Abstract
We performed this review to determine the weighted prevalence of equids parasitic infections in Ethiopia. Article searches on parasites of equids were conducted using PubMed, PubMed Central, Google Scholar, Science Direct, Web of Sciences, Scopus, AJOLs and Research Gate. A random effects model was used to estimate the weighted prevalence and to study heterogeneity. The primary searches generated, 3082 potential studies, of which 66 reports met the inclusion criteria and were included in the meta-analysis. There were 32 reports involving two or more equids species, eighteen on donkeys, and sixteen on horses. Moreover, fifty of the reports mentioned helminth infections in equids, thirteen on protozoans, and three on ectoparasites. The estimated weighted prevalence of parasitic infections in equids was 58.3% (95% CI 50.8–65.4%, I2 = 99%). Helminths were the most prevalent parasites in equids, accounting for 77.1% (95% CI 71.4%, 82%, I2 = 98.5%), followed by ectoparasites at 35.4% (95% CI 33.4–37.52%, I2 = 88.2%) and haemoparasites (protozoans) at 10.84% (95% CI 6.6%, 17.3%, I2 = 98.1%). Furthermore, with a prevalence of 82.3% (95% CI 75.9–87.3%, I2 = 97.4%), donkeys were the most affected equids with helminth parasites. From the reports, we found forty-three species of helminth parasites affecting equids, including thirty-four nematode species (Strongylus, Cyathostomum, Coronocyclus, Cylicocyclus, Cylicostephanus, Trichostrongylus, Oesophagodontus, Strongyloides, Triodontophorus, Gyalocephalus, Poteriostomum, Dictyocaulus, Oxyuris, Habronema, Draschia, Parascaris, Setaria, and Probstmayria species), three trematodes (two Fasciola and one Gastrodiscus species), three cestodes (two Anoplocephala and one Anoplocephaloides species) and three botfly larvae (one Rhinoestrus and two Gasterophilus species). Trypanosoma species (T. congolense, T. vivax, T. brucei, T. equiperdum, and T. evansi), piroplasms (Theileria equi and Babesia caballi), and Eimeria species have also been reported to affect equids. Ticks (Amblyomma variegatum, A. gemma, Rhipicephalus decoloratus, R. evertisi evertisi, R. pulchellus, R. muhsame, R. sanguineus, Hyalomma rufipes, and Hy. truncatum) and lice (Bovicola equi and Haematopinus asini) were the ectoparasites recorded to affect equids. The risk of publication bias across studies was likely to be high due to differences in publication year and diagnostic techniques. In conclusion, parasitic infections of equids are common in Ethiopia and are caused by a variety of parasite species, putting the performance and well-being of these packing animals at risk. Therefore, more research is needed to identify infection risk factors and raise awareness of the consequences of parasitism in order to provide strategies to mitigate the problems in equids.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12639-023-01598-3.
Keywords: Ethiopia, Equids, Meta-analysis, Parasites, Prevalence, Systematic review
Introduction
Ethiopia has 10.80 million donkeys, 2.15 million horses, and 0.33 million mules, accounting for roughly half of Africa's equids population (CSA 2021). Equids are important in Ethiopia's agricultural economy and are used for a variety of tasks in both rural and impoverished urban communities. They play an important role in transportation (riding, packing, and cart pulling), farming (tillage and threshing), weddings and tourism, water and milling, and so on (Kumsa et al. 2012; Seyoum et al. 2015). Equids have received far less attention in terms of health management than other livestock species, despite their significant contribution to the country's transportation and agricultural economies (Seyoum et al. 2015). This could be due to communities' long-held belief that equids are hard and tolerant, as well as the fact that they do not provide meat or milk (Kumsa et al. 2012). In Ethiopia, the main factors affecting equids health and performance are a lack of feed, working stress, and diseases (of parasitic, bacterial, viral, and nutritional origin) (Sheferaw and Alemu 2013).
Parasitic diseases are major threats to the health, welfare, and performance of equids, particularly in developing countries (Khamesipour et al. 2021). Endoparasites (helminths, protozoans, and arthropod larvae) and ectoparasites (ticks, mites, lice, and flies) are the two types of equids parasites (Khamesipour et al. 2021). Helminths are the most serious and well-known parasites in equids (Getachew et al. 2010; Valdéz-Cruz et al. 2013; Khamesipour et al. 2021). Trichostrongylus spp., Strongylus spp., Cyathostomins, Trichuris spp., Oxyuris equi, Parascaris equorum, Probstmayria spp., Paramphistomum spp., Fasciola spp., Dicrocoelium spp., Anoplocephala spp., Anoplocephaloides spp., Dictyocaulus arnfieldi, Gastrodiscus spp., Habronema spp., Draschia spp., Gasterophilus spp., Strongyloides spp. and Triodontophorus spp. are the common helminth parasites affecting equids (Getachew et al. 2010; Sheferaw and Alemu 2015; Khamesipour et al. 2021). Helminth infection rates in equids from various parts of the world have been estimated to range from 11.2 to 100% (Fikru et al. 2005; Valdéz-Cruz et al. 2013; Getachew et al. 2010; Sheferaw and Alemu 2015). According to reports, helminths can infect over 90% of equids in a given area (Fikru et al. 2005; Getachew et al. 2010). Helminth parasite studies in equids have revealed a wide range of helminth species (Hosseini et al. 2009; Getachew et al. 2010). Almost all equids have helminth parasites, which can impair the animal's performance if left untreated. Helminth parasites primarily affect equids' digestive systems, but the respiratory system and other organs may also be affected (Khamesipour et al. 2021). Ticks and lice are the most common ectoparasites found in equids (Kumsa et al. 2012; Tafese et al. 2014). Protozoa that commonly infect equids include Eimeria species, Theileria equi, Babesia caballi, and Trypanosoma species (Hagos et al. 2010a, b; Khamesipour et al. 2021).
In many agro-ecological zones, parasitic infestations pose serious threats to equids populations (Valdéz-Cruz et al. 2013). They affect animal health and welfare, limiting their working performance (Fesseha et al. 2022). Parasitism in equids causes diarrhoea, anaemia, fever, colic, reduced feed intake, emaciation, poor growth rate, poor body condition and traction power, loss of electrolytes and plasma proteins, protein metabolism alterations, increased susceptibility to other infectious diseases, and death (Valdéz-Cruz et al. 2013; Khamesipour et al. 2021). All of these clinical effects result in significant economic losses for farmers. Understanding the status of parasitic infections and the factors that influence epidemiology at the national level is therefore essential and a basic step in improving the health and welfare of equids and developing, implementing, and monitoring an efficient and effective parasitic control strategy.
Several factors have been identified as having an impact on the host-parasite pathogen, epidemiology, biology, and life cycle of equids parasites. These include host factors (age, gender, species, body condition score, and immune status), parasite factors (life cycle, mode of transmission, virulence, and parasite genotype), environmental factors (climate conditions, and geographic factors), and anthropogenic factors (socioeconomic status, community awareness/health care education level, and management practises) (Nielsen et al. 2007). As a result, parasitic infection control measures must rely on strong evidence of the risk factors that contribute to host transmission dynamics. Several studies on equids parasite infections in Ethiopia have been conducted, with prevalence ranging from 1.06 to 100% (Fikru et al. 2005; Getachew et al. 2010; Hagos et al. 2010a, b; Mekibib et al. 2010; Mkuria et al. 2010; Gizachew et al. 2013; Kumsa et al. 2012; Tafese et al. 2014; Getachew et al. 2014; Berhanu et al. 2014; Seyoum et al. 2015; Sheferaw and Alemu 2015; Fesseha et al. 2022). According to these works, equids parasites are the most commonly encountered concerns of working equids. Despite the fact that the prevalence of parasitic infections in equids varies in Ethiopia, the reports are fragmented and not systematically organised to show the national level and to be used as reference data by policymakers and veterinarians to plan strategic control and preventive measures. Furthermore, to the best of our knowledge, no published report on meta-analysis of prevalence estimates and associated risk factors exists. As a result, there is a knowledge gap regarding the trend and prevalence of parasitic infections in equids, as well as the associated risk factors. Detail information about the prevalence and risk factors of equids parasites across the country, on the other hand, is critical for developing strategic control measures and monitoring the economic impacts in developing countries like Ethiopia. Thus, this systematic review and meta-analysis of existing reports was conducted to provide information on the prevalence and species composition of parasites affecting equids in Ethiopia in order to align control policies.
Methodology
Between September 1, 2022 and November 30, 2022, a literature search was conducted to identify articles reporting parasitic infections of equids in Ethiopia. The current study follows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines (Moher et al. 2015). To ensure that all relevant information was included in the analysis, the PRISMA checklist was used.
Literature search
A literature search was carried out to identify articles published between the year of inception and November 30, 2022. The search for papers was carried out by using electronic databases and search engines. We planned to collect all English-language papers on the prevalence of helminths, haemoparasites (protozoans), and ectoparasites in equids throughout Ethiopia. The papers were found in PubMed, PubMed Central, Google Scholar, Science Direct, AJOLs, Web of Sciences, Scopus and Research Gate. There was no time limit set for the search strategy. To identify relevant articles or papers, we used various medical subject heading (MeSH) terms and a variety of relevant keywords such as “Epidemiology”, “Prevalence”, “Endoparasite”, “Ectoparasite”, “Nematode”, “Helminths”, “Gastrointestinal parasite”, “Tick”, “Mite”, “Lice”, “Equids”, “Horse”, “Mule”, “Donkey” and “Ethiopia”. Additionally, studies were identified by checking cross-references in collected articles. We used the “OR” and “AND” Boolean operators to find the aforementioned terms.
Inclusion criteria
Articles reporting parasitic infections in equids were retrieved, regardless of the diagnostic method used to confirm parasitic infections (coprological, serological, morphological, or molecular methods). The inclusion criteria for the studies were developed based on the study's objectives. As a result, the retrieved papers were considered for systematic review and meta-analysis based on the following inclusion criteria: studies that were (1) conducted in Ethiopia and focused on equids ectoparasite, endoparasite, or both; (2) original research articles and theses; (3) published in English between 2000 and 2022; (4) cross-sectional; (5) reported the prevalence or epidemiology and risk factors of endoparasite and ectoparasite; and (6) their target study population included equids species (greater or equal to 30 animals). Papers that did not meet the above criteria were rejected.
Study selection and data extraction
Two independent reviewers identified articles reporting parasitic infections in equids in Ethiopia based on the title and abstract of the paper. It was carried out in accordance with the predefined inclusion criteria. Before conducting the screening assessment, duplicates were initially removed. As a result, studies were excluded if the titles and abstracts were unrelated to the outcomes of interest or did not meet the eligibility criteria. Following that, the full texts of eligible studies were thoroughly reviewed to ensure their suitability for the analysis. A third reviewer was consulted in the event of a disagreement between the two reviewers. The third reviewer also validated the records. Finally, data was extracted using a standardised data collection form. The following information was collected from each selected study: first author, year of publication, study year, geographic location and region of the study, study design, study population, sample size, sampling method, diagnostic technique, type of parasite identified, host involved, risk factor identified, and event rate, which was then saved in an Excel spreadsheet. Figure 1 depicts the study's search strategies and exclusion criteria in detail.
Fig. 1.
PRISMA flow chart describing the selection process (Moher et al. 2015)
Quality assessment study
Two independent researchers assessed the quality of the selected studies using a quality assessment checklist (standard strengthening the Reporting of Observational Studies in Epidemiology checklist (STROBE)) (von Elm et al. 2007). This checklist includes 22 items that comprise various sections of the articles, including the title, abstract, introduction, methods, results, and discussion. The checklist included items assessing the studies' objectives, various method components (e.g., study design, sample size, study population, bias, statistical methods), results, limitations, and funding.
Data synthesis and statistical analysis
Data were extracted and stored in an Excel spreadsheet before being exported and analysed in STATA version 17 (Stata Corp., College Station, TX, USA). The meta-analysis was carried out in accordance with the protocol described by DerSimonian and Laird (1986) in order to examine the pooled prevalence of parasitic infections in equids using the eligible searches. In brief, helminths, protozoans, and ectoparasites of horses were studied separately. For each study, the parasitic infection rate and its 95% confidence interval (CI) in equids were calculated. Using the formula provided by Barendregt et al. (2013), the study level event rate was transformed to logit event rate. For ease of interpretation, the logit event rates were back transformed to prevalence event rates (prevalence estimates). In pooled prevalence analysis and 95% confidence intervals (95% CIs), the random-effects model with the Der-Simonian-Laird method of selection was used (Moher et al. 2015). To present the differences between studies, meta-analysis results displaying estimates of prevalence, and their corresponding confidence intervals (CIs) of all included studies with the pooled effect size, a Forest plot diagram was used. To determine heterogeneity and consistency among studies, the inverse variance index (I2) and Cochran's Q test were calculated, and I2 values of 25, 50, and 75% were regarded as indicating low, medium, and high heterogeneity, respectively (Higgins and Thompson 2002). To confirm the heterogeneity of study level estimates, a Galbraith plot was also constructed. Small study effects and the presence of publication bias were then visualised using funnel plot diagrams, Egger's and Begg's tests (Borenstein et al. 2009). The studies were also classified geographically (north, east, south, central, west, northeast, northwest, southeast, and southwest), and the total prevalence was assessed in six different regions. Figures and tables were used to display the summarised and descriptive results.
Results
Study characteristics and search results
The authors conducted a systematic review and meta-analysis on published and unpublished (thesis) reports of parasitic infestations of equids in Ethiopia. The period of literature search began in September 2022 and ended in November 2022. We initially identified 3082 reports, 2738 of which were rejected due to title screening indicating irrelevance to the review. The reference manager software (EndNote-TM) and manual confirmation were used to identify duplicates among the remaining 344 papers. Following that, 82 articles were evaluated based on eligibility criteria. Although 69 of these studies were suitable for systematic review, only 66 met the quantitative and meta-analysis requirements (Fig. 1, S1Table). As a result, 47 helminth studies, 16 protozoans (haemoparasites), and 3 ectoparasite studies were considered for meta-analysis. All of these studies were carried out between 2002 and 2021, and they all used a cross-sectional study design with purposive, simple, systematic, and multistage sampling techniques. The identified studies were carried out in various geographical areas of Ethiopia. Seventeen of the studies were conducted in Ethiopia's central, five in the northern, thirteen in the north-western, ten in the southern, four in the south-western, one in the western, two in the eastern, five in the north-eastern, and nine in the south-eastern regions. Furthermore, depending on the parasite type, coprological, morphological, post-mortem, serological, and molecular laboratory techniques were used. Fourteen reports of parasitic infections on the three equid species (donkeys, horses, and mules) were noted; thirteen on both donkeys and horses; four on both donkeys and mules; nineteen on donkeys alone and sixteen on horses alone (S1Table). The included studies in the quantitative and meta-analysis had a total sample size of 28,025 equids (12,299 donkeys, 14,228 horses and 1,498 mules). Sixteen thousand four equids were found to be infected with equids parasites. Helminth, haemoparasite (protozoan), and ectoparasite apparent infection rates were found in 72.5%, 18.3%, and 35.38% of equids, respectively.
Meta-analysis and bias evaluation
For the current quantitative and meta-analysis, 66 studies written in English were used. Fourteen of them were reported the parasitic infections in equids (donkeys, horses and mules) at a weighted (pooled) prevalence of 62% (95% CI 48–74.2%), thirteen on both donkeys and horses 75.6% (95% CI 64.7%, 83.9%), four on both donkeys and mules 72.7% (95% CI 55.9–84.9%), nineteen on donkeys alone 42.7% (95% CI 22.7–65.3%) and sixteen on horses alone 53.5% (95% CI 38.6–67.8%).
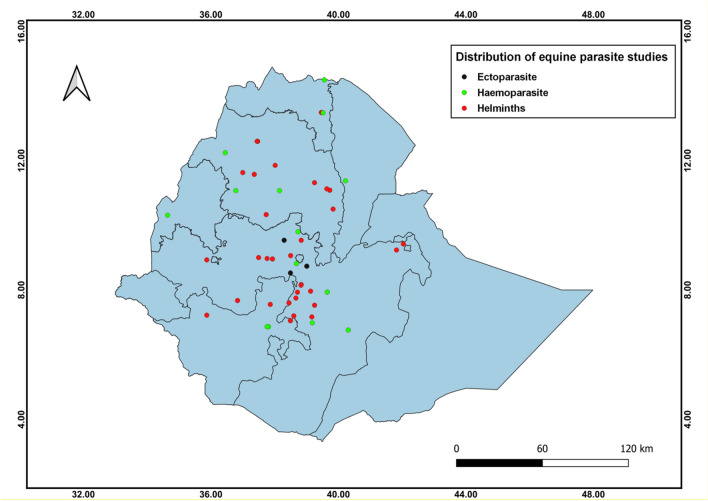
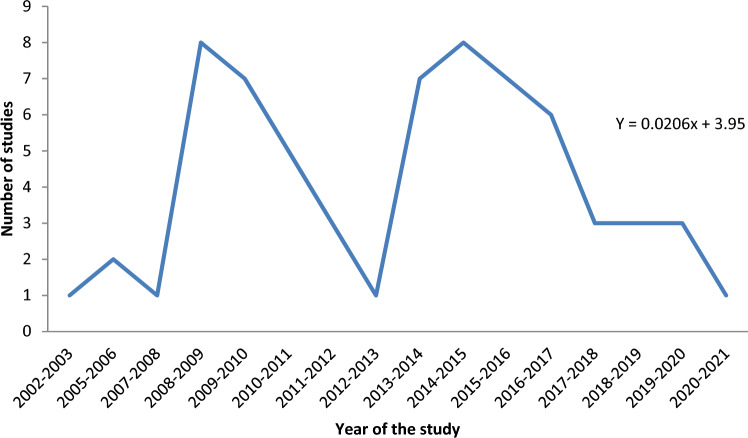
The studies retrieved were carried out between 2002 and 2021 in order to estimate the prevalence of parasitic infections in equids in various parts of Ethiopia. The geographic distribution of the studies in Ethiopia shows that seventeen studies were in the central at a pooled prevalence of 52% (95% CI 38–66%), thirteen in the north-western 59.6% (95% CI 38.4–77.7%), ten in the southern 76.1% (95% CI 58.8–87.6%), nine in the south-eastern 52% (95% CI 30.4–72.7%), five in the northern 25.9% (95% CI 13.1–44.7%), five in the north-eastern 69% (95% CI 58.3–77.5%), four in the south-western 42.8% (95% CI 16.1–74.5%), two in the eastern 87.1% (95% CI 59–97%) and one 92.8% (95% CI 89.7–95%)in the western parts of Ethiopia (S1Table). Figure 2 depicts the geographic distribution of studies by location. Similarly, the distribution of published studies retrieved from 2002 to 2021 depicted the temporal pattern of the study years. The majority of the retrieved studies (57.6%) were conducted after the year 2013 (Fig. 3). We confirmed a significant scarcity of studies between 2002 and 2010 (18.2%, or 12/66 studies). The investigation of a time series model to predict the trend of the number of studies over time revealed a slower increasing trend beginning in 2016 with a trend line of equation Y = 0.0206x + 3.95. The intercept value for the stated equation indicated a few on-going studies (0.0206), indicating a significant variation in the number of studies over time (Fig. 3).
Fig. 2.
Distribution of studies performed on equids parasites in Ethiopia
Fig. 3.
Trendline and equation depicting the growing trend of published articles over years
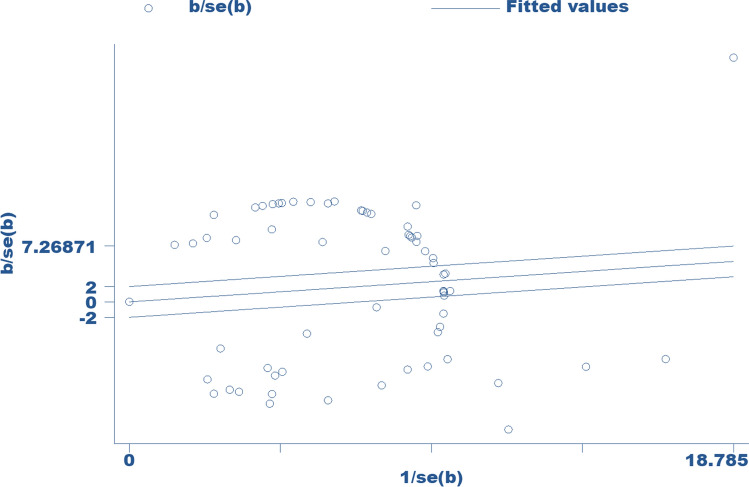
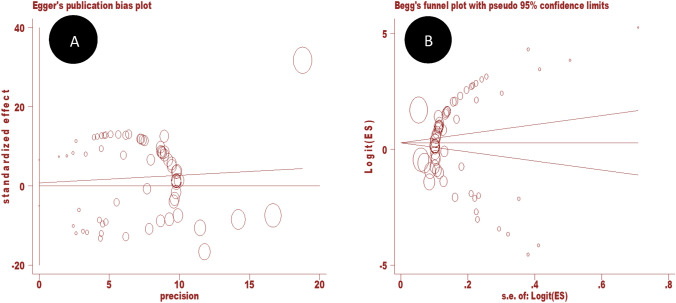
Overall, the apparent prevalence of parasitic infection in equids ranged from 1.06 to 100% (S1Table). The overall weighted prevalence of parasitic infections in equids was estimated to be 58.3% (95% CI 50.9–65.4%). There was significant heterogeneity in reports of parasitic infections in equids (I2 = 99%, Q-test = 6345.13, df = 65, p < 0.0001). Figure 4 depicts the forest-plot analysis for the study of parasitic infections in equids. The Galbraith plot analysis of studies on parasitic infections in equids (Fig. 5) revealed that almost all the included studies are located outside the 95% confidence limit, demonstrating the heterogeneity of the studies. Furthermore, for small study effects, publication bias was assessed using funnel plot observation and risk bias coefficient tests (Egger's and Begg's tests) for studies published on parasitic infections in equids in Ethiopia. The funnel plot observation results show a symmetrical observation (Fig. 6), indicating that smaller studies are not missed to be reported to the scientific community. Similarly, as shown in Fig. 7, the results of Egger's test plot revealed no significant publication bias (but not Begg's test plot) in estimating the prevalence of parasitic infections in equids (Egger's test: b = 0.78, 95% CI -5.02, 6.58, p = 0.79 and Begg's test: p = 0.038).
Fig. 4.
Forest plot of the logit event rate (lp) of parasitic infections in equids in Ethiopia
Fig. 5.
Galbraith plot for the prevalence of parasitic infections in equids in Ethiopia
Fig. 6.
Funnel plot with 95% confidence limits of the prevalence of parasitic infections in equids in Ethiopia
Fig. 7.
Egger’s publication bias plot (A) and Begg’s funnel plot (B) reports on equids parasites
Donkeys 65% (95% CI 54.4–74.4%, I2 = 98.5%) and mules 64.4% (95% CI 53–74.4%, I2 = 92.9%) had a higher weighted prevalence of parasitic infections than horses 58.8% (95% CI 50.2–66.8%, I2 = 98.6%). Helminths were the most widely prevalent parasites of equids and the weighted prevalence was 77.1% (95% CI 71.4%, 82%, I2 = 98.5%), followed by ectoparasites 35.4% (95% CI 33.4%, 37.5%, I2 = 88.2%) and haemoparasites (protozoans) 10.84% (95% CI 6.6%, 17.3%, I2 = 98.1%). Donkeys had the highest prevalence of helminth parasites at 82.3% (95% CI 75.9%, 87.3%, I2 = 97.4%), followed by horses at 66.2% (95% CI 57.9%, 73.7%, I2 = 97.9%) and mules at 64.4% (95% CI 53%, 74.4%, I2 = 92.9%). Horses, on the other hand, were the most affected equids with haemoparasite (protozoa), with a prevalence of 24.5% (95% CI 17%, 34%) compared to donkeys 6.1% (95% CI 1.92%, 17.64%).
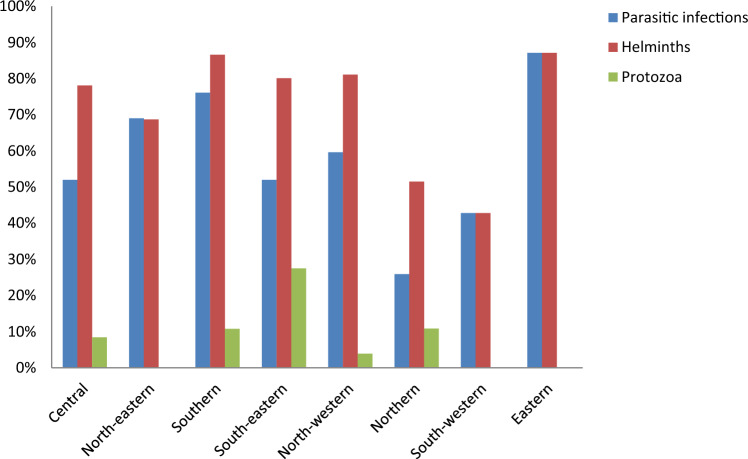
The prevalence of helminth infection was highest in the southern 86.6% (95% CI 78.02%, 92.2%) and eastern 87.1% (95% CI 59%, 97%) regions of Ethiopia, followed by the north-western 81.1% (95% CI 69.4%, 89%) and south-eastern 80.1% (95% CI 62.1–90.8%). The prevalence of protozoa infection, on the other hand, was higher in the country's south-eastern (27.5%) region (Fig. 8).
Fig. 8.
Pooled prevalence of parasitic infections of equids by geographic locations
We found forty-three species of helminth parasites affecting equids from the reports, including thirty-four nematode species (Strongylus, Cyathostomum, Coronocyclus, Cylicocyclus, Cylicostephanus, Trichostrongylus, Oesophagodontus, Strongyloides, Triodontophorus, Gyalocephalus, Poteriostomum, Dictyocaulus, Oxyuris, Habronema, Draschia, Parascaris, Setaria, and Probstmayria species), three trematodes (two Fasciola and one Gastrodiscus species), three cestodes (two Anoplocephala and one Anoplocephaloides species) and three botfly larvae (one Rhinoestrus and two Gasterophilus species) (S2Table). Equids have also been reported to be infected with Trypanosoma species (T. congolense, T. vivax, T. brucei, T. equiperdum, and T. evansi), piroplasms (Theileria equi and Babesia cabali), and Eimeria species (S3Table). Ticks (Amblyomma variegatum, A. gemma, Rhipicephalus decoloratus, R. evertisi evertisi, R. pulchellus, R. muhsame, R. sanguineus, Hyalomma rufipes, and Hy. truncatum) and lice (Bovicola equi and Haematopinus asini) were the observed ectoparasites in the equids population (S3Table).
Discussion
Ethiopia has diverse agroclimatic environments that support the survival and growth of various parasites affecting domestic animals, such as helminths and ectoparasites. Equids are frequently overlooked in domestic animal health management because they provide neither meat nor milk (Seyoum et al. 2015). This could also be due to the fact that equids are thought to be hardy and tolerant (Kumsa et al. 2012). Equids, on the other hand, can have a variety of health issues that limit their ability to participate in transportation and agricultural activities. The most serious of these are endo- and ecto- parasites (Getachew et al. 2010; Kumsa et al. 2012). Equids are also susceptible to parasites with varying life cycles, pathogenicity and epidemiology.
In light of the foregoing, it is critical to synthesise information from previous studies in order to depict the status of parasitic infection in equids. Estimating the national and regional pooled prevalence of parasite infection in equids could help Ethiopia develop appropriate control and prevention strategies. However, there is a scarcity of comprehensive, systematic data on the prevalence and species composition of equids parasites that contribute to parasite infection in equids. To the best of the authors' knowledge, this is the first systematic review and meta-analysis of equids parasite infections in Ethiopia. The current study collected weighted prevalence estimates, as well as the spatial and temporal distribution of parasitic infections in equids. The study included 66 original studies on the prevalence of parasite infections in equids. Parasitological analysis was performed on faeces, blood/serum, and macro-parasite samples. Coprological, serological, molecular, and morphological (parasitological) analyses were all performed to identify parasites. The central region of the country received the most reports (25.8%), followed by the north-western (20%) and southern (15.2%) regions.
This study found a high prevalence of parasitic infections in equids in Ethiopia. It also revealed polyparasitism with high infection intensity (Getachew et al. 2010; Seyoum et al. 2015; Tolossa and Ashenafi 2013; Sheferaw and Alemu 2015; Ayele et al. 2006; Berhanu et al. 2014; Abebe and Wolde 2010; Mkuria et al. 2010; Dagnachew et al. 2020; Fesseha et al. 2022). Helminths were the most diverse and prevalent parasites infecting equids in Ethiopia, with a prevalence ranging from 97.9 to 100% (Sheferaw and Alemu 2015; Naramo et al. 2016; Sankuro et al. 2018; Berhanu et al. 2014). The helminth parasites mentioned in this review were found in the gastrointestinal tracts of equids. In addition to helminths, various authors have documented protozoa infections ranging from 2.5 to 28.5% (Hagos et al. 2010a, b; Mekibib et al. 2010; Mkuria et al. 2010; Dagnachew et al. 2020). Furthermore, Ferede et al. (2010), Kumsa et al. (2012) and Tafese et al. (2014) found ectoparasite (tick and lice) infestations in equids ranging from 28.81 to 39.04%. As a result, it is possible to conclude that Ethiopia provided favourable environmental factors for the survival of various parasite species as well as their various stages.
According to the current meta-analysis, the weighted prevalence of parasitic infections in equids was 58.3%. The heterogeneity analysis using inverse variance indexes (I2) revealed that real differences between studies accounted for 99% of the observed variance. The weighted prevalence estimates of parasitic infections in equids reported in this study differ from those reported by Khamesipour et al. (2021), who reported a pooled prevalence of 28.8%. Similarly, the current study's findings are higher than those reported in Greece (Kouam et al. 2010). This variation could be attributed to differences in study areas' agroecological features, study period, livestock management systems, diagnostic techniques used, sample size, and geographical location.
Helminths are common parasites in equids and pose serious health and performance issues in Ethiopia (Fikru et al. 2005; Getachew et al. 2010; Sheferaw and Alemu 2015; Mathewos et al. 2021a, b, 2022; Fesseha et al. 2022). These are known to be transmitted to their hosts through the consumption of infective larvae from contaminated pastures (Taylor et al. 2007). Helminth parasites affecting equids have been widely reported in various agro-climatic zones of Ethiopia. These parasites can be identified by a variety of clinical signs, including malaise, slowed growth, abdominal discomfort, diarrhoea, colic or abdominal pain, weakness, weight loss, decreased productivity, and death (Seyoum et al. 2015). In the current meta-analysis, the pooled prevalence estimate of helminths was found to be 77.1%. This is in line with reports from Ethiopia and other parts of the world (Valdéz-Cruz et al. 2013; Tedla and Abichu 2018; Mathewos et al. 2021a). The findings of Uslu and Guclu (2007) from Turkey, Maria et al. (2012) from India and Devkota et al. (2021) from Nepal are also comparable. Ogbein et al. (2022) from Nigeria reported findings similar to the current report. The current report also corroborates previous reports published elsewhere (Seyoum et al. 2015; Oli and Subedi 2018). However, studies in Ethiopia and Mexico estimate that endoparasite infections affect more than 90% of equids (Getachew et al. 2010; Ismail et al. 2016; Sankuro et al. 2018; Imam et al. 2021; Fesseha et al. 2022; Mathewos et al. 2022). The seasonality of the study period, management practises, sample size, topography, animal working conditions, sampling techniques, and climatic factors can all be attributed to the variations in the reports. Overall, based on our findings and previous research, we can conclude that the tropical environment of Ethiopia is conducive to helminth parasite transmission dynamics in equids.
Donkeys were the most affected equids with helminth parasites in the current study, followed by horses and mules. This supports previous studies (Seyoum et al. 2015; Belay et al. 2016; Imam et al. 2021; Mathewos et al. 2021a, b, 2022), which found that donkeys are more susceptible to helminth parasite infections than horses and mules. This variation among equids species could be attributed to the fact that donkeys in Ethiopia are subjected to poor management and a heavy workload, as well as fewer deworming practises (Getachew et al. 2008; Seyoum et al. 2015). It might make them more vulnerable to parasite infestation. Furthermore, this could be due to the relatively good trend of farmers' horse and mule management practises, which has become a routine activity of horse and mule owners due to economic reasons and owners' awareness of their horses' and mules' welfare. These points, however, need to be emphasised and investigated further.
Besides, despite the small number of studies, ectoparasites (ticks and lice) were found in Ethiopia (Ferede et al. 2010; Kumsa et al. 2012; Tafese et al. 2014). These ectoparasites live as periodic or permanent parasites on their hosts' skin or skin surface, which can be harmful because they rely on their hosts for sustenance, maturation, and multiplication. Ticks and lice are a significant threat to cost-effective livestock production in many parts of the world, causing direct mechanical damage, anaemia, loss of condition, irritation, allergic reaction, toxicosis, morbidity, and mortality (Kumsa et al. 2012; Tafese et al. 2014). They are also indirectly involved in disease-causing pathogen transmission. Ticks, for example, have been identified as important vectors of animal diseases such as babesiosis, theileriosis, and anaplasmosis (Ferede et al. 2010; Kumsa et al. 2012). We observed tick-borne piroplasmosis caused by Theileria equi and Babesia caballi in our review (Mekibib et al. 2010; Tefera et al. 2011; Gizachew et al. 2013; Getachew et al. 2014). Control of equids ectoparasites is thus critical for the protection of equids' health and an increase in their productivity in the country.
Ticks and lice have been found in all agro-ecological zones of Ethiopia (Kumsa et al. 2012; Tafese et al. 2014; Kaba 2022). S3Table shows the tick species found in equids in Ethiopia: A. variegatum, A. gemma, R. decoloratus, R. e. evertisi, R. pulchellus, R. muhsame, and R. sanguineus (Ferede et al. 2010; Kumsa et al. 2012; Kaba 2022). Similarly, the lice species infecting horses are B. equi and H. asini (Tafese et al. 2014). The overall pooled prevalence of ectoparasites was found to be 35.4% in this review. This corresponds to the findings of Gharbi et al. (2018) and Tramboo et al. (2019). Payne et al. (2017) from Cameroon, and Dik et al. (2020) from Turkey, on the other hand, reported a higher prevalence of ectoparasite infestation with a prevalence ranging from 91.8 to 99.66%. The differences in prevalence observed between studies could be attributed to differences in agro-climatic and geographical conditions, study area, species involved in the study, livestock management system, study period season, breed, and anti-parasitic medication availability.
This review included reports of equids trypanosomosis and piroplasmosis caused by Trypanosoma species and piroplasms, respectively. Pathogens are either intracellular or extracellular protozoans that live in the bloodstream of vertebrate hosts, causing anaemia, icterus, fever, anorexia, weight loss, infertility, and death (Alaba et al. 2022; Nimako-Boateng et al. 2022). Protozoans found in equids in Ethiopia included Babesia caballi, Theileria equi, Trypanosoma vivax, T. brucei, T. congolense, T. evansi, and T. equiperdum. Trypanosoma equiperdum, a trypanosome, is known to be sexually transmitted in equids, causing tissue destruction (Yasine et al. 2019). The weighted prevalence estimates of haemoparasites in our meta-analysis were 10.84%. This supports the findings of Mahmoud et al. (2016) and Alaba et al. (2022).
Conclusion
The present meta-analysis and systematic review demonstrated that equids parasite infection is frequent in Ethiopia and is caused by a variety of parasite species (helminths, protozoa, ectoparasites, and bot fly larvae), compromising the animals' health, welfare, and productivity. The pooled prevalence of parasite infection was found to be 58.3%. Helminths were the most frequent parasites detected in equids, accounting for 77.1%. Donkeys had the highest helminth parasite infection rates among equids. However, the reports made no attempt to identify the risk factors for parasite infection and infestation in equids in order to propose effective control strategies. The current study had also limitations because there were lacks of studies in some regions of the country and ectoparasite studies. Studies on ectoparasites and risk factor analysis are therefore advised. More emphasis should be placed on raising awareness about animal health and welfare.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to study design and writing of the manuscript.
Funding
Not applicable.
Declarations
Ethical statement
Ethical approval is not applicable. No animal or human experimentation was undertaken.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Edom Mesafint, Email: eddiemes961@gmail.com.
Haileyesus Dejene, Email: haileyesus.dejene@uog.edu.et.
Moges Maru, Email: malemayehu19@yahoo.com.
Zewdu Seyoum Tarekegn, Email: zewdu.seyoum@uog.edu.et.
References
- Abebe R, Wolde A. A cross-sectional study of trypanosomosis and its vectors in donkeys and mules in Northwest Ethiopia. Parasitol Res. 2010;106(4):911–916. doi: 10.1007/s00436-010-1758-5. [DOI] [PubMed] [Google Scholar]
- Addis H, Gizaw TT, Minalu BA, Tefera Y. Cross-sectional study on the prevalence of equids strongyle infection in Mecha Woreda, Ethiopia. Int J Adv Res Biol Sci. 2017;4(8):68–77. doi: 10.22192/ijarbs.2017.04.08.011. [DOI] [Google Scholar]
- Adeba A, Kassa T, Teshale A. The Occurrence of gastro intestinal parasites of donkeys in and around Holeta Town, Oromia Regional State. Ethiopia Adv. 2022;3(3):73–80. doi: 10.11648/j.advances.20220303.15. [DOI] [Google Scholar]
- Alaba BA, Omoniwa DO, Olajide EO, Koleosho SA, Olaleye JT. Prevalence of haemoparasites and influence on haemato-biochemical parameters of polo horses in Ibadan, Nigeria. Sokoto J Vet Sci. 2022;20(3):205–211. doi: 10.4314/sokjvs.v20i3.7. [DOI] [Google Scholar]
- Alemayehu MT, Abebe BK, Haile SM. Investigation of strongyle prevalence and associated risk factors in Horses in and around Alage District, Ethiopia. J Parasitol Res. 2022;2022:3935008. doi: 10.1155/2022/3935008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambo K, Deneke Y, Ibrahim N. Prevalence of Equids helminthiasis in Gedeb Hasassa District, Arsi Zone, South Eastern Ethiopia. Global Veterinaria. 2017;18(6):406–413. doi: 10.5829/idosi.gv.2017.406.413. [DOI] [Google Scholar]
- Andarge B, Muhammed C, Tibesso G. Prevalence of major intestinal nematodes of Equids in Jimma Town, South Western Ethiopia. Int J Vet Sci Res. 2017;3(2):069–073. doi: 10.17352/ijvsr.000024. [DOI] [Google Scholar]
- Ayana D, Alemu A, Waktole H, Ashenafi H. Prevalence of Equids Gastro Intestinal Parasites in and Around Meki Town, Oromia, Ethiopia. Adv Biol Res. 2019;13(1):30–37. doi: 10.5829/idosi.abr.2019.30.37. [DOI] [Google Scholar]
- Ayele G, Feseha G, Bojia E, Joe A. Prevalence of gastro-intestinal parasites of donkeys in Dugda Bora District, Ethiopia. Livestock Res Rural Dev. 2006;18(10):14–21. [Google Scholar]
- Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- Bedada H, Dagnachew S. Study on the prevalence of donkey trypanosomosis in Awi zone northwest Ethiopia. Ethiop Vet J. 2012;16(2):65–76. doi: 10.4314/evj.v16i2.6. [DOI] [Google Scholar]
- Belay W, Teshome D, Abiye A. Study on the prevalance of gastrointestinal helminthes infection in equids in and around Kombolcha. J Vet Sci Technol. 2016;7(5):367–372. doi: 10.4172/2157-7579.1000372. [DOI] [Google Scholar]
- Belete S, Derso S. Prevalence of major gastrointestinal parasites of horses in and around Mekelle (Quiha and Wukro) World J Anim Sci Res. 2015;3(3):1–10. [Google Scholar]
- Berhanu T, Ibrahim N, Deressa B, Tolosa T. Prevalence of helminth parasites of horses in and around Hawassa town, southern Ethiopia. Acta Parasitol Globalis. 2014;5:7–11. doi: 10.5829/idosi.apg.2014.5.1.82300. [DOI] [Google Scholar]
- Bizuayehu F, Bedada H. Epidemiology of horse GIT Parasitism in Gondar Town and Wegera District. Afr J Basic Appl Sci. 2018;10(3):55–63. doi: 10.5829/idosi.ajbas.2018.55.63. [DOI] [Google Scholar]
- Bogale B, Sisay Z, Chanie M. Strongyle Nematode Infections of Donkeys and Mules in and Around Bahirdar, Northwest Ethiopia. Global Veterinaria. 2012;9(4):497–501. doi: 10.5829/idosi.gv.2012.9.4.65180. [DOI] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. 1. Chichester: Wiley; 2009. pp. 277–291. [Google Scholar]
- Chemeda R, Mekonnen N, Muktar Y, Terfa W. Study on prevalence of internal parasites of horses in and around Ambo town, Central Ethiopia. Am Eur J Agric Environ Sci. 2016;16(6):1051–1057. doi: 10.5829/idosi.aejaes.2016.16.6.10366. [DOI] [Google Scholar]
- CSA (2021) Federal democratic republic of Ethiopia central statistical agency agricultural sample survey 2020/21 [2013 E.C.] volume II report on livestock and livestock characteristics (private peasant holdings).
- Dagnachew S, Mohammed S, Dessie B, Tilahun M, Ayele A, Kefyalew H. Bovine and equids trypanosomosis in Northwest Ethiopia: Prevalence, density of vectors and control measures. Parasite Epidemiol Control. 2020;11:e00170. doi: 10.1016/j.parepi.2020.e00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debere D, Muktar Y, Shiferaw S, Belina D. Internal parasites of equids and associated risk factors in and around Guder town, West Shewa, central Ethiopia. Ethiop Vet J. 2018;22(2):36–52. doi: 10.4314/evj.v22i2.4. [DOI] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Devkota RP, Subedi JR, Wagley K. Prevalence of gastrointestinal parasites in equids of Mustang District, Nepal. Biodiversitas J Biol Divers. 2021;22(9):3958–3963. doi: 10.13057/biodiv/d220943. [DOI] [Google Scholar]
- Dibaba MD, Getachew AM, Assefa Z, Fanta A, Etana M, Firew S, Goshu L, Burden F. Seasonal variation of strongylosis in working donkeys of Ethiopia: a cross-sectional and longitudinal studies. Parasitol Res. 2017;116(7):2009–2015. doi: 10.1007/s00436-017-5485-z. [DOI] [PubMed] [Google Scholar]
- Dik B, Ceylan O, Ceylan C, Tekindal MA, Semassel A, Sönmez G, Derinbay Ekici Ö. Ectoparasites of feral horses [Equus ferus caballus (Linnaeus., 1758)] on Karadağ Mountain, Karaman, Turkey. J Parasitic Dis off Organ Indian Soc Parasitol. 2020;44(3):590–596. doi: 10.1007/s12639-020-01234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyob A, Mekuria S, Regassa A, Abebe R. A cross-sectional study of equids trypanosomosis and its vectors in Wolayta zone, Southern Ethiopia. J Vet Med Animal Health. 2011;3(2):21–26. [Google Scholar]
- Ferede B, Kumsa B, Bsrat A, Kalayou S. Ticks of donkeys in central Oromia regional state, Ethiopia. Revue De Médecine Vétérinaire. 2010;161(3):121–126. [Google Scholar]
- Fesseha H, Aliye S, Mathewos M, Nigusie K. Prevalence and risk factors associated with donkey gastrointestinal parasites in Shashemane and Suburbs, Oromia Region, Ethiopia. Heliyon. 2022;8(12):e12244. doi: 10.1016/j.heliyon.2022.e12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikru R, Reta D, Teshale S, Bizunesh M. Prevalence of equids gastrointestinal parasites in Western highlands of Oromia. Bull Anim Health Prod Afr. 2005;53(3):161–166. [Google Scholar]
- Gari FR, Ashenafi H, Tola A, Goddeeris BM, Claes F. Comparative diagnosis of parasitological, serological, and molecular tests in dourine-suspected horses. Trop Anim Health Prod. 2010;42(8):1649–1654. doi: 10.1007/s11250-010-9615-1. [DOI] [PubMed] [Google Scholar]
- Getachew M, Feseha G, Trawford A, Reid SW. A survey of seasonal patterns in strongyle faecal worm egg counts of working equids of the central midlands and lowlands. Ethiopia Trop Anim Health Prod. 2008;40(8):637–642. doi: 10.1007/s11250-008-9142-5. [DOI] [PubMed] [Google Scholar]
- Getachew M, Trawford A, Feseha G, Reid SW. Gastrointestinal parasites of working donkeys of Ethiopia. Trop Anim Health Prod. 2010;42(1):27–33. doi: 10.1007/s11250-009-9381-0. [DOI] [PubMed] [Google Scholar]
- Getachew M, Alemayehu F, Chala C, Amare B, Kassa D, Burden F, Wernery U. A cross-sectional sero-survey of some infectious diseases of working equids in Central Ethiopia. J Vet Med Anim Health. 2014;6(9):231–238. doi: 10.5897/JVMAH2014.0296. [DOI] [Google Scholar]
- Getahun TK, Kassa TZ. Prevalence and species of major gastrointestinal parasites of donkeys in Tenta Woreda, Amhara Regional State, Ethiopia. J Vet Med Anim Health. 2017;9(2):23–31. doi: 10.5897/JVMAH2016.0528. [DOI] [Google Scholar]
- Gharbi M, Drissi G, Darghouth MA. Population dynamics of ticks infesting horses in north-west Tunisia. Revue Scientifique Et Technique (int Office Epizoot) 2018;37(3):837–841. doi: 10.20506/rst.37.3.2890. [DOI] [PubMed] [Google Scholar]
- Gizachew A, Schuster RK, Joseph S, Wernery R, Georgy NA, Elizabeth SK, Wernery U. Piroplasmosis in donkeys a hematological and serological study in Central Ethiopia. J Equids Vet Sci. 2013;33(1):18–21. doi: 10.1016/j.jevs.2012.04.003. [DOI] [Google Scholar]
- Hagos A, Degefa G, Yacob H, Fikru R, Alemu T, Feseha G, Claes F, Goddeeris BM. Seroepidemiological survey of trypanozoon infection in horses in the suspected dourine-infected Bale highlands of the Oromia region, Ethiopia. Revue Scientifique Et Technique (int Office Epizoot) 2010;29(3):649–654. doi: 10.20506/rst.29.3.2005. [DOI] [PubMed] [Google Scholar]
- Hagos A, Abebe G, Büscher P, Goddeeris BM, Claes F. Serological and parasitological survey of dourine in the Arsi-Bale highlands of Ethiopia. Trop Anim Health Prod. 2010;42(4):769–776. doi: 10.1007/s11250-009-9485-6. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hosseini SH, Meshgi B, Eslami A, Bokai S, Sobhani M, Ebrahimi SR. Prevalence and biodiversity of helminth parasites in donkeys (Equus asinus) in Iran. Int J Vet Res. 2009;3(2):95–99. [Google Scholar]
- Ibrahim N, Berhanu T, Deressa B, Tolosa T. Survey of prevalence of helminth parasites of donkeys in and around Hawassa town, Southern Ethiopia. Global Veterinaria. 2011;6(3):223–227. [Google Scholar]
- Imam BH, Oladejo AO, Measho S, Tesfagaber W, Tsfamariam E, Tsegay G. Prevalence of gastrointestinal nematodes in donkeys and Mule's species in Anseba Region, Eritrea. J Vet Med Anim Health. 2021;13(4):177–184. doi: 10.5897/JVMAH2021.0952. [DOI] [Google Scholar]
- Ismail AA, Ahmed NK, Bashar AE, Seri HI, El Tigani-Asil TA, Abakar AD. A survey of seasonal gastrointestinal parasitic infections in Donkeys from a Semiarid Sub-Saharan Region, Sudan. J Pathogens. 2016;2016:4602751. doi: 10.1155/2016/4602751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaba T. Geographical distribution of ixodid ticks and tick-borne pathogens of domestic animals in Ethiopia: a systematic review. Parasit Vectors. 2022;15(1):108. doi: 10.1186/s13071-022-05221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamesipour F, Taktaz-Hafshejani T, Tebit KE, Razavi SM, Hosseini SR. Prevalence of endo- and ecto-parasites of equids in Iran: a systematic review. Vet Med Sci. 2021;7(1):25–34. doi: 10.1002/vms3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouam MK, Kantzoura V, Gajadhar AA, Theis JH, Papadopoulos E, Theodoropoulos G. Seroprevalence of equids piroplasms and host-related factors associated with infection in Greece. Vet Parasitol. 2010;169(3–4):273–278. doi: 10.1016/j.vetpar.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Kumsa B, Tamrat H, Tadesse G, Aklilu N, Cassini R. Prevalence and species composition of ixodid ticks infesting horses in three agroecologies in central Oromia, Ethiopia. Trop Anim Health Prod. 2012;44(1):119–124. doi: 10.1007/s11250-011-9897-y. [DOI] [PubMed] [Google Scholar]
- Mahmoud MS, El-Ezz NT, Abdel-Shafy S, Nassar SA, El Namaky AH, Khalil WK, Knowles D, Kappmeyer L, Silva MG, Suarez CE. Assessment of Theileria equi and Babesia caballi infections in equids populations in Egypt by molecular, serological and hematological approaches. Parasit Vectors. 2016;9:260. doi: 10.1186/s13071-016-1539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangassa B, Tafese W (2016) Prevalence of strongyle infection and associated risk factors in horse and donkeys in and around Batu Town, East Shoa, Oromia Regional State, Ethiopia. Food Sci Quality Manag 54
- Maria A, Shahardar RA, Bushra M. Prevalence of gastrointestinal helminth parasites of equids in central zone of Kashmir Valley. Indian J Anim Sci. 2012;82(11):1276–1280. [Google Scholar]
- Mathewos M, Fesseha H, Yirgalem M. Study on Strongyle Infection of Donkeys and Horses in Hosaena District, Southern Ethiopia. Vet Med (auckland, n.z.) 2021;12:67–73. doi: 10.2147/VMRR.S297951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewos M, Girma D, Fesseha H, Yirgalem M, Eshetu E. Prevalence of gastrointestinal helminthiasis in horses and Donkeys of Hawassa District, Southern Ethiopia. Vet Med Int. 2021;2021:6686688. doi: 10.1155/2021/6686688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewos M, Teshome D, Fesseha H. Study on gastrointestinal nematodes of equids in and around Bekoji, South Eastern Ethiopia. J Parasitol Res. 2022;2022:8210160. doi: 10.1155/2022/8210160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekibib B, Manegerew M, Tadesse A, Abuna F, Megersa B, Regassa A, Abebe R. Prevalence of haemoparasites and associated risk factors in working donkeys in Adigudem and Kwiha Districts of Tigray Region, Northern Ethiopia. J Anim Vet Adv. 2010;9(17):2249–2255. doi: 10.3923/javaa.2010.2249.2255. [DOI] [Google Scholar]
- Mekuria M. Seroprevalence of Trypanosoma equiperdum (Dourine) in and Around Asella, Oromia, Ethiopia. Int J Adv Res Biol Sci. 2020;7(10):74–83. doi: 10.22192/ijarbs. [DOI] [Google Scholar]
- Mkuria S, Eyob A, Regassa A, Tadesse A, Mekibib B, Abebe R. A cross-sectional study of equids trypanosomosis and its vectors in Wolayta zone, Southern Ethiopia. J Anim Vet Adv. 2010;9(15):2061–2066. doi: 10.3923/javaa.2010.2061.2066. [DOI] [Google Scholar]
- Mezgebu T, Tafess K, Tamiru F. Prevalence of gastrointestinal parasites of horses and donkeys in and around Gondar Town, Ethiopia. Open J Vet Med. 2013;3(06):267. doi: 10.4236/ojvm.2013.36043. [DOI] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4(1):1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed]
- Molla B, Worku Y, Shewaye A, Mamo A. Prevalence of strongyle infection and associated risk factors in equids in Menz Keya Gerbil District, North-Eastern Ethiopia. J Vet Med Anim Health. 2015;7(4):117–121. doi: 10.5897/JVMAH2014.0354. [DOI] [Google Scholar]
- Molla E, Selamu A, Nibret G. Study on Nematode Infections in Horses and Donkeys in and Around Bishoftu, Ethiopia. Acta Sci Vet Sci. 2022;4(5):78–83. doi: 10.31080/ASVS.2022.03.0257. [DOI] [Google Scholar]
- Muhammad A, Bashir R, Mahmood M, Afzal MS, Simsek S, Awan UA, Khan MR, Ahmed H, Cao J. Epidemiology of Ectoparasites (Ticks, Lice, and Mites) in the Livestock of Pakistan: a review. Front Vet Sci. 2021;8:780738. doi: 10.3389/fvets.2021.780738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramo M, Terefe Y, Kemal J, Merga T, Haile G, Dhaba M. Gastrointestinal nematodes of donkeys in and around Alage, South Western Ethiopia. Ethiop Vet J. 2016;20(2):87–97. doi: 10.4314/evj.v20i2.7. [DOI] [Google Scholar]
- Negasa T, Dilbato T, Gudeta D. Cross-sectional study on equids lung worm and associated risk factor in Ambo district, Oromia region, Ethiopia. Int J Res-Granthaalayah. 2017;5:312–319. doi: 10.5281/zenodo.802336. [DOI] [Google Scholar]
- Negash W, Erdachew Y, Dubie T. Prevalence of strongyle infection and associated risk factors in horses and Donkeys in and around Mekelle City, Northern Part of Ethiopia. Vet Med Int. 2021;2021:9430824. doi: 10.1155/2021/9430824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MK, Kaplan RM, Thamsborg SM, Monrad J, Olsen SN. Climatic influences on development and survival of free-living stages of equids strongyles: implications for worm control strategies and managing anthelmintic resistance. Vet J (lond, England) 2007;174(1):23–32. doi: 10.1016/j.tvjl.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Nigusu K, Mohammed T. Assessment of Gastrointestinal Tract Parasites of Equids in Asella Town, Ethiopia. East Afr J Vet Anim Sci. 2017;1(2):93–100. [Google Scholar]
- Nimako-Boateng MB, Boakye OD, Bediako OS, Asare DA, Emikpe BO. Prevalence of haemoparasites and effects on blood parameters of horses in the Ashanti Region of Ghana. Niger J Parasitol. 2022;43(2):253–259. doi: 10.4314/njpar.v43iXXXX. [DOI] [Google Scholar]
- Ogbein KE, Dogo AG, Oshadu DO, Edeh ER. Gastrointestinal parasites of horses and their socio-economic impact in Jos Plateau-Nigeria. Appl Vet Res. 2022;1(2):e2022010. doi: 10.31893/avr.2022010. [DOI] [Google Scholar]
- Oli N, Subedi JR. Prevalence of gastro-intestinal parasites of horse (equus caballus linnaeus, 1758) in seven village development committee of Rukum district, Nepal. J Inst Sci Technol. 2018;22(2):70–75. doi: 10.3126/jist.v22i2.19596. [DOI] [Google Scholar]
- Payne VK, Mbafor FL, Wabo Pone J, Tchoumboué J. Preliminary study of ectoparasites of horses in the western highlands of Cameroon. Vet Med Sci. 2017;3(2):63–70. doi: 10.1002/vms3.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankuro M, Elemo KK, Mekibib B. Prevalence, intensity and major species of gastrointestinal parasites of donkeys in Adami Tulu Jido Kombolcha District, Central Ethiopia. J Parasitol Vector Biol. 2018;10(5):58–65. doi: 10.5897/JPVB2017.0292. [DOI] [Google Scholar]
- Seyoum Z, Tesfaye M, Derso S. Prevalence, intensity and risk factors of infestation with major gastrointestinal nematodes in equids in and around Shashemane, Southern Ethiopia. Trop Anim Health Prod. 2015;47(8):1515–1521. doi: 10.1007/s11250-015-0893-5. [DOI] [PubMed] [Google Scholar]
- Sheferaw D, Alemu M. Epidemiological study of gastrointestinal helminths of equids in Damot-Gale district, Wolaita zone, Ethiopia. J Parasitic Dis off Organ Indian Soc Parasitol. 2015;39(2):315–320. doi: 10.1007/s12639-013-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sori G, Bekele T, Geso G, Ibrahim H, Gobena F, Jarso G, Melaku M, Shumet A. Prevalence of equids strongyle infection and its associated risk factors in Jimma Town, Southwest Ethiopia. Int J Livestock Prod. 2017;8(11):187–191. doi: 10.5897/IJLP2016.0325. [DOI] [Google Scholar]
- Sultan A, Ayele G, Tadesse B, Ahmed A. Prevalence of gastrointestinal parasites of horses and donkeys in Kurfa Chale District, East Hararghe, Ethiopia. Livestock Res Rural Dev. 2014;26(7):23–27. [Google Scholar]
- Taddie W. Prevalence of gastrointestinal nematode parasites in mules and donkeys in and around Debre-Tabor. Int J Vet Sci Anim Husbandry. 2016;1(4):15–19. [Google Scholar]
- Tafese A, Jibat T, Aklilu N, Zewdu H, Kumsa B. Lice infesting horses in three agroecological zones in central Oromia. J Parasitic Dis off Organ Indian Soc Parasitol. 2014;38(4):352–357. doi: 10.1007/s12639-013-0235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takele B, Nibret E. Prevalence of gastrointestinal helminthes of donkeys and mules in and around Bahir Dar, Ethiopia. Ethiop Vet J. 2013;17(1):13–30. doi: 10.4314/evj.v17i1.2. [DOI] [Google Scholar]
- Takele B, Sisay A. Prevalence of gastrointestinal helminths on equids in and around Debre Markos. Ethiopia Sci J Vet Adv. 2016;5(11):134–144. doi: 10.14196/sjva.v5i11.2304. [DOI] [Google Scholar]
- Tamene A, Bayu Y, Wondimu A. Prevalence of gastrointestinal parasites of equids in and Around Gondar town, Amhara Regional State, Ethiopia. J Vet Sci Technol. 2019;10(2):573. doi: 10.5897/JVMAH2016.0528. [DOI] [Google Scholar]
- Taye A. Epidemiological study on equids gastrointestinal helminth parasites in Mekelle, North Ethiopia. Open J Vet Med. 2017;7(10):121. doi: 10.4236/ojvm.2017.710012. [DOI] [Google Scholar]
- Taylor MA, Coop RL, Wall RL. Veterinary parasitology. London: Wiley-Blackwell; 2007. [Google Scholar]
- Tedla M, Abichu B. Cross-sectional study on gastro-intestinal parasites of equids in South-western Ethiopia. Parasite Epidemiol Control. 2018;3(4):e00076. doi: 10.1016/j.parepi.2018.e00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefera M, Worku A, Tolosa T, Bitew M. Prevalence and risk factors for donkey babesiosis in and around Debre Zeit, Central Ethiopia. Vet Res. 2011;4:56–60. [Google Scholar]
- Tesfu N, Asrade B, Abebe R, Kasaye S. Prevalence and risk factors of gastrointestinal nematode parasites of horse and donkeys in Hawassa town, Ethiopia. J Vet Sci Technol. 2014;5(5):2157–7579. doi: 10.4172/2157-7579.1000210. [DOI] [Google Scholar]
- Tirosh-Levy S, Gottlieb Y, Apanaskevich DA, Mumcuoglu KY, Steinman A. Species distribution and seasonal dynamics of equids tick infestation in two Mediterranean climate niches in Israel. Parasit Vectors. 2018;11(1):546. doi: 10.1186/s13071-018-3093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolossa YH, Ashenafi H. Epidemiological study on gastrointestinal helminths of horses in Arsi-Bale highlands of Oromiya Region, Ethiopia. Ethiop Vet J. 2013;17(2):51–62. doi: 10.4314/evj.v17i2.4. [DOI] [Google Scholar]
- Tramboo SR, Shahardar RA, Allaie IM, Bulbul KH, Khan AA, Mir MS, Hussain I, Rather MA, Ahanger AA. Prevalence of ectoparasites in equids of Kashmir valley. J Entomol Zool Stud. 2019;7(6):528–534. [Google Scholar]
- Tsegaye B, Chala A. Prevalence of endoparasitic helminths of donkeys in and around Haramaya district, Eastern Ethiopia. J Vet Med Anim Health. 2015;7(6):221–224. doi: 10.5897/JVMAH2015.0383. [DOI] [Google Scholar]
- Tullu DG (2008) A cross sectional study of dourine in selected horse breeding districts of bale highlands of Oromia regional state of Ethiopia. MSc thesis submitted to School of Graduate Studies of Addis Ababa University, Addis Ababa, Ethiopia. http://etd.aau.edu.et/handle/123456789/21657
- Uslu U, Guclu F. Prevalence of endoparasites in horses and donkeys in Turkey. Bull Vet Inst Pulawy. 2007;51(2):237. [Google Scholar]
- Valdéz-Cruz MP, Hernández-Gil M, Galindo-Rodríguez L, Alonso-Díaz MA. Gastrointestinal nematode burden in working equids from humid tropical areas of central Veracruz, Mexico, and its relationship with body condition and haematological values. Trop Anim Health Prod. 2013;45(2):603–607. doi: 10.1007/s11250-012-0265-3. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative STROBE. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (london, England) 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- Wondimu A, Sharew G. Gastrointestinal nematodes of donkeys and horses in Gondar town northwest, Ethiopia. J Vet Med Anim Health. 2017;9(5):88–91. doi: 10.5897/JVMAH2012.030. [DOI] [Google Scholar]
- Worku S, Afera B (2012) Prevalence of equids nematodes in and around Kombolcha south Wollo, Ethiopia. REDVET 13(9)
- Yasine A, Daba M, Ashenafi H, Geldhof P, Van Brantegem L, Vercauteren G, Demissie T, Bekana M, Tola A, Van Soom A, Duchateau L, Goddeeris B, Govaere J. Tissue (re)distribution of Trypanosoma equiperdum in venereal infected and blood transfused horses. Vet Parasitol. 2019;268:87–97. doi: 10.1016/j.vetpar.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Zeryehun T, Tsegaw F. Endoparasites of donkeys in Dessie and its surroundings, Northeastern Ethiopia. Ethiop Vet J. 2016;20(1):79–90. doi: 10.4314/evj.v20i1.6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.