Abstract
Congenital anomalies of the kidney and urinary tract (CAKUT) are among the most common birth defects worldwide and a major cause of kidney failure in children. Extra-renal manifestations are also common. This study reviewed diseases associated with the Genomics England CAKUT-associated gene panel for ocular anomalies. In addition, each gene was examined for expression in the human retina and an ocular phenotype in mouse models using the Human Protein Atlas and Mouse Genome Informatics databases, respectively. Thirty-four (54%) of the 63 CAKUT-associated genes (55 ‘green’ and 8 ‘amber’) had a reported ocular phenotype. Five of the 6 most common CAKUT-associated genes (PAX2, EYA1, SALL1, GATA3, PBX1) that represent 30% of all diagnoses had ocular features. The ocular abnormalities found with most CAKUT-associated genes and with five of the six commonest were coloboma, microphthalmia, optic disc anomalies, refraction errors (astigmatism, myopia, and hypermetropia), and cataract. Seven of the CAKUT-associated genes studied (11%) had no reported ocular features but were expressed in the human retina or had an ocular phenotype in a mouse model, which suggested further possibly-unrecognised abnormalities. About one third of CAKUT-associated genes (18, 29%) had no ocular associations and were not expressed in the retina, and the corresponding mouse models had no ocular phenotype. Ocular abnormalities in individuals with CAKUT suggest a genetic basis for the disease and sometimes indicate the affected gene. Individuals with CAKUT often have ocular abnormalities and may require an ophthalmic review, monitoring, and treatment to preserve vision.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00467-023-06068-9.
Keywords: Congenital anomalies of the kidney and urinary tract (CAKUT), Coloboma, Microphthalmia, Optic disc anomalies, Refraction errors, Cataract
CAKUT
Genetic kidney disease comprises congenital anomalies of the kidney and urinary tract (CAKUT), cystic kidney diseases and ciliopathies, glomerulopathies such as Alport syndrome, complementopathies, and focal and segmental glomerulosclerosis (FSGS), as well as the tubulopathies.
CAKUT are a diverse group of developmental anomalies and the most common (20–30%) of all birth defects [1]. CAKUT is found in nearly 1% of live births worldwide, and is the cause in almost half the children who develop kidney failure [1].
The kidney phenotypes of CAKUT include agenesis, hypoplasia, dysplasia, cysts, ectopia, fusion, hydronephrosis, urinary tract agenesis, duplication, megaureter, ureteropelvic junction obstruction, vesicoureteral reflux, and posterior urethral valves. Defects may be unilateral or bilateral. Some changes, such as bilateral kidney agenesis, are almost always fatal [2]. Some progress to kidney failure necessitating dialysis or transplantation within the first few years of life or later [3]. Some have an excellent prognosis [3]. At present, many individuals with CAKUT may be diagnosed antenatally with ultrasound but others are found incidentally after imaging, repeated urinary tract infections, or the detection of impaired kidney function.
CAKUT occurs in isolation or together with defects in other organ systems in syndromic disease. Many cases are sporadic [4], with no identifiable cause, and probably result from both genetic and environmental factors, including maternal diabetes or obesity [5]. However, familial CAKUT consistent with a genetic basis still represents at least 10–20% of cases [4].
More than 150 genes are associated with CAKUT and more remain to be identified. Many of these genes are transcription factors that are important in embryonic development. Six are common (PAX2, HNF1B, EYA1, SALL1, GATA3, PBX1) but many of the others are found only in individual families [6]. Inheritance is usually autosomal dominant (AD) with incomplete penetrance and variable expression. This means that clinical features vary even within a family, and one affected family member may have a single kidney, another has vesicoureteric reflux and another affected family member has normal kidneys. CAKUT may be misdiagnosed as FSGS since some genes result in a reduced nephron number. In addition, the diagnosis of CAKUT may be overlooked when it results from copy number variants that are difficult to detect with Whole Exome Sequencing.
Ocular anomalies occur in different forms of CAKUT. This is because of the developmental and structural overlap between the kidney and the eye despite their different functions. The kidney and the eye develop embryologically at about the same time (5th to 12th weeks of gestation) and under the control of some of the same transcription factors, including BMP7, EYA1, FOXC1, PAX, and WNT1 [7]. Surprisingly, the kidney and the eye also share structural features such as the epithelial cell barrier, basement membrane and capillary network in the glomerular filter and in the retinal pigment epithelial cells, Bruch’s membrane and choriocapillaris [7]. In addition, the glomerular and retinal basement membranes both mainly comprise the collagen IV α3α4α5 network. Importantly the kidney and the retina also depend on ciliated cells for their functions.
This review examines monogenic causes of CAKUT for their ocular associations. Identifying ocular features suggests that the disease has a genetic basis, and may indicate the affected gene and the need for treatment or ophthalmic monitoring to prevent complications.
Search strategy for ocular features in CAKUT
Sixty-three genes associated with CAKUT from the ‘green’ (n = 55) or ‘amber’ lists (n = 8) of the Genomics England CAKUT panel (https://panelapp.genomicsengland.co.uk/) were searched for ocular manifestations in the Online Mendelian Inheritance in Man (OMIM, https://www.omim.org/), PubMed and Google Scholar (https://scholar.google.com/) databases between July 2022 and March 2023. Genomics England uses a traffic light system where green genes have a high level of evidence for a disease association (reported in 3 unrelated families or 2 families with additional strong evidence) as decided by an expert panel. These panels represent the genes often examined in a diagnostic genetic laboratory. Amber and red genes have borderline and low levels of evidence for disease association.
The 63 genes were also examined for mRNA expression in the retina in The Human Protein Atlas (https://www.proteinatlas.org), and for an ocular phenotype in mouse models in the Mouse Genome Informatics (MGI) database (http://www.informatics.jax.org/).
Ocular features associated with CAKUT
About half of the 63 CAKUT-associated genes in the Genomics England panels (34, 54%) have reported ocular abnormalities (Supplementary Table 1). About one third of CAKUT genes (18, 29%) have no reported ocular associations, are not expressed in the human retina and have no ocular features in a mouse model. Seven further genes (7, 11%) have no reported ocular phenotype in human disease but are expressed in the human retina or the mouse models have ocular features. Thus, further ocular abnormalities may yet be described with these other genes.
Five of the 6 most common CAKUT genes that represent 30% of all cases (PAX2, EYA1, SALL1, GATA3, PBX1) have an ocular phenotype (Table 1). There are no reported ocular features for the HNF1B gene which is affected in HNF1B-nephropathy, formerly known as renal cysts and diabetes syndrome.
Table 1.
Most common CAKUT genes, their ocular associations, retinal expression, and ocular phenotypes in mouse models
| Gene | Disease, OMIM number | Renal features | Extrarenal features (OMIM) | Ocular features | mRNA expression (Human Protein Atlas) | Knockout mouse model (MGI) | Reference |
|---|---|---|---|---|---|---|---|
| HNF1B (189907) | Renal cysts and diabetes syndrome (AD);137920 | Renal agenesis, hypoplasia, dysplasia, cysts, horseshoe kidney; ureteropelvic junction obstruction | Gout, diabetes, hypomagnesemia | None reported | NA | None noted | Bellanne-Chantelot et al. (2004) [8]; Kaplan et al. (1989) [9]; Nakayama et al. (2010) [10]; Rizzoni et al. (1982) [11] |
| PAX2 (167409) | Papillorenal syndrome (renal-coloboma syndrome) (AD); 120330 | Kidney agenesis, hypoplasia, dysplasia, cysts, malrotation, horseshoe kidney; pyeloureteric duplication; hydro-nephrosis, ureteropelvic junction obstruction, ureterovesical junction obstruction, vesicoureteric reflux | Hearing loss, joint laxity | Reduced visual acuity, visual field defects; microphthalmia; coloboma (retina, optic nerve); microcornea; lens opacity, posterior lens subluxation; retinal staphyloma, retinal hypoplasia, abnormal retinal pigment epithelium, macular hyperpigmentation, chorioretinal degeneration, cystic macular degeneration, retinal detachment; optic disc dysplasia, ‘morning glory’ anomaly, optic pit, rudimentary/ absent central retinal vessels, optic disc hypoplasia, aplasia, hyperplasia | 1.2 TPM | Abnormal retinal vasculature, abnormal optic nerve, optic disc coloboma, abnormal retinal pigmentation, abnormal retinal nerve fibre layer | Eccles and Schimmenti (1999) [12]; Schimmenti (2011) [13] |
| EYA1 (601653) | Branchiootorenal syndrome 1, with or without cataract(AD) or Anterior segment anomalies with or without cataract; 113650 | Kidney agenesis, hypoplasia, dysplasia, cysts, crossed ectopia, malrotation; distorted pelvicalyceal system, bifid kidney pelvis, narrowed ureteropelvic junction; ureteral duplication; vesicoureteric reflux | Hearing loss, preauricular pits, abnormal ears, cochlear malformation, high arched palate | Congenital nuclear-type cataracts; nystagmus, esotropia; reduced visual acuity; central corneal opacity (Peters’ anomaly); abnormality of the lacrimal ducts | 0.4 TPM | Eyelids open at birth | Azuma et al. (2000) [14]; Carmi et al. (1983) [15]; Chitayat et al. (1992) [16]; Fraser et al. (1978) [17]; Fraser et al. (1983) [18]; Legius et al. (1990) [19]; Melnick et al. (1975) [20]; Melnick et al. (1976) [21] |
| SALL1 (602218) | Townes-Brocks branchio-oto-renal-like syndrome (AD);107480 | Kidney agenesis, hypoplasia, dysplasia, cysts, ectopia (pelvic kidney); vesicoureteric reflux; urethral valves, stenosis | Skeletal, facial, cardiac, gastrointestinal anomalies; hearing loss | Unilateral microphthalmia; coloboma (iris, choroid, retina); congenital lamellar cataract; optic nerve atrophy; Duane anomaly (limited horizontal eye movement); orbital dermoid; crocodile tears | 4.6 TPM | None noted | Blanck et al. (2000) [22]; Botzenhart et al. (2005) [23]; Botzenhart et al. (2007) [24]; Ferraz et al. (1989) [25]; Johnson et al. (1996) [26]; Kohlhase et al. (1999) [27]; Kurnit et al. (1978) [28]; Rossmiller and Pasic (1994) [29] |
| GATA3 (131320) | Hypoparathyroidism, sensorineural deafness, and renal dysplasia (AD); 146255 | Kidney agenesis, hypoplasia, dysplasia; vesicoureteric reflux | Hearing loss, abnormal female genitalia | Inherited retinal degeneration; pseudopapilloedema; horizontal nystagmus | NA | Narrow eye opening | Barakat et al. (2018) [30]; Bilous et al. (1992) [31]; Ferraris et al. (2009) [32]; Hasegawa et al. (1997) [33] |
| PBX1 (176310) | CAKUT with or without hearing loss, abnormal ears, or developmental delay (AD); 617641 | Kidney hypoplasia, dysplasia, cysts, ectopia, horseshoe kidney; dilated kidney/ calyces/pelvis, dilated ureter; bifid ureter; vesicoureteric reflux; urethral valve | Hearing loss; facial anomalies; ear anomalies including malformed pinnae; short stature; cardiac anomalies, abnormal genitalia developmental delay | Strabismus; corneal dystrophy and clouding present from birth; glaucoma; iris abnormalities | 17.1 TPM | Eye lids open at birth | Heidet et al. (2017) [34]; Le Tanno et al. (2017) [35]; Slavotinek et al. (2017) [36]; Murphy et al. (2010) [37]; Safgren et al. (2021) [38] |
TPM, transcripts per million; NA not available
The most common ocular abnormalities associated with CAKUT-associated genes are coloboma, optic disc (‘morning glory’) anomalies, microphthalmia, refraction errors (astigmatism, myopia, and hypermetropia), and cataracts (Table 2, Fig. 1).
Table 2.
Common ocular abnormalities associated with CAKUT-causing genes
| Ophthalmic Finding | Genes and Syndromes |
|---|---|
| Refractive error | |
| Astigmatism | CENPF (Stromme syndrome) |
| Hypermetropia | CHD7 (CHARGE syndrome); DDX6 (Intellectual developmental disorder with impaired language and dysmorphic facies) |
| Myopia | BMP4 (Microphthalmia, syndromic 6); CHD7 (CHARGE syndrome); JAG1 (Alagille syndrome 1); KMT2D (Kabuki syndrome 1); NIPBL (Cornelia de Lange syndrome 1); PAX2 (Papillorenal syndrome) |
| Anomalies of globe size | |
| Microphthalmia/anophthalmia | BMP4 (Microphthalmia, syndromic 6); CHD7 (CHARGE syndrome); DHCR7 (Smith-Lemli-Opitz syndrome); EP300 (Rubinstein-Taybi syndrome 2); FRAS1 (Fraser syndrome 1); FREM1 (Manitoba oculotrichoanal syndrome); GLI3 (Pallister-Hall syndrome); PAX2 (Papillorenal syndrome); SALL1 (Townes-Brocks syndrome 1; Townes-Brocks branchiootorenal-like syndrome); STRA6 (Microphthalmia, syndromic 9; Microphthalmia, isolated, with coloboma 8); TFAP2A (Branchiooculofacial syndrome) |
| Coloboma | |
| Iris | BMP4 (Microphthalmia, syndromic 6); CENPF (Stromme syndrome); CHD7 (CHARGE syndrome); SALL1 (Townes-Brocks syndrome 1; Townes-Brocks branchiootorenal-like syndrome); STRA6 (Microphthalmia, syndromic 9; Microphthalmia, isolated, with coloboma 8); TFAP2A (branchiooculofacial syndrome) |
| Retina and choroid | BMP4 (Microphthalmia, syndromic 6); CHD7 (CHARGE syndrome); KMT2D (Kabuki syndrome 1); KRAS (Schimmelpenning-Feuerstein-Mims syndrome); PAX2 (Papillorenal syndrome); SALL1 (Townes-Brocks syndrome 1; Townes-Brocks branchiootorenal-like syndrome); SALL4 (Duane-radial ray syndrome); STRA6 (Microphthalmia, syndromic 9; Microphthalmia, isolated, with coloboma 8); TFAP2A (branchiooculofacial syndrome) |
| Optic nerve | CHD7 (CHARGE syndrome); PAX2 (Papillorenal syndrome); SALL4 (Duane-radial ray syndrome); TFAP2A (Branchiooculofacial syndrome) |
| Anterior segment | |
| Cataract | CENPF (Stromme syndrome); CHD7 (CHARGE syndrome); DHCR7 (Smith-Lemli-Opitz syndrome); EYA1 (Branchiootorenal syndrome 1, with or without cataracts; Anterior segment anomalies with or without cataract); GPC3 (Simpson-Golabi-Behmel syndrome, type 1); JAG1 (Alagille syndrome 1; LRP4 (Cenani-Lenz syndactyly syndrome); PAX2 (Papillorenal syndrome); SALL1 (Townes-Brocks syndrome 1; Townes-Brocks Branchiootorenal-like syndrome); SALL4 (Duane-radial ray syndrome); TFAP2A (Branchiooculofacial syndrome) |
| Ectopia lentis (dislocated lens) | CHD7 (CHARGE syndrome); PAX2 (Papillorenal syndrome) |
| Iris hypoplasia | CENPF (Stromme syndrome) |
| Iris cyst | PLVAP (Diarrhea 10, protein-losing enteropathy type); TFAP2A (Branchiooculofacial syndrome) |
| Microcornea | CENPF (Stromme syndrome); CHD7 (CHARGE syndrome); JAG1 (Alagille syndrome 1); SALL4 (Duane-radial ray syndrome) |
| Corneal opacity | CENPF (Stromme syndrome); EYA1 (Anterior segment anomalies with or without cataract); JAG1 (Alagille syndrome 1) |
| Peters’ anomaly (thinning and clouding of the cornea, and attachment to iris) | CENPF (Stromme syndrome); EYA1 (Anterior segment anomalies with or without cataract); NIPBL (Cornelia de Lange syndrome 1) |
| Sclerocornea | CENPF (Stromme syndrome); STRA6 (Microphthalmia, syndromic 9; Microphthalmia, isolated, with coloboma 8) |
| Corectopia (off-centre pupil) | CENPF (Stromme syndrome); JAG1 (Alagille syndrome 1) |
| Polycoria (two pupils) | TFAP2A (Branchiooculofacial syndrome) |
| Posterior embryotoxon (peripheral corneal stroma) | JAG1 (Alagille syndrome 1); NOTCH2 (Alagille syndrome 2) |
| Axenfeld-Rieger anomaly (posterior embryotoxon plus congenital iris anomalies) | JAG1 (Alagille syndrome 1) |
| Posterior segment | |
| Inherited retinal degeneration | BMP4 (Microphthalmia, syndromic 6); GATA3 (Hypoparathyroidism, sensorineural deafness, and renal dysplasia) PAX2 (Papillorenal syndrome) |
| Chorioretinal scar | SALL4 (Duane-radial ray syndrome) |
| Choroidal folds | JAG1 (Alagille syndrome 1) |
| Glaucoma | DHCR7 (Smith-Lemli-Opitz syndrome) |
| Optic atrophy | DHCR7 (Smith-Lemli-Opitz syndrome); SALL1 (Townes-Brocks syndrome 1; Townes-Brocks branchiootorenal-like syndrome) |
| Optic nerve aplasia, dysplasia, hypoplasia | PAX2 (Papillorenal syndrome); STRA6 (Microphthalmia, syndromic 9; Microphthalmia, isolated, with coloboma 8); SALL4 (Duane-radial ray syndrome); CENPF (Stromme syndrome); DHCR7 (Smith-Lemli-Opitz syndrome); |
| Optic disc drusen | JAG1 (Alagille syndrome 1) |
| Persistent foetal vasculature | CHD7 (CHARGE syndrome) |
| Retinal dysplasia, detachment | PAX2 (Papillorenal syndrome) |
| Retinal hamartoma | TFAP2A (Branchiooculofacial syndrome) |
| Neuro-ophthalmic abnormalities | |
| Strabismus | DHCR7 (Smith-Lemli-Opitz syndrome); EYA1 (Branchiootorenal syndrome 1, with or without cataracts); JAG1 (Alagille syndrome 1); KMT2D (Kabuki syndrome 1); NIPBL (Cornelia de Lange syndrome 1); PBX1 (CAKUT with or without hearing loss, abnormal ears, or developmental delay); SALL4 (Duane-radial ray syndrome); TFAP2A (Branchiooculofacial syndrome) |
| Nystagmus | EYA1 (Branchiootorenal syndrome 1, with or without cataracts); GATA3 (Hypoparathyroidism, sensorineural deafness, and renal dysplasia); NIPBL (Cornelia de Lange syndrome 1) |
| Duane anomaly (strabismus with limited horizontal eye movement) | SALL1 (Townes-Brocks syndrome 1; Townes-Brocks branchiootorenal-like syndrome); SALL4 (Duane-radial ray syndrome) |
| Nocturnal lagophthalmos (eyelids do not close fully during sleep) | KMT2D (Kabuki syndrome 1) |
Fig. 1.
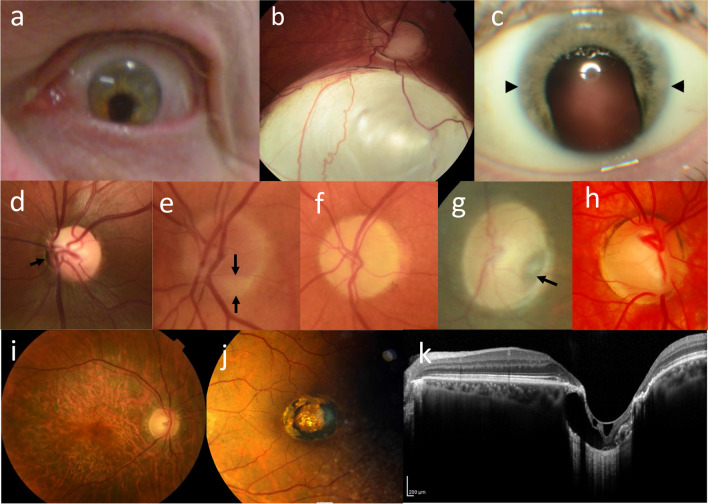
Common ocular abnormalities associated with CAKUT: a iris coloboma that resembles a ‘keyhole’ inferiorly; b large chorioretinal coloboma; c inferior iris coloboma with microphthalmia (diameter between arrows 7 mm); d subtle papilloretinal syndrome with vessels emerging from the periphery of the disc (arrow); e optic disc hypoplasia, arrows indicate area of missing disc; f optic atrophy; g optic disc pit; h optic disc coloboma; i inherited retinal dystrophy; j macular coloboma with excavated lesion and surrounding pigmented margin; and k optical coherence tomography scan of the fundus in j confirming macular coloboma with excavation, absent retina and choroid, and thinned residual sclera, but more distant normal retina and disc.
Ocular coloboma (CHD7, KMT2D, PAX2, PLVAP, SALL1, STRA6, TFAP2A, BMP4, CENPF, SALL4)
Ocular coloboma affect one in 2000 live births [39], and most have a genetic basis [40].
They derive from incomplete closure of the choroidal fissure during embryonic development at about week 7.
Coloboma in CAKUT affect the iris (anterior segment), or choroid, retina and optic nerve (posterior segment). They affect the anterior and posterior segments equally often [39], and both segments are affected in about one quarter of patients. The extent of the defect depends on the stage at which the fissure fails to close, with a late failure-to-close resulting in only an iris defect [41]. Coloboma are usually unilateral but bilateral in one third of cases [39]. Ocular coloboma are also associated with developmental delay [39], and other neurological, skeletal, and craniofacial, as well as kidney anomalies [41].
Coloboma are often associated with microphthalmia, and are present from birth. The visual prognosis depends mainly on the involvement of the macula and optic nerve. Isolated coloboma of the iris do not affect vision and rarely require surgical treatment, for cosmetic reasons [42]. Chorioretinal coloboma are usually asymptomatic, require a formal ophthalmic examination for their demonstration, but may result in a visual field defect [41]. Unilateral cases may develop strabismus. Bilateral cases often present in infancy with poor vision and nystagmus.
At diagnosis, the extent of the coloboma and associated abnormalities such as microphthalmia, amblyopia, squint, and refractive errors should be identified. Direct and indirect ophthalmoscopy, accurate refraction, assessment for microphthalmia, and visual fields should be performed. Evaluation may be difficult in an infant. Individuals with a coloboma, especially of the posterior segment, require monitoring by an ophthalmologist.
In general, coloboma cannot be corrected, and their management focuses on monitoring for and treating complications such as cataract, glaucoma, retinal detachment and subretinal neovasculariation [42–46]. More than half of children with a coloboma develop another ocular abnormality including amblyopia or strabismus over a 9-year period [39]. Amblyopia may be helped with part-time occlusion, strabismus can be treated surgically and spectacles may help. Chorioretinal coloboma are associated with retinal detachments in up to 40% of affected individuals [41]. These may be overlooked because of already-limited vision. Subretinal neovascularisation at the coloboma edge may be managed with antiVEGF treatment.
The patient with CAKUT and a coloboma must also be assessed for other syndromic features, and first-degree relatives examined for coloboma, other ocular features and for CAKUT.
Optic nerve dysplasia (PAX2)
Optic nerve dysplasia also described as ‘optic nerve coloboma’ or a ‘morning glory disc anomaly’ is typical of papillorenal syndrome due to PAX2 pathogenic variants. It occurs in the majority of cases [47], is often bilateral and is characterised by the emergence of the retinal vessels from the periphery rather than the centre of the optic disc.
Microphthalmia (CHD7, DHCR7, FRAS1, GLI3, PAX2, SALl1, STRA6, TFAP2A, BMP4, CENPF, SALL4)
This is a rare developmental disorder affecting one in 5000 individuals [48] where one or both eyes are abnormally small with an axial diameter less than 2SD below the mean for age. Microphthalmia may be associated with microcornea, aniridia, cataract, and retinal degeneration [49]. It usually affects vision, and treatment may be required for the more severe forms [49].
Cataracts (CHD7, DHCR7, EYA1, GPC3, JAG1, KMT2D, LRP4, PAX2, RET, SALL1, TFAP2A, CENPF, HS2ST1, SALL4)
Many of these cataracts are present at birth. Again, these may be unilateral or bilateral, are detected on examination for a red reflex and those more than 3 mm in diameter affect vision. Visually-significant cataracts should be removed surgically within weeks of birth.
Inherited retinal degeneration (GATA3, NPHP3, PAX2, BMP4)
Inherited retinal degeneration is a diverse group of diseases characterised by photoreceptor cell death and progressive loss of vision, and encompassing the retinal dystrophies, including retinitis pigmentosa. It usually reflects ciliary dysfunction which is more commonly associated with the renal ciliopathies and cystic kidney disease. The diagnosis is usually made on the basis of clinical history, fundus examination, and electroretinography. In retinitis pigmentosa, the fundus characteristically demonstrates the triad of 'bone spicules', pale optic disc, and arteriolar attenuation bilaterally.
Hypertelorism (WNT5A, CTU2, LRP4, ZMYM2, H2ST1)
Hypertelorism is an abnormally-increased distance between the orbits which may be corrected surgically between the ages of 5 and 8.
Posterior embryotoxon (NOTCH2, JAG1, HS2ST1) and Axenfeld anomaly (JAG1)
Posterior embryotoxon and Axenfeld anomaly is rare, occurring in one in 200,000 live births. It is usually diagnosed in childhood, where there is a membrane extending from the cornea onto the iris surface in both eyes. It may be associated with iris atrophy and later, glaucoma.
Peter’s anomaly (CENPF, EYA1, NIPBL) is where a thinned and clouded cornea attaches to the iris and results in blurred vision. It is often associated with cataracts and glaucoma.
Refractive errors (CHD7, NIPBL, BMP4), such as myopia and hypermetropia, correspond to short- and long-sightedness, are common, and result from defects in the shape of the eyeball, cornea, or lens.
Neuro-ophthalmic disorders (ANOS1, CHRNA3, DHCR7, EYA1, FAM58A, JAG1, KDM6A, KMT2D, NIPBL, NPHP3, PBX1, SALL1, SALL4) include a poor light reflex, nystagmus, and strabismus.
Duane anomaly (FAM58A) is a congenital anomaly where the horizontal eye movement is limited in abduction, adduction, or both.
Some forms of CAKUT and their ocular associations
Papillorenal (renal-coloboma) syndrome (PAX2)
Papillorenal syndrome is associated with malformations of the kidneys and eyes. More than 250 affected individuals have been reported but many are unrecognised so that population frequencies are likely to be underestimates. A pathogenic variant in PAX2 is identified in nearly 50% of cases [50]. Kidney anomalies occur in 90% of cases, with hypodysplasia in 65%, multicystic dysplasia in 10%, and vesicoureteric reflux in 14% [47, 51]. The principal ocular manifestation is a dysplastic optic nerve, which is usually bilateral [47]. This appears as an excavated optic disc, with the retinal vessels emerging at the periphery rather than centrally (Fig. 1). This is often described as an ‘optic nerve coloboma’ or ‘morning glory disc anomaly’ because of its resemblance to the flower. Detection requires a careful dilated fundus examination. Visual acuity is reduced in at least one eye in 75% of cases but may be normal. Further visual loss occurs with retinal detachment [46]. This risk necessitates close monitoring by an ophthalmologist. The ocular phenotype may differ even among family members with the same variant.
Branchiootorenal syndrome 1 with or without cataracts (EYA1)
Branchiootorenal syndrome 1 is an AD-inherited disease characterised by branchial cysts or fistulae, structural defects and an abnormal shape of the outer, middle or inner ear, preauricular pits, and hearing loss [52]. It affects about one in 40,000 of the population. Kidney abnormalities including agenesis, hypoplastic and cystic kidneys, pelviureteric obstruction, bifid ureters and kidney failure occur in about two-thirds of affected individuals. The ocular associations include congenital anterior segment anomalies including cataract. However, these features are not common [14], and sometimes the kidneys are normal.
Townes-Brocks syndrome 1 (SALL1)
Townes-Brocks syndrome 1 is an AD-inherited disease characterised by kidney, limb and ear anomalies as well as an imperforate anus, rectal atresia, polydactyly, and a triphalangeal thumb [50]. Other features include preauricular tags, overfolded helices, cardiac anomalies, hypospadias, and impaired kidney function. Coloboma and the Duane anomaly are rare. The features may overlap with branchiootorenal syndrome since the EYA1 and SALL1 genes are involved in the same biochemical pathways.
Hypoparathyroidism, sensorineural deafness and renal dysplasia (HDR syndrome) (GATA3)
GATA3 is a zinc-finger transcription factor that binds to the enhancer elements (A/T)GATA (A/G) of all four T cell antigen receptors [53], and is required for the embryonic development of the parathyroids, hearing, and kidneys. Hypoparathyroidism in HDR syndrome ranges from asymptomatic disease to features including myalgia, sensory problems, and tetany from hypocalcaemia. Parathyroid hormone levels vary from low to high. Hearing loss is obvious early. Kidney problems include developmental abnormalities such as dysplasia and hypoplasia but also cystic kidneys, vesicoureteric reflux, proteinuria, renal tubular acidosis, nephrocalcinosis, and kidney failure. Penetrance varies in different family members. Other manifestations include pyloric stenosis and female genital tract malformations [54]. Hearing loss ranges from mild to severe and is usually bilateral. The prognosis depends on disease severity. Ophthalmic features include band keratopathy and inherited retinal degeneration [55].
CAKUT with or without hearing loss, abnormal ears or developmental delay (PBX1)
PBX1 is a transcription factor that regulates morphologic patterning, organogenesis, and haematopoiesis in the embryo. It does this through modulation of the HOX protein [34]. This syndrome is sometimes referred to as CAKUTHED (Hearing loss, abnormal ears, and developmental delay). The disease usually presents in childhood. Ocular features include strabismus, corneal clouding, iris abnormalities, and glaucoma [38].
Other forms of CAKUT with interesting ocular manifestations include the following.
CHARGE syndrome (CHD7)
CHARGE syndrome (Coloboma of the eye, Heart defects, nasal choanae, growth Retardation, and Genital and urinary tract abnormalities and Ear anomalies with deafness) affects one in 10,000 births [56], and results from a pathogenic variant in CHD7 in two-thirds of cases [57]. However, these are almost all de novo so that there is often no family history [58]. Inheritance is otherwise AD and there is phenotypic variation even between affected family members. The kidney manifestations include unilateral agenesis, fusion, ectopia, and malrotation, duplex collecting systems and ureteral agenesis. The other classical features are choanal atresia, developmental delay, hypoplasia or aplasia of the semicircular canals, and cardiac defects in 80% of cases [59].
Coloboma are characteristic and seen in nearly all affected individuals [60]. They are typically chorioretinal, but may also affect the iris or optic nerve [61]. They are usually bilateral. Other reported ocular anomalies include microphthalmia and anophthalmia, microcornea, cataract, ectopia lentis, and persistent foetal vasculature [62]. There is an association with high myopia, but overall, visual acuity ranges from near normal to an absence of light perception [62]. Patients require management by an ophthalmologist, because of the risk of retinal detachment [63], and to ensure refractive errors are corrected.
Alagille syndrome (JAG1, NOTCH2)
Alagille syndrome results from a pathogenic variant in JAG1 in 97% of cases and NOTCH2 in fewer than 1% [64]. The population frequency is one in 70,000 but this is probably an underestimate [64]. Inheritance is AD. With a JAG1 variant, kidney anomalies occur in about 40% [65], with dysplasia being the most common, and others including vesicoureteral reflex and ureteropelvic junction obstruction [65]. Posterior embryotoxon, an anteriorly displaced and thickened Schwalbe’s line, is a cardinal feature of Alagille syndrome, occurring in 95% of cases [66]. It does not affect vision but is helpful diagnostically. Optic disc drusen are also common and usually occur bilaterally [67]. Other common ocular anomalies include those of the optic disc (76%), diffuse fundus hypopigmentation (57%), and a speckled retinal pigment epithelium (33%) [66]. The Axenfeld anomaly occurs in 13% [68].
Rubinstein-Taybi syndrome (CREBBP, EP300)
Rubinstein-Taybi syndrome is characterised by short stature, microcephaly, intellectual disability, dysmorphic facial features, broad thumbs and big toes and cryptorchidism. It affects about one in 100,000 births and has AD inheritance. Pathogenic variants in CREBBP account for 60% of cases, EP300 for 10% and the other genes are not known [69, 70]. Kidney malformations occur in half the cases, including kidney agenesis, duplication, hypoplasia, hydronephrosis, and vesicoureteric reflux.
More than half the affected individuals have ocular abnormalities [71]. These include strabismus (in 70%); refractive error (60%) including high myopia, in 25%; and coloboma affecting the iris, retina, choroid, or optic nerve in 10% [71–73]. Congenital and juvenile glaucoma, and congenital cataract occur [72]. Inherited retinal degeneration may be present with an abnormal retinal pigment epithelium, absent foveal reflex, and electroretinogram findings suggesting cone or cone-rod dysfunction [72], as well as peripheral retinal avascularity [74].
Discussion
This study found that about half the genes associated with CAKUT have ocular features and that more may be expected based on the retinal expression data and the phenotypes of mouse models. There is, however, little information on the proportion of individuals with each form of CAKUT who have ocular abnormalities.
Coloboma are one of the ocular features associated with the most CAKUT genes. They are typically present from birth and do not progress during life, but complications such as amblyopia, strabismus, retinal detachment, cataract and subretinal neovascularisation may occur. Coloboma are also sometimes seen in other genetic kidney diseases such as the ciliopathies, focal and segmental glomerulosclerosis, and tubulopathies, as well as genetic diseases that do not affect the kidney, and sometimes without an obvious cause.
Many ocular abnormalities, such as coloboma, microphthalmia or strabismus, are obvious to a renal physician and should prompt a formal ophthalmological review. However, the diagnosis of CAKUT itself indicates that screening for syndromic features including an ophthalmological examination is warranted. The input from an interested ophthalmologist is important in assessment of an ocular phenotype, and in deciding on the need for active treatment or ongoing monitoring.
The demonstration of a coloboma or other ocular abnormality in a person with a structural kidney disease suggests a genetic cause, and in some cases a specific gene. However, more than 150 genes have been identified in CAKUT and the genetic detection rate is less than 20% so that testing for a variant is only advocated where a defect in a specific gene is suspected, such as for PAX2 with optic disc dysplasia [75].
Most genes causing CAKUT demonstrate AD inheritance and first-degree family members should also be examined. However, clinical features are often incompletely penetrant and even affected family members may have no phenotype. The variable penetrance means that it is often not possible to accurately predict the kidney and visual consequences for a future child.
This study’s strengths were the use of the Genomics England CAKUT panel, and of curated retinal expression and mouse model databases to identify the ocular associations. The study’s major limitations were that individual forms of CAKUT diseases are rare, data is limited on how often ocular features occur with different forms of CAKUT, affected individuals have not necessarily undergone an ophthalmic examination, and reported ocular features may have been coincidental. The list of CAKUT genes is not exhaustive but nevertheless represents those considered by many laboratories in their search for pathogenic variants and is representative of the genes causing CAKUT and their ocular phenotypes.
Thus, clinicians should consider the possibility of ocular disease in patients with CAKUT, since these can be helpful diagnostically and may require further ophthalmic management. The ocular abnormalities in CAKUT generally do not progress over time, but complications may occur, and monitoring and treatment may be necessary.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the many patients who have participated in our studies and their referring clinicians. We would also like to particularly acknowledge the use of the Genomics England CAKUT Panel, and the OMIM, the Human Protein Atlas and the Mouse Genome Informatics databases.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Data Availability
All relevant data is included in the manuscript or in the Supplementary Information.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Loane M, Dolk H, Kelly A, Teljeur C, Greenlees R, Densem J, EUROCAT Working Group Paper 4: EUROCAT statistical monitoring: identification and investigation of ten year trends of congenital anomalies in Europe. Birth Defects Res A Clin Mol Teratol. 2011;91(Suppl 1):S31–S43. doi: 10.1002/bdra.20778. [DOI] [PubMed] [Google Scholar]
- 2.Wuhl E, van Stralen KJ, Verrina E, Bjerre A, Wanner C, Heaf JG, Zurriaga O, Hoitsma A, Niaudet P, Palsson R, Ravani P, Jager KJ, Schaefer F. Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol. 2013;8:67–74. doi: 10.2215/CJN.03310412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riddle S, Habli M, Tabbah S, Lim FY, Minges M, Kingma P, Polzin W. Contemporary outcomes of patients with isolated bilateral renal agenesis with and without fetal intervention. Fetal Diagn Ther. 2020;47:675–681. doi: 10.1159/000507700. [DOI] [PubMed] [Google Scholar]
- 4.Capone VP, Morello W, Taroni F, Montini G. Genetics of congenital anomalies of the kidney and urinary tract: the current state of play. Int J Mol Sci. 2017;18:796. doi: 10.3390/ijms18040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macumber I, Schwartz S, Leca N. Maternal obesity is associated with congenital anomalies of the kidney and urinary tract in offspring. Pediatr Nephrol. 2017;32:635–642. doi: 10.1007/s00467-016-3543-x. [DOI] [PubMed] [Google Scholar]
- 6.Kagan M, Pleniceanu O, Vivante A. The genetic basis of congenital anomalies of the kidney and urinary tract. Pediatr Nephrol. 2022;37:2231–2243. doi: 10.1007/s00467-021-05420-1. [DOI] [PubMed] [Google Scholar]
- 7.Izzedine H, Bodaghi B, Launay-Vacher V, Deray G. Eye and kidney: from clinical findings to genetic explanations. J Am Soc Nephrol. 2003;14:516–529. doi: 10.1097/01.asn.0000051705.97966.ad. [DOI] [PubMed] [Google Scholar]
- 8.Bellanne-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm JM, Boitard C, Noel LH, Velho G, Timsit J. Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med. 2004;140:510–517. doi: 10.7326/0003-4819-140-7-200404060-00009. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan BS, Gordon I, Pincott J, Barratt TM. Familial hypoplastic glomerulocystic kidney disease: a definite entity with dominant inheritance. Am J Med Genet. 1989;34:569–573. doi: 10.1002/ajmg.1320340423. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama M, Nozu K, Goto Y, Kamei K, Ito S, Sato H, Emi M, Nakanishi K, Tsuchiya S, Iijima K (2010) HNF1B alterations associated with congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 25:1073–1079 [DOI] [PubMed]
- 11.Rizzoni G, Loirat C, Levy M, Milanesi C, Zachello G, Mathieu H. Familial hypoplastic glomerulocystic kidney. A new entity? Clin Nephrol. 1982;18:263–268. [PubMed] [Google Scholar]
- 12.Eccles MR, Schimmenti LA. Renal-coloboma syndrome: a multi-system developmental disorder caused by PAX2 mutations. Clin Genet. 1999;56:1–9. doi: 10.1034/j.1399-0004.1999.560101.x. [DOI] [PubMed] [Google Scholar]
- 13.Schimmenti LA. Renal coloboma syndrome. Eur J Hum Genet. 2011;19:1207–1212. doi: 10.1038/ejhg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azuma N, Hirakiyama A, Inoue T, Asaka A, Yamada M. Mutations of a human homologue of the Drosophila eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum Mol Genet. 2000;9:363–366. doi: 10.1093/hmg/9.3.363. [DOI] [PubMed] [Google Scholar]
- 15.Carmi R, Binshtock M, Abeliovich D, Bar-Ziv J. The branchio-oto-renal (BOR) syndrome: report of bilateral renal agenesis in three sibs. Am J Med Genet. 1983;14:625–627. doi: 10.1002/ajmg.1320140405. [DOI] [PubMed] [Google Scholar]
- 16.Chitayat D, Hodgkinson KA, Chen MF, Haber GD, Nakishima S, Sando I. Branchio-oto-renal syndrome: further delineation of an underdiagnosed syndrome. Am J Med Genet. 1992;43:970–975. doi: 10.1002/ajmg.1320430613. [DOI] [PubMed] [Google Scholar]
- 17.Fraser FC, Ling D, Clogg D, Nogrady B. Genetic aspects of the BOR syndrome–branchial fistulas, ear pits, hearing loss, and renal anomalies. Am J Med Genet. 1978;2:241–252. doi: 10.1002/ajmg.1320020305. [DOI] [PubMed] [Google Scholar]
- 18.Fraser FC, Ayme S, Halal F, Sproule J. Autosomal dominant duplication of the renal collecting system, hearing loss, and external ear anomalies: a new syndrome? Am J Med Genet. 1983;14:473–478. doi: 10.1002/ajmg.1320140311. [DOI] [PubMed] [Google Scholar]
- 19.Legius E, Fryns JP, Van den Berghe H. Dominant branchial cleft syndrome with characteristics of both branchio-oto-renal and branchio-oculo-facial syndrome. Clin Genet. 1990;37:347–350. doi: 10.1111/j.1399-0004.1990.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 20.Melnick M, Bixler D, Silk K, Yune H, Nance WE. Autosomal dominant branchiootorenal dysplasia. Birth Defects Orig Artic Ser. 1975;11:121–128. [PubMed] [Google Scholar]
- 21.Melnick M, Bixler D, Nance WE, Silk K, Yune H. Familial branchio-oto-renal dysplasia: a new addition to the branchial arch syndromes. Clin Genet. 1976;9:25–34. doi: 10.1111/j.1399-0004.1976.tb01546.x. [DOI] [PubMed] [Google Scholar]
- 22.Blanck C, Kohlhase J, Engels S, Burfeind P, Engel W, Bottani A, Patel MS, Kroes HY, Cobben JM. Three novel SALL1 mutations extend the mutational spectrum in Townes-Brocks syndrome. J Med Genet. 2000;37:303–307. doi: 10.1136/jmg.37.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Botzenhart EM, Green A, Ilyina H, Konig R, Lowry RB, Lo IF, Shohat M, Burke L, McGaughran J, Chafai R, Pierquin G, Michaelis RC, Whiteford ML, Simola KO, Rosler B, Kohlhase J. SALL1 mutation analysis in Townes-Brocks syndrome: twelve novel mutations and expansion of the phenotype. Hum Mutat. 2005;26:282. doi: 10.1002/humu.9362. [DOI] [PubMed] [Google Scholar]
- 24.Botzenhart EM, Bartalini G, Blair E, Brady AF, Elmslie F, Chong KL, Christy K, Torres-Martinez W, Danesino C, Deardorff MA, Fryns JP, Marlin S, Garcia-Minaur S, Hellenbroich Y, Hay BN, Penttinen M, Shashi V, Terhal P, Van Maldergem L, Whiteford ML, Zackai E, Kohlhase J. Townes-Brocks syndrome: twenty novel SALL1 mutations in sporadic and familial cases and refinement of the SALL1 hot spot region. Hum Mutat. 2007;28:204–205. doi: 10.1002/humu.9476. [DOI] [PubMed] [Google Scholar]
- 25.Ferraz FG, Nunes L, Ferraz ME, Sousa JP, Santos M, Carvalho C, Maroteaux P. Townes-Brocks syndrome. Report of a case and review of the literature. Ann Genet. 1989;32:120–123. [PubMed] [Google Scholar]
- 26.Johnson JP, Poskanzer LS, Sherman S. Three-generation family with resemblance to Townes-Brocks syndrome and Goldenhar/oculoauriculovertebral spectrum. Am J Med Genet. 1996;61:134–139. doi: 10.1002/(SICI)1096-8628(19960111)61:2<134::AID-AJMG6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 27.Kohlhase J, Taschner PE, Burfeind P, Pasche B, Newman B, Blanck C, Breuning MH, ten Kate LP, Maaswinkel-Mooy P, Mitulla B, Seidel J, Kirkpatrick SJ, Pauli RM, Wargowski DS, Devriendt K, Proesmans W, Gabrielli O, Coppa GV, Wesby-van Swaay E, Trembath RC, Schinzel AA, Reardon W, Seemanova E, Engel W. Molecular analysis of SALL1 mutations in Townes-Brocks syndrome. Am J Hum Genet. 1999;64:435–445. doi: 10.1086/302238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurnit DM, Steele MW, Pinsky L, Dibbins A. Autosomal dominant transmission of a syndrome of anal, ear, renal, and radial congenital malformations. J Pediatr. 1978;93:270–273. doi: 10.1016/s0022-3476(78)80518-6. [DOI] [PubMed] [Google Scholar]
- 29.Rossmiller DR, Pasic TR. Hearing loss in Townes-Brocks syndrome. Otolaryngol Head Neck Surg. 1994;111:175–180. doi: 10.1177/01945998941113P103. [DOI] [PubMed] [Google Scholar]
- 30.Barakat AJ, Raygada M, Rennert OM. Barakat syndrome revisited. Am J Med Genet A. 2018;176:1341–1348. doi: 10.1002/ajmg.a.38693. [DOI] [PubMed] [Google Scholar]
- 31.Bilous RW, Murty G, Parkinson DB, Thakker RV, Coulthard MG, Burn J, Mathias D, Kendall-Taylor P. Brief report: autosomal dominant familial hypoparathyroidism, sensorineural deafness, and renal dysplasia. N Engl J Med. 1992;327:1069–1074. doi: 10.1056/NEJM199210083271506. [DOI] [PubMed] [Google Scholar]
- 32.Ferraris S, Del Monaco AG, Garelli E, Carando A, De Vito B, Pappi P, Lala R, Ponzone A. HDR syndrome: a novel “de novo” mutation in GATA3 gene. Am J Med Genet A. 2009;149A:770–775. doi: 10.1002/ajmg.a.32689. [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa T, Hasegawa Y, Aso T, Koto S, Nagai T, Tsuchiya Y, Kim KC, Ohashi H, Wakui K, Fukushima Y. HDR syndrome (hypoparathyroidism, sensorineural deafness, renal dysplasia) associated with del(10)(p13) Am J Med Genet. 1997;73:416–418. doi: 10.1002/(sici)1096-8628(19971231)73:4<416::aid-ajmg9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 34.Heidet L, Moriniere V, Henry C, De Tomasi L, Reilly ML, Humbert C, Alibeu O, Fourrage C, Bole-Feysot C, Nitschke P, Tores F, Bras M, Jeanpierre M, Pietrement C, Gaillard D, Gonzales M, Novo R, Schaefer E, Roume J, Martinovic J, Malan V, Salomon R, Saunier S, Antignac C, Jeanpierre C. Targeted exome sequencing identifies PBX1 as involved in monogenic congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol. 2017;28:2901–2914. doi: 10.1681/ASN.2017010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Tanno P, Breton J, Bidart M, Satre V, Harbuz R, Ray PF, Bosson C, Dieterich K, Jaillard S, Odent S, Poke G, Beddow R, Digilio MC, Novelli A, Bernardini L, Pisanti MA, Mackenroth L, Hackmann K, Vogel I, Christensen R, Fokstuen S, Bena F, Amblard F, Devillard F, Vieville G, Apostolou A, Jouk PS, Guebre-Egziabher F, Sartelet H, Coutton C. PBX1 haploinsufficiency leads to syndromic congenital anomalies of the kidney and urinary tract (CAKUT) in humans. J Med Genet. 2017;54:502–510. doi: 10.1136/jmedgenet-2016-104435. [DOI] [PubMed] [Google Scholar]
- 36.Slavotinek A, Risolino M, Losa M, Cho MT, Monaghan KG, Schneidman-Duhovny D, Parisotto S, Herkert JC, Stegmann APA, Miller K, Shur N, Chui J, Muller E, DeBrosse S, Szot JO, Chapman G, Pachter NS, Winlaw DS, Mendelsohn BA, Dalton J, Sarafoglou K, Karachunski PI, Lewis JM, Pedro H, Dunwoodie SL, Selleri L, Shieh J. De novo, deleterious sequence variants that alter the transcriptional activity of the homeoprotein PBX1 are associated with intellectual disability and pleiotropic developmental defects. Hum Mol Genet. 2017;26:4849–4860. doi: 10.1093/hmg/ddx363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy MJ, Polok BK, Schorderet DF, Cleary ML. Essential role for Pbx1 in corneal morphogenesis. Invest Ophthalmol Vis Sci. 2010;51:795–803. doi: 10.1167/iovs.08-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Safgren SL, Olson RJ, Pinto EVF, Bothun ED, Hanna C, Klee EW, Schimmenti LA. De novo PBX1 variant in a patient with glaucoma, kidney anomalies, and developmental delay: an expansion of the CAKUTHED phenotype. Am J Med Genet A. 2022;188:919–925. doi: 10.1002/ajmg.a.62576. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura KM, Diehl NN, Mohney BG. Incidence, ocular findings, and systemic associations of ocular coloboma: a population-based study. Arch Ophthalmol. 2011;129:69–74. doi: 10.1001/archophthalmol.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory-Evans CY, Williams MJ, Halford S, Gregory-Evans K. Ocular coloboma: a reassessment in the age of molecular neuroscience. J Med Genet. 2004;41:881–891. doi: 10.1136/jmg.2004.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al AS, Gregory-Evans CY, Gregory-Evans K. An update on the genetics of ocular coloboma. Hum Genet. 2019;138:865–880. doi: 10.1007/s00439-019-02019-3. [DOI] [PubMed] [Google Scholar]
- 42.Onwochei BC, Simon JW, Bateman JB, Couture KC, Mir E. Ocular colobomata. Surv Ophthalmol. 2000;45:175–194. doi: 10.1016/s0039-6257(00)00151-x. [DOI] [PubMed] [Google Scholar]
- 43.Hussain RM, Abbey AM, Shah AR, Drenser KA, Trese MT, Capone A., Jr Chorioretinal coloboma complications: retinal detachment and choroidal neovascular membrane. J Ophthalmic Vis Res. 2017;12:3–10. doi: 10.4103/2008-322X.200163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bavbek T, Ogut MS, Kazokoglu H. Congenital lens coloboma and associated pathologies. Doc Ophthalmol. 1993;83:313–322. doi: 10.1007/BF01204333. [DOI] [PubMed] [Google Scholar]
- 45.Bron AJ, Burgess SE, Awdry PN, Oliver D, Arden G. Papillo-renal syndrome. An inherited association of optic disc dysplasia and renal disease. Report and review of the literature. Ophthalmic Paediatr Genet. 1989;10:185–198. doi: 10.3109/13816818909009875. [DOI] [PubMed] [Google Scholar]
- 46.Jesberg DO, Schepens CL. Retinal detachment associated with coloboma of the choroid. Arch Ophthalmol. 1961;65:163–173. doi: 10.1001/archopht.1961.01840020165003. [DOI] [PubMed] [Google Scholar]
- 47.Bower M, Salomon R, Allanson J, Antignac C, Benedicenti F, Benetti E, Binenbaum G, Jensen UB, Cochat P, DeCramer S, Dixon J, Drouin R, Falk MJ, Feret H, Gise R, Hunter A, Johnson K, Kumar R, Lavocat MP, Martin L, Moriniere V, Mowat D, Murer L, Nguyen HT, Peretz-Amit G, Pierce E, Place E, Rodig N, Salerno A, Sastry S, Sato T, Sayer JA, Schaafsma GC, Shoemaker L, Stockton DW, Tan WH, Tenconi R, Vanhille P, Vats A, Wang X, Warman B, Weleber RG, White SM, Wilson-Brackett C, Zand DJ, Eccles M, Schimmenti LA, Heidet L. Update of PAX2 mutations in renal coloboma syndrome and establishment of a locus-specific database. Hum Mutat. 2012;33:457–466. doi: 10.1002/humu.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ragge NK, Subak-Sharpe ID, Collin JR. A practical guide to the management of anophthalmia and microphthalmia. Eye (Lond) 2007;21:1290–1300. doi: 10.1038/sj.eye.6702858. [DOI] [PubMed] [Google Scholar]
- 49.Verma AS, Fitzpatrick DR. Anophthalmia and microphthalmia. Orphanet J Rare Dis. 2007;2:47. doi: 10.1186/1750-1172-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bower MA, Schimmenti LA, Eccles MR. PAX2-related disorder (updated 2018) In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet] Seattle: University of Washington; 1993. [Google Scholar]
- 51.Fletcher J, Hu M, Berman Y, Collins F, Grigg J, McIver M, Juppner H, Alexander SI. Multicystic dysplastic kidney and variable phenotype in a family with a novel deletion mutation of PAX2. J Am Soc Nephrol. 2005;16:2754–2761. doi: 10.1681/ASN.2005030239. [DOI] [PubMed] [Google Scholar]
- 52.Melnick M, Hodes ME, Nance WE, Yune H, Sweeney A. Branchio-oto-renal dysplasia and branchio-oto dysplasia: two distinct autosomal dominant disorders. Clin Genet. 1978;13:425–442. doi: 10.1111/j.1399-0004.1978.tb04142.x. [DOI] [PubMed] [Google Scholar]
- 53.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406:419–422. doi: 10.1038/35019088. [DOI] [PubMed] [Google Scholar]
- 54.Shim YS, Choi W, Hwang IT, Yang S. Hypoparathyroidism, sensorineural deafness, and renal dysgenesis syndrome with a GATA3 mutation. Ann Pediatr Endocrinol Metab. 2015;20:59–63. doi: 10.6065/apem.2015.20.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim C, Cheong HI, Kim JH, Yu YS, Kwon JW. Presumed atypical HDR syndrome associated with Band Keratopathy and pigmentary retinopathy. J Pediatr Ophthalmol Strabismus. 2011;48:e1–3. doi: 10.3928/01913913-20090616-08. [DOI] [PubMed] [Google Scholar]
- 56.Blake KD, Davenport SL, Hall BD, Hefner MA, Pagon RA, Williams MS, Lin AE, Graham JM., Jr CHARGE association: an update and review for the primary pediatrician. Clin Pediatr (Phila) 1998;37:159–173. doi: 10.1177/000992289803700302. [DOI] [PubMed] [Google Scholar]
- 57.Janssen N, Bergman JE, Swertz MA, Tranebjaerg L, Lodahl M, Schoots J, Hofstra RM, van Ravenswaaij-Arts CM, Hoefsloot LH. Mutation update on the CHD7 gene involved in CHARGE syndrome. Hum Mutat. 2012;33:1149–1160. doi: 10.1002/humu.22086. [DOI] [PubMed] [Google Scholar]
- 58.Sanlaville D, Etchevers HC, Gonzales M, Martinovic J, Clement-Ziza M, Delezoide AL, Aubry MC, Pelet A, Chemouny S, Cruaud C, Audollent S, Esculpavit C, Goudefroye G, Ozilou C, Fredouille C, Joye N, Morichon-Delvallez N, Dumez Y, Weissenbach J, Munnich A, Amiel J, Encha-Razavi F, Lyonnet S, Vekemans M, Attie-Bitach T. Phenotypic spectrum of CHARGE syndrome in fetuses with CHD7 truncating mutations correlates with expression during human development. J Med Genet. 2006;43:211–217. doi: 10.1136/jmg.2005.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blake KD, Prasad C. CHARGE syndrome. Orphanet J Rare Dis. 2006;1:34. doi: 10.1186/1750-1172-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell-Eggitt IM, Blake KD, Taylor DS, Wyse RK. The eye in the CHARGE association. Br J Ophthalmol. 1990;74:421–426. doi: 10.1136/bjo.74.7.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Metlay LA, Smythe PS, Miller ME. Familial CHARGE syndrome: clinical report with autopsy findings. Am J Med Genet. 1987;26:577–581. doi: 10.1002/ajmg.1320260311. [DOI] [PubMed] [Google Scholar]
- 62.Nishina S, Kosaki R, Yagihashi T, Azuma N, Okamoto N, Hatsukawa Y, Kurosawa K, Yamane T, Mizuno S, Tsuzuki K, Kosaki K. Ophthalmic features of CHARGE syndrome with CHD7 mutations. Am J Med Genet A. 2012;158A:514–518. doi: 10.1002/ajmg.a.34400. [DOI] [PubMed] [Google Scholar]
- 63.McMain K, Blake K, Smith I, Johnson J, Wood E, Tremblay F, Robitaille J. Ocular features of CHARGE syndrome. J AAPOS. 2008;12:460–465. doi: 10.1016/j.jaapos.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Turnpenny PD, Ellard S. Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2012;20:251–257. doi: 10.1038/ejhg.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamath BM, Podkameni G, Hutchinson AL, Leonard LD, Gerfen J, Krantz ID, Piccoli DA, Spinner NB, Loomes KM, Meyers K. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A. 2012;158A:85–89. doi: 10.1002/ajmg.a.34369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hingorani M, Nischal KK, Davies A, Bentley C, Vivian A, Baker AJ, Mieli-Vergani G, Bird AC, Aclimandos WA. Ocular abnormalities in Alagille syndrome. Ophthalmology. 1999;106:330–337. doi: 10.1016/S0161-6420(99)90072-6. [DOI] [PubMed] [Google Scholar]
- 67.Nischal KK, Hingorani M, Bentley CR, Vivian AJ, Bird AC, Baker AJ, Mowat AP, Mieli-Vergani G, Aclimandos WA. Ocular ultrasound in Alagille syndrome: a new sign. Ophthalmology. 1997;104:79–85. doi: 10.1016/s0161-6420(97)30358-3. [DOI] [PubMed] [Google Scholar]
- 68.Emerick KM, Rand EB, Goldmuntz E, Krantz ID, Spinner NB, Piccoli DA. Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology. 1999;29:822–829. doi: 10.1002/hep.510290331. [DOI] [PubMed] [Google Scholar]
- 69.Bartsch O, Schmidt S, Richter M, Morlot S, Seemanova E, Wiebe G, Rasi S. DNA sequencing of CREBBP demonstrates mutations in 56% of patients with Rubinstein-Taybi syndrome (RSTS) and in another patient with incomplete RSTS. Hum Genet. 2005;117:485–493. doi: 10.1007/s00439-005-1331-y. [DOI] [PubMed] [Google Scholar]
- 70.Negri G, Milani D, Colapietro P, Forzano F, Della Monica M, Rusconi D, Consonni L, Caffi LG, Finelli P, Scarano G, Magnani C, Selicorni A, Spena S, Larizza L, Gervasini C. Clinical and molecular characterization of Rubinstein-Taybi syndrome patients carrying distinct novel mutations of the EP300 gene. Clin Genet. 2015;87:148–154. doi: 10.1111/cge.12348. [DOI] [PubMed] [Google Scholar]
- 71.Milani D, Manzoni FM, Pezzani L, Ajmone P, Gervasini C, Menni F, Esposito S. Rubinstein-Taybi syndrome: clinical features, genetic basis, diagnosis, and management. Ital J Pediatr. 2015;41:4. doi: 10.1186/s13052-015-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Genderen MM, Kinds GF, Riemslag FC, Hennekam RC. Ocular features in Rubinstein-Taybi syndrome: investigation of 24 patients and review of the literature. Br J Ophthalmol. 2000;84:1177–1184. doi: 10.1136/bjo.84.10.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubinstein JH. Broad thumb-hallux (Rubinstein-Taybi) syndrome 1957–1988. Am J Med Genet Suppl. 1990;6:3–16. doi: 10.1002/ajmg.1320370603. [DOI] [PubMed] [Google Scholar]
- 74.Jacobs DJ, Sein J, Berrocal AM, Grajewski AL, Hodapp E. Fluorescein angiography findings in a case of Rubinstein-Taybi syndrome. Clin Ophthalmol. 2012;6:1369–1371. doi: 10.2147/OPTH.S31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knoers N, Antignac C, Bergmann C, Dahan K, Giglio S, Heidet L, Lipska-Zietkiewicz BS, Noris M, Remuzzi G, Vargas-Poussou R, Schaefer F. Genetic testing in the diagnosis of chronic kidney disease: recommendations for clinical practice. Nephrol Dial Transplant. 2022;37:239–254. doi: 10.1093/ndt/gfab218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data is included in the manuscript or in the Supplementary Information.