Keywords: bimanual coordination, decision-making, force distribution, stroke, upper extremity
Abstract
Large bilateral asymmetry and task deficits are typically observed during bimanual actions of stroke survivors. Do these abnormalities originate from unilateral impairments affecting their more-impaired limb, such as weakness and abnormal synergy, or from bilateral impairments such as incoordination of two limbs? To answer this question, 23 subjects including 10 chronic stroke survivors and 13 neurologically intact subjects participated in an experiment where they produced bimanual forces at different hand locations. The force magnitude and directional deviation of the more-impaired arm were measured for unilateral impairments and bimanual coordination across locations for bilateral impairments. Force asymmetry and task error were used to define task performance. Significant unilateral impairments were observed in subjects with stroke; the maximal force capacity of their more-impaired arm was significantly lower than that of their less-impaired arm, with a higher degree of force deviation. However, its force contribution during submaximal tasks was greater than its relative force capacity. Significant bilateral impairments were also observed, as stroke survivors modulated two forces to a larger degree across hand locations but in a less coordinated manner than control subjects did. But only unilateral, not bilateral, impairments explained a significant amount of between-subject variability in force asymmetry across subjects with stroke. Task error, in contrast, was correlated with neither unilateral nor bilateral impairments. Our results suggest that unilateral impairments of the more-impaired arm of stroke survivors mainly contribute to its reduced recruitment, but that the degree of its participation in bimanual task may be greater than their capacity as they attempt to achieve symmetry.
NEW & NOTEWORTHY We studied how unilateral and bilateral impairments in stroke survivors affect their bimanual task performance. Unilateral impairments of the more-impaired limb, both weakness and loss of directional control, mainly contribute to bimanual asymmetry, but stroke survivors generally produce higher force with their more-impaired limb than their relative capacity. Bilateral force coordination was significantly impaired in stroke survivors, but its degree of impairment was not related to their unilateral impairments.
INTRODUCTION
Significant functional impairment of upper extremity (UE) is prevalent after stroke (1), and various patterns of behavioral (mal)adaptation of UE function emerge; for unimanual tasks that primarily require the use of a single arm, use of compensatory strategies (2) or even behavioral suppression of the use of more-affected arm (“learned nonuse”; 3), is commonly observed among chronic stroke survivors. For activities that require concurrent use of both arms, significant impairments in their movements are commonly observed, such as temporal lag between the movements of two arms and delayed task completion (4).
The question remains as to where the observed impairments originate from. In bimanual force production, task performance of stroke survivors is generally poor (5, 6), as they exhibit increased asymmetry and reduced coordination (5, 7). Do such impaired performances mainly reflect unilateral impairments of the more-impaired limb, or do they rather represent a separate impairment in bilateral coordination? For instance, is the force asymmetry in bimanual task merely due to (unilateral) weakness of more-impaired arm, or does it rather represent a distinct feature of altered bilateral motor control following stroke?
Bimanual force production is a decision-making process that considers the ability of each arm to perform the task in terms of reliability, as measured via variance, as well as its effort cost in terms of forces required with respect to maximum capability (8). It is possible that task participation (force production) of the more-impaired arm of stroke survivors is further discouraged, beyond its weakness, because of impaired force control (9). Conversely, stroke survivors may “choose” to increase the contribution of the more-impaired arm, more than its relative capacity, to achieve bilateral symmetry (10). To properly answer these questions, bilateral impairments of stroke survivors should be examined in relation to their unilateral impairments such as weakness and loss of control.
As of yet, relationships between the unilateral/bilateral impairments of stroke survivors and their bimanual task performance have not been well described. Bilateral force asymmetry of stroke survivors has not been viewed as an outcome of the decision-making (“choice” of allocating forces to two arms) process, affected by unilateral impairments, but rather regarded as a discrete motor impairment that emerges after stroke. Previous studies, for instance, primarily focused on elucidating between-group differences (i.e., controls vs. stroke) in their performance measures, such as force asymmetry and temporal force coordination between the limbs (5–7, 11) during bimanual tasks, whereas their relationship with unilateral impairments such as weakness remains largely unexplained. It is possible that bilateral and unilateral impairments of stroke survivors are related to each other as both bilateral and unilateral impairments are determined by stroke characteristics such as lesion location (12–14) or hemisphere of damage (15, 16). However, this remains a relatively underexplored area of work, particularly with respect to how motor costs associated with task conditions might influence their intrinsic decision-making processes.
To quantify how impairments of stroke survivors (e.g., weakness, loss of control) affect their intrinsic decision-making process, we should clarify how motor costs associated with the task affect the decision. This relationship can be explored by inducing systematic changes in the task conditions (such as arm postures), which could affect the motor costs associated with the two arms differently (e.g., Ref. 17).
In this study, we examined how unilateral and bilateral impairments of stroke survivors affected their decision-making process during bimanual task. Chronic stroke survivors and neurologically intact subjects participated in an experiment in which they produced bimanual forces at different arm configurations. We examined two measures of unilateral impairments (force magnitude/angle of the more-impaired arm) and one measure of bilateral impairments (bilateral force redistribution across postures) of stroke survivors.
We hypothesized that the weakness of the paretic arm will primarily explain the participation (force contribution) of the more-impaired arm, due to its functional significance (18, 19). We expected that force deviation, caused by abnormal synergy affecting control of the paretic arm, will also correlate with its force contribution, but to a lesser degree, as directional control of arm movements of many stroke survivors is found largely preserved (20). We also anticipated that stroke survivors will have difficulty in adjusting force contribution of two arms across postures, as postural changes could have a greater impact on volitional muscle activities for stroke survivors when compared with controls (21); such impairments at a higher-level control would be signified by impaired correlation between the two forces across conditions (e.g., postures). We expected that the severity of bilateral impairments and that of unilateral impairments will be correlated with each other. Finally, we expected to observe a significant difference in the motor control of both arms during bilateral task between the subjects with right-hemisphere stroke and those with left-hemisphere stroke, as shown in previous studies (15, 16, 22, 23).
METHODS
Subjects
Twenty-three subjects, including 10 chronic stroke survivors (age: 36–65 yr, mean 58 ± 13 yr; Fugl-Meyer upper extremity score = 37 ± 9; Table 1) and 13 right-handed control subjects (age: 22–73 yr, mean 39 ± 18 yr; 7 female), participated in the study. The inclusion criterion was the ability to perform the target task, i.e., to place hands at the 10 target locations (shown in Fig. 1) and produce voluntary bimanual forces in the three target directions. The experimental protocol was approved by the Institutional Review Boards of the Catholic University of America and the MedStar Health Research Institute, and written informed consent was obtained from each subject before participation.
Table 1.
Characteristics of subjects with stroke
| No. | Age, yr | Sex | Side of hemiplegia | Time since stroke, yr | FMUE (66) | ARAT (57) |
|
|---|---|---|---|---|---|---|---|
| Left Side | Right Side | ||||||
| 1 | 58 | Male | Left | 2 | 28 | 9 | 50 |
| 2 | 65 | Male | Left | 4 | 29 | 32 | 57 |
| 3 | 57 | Male | Left | 2 | 49 | 32 | 50 |
| 4 | 58 | Female | Left | 4 | 36 | 38 | 57 |
| 5 | 71 | Male | Right | 30 | 43 | 54 | 48 |
| 6 | 38 | Male | Right | 10 | 45 | 55 | 37 |
| 7 | 72 | Female | Right | 3 | 44 | 57 | 48 |
| 8 | 36 | Female | Right | 5 | 37 | 56 | 23 |
| 9 | 65 | Male | Right | 3 | 22 | 49 | 47 |
| 10 | 65 | Male | Right | 3 | 37 | 38 | 57 |
ARAT, action research arm test; FMUE, Fugl-Meyer assessment of motor recovery for the upper extremity.
Figure 1.
Experimental setup. A: custom-made apparatus that placed two load cells at 10 locations. B: 10 target locations determined by anthropometric measurements and voluntary reaching distance of each subject. The target force and bimanual force produced by subjects were displayed on the computer screen.
Instrumentation
A custom apparatus was used to place two load cells (Delta SI-330-30; ATI Industrial Automation Inc., Apex, NC) at 10 locations within the workspace of each subject, whose boundaries were determined during experiment (Fig. 1). The apparatus was rigidly connected to the chair that provided back support and stability for the patients. A computer screen was placed in front of the subjects, which displayed the sum of the force produced by the two hands toward the target direction and the target force. A colored time bar graph was also shown to indicate the timing of the task.
Experimental Protocol
Subjects were seated on the chair, to which their torso was strapped. The maximal reach distance of their paretic arm in the anterior direction was measured to determine the voluntary workspace of each subject. Maximal voluntary forces (MVFs) in three directions (anterior, lateral, and superior) were then recorded at the center location (hand location 1 in Fig. 1), and the target force level was set to 40% of MVF in each direction.
Subjects held the handles placed at one of the 10 target locations within their workspace, whose boundaries were determined by their shoulder width (lateral), shoulder and waist height levels (superior), and 40% and 80% of their voluntary reaching distance (anterior) (Fig. 1). The order of the locations to be tested was randomized across subjects.
At each location, subjects produced bimanual forces toward each of the three target directions (anterior, lateral, and superior). They were informed that the sum of the forces produced by their two arms would be displayed on the screen. For the lateral force, stroke survivors produced forces toward their more-impaired side and control subjects toward the right side. For each direction, the target force magnitude was set as 40% of their maximum voluntary force, and the target force and the sum of forces produced by two arms toward the target direction were displayed on the computer screen as vertical bars. Each trial lasted 6 s, and three trials were performed for each force direction at each target location. A 1-min rest period was provided between each trial, and a 5-min rest period was administered between the various locations.
Data Analysis
1) Task performance: The task performance was evaluated by the force error (FE), difference between the target force, and the bimanual force produced in the target direction. For each trial, a 1-s moving window was applied to find the time during which the task error, defined as a root-mean-square-error (RMSE) between the target force and the bimanual force (sum of the two forces in the target direction), was minimized. FE was defined as the RMSE value during the 1-s period with the lowest error.
2) Motor control measures: From the force data recorded by the load cells, the following measures that quantify motor control characteristics of bimanual performance were computed.
First, for the MVF trials, the force vectors of the two arms were computed when the maximum force was produced. A 0.5-s sliding window was applied to find the period where the force magnitude was the largest. These maximum force vectors are used to compute force ratios (OFRmax and TFRmax).
Then, for each trial of task performance, the average force vectors of the two arms were first computed for the 1-s period where the force error was minimized; control: FD (dominant) and FND (nondominant); stroke: FLI (less-impaired) and FMI (more-impaired). These force vectors were used to compute force ratios between two limbs, deviations from the target direction, and the degree of postural adjustment due to the change in target locations.
To compute the outcome measures, first, the overall magnitudes of the force vectors (F) and the magnitudes of the force component in the target force direction (Fk) were computed, which were used to calculate two force ratios, overall force ratio (OFR) and target force ratio (TFR). OFR denotes the ratio of total force magnitudes produced by the two arms, and TFR is the ratio of their actual task contributions (i.e., ratio of the force magnitudes in the target direction).
Here, denotes a two-norm of the force vector F (ND: nondominant, D: dominant; MI: more-impaired, LI: less-impaired), and the kth component of the vector F that contributes to the target force (k = 1: anterior, 2: lateral, 3: superior).
Then, for each of the three directions (anterior, lateral, and superior), the amount of deviation of the force vectors from the target direction was quantified by the force deviation angles (FDAs) and the angles between the three force vectors (F1, F2, F3): 1) force vector produced by the less-used arm (F1: FND or FMI), 2) force vector produced by the more-used arm (F2: FND or FMI), and 3) the target force vector (F3: Ft).
For the impairment of bilateral coordination, the correlation coefficients (r values) between the force produced by the more-used arm (FND or FMI) and that by the less-used arm (FD or FLI) force across postures were computed for each target force direction.
Lastly, the postural impact of the bimanual force production was quantified by the modulation range (MR) percentage in the force magnitudes across the target locations. For a given force direction, the maximum and minimum forces produced by each arm were identified across 10 target locations (i.e., 10 arm postures), and the force MR percentage of each arm was computed by subtracting its minimum force magnitude from the maximum magnitude (i.e., force range), normalized by the target force magnitude. The MR values quantify the degree of changes in the force magnitude of each arm across the target locations.
where i denotes the side (ND/MI or D/LI), and j the target location (1–10).
3) Statistical analysis: Four types of statistical analyses were performed. First, to examine how task performance and motor control measures are different between groups (control vs. stroke: between-subject) and across task types (target force direction: within-subject), force error (FE), force ratios (OFR and TFR), and the force deviation angle (FDA) for the three target force directions were compared using univariate (FE and FDA) or multivariate (OFR/TFR) analyses of variance (ANOVA/MANOVA) with the subject group (control/stroke) as a between-subject factor and the target force direction as a within-subject factor (SPSS Statistics ver. 27; IBM Corp., Armonk, NY). Follow-up analyses (post hoc comparisons) were conducted on the variables with significant differences found between the force directions, with Bonferroni adjustments for multiple comparisons.
Second, to examine the impact of (intrinsic) changes in task conditions, i.e., postural changes across target locations, the normalized modulation ranges (MRs) of the three forces for each arm was compared between the subject groups via MANOVA. Post hoc comparisons for each force direction were performed on the variables with significant differences, with Bonferroni adjustments.
Third, two correlation analyses were performed to examine the relationships between task contribution and impairments in motor control of stroke survivors. The first correlation analyses were performed between the force magnitudes of two arms across 10 target locations to evaluate subjects’ ability to modulate two forces upon postural changes that affected biomechanical efficiency of two arms differently. For each subject, the correlation coefficient (r value) and the slope of the dominant/less-impaired force and the nondominant/more-impaired force relation was computed for a given target force direction. The second correlation analysis was performed between the asymmetry in force contribution (TFR). The following motor control impairment measures of the more-impaired or nondominant arm were used to represent the unilateral impairments: 1) relative force capacity (TFRmax: TFR values estimated during maximum force production), 2) force magnitude averaged across postures (FND: control or FMI: stroke), and 3) force deviation angle (FDA) averaged across postures (θND: control or θMI: stroke), which were computed for each subject and each target force direction (control: 13 subjects × 3 force directions = 39 data points; stroke: 10 subjects × 3 force directions = 30 data points). These measures represent force capacity (TFRmax), average force generated (FND/FMI), and average force deviation (θND/θMI) of the more-impaired/nondominant arm of each subject. For the bilateral impairment, the correlation coefficients (r values) between the dominant/less-impaired force and the nondominant/more-impaired force across postures were used. These measures were also computed for each subject and each target force direction (control: 39 data points; stroke: 30 data points). The purpose was to examine how different motor impairments of more-impaired (or nondominant) arms affect their task participation.
Lastly, we examined the effects of the lesion side on bilateral coordination. Previous studies showed that different patterns of impairments in the bilateral coordination were observed between those with the right-side and the left-side impaired (16). Therefore, we compared the task performance and motor control measures (OFR, TFR, and force deviation) between the two groups (right-impaired vs. left-impaired) in a post hoc analysis.
RESULTS
Different patterns of bimanual task performance were observed between the control subjects and subjects with stroke. During maximal force production (Fig. 2), control subjects generally produced a similar level of force with their two arms (Fig. 2A1), whereas subjects with stroke produced much smaller force with their more-impaired arm (Fig. 2B1). During submaximal (target) force production, subjects with stroke typically produced a larger force with their more-impaired arm (Fig. 2B2) than their capacity ratio (observed in maximal force; Fig. 2B1), but its contribution was further reduced at certain target locations (Fig. 2B3). In contrast, the magnitudes of the forces produced by the two arms of control subjects during submaximal force production were similar across different target location (Figs. 2, A2 and A3).
Figure 2.
Representative cases of bimanual force production. A: control subject (C5). B: subject with stroke (S4). Control subjects produced a similar level of forces with their two arms during the maximum voluntary force production and during the target force production (A1–A3). In contrast, subjects with stroke produced significantly smaller forces with their more-impaired arm during maximal force production (B1). However, at the same target location, when they produced submaximal forces, the contribution of the more-impaired arm was generally higher (B2). But the force produced by the more-impaired arm often decreased significantly when the target location changed (B3).
Unilateral Impairments: Weakness and Force Deviation
We observed a significant between-group difference in the force ratios during maximal force production (F2,59 = 6.90; ηp2 = 0.19; P < 0.01). Control subjects produced approximately the same amounts of force with their arms (OFRmax means ± SD = 1.06 ± 0.36), but stroke survivors produced significantly smaller forces with their more-impaired arms compared with the control subjects (OFRmax means ± SD = 0.43 ± 0.36) (F1,59 = 11.49; ηp2 = 0.16; P < 0.01) (Fig. 3A1). Contribution of the more-impaired arms toward the target force direction, quantified by the TFRmax values, was also significantly lower across all directions during maximal force production (means ± SD = 1.01 ± 0.35 vs. 0.46 ± 0.33) (F1,59 = 9.93; ηp2 = 0.14; P < 0.01) (Fig. 3A2). Post hoc comparisons showed that the between-group difference in the TFRmax values was significant in all directions. Thus, subjects with stroke exhibited weakness in their more-impaired arm.
Figure 3.
Task performance and force asymmetry: A and B: overall force ratios (OFRs) and target force ratios (TFRs) during maximal force production (A1: OFRmax, A2: TFRmax) or task performance (B1: OFR, B2: TFR). C: force deviation of the two arms (C1: more-impaired/nondominant, C2: less-impaired/dominant). D: force error during task performance.
During targeted task performance (submaximal force production), the between-group differences in the force ratios became smaller than those observed in maximal force production (F2,59 = 4.64; ηp2 = 0.14; P = 0.02) (Fig. 3B1). The OFR values of stroke survivors were generally smaller, but in the post hoc comparisons, a statistically significant difference was found only in the superior direction. The TFR values of stroke survivors were overall smaller than those of control subjects, but a statistically significant difference was found only in the anterior direction (Fig. 3B2). Thus, during submaximal force production, the contribution of the less-used arm in the patients with stroke was greater than would be expected from the weakness that they exhibited in that arm.
The degree of force deviation from the target direction was significantly greater in stroke survivors, in both their less-used arm (FDA13: F1,59 = 39.00; ηp2 = 0.40; P < 0.01) and their more-used arm (FDA23: F1,59 = 8.07; ηp2 = 0.12; P < 0.01) (Fig. 3, C1 and C2). The angle between the forces of the two arms of stroke survivors was also significantly greater than that of control subjects (FDA12: F1,59 = 49.47; ηp2 = 0.46; P < 0.01) (Fig. 3C3). The post hoc comparisons showed that the group difference in the force direction of the more-used arm (dominant vs. less-impaired) was mainly due to the difference observed in the lateral force production, during which the force direction of the less-impaired arm of stroke survivors was similar to that of their more-impaired arm (Fig. 3C3). Thus, the subjects with stroke had greater errors in the direction of force that they produced with each arm, with this error being greater in their more-impaired arm.
A significantly larger amount of force-matching errors (difference between the target and actual forces) was observed in subjects with stroke, when compared with control subjects, particularly in the superior and anterior directions (P < 0.05; Fig. 3D) during their target task performance.
Therefore, although the maximal force capacity of the more-impaired arm of stroke survivors was significantly lower than that of their less-impaired arm, with a higher degree of force deviation, its force contribution during submaximal tasks was greater than its relative force capacity.
Bilateral Impairments: Impaired Bimanual Force Coordination across Postures
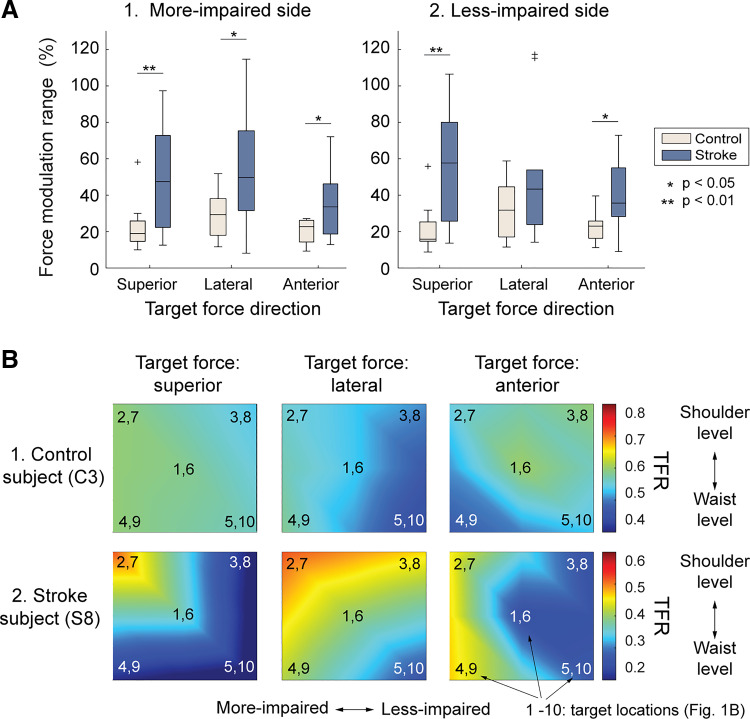
The impact of postural change on bimanual coordination was greater for stroke survivors, as the force modulation ranges (MRs) of both arms of subjects with stroke (across target locations) were significantly greater than those of control subjects (both P values < 0.01; Fig. 4A). Generally, the contribution of the more-impaired (stroke) arms increased for the targets located on the more-impaired side (Fig. 4B).
Figure 4.
Changes in force contribution of the less-preferred limb across target locations. A: modulation range (%) of the force produced by less-preferred limbs (A1: control; A2: stroke). B: representative plots of relative force contribution of nondominant/more-impaired arms (TFR) at five target locations in the frontal plane, averaged across anterior directions (control subject C3; stroke subject S8). The modulation ranges of subjects with stroke were significantly greater than those of control subjects except the lateral force of the more-impaired side. The force contribution of more-impaired arm increased at the targets located in the more-impaired side. TFR, target force ratio.
Furthermore, stroke survivors were not able to efficiently adjust the magnitudes of forces produced by two limbs across different target locations as control subjects do. For control subjects, forces produced by the dominant arm and by the nondominant arm exhibited a strong negative correlation (means ± SD: r = −0.94 ± 0.13) across the 10 target locations, with a slope close to −1 (nondominant/dominant; means ± SD: s = −0.95 ± 0.12), indicating the rates of changes for the two forces were approximately the same across postures (i.e., different target locations) (Fig. 5A; Table 2).
Figure 5.
Representative relationships between the force magnitude of the two limbs. A: control subject (C8): dominant and nondominant side forces. B: stroke survivor (S7): less-impaired and more-impaired side forces.
Table 2.
Correlation coefficients and slope
| Group | Direction | Correlation Coefficient |
Slope |
|---|---|---|---|
| Means (SD) | Means (SD) | ||
| Control | Anterior | −0.89 (0.19) | −0.87 (0.22) |
| Lateral | −0.83 (0.25) | −0.85 (0.20) | |
| Superior | −0.95 (0.08) | −0.99 (0.10) | |
| Mean | −0.90 (0.19) | −0.91 (0.19) | |
| Stroke | Anterior | −0.74 (0.38) | −0.77 (0.41) |
| Lateral | −0.57 (0.64) | −0.60 (0.53) | |
| Superior | −0.80 (0.20) | −0.84 (0.23) | |
| Mean | −0.69 (0.44) | −0.73 (0.41) |
Means (SD) values of the correlation coefficient and slope.
In contrast, the forces by the more-impaired and less-impaired sides of stroke survivors showed a weaker correlation across postures (means ± SD: r = −0.69 ± 0.50) with a smaller slope (more-impaired/less-impaired; means ± SD: s = −0.73 ± 0.54); subjects with stroke had difficulty distributing two forces properly upon postural changes (smaller r values), and that they relied more on their less-impaired limb to adapt to the postural changes (low slopes) (Fig. 5B; Table 2).
Thus, stroke survivors exhibited a higher level of bilateral impairment, as they modulated two forces to a larger degree across hand locations but in a less coordinated manner than control subjects did.
Relationship between Unilateral Impairments, Bilateral Impairments, and Task Performance
The question remains whether the observed bilateral asymmetry of subjects with stroke is related to their weakness, loss of directional control, or impaired bimanual coordination. How much of the observed bilateral asymmetry of stroke survivors can be explained by the their unilateral and/or bilateral impairments?
Among the unilateral and bilateral impairments of stroke survivors (unilateral: relative and absolute strength, and force deviation of the more-impaired/nondominant arm; bilateral: force coordination across target locations), only the unilateral impairments of the more-impaired arm, i.e., relative strength and force directional deviation, were significantly correlated with its task participation. Namely, the relative contribution of their more-impaired arm (TFR) was found to significantly correlate with the relative maximum force ratio (OFRmax; r = 0.65; P < 0.01) and negatively correlate with the force deviation of the more-impaired (MI) side (r = −0.46; P = 0.02), but not with its absolute force magnitude () (Fig. 6B). The two unilateral impairments of stroke survivors (OFRmax and θMI) were also correlated with each other (r = −0.56; P = 0.01) (Table 3).
Figure 6.
Correlation between target force contribution (TFR) and unilateral impairments of the more-impaired/nondominant limb. A: control subjects. B: stroke survivors. Maximum force ratio (OFR: 1st column); force magnitude of nondominant (FND: control) or more-impaired (FMI: stroke) limb (2nd column); and force deviation of nondominant (θND: control) or more-impaired (θMI: stroke) limb (3rd column). In each graph, 39 data points (13 control subjects × 3 force directions) or 30 points (10 stroke subjects × 3 force directions) were included. Contribution of nondominant arm (TFR) of control subjects was not affected by any of the factors examined, but remained similar to that of dominant arm across conditions (TFR ≈ 1). TFR of stroke survivors, on the other hand, was found to significantly correlate with the relative force capacity (OFRmax) and force deviation of their more-impaired arm (θMI). OFR, overall force ratio.
Table 3.
Correlation coefficient and P values between variables that measured unilateral/bilateral impairments and task performance of stroke survivors
| Impairments |
Task Performance |
||||
|---|---|---|---|---|---|
| Unilateral |
Bilateral |
||||
| Weakness (OFRmax) | Loss of control (θMI) | Bimanual Incoordination (r Value)* | Force Asymmetry (TFR) | Force Error (FE) | |
| Impairments | |||||
| Weakness | 1 |
r = −0.56
(P < 0.01) |
r = −0.01 (P = 0.94) |
r = 0.65
(P < 0.01) |
r = −0.10 (P = 0.62) |
| Loss of control |
1 |
r = −0.01 (P = 0.98) |
r = −0.46
(P = 0.02) |
r = 0.00 (P = 0.99) |
|
| Bimanual incoordination | 1 |
r = 0.10 (P = 0.61) |
r = −0.25 (P = 0.17) |
||
| Task performance | |||||
| Force asymmetry | 1 |
r = 0.02 (P = 0.93) |
|||
| Force error | 1 | ||||
*Boldface denotes significant correlations. Bimanual incoordination, correlation between two forces across postures; FE, force error; OFRmax, overall force ratio during maximal force production; r, correlation coefficient; TFR, target force ratio; θMI, force angle of the more-impaired/MI arm.
In contrast, TFR values of control subjects were correlated with none of the three factors examined (Fig. 6A).
In addition, no significant impact of lesion side on the bimanual performance was found, as all P values for the five outcome measures (OFRmax, TFRmax, OFR, TFR, and θMI) were greater than 0.05.
DISCUSSION
Both weakness and abnormal synergy are commonly observed among chronic stroke survivors, each of which could contribute to their functional deficits (1, 24–26). Our results show that reduced use of the more-impaired arm during bimanual force production of stroke survivors could be related to their unilateral impairments, weakness (which limited force magnitude) and abnormal synergy (which led to larger force deviation) of their more-impaired arm, but not to their bilateral impairments (i.e., bilateral incoordination).
Impact of Weakness on Task Participation
During the submaximal bimanual force production, the force magnitudes produced by the two arms were significantly different for stroke survivors, whereas the magnitude of the forces were similar for control subjects. Note that the target tasks in our experiment, i.e., force production in anterior, superior, and lateral directions, required mostly shoulder flexion/abduction, and/or elbow extension, all of which were found particularly weakened following stroke (25, 27). Thus, the observed difference in the force magnitudes between the arms of stroke survivors appears to reflect their weakness.
However, the level of task participation of the more-impaired arm of stroke survivors was not simply proportionate to their relative strength. The magnitude of the force produced by their more-impaired arm was ∼80% of their less-impaired arm during the task (mean OFR = 0.79), which was considerably higher than their relative maximal capacity, estimated from the maximum force production trials (mean OFRmax = 0.49). The relative force magnitude of the more-impaired arm of stroke survivors was also higher than previously reported values of force-generating capacities of the more-impaired arm; the maximum joint moments of the more-impaired arm of chronic stroke survivors was found to be ∼60% of those of their less-impaired side for the shoulder joints (28, 29), and ∼70% for the elbow joints (30, 31).
It is possible that stroke survivors attempted to maintain force symmetry, thereby producing a larger force than their relative capacity on their more-impaired side. During bimanual tasks, the task contribution of paretic arm was previously shown to increase under symmetric condition (i.e., performing the same task adopting similar postures; 10). It appears that weakness may have limited force-generating capacity of the more-impaired arms of stroke survivors, but they still attempted to achieve more symmetry during bimanual force production. Note that, similarly, some control subjects with asymmetric force capacity achieved higher force symmetry during task (Fig. 6A).
Impact of Force Deviation on Task Participation
The force deviation of the more-impaired arm of stroke survivors also had a large impact on the task contribution, as the target force contribution of their more-impaired arms (mean TFR = 0.47) was significantly smaller than their relative force magnitude (mean OFR = 0.79), mainly due to the large force deviation from the target direction (Fig. 2C). Such deviation can be explained by their abnormal muscle synergy (32), contributing to a loss of independent muscle control required for directional control (20).
Results from the correlation analyses indicate that between-subject variability of stroke survivors in their task participation (force asymmetry) is explained by both their weakness (OFRmax) and loss of directional control (θMI). We thus posit that task participation/contribution of the more-impaired arm during the current task should be understood as a choice, implicitly made by each subject, which can be influenced by interaction between effort and reward (in this case, force symmetry and task efficiency) (33). Although subjects with stroke attempted to achieve force symmetry during task performance, possibly to achieve “perceptual” symmetry despite their “motoric” asymmetry (34), they also considered task efficiency in producing bimanual force, which was affected by force deviation. As the degree of force deviation increased for some subjects with stroke, conversion of the effort (energy expenditure) to the target goal (target force increase) should have become smaller, discouraging them from engaging their more-impaired arm in the task (i.e., participation). For an explicit choice task (i.e., reaching task for which the arm to be used can be chosen), the arm choice of stroke survivors is affected by the expected level of task success (35). A recent study also showed that the Fugl-Meyer upper extremity score, which includes items that quantify the degree of abnormal synergy (multijoint control) of stroke survivors, is a good predictor of their arm use in daily activities (36). Note that, to a certain degree, stroke survivors retain the ability to modulate the task performance of their more-impaired limb depending on the task context (37). Therefore, it is possible that stroke survivors also retain their ability to detect “efficacy” of their more-impaired arm (discounted by force deviation) and choose to get their “more-efficient” arm to be more involved in the task in case their more-impaired arm becomes less effective.
Here, we also postulate that the observed impairments in the force directional control could be in part due to our experimental task conditions, in which only visual feedback of the target force magnitude was provided to subjects. In dynamic conditions (i.e., reaching movements), directional control of stroke survivors (with moderate or mild impairments) was found to be largely preserved (20, 38). During our experiment, it is possible that stroke survivors could not rectify the impact of abnormal synergy due to the lack of proprioceptive (no movements) or visual (no feedback regarding force direction) feedback. Correction of the abnormal force direction, which should have been caused mainly by abnormal muscle activation, could have been difficult for most stroke survivors as they commonly experience sensory impairment, especially of tactile discrimination (39) that would be required for detecting isometric force directions. Here, our experimental setup (i.e., isometric) does not provide proprioceptive feedback, which was also found to play an important role in increasing coordination and neural coupling between the two arms during bimanual coordination (40). Note that directional force control of the ipsilesional side of stroke survivors is also impaired (41), particularly for the mediolateral directions, which could have contributed to the large force deviation of the less-impaired arm (Fig. 3C).
Implications
Despite weakness affecting their more-impaired limb, stroke survivors may attempt to achieve/improve force symmetry during bimanual task, as the contribution of their more-impaired arm is greater than its relative strength (defined by OFRmax). This suggests that bimanual training of symmetric movements (or symmetric force production tasks) could be beneficial in promoting task participation of more-impaired arm in stroke rehabilitation. Previous studies indeed found that bilateral training for upper extremity could be more effective than unilateral training in inducing functional improvement (42) and facilitate cortical remodeling better than unilateral training (43).
Our results also emphasize the importance of directional control of more-impaired arm of stroke survivors during bimanual tasks in their arm use/participation. Note that, in dynamic tasks, proprioceptive deficits that degrade directional control of movements were found to significantly degrade task performance (10). Therefore, restoring both strength and directional control of more-impaired arms should be emphasized in bimanual training, as it could lead to increased task participation of their more-impaired arms.
The outcome of this study can also be interpreted as evidence that the relationships between the effort and reward (or task achievement) in the bimanual task may change after stroke. For neurotypical subjects, arm use is determined by the motor cost (effort) and task performance such as task success (44) or task variability (8). Our results suggest that stroke survivors may attempt to achieve force symmetry by producing higher force with their more-impaired limb than their relative capacity, which would require more effort/energy. Bimanual force production could thus promote the use of the more-impaired arm of stroke survivors during training, providing additional rehabilitative benefits.
Limitations of the Study
Subjects produced bimanual force toward only three directions, out of six possible directions, which were chosen to represent multijoint movement patterns that are typically difficult for stroke survivors to perform (i.e., requiring mostly out-of-synergy joint coordination patterns). Further studies, however, are warranted to confirm similar findings in the bimanual force production toward other directions. In addition, control subjects produced lateral forces toward their dominant (more-used) side, whereas the target force direction for subjects with stroke was their more-impaired (less-used) side. This could have contributed to the between-group difference in the motor control measures.
Subjects with stroke were generally older than control subjects, which could have contributed to the observed between-group differences in the outcome measures. However, a previous study (45) also showed that motor adaptation during bimanual coordination by older subjects becomes similar to that of younger subjects with sufficient amount of visual feedback provided. Vieluf et al. (46) also showed that, while temporal coordination of bimanual coordination by older subjects is degraded, spatial bimanual coordination is generally preserved.
Some of the findings of this study may not be generalizable due to our subject characteristics. Subjects with stroke who participated in the study, due to our exclusion criteria, were limited to those with moderate to mild motor impairments, excluding those with severe or mild impairments (i.e., FMUE <16 or >53; 47). The findings of this study, therefore, may not apply to those with more severe and/or mild impairments. We also did not control lesion locations when recruiting subjects with stroke, which could have contributed to the between-subject variability of the outcome measures; for instance, previous studies showed that those with a lesion close to the corpus callosum or supplementary motor area may exhibit profound bimanual performance deficits while showing only mild unilateral deficits (12, 13). In addition, a relatively small number of subjects could have resulted in a type II error, specifically for the impact of lesion side, as we only recruited four left-impaired subjects.
Some subjects adopted compensatory strategies, involving torso movements (mainly tilting and twisting, as the torso was strapped to the chair), during force production. Experimenters instructed subjects not to use such strategy, whenever detected, but it is possible that stroke survivors used torso movements to a certain degree during task; this could have affected their bimanual force production.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
H.N., T.P., and S.W.L. were supported by grants from the National Institute on Disability, Independent Living, and Rehabilitation Research (90REGE0004 and 90REMM0001). R.S. was supported by grants from the National Institutes of Health (R01-NS078311, R01-EB028156, and R01-NS096083) and the National Science Foundation (CNS-1714623).
DISCLOSURES
R. Shadmehr is an editor of Journal of Neurophysiology and was not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
T.P., R.S., and S.W.L. conceived and designed research; H.N., T.P., and S.W.L. performed experiments; H.N., T.P., and S.W.L. analyzed data; H.N., T.P., R.S., and S.W.L. interpreted results of experiments; T.P. and S.W.L. prepared figures; H.N. drafted manuscript; H.N., T.P., R.S., and S.W.L. edited and revised manuscript; H.N., T.P., R.S., and S.W.L. approved final version of manuscript.
REFERENCES
- 1. Raghavan P. Upper limb motor impairment post stroke. Phys Med Rehabil Clin N Am 26: 599–610, 2015. doi: 10.1016/j.pmr.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain 123: 940–953, 2000. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 3. Taub E, Uswatte G, Mark VW, Morris DMM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys 42: 241–256, 2006. [PubMed] [Google Scholar]
- 4. Kantak S, Jax S, Wittenberg G. Bimanual coordination: a missing piece of arm rehabilitation after stroke. Restor Neurol Neurosci 35: 347–364, 2017. doi: 10.3233/RNN-170737. [DOI] [PubMed] [Google Scholar]
- 5. Kang N, Cauraugh JH. Force control improvements in chronic stroke: bimanual coordination and motor synergy evidence after coupled bimanual movement training. Exp Brain Res 232: 503–513, 2014. doi: 10.1007/s00221-013-3758-z. [DOI] [PubMed] [Google Scholar]
- 6. Patel P, Lodha N. Dynamic bimanual force control in chronic stroke: contribution of non-paretic and paretic hands. Exp Brain Res 237: 2123–2133, 2019. doi: 10.1007/s00221-019-05580-5. [DOI] [PubMed] [Google Scholar]
- 7. Lodha N, Coombes SA, Cauraugh JH. Bimanual isometric force control: asymmetry and coordination evidence post stroke. Clin Neurophysiol 123: 787–795, 2012. doi: 10.1016/j.clinph.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 8. Salimpour Y, Shadmehr R. Motor costs and the coordination of the two arms. J Neurosci 34: 1806–1818, 2014. doi: 10.1523/JNEUROSCI.3095-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lum PS, Burgar CG, Kenney DE, Van der Loos HM. Quantification of force abnormalities during passive and active-assisted upper-limb reaching movements in post-stroke hemiparesis. IEEE Trans Biomed Eng 46: 652–662, 1999. doi: 10.1109/10.764942. [DOI] [PubMed] [Google Scholar]
- 10. Kantak SS, Zahedi N, McGrath RL. Task-dependent bimanual coordination after stroke: relationship with sensorimotor impairments. Arch Phys Med Rehabil 97: 798–806, 2016. doi: 10.1016/j.apmr.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 11. Chang S-H, Durand-Sanchez A, Ditommaso C, Li S. Interlimb interactions during bilateral voluntary elbow flexion tasks in chronic hemiparetic stroke. Physiol Rep 1: e00010, 2013. doi: 10.1002/phy2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brainin MA, Seiser A, Matz K. The mirror world of motor inhibition: the alien hand syndrome in chronic stroke. J Neurol Neurosurg Psychiatry 79: 246–252, 2008. doi: 10.1136/jnnp.2007.116046. [DOI] [PubMed] [Google Scholar]
- 13. Brugger F, Galovic M, Weder BJ, Kägi G. Supplementary motor complex and disturbed motor control—a retrospective clinical and lesion analysis of patients after anterior cerebral artery stroke. Front Neurol 6: 209, 2015. doi: 10.3389/fneur.2015.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Konczak J, Pierscianek D, Hirsiger S, Bultmann U, Schoch B, Gizewski ER, Timmann D, Maschke M, Frings M. Recovery of upper limb function after cerebellar stroke: lesion symptom mapping and arm kinematics. Stroke 41: 2191–2200, 2010. doi: 10.1161/STROKEAHA.110.583641. [DOI] [PubMed] [Google Scholar]
- 15. Mani S, Mutha PK, Przybyla A, Haaland KY, Good DC, Sainburg RL. Contralesional motor deficits after unilateral stroke reflect hemisphere-specific control mechanisms. Brain 136: 1288–1303, 2013. doi: 10.1093/brain/aws283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schaffer JE, Maenza C, Good DC, Przybyla A, Sainburg RL. Left hemisphere damage produces deficits in predictive control of bilateral coordination. Exp Brain Res 238: 2733–2744, 2020. doi: 10.1007/s00221-020-05928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Córdova Bulens D, Crevecoeur F, Thonnard J-L, Lefèvre P. Optimal use of limb mechanics distributes control during bimanual tasks. J Neurophysiol 119: 921–932, 2018. doi: 10.1152/jn.00371.2017. [DOI] [PubMed] [Google Scholar]
- 18. Chae J, Yang G, Park BK, Labatia I. Muscle weakness and cocontraction in upper limb hemiparesis: relationship to motor impairment and physical disability. Neurorehabil Neural Repair 16: 241–248, 2002. doi: 10.1177/154596830201600303. [DOI] [PubMed] [Google Scholar]
- 19. Harris JE, Eng JJ. Paretic upper-limb strength best explains arm activity in people with stroke. Phy Ther 87: 88–97, 2007. doi: 10.2522/ptj.20060065. [DOI] [PubMed] [Google Scholar]
- 20. Reinkensmeyer DJ, McKenna Cole A, Kahn LE, Kamper DG. Directional control of reaching is preserved following mild/moderate stroke and stochastically constrained following severe stroke. Exp Brain Res 143: 525–530, 2002. doi: 10.1007/s00221-002-1055-3. [DOI] [PubMed] [Google Scholar]
- 21. Lewek MD, Schmit BD, Hornby TG, Dhaher YY. Hip joint position modulates volitional knee extensor muscle activity after stroke. Muscle Nerve 34: 767–774, 2006. doi: 10.1002/mus.20663. [DOI] [PubMed] [Google Scholar]
- 22. Kang N, Cauraugh JH. Right hemisphere contributions to bilateral force control in chronic stroke: a preliminary report. J Stroke Cerebrovasc Dis 27: 3218–3223, 2018. doi: 10.1016/j.jstrokecerebrovasdis.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 23. Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia 47: 2953–2966, 2009. [Erratum in Neuropsychologia 48: 1178–1180, 2010]. doi: 10.1016/j.neuropsychologia.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ada L, O'Dwyer N, Green J, Yeo W, Neilson P. The nature of the loss of strength and dexterity in the upper limb following stroke. Hum Mov Sci 15: 671–687, 1996. doi: 10.1016/0167-9457(96)00015-2. [DOI] [Google Scholar]
- 25. Canning CG, Ada L, Adams R, O'Dwyer NJ. Loss of strength contributes more to physical disability after stroke than loss of dexterity. Clin Rehabil 18: 300–308, 2004. doi: 10.1191/0269215504cr715oa. [DOI] [PubMed] [Google Scholar]
- 26. Dewald JP, Sheshadri V, Dawson ML, Beer RF. Upper-limb discoordination in hemiparetic stroke: implications for neurorehabilitation. Top Stroke Rehabil 8: 1–12, 2001. doi: 10.1310/WA7K-NGDF-NHKK-JAGD. [DOI] [PubMed] [Google Scholar]
- 27. Sukal TM, Ellis MD, Dewald JP. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res 183: 215–223, 2007. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCrea PH, Eng JJ, Hodgson AJ. Time and magnitude of torque generation is impaired in both arms following stroke. Muscle Nerve 28: 46–53, 2003. doi: 10.1002/mus.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwerin S, Dewald JP, Haztl M, Jovanovich S, Nickeas M, MacKinnon C. Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp Brain Res 185: 509–519, 2008. doi: 10.1007/s00221-007-1169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ekstrand E, Lexell J, Brogårdh C. Isometric and isokinetic muscle strength in the upper extremity can be reliably measured in persons with chronic stroke. J Rehabil Med 47: 706–713, 2015. doi: 10.2340/16501977-1990. [DOI] [PubMed] [Google Scholar]
- 31. Mercier C, Bertrand AM, Bourbonnais D. Differences in the magnitude and direction of forces during a submaximal matching task in hemiparetic subjects. Exp Brain Res 157: 32–42, 2004. doi: 10.1007/s00221-003-1813-x. [DOI] [PubMed] [Google Scholar]
- 32. Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain 118: 495–510, 1995. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 33. Shadmehr R, Reppert TR, Summerside EM, Yoon T, Ahmed AA. Movement vigor as a reflection of subjective economic utility. Trends Neurosci 42: 323–336, 2019. doi: 10.1016/j.tins.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mechsner F, Kerzel D, Knoblich G, Prinz W. Perceptual basis of bimanual coordination. Nature 414: 69–73, 2001. doi: 10.1038/35102060. [DOI] [PubMed] [Google Scholar]
- 35. Kim S, Han CE, Kim B, Winstein CJ, Schweighofer N. Effort, success, and side of lesion determine arm choice in individuals with chronic stroke. J Neurophysiol 127: 255–266, 2022. doi: 10.1152/jn.00532.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lundquist CB, Nielsen JF, Brunner IC. Prediction of upper limb use three months after stroke: a prospective longitudinal study. J Stroke Cerebrovasc Dis 30: 106025, 2021. doi: 10.1016/j.jstrokecerebrovasdis.2021.106025. [DOI] [PubMed] [Google Scholar]
- 37. Ranganathan R, Gebara R, Andary M, Sylvain J. Chronic stroke survivors show task-dependent modulation of motor variability during bimanual coordination. J Neurophysiol 121: 756–763, 2019. doi: 10.1152/jn.00218.2018. [DOI] [PubMed] [Google Scholar]
- 38. Reisman DS, Scholz JP. Aspects of joint coordination are preserved during pointing in persons with post-stroke hemiparesis. Brain 126: 2510–2527, 2003. doi: 10.1093/brain/awg246. [DOI] [PubMed] [Google Scholar]
- 39. Carey LM, Matyas TA, Oke LE. Sensory loss in stroke patients: effective training of tactile and proprioceptive discrimination. Arch Phys Med Rehabil 74: 602–611, 1993. doi: 10.1016/0003-9993(93)90158-7. [DOI] [PubMed] [Google Scholar]
- 40. Nguyen HB, Lee SW, Harris-Love ML, Lum PS. Neural coupling between homologous muscles during bimanual tasks: effects of visual and somatosensory feedback. J Neurophysiol 117: 655–664, 2017. doi: 10.1152/jn.00269.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pellegrino L, Coscia M, Giannoni P, Marinelli L, Casadio M. Stroke impairs the control of isometric forces and muscle activations in the ipsilesional arm. Sci Rep 11: 18533, 2021. doi: 10.1038/s41598-021-96329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stoykov ME, Lewis GN, Corcos DM. Comparison of bilateral and unilateral training for upper extremity hemiparesis in stroke. Neurorehabil Neural Repair 23: 945–953, 2009. doi: 10.1177/1545968309338190. [DOI] [PubMed] [Google Scholar]
- 43. Whitall J, Waller SM, Sorkin JD, Forrester LW, Macko RF, Hanley DF, Goldberg AP, Luft A. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single-blinded randomized controlled trial. Neurorehabil Neural Repair 25: 118–129, 2011. doi: 10.1177/1545968310380685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schweighofer N, Xiao Y, Kim S, Yoshioka T, Gordon J, Osu R. Effort, success, and nonuse determine arm choice. J Neurophysiol 114: 551–559, 2015. doi: 10.1152/jn.00593.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu X, Newell KM. Aging, visual information, and adaptation to task asymmetry in bimanual force coordination. J Appl Physiol (1985) 111: 1671–1680, 2011. doi: 10.1152/japplphysiol.00760.2011. [DOI] [PubMed] [Google Scholar]
- 46. Vieluf S, Godde B, Reuter E-M, Temprado JJ, Voelcker-Rehage C. Practice effects in bimanual force control: does age matter? J Mot Behav 47: 57–72, 2015. doi: 10.1080/00222895.2014.981499. [DOI] [PubMed] [Google Scholar]
- 47. Woytowicz EJ, Rietschel JC, Goodman RN, Conroy SS, Sorkin JD, Whitall J, McCombe Waller S. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer assessment of the upper extremity in chronic stroke. Arch Phys Med Rehabil 98: 456–462, 2017. doi: 10.1016/j.apmr.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.