserted, SA and EE take about 2 h to be absorbed into systemic circulation. In each subsequent cycle, the peak levels of SA and EE decrease. 21 SA and EE are metabolized by the cytochrome P450 3A4 isoenzyme. SA produces two oxidative metabolites that are active metabolites that bind to progesterone receptors. EE is metabolized mostly by aromatic hydroxylation to produce hydroxylated and methylated metabolites that have weak estrogenic activity. 21 EE is excreted in the urine and feces and undergoes enterohepatic recirculation. The half-life of SA is 4.5 h and EE is 15.1 h. 21
A study was performed by Liu et al. to assess serum SA and EE levels after continuous use of the SA/EE ring. A linear regression model was performed to predict daily serum SA and EE levels after 364 days. The predicted level was comparable to reported levels that have prevented pregnancy (>100 pmol/L). This is favorable for the use of the SA/EE ring to be used continuously; however, further clinical trials are necessary. 22
Mechanism of action
The mechanism of action of the SA/EE ring is the same as the ENG/EE ring because they are both combination of hormonal contraceptives. These methods of contraception reduce the risk of pregnancy by suppressing ovulation, which is described in detail above (see ENG/EE Ring Mechanism of Action). 1
Usage
The SA/EE ring is placed inside the vagina and remains in place for 21 days. The device is removed during the next 7 days to allow for withdrawal bleeding. During this time, the ring is cleaned and stored in its case. After the 7-day period, the same ring is replaced inside the vagina for the next cycle. The SA/EE ring was designed to provide contraception for 1 year or 13 cycles. The method of insertion of the SA/EE ring is identical to the ENG/EE ring. The important point to emphasize with patients is that the device should be entirely inside the vagina, behind the pelvic bone. 21
If a patient has regular periods, the SA/EE ring can be initiated between Day 2 and Day 5 of the menstrual cycle without the need for back-up contraception. If the patient has irregular periods or if the ring is inserted at any other time of a patient’s regular cycle, the ring will not be effective for the first 7 days of use and a back-up method is required. 21
Efficacy and advantages
The efficacy of the SA/EE ring was studied in two multicenter, open-label, single-arm, phase III trials with identical protocols performed by Archer et al. in community and academic centers in the United States and internationally. 23 The participants were between the ages of 18 and 40 years, who were sexually active, healthy, non-sterilized, and not pregnant. The participants followed the recommended regimen of placing the ring for 21 days and removing the system for 7 days, each cycle for 13 cycles. 23
The efficacy analysis included 2265 participants and about 57.5% of participants completed the 13 cycles. The Pearl Index for the primary efficacy group was 2.98, which was within range of efficacy for a patient-controlled contraceptive system. An ITT life table analysis was performed which showed 97.5% efficacy in preventing pregnancy. Furthermore, there was no change in efficacy across 13 cycles. The Pearl Index for good use was 2.98 (95% CI 2.13–4.06) per 100 woman-years. If participants removed the vaginal ring for longer than 2 h, the Pearl Index increased to 5.89, which indicates reduced efficacy with improper use. The youngest participants in the study had the highest rate of contraceptive failure. 23
Another consideration when prescribing this vaginal contraceptive ring are medications that can be used in combination with this product. A study done by Simmons et al. examined the effects of vaginal miconazole treatment when used with the SA/EE ring. This study found that the systemic levels of SA and EE were elevated when a miconazole suppository was placed with the vaginal contraceptive ring. However, when miconazole cream was administered with the vaginal contraceptive ring, there was no effect on the hormone levels. 24
Adverse reactions
The most serious adverse reactions with Annovera cited by the FDA label include venous thromboembolic events, psychiatric events, drug hypersensitivity reactions, and spontaneous abortions. Furthermore, the most common adverse drug reaction reported by patients is headache, noted in 38.6% of patients. Other common adverse drug reactions were nausea/vomiting (25.0%), vulvovaginal mycotic infection (14.5%), abdominal pain (13.3%), dysmenorrhea (12.5%), vaginal discharge (11.8%), urinary tract infection (10%), breast pain (9.5%), diarrhea (7.2%), and general pruritis (5.5). 21
A safety evaluation was recently published by Gemezell-Danielsson et al. in 2020. This study included clinical safety data from nine studies for a total of 2308 persons who received the final manufactured vaginal contraceptive ring and 999 patients who completed 13 cycles of use. The most common adverse event was headache, which was consistent with previous data. However, it was reported in 26% of patients. Nausea was reported in 18% of individuals; vaginal discharge/mycotic infection was reported in 10%; and abdominal pain was reported in 10%. 25
The most common adverse reaction to lead to discontinuation was metrorrhagia or menorrhagia, which was reported by 1.7% of patients. 21 Other adverse reactions that lead to discontinuation of the SA/EE ring is headache (1.3%), vaginal discharge or mycotic infections (1.3%), and nausea/vomiting (1.2%). 21 The rate of partial expulsion of the device is 19.8% and the rate of complete expulsion is 7%. 25
It is important to counsel patients regarding the risk of venous thromboembolism (VTE). In a clinical safety study that combines data from four large datasets from patients using the SA/EE ring, four (0.2%) of 999 women who completed 13 cycles of use experienced VTE. Of note, three had other risk factors for thrombosis including Factor V Leiden mutation (one patient) and two with a BMI greater than 29 kg/m2. 25
Contraindications
The SA/EE ring is contraindicated in those with a hypersensitivity to any of the components of the ring. It is also contraindicated in those who are at high risk of arterial or venous thrombotic events. This includes individuals above the age of 35 who smoke, and those with a history of deep vein thrombosis, pulmonary embolism, cerebrovascular disease, coronary artery disease, inherited or acquired hypercoagulopathies, and uncontrolled diabetes mellitus or hypertension. In addition, individuals with headache with focal neurological symptoms and migraine headaches with aura are also at high risk for thrombotic events. 21
This product should not be given to patients with a history of breast cancer or other estrogen- or progesterone-sensitive cancers. In addition, this product should be avoided in patients with abnormal uterine bleeding of unknown origin, due to the risk of undiagnosed endometrial hyperplasia. 21
The SA/EE ring is contraindicated in those with liver tumors, acute hepatitis, or severe cirrhosis. This is due to the risk of liver damage which could lead to elevated liver enzymes and jaundice. The use of Hepatitis C drug combinations with the SA/EE ring is contraindicated due to the risk of alanine transaminase elevations. Development of hepatic adenomas is a known risk of hormonal contraceptive use. These tumors have the potential to grow with SA/EE rings, which may lead to death by intra-abdominal hemorrhage. 21
Cost-effectiveness
In 2018, the cost of a single Annovera is $2000, which lasts for 1 year. 26 The cost of Annovera with insurance is $0.00 and the manufacturer has coupons for uninsured to reduce price to $60.00 per year. 27 The cost of a 1-year supply of generic oral contraceptive pills or a 1-year supply of the NuvaRing is less than this. 26 For example, per Trussell et al., oral contraceptives (ortho Novum) average $52.81 per month. 28 Therefore, it is important to determine if this product is covered by the patient’s health insurance. 26
Conclusions
Clinical implications
The nuances that differentiate the ENG/EE ring and the SA/EE ring should be discussed with patients who are considering a vaginal contraceptive ring and are summarized in Table 1. When counseling patients on the use of each vaginal contraceptive ring, proper usage should be emphasized to ensure efficacy. Patients should be instructed to check ring placement before and after sexual intercourse. The device may be expelled accidentally while removing a tampon, during intercourse or straining with a bowel movement. In this case, the device can be rinsed with lukewarm water and re-inserted as soon as possible.
Table 1.
Vaginal contraceptive rings comparison.
| Characteristic | ENG/EE ring | SA/EE ring |
|---|---|---|
| Size | Outer diameter: 54 mm Inner diameter: 50 mm |
Diameter 56 mm |
| Dose | 120 µg of ENG 15 µg EE |
103 mg of SA |
| FDA approval | 2001 | 2018 |
| Usage | Place for 3 weeks Remove for 7 days Dispose |
Place for 3 weeks Remove for 7 days Replace. Reuse for 13 cycles |
| Pearl Index | 0.65–1.18 | 2.98 |
| Most common adverse reactions | Vaginitis (13.8%) Headache/migraine (11.2%) Mood changes (6.4%) |
Headache (26%) Nausea (18%) Vaginitis (10%) Abdominal pain (10%) |
EE, ethinyl estradiol; ENG, etonogestrel; FDA, Food and Drug Administration; SA, segesterone acetate.
A study was performed by Stifani et al. to examine the factors associated with non-adherence to instructions for using the SA/EE contraceptive vaginal ring. Data were collected from previous phase III clinical trial data to identify the causes of dissatisfaction. It was noted that factors associated with non-adherence were removing the ring for washing and before intercourse. As a result, providers are encouraged to counsel patients on use during intercourse and inquire of patients regarding their vaginal hygiene practices. This is suggested to decrease the risk of discontinuation, dissatisfaction, and risk of pregnancy. 29
If the ring has been out of place for greater than 3 h, then after the ring is replaced, the patient should use a backup contraceptive method (condoms or spermicide) for the next 7 days. 2 When removing the ring, patient should also try to keep similar times in which it was inserted. 30
It is also important to consider the appropriate time to initiate usage of the vaginal contraceptive ring. A patient may start using both vaginal contraceptive rings within the first 5 days following a complete first trimester abortion or miscarriage. Following childbirth, the ENG/EE ring should not be initiated until 4 weeks postpartum due to the increased risk of thromboembolism.2,21 If patients desire to change their contraceptive method, the vaginal contraceptive ring can be placed on the day of Depo Provera, Nexplanon, and IUD renewal. 21
Patient satisfaction
In two multicenter open-year study questionnaires by Szarewski, patients had a greater than 90% satisfaction rate with vaginal ring contraceptive. 31 A qualitative analysis was performed after in-depth interviews with 32 patients of various racial/ethnic minoritized groups. Overall, the patients initially had concerns about insertion and removal, leaving the device inside the vagina, interference with sex, and the size of the device; however, many patients were able to overcome their concerns with counseling from providers and positive experiences. Most patients stated that they would recommend it to friends.32–34
Summary
There are two widely used vaginal contraceptive rings: ENG/EE estradiol ring and the SA/EE ring. The two rings have similar side effect profiles and efficacy. Overall, the vaginal contraceptive ring is well tolerated and liked by patients. Patients should be well counseled on known severe adverse reactions. The vaginal contraceptive ring is more expensive than other forms of contraception and this should be an important point of discussion with the patient.
Acknowledgments
None.
Contributor Information
Sara Al-Haddad, Department of Obstetrics and Gynecology, Downstate Health Sciences University, 450 Clarkson Avenue, Brooklyn, NY 11203-2098, USA.
Ki’ara K. R. Branham, Department of Obstetrics and Gynecology, Meharry Medical College, Nashville, TN, USA
Camille A. Clare, Department of Obstetrics and Gynecology, Downstate Health Sciences University, Brooklyn, NY, USA
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Sara Al-Haddad: Conceptualization; Investigation; Writing – original draft; Writing – review & editing.
Ki’ara K.R. Branham: Investigation; Methodology; Writing – original draft.
Camille A. Clare: Conceptualization; Resources; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1.Hoffman BL, Schorge JO, Bradshaw KD, et al. Williams gynecology. 4th ed.New York, NY: McGraw-Hill Education LLC, 2020. [Google Scholar]
- 2.NuvaRing [Package Insert]. Whitehouse Station, NJ: Merck & Co., INC., 2013. [Google Scholar]
- 3.Roumen FJ.Review of the combined contraceptive vaginal ring, NuvaRing. Ther Clin Risk Manag 2008; 4: 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wieder DR, Pattimakiel L.Examining the efficacy, safety, and patient acceptability of the combined contraceptive vaginal ring (NuvaRing). Int J Womens Health 2010; 2: 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnhart KT, Timbers K, Pretorius ES, et al. In vivo assessment of NuvaRing placement. Contraception 2005; 72: 196–199. [DOI] [PubMed] [Google Scholar]
- 6.Rowland K, Schumann SA.When to suggest this OC alternative. J Fam Pract 2009; 58: 207–210. [PMC free article] [PubMed] [Google Scholar]
- 7.Roumen FJME, Apter D, Mulders TMT, et al. Efficacy, tolerability and acceptability of a novel contraceptive vaginal ring releasing etonogestrel and ethinyl oestradiol. Hum Reprod 2001; 16: 469–475. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber C, Barnhart K.CHAPTER 34 – Contraception. In: Strauss JF, Barbieri RL. Yen and Jaffe’s Reproductive Endocrinology. 6th ed.Elsevier, 2009, pp. 873–892. https://www.sciencedirect.com/science/article/pii/B9781416049074000346 (accessed March 13, 2023). [Google Scholar]
- 9.Dieben TO, Roumen FJ, Apter D.Efficacy, cycle control, and user acceptability of a novel combined contraceptive vaginal ring. Obstet Gynecol 2002; 100: 585–593. [DOI] [PubMed] [Google Scholar]
- 10.Haring T, Mulders TM.The combined contraceptive ring NuvaRing and spermicide co-medication. Contraception 2003; 67: 271–272. [DOI] [PubMed] [Google Scholar]
- 11.Verhoeven CH, Dieben TO.The combined contraceptive vaginal ring, NuvaRing, and tampon co-usage. Contraception 2004; 69: 197–199. [DOI] [PubMed] [Google Scholar]
- 12.Roumen FJ, Apter D, Mulders TM, et al. Efficacy, tolerability and acceptability of a novel contraceptive vaginal ring releasing etonogestrel and ethinyl oestradiol. Hum Reprod 2001; 16: 469–475. [DOI] [PubMed] [Google Scholar]
- 13.Selvan P, Piran P, Balucani C, et al. Stroke and etonogestrel/ethinyl estradiol ring (nuvaring): clinical, radiological, and prognostic features. J Stroke Cerebrovasc Dis 2017; 26: 608–617. [DOI] [PubMed] [Google Scholar]
- 14.Selvan P, Piran P, Balucani C, et al. Stroke and etonogestrel/ethinyl estradiol ring (NuvaRing): clinical, radiological, and prognostic features. J Stroke Cerebrovasc Dis 2017; 26: 608–617. [DOI] [PubMed] [Google Scholar]
- 15.Etminan M, Khosrow-Khavar F, Sodhi M, et al. Pseudotumor cerebri syndrome with different types of hormonal contraceptives in women of child-bearing age. Eur J Neurol 2020; 27: 2625–2629. [DOI] [PubMed] [Google Scholar]
- 16.Bailey KE, Tranovich MJ.Portal venous thrombosis associated with use of etonogestrel/ethinyl estradiol vaginal ring. Clin Pract Cases Emerg Med 2020; 4: 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selim M, Rakhra A, Kassim T, et al. Cerebral sinus venous thrombosis in a patient using etonogestrel/ethinyl estradiol vaginal ring. Cureus 2018; 10: e3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merck Stands Behind the Safety Profile of NuvaRing® (etonogestrel/ethinyl estradiol vaginal ring). Merck.com, https://www.merck.com/news/merck-stands-behind-the-safety-profile-of-nuvaring-etonogestrel-ethinyl-estradiol-vaginal-ring/ (accessed 14 March 2023).
- 19.Crespi S, Kerrigan M, Sood V.Budget impact analysis of 8 hormonal contraceptive options. Am J Manag Care 2013; 19: e249–e255. [PubMed] [Google Scholar]
- 20.Population Counsil. Population council. The Nestorone/ethinyl estradiol one year vaginal contraceptive system, https://popcouncil.org/project/the-nestorone-ethinyl-estradiol-one-year-vaginal-contraceptive-system/ (accessed 14 March 2023).
- 21.Annovera [Package Insert]. New York, NY: Population Council; 2018. [Google Scholar]
- 22.Liu JH, Plagianos M, Archer DF, et al. Segesterone acetate serum levels with a regression model of continuous use of the segesterone acetate/ethinyl estradiol contraceptive vaginal system. Contraception 2021; 104: 229–234. [DOI] [PubMed] [Google Scholar]
- 23.Archer DF, Merkatz RB, Bahamondes L, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiolcontraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health 2019; 7: e1054–e1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons KB, Kumar N, Plagianos M, et al. Effects of concurrent vaginal miconazole treatment on the absorption and exposure of Nestorone® (segesterone acetate) and ethinyl estradiol delivered from a contraceptive vaginal ring: a randomized, crossover drug-drug interaction study. Contraception 2018; 97: 270–276. [DOI] [PubMed] [Google Scholar]
- 25.Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception 2019; 99: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Population Counsil, 2018. C. Population council. The Nestorone/ethinyl estradiol one year vaginal contraceptive system, https://www.popcouncil.org/research/one-year-contraceptive-vaginal-ring (2018, accessed 14 March 2023).
- 27.The annovera birth control ring: is it right for you? The Pill Club, https://thepillclub.com/blog/the-annovera-birth-control-ring-pros-cons (accessed 20 March 2023).
- 28.Trussell J, Lalla AM, Doan QV, et al. Cost effectiveness of contraceptives in the United States. Contraception. 2009; 79: 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stifani BM, Plagianos M, Vieira CS, et al. Factors associated with nonadherence to instructions for using the Nestorone®/ethinyl estradiol contraceptive vaginal ring. Contraception 2018; 97: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frequently Asked Questions About NuvaRing® (etonogestrel/ethinyl estradiol vaginal ring). www.nuvaring.com, https://www.nuvaring.com/frequently-asked-questions/ (accessed February 2023).
- 31.Szarewski A.High acceptability and satisfaction with NuvaRing use. Eur J Contracept Reprod Health Care 2002; 7(Suppl. 2): 31–39. [PubMed] [Google Scholar]
- 32.Epstein LB, Sokal-Gutierrez K, Ivey SL, et al. Adolescent experiences with the vaginal ring. J Adolesc Health 2008; 43: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figure 2. Percent distribution of women aged 15–49, by current contraceptive status: United States, 2017–2019. https://www.cdc.gov/nchs/products/databriefs/db388.htm#fig1 (accessed 13 March 2013).
- 34.Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring: a randomized controlled trial. Obstet Gynecol 2008; 112: 563–571. [DOI] [PubMed] [Google Scholar]