Abstract
Neurexin-3 is primarily localized in the presynaptic membrane and forms complexes with various ligands located in the postsynaptic membrane. Neurexin-3 has important roles in synapse development and synapse functions. Neurexin-3 mediates excitatory presynaptic differentiation by interacting with leucine-rich-repeat transmembrane neuronal proteins. Meanwhile, neurexin-3 modulates the expression of presynaptic α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors and γ-aminobutyric acid A receptors by interacting with neuroligins at excitatory and inhibitory synapses. Numerous studies have documented the potential contribution of neurexin-3 to neurodegenerative and neuropsychiatric disorders, such as Alzheimer's disease, addiction behaviors, and other diseases, which raises hopes that understanding the mechanisms of neurexin-3 may hold the key to developing new strategies for related illnesses. This review comprehensively covers the literature to provide current knowledge of the structure, function, and clinical role of neurexin-3.
Keywords: Excitatory synapses, Inhibitory synapses, Neural cell adhesion molecules, Neurexin-3, Neurodegenerative diseases, Neuropsychiatric diseases
Introduction
Synapses are key components of information transmission between neurons.1 Almost all information transmission in the brain depends on synapses through the process of integrating and processing various sensory signals to produce appropriate motor signals. A synapse is mainly comprised of three parts: presynaptic membrane, synaptic cleft and postsynaptic membrane. Signal transduction at synapses is achieved by the release of neurotransmitters and changes in the function of neurotransmitter receptors. Synaptic plasticity, a significant feature of the neural system that refers to the changes in the structure, number and function of synapses under the influence of continuous neuronal activity, is the basis of the cellular biology of cognitive function and includes long-term synaptic plasticity and short-term synaptic plasticity. Long-term synaptic plasticity mainly includes long-term potentiation (LTP) and long-term depression (LTD), while short-term synaptic plasticity mainly consists of facilitation and depression.2,3 The formation and regulation of synapses rely on signal transduction between presynaptic and postsynaptic membrane proteins, especially neural cell adhesion molecules (NCAMs).4 NCAMs are cell surface proteins that promote neuronal adhesion, increase axon growth and alter synaptic plasticity.5
Neurexin-3 belongs to the neurexin family, which was discovered in the process of studying α-latrotoxin. α-Latrotoxin is a component of the venom of black widow spider that results in the release of neurotransmitters from the presynaptic membrane by binding to a member of the neurexin family.6 Three neurexin genes (Nrxn1, Nrxn2, and Nrxn3) have been identified in the mammalian genome, and each encodes a longer α-protein and a shorter β-protein from separate promoters. Taken together, the neurexin family contains six main members, including neurexin-1α, neurexin-1β, neurexin-2α, neurexin-2β, neurexin-3α and neurexin-3β.7 Neurexin-1 and neurexin-2 have been characterized in detail. In this article, we summarize the structure and function and discuss the latest knowledge on the clinical role of neurexin-3.
The structure of neurexin-3
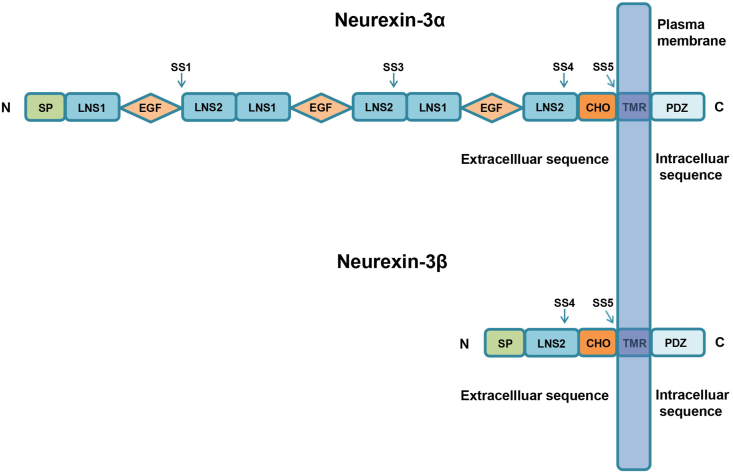
Neurexins have different expression patterns during early development of the human cerebral cortex, in which neurexin-3 is mainly expressed in the cortical plate, and its expression in the ventricular zone increases with age.8 Early studies suggested that neurexin-3 was specifically expressed in vertebrate brain tissues9; however, neurexin-3 expression is also detected in other tissues, including the lung, pancreas, heart, liver, and kidney.10 Neurexin-3α and neurexin-3β are both type 1 membrane proteins, but they have distinct domain structures. Neurexin-3α consists of a classic N-terminal signal peptide (SP) followed by three copies of a long repeat domain that contains a central epidermal growth factor (EGF)-like domain with bilateral LNS domains (laminin, neurexin, sex-hormone-binding globulin domains), whereas neurexin-3β contains an N-terminal SP followed by a short sequence that is unique to β-neurexins and then splices into the right LNS domain of the third repeat of neurexin-3α (Fig. 1). The LNS domains of neurexin-3α and neurexin-3β are followed by a sequence that consists of large numbers of threonine and serine residues, and the sequence may putatively represent the carbohydrate attachment sequence (CHO). Neurexin-3 is mainly anchored in the presynaptic membrane via a single transmembrane region (TMR) and ends in a short intracellular sequence (Fig. 2).11,12
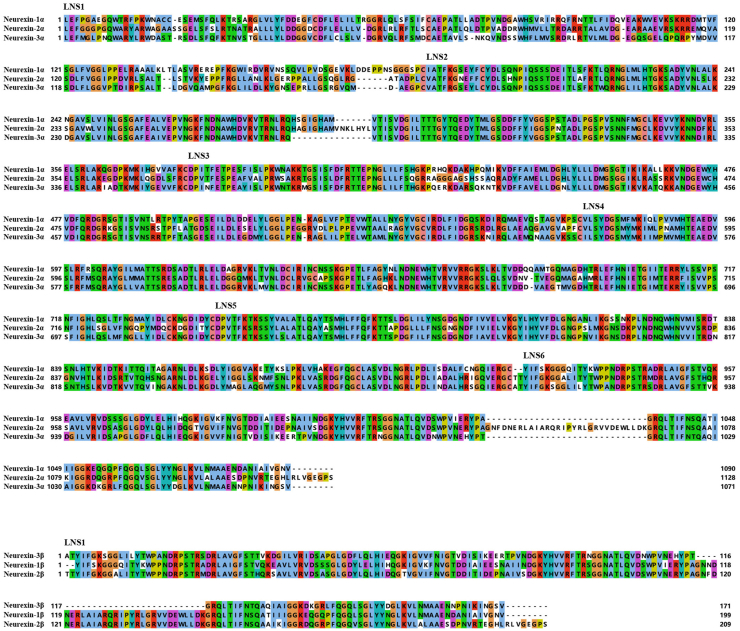
Figure 1.
Amino acid sequence alignment of the LNS domains of the neurexin family. The LNS domains of neurexin-3α and neurexin-3β are followed by a sequence that consists of large numbers of threonines and serines, and the sequence may putatively represent the carbohydrate attachment sequence (CHO).
Figure 2.
Structures of neurexin-3α and neurexin-3β. The extracellular sequence of neurexin-3α comprises an N-terminal signal peptide (SP) followed by three copies of a long repeat that contains a central epidermal growth factor (EGF)-like domain flanked by LNS domains (laminin, neurexin, sex-hormone-binding globulin domains), and the LNS domain of neurexin-3α is followed by a sequence that consists of large numbers of threonines and serines. This sequence may putatively represent the carbohydrate attachment sequence (CHO). Neurexin-3α is mainly anchored in the presynaptic membrane through a single transmembrane region (TMR) and ends in a short intracellular sequence that includes the PDZ II interaction site. Neurexin-3α contains four alternative splice sites (SS1, SS3, SS4, and SS5). The extracellular region of neurexin-3β contains an SP, an LNS domain and a CHO. Neurexin-3β is also anchored in the presynaptic membrane by a TMR and ends in a short tail. Neurexin-3β consists of only two alternative splice sites (SS4 and SS5).
Neurexin-3 is highly homologous to neurexin-1 and neurexin-2; however, neurexin-3α includes four alternative splicing sites (SS1, SS3, SS4, and SS5), while neurexin-3β only includes two alternative splicing sites (SS4 and SS5). Through the analysis of neurexin-3 transcriptional precursors, different alternative splice sites produce a variety of transcripts and protein subtypes. The effective cellular mechanism mediated by alternative splicing constructs involves many different NCAMs and signaling molecules that are located at the synapses and contribute to increasing the specificity and diversity of interactions between cells.13 At least 12 variations in the C-terminal splice site of neurexin-3 have been detected, where a stop codon can even be inserted to delete the TMR region and tail.14
The function of neurexin-3
Neurexin-3 plays important roles in synapse formation, differentiation, maturation and stability, as well as neuromuscular junctions (NMJs). Deletion of neurexin-2α and/or neurexin-3α reduces neurotransmitter release, and adult α-neurexin double-knockout mutant mice die prematurely, although the junction structure of the diaphragm remained normal, indicating that neurexin-3α plays an important role in NMJs.15 α-Dystroglycan (α-DG) is involved in multiple forms of congenital muscular dystrophy (CMD) associated with improper glycosylation.16 Interestingly, a recent study showed that neurexin-3 is also the same O-mannosylated glycoprotein as α-DG. Neurexin-3 may be a potential target for the diseases mentioned above; however, compared with neurexin-1, more specific mechanisms of neurexin-3 at NMJs are still unknown.17,18
Neurexin-3 and synapse development
Correct synaptic development is indispensable for normal brain functions. Synaptic development includes the whole process from synaptogenesis to the complete establishment of synaptic function and synaptic structure. Numerous studies have been undertaken to reveal the interaction of neurexin-3 and other molecular signaling pathways. Neurexin-3 is expressed in the cerebral cortex of early humans, where the levels of proteins related to synapse formation increase accordingly, such as vesicular GABA transporter and Ca2+/calmodulin-dependent Ser/Thr kinase (CASK).8 In addition, the levels of postsynaptic proteins, such as neuroligin-2 and LRRTM2, also increase during this period. These proteins have been shown to be related to synapse development, and thus neurexin-3 expression may reflect synapse formation.
Neurexin-3 in the presynaptic membrane binds to leucine-rich-repeat transmembrane neuronal proteins (LRRTMs) and neuroligins to form synaptic complexes that are essential for synapse development.19, 20, 21 The neurexin-3-LRRTMS complexes induce glutamatergic synapse development, while the neurexin-3-neuroligin complexes play a role in both glutamatergic and GABAergic synapse development.20,22 The specific mechanisms are discussed in the next section.
Neurexin-3 and leucine-rich-repeat transmembrane neuronal proteins
LRRTM is a synapse-organizing protein that is mainly located in the postsynaptic membrane.23,24 LRRTM genes are exclusively expressed in vertebrates. Four genes that encode LRRTMs have been identified in the human genome, namely Lrrtm1, Lrrtm2, Lrrtm3, and Lrrtm4.25 LRRTM is also a type I membrane protein, including an extracellular sequence, a single TMR and a relatively short cytoplasmic tail. The tail binds to postsynaptic signaling and scaffolding proteins.20,26 LRRTMs play an important role in synaptic development.20 LRRTMs promote the development of excitatory synapses without affecting inhibitory synapses.27,28 LRRTMs stabilize and increase the levels of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) expressed in the postsynaptic membrane, which are related to LTP.29, 30, 31 In addition, LRRTMs are associated with a variety of neuropsychiatric diseases, such as bipolar disorder and schizophrenia.32,33
LRRTM1, LRRTM2 and LRRTM3 induce presynaptic development by binding to neurexin-3 20,34. LRRTMs promote the presynaptic differentiation of glutamatergic neurons, but GABAergic neurons are not affected.20 When all neurexins are knocked out (KO), LRRTMs do not function properly, indicating that neurexins mediate the function of LRRTMs.20 In the hippocampus of mice lacking LRRTM1, the distribution of vesicular glutamate transporters is increased.27 However, the number of synapses is not affected, which may be related to the increased expression of LRRTM2, suggesting that LRRTM1 and LRRTM2 bind to the same neurexin, such as neurexin-3, to perform some similar functions.27,28 In addition, they have the same ability to induce synapse development.27 Although triple knockdown (KD) of neurexin-3 and both LRRTMs in neurexin-1 KO mice yield substantial reductions in excitatory synaptic transmission when synapses are forming,35 LRRTM1 and LRRTM2 KD alone or in combination do not reduce the number of excitatory synapses.36 In another study, overexpression of neuroligin1 or LRRTM2 resulted in strong synaptogenic activity during neuronal maturation.37 This evidence suggests that LRRTMs and neuroligins perform some overlapping functions during the early development of excitatory synapses.
In a fibroblast-neuron coculture experiment, LRRTM2 containing a point mutation in the extracellular domain did not bind to neurexins and did not induce presynaptic differentiation, indicating that LRRTMs promote presynaptic differentiation mediated by neurexins.28 Meanwhile, LRRTM3 is reported to induce presynaptic recruitment.27 The number of excitatory synapses is decreased in mice lacking LRRTM3.31 As mentioned above, neurexin-3 and LRRTMs are involved in synapse development. However, more research is needed to understand the specific mechanisms.
Neurexin-3 and neuroligins
Neurexins and neuroligins are a pair of NCAMs that are very important for synapse development and functions.38 Different cellular and molecular mechanisms, including alternative splicing and calcium binding, regulate the neurexin-neuroligin interactions to promote differentiation, maturation, stability and plasticity of inhibitory and excitatory synapses.13 Neuroligin is also a type I membrane protein that is mainly located in the postsynaptic membrane. Neuroligin consists of a short cytoplasmic tail, a TMR and an extracellular sequence.39 Three genes that encode neuroligins have been detected in mice and rats, namely Nlgn1, Nlgn2 and Nlgn3, while five genes that encode neuroligins have been identified in the human genome, namely Nlgn1, Nlgn2, Nlgn3, Nlgn4X, and Nlgn4Y.40,41 Neuroligins are expressed in different sites of the central nervous system (CNS). Neuroligin-1 targets excitatory synapses, and neuroligin-2 targets inhibitory, cholinergic and dopaminergic synapses.42, 43, 44, 45 Neuroligin-3 is mainly localized at excitatory and inhibitory synapses,46 while neuroligin-4 is mainly localized at glycinergic synapses.47 In addition to the CNS, neuroligins are also expressed in the pancreas, pulmonary endothelial cells, colon and heart.48,49
Neuroligins function at excitatory and inhibitory synapses. Overexpressed neuroligins increase synaptic transmission and the synapse density in transfected neurons and the number of synapses in the spine.50,51 Overexpression of monomeric or dimerized neuroligin-1 increases the number of synapses in neurons, while overexpression of neuroligin-3 increases the synapse number of wild-type neurons.51 When neuroligin-3 is unable to form dimers with neuroligin-1 or neuroligin-2, neuroligin-3 is unable to promote synapse development, suggesting that neuroligin-1 and neuroligin-2 are very important for synaptic development.51 In addition to modulating the number of synapses, neuroligins lead to changes in excitatory and inhibitory receptors. Neuroligin-1 deletion reduces the excitatory synaptic response mediated by AMPARs, while inhibitory synapses are not affected.50,52 Compared with neuroligin-1, neuroligin-2 deletion reduces inhibitory synaptic responses instead of the responses of excitatory synapses.53,54
Neurexin-3 interact with neuroligin-1, neuroligin-2, neuroligin-3 and neuroligin-4X.34 In distinct classes of neurons, neurexin-3 performs different functions rather than a classical function in all neurons. Two dramatic phenotypes caused by conditional deletion of neurexin-3 are observed in the hippocampal CA1 region. First, the excitatory responses mediated by AMPARs are decreased (∼40%) due to a loss of postsynaptic AMPARs. Meanwhile, LTP mediated by postsynaptic NMDARs is blocked completely. However, in the olfactory bulb, conditional deletion of neurexin-3 caused a completely distinct phenotype: the inhibitory responses mediated by γ-aminobutyric acid receptors (GABARs) are decreased (∼60%).55,56 In these processes, neuroligins mediate the function of neurexin-3. Neurexin-3 actively signals through neuroligins to promote postsynaptic differentiation, including the aggregation of excitatory and inhibitory receptors.19,22,57 These two signaling pathways underscore the role of neurexin-3 in synapse development.
The neurexin-3-neuroligin-1-AMPARs interaction
Glutamatergic neurons are some of the most important excitatory neurons and play a crucial role in the regulation of the CNS. Glutamate receptors include three types of ionic and metabolic receptors. AMPARs are ionic receptors with a substantial regulatory effect on synaptic plasticity. AMPARs that are widely distributed in the CNS are primarily located in the postsynaptic membrane and play an important role in the transmission of postsynaptic excitatory currents. AMPARs bind to glutamate released from the presynaptic membrane and then change its configuration to activate the ion channel. Four genes encoding four subunits have been identified for AMPARs, and these subunits form ion channels with distinct functions by combining in different stoichiometries.58 The expression of these subunits is highly regulated and is specific to different brain regions.59 The transport of AMPARs includes insertion, diffusion, immobilization, endocytosis, and cycling or degradation.60 AMPARs endocytosis is one of the biological bases of LTD.61 Endocytosis of AMPARs is characterized by a decrease in the number and density of AMPARs in the membrane, which may lead to LTD, memory loss and motor learning deficits.62,63 AMPARs endocytosis is associated with the progression of several diseases, such as Alzheimer's disease (AD). Inhibition of AMPARs endocytosis slows the speed of forgetting in animal models of AD and strengthens memory in normal animals.6 Endocytosis of AMPARs and synaptic dysfunction may be common pathophysiological processes in many diseases characterized by cognitive impairment, including AD.6,64
All α- and β-neurexins have selective splice site 4 (SS4), which includes or excludes highly conserved 90 bp exons that generate SS4- (exon-included isoforms) and SS4+ (exon-excluded isoforms) variants after splicing.7 Neurexin-3β (SS4+) interacts with neuroligin-1 to regulate excitatory synapses.20,65 Neuroligin-1 overexpression increases excitatory postsynaptic currents mediated by N-methyl-d-aspartic acid receptors (NMDARs) and AMPARs.50 Neuroligin-1 recruits postsynaptic density-95 (PSD-95) and binds to the third PDZ domain of PSD-95, which is an important component of the postsynaptic density.66 PSD-95 contains three PDZ domains, one SH3 domain and one guanylate kinase (GK) domain, each with its own function.67 PSD-95 binds to AMPARs through stargazin/TARPs, in addition to recruiting other postsynaptic components.4,67,68 PSD-95 also binds to guanylate kinase-associated protein (GKAP), which is an adaptor protein that then binds to SHANKs. In cultured neurons, the expression of dominant-negative neuroligin-1, which lacks the last four necessary amino acids of the C-terminal to bind to PSD-95, significantly decreases the number of postsynaptic components, including PSD-95 and AMPARs.12,57
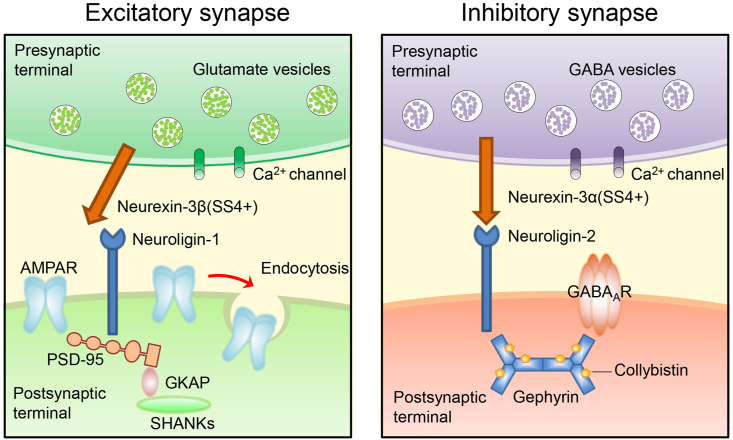
Compared with neurexin-1β (SS4+) and neurexin-2β (SS4+), neurexin-3β (SS4+) plays a completely different role in the regulation of excitatory synapses. Neurexin-3β (SS4+) prevents neuroligin-1 pairing, decreases the number of postsynaptic AMPARs and increases AMPARs endocytosis in mouse hippocampal synapses.13,69 These changes caused by neurexin-3β (SS4+) are reversed by removing SS4 (Fig. 3).56
Figure 3.
Two signaling pathways reported based on synaptic junctions formed by neurexin-3 that alter synaptic function. To date, the complete signaling pathway responsible for the change in synaptic function in response to the expression of neurexin-3 has not been fully explained; however, the association of neurexin-3 with AMPARs and GABAARs signaling pathways has been observed. At excitatory synapses, by blocking neuroligin-1 pairing, neurexin-3β (SS4+) prevents postsynaptic density-95 (PSD-95) from recruiting specific postsynaptic proteins that are present in glutamate synapses, including AMPARs. PSD-95 binds to guanylate kinase-associated protein (GKAP), which is an adaptor protein, and GKAP binds to SHANKs. Moreover, neurexin-3β (SS4+) promotes the endocytosis of AMPARs in the postsynaptic membrane. At inhibitory synapses, neurexin-3α (SS4+) interacts with neuroligin-2 and specifically activates collybistin. Neuroligin-2 and activated collybistin are involved in the recruitment of gephyrin, which then recruits GABAARs to the postsynaptic membrane. In addition, neurexin-3α located in the presynaptic membrane acts directly on postsynaptic GABAARs.
The neurexin-3-neuroligin-2-GABAA receptors interaction
GABAergic neurons are some of the most important inhibitory neurons and play important roles in the CNS. GABARs mainly consist of three types: GABAA receptors (GABAARs), GABAB receptors (GABABRs) and GABAC receptors (GABACRs).70 GABARs located in the postsynaptic membrane interact with GABA released from the presynaptic membrane to cause changes in the configuration of GABARs and activate ion channels. The activated ion channels facilitate the hyperpolarization of postsynaptic neurons, which blocks the conduction of nerve impulses. Different types of GABARs have been discovered in different brain regions, but all types exert inhibitory effects on learning and memory through different mechanisms. Antagonists of GABARs ameliorate the inhibition of learning and memory.
Among all types of GABARs, neurexin-3 significantly affects GABAARs. GABAARs distributed at synaptic and extrasynaptic sites are ligand-gated ion channel receptors that mediate phasic and tonic inhibition.71 GABAARs located at synapses directly control the efficiency of GABA transmission and inhibit the conduction of nerve impulses. GABAARs not only inhibit learning and memory but also participate in brain development.72 Studies have shown that a variety of neuropsychiatric disorders are associated with changes in GABAARs. For instance, abnormal GABAARs are detected in the brain tissues of patients with schizophrenia.73 GABAARs blockade enhances hippocampal synaptic plasticity in mice with AD.74
Neurexin-3α (SS4+) promotes postsynaptic differentiation at inhibitory synapses by binding to neuroligin-2.12,75 Neuroligin-2 participates in recruiting postsynaptic membrane scaffold proteins, including collybistin and gephyrin. Neuroligin-2 specifically activates collybistin.46 Activated collybistin is also involved in the recruitment of gephyrin.76, 77, 78 Neuroligin-2 binds to gephyrin through its cytoplasmic motif.46 Gephyrin is an important component of GABAergic synapses and recruits GABAARs.79, 80, 81, 82 Inhibitory neurotransmitter receptors, including GABAARs, are then recruited to the postsynaptic membrane through the action of the complex that comprises neuroligin-2, gephyrin and collybistin (Fig. 3). Animal experiments have revealed that a decrease in the level of neuroligin-2 in mice causes a loss of postsynaptic specificity of inhibitory synapses.43 In Purkinje cell-specific neuroligin-2 KO mice, the inhibitory postsynaptic current (IPSC) amplitude and the frequency of miniature IPSCs (mIPSCs) are decreased.54 In transgenic mice overexpressing neuroligin-2, GABAARs expression in the frontal cortex is increased, the frequency of mIPSCs is increased, the excitatory inhibition rate is decreased, and anxiety and social phobia are observed.53 In addition, neurexin-3 located in the presynaptic membrane acts directly on the GABAARs located in the postsynaptic membrane and may influence the excitatory/inhibitory synaptic balance.83 Meanwhile, ectopically expressed neurexin-3α at excitatory synapses interacts with the GABARs complex and recruit GABAARs to the postsynaptic membrane.84 In general, molecules and mechanisms that are involved in regulating GABAARs in the CNS may be far more complex than previously known.
Neurexin-3 and synapse functions
Neurexin-3 plays a critical role in regulating synaptic properties. In addition to binding to LRRTMs and NLGNs, neurexin-3 interacts with CASK, cerebellin (Cbln), SHANK2 and Discs-large4 (DLG4).34 The levels of these proteins increase to varying degrees in the early human cerebral cortex.8 CASK is mainly located in the presynaptic terminal and is considered a multidomain scaffold protein.85 Within the presynaptic terminal, CASK binds to neurexin-3β to promote the accumulation of vesicles. In addition, it interacts with VELIs and MINT1 to form the basic framework of the synaptic vesicle fusion mechanism.7,86, 87, 88 Cblns are a family of secreted glycoproteins that are located at excitatory and inhibitory synapses. Cblns simultaneously bind to presynaptic neurexin-3 and postsynaptic ionotropic glutamate delta receptors (GluD) to mediate synapse function.89 At hippocampal synapses, by activating postsynaptic GluD1, the presynaptic neurexin-3 (SS4+)-Cbln2 complex regulates the activity of AMPARs, but the number of AMPARs is normal.90 SHANK2 is a scaffold protein that is required for synapse formation and function. In humans, some neuropsychiatric and neurodevelopmental diseases, including autism spectrum disorders (ASD) and schizophrenia, are mediated by different SHANK2 variants.91 DLG4 (also known as PSD-95) is a scaffold protein that plays a role in the assembly of large signal transduction networks at specific sites within the cell.92 By interacting directly with neuroligin-1 and indirectly with PSD-95, neurexin-3 regulates postsynaptic AMPARs.55,67
In cultured neurons in vitro and in the hippocampus in vivo, conditional constitutive KOs (cKOs) of neurexin-1β, neurexin-2β and neurexin-3β reduce the release of transmitters at excitatory synapses. A major synaptic phenotype caused by the deletion of β-neurexins is observed; however, survival is normal.93 All three α-neurexins are also important for neurotransmitter release. Deletion of all three α-neurexins interferes with synaptic Ca2+ channel function, which leads to a decrease in Ca2+-triggered neurotransmitter release, but the number of Ca2+ channels remain normal.94 Neurexin-3 binds to the extracellular junction proteins C1ql2 and C1ql3 and then forms a transsynaptic complex with postsynaptic kainate receptors (Gluk2 and Gluk4) at the synapse of hippocampal mossy fibers.95 In addition to regulating the expression of postsynaptic receptors, neurexin-3 affects presynaptic receptors. Deletion of neurexin-1, neurexin-2 and neurexin-3 leads to the loss of presynaptic GABABRs at calyx synapses.96 Sufficient evidence suggests the important role of neurexin-3 in synaptic signaling.
The correlation of neurexin-3 with neurodegenerative and neuropsychiatric diseases
Neurexin-3 and AD
AD is one of the most common neurodegenerative diseases and is characterized clinically by progressive cognitive dysfunction and behavioral impairment.97 The presence of amyloid plaques, tau tangles and neuronal loss are the main pathological features of AD.98 Amyloid β (Aβ), which is considered to induce the pathogenesis of AD, is a marker protein of AD that causes synaptic dysfunction, neuroinflammation and neurodegeneration.98,99 Studies have found that synaptic dysfunction often occurs in the first stage of AD and affects multiple brain regions.100, 101, 102, 103 The levels of various proteins are decreased in the frontal cortex of patients with AD.104,105
Neurexin-3 may have a critical role in the early stage of AD. The expression levels of neurexin-3α and neurexin-3β are not significantly altered in cerebrospinal fluid (CSF) from patients with AD but are increased in patients with mild cognitive impairment (MCI).106, 107, 108 Neuroligin-1 and PSD-95 levels are decreased in the hippocampus of patients with AD and MCI, but soluble Aβ levels are increased. Importantly, a reduced level of neuroligin-1 is associated with an elevated level of soluble Aβ and disease progression.109 Aβ deposition inhibits the expression of neuroligin-1 and impairs synaptic function and memory.110 Therefore, the neurexin-3β-neuroligin-1-AMPARs pathway may lead to synaptic dysfunction to promote the development of AD by blocking the recruitment of AMPARs and increasing the endocytosis of AMPARs.
The neurexin-3α-neuroligin-2-GABAARs pathway may be an additional mechanism involved in the pathogenesis of AD. Elevated levels of neurexin-3α and neuroligin-2 have been detected in the CSF of individuals in the preclinical stage of AD,108 which may further lead to the upregulation of GABAARs. The increase in GABAARs affects information transmission at inhibitory synapses, synaptic plasticity, learning and memory and promotes the progression of AD.
Neurexin-3 may be associated with susceptibility to AD in males, but the mechanism is unclear.111 Neurexin-3β is cleaved into an N-terminal extracellular domain and a C-terminal transmembrane fragment (CTF) by α-secretase. The CTF is processed into a smaller intracellular domain (ICD) by γ-secretase. Presenilin1 (PS1) is the catalytic subunit of γ-secretase, and mutations in PS1 will cause γ-secretase inactivation, which lead to an increase in CTF levels and a decrease in ICD levels.112 The CTF is similar to amyloid precursor protein (APP), which is cleaved to promote the deposition of Aβ. Therefore, researchers have speculated that neurexin-3 may participate in the pathological process of AD through proteolytic processing to generate the CTF in subjects with a PS1 mutation.113
Neurexin-3 and addictive behaviors
Addiction is a public health problem of great concern that is related to the euphoria and rewards caused by substance abuse.114 In fact, addiction is a complex disease affected by heredity and the environment.115 Many proteins, including neurexin-3, are associated with addictive behaviors, such as opioid abuse, nicotine dependence and alcohol dependence.116, 117, 118 Neurexin-3 is expressed in brain regions related to addictive behaviors, such as the striatum and nucleus accumbens.119
Nrxn3 has been considered a potential candidate gene for drug addiction.120 In European and African American samples, 38 loci containing single nucleotide polymorphisms (SNPs) were strongly associated with amount of opioid abuse, including Nrxn3.116 An animal experiment found that neurexin-3 expression is increased in the globus pallidus of short-term cocaine-addicted mice.121
Nrxn3 is a potential factor related to nicotine dependence. A Nrrxn3 SNP (rs1004212) is associated with the degree of smoking in patients with schizophrenia.117 Meanwhile, homozygous individuals with the C allele of rs1004212 smoked more often every day than heterozygous individuals.117 Six SNPs in Nrxn3 are associated with the maintenance of smoking.122 Heterozygosity in the C allele of rs1004212 or the G allele of rs11624704 are associated with nicotine use disorder in Turkish individuals.123 However, some Nrxn3 SNPs exert the opposite effect. A 16 KB block of Nrxn3 that includes two SNPs (rs221473 and rs221497) is related to a lower risk of smoking among Spanish Caucasians, suggesting that different SNPs may lead to completely different effects.124
Ethanol not only affects ion channels and neurotransmitter receptors but also changes synaptic adhesion.125 Sufficient evidence suggests that neurexin-3 is associated with alcohol dependence.118 The SNP (rs8019381) at the SS5 exon 23 donor sites of Nrxn3 has been shown to be associated with alcohol dependence.126 Compared with homozygous individuals with the addiction-related rs8019381 SNP, heterozygous individuals express lower levels of two splice variants that encode transmembrane neurexin-3 isoforms in the cerebral cortex,126 suggesting that alcohol dependence may be associated with alternative splicing of exon 23 and changes in synaptic connections. In men, the SNP rs1004212 is also associated with alcohol problems, but women are not affected, indicating that the association between alcohol use problems and Nrxn3 SNPs may be related to sex.127
Neurexin-3 and autism
Autism is a neurodevelopmental disorder, and impaired social interaction, limited interests, and restricted and repetitive behaviors are all typical features of autism. The disease starts in early childhood, and most of the pediatric patients exhibit varying degrees of mental retardation.128 The prevalence of autism is approximately 15–20 per 10,000 and four times lower in women than in men.129 The pathogenesis of autism is associated with genetic factors, but the specific mechanism of autism remains unclear.
Rare genetic or neonatal microdeletions at 14q24.3–31.1, which overlap exons of the α and/or β subtypes of neurexin-3, have been identified in some Canadian patients diagnosed with autism. A clinical study reported that the deletions of neurexin-3 are present in carrier parents who have not been formally diagnosed with autism and a father confirmed to have subclinical autism.130 In addition, a rare exonic microdeletion of Nrxn3 was detected in a three-generation Chinese family using a chromosomal microarray analysis (CMA). The proband has a normal karyotype, but the microdeletion that contains an exon of Nrxn3 at 14q24.3–q31.1 was identified using CMA. Both the mother and the maternal grandfather possess the same microdeletion. Based on clinical observations of the clinical symptoms of members of this family, facial dysmorphism and schizophrenia are potential new clinical characteristics of Nrxn3 haploinsufficiency.131
Other diseases
Accumulating clinical studies have shown that neurexin-3 is associated with other neurological disorders. Neurexin-3 is related to schizophrenia in the Chinese Han population; among seven SNPs, three (rs7157669, rs724373 and rs7154021) are associated with schizophrenia.132 Anti-neurexin-3 antibody may be related to potential autoimmune encephalitis associated with yellow fever vaccine.133 Proteomic analysis revealed a decrease in neurexin-3 levels in the CSF of patients with major depressive disorder.134 Delayed encephalopathy after acute carbon monoxide poisoning is associated with two SNPs (rs2196447 and rs11845632) located in Nrxn3.135 Some Nrxn3 SNPs may be associated with borderline personality disorder phenotypes. Three SNPs (rs10144398, rs10151731, and rs10083466) are associated with identity disturbance, emotional instability and positive screening.136 The Nrxn3 rearrangement may be related to cluster headache (CH) in some cases.137
Accumulating evidence from animal models also suggests that neurexin-3 may be involved in the various pathophysiological mechanisms. In an experimental autoimmune encephalopathy (EAE) mouse model that represents multiple sclerosis, an increase in SS4 exon inclusion in Nrxn3 was detected in the mouse prefrontal cortex, consistent with IL-1β expression, indicating that neuroinflammation may regulate the alternative splicing of Nrxn3.138 Circular RNA-0001367 (circ_0001367) is a differentially expressed circRNA that indirectly regulates the expression and function of neurexin-3 through microRNA-431 (miR-431) to prevent the development of glioma, suggesting that neurexin-3 may be involved in the pathogenesis of glioma.139 Neurexin-3 may be correlated with empathy-related fear. Selective loss of neurexin-3 in somatostatin-expressing (SST+) interneurons in the anterior cingulate cortex (ACC) damages inhibitory synaptic transmission in SST + neurons and significantly enhances observational fear.140 The neurexin-3α (SS4+) mRNA level in rat hippocampal CA1 neurons is increased following transient global ischemia.141
Conclusions and future perspectives
While the structure of neurexin-3 and its roles in synapse development and functions are well established, the key molecular events involved in neurodegenerative and neuropsychiatric diseases remain unclear. Further studies are needed to determine the roles of neurexin-3 in various diseases and to understand the specific mechanisms, with the exception of some Nrxn3 SNPs. In particular, we must understand how the neurexin-3β (SS4+)-neuroligin-1-AMPARs and neurexin-3α (SS4+)-neuroligin-2-GABAARs pathways dictate or coordinate the function of excitatory and inhibitory synapses in the CNS, respectively. This information may provide opportunities to develop new interventions for AD, autism and other related diseases.
Conflict of interests
The authors have no conflict of interests to declare.
Funding
This work was supported by the Science and Technology Innovation Project of Chongqing Education Commission (China) (No. KJCXZD2020021).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
YuanJie Liu, Email: liuyuanjie@cqmu.edu.cn.
GuiQiong He, Email: guiqionghe@cqmu.edu.cn.
References
- 1.Südhof T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455(7215):903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poo M.M., Pignatelli M., Ryan T.J., et al. What is memory? The present state of the engram. BMC Biol. 2016;14:40. doi: 10.1186/s12915-016-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peineau S., Rabiant K., Pierrefiche O., Potier B. Synaptic plasticity modulation by circulating peptides and metaplasticity: involvement in Alzheimer's disease. Pharmacol Res. 2018;130:385–401. doi: 10.1016/j.phrs.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Chih B., Engelman H., Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307(5713):1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 5.Missaire M., Hindges R. The role of cell adhesion molecules in visual circuit formation: from neurite outgrowth to maps and synaptic specificity. Dev Neurobiol. 2015;75(6):569–583. doi: 10.1002/dneu.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Z., Han H., Li H., et al. Long-term potentiation decay and memory loss are mediated by AMPAR endocytosis. J Clin Invest. 2015;125(1):234–247. doi: 10.1172/JCI77888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabuchi K., Südhof T.C. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79(6):849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- 8.Harkin L.F., Lindsay S.J., Xu Y., et al. Neurexins 1-3 each have a distinct pattern of expression in the early developing human cerebral cortex. Cerebr Cortex. 2017;27(1):216–232. doi: 10.1093/cercor/bhw394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ushkaryov Y.A., Petrenko A.G., Geppert M., Südhof T.C. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257(5066):50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- 10.Occhi G., Rampazzo A., Beffagna G., Antonio Danieli G. Identification and characterization of heart-specific splicing of human neurexin 3 mRNA (NRXN3) Biochem Biophys Res Commun. 2002;298(1):151–155. doi: 10.1016/s0006-291x(02)02403-8. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich B., Ushkaryov Y.A., Südhof T.C. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 1995;14(3):497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 12.Bang M.L., Owczarek S. A matter of balance: role of neurexin and neuroligin at the synapse. Neurochem Res. 2013;38(6):1174–1189. doi: 10.1007/s11064-013-1029-9. [DOI] [PubMed] [Google Scholar]
- 13.Sindi I.A., Tannenberg R.K., Dodd P.R. Role for the neurexin-neuroligin complex in Alzheimer's disease. Neurobiol Aging. 2014;35(4):746–756. doi: 10.1016/j.neurobiolaging.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Ushkaryov Y.A., Südhof T.C. Neurexin III alpha: extensive alternative splicing generates membrane-bound and soluble forms. Proc Natl Acad Sci U S A. 1993;90(14):6410–6414. doi: 10.1073/pnas.90.14.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sons M.S., Busche N., Strenzke N., et al. alpha-Neurexins are required for efficient transmitter release and synaptic homeostasis at the mouse neuromuscular junction. Neuroscience. 2006;138(2):433–446. doi: 10.1016/j.neuroscience.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Bartels M.F., Winterhalter P.R., Yu J., et al. Protein O-mannosylation in the murine brain: occurrence of mono-O-mannosyl glycans and identification of new substrates. PLoS One. 2016;11(11):e0166119. doi: 10.1371/journal.pone.0166119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey H., Bourahmoune K., Honda T., et al. Genetic interaction of DISC1 and Neurexin in the development of fruit fly glutamatergic synapses. NPJ Schizophr. 2017;3(1):39. doi: 10.1038/s41537-017-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee S., Riordan M. Coordinated regulation of axonal microtubule organization and transport by Drosophila neurexin and BMP pathway. Sci Rep. 2018;8(1):17337. doi: 10.1038/s41598-018-35618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig A.M., Kang Y. Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol. 2007;17(1):43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roppongi R.T., Karimi B., Siddiqui T.J. Role of LRRTMs in synapse development and plasticity. Neurosci Res. 2017;116:18–28. doi: 10.1016/j.neures.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Gomez A.M., Traunmuller L., Scheiffele P. Neurexins: molecular codes for shaping neuronal synapses. Nat Rev Neurosci. 2021;22(3):137–151. doi: 10.1038/s41583-020-00415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graf E.R., Zhang X., Jin S.X., Linhoff M.W., Craig A.M. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119(7):1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karki S., Maksimainen M.M., Lehtiö L., Kajander T. Inhibitor screening assay for neurexin-LRRTM adhesion protein interaction involved in synaptic maintenance and neurological disorders. Anal Biochem. 2019;587:113463. doi: 10.1016/j.ab.2019.113463. [DOI] [PubMed] [Google Scholar]
- 24.Liouta K., Chabbert J., Benquet S., et al. Role of regulatory C-terminal motifs in synaptic confinement of LRRTM2. Biol Cell. 2021;113(12):492–506. doi: 10.1111/boc.202100026. [DOI] [PubMed] [Google Scholar]
- 25.Ko J., Fuccillo M.V., Malenka R.C., Südhof T.C. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009;64(6):791–798. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minatohara K., Murata Y., Fujiyoshi Y., Doi T. An intracellular domain with a novel sequence regulates cell surface expression and synaptic clustering of leucine-rich repeat transmembrane proteins in hippocampal neurons. J Neurochem. 2015;134(4):618–628. doi: 10.1111/jnc.13159. [DOI] [PubMed] [Google Scholar]
- 27.Linhoff M.W., Laurén J., Cassidy R.M., et al. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61(5):734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiqui T.J., Pancaroglu R., Kang Y., Rooyakkers A., Craig A.M. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J Neurosci. 2010;30(22):7495–7506. doi: 10.1523/JNEUROSCI.0470-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siddiqui T.J., Tari P.K., Connor S.A., et al. An LRRTM4-HSPG complex mediates excitatory synapse development on dentate gyrus granule cells. Neuron. 2013;79(4):680–695. doi: 10.1016/j.neuron.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Soler-Llavina G.J., Arstikaitis P., Morishita W., Ahmad M., Südhof T.C., Malenka R.C. Leucine-rich repeat transmembrane proteins are essential for maintenance of long-term potentiation. Neuron. 2013;79(3):439–446. doi: 10.1016/j.neuron.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Um J.W., Choi T.Y., Kang H., et al. LRRTM3 regulates excitatory synapse development through alternative splicing and neurexin binding. Cell Rep. 2016;14(4):808–822. doi: 10.1016/j.celrep.2015.12.081. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig K.U., Mattheisen M., Mühleisen T.W., et al. Supporting evidence for LRRTM1 imprinting effects in schizophrenia. Mol Psychiatr. 2009;14(8):743–745. doi: 10.1038/mp.2009.28. [DOI] [PubMed] [Google Scholar]
- 33.Malhotra D., McCarthy S., Michaelson J.J., et al. High frequencies of de novo CNVs in bipolar disorder and schizophrenia. Neuron. 2011;72(6):951–963. doi: 10.1016/j.neuron.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuttler K., Hassan M., Carr J., Cloete R., Bardien S. Emerging evidence implicating a role for neurexins in neurodegenerative and neuropsychiatric disorders. Open Biol. 2021;11(10):210091. doi: 10.1098/rsob.210091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soler-Llavina G.J., Fuccillo M.V., Ko J., Südhof T.C., Malenka R.C. The neurexin ligands, neuroligins and leucine-rich repeat transmembrane proteins, perform convergent and divergent synaptic functions in vivo. Proc Natl Acad Sci U S A. 2011;108(40):16502–16509. doi: 10.1073/pnas.1114028108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko J., Soler-Llavina G.J., Fuccillo M.V., Malenka R.C., Südhof T.C. Neuroligins/LRRTMs prevent activity- and Ca2+/calmodulin-dependent synapse elimination in cultured neurons. J Cell Biol. 2011;194(2):323–334. doi: 10.1083/jcb.201101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dagar S., Gottmann K. Differential properties of the synaptogenic activities of the neurexin ligands Neuroligin1 and LRRTM2. Front Mol Neurosci. 2019;12:269. doi: 10.3389/fnmol.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Südhof T.C. Synaptic neurexin complexes: a molecular code for the logic of neural circuits. Cell. 2017;171(4):745–769. doi: 10.1016/j.cell.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen T., Südhof T.C. Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J Biol Chem. 1997;272(41):26032–26039. doi: 10.1074/jbc.272.41.26032. [DOI] [PubMed] [Google Scholar]
- 40.Ichtchenko K., Nguyen T., Südhof T.C. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271(5):2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- 41.Ylisaukko-oja T., Rehnström K., Auranen M., et al. Analysis of four neuroligin genes as candidates for autism. Eur J Hum Genet. 2005;13(12):1285–1292. doi: 10.1038/sj.ejhg.5201474. [DOI] [PubMed] [Google Scholar]
- 42.Song J.Y., Ichtchenko K., Südhof T.C., Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96(3):1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poulopoulos A., Aramuni G., Meyer G., et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63(5):628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 44.Takács V.T., Freund T.F., Nyiri G. Neuroligin 2 is expressed in synapses established by cholinergic cells in the mouse brain. PLoS One. 2013;8(9):e72450. doi: 10.1371/journal.pone.0072450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchigashima M., Ohtsuka T., Kobayashi K., Watanabe M. Dopamine synapse is a neuroligin-2-mediated contact between dopaminergic presynaptic and GABAergic postsynaptic structures. Proc Natl Acad Sci U S A. 2016;113(15):4206–4211. doi: 10.1073/pnas.1514074113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchigashima M., Cheung A., Futai K. Neuroligin-3: a circuit-specific synapse organizer that shapes normal function and autism spectrum disorder-associated dysfunction. Front Mol Neurosci. 2021;14:749164. doi: 10.3389/fnmol.2021.749164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoon M., Soykan T., Falkenburger B., et al. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc Natl Acad Sci U S A. 2011;108(7):3053–3058. doi: 10.1073/pnas.1006946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolliger M.F., Frei K., Winterhalter K.H., Gloor S.M. Identification of a novel neuroligin in humans which binds to PSD-95 and has a widespread expression. Biochem J. 2001;356(Pt 2):581–588. doi: 10.1042/0264-6021:3560581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varoqueaux F., Jamain S., Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83(9):449–456. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 50.Chubykin A.A., Atasoy D., Etherton M.R., et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54(6):919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chanda S., Hale W.D., Zhang B., Wernig M., Südhof T.C. Unique versus redundant functions of neuroligin genes in shaping excitatory and inhibitory synapse properties. J Neurosci. 2017;37(29):6816–6836. doi: 10.1523/JNEUROSCI.0125-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang M., Polepalli J., Chen L.Y., Zhang B., Südhof T.C., Malenka R.C. Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autonomous NMDA receptor-independent mechanism. Mol Psychiatr. 2017;22(3):375–383. doi: 10.1038/mp.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hines R.M., Wu L., Hines D.J., et al. Synaptic imbalance, stereotypies, and impaired social interactions in mice with altered neuroligin 2 expression. J Neurosci. 2008;28(24):6055–6067. doi: 10.1523/JNEUROSCI.0032-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang B., Chen L.Y., Liu X., et al. Neuroligins sculpt cerebellar Purkinje-cell circuits by differential control of distinct classes of synapses. Neuron. 2015;87(4):781–796. doi: 10.1016/j.neuron.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aoto J., Martinelli D.C., Malenka R.C., Tabuchi K., Südhof T.C. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell. 2013;154(1):75–88. doi: 10.1016/j.cell.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aoto J., Földy C., Ilcus S.M., Tabuchi K., Südhof T.C. Distinct circuit-dependent functions of presynaptic neurexin-3 at GABAergic and glutamatergic synapses. Nat Neurosci. 2015;18(7):997–1007. doi: 10.1038/nn.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nam C.I., Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102(17):6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hollmann M., Maron C., Heinemann S. N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron. 1994;13(6):1331–1343. doi: 10.1016/0896-6273(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 59.Shepherd J.D., Huganir R.L. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 60.Bassani S., Folci A., Zapata J., Passafaro M. AMPAR trafficking in synapse maturation and plasticity. Cell Mol Life Sci. 2013;70(23):4411–4430. doi: 10.1007/s00018-013-1309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashby M.C., De La Rue S.A., Ralph G.S., Uney J., Collingridge G.L., Henley J.M. Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J Neurosci. 2004;24(22):5172–5176. doi: 10.1523/JNEUROSCI.1042-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kakegawa W., Katoh A., Narumi S., et al. Optogenetic control of synaptic AMPA receptor endocytosis reveals roles of LTD in motor learning. Neuron. 2018;99(5):985–998. doi: 10.1016/j.neuron.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 63.Awasthi A., Ramachandran B., Ahmed S., et al. Synaptotagmin-3 drives AMPA receptor endocytosis, depression of synapse strength, and forgetting. Science. 2019;363(6422):eaav1483. doi: 10.1126/science.aav1483. [DOI] [PubMed] [Google Scholar]
- 64.Teravskis P.J., Covelo A., Miller E.C., et al. A53T mutant alpha-synuclein induces tau-dependent postsynaptic impairment independently of neurodegenerative changes. J Neurosci. 2018;38(45):9754–9767. doi: 10.1523/JNEUROSCI.0344-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boucard A.A., Chubykin A.A., Comoletti D., Taylor P., Südhof T.C. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48(2):229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 66.Irie M., Hata Y., Takeuchi M., et al. Binding of neuroligins to PSD-95. Science. 1997;277(5331):1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 67.Coley A.A., Gao W.J. PSD95: a synaptic protein implicated in schizophrenia or autism? Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;82:187–194. doi: 10.1016/j.pnpbp.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ichtchenko K., Hata Y., Nguyen T., et al. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81(3):435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 69.Dai J., Aoto J., Südhof T.C. Alternative splicing of presynaptic neurexins differentially controls postsynaptic NMDA and AMPA receptor responses. Neuron. 2019;102(5):993–1008. doi: 10.1016/j.neuron.2019.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bormann J. ‘The ABC’ of GABA receptors. Trends Pharmacol Sci. 2000;21(1):16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- 71.Wu X., Wu Z., Ning G., et al. γ-Aminobutyric acid type A (GABAA) receptor α subunits play a direct role in synaptic versus extrasynaptic targeting. J Biol Chem. 2012;287(33):27417–27430. doi: 10.1074/jbc.M112.360461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luscher B., Fuchs T., Kilpatrick C.L. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70(3):385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidt M.J., Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190–206. doi: 10.1038/npp.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kadoyama K., Matsuura K., Takano M., et al. Proteomic analysis involved with synaptic plasticity improvement by GABAA receptor blockade in hippocampus of a mouse model of Alzheimer's disease. Neurosci Res. 2021;165:61–68. doi: 10.1016/j.neures.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Ali H., Marth L., Krueger-Burg D. Neuroligin-2 as a central organizer of inhibitory synapses in health and disease. Sci Signal. 2020;13(663):eabd8379. doi: 10.1126/scisignal.abd8379. [DOI] [PubMed] [Google Scholar]
- 76.Kins S., Betz H., Kirsch J. Collybistin, a newly identified brain-specific GEF, induces submembrane clustering of gephyrin. Nat Neurosci. 2000;3(1):22–29. doi: 10.1038/71096. [DOI] [PubMed] [Google Scholar]
- 77.Harvey K., Duguid I.C., Alldred M.J., et al. The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J Neurosci. 2004;24(25):5816–5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kalscheuer V.M., Musante L., Fang C., et al. A balanced chromosomal translocation disrupting ARHGEF9 is associated with epilepsy, anxiety, aggression, and mental retardation. Hum Mutat. 2009;30(1):61–68. doi: 10.1002/humu.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prior P., Schmitt B., Grenningloh G., et al. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron. 1992;8(6):1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- 80.Essrich C., Lorez M., Benson J.A., Fritschy J.M., Lüscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1(7):563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 81.Feng G., Tintrup H., Kirsch J., et al. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282(5392):1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- 82.Moss S.J., Smart T.G. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2(4):240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- 83.Zhang C., Atasoy D., Araç D., et al. Neurexins physically and functionally interact with GABA(A) receptors. Neuron. 2010;66(3):403–416. doi: 10.1016/j.neuron.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miyazaki T., Morimoto-Tomita M., Berthoux C., et al. Excitatory and inhibitory receptors utilize distinct post- and trans-synaptic mechanisms in vivo. Elife. 2021;10:e59613. doi: 10.7554/eLife.59613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsueh Y.P. The role of the MAGUK protein CASK in neural development and synaptic function. Curr Med Chem. 2006;13(16):1915–1927. doi: 10.2174/092986706777585040. [DOI] [PubMed] [Google Scholar]
- 86.Hata Y., Butz S., Südhof T.C. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16(8):2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Butz S., Okamoto M., Südhof T.C. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94(6):773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- 88.Biederer T., Südhof T.C. Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J Biol Chem. 2000;275(51):39803–39806. doi: 10.1074/jbc.C000656200. [DOI] [PubMed] [Google Scholar]
- 89.Seigneur E., Wang J., Dai J., Polepalli J., Südhof T.C. Cerebellin-2 regulates a serotonergic dorsal raphe circuit that controls compulsive behaviors. Mol Psychiatr. 2021;26(12):7509–7521. doi: 10.1038/s41380-021-01187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai J., Patzke C., Liakath-Ali K., Seigneur E., Südhof T.C. GluD1 is a signal transduction device disguised as an ionotropic receptor. Nature. 2021;595(7866):261–265. doi: 10.1038/s41586-021-03661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monteiro P., Feng G. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci. 2017;18(3):147–157. doi: 10.1038/nrn.2016.183. [DOI] [PubMed] [Google Scholar]
- 92.Roberts S., Delury C., Marsh E. The PDZ protein discs-large (DLG): the ‘Jekyll and Hyde' of the epithelial polarity proteins. FEBS J. 2012;279(19):3549–3558. doi: 10.1111/j.1742-4658.2012.08729.x. [DOI] [PubMed] [Google Scholar]
- 93.Anderson G.R., Aoto J., Tabuchi K., et al. β-Neurexins control neural circuits by regulating synaptic endocannabinoid signaling. Cell. 2015;162(3):593–606. doi: 10.1016/j.cell.2015.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Missler M., Zhang W., Rohlmann A., et al. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423(6943):939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 95.Matsuda K., Budisantoso T., Mitakidis N., et al. Transsynaptic modulation of kainate receptor functions by C1q-like proteins. Neuron. 2016;90(4):752–767. doi: 10.1016/j.neuron.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 96.Luo F., Sclip A., Merrill S., Südhof T.C. Neurexins regulate presynaptic GABAB-receptors at central synapses. Nat Commun. 2021;12(1):2380. doi: 10.1038/s41467-021-22753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Celone K.A., Calhoun V.D., Dickerson B.C., et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu Y., Zhao M., Han Y., Zhang H. GABAergic inhibitory interneuron deficits in Alzheimer's disease: implications for treatment. Front Neurosci. 2020;14:660. doi: 10.3389/fnins.2020.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu J., Cho E., Kwon H., et al. Akt and calcium-permeable AMPA receptor are involved in the effect of pinoresinol on amyloid beta-induced synaptic plasticity and memory deficits. Biochem Pharmacol. 2021;184:114366. doi: 10.1016/j.bcp.2020.114366. [DOI] [PubMed] [Google Scholar]
- 100.Perdahl E., Adolfsson R., Alafuzoff I., et al. Synapsin I (protein I) in different brain regions in senile dementia of Alzheimer type and in multi-infarct dementia. J Neural Transm. 1984;60(2):133–141. doi: 10.1007/BF01245030. [DOI] [PubMed] [Google Scholar]
- 101.Honer W.G., Dickson D.W., Gleeson J., Davies P. Regional synaptic pathology in Alzheimer's disease. Neurobiol Aging. 1992;13(3):375–382. doi: 10.1016/0197-4580(92)90111-a. [DOI] [PubMed] [Google Scholar]
- 102.Bancher C., Braak H., Fischer P., Jellinger K.A. Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer's and Parkinson's disease patients. Neurosci Lett. 1993;162(1–2):179–182. doi: 10.1016/0304-3940(93)90590-h. [DOI] [PubMed] [Google Scholar]
- 103.Sze C.I., Troncoso J.C., Kawas C., Mouton P., Price D.L., Martin L.J. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(8):933–944. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 104.Lassmann H., Weiler R., Fischer P., et al. Synaptic pathology in Alzheimer's disease: immunological data for markers of synaptic and large dense-core vesicles. Neuroscience. 1992;46(1):1–8. doi: 10.1016/0306-4522(92)90003-k. [DOI] [PubMed] [Google Scholar]
- 105.Tannenberg R.K., Scott H.L., Tannenberg A.E., Dodd P.R. Selective loss of synaptic proteins in Alzheimer's disease: evidence for an increased severity with APOE varepsilon4. Neurochem Int. 2006;49(7):631–639. doi: 10.1016/j.neuint.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 106.Brinkmalm G., Sjödin S., Simonsen A.H., et al. A parallel reaction monitoring mass spectrometric method for analysis of potential CSF biomarkers for Alzheimer's disease. Proteonomics Clin Appl. 2018;12(1):e1700131. doi: 10.1002/prca.201700131. [DOI] [PubMed] [Google Scholar]
- 107.Duits F.H., Brinkmalm G., Teunissen C.E., et al. Synaptic proteins in CSF as potential novel biomarkers for prognosis in prodromal Alzheimer's disease. Alzheimer's Res Ther. 2018;10(1):5. doi: 10.1186/s13195-017-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lleó A., Núñez-Llaves R., Alcolea D., et al. Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer's disease cerebrospinal fluid. Mol Cell Proteomics. 2019;18(3):546–560. doi: 10.1074/mcp.RA118.001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dufort-Gervais J., Provost C., Charbonneau L., et al. Neuroligin-1 is altered in the hippocampus of Alzheimer's disease patients and mouse models, and modulates the toxicity of amyloid-beta oligomers. Sci Rep. 2020;10(1):6956. doi: 10.1038/s41598-020-63255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bie B., Wu J., Yang H., Xu J.J., Brown D.L., Naguib M. Epigenetic suppression of neuroligin 1 underlies amyloid-induced memory deficiency. Nat Neurosci. 2014;17(2):223–231. doi: 10.1038/nn.3618. [DOI] [PubMed] [Google Scholar]
- 111.Martinez-Mir A., González-Pérez A., Gayán J., et al. Genetic study of neurexin and neuroligin genes in Alzheimer's disease. J Alzheim Dis. 2013;35(2):403–412. doi: 10.3233/JAD-122257. [DOI] [PubMed] [Google Scholar]
- 112.Bot N., Schweizer C., Ben Halima S., Fraering P.C. Processing of the synaptic cell adhesion molecule neurexin-3beta by Alzheimer disease alpha- and gamma-secretases. J Biol Chem. 2011;286(4):2762–2773. doi: 10.1074/jbc.M110.142521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saura C.A., Servián-Morilla E., Scholl F.G. Presenilin/γ-secretase regulates neurexin processing at synapses. PLoS One. 2011;6(4):e19430. doi: 10.1371/journal.pone.0019430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nestler E.J. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 115.Liu Q.R., Drgon T., Johnson C., Walther D., Hess J., Uhl G.R. Addiction molecular genetics: 639,401 SNP whole genome association identifies many "cell adhesion" genes. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(8):918–925. doi: 10.1002/ajmg.b.30436. [DOI] [PubMed] [Google Scholar]
- 116.Liu Q.R., Drgon T., Walther D., et al. Pooled association genome scanning: validation and use to identify addiction vulnerability loci in two samples. Proc Natl Acad Sci U S A. 2005;102(33):11864–11869. doi: 10.1073/pnas.0500329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Novak G., Boukhadra J., Shaikh S.A., Kennedy J.L., Le Foll B. Association of a polymorphism in the NRXN3 gene with the degree of smoking in schizophrenia: a preliminary study. World J Biol Psychiatr. 2009;10(4 Pt 3):929–935. doi: 10.1080/15622970903079499. [DOI] [PubMed] [Google Scholar]
- 118.Sasabe T., Ishiura S. Alcoholism and alternative splicing of candidate genes. Int J Environ Res Publ Health. 2010;7(4):1448–1466. doi: 10.3390/ijerph7041448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lein E.S., Hawrylycz M.J., Ao N., et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 120.Lachman H.M., Fann C.S., Bartzis M., et al. Genomewide suggestive linkage of opioid dependence to chromosome 14q. Hum Mol Genet. 2007;16(11):1327–1334. doi: 10.1093/hmg/ddm081. [DOI] [PubMed] [Google Scholar]
- 121.Kelai S., Maussion G., Noble F., et al. Nrxn3 upregulation in the globus pallidus of mice developing cocaine addiction. Neuroreport. 2008;19(7):751–755. doi: 10.1097/WNR.0b013e3282fda231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wolock S.L., Yates A., Petrill S.A., et al. Gene x smoking interactions on human brain gene expression: finding common mechanisms in adolescents and adults. J Child Psychol Psychiatry. 2013;54(10):1109–1119. doi: 10.1111/jcpp.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Güleç G., Coşan D.T., Şahin F.M., et al. Association of nicotine use disorder with Neurexin 3 gene polymorphisms. Türk Psikiyatri Derg. 2021;32(3):160–166. doi: 10.5080/u25686. [DOI] [PubMed] [Google Scholar]
- 124.Docampo E., Ribasés M., Gratacòs M., et al. Association of neurexin 3 polymorphisms with smoking behavior. Gene Brain Behav. 2012;11(6):704–711. doi: 10.1111/j.1601-183X.2012.00815.x. [DOI] [PubMed] [Google Scholar]
- 125.Charness M.E., Safran R.M., Perides G. Ethanol inhibits neural cell-cell adhesion. J Biol Chem. 1994;269(12):9304–9309. [PubMed] [Google Scholar]
- 126.Hishimoto A., Liu Q.R., Drgon T., et al. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Hum Mol Genet. 2007;16(23):2880–2891. doi: 10.1093/hmg/ddm247. [DOI] [PubMed] [Google Scholar]
- 127.Stoltenberg S.F., Lehmann M.K., Christ C.C., Hersrud S.L., Davies G.E. Associations among types of impulsivity, substance use problems and neurexin-3 polymorphisms. Drug Alcohol Depend. 2011;119(3):e31–e38. doi: 10.1016/j.drugalcdep.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Autism Genome Project Consortium. Szatmari P., Paterson A.D., et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39(3):319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chakrabarti S., Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatr. 2005;162(6):1133–1141. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- 130.Vaags A.K., Lionel A.C., Sato D., et al. Rare deletions at the neurexin 3 locus in autism spectrum disorder. Am J Hum Genet. 2012;90(1):133–141. doi: 10.1016/j.ajhg.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yuan H., Wang Q., Liu Y., et al. A rare exonic NRXN3 deletion segregating with neurodevelopmental and neuropsychiatric conditions in a three-generation Chinese family. Am J Med Genet B Neuropsychiatr Genet. 2018;177(6):589–595. doi: 10.1002/ajmg.b.32673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu X., Zhang J., Jin C., et al. Association study of NRXN3 polymorphisms with schizophrenia and risperidone-induced bodyweight gain in Chinese Han population. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;43:197–202. doi: 10.1016/j.pnpbp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 133.Guedes B.F., Ribeiro A.F., Pinto L.F., et al. Potential autoimmune encephalitis following yellow fever vaccination: a report of three cases. J Neuroimmunol. 2021;355:577548. doi: 10.1016/j.jneuroim.2021.577548. [DOI] [PubMed] [Google Scholar]
- 134.Al Shweiki M.R., Oeckl P., Steinacker P., et al. Proteomic analysis reveals a biosignature of decreased synaptic protein in cerebrospinal fluid of major depressive disorder. Transl Psychiatry. 2020;10(1):144. doi: 10.1038/s41398-020-0825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li W., Zhang Y., Gu R., et al. DNA pooling base genome-wide association study identifies variants at NRXN3 associated with delayed encephalopathy after acute carbon monoxide poisoning. PLoS One. 2013;8(11):e79159. doi: 10.1371/journal.pone.0079159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Panagopoulos V.N., Trull T.J., Glowinski A.L., et al. Examining the association of NRXN3 SNPs with borderline personality disorder phenotypes in heroin dependent cases and socio-economically disadvantaged controls. Drug Alcohol Depend. 2013;128(3):187–193. doi: 10.1016/j.drugalcdep.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zarrilli F., Tomaiuolo R., Ceglia C., et al. Molecular analysis of cluster headache. Clin J Pain. 2015;31(1):52–57. doi: 10.1097/AJP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 138.Marchese E., Valentini M., Di Sante G., et al. Alternative splicing of neurexins 1-3 is modulated by neuroinflammation in the prefrontal cortex of a murine model of multiple sclerosis. Exp Neurol. 2021;335:113497. doi: 10.1016/j.expneurol.2020.113497. [DOI] [PubMed] [Google Scholar]
- 139.Liu L., Zhang P., Dong X., et al. Circ_0001367 inhibits glioma proliferation, migration and invasion by sponging miR-431 and thus regulating NRXN3. Cell Death Dis. 2021;12(6):536. doi: 10.1038/s41419-021-03834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keum S., Kim A., Shin J.J., Kim J.H., Park J., Shin H.S. A missense variant at the Nrxn3 locus enhances empathy fear in the mouse. Neuron. 2018;98(3):588–601. doi: 10.1016/j.neuron.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 141.Sun H.B., Yokota H., Chi X.X., Xu Z.C. Differential expression of neurexin mRNA in CA1 and CA3 hippocampal neurons in response to ischemic insult. Brain Res Mol Brain Res. 2000;84(1–2):146–149. doi: 10.1016/s0169-328x(00)00237-0. [DOI] [PubMed] [Google Scholar]