Abstract
The decline in honey bee colonies in different parts of the world in recent years is due to different reasons, such as agricultural practices, climate changes, the use of chemical insecticides, and pests and diseases. Viral infections are one of the main causes leading to honey bee population declines, which have a major economic impact due to honey production and pollination. To investigate the presence of viruses in bees in southern Brazil, we used a metagenomic approach to sequence adults’ samples of concentrated extracts from Apis mellifera collected in fifteen apiaries of six municipalities in the Rio Grande do Sul state, Brazil, between 2016 and 2017. High-throughput sequencing (HTS) of these samples resulted in the identification of eight previously known viruses (Apis rhabdovirus 1 (ARV-1), Acute bee paralysis virus (ABPV), Aphid lethal paralysis virus (ALPV), Black queen cell virus (BQCV), Bee Macula-like virus (BeeMLV), Deformed wing virus (DWV), Lake Sinai Virus NE (LSV), and Varroa destructor virus 3 (VDV-3)) and a thogotovirus isolate. This thogotovirus shares high amino acid identities in five of the six segments with Varroa orthomyxovirus 1, VOV-1 (98.36 to 99.34% identity). In contrast, segment 4, which codes for the main glycoprotein (GP), has no identity with VOV-1, as observed for the other segments, but shares an amino acid identity of 34–38% with other glycoproteins of viruses from the Orthomyxoviridae family. In addition, the putative thogotovirus GP also shows amino acid identities ranging from 33 to 41% with the major glycoprotein (GP64) of insect viruses of the Baculoviridae family. To our knowledge, this is the second report of a thogotovirus found in bees and given this information, this thogotovirus isolate was tentatively named Apis thogotovirus 1 (ATHOV-1). The detection of multiple viruses in bees is important to better understand the complex interactions between viruses and their hosts. By understanding these interactions, better strategies for managing viral infections in bees and protecting their populations can be developed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01078-z.
Keywords: Honey bee viruses, Metagenomic approach, High-throughtput sequence, New togotovirus isolate, Bee population declines
Introduction
Increased awareness of the significance of insect-mediated pollination of plants in different ecosystems and global food security has caused a surge in research regarding pollinator health, especially for the honeybee, Apis mellifera [1–3]. A. mellifera is naturally found in Europe, Western Asia, and Africa. After spreading all over the world, it is responsible for more than 80% of the agricultural pollination, covering all types of crops [4]. Unfortunately, in the last decade, multiple stressors have worked together to enhance A. millifera population decline. These stressors include an increase in parasite and pathogen incidence, chemical insecticide abuse in agriculture, decrease in food diversity, poor nutrition, climate change, and beekeeper management [5–7]. A significant cause of this decline could be directly related to the increase in the incidence of the parasitic mite Varroa destructor (Acari: Varroidae). This mite feeds on the larvae and adults of developing honeybees and vectors different types of pathogenic viruses [8].
Several honeybee pathologies are associated with viruses that act in a synergistic manner with other biotic and abiotic stressors, likely contributing to the collapse of bee colonies [5–7]. Besides being vectored by parasites like V. destructor, honeybee viruses can be transmitted horizontally and vertically and spread during colony management [9]. Most known and described honeybee viruses are positive-sense single-stranded RNA viruses that can infect all developmental stages of this insect, including eggs, brood (larvae and pupae), and adults. These viruses include members of the families Dicistroviridae, Iflaviridae, Rhabdoviridae, Tymoviridae, Sinhaliviridae, and Orthomyxoviridae. During infection, these viruses may cause a range of clinical signs, such as wing deformities, hair loss, yellow dead larvae in the honeycomb, and behavioral disorders like trembling, paralysis, and disorientation [10]. However, viruses may persist covertly in some honeybee populations, leading to no explicit clinical signs [9], and are believed to contribute to the decrease in their survival time [4, 9].
Several recent studies on the prevalence of viruses in honeybee colonies have mainly been performed using high-throughput sequencing (HTS) [9]. This technology has improved our knowledge about virus covert infections by discovering an impressive diversity of hitherto unknown viruses, which outnumber known disease-causing viruses and asymptomatic viruses [11]. Identifying new insect viruses is of utmost importance in understanding the evolutionary processes that may play a crucial role in virus-pathogen interaction [12]. Therefore, we characterized the viral landscape of A. mellifera honeybees collected in southern Brazil using an HTS approach. Our investigation revealed a diverse array of viruses already described worldwide. Notably, we identified a multipartite negative-sense single-stranded RNA virus isolate belonging to the genus Thogotovirus, which we have designated as Apis thogotovirus 1 (ATHOV-1). Intriguingly, five out of six segments of ATHOV-1 exhibited substantial amino acid similarities to the corresponding segments of the of Varroa orthomyxovirus 1, VOV-1.
Methods
Honeybee sampling
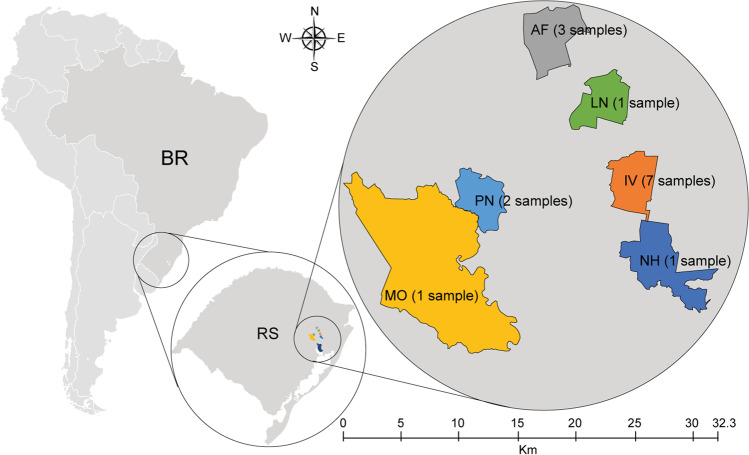
The samples of Apis mellifera were collected in fifteen apiaries in six municipalities (Montenegro, Pareci Novo, Alto Feliz, Linha Nova, Ivoti, and Novo Hamburgo) of the Rio Grande do Sul state in Brazil (map) between 2016 and 2017. The samples contained larvae and adults (Fig. 1). For each of the 15 apiaries, 3 individuals at the larval stage and 3 workers were collected to generate two pools of larvae and adults. The honeybees were sampled alive, directly transported to the laboratory in a cage, euthanized by freezing at − 80 °C, and kept frozen until further analyses. No symptoms of infection were observed in the collected insects.
Fig. 1.
Maps and location of the six municipalities in the Brazilian state of Rio Grande do Sul. The samples were taken between 2016 and 2017. The maps highlight the political demarcation of each city and the number of apiaries targeted by the study: samples from 1 apiary at Montenegro (MO); samples from 2 apiaries at Pareci Novo (PN); samples from 3 apiaries at Alto Feliz (AF); sample from 1 apiary in Linha Nova (LN); samples from 7 apiaries at Ivoti (IV); and samples from 1 apiary in Novo Hamburgo (NH)
RNA extraction and genome sequencing, assembly, and analysis
Individual honeybee pooled samples (200 mg) of adults were homogenized in SM buffer (100 mM NaCl, 8 mM MgSO4, 50 mM Tris–Cl, pH 7.5). The homogenate was filtered once through cheesecloth and centrifuged three times at 4000 × g for 10 min for clarification. The supernatant was filtered through a 0.2-µm filter and centrifuged at 63,000 × g for 75 min in a 38-mL polyallomer ultracentrifuge tube (Beckman). The pellet was resuspended in RNase-free water. RNA was extracted using the ZR Soil/Fecal RNA MicroPrep™ (Zymo Research) according to the manufacturer’s instructions. RNA samples were processed for rRNA removal using the Ribo-Zero rRNA removal kit (Illumina, San Diego, CA, USA) and cDNA library construction using the TruSeq RNA library preparation kit (Illumina). The samples were sequenced at Macrogen (Seoul, Republic of Korea) using the Illumina HiSeq 2000 paired-end method. The raw reads were quality trimmed and de novo assembled using the CLC Genome Workbench 6.5.2 (CLC bio, Qiagen). To identify and classify the contigs associated with the virus, a BLASTx analysis was conducted against a viral RefSeq database obtained from the public database at NCBI, utilizing the software Geneious 11.1.5 [13]. After that, in order to extend the assembled sequences as far as possible, the generated and trimmed reads were mapped back to the respective viral genomes using the same software. Genome annotation was also performed using the same software, in which the open reading frames (ORFs) were assigned using BLASTx searches against the NCBI non-redundant protein database.
Validation of viral sequences
To validate the presence of viral sequences, specifically ATHOV-1 and VOV-1, within the different RNA pools, a reverse transcription (RT) reaction followed by a polymerase chain reaction (PCR) was conducted using segment-specific oligonucleotide primers (Fig. S1). For the detection of ATHOV-1, the primers ATHO-1 (S1) F (5′- TAGAGTCGGTGGTGGAGGAA-3′) and ATHO-1 (S1) R (5′- TGGTAATTGGCGTCCTAGGG-3′) were utilized to amplify a 150-bp fragment from segment 1 (polymerase subunit PB2). Furthermore, the primers ATHO-1 (S4) R (5′- TGGAAGTGGAGCTCTCGGTT-3′) and ATHO-1 (S4) F (5′- TGGTGCCAGCAAGGTGATAA-3′) were employed to amplify a 200-bp fragment from segment 4 (glycoprotein) of the thogotovirus under investigation in this study. For the detection of the VOV-1, specific primers VOV-1 (S4) F (5′- CGCTAGTTCGGGAAGTCTCA – 3′) and VOV-1 (S4) R (3′- AGCCCCTGAGGTACACAAAA – 3′) were designed and used to amplify a 250-bp fragment from the glycoprotein region of segment 4 of the Varroa orthomyxovirus-1 (GenBank accession number MK032468). The selection of the orthomyxovirus sequence as e reference sequence is warranted due to the concerns that the deposited sequence of segment 4 of VOV-1 may potentially be from a rhabdovirus (see the “Results” section).
Phylogenetic analysis
For the phylogenetic analyses, a multiple alignment, using fast Fourier transform method [14], was carried out with amino acid sequences of all major Thogotovirus ORFs available in the Genbank database (Table S2). In the specific case of segment 4, the analysis was performed only with the proteins with the best hits on the BLASTx non-redundant protein database search. Only those hits with e-values of less than 1e − 20 were used. Redundant sequences (with an identity higher than 95%) were omitted from our analysis for clarity. A maximum-likelihood tree was inferred using the Fast-tree method [15], implemented in Geneious R11, and the branch support was estimated by a Shimodaira-Hasegawa-like test [16].
Putative orthomyxovirus ATHOV-1 glycoprotein in silico characterization
The deduced amino acid sequence of the ATHOV-1 segment 4 (S4) was characterized in silico through comparison with glycoproteins from Autographa californica multiple nucleopolyhedrovirus (AcMNPV) (PDB accession number 3DUZ), Dhori virus (PDB accession number 5XEB), Bourbon virus (PDB accession number 5ZKX), and Thogoto virus (PDB accession number 5XEA). First, homology studies using the full S4 protein sequence were performed by protein–protein BLAST (blastp) against the non-redundant sequence database (http://www.ncbi.nlm.nih.gov). From the homologs containing PDB structures, an alignment was performed using ClustalW [17] and further visualized using ESPRIPT software [18] and the Thogoto thogotovirus glycoprotein as a secondary structure protein model. The conservation of its secondary and tertiary structure was evaluated based on a homology model. The best template for the three-dimensional (3D) structure prediction was searched in Expasy SWISS-MODEL Server 59 [19] using the predicted amino acid sequence as a reference. The structural model was obtained from a crystallized model (PDB accession number 5XEA) and validated by SAVES v. 5.0 (http://servicesn.mbi.ucla.edu/SAVES/) to obtain the Ramachandran plot.
Results
High throughput sequencing (HTS) analyses
After conducting RNA sequencing, a pool of adult samples generated a total of 29,070,860 total reads. These reads were subsequently processed using the Kaiju program for rapid taxonomic classification [20]. The results revealed that 21,922,313 reads were classified as viruses, while 21,194 remained unassigned within the viral category. Approximately 75% of the total reads were attributed to either classified or unclassified viruses. Furthermore, 1,618,329 reads were classified as bacteria/fungi, and 19,628 were unassigned within this category, accounting for 6% of the total reads. Additionally, 5,529,228 did not match any microorganism classification, comprising a total of 19% of the reads. To further analyze the data, we employed de novo assembly, resulting in a total of 4547 generated contigs. These contigs were subsequently compared to a Viral RefSeq database using a local BlastX for further analysis. We found contigs with nucleotide identities to nine RNA virus sequences already reported in honeybees, including Apis rhabdovirus 1, Acute bee paralysis virus, Aphid lethal paralysis virus, Black queen cell virus, Bee Macula-like virus, Deformed wing virus, Lake Sinai Virus, Varroa destructor virus 3, and a putative thogotovirus. These RNA viruses were assigned to seven different families, including Rhabdoviridae, Dicistroviridae, Iflaviridae, Tymoviridae, Sinhaliviridae, and Orthomyxoviridae. Table S1 summarizes features observed for the contigs together with genome coverage and identity.
To ensure that only total RNA from the bee species was sequenced and that there was no contamination by mite RNA (such as Varroa destructor), confirmation was carried out given that some of these viruses were reported only in mites, which are present as parasites in most bee colonies. All reads that were sequenced were compared with cytochrome oxidase subunit 1 (COI) records derived from the Barcode of Life Data (BOLD) Systems (http://www.boldsystems.org/), and subsequently, the potential honeybee (Apis mellifera) COI sequence was extracted. The potential honeybee COI sequence was then compared with the nucleotide (nt) database in NCBI, which showed high homology (99.86% identity) to the COI of Apis mellifera scutellata (Accession number: MG552702.1). However, no reads were detected for mites, confidently indicating that the total RNA sequenced was only from bees of the species Apis mellifera scutellata.
Orthomyxovirus
Orthomyxoviridae family of viruses is divided into nine genera: Alphainfluenzavirus, Betainfluenzavirus, Deltainfluenzavirus, Gammainfluenzavirus, Isavirus, Mykissvirus, Quaranjavirus, Srdinovirus, and Thogotovirus [21]. Its members have a negative-sense, single-stranded, and segmented RNA genome, and many putative members were recently found by metagenomic analyses of various invertebrates, and almost all of which remain unclassified and uncharacterized [22].
The genus Thogotovirus comprises a group of arthropod-borne viruses, of which most members are transmitted by ticks of the families Ixodidae (hard ticks) and Argasidae (soft ticks) [23]. In general, the thogotovirus genome consists of six (or seven) segments encoding each of the following viral proteins: polymerase basic subunit 1 (PB1), polymerase basic subunit 2 (PB2), polymerase acidic subunit (PA), surface glycoprotein (GP), nucleoprotein (NP), and matrix protein (M) [24]. The GPs of thogotoviruses are not related to the GPs of influenza viruses, showing no amino acid homology. On the other hand, they show amino acid homology to the major envelope glycoprotein (GP64) of group I alphabaculoviruses (Baculoviridae family) [25–29]. The ATHOV-1 genome found in this work presented six RNA segments with complete open reading frames (ORFs): a putative PB2, polymerase subunit (segment 1, coding for a protein of 759 aa), PBI, polymerase subunit (segment 2, coding for a protein of 711 aa), PA, polymerase subunit (segment 3, coding for a protein of 648 aa), GP, glycoprotein (segment 4, coding for a protein of 524 aa), NP, nucleoprotein (segment 5, coding for a protein of 457 aa), and M, matrix protein (segment 6, coding for a protein of 273 aa) (Fig. 2). When compared to the Thogotovirus amino acid sequences deposited in the GenBank, the ATHOV-1 segments share high amino acid identities in five of the six segments with Varroa orthomyxovirus 1 (VOV-1) (Taxonomy ID: 2,510,845) (98.36 to 99.34% identity). In contrast, segment 4, which codes for the GP, has no identity with VOV-1, as observed for the other segments, but shares an amino acid identity of 34–38% with other viruses of the same family (e.g., Oz virus (YP_009553284.1), Bourbon virus (AMN92169.1), Thailand tick thogotovirus (QFR36191.1), Dhori thogotovirus (QBQ64970.1), Jos virus (AED98372.1), Batken virus (AHX03161.1), and Sinu virus (APP91608.1)). The ATHOV-1 GP also shows amino acid identities ranging from 33 to 41% with the major glycoprotein (GP64) of insect viruses of the Baculoviridae family.
Fig. 2.
The genome organization and read coverage of the putative Apis thogotovirus 1 (ATHOV-1). The reads in the sequence library were aligned with the genome (segments) as shown in blue
Rhabdovirus
Members of the family Rhabdoviridae are divided into 30 genera and 192 species. These viruses have a genome composed of negative-sense, single-stranded RNA of approximately 10–16 kb. Virions are normally enveloped with bullet-shaped or bacilliform morphology. The genomes are usually single RNA molecules with partially complementary termini. Most rhabdovirus genomes have five genes that encode: an RNA-dependent RNA polymerase (L), a phosphorylated phosphoprotein (P), a nucleoprotein (N), a matrix protein (M), and an envelope glycoprotein (G) [30]. The family is ecologically diverse, with members infecting plants and animals, including mammals, birds, reptiles, and fish. Rhabdoviruses are also found infecting arthropods, which serve as unique hosts or as vectors for transmission to other animals or plants. Rhabdoviruses are important pathogens of humans, livestock, fish, and crops [31, 32].
The rhabdovirus identified in this work has a genome of 14,602 nt in length, with the most common five ORFs (N, P, M, G, and L) [33] (Fig. S2). The most conserved ORF encodes a 2143 amino acid (aa) protein containing the RdRp domain RdRp, and all predicted ORFs showed high amino acid identity (99.63–100%) to those of Apis rhabdovirus 1 (ARV-1) (AYP65058.1).
Dicistrovirus
Dicistroviridae is a family of small, non-enveloped viruses found infecting arthropod hosts with a linear single-stranded positive-sense RNA genome of approximately 8–10 kb [34]. The Dicistrovirus RNA genome contains two non-overlapping ORFs separated by an intergenic region (IGR) that contains an internal ribosome entry site (IRES). The ORF-1 encodes for the non-structural proteins (RdRp), whereas the ORF-2 encodes for structural proteins (VP1, VP2, and VP3 capsid proteins in most species) (Fig. S2). The family Discistroviridae comprises three different genera: Cripavirus, Aparavirus, and Triatovirus [34]. Three known dicistrovirus genome sequences were found in the RNA pools of this work by HTS: Acute bee paralysis virus (ABPV), Aphid lethal paralysis virus (ALPV), and Black queen cell virus (BQCV), from the genera Aparavirus, Cripavirus, and Triatovirus, respectively (Fig. S2). The ABPV assembled in this work has a genome of 9664 nt in length with 2 ORFs encoding non-structural (ORF 1) and structural proteins (ORF 2). The RdRp ORF encodes 1908 aa with 95.91% identity with ABPV (GenBank accession number QGX47957.1), and a capsid protein (CP) ORF has 914 aa with 97.92% identity with ABPV (GenBank accession number QGX47958.1). The ALPV found in this work has a genome of 9527 nt in length, containing two ORFs, the RdRp coding for 2035 aa with 97.18% identity with ALPV (GenBank accession number APG77439.1), and a capsid protein (CP) coding for 798 aa with 69.11% identity with ALPV (GenBank accession number QGX47960.1). The BQCV found in this work has a genome of 8501 nt in length, containing two ORFs, a RdRp coding for 1654 aa with 97.41% identity with BQCV (GenBank accession number ABS82435.1), and a capsid protein (CP) coding for 826 aa with 97.34% identity with BQCV (GenBank accession number AYP65062.1).
Sinaivirus
Sinaivirus is a virus genus that belongs to the family Sinhaliviridae, with non-enveloped virions and a linear single-stranded positive-sense RNA genome of about 5.6 kb in length. Sinaivirus genome encodes three genes: ORF1, RdRP, and capsid with overlapping fragments (Fig. S2). Although the function of ORF1 remains unclear, it contains a domain homologous to the Alphavirus methyltransferase-guanylyltransferase, a putative membrane protein [35–37].
A virus genome sequence similar to Lake Sinai Virus NE (GenBank accession number AYP65062.1 NC_035113), which was only recently discovered [37], was assembled. This genome has 5921 nt in length coding for 4 open reading frames (ORFs) (Fig. S2), similarly to previously characterized LSV-1–2 and LSV-NE genomes, ranging from 99 to 100% amino acid identities [35, 37, 38].
Iflavirus
These viruses are members of the Iflaviridae family, classified under the order Picornavirales that has a positive-sense single-stranded RNA of 9–11-kb length size genome and comprises species isolated from insects and parasitic mites [39]. Iflavirus have non-enveloped virions of 30 nm in diameter that contains one linear molecule. The genome contains a single large ORF encoding the capsid proteins at the 5′ end and the non-structural proteins at the 3′ end. The 5′ end of the genome is covalently linked to a small peptide, VPg, which plays an important role in RNA replication [40]. The sequencing analysis resulted in one contig of 8718 nucleotides with 96.6% of amino acid identity with Deformed wing virus, DWV (GenBank accession number NC_004830) (Fig. S2).
Tymovirus
The family Tymoviridae consist of three genera (Tymovirus, Marafivirus, Maculavirus) in the family Tymoviridae [41]. Viruses in this family have a positive-sense, single-stranded RNA genome with a cytosine content ranging from 32 to 50%, which is unusually high. The genome length ranges from 6.0 to 7.5 kb, with capping at the 5′-terminus and the presence of ORFs that encode replication-related proteins analogous to those of other taxa in the “alpha-like” supergroup of ssRNA viruses [41]. The genomic organization and number of ORFs in the family Tymoviridae depend on the genus or individual viral species. In this work, we assembled a genome of 6324 nt with three putative genes (polyprotein, coat protein, p15) and sub-genomic RNA (Fig. S2). The species demarcation criteria in the genus are based on the amino acid identity of the coat protein, with less than 90% identity indicating separate species. The coat protein of the putative virus assembled in this work showed a 94.27% amino acid identity with Bee macula-like virus (GenBank accession number NC_027631).
Unclassified virus
We have partially assembled the genome of virus with high amino acid identity to Varroa destructor virus 3 (GenBank accession number NC_040313), which represents 60.3% of the complete genome (4146 bp) with 7 contigs (ranging in size from 105 to 1462 nt). These contigs map to ORF1, ORF2, and ORF3, with identity varying from 95 to 100%. This result confirms that these contigs represent an isolate of the Varroa destructor 3 virus (Fig. S2). Due to the large number and diversity of viral sequences identified in metagenomic data, they far exceed experimentally characterized virus isolates. Many have been designated as members of species in different genera and families. However, several relatively well-characterized viruses remain unclassified. In some cases, this is because they are distinguished from recognized members of existing taxa, and are known only for their genome sequence. There is a lack of knowledge about their biology or pathogenicity to suggest that they form members of new genera or families [42, 43].
Thogotovirus (ATHOV-1) RNA detection in total RNA from larvae, adults’ pools, and a queen bee
Total RNA was extracted from pools of larvae and adult bees, as well as a queen bee (not shown) for the detection of ATHOV-1 using RT-PCR. Two set of primers were employed to amplify specific fragments: a 150-bp (889–1038) fragment of the of the S1 segment (polymerase subunit PB2) and a 200-bp (778–977) fragment of the S4 segment (GP). These amplifications confirmed the presence of the virus in both larvae and adult bee samples. In addition, a primer pair was designed to specifically bind to positions 1051–1300 of segment 4 (GP) of Varroa orthomyxovirus 1 (VOV-1) (Genbank accession number MK032468). This primer pair was utilized to detect this particular segment in both RNA pools of larvae and adult bees (Fig. 3). In the RT-PCR reaction, a distinct DNA band of 250 bp was observed in the using in the larvae RNA pool, while a faint band was detected in the adult bee pool. This finding was unexpected, as we had not identified this sequence during our sequencing of the adult RNA pool. However, due to the faint intensity of the amplified DNA band in the adult pool, we speculate that this sequence was present in low concentration and did not generate sufficient reads to be detected in our analysis. Furthermore, no DNA amplification was detected in the RNA sample from the queen bee (Fig. 3).
Fig. 3.
Detection of the thogotovirus ATHOV-1 by RT-PCR in the pooled RNA for sequencing (larvae, adults, and queen bee). The expected sizes of the polymerase subunit PB2 and GP of the ATHOV-1 were confirmed the amplified DNA fragments of 150 bp (lanes 1 and 2 of S1 segment) and 200 bp (lanes 1 and 2 of S4 segment). Additionally, the amplified DNA fragment of 250 bp (lanes 1 and 2 of MK032468) corresponding to the expected of the GP of the Varroa destructor othomyxovirus (VOV-1). PCR products (5 µl) were loaded into a 1.2% agarose gel. M (100-bp molecular marker); S1 (polymerase subunit PB2); S4 (glycoprotein); 1 (larvae); 2 (adults); and 3 (queen bee). No virus was detected in a sample or RNA from the queen bee (lane 3 of S1, S4, and MK032468)
ATHOV-1 phylogenetic relationships
Amino acid sequence similarities of the five viral proteins PB2, PB1, PA, NP, and M were used to analyze the phylogenetic relationships among ATHOV-1 and other genera of orthomyxoviruses. The phylogenetic trees of five viral proteins showed that ATHOV-1 formed a robust clade with VOV-1 and both formed a subclade distinct from other orthomyxoviruses (Fig. S3). These results indicate that ATHOV-1 is a member of the genus Thogotovirus and is closely related to VOV-1. Interestingly, the phylogenetic tree for GP demonstrated that ATHOV-1 is an unrecognized ancestral member of the Thogotovirus genus and is related to the GP64 protein of members of the Baculoviridae family. Although the segment encoding the GP had no similarity to VOV-1, as observed for the other proteins, it was closely related to VOV-1 for the other five viral proteins (Fig. 4).
Fig. 4.
Phylogenies of deduced amino acid sequences of glycoprotein (segment 4) of ATHOV-1 in comparison to homologous sequences of selected viruses, using the maximum-likelihood method. Amino acid sequences of virus proteins used in this study were obtained from GenBank. The accession numbers of all viral sequences are presented in Supplementary Table S2
In silico characterization of ATHOV-1 glycoprotein (S4-coding gene)
An annotation of domains carried out with the S4-coding protein gene of ATHOV-1 indicated that this protein belongs to the same family of viral glycoprotein fusion proteins as the baculovirus GP64 envelope glycoprotein (class III viral fusion proteins) [44]. To further analyze the predicted amino acid sequence of S4-coding gene, we aligned it against homologs with solved crystal structures, including Thogoto thogotovirus (PDB accession number 5XEA), Bourbon virus (PDB accession number 5ZKX), Dhori virus (PDB accession number 5XEB), and GP64 of AcMNPV (PDB accession number 3DUZ) (Fig. 5). Comparison with these four crystalized structures revealed aspects of residue conservation and the presence of predicted secondary structures, such as the helix which belongs to Domain III. Leu 87 of fusion loop (L1) and Phe 157 and Ala 158 of fusion loop 2 (L2), which form an exposed hydrophobic patch that presents fusion activity, are conserved in S4. The structure of the GP protein of Thogo thogotovirus virus was deduced to be the best model for the three-dimensional structure of glycoprotein encoded by the S4 gene (Fig. S4A). The domain I contains the conserved fusion loops mentioned above, while domain III, composed of long α-helix in the center of the trimer, has a high number of charged amino acids (Fig. S4B). The identity between the viral sequence and its homolog was 31.94%. The model was checked using the SAVES software v5.0, which showed that 87.4% of the amino acid residues were in most favored regions, and only 0.3% of the residues were in the disallowed region (Fig. S4). In the trimer structure model, it is possible to clearly visualize domains I and III (with lower Qmean values) from the 5-domain structure previously demonstrated [45, 46].
Fig. 5.
Sequence alignment of ATHOV-1 segment 4 with Thogoto thogotovirus (PDB accession number 5XEA), Bourbon virus (PDB accession number 5ZKX), Dhori virus (PDB accession number 5XEB), and AcMNPV (PDB accession number 3DUZ) glycoproteins. Residues that are 100% conserved are in solid red boxes. Those with similarity > 70% are labeled in red. The secondary structures are indicated and labeled above the sequences
Discussion
This study employed a high-throughput sequencing (HTS) approach to examine the diversity of bee viruses in bee colonies located in the southern Brazil. RNA samples from Apis melifera adult honeybees were analyzed, revealing the presence of nine different viruses. Eight of these viruses were previously characterized, namely Apis rhabdovirus 1, Acute bee paralysis virus, Aphid lethal paralysis virus, Black queen cell virus, Bee Macula-like virus, Deformed wing virus, Lake Sinai Virus, and Varroa destructor virus 3). Additionally, a thogotovirus isolate was also identified and named Apis thogotovirus 1 (ATHOV-1). Among these viruses, the Lake Sinai Virus exhibited the highest abundance, with a coverage of over 335,000 × , followed by the Acute bee paralysis virus, with a genome coverage of more than 48,000 × . The genome coverage of other viruses ranged from 2.5 × (Varroa destructor virus-3) to 99 × (Varroa orthomyxovirus-1 and Apis rhabdovirus 1) (see Table S1). Mixed infections involving multiple virus species from different families and genera are commonly observed in honeybees [47–51]. ATHOV-1, classified within the Orthomyxoviridae family, possesses a single-stranded negative-sense RNA genome and belongs to the Thogotovirus genus. Most Thogotoviruses have been associated with ticks [52], while only a limited number of them have been described in Acari or other hematophagous arthropods and honeybees [52–54]. ATHOV-1 consisting of six segments represents only the second documented instance of a complete genome of a single-stranded negative-sense RNA segmented virus found in honeybees [55].
Genetic and phylogenetic analyses conducted in all segments of ATHOV-1 have revealed significant similarity to the Thogotovirus genus. Most segments show a high degree of similarity to Varroa orthomyxovirus 1 (VOV-1) (NCBI taxonomy ID: 2,510,845) [55], with identities ranging from 98.36 to 99.34%, except for segment 4. Interestingly, segment 4 of VOV-1 is distinct and does not exhibit similarity to ATHOV-1. To further investigate this finding, we performed an analysis (BLAST) specifically focused on segment 4 of VOV-1. Our analysis revealed that this segment encodes a protein that shares 99.18% and 99.62% nucleotide and amino acid identity, respectively, with Apis rhabdovirus 2 (Genbank accession number KY354234.1) (Fig. 6). Surprisingly, the VOV-1 glycoprotein DNA fragment was successfully amplified from our samples, which had been previously associated with an orthomyxovirus [55]. However, this sequence was not identified during our sequencing of the adult RNA pool. Based on the high conservation of glycoproteins among rhabdoviruses [56], it is more likely that VOV-1 segment 4 is derived from a rhabdovirus. Furthermore, our phylogenetic analyses support the previous assessment suggesting a possible error in the deposition of the VOV-1 segment 4 sequence. This is evident as segment 4 of VOV-1 encodes a glycoprotein typically associated with Rhabdovirus rather than Orthomyxovirus (see Fig. 6).
Fig. 6.
Phylogenies of deduced amino acid sequences of glycoprotein (segment 4) of VOV-1 in comparison to best hits on BLASTx non-redundant protein database search, using the maximum-likelihood method. The accession numbers of all viral sequences are presented in Supplementary Table S2. The blue box shows segment S4 of VOV-1 clustered with Apis rabdovirus 2 (KY354234.1) and other orthomyxo-like viruses
Despite the low amino acid sequence identity of the glycoprotein (GP) in ATHOV-1 segment 4 with other glycoproteins of other viral families (below 36%), a structural analysis using the Thogoto thogotovirus glycoprotein as a model suggests that ATHOV-1 likely belongs to the class III viral fusion proteins. It is worth noting that class III viral fusion protein genes are found in different viral families and are believed to have been horizontally transferred [57]. Furthermore, the proposed model of ATHOV-1 GP reveals regions of conserved amino acids (domain I) and charged amino acids (domain III), which are responsible for cell attachment and trimer conformation. These regions bears similarities to the GP of Thogoto thogotovirus45, 46.
The identification of multiple viruses infecting Apis mellifera provides valuable insights into the intricate diversity of interactions between viral parasites and bee colonies. However, this research also raises several questions regarding how host–pathogen relationships can impact bee colony dynamics. Currently, it remains uncertain whether ATHOV-1 is associated with any pathology or signs of infection. Additionally, the mechanism of virus transmission, including potential involvement of parasitic mite vectors like Varroa destructor or by horizontal/vertical transmission, requires further investigation. Moreover, the interactions among co-infecting viruses within their hosts pose intriguing questions. It is unclear whether competitive or symbiotic relationships exist among these viruses. Additionally, there is a need to determine if individuals infected by a single virus multiple viruses simultaneously exhibit different pathologies. To gain a comprehensive understanding of the role played by viral pathogens in bee colonies, additional studies are necessary to address these inquiries and expand our knowledge in this field.
Conclusion
In this work, we investigated the presence of the several viruses in bees from southern Brazil. The complete virus sequences obtained from honeybees were closely related to sequences described in previous studies exploring virus diversity in honeybee populations, suggesting that these viruses are globally distributed. However, further research is required to investigate the dynamics of infection and the potential for virus transmission between bees and their vectors.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Bergmann Morais Ribeiro, Lidia Mariana Fiuza, and Daniel Mendes Pereira Ardisson-Araújo designed the study. Leonardo Assis da Silva, Brenda Rabello de Camargo, Bruno Milhomem Pilati Rodrigues, and Diouneia Lisiane Berlitz performed the research. Leonardo Assis da Silva, Daniel Mendes Pereira Ardisson-Araújo, and Bergmann Morais Ribeiro performed the data analysis. Leonardo Assis da Silva, Daniel Mendes Pereira Ardisson-Araújo, and Bergmann Morais Ribeiro wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Grant number 304223/2021–2) and Fundação de apoio à pesquisa do Distrito Federal (FAPDF, Grant number 193001532/2016).
Data availability
All data are available under request.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Responsible Editor: María Martha Martorell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Daniel Mendes Pereira Ardisson-Araújo, Email: araujo.daniel@unb.br.
Bergmann Morais Ribeiro, Email: bergmann@unb.br.
References
- 1.Vanbergen AJ, Garratt MP, Vanbergen AJ, et al (2013) Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ10.1890/120126
- 2.Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science (80). 10.1126/science.1255957 [DOI] [PubMed]
- 3.Klein AM, Vaissière BE, Cane JH, et al. Importance of pollinators in changing landscapes for world crops. Proc R Soc B Biol Sci. 2007;274(1608):303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hung KLJ, Kingston JM, Albrecht M, Holway DA. Kohn JR (2018) The worldwide importance of honey bees as pollinators in natural habitats. Proc R Soc B Biol Sci. 1870;285:20172140. doi: 10.1098/RSPB.2017.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Miranda JR, Bailey L, Ball B V et al (2013) Standard methods for virus research in Apis mellifera. J Apic Res. 10.3896/IBRA.1.52.4.22
- 6.Doublet V, Labarussias M, de Miranda JR, Moritz RFA, Paxton RJ (2015) Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ Microbiol. 10.1111/1462-2920.12426 [DOI] [PubMed]
- 7.Degrandi-Hoffman G, Chen Y, Watkins Dejong E, Chambers ML, Hidalgo G (2015) Effects of oral exposure to fungicides on honey bee nutrition and virus levels. J Econ Entomol. 10.1093/jee/tov251 [DOI] [PubMed]
- 8.Traynor KS, Mondet F, de Miranda JR, et al (2020) Varroa destructor: a complex parasite, crippling honey bees worldwide. Trends Parasitol. 10.1016/j.pt.2020.04.004 [DOI] [PubMed]
- 9.Beaurepaire A, Piot N, Doublet V, et al (2020) Diversity and global distribution of viruses of the western honey bee, Apis mellifera. Insects. 10.3390/insects11040239 [DOI] [PMC free article] [PubMed]
- 10.Matthijs S, Regge N De (2020) Nationwide screening for important bee viruses in Belgian honey bees. Proceedings. 10.3390/proceedings2020050054 [DOI] [PMC free article] [PubMed]
- 11.Shi M, Lin XD, Tian JH, et al. Redefining the invertebrate RNA virosphere. Nature. 2016;540(7634):539–543. doi: 10.1038/nature20167. [DOI] [PubMed] [Google Scholar]
- 12.Páez DJ, Fleming-Davies AE. Understanding the evolutionary ecology of host-pathogen interactions provides insights into the outcomes of insect pest biocontrol. Viruses. 2020;12(2):141. doi: 10.3390/v12020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearse M, Moir R, Wilson A, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Renner S, ed. Syst Biol. 2008;57(5):758–771. 10.1080/10635150802429642 [DOI] [PubMed]
- 16.Anisimova M, Gil M, Dufayard JF, Dessimoz C, Gascuel O. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst Biol. 2011;60(5):685–699. doi: 10.1093/sysbio/syr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bin Li K, ClustalW MPI. ClustalW analysis using distributed and parallel computing. Bioinformatics. 2003;19(12):1585–1586. doi: 10.1093/bioinformatics/btg192. [DOI] [PubMed] [Google Scholar]
- 18.Gouet P, Courcelle E, Stuart DI, Métoz F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15(4):305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
- 19.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31(13):3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menzel P, Ng KL, Krogh A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat Commun. 2016;7(1):1–9. doi: 10.1038/ncomms11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payne S (2023) Family Polyomaviridae. In: Viruses. Academic Press; 321–325. 10.1016/b978-0-323-90385-1.00043-1
- 22.Shi M, Lin XD, Tian JH, et al. Redefining the invertebrate RNA virosphere. Nature. 10.1038/nature20167 [DOI] [PubMed]
- 23.Hubálek Z, Rudolf I. Tick-borne viruses in Europe. Parasitol Res. 10.1007/s00436-012-2910-1 [DOI] [PubMed]
- 24.Payne S (2017) Family Orthomyxoviridae. In: Viruses. 10.1016/b978-0-12-803109-4.00023-4
- 25.Portela A, Jones LD, Nuttall P. Identification of viral structural polypeptides of Thogoto virus (a tick-borne orthomyxo-like virus) and functions associated with the glycoprotein. J Gen Virol. 1992;73(11):2823–2830. doi: 10.1099/0022-1317-73-11-2823. [DOI] [PubMed] [Google Scholar]
- 26.Morse MA, Marriott AC, Nuttall PA (1992) The glycoprotein of Thogoto virus (a tick-borne orthomyxo-like virus) is related to the baculovirus glycoprotein GP64. Virology. 10.1016/0042-6822(92)90030-S [DOI] [PubMed]
- 27.Leahy MB, Dessens JT, Weber F, Kochs G, Nuttall PA (1997) The fourth genus in the Orthomyxoviridae: sequence analyses of two Thogoto virus polymerase proteins and comparison with influenza viruses. Virus Res. 10.1016/S0168-1702(97)00072-5 [DOI] [PubMed]
- 28.Orthomyxoviridae - negative sense RNA viruses - negative sense RNA viruses (2011) - International Committee on Taxonomy of Viruses (ICTV). Accessed September 4, 2020. https://talk.ictvonline.org/ictv-reports/ictv_9th_report/negative-sense-rna-viruses-2011/w/negrna_viruses/209/orthomyxoviridae
- 29.Lung O, Westenberg M, Vlak JM, Zuidema D, Blissard GW. Pseudotyping Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV): F proteins from group II NPVs are functionally analogous to AcMNPV GP64. J Virol. 2002;76(11):5729–5736. doi: 10.1128/JVI.76.11.5729-5736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuzmin IV, Novella IS, Dietzgen RG, Padhi A, Rupprecht CE. The rhabdoviruses: biodiversity, phylogenetics, and evolution. Infect Genet Evol. 2009;9(4):541–553. doi: 10.1016/j.meegid.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Walker PJ, Blasdell KR, Calisher CH, et al. ICTV virus taxonomy profile: Rhabdoviridae. J Gen Virol. 2018;99(4):447–448. doi: 10.1099/jgv.0.001020. [DOI] [PubMed] [Google Scholar]
- 32.Kuzmin IV, Novella IS, Dietzgen RG, Padhi A, Rupprecht CE. The rhabdoviruses: biodiversity, phylogenetics, and evolution. Infect Genet Evol. 2009;9(4):541–553. doi: 10.1016/j.meegid.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Walker PJ, Firth C, Widen SG, et al (2015) Evolution of genome size and complexity in the Rhabdoviridae. PLoS Pathog. 10.1371/journal.ppat.1004664 [DOI] [PMC free article] [PubMed]
- 34.Valles SM, Chen Y, Firth AE, et al. ICTV virus taxonomy profile: Dicistroviridae. J Gen Virol. 2017;98(3):355–356. doi: 10.1099/jgv.0.000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Runckel C, Flenniken ML, Engel JC, et al. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS One. 2011;6(6):e20656. doi: 10.1371/journal.pone.0020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daughenbaugh KF, Martin M, Brutscher LM, et al. Honey bee infecting Lake Sinai viruses. Viruses. 2015;7(6):3285–3309. doi: 10.3390/v7062772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remnant EJ, Shi M, Buchmann G, et al. A diverse range of novel RNA viruses in geographically distinct honey bee populations. J Virol. 2017;91(16):158–175. doi: 10.1128/jvi.00158-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daughenbaugh KF, Martin M, Brutscher LM, et al (2015) Honey bee infecting Lake Sinai viruses. Viruses. 10.3390/v7062772 [DOI] [PMC free article] [PubMed]
- 39.Valles SM, Chen Y, Firth AE, et al (2017) ICTV virus taxonomy profile: Iflaviridae. J Gen Virol. 10.1099/jgv.0.000757 [DOI] [PMC free article] [PubMed]
- 40.Oers MM va. Genomics and biology of lflaviruses. Insect Virol. Published online 2010.
- 41.Martelli GP, Sabanadzovic S, Sabanadzovic NAG, Edwards MC, Dreher T. The family Tymoviridae. Arch Virol. 2002;147(9):1837–1846. doi: 10.1007/s007050200045. [DOI] [PubMed] [Google Scholar]
- 42.Simmonds P, Adams MJ, Benk M, et al (2017) Consensus statement: Virus taxonomy in the age of metagenomics. Nat Rev Microbiol. 10.1038/nrmicro.2016.177 [DOI] [PubMed]
- 43.Unclassified viruses - unclassified viruses - unclassified viruses - ICTV. Accessed March 23, 2021. https://talk.ictvonline.org/ictv-reports/ictv_online_report/unclassified-viruses/w/unclassified-viruses
- 44.Backovic M, Jardetzky TS. Class III viral membrane fusion proteins. Curr Opin Struct Biol. 2009;19(2):189–196. doi: 10.1016/J.SBI.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng R, Zhang S, Cui Y, Shi Y, Gao GF, Qi J. Structures of human-infecting Thogotovirus fusogens support a common ancestor with insect baculovirus. Proc Natl Acad Sci U S A. 2017;114(42):E8905–E8912. doi: 10.1073/pnas.1706125114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai C, Qi J, Wu Y, et al. Postfusion structure of human-infecting Bourbon virus envelope glycoprotein. J Struct Biol. 2019;208(2):99–106. doi: 10.1016/j.jsb.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Granberg F, Vicente-Rubiano M, Rubio-Guerri C, et al. Metagenomic detection of viral pathogens in Spanish honeybees: co-infection by Aphid lethal paralysis, Israel acute paralysis and Lake Sinai viruses. PLoS One. 2013;8(2):e57459. doi: 10.1371/journal.pone.0057459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh R, Levitt AL, Rajotte EG, et al. RNA viruses in hymenopteran pollinators: evidence of inter-taxa virus transmission via pollen and potential impact on non-Apis hymenopteran species. PLoS One. 2010;5(12):e14357. doi: 10.1371/journal.pone.0014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ravoet J, Maharramov J, Meeus I, et al. Comprehensive bee pathogen screening in Belgium reveals Crithidia mellificae as a new contributory factor to winter mortality. PLoS One. 2013;8(8):e72443. doi: 10.1371/journal.pone.0072443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forgách P, Bakonyi T, Tapaszti Z, Nowotny N, Rusvai M. Prevalence of pathogenic bee viruses in Hungarian apiaries: situation before joining the European Union. J Invertebr Pathol. 2008;98(2):235–238. doi: 10.1016/j.jip.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 51.McMenamin AJ, Flenniken ML. Recently identified bee viruses and their impact on bee pollinators. Curr Opin Insect Sci. 10.1016/j.cois.2018.02.009 [DOI] [PubMed]
- 52.Contreras-Gutiérrez MA, Nunes MRT, Guzman H, et al. Sinu virus, a novel and divergent orthomyxovirus related to members of the genus Thogotovirus isolated from mosquitoes in Colombia. Virology. 2017;501:166–175. doi: 10.1016/j.virol.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bolling BG, Weaver SC, Tesh RB, Vasilakis N. Insect-specific virus discovery: significance for the arbovirus community. Viruses. 2015;7(9):4911–4928. doi: 10.3390/v7092851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levin S, Sela N, Chejanovsky N. Two novel viruses associated with the Apis mellifera pathogenic mite Varroa destructor. Sci Rep. 2016;6(1):37710. doi: 10.1038/srep37710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levin S, Sela N, Erez T, et al. New viruses from the ectoparasite mite Varroa destructor infesting Apis mellifera and Apis cerana. Viruses. 2019;11(2):94. doi: 10.3390/v11020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coll JM. The glycoprotein G of rhabdoviruses. Arch Virol. 1995;140(5):827–851. doi: 10.1007/BF01314961. [DOI] [PubMed] [Google Scholar]
- 57.Kadlec J, Loureiro S, Abrescia NGA, Stuart DI, Jones IM. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nat Struct Mol Biol. 2008;15(10):1024–1030. doi: 10.1038/nsmb.1484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available under request.
Not applicable.