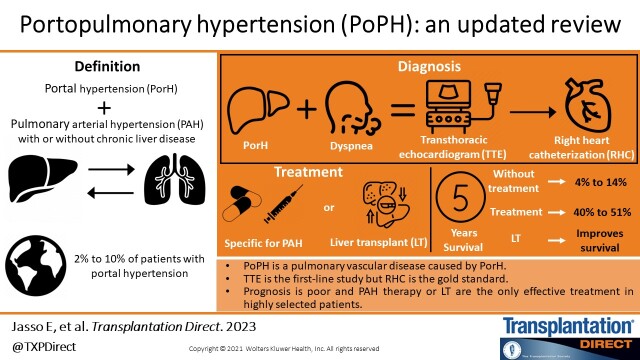
Abstract
Portal hypertension may have major consequences on the pulmonary vasculature due to the complex pathophysiological interactions between the liver and lungs. Portopulmonary hypertension (PoPH), a subset of group 1 pulmonary hypertension (PH), is a serious pulmonary vascular disease secondary to portal hypertension, and is the fourth most common subtype of pulmonary arterial hypertension. It is most commonly observed in cirrhotic patients; however, patients with noncirrhotic portal hypertension can also develop it. On suspicion of PoPH, the initial evaluation is by a transthoracic echocardiogram in which, if elevated pulmonary pressures are shown, patients should undergo right heart catheterization to confirm the diagnosis. The prognosis is extremely poor in untreated patients; therefore, management includes pulmonary arterial hypertension therapies with the aim of improving pulmonary hemodynamics and moving patients to orthotopic liver transplantation (OLT). In this article, we review in detail the epidemiology, pathophysiology, process for diagnosis, and most current treatments including OLT and prognosis in patients with PoPH. In addition, we present a diagnostic algorithm that includes the current criteria to properly select patients with PoPH who are candidates for OLT.
Pulmonary arterial hypertension (PAH) is a chronic and progressive disease characterized by elevated pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR), which eventually leads to right cardiac insufficiency.1
PAH associated with portal hypertension (PorH) in patients with cirrhosis and those without cirrhosis is known as portopulmonary hypertension (PoPH). The prevalence of PoPH ranges between 5.3% and 10.0% of patients with PAH and 2% and 10% of patients with PorH, in patients who are candidates or have already undergone orthotopic liver transplantation (OLT). It is an important factor that impacts the prognosis before, during, or after transplantation.1,2
PoPH has 3 major pathogenic features: vasoconstriction of the pulmonary artery secondary to a proliferation of the inner wall; expansion of smooth muscle, leading to hypertrophy of the medial wall and development of plexiform arteriopathy; and finally, platelet aggregation and thrombosis in situ.3
Early detection of PoPH by transthoracic echocardiography (TTE) is necessary for symptomatic patients and patients undergoing liver transplant evaluation. The utility of cardiac catheterization is to confirm the diagnosis and exclude other causes of elevated pulmonary pressures.4
The treatment is complex and requires a multidisciplinary approach. It includes specific therapies for PoPH and supportive management to improve pulmonary hemodynamic and functional capacity and can serve as a bridge therapy to transplantation for those patients who respond to the treatment.5,6
Patients with PoPH have a worse 2- and 5-y prognosis compared with idiopathic PAH. In carefully selected patients, OLT improves survival.7
DEFINITIONS
Pulmonary hypertension (PH) was defined at the first world symposium on PH (Geneva, Switzerland, 1973) as a mean pulmonary arterial pressure (MPAP) ≥25 mm Hg at rest, measured through the RHC.8 At the world symposium in Nice, France, in 2018, the cutoff for PH was redefined to >20 mm Hg. It is essential to include PVR and pulmonary arterial wedge pressure in the definition of precapillary PH, to discriminate elevated PAP due to pulmonary vascular disease from that due to left heart disease. According to the latest guidelines, precapillary PH is characterized by a pulmonary arterial wedge pressure <15 mm Hg and a PVR >2 Wood units (WU) (Table 1).9 Despite significant changes to PH definitions, the risk stratification guidelines, treatment selection, and response evaluation in PoPH were not different from the other subtypes.10
TABLE 1.
Hemodynamic definitions of PH
| Definitions | Characteristics | Clinical groups |
|---|---|---|
| Precapillary PH | MPAP > 20 mm HgPAWP ≤ 15 mm HgPVR ≥ 2 WU | 1,3,4,5 |
| Isolated postcapillary PH | MPAP >20 mm HgPAWP > 15 mm HgPVR ≤ 2 WU | 2,5 |
| Combined precapillary and postcapillary PH | MPAP > 20 mm HgPAWP > 15 mm HgPVR ≥ 2 UW | 2,5 |
MPAP, mean pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; WU, wood units
In cirrhotic patients, PorH is a common finding that is characterized by an increase in the pressure gradient between the portal vein and the inferior vena cava, which is hemodynamically defined by a hepatic venous pressure gradient (HVPG) up to 5 mm Hg, trans-splenic pressure ≥15 mm Hg, and a portal vein pressure ≥21 mm Hg.11
Cirrhotic patients can present PH with a hyperdynamic state, volume overload, and recurring venous thromboembolic events. Until now, the association between PorH and PAH with or without chronic liver disease (periportal fibrosis, portal vein thrombosis, hepatic vein sclerosis, and congenital anomalies of portal circulation) is known as PoPH, which corresponds to the first group of the updated PH classification (Table 2).1,11-13
TABLE 2.
Diagnostic classification of PH
| 1. Pulmonary arterial hypertension | 1.1 Idiopathic1.2 Hereditary1.2.1 BMP-21.2.2 ALK-1, endoglin (with or without hereditary telangiectasias)1.3 Induced by drugs and toxins1.4 Associated with:1.4.1 Connective tissue disease1.4.2 HIV infection1.4.3 Portal hypertension1.4.4 Congenital heart disease1.4.5 Schistosomiasis1.5 Persistent PH of the newborn |
| 2. PH due to left heart disease | 2.1 Systolic dysfunction2.2 Diastolic dysfunction2.3 Valve disease |
| 3. PH due to pulmonary diseases or hypoxia | 3.1 Chronic obstructive pulmonary disease3.2 Interstitial lung disease 3.3 Other lung diseases with a mixed restrictive and obstructive pattern3.4 Respiratory sleep disorders3.5 Alveolar hypoventilation disorders3.6 Chronic exposure to an elevated altitude3.7 Lung development diseases |
| 4. Chronic thromboembolic PH and other pulmonary artery obstructions | 4.1 Chronic pulmonary embolus4.2 Other pulmonary artery obstructions:4.2.1 Angiosarcomas4.2.2 Intravascular tumors4.2.3 Arteritis4.2.4 Congenital stenosis of pulmonary arteries4.2.5 Parasites (hydatid disease) |
| 5. PH with less known multifactorial mechanisms | 5.1 Hematologic disorders: chronic hemolytic anemia, myeloproliferative diseases, splenectomy5.2 Systemic disorders: sarcoidosis, pulmonary histiocytosis, lymphangioleiomyomatosis, neurofibromatosis5.3 Metabolic disorders: glycogen storage diseases, Gaucher disease, thyroid diseases5.4 Others: neoplasm obstruction, fibrosing mediastinitis, chronic renal disease (with or without dialysis), segmental PH |
BMP-2, bone morphogenetic protein receptor 2; PH, pulmonary hypertension
EPIDEMIOLOGY
PoPH is described among 2%–10% of patients with portal hypertension, representing around 5.3%–10 % of all PAH cases14; nonetheless, the reported prevalence depends on the characteristics of the population and the hemodynamic definitions used to represent PoPH. There does not seem to be a link between the presence and severity of PoPH with the degree of underlying liver dysfunction or HVPG. Most studies show that autoimmune hepatitis and female sex are risk factors.15-17
PoPH is associated with significant morbidity, leading to right heart insufficiency and death. Patients seem to have a lower survival rate in comparison with those with other causes of PH.
The REVEAL study found a 2- and 5-y survival rate of patients with PoPH at 67% and 40% versus patients with idiopathic or familial PAH at 85% and 64%, respectively. Regardless, this difference could be explained by deaths of hepatic origin more than vascular issues.5,13
The survival of patients with untreated PoPH is poor. In untreated patients at 5 y, it is 14%, in patients with treatment for PH without liver transplantation at 5 y, it is 45%, and in patients with treatment for PH and transplantation it is 67%.18
PATHOPHYSIOLOGY
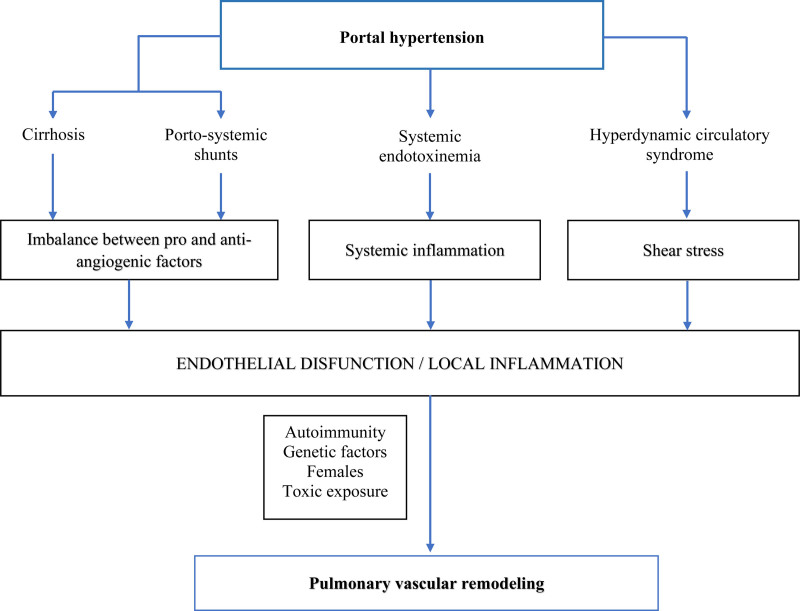
PoPH is defined as PAH in the presence of portal hypertension.19 The pathophysiology of PoPH is not yet well understood, and it is, however, an active research topic.20 PoPH occurs due to blood flow obstruction in the pulmonary arteries. There are several causes of blood flow obstruction, such as vasoconstriction, endothelium or smooth muscle proliferation, and platelet aggregation. In PoPH endothelin-1, circulating estradiol and prostacyclin synthase deficiency of pulmonary endothelial cells are the associated mediators of PoPH.20 Another hypothesis is that dysfunctional liver and portosystemic shunts expose the pulmonary vessels to harmful factors, negatively affecting the vasculature through abnormal estrogen signaling in genetically susceptible individuals.20
Liver cirrhosis and portal hypertension favor splenic vasodilation and the formation of portosystemic shunts, which contribute to the pathogenesis of PoPH in multiple ways.21 At the molecular level, the presence of a venous-venous derivation and a surgical porta-cava shunt allows the vast majority of portal blood flow to divert away from the liver vasoactive molecules and evade hepatic metabolism, leading to a reduction of peripheral vascular resistance as well as indirect vasodilation through vasoactive intestinal molecules, causing a hyperdynamic state.22-24 Furthermore, the disequilibrium between the circulating pulmonary vasoactive molecules (including pulmonary vasodilators: nitrous oxide and prostacyclin, along with pulmonary vasoconstrictors such as endothelin-1, thromboxane A2, and serotonin) results in overall vasoconstriction with the elevation of PVR25-27; this is acknowledged as a fundamental mechanism in the development of PoPH.28
All of the above, produce shear stress with endothelial cell injury, activation and suppression of specific genes that participate in the vascular remodeling process,29,30 which in turn leads to smooth muscle proliferation and thickening of the tunica intima, media, and external within pulmonary vasculature,31 resulting in a poor pulmonary arterial circulation, platelet aggregation, and thrombosis.32
As a result, a series of vascular mediators, proinflammatory cytokines, proangiogenic factors, and bacterial endotoxins can directly translocate from the intestines to the lungs, thus avoiding inactivation by liver metabolism; therefore, all these factors are harmful to pulmonary vascular endothelium, promoting the proliferation of endothelial cells, smooth muscle hypertrophy, and in situ thrombosis, favoring the development of PoPH.33 In addition, portal vein thrombosis can reach pulmonary circulation through a venous-venous shunt contributing to the enhancement of PH.34
There are others contributing factors to the pathogenesis of this entity, such as hereditary factors; however, only a small percentage of cirrhotic patients with portal hypertension (4%–15%) develop PoPH, and this can be the leading cause of the missing genetic predisposition.34 Signaling mediated by bone morphogenetic protein receptor 2 (BMP-2), a member of the transforming growth factor-beta family, has been shown to play a critical role in familial PH; studies have found mutations in the genes that code for this receptor on 15%–40% of PAH idiopathic cases,35 along with significantly lower circulating levels of BMP-2 in comparison to control patients with advanced-stage liver disease without evidence of PH.36,37
Similarly, it has been shown that the activation of toll-like receptors by the bacterial lipopolysaccharides (LPSs) from bacterial translocations has implications on the pathogenesis of PoPH,38 along with hormones, such as estrogens, which can pertain to the pathophysiology, by joining the promoting region of the BMP-2 gene and regulating its expression.39 The polymorphisms on CYP1B1, which metabolizes estrogens, have been associated with the penetration of PAH in women with BMP-2 mutations but not in men.40 Al-Naamani et al proved that in patients with PoPH, the high-risk allele rs7175922 of CYP19A1 was associated with significantly higher levels of estradiol, 2-hydroxy estrogen/16-α-hydroxy estrone (2-OHE1/16α-OHE1) in urine, plasmatic levels of dehydroepiandrosterone sulfate and plasmatic levels of 16-α-hydroxy estradiol (16α-OHE2) in comparison to patients with liver disease that do not have PoPH.41
Bone morphogenetic protein 9 (BMP9) and BMP10 are produced in the liver (and for BMP10, right atrium) and circulate either as homodimers or heterodimers. BMP9 and BMP10 are ligands for BMP receptor type II, activin A receptor-like type 1 and endoglin receptor complex. In addition, studies show that abnormal BMP9 signaling or decreased circulating BMP9 causes PH.42
In summary, the pathophysiology of portopulmonary hypertension is complex and still unclear. Further research is urgently needed to understand the PoPH.34 Figure 1 summarizes the pathophysiology of PoPH.
Figure 1.
Proposed pathophysiological mechanisms of portopulmonary hypertension. Adapted from Savale et al.4
EVALUATION AND DIAGNOSIS
It is unclear if the severity of liver disease or the level of portal hypertension is correlated with the severity of PoPH.5 Female sex, autoimmune liver diseases, elevated estradiol levels, and splenectomy have been linked as plausible risk factors for developing PoPH.3 Most patients remain asymptomatic or develop unspecific symptoms such as dyspnea and fatigue, which can be indistinguishable from cardiopulmonary or liver disease; nonetheless, as the disease progresses, exertional dyspnea becomes nearly universal.3,20,43 In dyspneic patients with underlying liver disease, hepatopulmonary syndrome should always be considered as a differential diagnosis.44
The first step for the diagnosis of PoPH is the documentation of PH and PorH, either clinically, by imaging, or with invasive measures such as HVPG. Clinically significant portal hypertension is defined when HVPG is >10 mm Hg; imaging studies may usually show collateral splenic circulation and/or splenomegaly; endoscopy may show esophageal or gastric varices. Currently, this can be achieved with a noninvasive test such as hepatic elastography, since the cut-off point depend on the etiology of the chronic liver disease, they should be interpreted in accordance with the manufacturer suggestion, also the absence of precise PorH data or decompensating values >25 kilopascals are suggestive of clinically significant PorH by elastography but it must be validated on patients with PoPH.5,45,46
A physical examination can imitate other forms of PAH, including a second, accentuated, and divided heart sound, proper side S3 gallop, midaxillary line displacement of the apex shock, lower left parasternal systolic upbringing, palpation of the pulmonary valve closure on the second left intercostal space, a continuous unfolding of the second heart sound on the pulmonic area, diastolic murmur on the pulmonic site, jugular vein distension, ascites, and edema.11,20 Other unspecific symptoms include chest pain, palpitations, presyncope, and syncope.3 A subtle hypoxemia is a common find, without being high-risk as in hepatopulmonary syndrome. Chest radiographs may show cardiomegaly and enlargement of the central pulmonary arteries.3 Pulmonary function tests may reveal pulmonary diffusion. Equally important, patients may experience lower exercise tolerance and reduction on the 6-min walk test.47 On the electrocardiogram, there can be right atrial enlargement, right ventricular hypertrophy, and axis deviation to the right and right branch block.48
The REVEAL Registry of the United States found that patients with portopulmonary syndrome had less exertional dyspnea (81% versus 86%), more fatigue (31% versus 23%), more edema (33% versus 21%), and more abdominal distension (12% versus 3%) than patients with idiopathic PAH or familial PAH.13
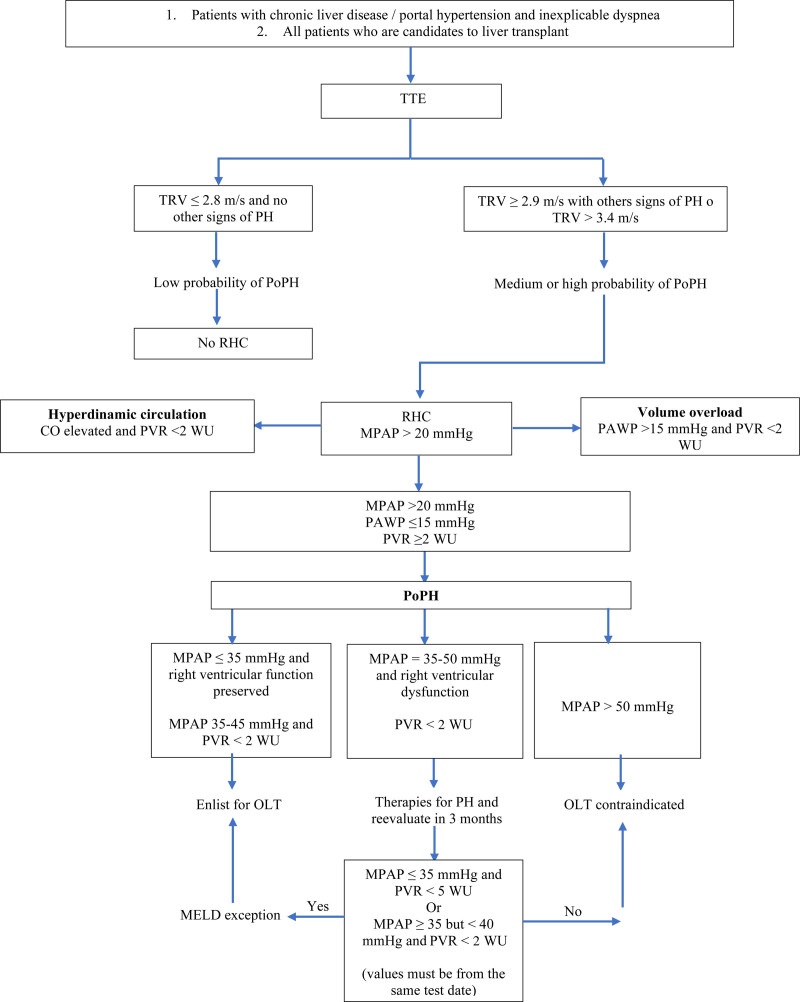
The TTE is the first-line noninvasive screening tool widely available for PoPH.44 The TTE has an 85% sensitivity and 83% specificity for the detection of PoPH.49 Therefore, it should be done on all patients with chronic liver disease that present dyspnea or inexplicable hypoxemia, as well as all patients under liver transplantation evaluation.3,44 When measuring the maximum tricuspid regurgitation velocity, an echocardiographic approximation of the right systolic ventricular pressure (RSVP) is common. TTE aims to identify patients who must be referred to RHC, the gold standard to obtain direct pulmonary hemodynamic measurements and confirm the PoPH diagnosis. Values of tricuspid regurgitation velocity above 3.4 m/s or ranges between 2.9 and 3.4 m/s followed by echocardiographic sounds of PH provide a high probability of PH. There is an ongoing debate on whether RSVP requires a different cutoff point in cirrhotic patients, because there is an increased MPAP in 20% of this population, due to a high flow state.3,44
Currently, the American Association for the Study of Liver Diseases (AASLD) recommends RHC in patients with RSVP ≥45 mm Hg and/or evidence of high MPAP founded on TTE (pulmonary artery dilation, right atrium dilation, and diastolic dysfunction). Meanwhile, the European Respiratory Society and the Mayo Clinic use RSVP ≥50 mm Hg. Using a value of 50 mm Hg of RSVP has a sensitivity and specificity of 97% and 77%, respectively, to identify patients with moderate to severe PoPH. An RSVP ≥35 mm Hg generally reflects an MPAP above 25 mm Hg, whereas an RSVP <30 mm Hg excludes PoPH41. The severity of PoPH is based on the RHC results and is classified as mild (MPAP 20–35 mm Hg), moderate (MPAP 35–45 mm Hg), or severe (MPAP >45 mm Hg).3,44 Other causes of PH should be ruled out as well.
Additionally, there must be an extensive evaluation of the hemodynamics perceived on the RHC since these are crucial to distinguish between PoPH and different clinical conditions in patients with liver disease that can elevate MPAP. These conditions can be classified into 3 categories: hyperdynamic state, volume overload, and portopulmonary hypertension (Table 3). The hyperdynamic state may be intrinsic to the liver disease, or it may be caused by other medical conditions such as anemia, arteriovenous shunts, or hyperthyroidism. Volume overload should be cautiously handled due to the high risk it conveys to patients with liver disease to develop kidney injury.1
TABLE 3.
Hemodynamic profiles with right heat catheterization on patients with an elevated mean pulmonary arterial pressure
| Mean pulmonary arterial pressure | Cardiac output | Pulmonary vascular resistance | Pulmonary arterial wedge pressure | |
|---|---|---|---|---|
| Hyperdynamic state | Elevated | Elevated | Low | Low |
| Volume overload | Elevated | Elevated | Elevated/normal | Elevated |
| Portopulmonary hypertension | Elevated | Low/normal | Elevated | Low/normal |
The pulmonary hemodynamic conditions can change throughout time in cases of PoPH; the evaluation with RHC should be done every 12 mo on candidates for OLT.11
Figure 2 shows our proposal diagnostic algorithm for portopulmonary hypertension.
Figure 2.
Diagnostic algorithm for PoPH. CO, cardiac output; MELD, Model for End-stage Liver Disease; MPAP, mean pulmonary arterial pressure; OLT, orthotopic liver transplantation; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PoPH, portopulmonary hypertension; PVR, pulmonary vascular resistance; RHC, right heart catheterization; TTE, transthoracic echocardiogram; WU, wood unit.
TREATMENT
The basis of treatment for PoPH rests on 3 therapeutic pillars: general measures, support treatment, and specific treatment for PAH, including LT.
General Measures
The general measures include avoiding pregnancy due to the inherent hemodynamic changes caused by this condition and yearly immunization against the influenza virus and pneumococcus according to each region’s guidelines.11 Likewise, the patients should be followed-up by medical professionals for the underlying complications of portal hypertension and the direct complications of PAH, such as right heart failure.
Supportive Treatment
The supportive treatment includes:
Anticoagulation, which according to the COMPARE study, there has not been an established benefit on the survival rate to 3 y for patients with PoPH with this therapy.50 It is not recommended in PoPH as per ILTS guidelines.51
Supplementary oxygen is recommended for patients with oxygen saturation <89%, as per ILTS guidelines.51
Diuretics, which are frequently used for the treatment of portal hypertension, are important in the management of PH and volume overload.9
In addition to supportive treatment, there is some medication to avoid. It is essential to highlight the deleterious effects of beta-blockers; they are commonly used for their primary and secondary prophylaxis effects on hemorrhagic portal hypertension. In patients with PoPH, beta-blockers can have a negative clinical effect due to the right heart failure that can be present in these patients, which will cause a reduction of cardiac output with increased PVR.52 However, there are studies in animal and human models with stable PoPH in which beta-blockers such as carvedilol can have a positive effect at 6 mo, although their recommendation is not yet specific.53 The use of calcium antagonists should also be avoided given the adverse effects they have on patients with PoPH, including hypotension and splenic vasodilation. PoPH patients are susceptible to hypotension because of the lower systemic vascular resistance present during chronic liver disease, conditioning a significant reduction in the right ventricular filling and aggravating the functional class.54
Specific Treatment for PAH
The most frequently used therapeutic arsenal are prostacyclin agonists, endothelin receptor antagonists, phosphodiesterase inhibitors, and guanylate cyclase stimulants; reducing the MPAP and vascular resistance is the main therapeutic goal.
The prostacyclin agonists (prostanoids) are reserved for patients with a type IV functional class according to the World Health Organization or any patient with PH not meeting low-risk criteria.9 They can be used as bridge therapy for patients on the transplant list. Generally, prostacyclin agonists have shown an improvement in pulmonary hemodynamics, functional class, and the ability to exercise. Epoprostenol is a potent vasodilator with antiplatelet aggregation and antiproliferation properties for patients with PAH. It also appears to significantly improve pulmonary hemodynamics for patients with moderate to severe PAH and reduce the mean arterial pulmonary pressure and PVR.55,56 Efficacy of sildenafil added to treprostinil has been studied in a series of cases with a retrospective design, reporting an improvement in the mean arterial pulmonary pressure, peripheric vascular resistances, cardiac output, and the transpulmonary gradient57; finally, iloprost administered via inhalation, has been reported to improve the survival rate to 1 y 77%, 2 y 62%, and 3 y 46% on a cohort of 13 patients who were analyzed through a retrospective manner.58
The endothelin receptor antagonists are targeted for patients with a functional class of II and III, whether as monotherapy or in conjunction with phosphodiesterase inhibitors as a combined therapy. They can also used as triple therapy in FC IV patients. Bosentan and Macitentan are endothelin A and B receptor antagonists, and Ambrisentan is a selective endothelin receptor A antagonist.59 Monthly vigilance with hepatic biochemical tests should be performed when treated with bosentan; their use is not recommended on patients with transaminase levels 3 times above the upper limit. Bosentan has demonstrated its efficacy with an improvement of the functional class (6-min walking test) and pulmonary hemodynamics.60 Ambrisentan has been shown to improve pulmonary dynamics, with a significant decrease of MPAP from a baseline median of 58 to 41 mm Hg, without adverse effects on hepatic function.61 Macitentan has been the only drug whose pulmonary vasodilator effects have been evaluated prospectively in patients with PoPH. The PORTICO study randomized 85 patients from 36 medical centers in 7 countries and evaluated the efficacy and security of Macitentan versus placebo. Macitentan improved PVR by 35% of patients with minimal effects on liver function tests. This study excluded patients with advanced liver disease (Child-Pugh C or Model for End-Stage Liver Disease [MELD] >19 points).62
Phosphodiesterase 5 (PDE 5) inhibitors block the degrading activity of cGMP, thus increasing serum levels. Their use in the first group of PAH was investigated, and the results manifested an enhancement in the functional class and pulmonary hemodynamics.63 One observational study with a population of 14 patients ranging from moderate to severe PoPH, using sildenafil as monotherapy with doses of 50 mg 3 times per day for 3 mo, found an improvement in the reduction of mean arterial pulmonary pressure and PVR, at the same time there was a better performance on the 6-min walking test.64 Regarding the guanylate cyclase stimulants, riociguat is the drug prototype whose benefit has been proven in chronic PAH secondary to pulmonary thromboembolism. Nonetheless, a post hoc analysis of the PATENT-1 study included 13 patients with PoPH, of whom 11 were randomized to receive 2.5 mg of riociguat 3 times a day max, and the remaining 2 were prescribed placebos for 12 ks. Riociguat was well tolerated and showed an improvement on the 6-min walking test and an overall benefit of the functional class, which remained steady for 2 y in an open-label study, PATENT-2; alternatively, there are no data related to OLT.65 Table 4 summarizes the drugs used as a treatment for PoPH.
TABLE 4.
Drugs used for portopulmonary hypertension
| Group | Drug | Indications according to CPT | Dosage | Changes in pulmonary hemodynamics | Adverse effects |
|---|---|---|---|---|---|
| Prostanoids | Epoprostenol | A, B, C | 2–4 ng/kg/IV, increasing in 1–2 ng/kg/min with 15-min intervals | FC improvement | Headache, influenza like-symptoms, splenomegaly, and thrombocytopenia |
| Iloprost | A | 2.5–5 µg inhaled (6–9 times per day) | Increases exercise tolerance (6MWT)MPAP and PVR Improvement | Cough, tongue, pain, and syncope | |
| Treprostinil | A | 0.5 ng/kg/minSC | FC improvement | Pain, cutaneous reactions on puncture site | |
| ERA | Bosentan | A | 62.5 mg PO for 4 wks, increase to 125 mg PO every 12 h without hepatotoxicity | Increases exercise tolerance (6MWT) | Hepatotoxicity, peripheral edema headache, vertigo |
| Ambrisentan | A | 5–10 mg PO every 24 h | MPAP, PVR, and CO improvement | Nasal congestion | |
| Macitentan | A, B | 10 mg PO every 24 h | PVR improvement | Peripheral edema, headache | |
| PDE-5 | Sildenafil | B, C | 20 mg PO every 8 h | Increases exercise tolerance (6MWT), MPAP, and PVR reduction | Headache, myalgias, priapism, diarrhea, variceal hemorrhage |
| AGC | Riociguat | A, B | 0.5-2.5 mg PO every 8 h | Increases exercise tolerance (6MWT) | Headache, dyspepsia, nausea, vomiting, diarrhea, arterial hypertension |
6MWT, 6-min walking test; AGC, guanylate cyclase agonist; CO, cardiac output; CPT, Child-Pugh Turcotte; ERA, endothelin receptor antagonist; FC, functional capacity; IV, intravenous; MPAP, mean pulmonary arterial pressure; PDE-5, phosphodiesterase 5 inhibitor; PO, by mouth; PVR, pulmonary vascular resistance; SC, subcutaneous.
PROGNOSIS AND IMPACT OF LIVER TRANSPLANT
Generally, the prognosis is poor and PAH therapy and OLT are the only effective treatment for PoPH in highly selected patients. Cohort studies have demonstrated that patients with PoPH have a worse prognosis compared with patients with idiopathic PH, independently of having better hemodynamics during the diagnosis.66 In a study, Aggarthel and collaborators evaluated survival predictors of PoPH in 80 patients for 20 y, of whom 63 (78.5%) began specific treatment for PAH within the first 6 mo of their diagnosis, and 62.5% of patients treated with monotherapy showed a survival rate to 1, 3, and 5 y of 77%, 52%, and 34%, respectively. Only the MELD-sodium (MELD-Na), resting cardiac frequency, and presence of hepatic encephalopathy were independent survival factors, revealing that the survival rate is predominantly caused by the severity of the subjacent liver disease and not the severity of the PoPH hemodynamics; in addition, PAH-specific therapies within the first 6 mo of PoPH diagnosis did not significantly impact the natural history of the disease; therefore, the ongoing efforts should focus on liver transplantation for these patients.67
PoPH is a challenge for the anesthesiologist since it significantly increases trans-surgical and postsurgical risks during the OLT.10 Until the middle of the year 2000, it was not rare for physicians to diagnose PoPH for the first time while in the operating room. However, this event is uncommon nowadays.51 Hemodynamic monitoring is possible by placing a catheter on the pulmonary artery. A liver transplant can be suspended in the operating room if an MPAP >50 mm Hg is observed without treatment or when the treatment cannot achieve an MPAP reduction of <40 mm Hg.51 The reperfusion syndrome (cardiovascular collapse after hepatic reperfusion posterior to the transplant) can be observed in up to 30% of cases. It becomes relevant since this can lead to an acute increase in PAPs, resulting in right ventricular and pulmonary artery injury or graft failure. More severe cases have been successfully treated with varying progress with trans-surgical nitrous oxide, prostacyclin, milrinone, and even extracorporeal membrane oxygenation.11,20,51
Without medical treatment or transplantation, patients have a 1-y survival rate of 35%–46% and a 5-y survival rate of 4%–14%. The causes of death are divided between heart failure and subjacent liver disease complications.3,68 In 2012 (a flourishing era for the treatment of PH), the REVEAL study associated PoPH with a 40% 5-y survival rate.13
The Spanish study REHAP followed 237 patients with PoPH, the 1, 3, and 5 y survival rates from the date of diagnosis were 79.6%, 65.3%, and 49.3%, respectively, and patients who received specific therapy for PH had better survival aside from having poor hemodynamics. The study also revealed that advanced age and clinical presentation of ascites increased the risk of death, whereas the first-line treatment with oral monotherapy was associated with better disease evolution. Of the 237 patients, 8 received OLT, 1 died during the perioperative period, 3 improved PH after transplantation, 1 died from posttransplant complications, and 4 remained with persistent PH, with 2 patients dying during follow-up.66
Krowka et al showed fatal outcomes after OLT in a 36-patient cohort with PoPH without any treatment. The mortality rate after OLT was 100%, 50%, and 0% in patients with MPAP >50, 35–50, and <35 mm Hg, respectively.69 Khaderi and collaborators evaluated the long-term results of 7 patients with OLT after successfully reducing the MPAP <35 mmHg. The survival rates of the graft and patients were 7.8 y.70
Compared with hepatopulmonary syndrome, the indications of OLT in PoPH are controversial since the posttransplantation hemodynamic response is unpredictable.11 On the other hand, a liver transplantation should be considered for patients with mild PoPH or those with an MPAP between 35 and 45 mm Hg that can successfully lower the MPAP <35 mm Hg post–vasodilation therapy.
The AASLD guidelines suggest that OLT can be offered to patients with mild PoPH and those with an excellent response to medical treatment (MPAP after treatment <35 mm Hg and peripheral vascular resistance <3 WU).71 The guidelines of the International Society of Liver Transplantation, added to the recommendations of the AASLD, considers OLT for patients with MPAP that does not lower beneath <35 mm Hg with treatment, but there is, however, normalization of the peripheral vascular resistance (<3 WU).72
Currently, patients with PoPH that have an adequate hemodynamic response to the medical therapy evidenced on the 3-mo follow-up heart catheterization after starting treatment can receive MELD exception points with a Median MELD at Transplant minus 3 points, according to the actual policy of the Organ Procurement Transplantation Network.68,73
The cohort study by Savale and collaborators evaluated long-term results of OLT in a period wherein the current therapies of PH revealed a survival rate of 80%, 77%, and 77% in 6 mo, 1 y, and 3 y after OLT, respectively. In addition, 45% of patients who underwent OLT had PH resolutions. Meanwhile, 55% required ongoing pulmonary vasodilation therapy.74
In another study by Savale and collaborators, 637 patients with PoPH were evaluated; 90% received immediate treatment for PH (monotherapy, bitherapy, and triple therapy) and showed a survival to 5 y of 51%, with the principal prognostic factor being severity of the basal liver disease.5 Baseline PVR has been associated with worse mortality.68 Subsequently, Savale et al disclosed a higher survival in OLT patients (92%, 83%, and 81% to 1, 3, and 5 y, respectively) compared with patients who do not receive a liver transplant.5 Moreover, the survival of OLT patients with PoPH has proved to be superior compared with patients with OLT from autoimmune diseases (79% to 5 y),75 alcoholic liver disease (79% to 5 y),76 and fatty liver disease (80% to 5 y).77
A retrospective study of the Chinese population compared PoPH patients who did not receive vasodilation therapy before or after OLT versus patients without PoPH. The results showed a survival rate significantly lower after transplantation in patients with PoPH, the mortality was 57%; of which, 50% died during the first-year post-OLT, with a mean survival of 11.4 mo being lung infection the primary cause of death. Compared with those who did not present PoPH, the mortality was only 18%. The conclusion was that PoPH patients without vasodilation treatment have higher mortality despite being subjected to an OLT.78
The impact of OLT on PoPH is unpredictable. A retrospective study in France included 8 centers and indicated that 17.4% of patients are freed of pulmonary hypertensive therapy after 1 y post-OLT, 60.7% normalized their MPAP with medical treatment, only 8.7% had an increase in MPAP, and the last 30.4% remained stable.79
OLT can resolve portal hypertension and is an effective treatment for PoPH. The percentage of patients who remain with pulmonary hypertensive treatment after OLT varies between 18% and 55%. Conversely, without specific therapy for PH, patients with portopulmonary hypertension have a worse prognosis after OLT. In cases wherein PoPH does not resolve after OLT, there are suggestions for irreversible remodeling of the pulmonary arterial walls before PH therapy. In recent years, studies have shown that patients with PoPH benefit from vasodilation treatment, and those who underwent OLT had excellent long-term survival; therefore, vasodilation therapy should be considered in all patients with PoPH.5,66,78
Table 5 summarizes the results of liver transplantation in patients with portopulmonary hypertension.
TABLE 5.
Results of liver transplantation in patients with portopulmonary hypertension
| Publication | Patients treated with OLT | Mean MELD | 1-y survival, % | 3-y survival. % | 5-y survival | Posttrasplant mortality |
|---|---|---|---|---|---|---|
| Savale et al5Prospective | N = 63 | 11 | 92 | 83 | 81% | (12 of 63)Within 1 mo |
| Swanson et al18Single-center retrospective | N = 12 | 13 | NA | NA | Without PH treatment prior OLT25%.With PH treatment prior OLT67% | (5 of 12) Within 1 mo(2 of 5)Intraoperativedeath |
| DuBrock et al68Retrospective | N = 103 | 12 | 86 | NA | NA | (18 of 103)Within 1 y |
| Savale et al(2017)74Retrospective | N = 35 | 20 | 77 | 77 | NA | (8 of 35)Within 6 mo(5 of 8 PoPH) |
| Reymond et al79Multicenter retrospective | N = 23 | 17.5 | 83 | 83 | NA | (2 of 23)Within 1 wkAcute right heart failure |
| Ashfaq et al80Single-center retrospective | N = 11 | 18 | 90.9 | 80 | 67% | (4 of 11)Intraabdominal hemorrhage (week 2)Infectious pericarditis(month 6)Recurrent hepatitis C(month 13)Sepsis(month 63) |
| Salgia et al81Retrospective | N = 78 | 14 | 85 | 81 | NA | NA |
| Rajaram et al82Single-center retrospective | N = 13 | 21 | 69 | NA | NA | NA |
| Verma et al83Multicenterretrospective | N = 28 | 18 | 63 | 59 | 54% | (12 of 28)Within 6 mo(3 of 12)right heart failure(2 of 12)sepsis |
| Cartin-Ceba et al84Multicenterretrospective | N = 50 | 15 | 73 | 63 | 60% | (2 of 24 Intraoperative death)(4 of 24 progressive RV failure within 4 mo)(5 of 24 graft dysfunction)(3 of 24 malignancy)(8 of 24 sepsis)(2 of 24others) |
| Sadd et al85Single-centerretrospective | N = 24 | 12.5 | 87 | 87 | 87% | (3 of 24)within 6 moinfectious complications |
MELD, Model of End-Stage Liver Disease; NA, not available; OLT, orthotopic liver transplantation; PH, pulmonary hypertension; PoPH, portopulmonary hypertension.
CONCLUSION
PoPH is a pulmonary vascular disease caused by portal hypertension (with or without underlying liver disease) leading to right heart failure. For the evaluation of patients with PoPH, TTE is the first-line study for screening. However, RHC remains the gold standard. Five-year survival without treatment ranges from 4% to 14% but increases to 40% to 51% with specific treatment. OLT has shown excellent long-term survival in appropriately selected patients who received vasodilator therapy as a bridge to transplantation.
PoPH patients are an active research area and although many barriers and challenges still exist, the pathophysiological mechanisms understanding and studies exploring the combined treatment with OLT will improve the management of these patients.
Supplementary Material
Footnotes
E.A.J.-B., G.A.P.-A., and I.G.-J.: conception, search for information, writing, and revision of the article. J.A.-V., I.R., B.P.-R., J.V.J., C.J.G.-C., F.E.R.-L., J.R.-A., F.C.L.-L., J.L.H.-O., J.A.T.-D., E.K.-O., J.R.-M., P.H.-R., J.Z.-B., C.A.R.-O., T.P., and S.M.-M.: writing and revision of the article.
Supplemental Visual Abstract statement: http://links.lww.com/TP/C823.
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.Thomas C, Glinskii V, de Jesus Perez V, et al. Portopulmonary hypertension: from bench to bedside. Front Med (Lausanne). 2020;7:569413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navarro-Vergara DI, Roldan-Valadez E, Cueto-Robledo G, et al. Portopulmonary hypertension: prevalence, clinical and hemodynamic features. Curr Probl Cardiol. 2021;46:100747. [DOI] [PubMed] [Google Scholar]
- 3.Weinfurtner K, Forde K. Hepatopulmonary syndrome and portopulmonary hypertension: current status and implications for liver transplantation. Curr Hepatol Rep. 2020;19:174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savale L, Watherald J, Sitbon O. Portopulmonary hypertension. Semin Respir Crit Care Med. 2017;38:651–661. [DOI] [PubMed] [Google Scholar]
- 5.Savale L, Guimas M, Ebstein N, et al. Portopulmonary hypertension in the current era of pulmonary hypertension management. J Hepatol. 2020;73:130–139. [DOI] [PubMed] [Google Scholar]
- 6.AbuHalimeh B, Krowka MJ, Tonelli AR. Treatment barriers in portopulmonary hypertension. Hepatology. 2019;69:431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahay S, Al Abdi S, Melillo C, et al. Causes and circumstances of death in portopulmonary hypertension. Transplant Direct. 2021;7:e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thévenot T, Savale L, Sitbon O. Portopulmonary hypertension: an unfolding story. Clin Res Hepatol Gastroenterol. 2021;45:101492. [DOI] [PubMed] [Google Scholar]
- 9.Humbert M, Kovacs G, Hoeper MM, et al. ; ESC/ERS Scientific Document Group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension [published correction appears in Eur Heart J. 2023 Apr 17;44(15):1312]. Eur Heart J. 2022;43:3618–3731.36017548 [Google Scholar]
- 10.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Almendros N, Toapanta-Yanchapaxi LN, Aguirre Valadez J, et al. Hipertensión portopulmonar: revisión actualizada [portopulmonary hypertension: updated review]. Arch Cardiol Mex. 2018;88:25–38. [DOI] [PubMed] [Google Scholar]
- 12.Ruopp NF, Cockrill BA. Diagnosis and treatment of pulmonary arterial hypertension: a review. JAMA. 2022;327:1379–1391. [DOI] [PubMed] [Google Scholar]
- 13.Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest. 2021;141:906–915. [DOI] [PubMed] [Google Scholar]
- 14.Shao Y, Yin X, Qin T, et al. Prevalence and associated factors of portopulmonary hypertension in patients with portal hypertension: a case-control study. Biomed Res Int. 2021;2021:5595614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawut SM, Krowka MJ, Trotter JF, et al. ; Pulmonary Vascular Complications of Liver Disease Study Group. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savale L, Watherald J, Sitbon O. Portopulmonary hypertension. Semin Respir Crit Care Med. 2017;38:651–661. [DOI] [PubMed] [Google Scholar]
- 17.Sithamparanathan S, Nair A, Thirugnanasothy L, et al. ; National Pulmonary Hypertension Service Research Collaboration of the United Kingdom and Ireland. Survival in portopulmonary hypertension: outcomes of the United Kingdom National Pulmonary Arterial Hypertension Registry. J Heart Lung Transplant. 2017;36:770–779. [DOI] [PubMed] [Google Scholar]
- 18.Swanson KL, Wiesner RH, Nyberg SL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445–2453. [DOI] [PubMed] [Google Scholar]
- 19.Raevens S, Geerts A, Devisscher L, et al. Recent advances in the approach to hepatopulmonary syndrome and portopulmonary hypertension. Acta Gastroenterol Belg. 2021;84:95–99. [DOI] [PubMed] [Google Scholar]
- 20.Iqbal S, Smith KA, Khungar V. Hepatopulmonary syndrome and portopulmonary hypertension: implications for liver transplantation. Clin Chest Med. 2017;38:785–795. [DOI] [PubMed] [Google Scholar]
- 21.Bolognesi M, Di Pascoli M, Verardo A, et al. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J Gastroenterol. 2014;20:2555–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talwalkar JA, Swanson KL, Krowka MJ, et al. Prevalence of spontaneous portosystemic shunts in patients with portopulmonary hypertension and effect on treatment. Gastroenterology. 2011;141:1673–1679. [DOI] [PubMed] [Google Scholar]
- 23.Zardi EM, Uwechie V, Caccavo D, et al. Portosystemic shunts in a large cohort of patients with liver cirrhosis: detection rate and clinical relevance. J Gastroenterol. 2009;44:76–83. [DOI] [PubMed] [Google Scholar]
- 24.Van der Linden P, Le Moine O, Ghysels M, et al. Pulmonary hypertension after transjugular intrahepatic portosystemic shunt: effects on right ventricular function. Hepatology. 1996;23:982–987. [DOI] [PubMed] [Google Scholar]
- 25.Huertas A, Guignabert C, Barberà JA, et al. Pulmonary vascular endothelium: the orchestra conductor in respiratory diseases: highlights from basic research to therapy. Eur Respir J. 2018;51:1700745. [DOI] [PubMed] [Google Scholar]
- 26.Shenoda B, Boselli J. Vascular syndromes in liver cirrhosis. Clin J Gastroenterol. 2019;12:387–397. [DOI] [PubMed] [Google Scholar]
- 27.Porres-Aguilar M, Altamirano JT, Torre-Delgadillo A, et al. Portopulmonary hypertension and hepatopulmonary syndrome: a clinician-oriented overview. Eur Respir Rev. 2012;21:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology. 2006;44:1502–1510. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Roisin R, Krowka MJ, Hervé P, et al. ; ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J. 2004;24:861–880. [DOI] [PubMed] [Google Scholar]
- 30.Baeyens N, Bandyopadhyay C, Coon BG, et al. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest. 2016;126:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards BS, Weir EK, Edwards WD, et al. Coexistent pulmonary and portal hypertension: morphologic and clinical features. J Am Coll Cardiol. 1987;10:1233–1238. [DOI] [PubMed] [Google Scholar]
- 33.Talwalkar JA, Swanson KL, Krowka MJ, et al. Prevalence of spontaneous portosystemic shunts in patients with portopulmonary hypertension and effect on treatment. Gastroenterology. 2011;141:1673–1679. [DOI] [PubMed] [Google Scholar]
- 34.Zardi EM, Uwechie V, Caccavo D, et al. Portosystemic shunts in a large cohort of patients with liver cirrhosis: detection rate and clinical relevance. J Gastroenterol. 2009;44:76–83. [DOI] [PubMed] [Google Scholar]
- 35.Zardi EM, Zardi DM, Giorgi C, et al. Portopulmonary hypertension and hepatorenal syndrome. Two faces of the same coin. Eur J Intern Med. 2017;43:22–27. [DOI] [PubMed] [Google Scholar]
- 36.Lane KB, Machado RD, Pauciulo MW, et al. ; International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. [DOI] [PubMed] [Google Scholar]
- 37.Thomson JR, Machado RD, Pauciulo MW, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet. 2000;37:741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rochon ER, Krowka MJ, Bartolome S, et al. BMP9/10 in pulmonary vascular complications of liver disease. Am J Respir Crit Care Med. 2020;201:1575–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer EM, Chanthaphavong RS, Sodhi CP, et al. Genetic deletion of toll-like receptor 4 on platelets attenuates experimental pulmonary hypertension. Circ Res. 2014;114:1596–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin ED, Hamid R, Hemnes AR, et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ. 2012;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Austin ED, Cogan JD, West JD, et al. Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J. 2009;34:1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodgson J, Swietlik EM, Salmon RM, et al. Characterization of GDF2 mutations and levels of BMP9 and BMP10 in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2020;201:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sendra C, Carballo-Rubio V, Sousa JM. Hepatopulmonary syndrome and portopulmonary hypertension: management in liver transplantation in the horizon 2020. Transplant Proc. 2020;52:1503–1506. [DOI] [PubMed] [Google Scholar]
- 44.Soulaidopoulos S, Goulis I, Cholongitas E. Pulmonary manifestations of chronic liver disease: a comprehensive review. Ann Gastroenterol. 2020;33:237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Franchis R, Bosch J, Garcia-Tsao G, et al. ; Baveno VII Faculty. Baveno VII - renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pons M, Augustin S, Scheiner B, et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2021;116:723–732. [DOI] [PubMed] [Google Scholar]
- 47.Xu H, Cheng B, Wang R, et al. Portopulmonary hypertension: current developments and future perspectives. Liver Res. 2022;6:10–20. [Google Scholar]
- 48.Benz F, Mohr R, Tacke F, et al. Pulmonary complications in patients with liver cirrhosis. J Transl Int Med. 2020;8:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin X, Shao Y, Zhang Y, et al. Role of echocardiography in screening for portopulmonary hypertension in liver transplant candidates: a meta-analysis. PeerJ. 2020;8:e9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olsson KM, Delcroix M, Ghofrani HA, et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Circulation. 2014;129:57–65. [DOI] [PubMed] [Google Scholar]
- 51.Krowka MJ, Fallon MB, Kawut SM, et al. International Liver Transplant Society Practice Guidelines: diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation. 2016;100:1440–1452. [DOI] [PubMed] [Google Scholar]
- 52.Provencher S, Herve P, Jais X, et al. Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology. 2006;130:120–126. [DOI] [PubMed] [Google Scholar]
- 53.Farha S, Saygin D, Park MM, et al. Pulmonary arterial hypertension treatment with carvedilol for heart failure: a randomized controlled trial. JCI Insight. 2017;2:e95240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montani D, Savale L, Natali D, et al. Long-term response to calcium-channel blockers in non-idiopathic pulmonary arterial hypertension. Eur Heart J. 2010;31:1898–1907. [DOI] [PubMed] [Google Scholar]
- 55.McLaughlin VV, Genthner DE, Panella MM, et al. Compassionate use of continuous prostacyclin in the management of secondary pulmonary hypertension: a case series. Ann Intern Med. 1999;130:740–743. [DOI] [PubMed] [Google Scholar]
- 56.Krowka MJ, Frantz RP, McGoon MD, et al. Improvement in pulmonary hemodynamics during intravenous epoprostenol (prostacyclin): a study of 15 patients with moderate to severe portopulmonary hypertension. Hepatology. 1999;30:641–648. [DOI] [PubMed] [Google Scholar]
- 57.Hollatz TJ, Musat A, Westphal S, et al. Treatment with sildenafil and treprostinil allows successful liver transplantation of patients with moderate to severe portopulmonary hypertension. Liver Transpl. 2012;18:686–695. [DOI] [PubMed] [Google Scholar]
- 58.Hoeper MM, Seyfarth HJ, Hoeffken G, et al. Experience with inhaled iloprost and bosentan in portopulmonary hypertension. Eur Respir J. 2007;30:1096–1102. [DOI] [PubMed] [Google Scholar]
- 59.Halank M, Miehlke S, Hoeffken G, et al. Use of oral endothelin-receptor antagonist bosentan in the treatment of portopulmonary hypertension. Transplantation. 2004;77:1775–1776. [DOI] [PubMed] [Google Scholar]
- 60.Savale L, Magnier R, Le Pavec J, et al. Efficacy, safety and pharmacokinetics of bosentan in portopulmonary hypertension. Eur Respir J. 2013;41:96–103. [DOI] [PubMed] [Google Scholar]
- 61.Cartin-Ceba R, Swanson K, Iyer V, et al. Safety and efficacy of ambrisentan for the treatment of portopulmonary hypertension. Chest. 2011;139:109–114. [DOI] [PubMed] [Google Scholar]
- 62.Sitbon O, Bosch J, Cottreel E, et al. Macitentan for the treatment of portopulmonary hypertension (PORTICO): a multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet Respir Med. 2019;7:594–604. [DOI] [PubMed] [Google Scholar]
- 63.Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361:1864–1871. [DOI] [PubMed] [Google Scholar]
- 64.Chua R, Keogh A, Miyashita M. Novel use of sildenafil in the treatment of portopulmonary hypertension. J Heart Lung Transplant. 2005;24:498–500. [DOI] [PubMed] [Google Scholar]
- 65.Cartin-Ceba R, Halank M, Ghofrani HA, et al. Riociguat treatment for portopulmonary hypertension: a subgroup analysis from the PATENT-1/-2 studies. Pulm Circ. 2018;8:2045894018769305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lazaro Salvador M, Quezada Loaiza CA, Rodríguez Padial L, et al. ; REHAP Investigators. Portopulmonary hypertension: prognosis and management in the current treatment era—results from the REHAP registry. Intern Med J. 2021;51:355–365. [DOI] [PubMed] [Google Scholar]
- 67.Aggarwal M, Li M, Bhardwaj A, et al. Predictors of survival in portopulmonary hypertension: a 20-year experience. Eur J Gastroenterol Hepatol. 2022;34:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DuBrock HM, Goldberg DS, Sussman NL, et al. Predictors of waitlist mortality in portopulmonary hypertension. Transplantation. 2017;101:1609–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443–450. [DOI] [PubMed] [Google Scholar]
- 70.Khaderi S, Khan R, Safdar Z, et al. Long-term follow-up of portopulmonary hypertension patients after liver transplantation. Liver Transpl. 2014;20:724–727. [DOI] [PubMed] [Google Scholar]
- 71.Martin P, DiMartini A, Feng S, et al. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144–1165. [DOI] [PubMed] [Google Scholar]
- 72.Sendra C, Carballo-Rubio V, Sousa JM. Hepatopulmonary syndrome and portopulmonary hypertension: management in liver transplantation in the horizon 2020. Transplant Proc. 2020;52:1503–1506. [DOI] [PubMed] [Google Scholar]
- 73.DuBrock HM, Del Valle KT, Krowka MJ. Mending the model for end-stage liver disease: an in-depth review of the past, present, and future portopulmonary hypertension model for end-stage liver disease exception. Liver Transpl. 2022;28:1224–1230. [DOI] [PubMed] [Google Scholar]
- 74.Savale L, Sattler C, Coilly A, et al. Long-term outcome in liver transplantation candidates with portopulmonary hypertension. Hepatology. 2017;65:1683–1692. [DOI] [PubMed] [Google Scholar]
- 75.Heinemann M, Adam R, Berenguer M, et al. ; all the Other Contributing Centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Longterm survival after liver transplantation for autoimmune hepatitis: results from the European Liver Transplant Registry. Liver Transpl. 2020;26:866–877. [DOI] [PubMed] [Google Scholar]
- 76.Lee BP, Vittinghoff E, Dodge JL, et al. National trends and long-term outcomes of liver transplant for alcohol-associated liver disease in the United States. JAMA Intern Med. 2019;179:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burra P, Becchetti C, Germani G. NAFLD and liver transplantation: disease burden, current management and future challenges. JHEP Rep. 2020;2:100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J, Zhuang Q, Zhang X, et al. Prevalence and prognosis of portopulmonary hypertension in 223 liver transplant recipients. Can Respir J. 2018;2018:9629570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reymond M, Barbier L, Salame E, et al. Does portopulmonary hypertension impede liver transplantation in cirrhotic patients? A French multicentric retrospective study. Transplantation. 2018;102:616–622. [DOI] [PubMed] [Google Scholar]
- 80.Ashfaq M, Chinnakotla S, Rogers L, et al. The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant. 2007;7:1258–1264. [DOI] [PubMed] [Google Scholar]
- 81.Salgia RJ, Goodrich NP, Simpson H, et al. Outcomes of liver transplantation for porto-pulmonary hypertension in model for end-stage liver disease era. Dig Dis Sci. 2014;59:1976–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rajaram P, Parekh A, Fisher M, et al. Comparison of post-liver transplantation outcomes in portopulmonary hypertension and pulmonary venous hypertension: a single-center experience. Transplant Proc. 2017;49:338–343. [DOI] [PubMed] [Google Scholar]
- 83.Verma S, Hand F, Armstrong MJ, et al. Portopulmonary hypertension: still an appropriate consideration for liver transplantation? Liver Transpl. 2016;22:1637–1642. [DOI] [PubMed] [Google Scholar]
- 84.Cartin-Ceba R, Burger C, Swanson K, et al. Clinical outcomes after liver transplantation in patients with portopulmonary hypertension. Transplantation. 2021;105:2283–2290. [DOI] [PubMed] [Google Scholar]
- 85.Sadd CJ, Osman F, Li Z, et al. Long-term outcomes and survival in moderate-severe portopulmonary hypertension after liver transplant. Transplantation. 2021;105:346–353. [DOI] [PubMed] [Google Scholar]