Highlights
-
•
We found that APE1 was significantly upregulated in LUAD tissues compared to the para-carcinoma tissues, and its high expression promoted the proliferation and invasion of the LUAD cells in vitro and in vivo.
-
•
Mechanistically, APE1 inhibited pyroptosis by inactivating the STING pathway via direct interaction with AIM2 and DDX41.
-
•
APE1 protects LUAD cells against radiation-induced damage and induces radio-resistance by targeting the STING/DDX41 pathway.
-
•
APE1 inhibitors should be considered for enhancing the radiosensitivity of LUAD cells and improving patient prognosis and therapeutic outcomes. We believe that the findings of this study are relevant to the scope of your journal and will be of interest to its readership.
Keywords: Radiosensitivity, LUAD, APE1, STING, Pyroptosis
Abstract
Mammalian apurinic/apyrimidinic endonuclease 1 (APE1, APEX1) is a multifunctional enzyme that maintains cellular homeostasis. It is involved in the base excision repair (BER) pathway and plays a key role in radiation-induced DNA damage response. However, the relationship between APE1-driven radiation resistance and pyroptosis in lung adenocarcinoma (LUAD) cells and the underlying molecular mechanisms remain unclear. We found that APE1 was significantly upregulated in LUAD tissues compared to para-carcinoma tissues and promoted the proliferation and invasion of LUAD cells in vitro and in vivo. Mechanistically, APE1 inhibited pyroptosis by inactivating the interferon gene stimulator (STING) pathway via direct interaction with AIM2 and DDX41, as detected by RNA-seq and co-immunoprecipitation. APE1 protects LUAD cells against radiation-induced damage and induces radio-resistance by targeting the STING pathway. It can induce pyroptosis and is negatively regulated by interactions with AIM2 and DDX41. Therefore, APE1 inhibitors should be considered to enhance the radiosensitivity of LUAD cells and improve patient prognosis and therapeutic outcomes. Thus, APE1 play a role in the tumor immune microenvironment and in tumor immunotherapy.
Introduction
Lung cancer is a commonly diagnosed malignancy worldwide with high morbidity and mortality rates. Lung adenocarcinoma (LUAD) is the major histological types of lung cancer, and accounts for about 40%−50% of all cases [1]. Although surgery is routinely performed for early-stage LUAD, many patients are initially diagnosed at advanced stage of the disease, which precludes the possibility of surgical resection. Radiotherapy can improve the survival rate of patients with advanced LUAD [2], and the combination of radiation therapy with other treatments has increased the 2-year survival rate from 30% in 2009–2010 to 36% in 2015–2016. In addition, the 2-year survival rate of approximately 80% of patients with non-small cell lung cancer (NSCLC) has increased from 34% to 42% following the introduction of combination therapies [3]. Nevertheless, the efficacy of radiotherapy is frequently limited by the resistance, which in turn affects patient survival. Therefore, it is crucial to explore the molecular mechanisms underlying radiotherapy resistance in LUAD cells in order to develop more effective treatment methods.
Pyroptosis is a newly discovered form of programmed cell death, which can also be termed as inflammatory necrosis [4,5], and it is characterized by the continuous expansion of the cell until the cell membrane ruptures and the cellular contents are released. It is mediated via classical caspase-1 dependent pathway and non-caspase-1 dependent pathways, which culminate in the release of the proinflammatory cytokines IL-1β and IL-18, resulting in local and even systemic inflammation [6,7]. Recent evidence has indicated that pyroptosis may play an important role in radiation [8]. For instance, pyroptosis is induced in liver cells and bone marrow-derived cells through NLRP3 inflammasome activation following radiotherapy [9]. In addition, lncRNA NEAT1 regulates radiation-induced pyroptosis in colon cancer cells. However, little is known about its role in radiotherapy of lung cancer cells.
The small molecule inhibitor of Mammalian apurinic/apyrimidinic endonuclease 1(APE1) can induce pyroptosis in NSCLC cells [10]. APE1, also known as APEX1, is a multifunctional enzyme involved in BER pathway and transcriptional regulation [11]. It cleaves the DNA skeleton at the damaged AP site, recruits other enzymes to remove the damaged base, generates the correct base and ligates the cleavage site to the DNA strand [12]. Furthermore, APE1 also functions as a transcriptional cofactor as well as an oxidation–reduction signaling factor that stimulates the DNA-binding activity of other transcription factors. It plays an important role in repairing radiation-induced DNA damage, and functional single nucleotide polymorphisms in APE1 are associated with radiotherapy-associated pneumonia [13]. Therefore, we hypothesized that silencing APE1 in LUAD cells may enhance radiosensitivity through pyroptosis-associated pathways.
STING is a 42-kDa "dimer adaptor protein" that is localized to the endoplasmic reticulum and acts as a cytoplasmic DNA sensor, which is an important discovery in the field of immunology research and cancer immunotherapy [14]. It is usually activated after DNA damage by the DNA sensor calcitonin gene-related peptide (cGAS) and second messenger cGAMP and translocates to the Golgi apparatus to recruit TBK1 to activate IRF3, thereby inducing the expression of interferon and chemokines [15]. Studies show that cGAS-STING signaling is not only involved in host defense against microbial infection but also regulates the tumor microenvironment (TME). DNA damage induced by radiotherapy and chemotherapy releases double-stranded DNA (dsDNA) fragments that are transferred to the micronucleus or cytoplasm, thereby activating the cGAS-STING pathway [16,17]. The absence of STING in tumors may be partly responsible for radiation resistance, although ionizing radiation can inhibit tumor cells by stimulating a STING-dependent immune response. Currently, the upstream mechanism of STING regulation in LUAD cells is not completely clear.
In this study, we found that APE1 activated the cGAS-STING pathway through DDX41 in LUAD cells, which may be the mechanism underlying resistance to radiotherapy. The aim of this study was to explore the underlying cellular and molecular mechanisms of the connection in APE1, radiation sensitivity and pyroptosis in LUAD. It was expected that the results could guide the clinical application.
Material and methods
LUAD cell lines
HBE, A549, LETP-a2, NCI-H1650, NCI-H1299 and PC9 cell lines were acquired from the Heilongjiang Cancer Institute (Harbin, China). PC9 cells were cultured in RPMI-DMEM (Sevenbio, Beijing, China), and the other cell lines were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS). All cell lines were maintained at 37°C in a humidified incubator under 5% CO2.The related consumables were obtained from Jet Biofil. All experiments were performed using mycoplasma-free cells [18].
Cell transfection
A549 and H1650 cells were seeded in 6-well plates, and transiently transfected with APE1-specific siRNA purchased from HANBIO (Shanghai, China). The sequences were as follows: siRNA-1 (5′-GCAAACCUGCCACACUCAATT-3′), siRNA-2 (5′-CCUGGACUCUCUCAUCAAUTT-3′) and siRNA-3 (5′-GCAGUGAUCACUGUCCUAUTT-3′). APE1 mRNA expression levels in the transfected cells were measured using qRT-PCR. Based on these results, siRNA-3 was selected to produce lentiviruses to establishing stably transfected cell lines that were screened using puromycin [19].
RNA-sequencing
RNA-seq was performed on suitably transfected and irradiated A549 cells according to standard protocols, including A549/Vector, A549/shRNA, A549/Vector and A549/shRNA cells irradiated by 6Gy. The sequencing data were provided by BGI-Tech and filtered with SOAPnuke(v1.5.2) by removing reads containing sequencing adapter. The clean reads were mapped to the reference genome using HISAT2(v2.0.4). Bowtie(v2.2.5) was used to align the clean reads to the reference coding gene set, and gene expression levels of gene were calculated by RSEM(v1.2.12). Additional details were provided in the supplemental information [20].
Quantitative real-time PCR
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen) and reverse-transcribed to cDNA using the Transcription First Strand cDNA Synthesis Kit (TIANGEN). QRT-PCR was performed using SYBR qPCR Mix reagent (TOYOBO) on an ABI Step One Plus machine (Applied Biosystems) with GAPDH as the housekeeping gene [21].
Western blot
Proteins were extracted from suitably treated cells using RIPA lysis buffer. Forty micrograms of protein per sample was separated by SDS-PAGE (10%gel) and transferred to a 0.45 μm polyvinylidene fluoride (PVDF) membrane. The blots were probed with antibodies against APE1 (10,203–1-AP, Proteintech), beta Tubulin (Abways Technology, China), STING, p-STING, TBK1, p-TBK1, IRF-3 and p-IRF-3 (38866T, Cell Signaling Technology KIT), and the positive bands were detected using the Super ECL Plus reagent [22].
Irradiation
The cell culture dishes were sealed with parafilm and irradiated by a 6MV X-ray beam at a dose rate of 400cGy/min for a total dose of 0–10Gy using a linear accelerator (Elekta) from a distance of 100cm (SSD=100cm) [23].
Clone formation assay
Cells were seeded in 6-well plates and irradiated by 0, 2, 4, 6, 8 and 10Gy X-rays as described above, and then incubated at 37°C for 10–14 days. The ensuing clones were fixed with methanol and stained with crystal violet. Colonies with more than 50 cells were counted under a microscope. The percentage of surviving colonies was calculated relative to that in the 0Gy control group. The data were analyzed by selecting the multi-objective model, and survival curves were plotted for the logarithm of the surviving fraction versus the radiation dose [24].
Flow cytometry
Cells stably transfected with lentivirus were irradiated by 6Gy X-rays, harvested and stained with 5μL Annexin V-FITC and 5μL propidium iodide (PI) provided in the Annexin V-FITC/PI Apoptosis Detection Kit (KGA105-KGA108, KeyGEN BioTECH). For cell-cycle analysis, cells were washed with precooled PBS and fixed overnight with 70% ethanol at 4°C. After washing with PBS, cells were incubated with PI/RNase for 30min at room temperature. The samples were analyzed by flow cytometry (Becton Dickinson), and the percentage of apoptotic cells and the cell cycle distribution were analyzed using FlowJo software [24].
EdU proliferation assay
The siRNA transfected LUAD cells and their Vector cells were seeded into 96-well plates and stained using EdU Apollo567 and EdU Apollo488 kits (C10310–1 and C10310–3, RiboBio, China) according the manufacturer's instructions. These three cell lines were labelled with EdU for approximately about 2h [25].
Scratch assays and Transwell assays
The cells were seeded in 6-well plates in complete medium, and cultured for 24h until they reached 80% confluency. The monolayer was scratched longitudinally with a sterile 200μL pipette tip and washed once to remove the dislodged cells. The scratched area was photographed at 0h and 48h, and the extent of coverage was measured to determine cell migration.
The cells were seeded in the uncoated or Matrigel-coated upper chambers of Transwell inserts (corning, USA) in 300μL serum-free medium at density of 5×104 cells per well. The lower chambers were filled with 700µL complete medium supplemented with 10% FBS. After culturing for 24 or 48h, the cells remaining on the top surface of the microporous membrane were removed with a cotton bud, and the cells that invaded the Matrigel were fixed and stained. The number of invading cells was counted in three random fields per sample. The experiment was repeated thrice [26].
Immunofluorescence
The cells were irradiated by 6Gy X-rays in their logarithmic growth phase, cultivated for one hour then fixed and permeabilized in 4% paraformaldehyde containing 0.2% Triton X-100 for 30min. Following overnight incubation with rabbit monoclonal anti-γ-H2AX (AB81299, Abcam;1:100) and rabbit monoclonal anti-53BP1 (ABCAM175933, Abcam;1:100) primary antibodies at 4°C overnight. The cells were probed with a fluorescent secondary antibody for 1h at room temperature the following day. The cells were counter stained with DAPI for 5–8min, and the γ-H2AX and 53BP1 foci were counted and photographed using a laser confocal microscope (Leica Microsystems, USA). The images were taken by a Zeiss LSM 800 Laser Confocal Scanning Microscope (Axio-Imager_LSM-800) with the oil in the objective [24].
Scanning electron microscope
The cells were washed with PBS, fixed overnight with 3% glutaraldehyde at 4°C, then washed thrice with precooled PBS. The samples were dehydrated through an ethanol gradient (30%, 50%, 70%, 95%, and 100%) and dried using tert‑butanol. The samples were imaged using a ZEISS Gemini 560 emission scanning electron microscope at 1kV [27].
Co-immunoprecipitation
The co-immunoprecipitations (co-IP) assay was performed by the Thermo Scientific Pierce Crosslink Magnetic IP/Co-IP Kit according to the manufacturer's instructions. Briefly, the cells were washed with cold PBS. The cell suspension was lysed using immunoprecipitation lysis buffer containing protease inhibitors at 4°C. For each lysate, 500μL of (containing 200–1000μg of total protein) was incubated overnight with magnetic bead-conjugated antibodies at 4°C. The precipitated complex samples were analyzed by western blot the following day. The experiment was performed in triplicate [28].
In vivo radiosensitivity assay
Female BALB/C nude mice (4–5 weeks, 13–15g) were purchased from the Beijing Vital River Laboratory. All animal experiments were performed in accordance with regulations and guidelines for animal care at the Animal Laboratory of the Second Affiliated Clinical Medical College of Harbin Medical University. To establish the xenograft tumor model, 2×107/100µL control or APE1 knockdown A549 stable cells (A549/Vector cells and A549/shRNA cells) were subcutaneously injected into the armpits of the mice. After one week, the length and width of the tumors were measured every four days with calipers. The tumor volume (mm3) was calculated by the formula: V=0.5× (length) × (width)2. The mice were randomly divided into four groups (Vector, sh-APE1, Vector+IR and sh-APE1+IR) with six mice in each group (n=6). Once the tumors grew to 0.4 to 0.6 cm in diameter, the mice in the two groups were irradiated by a total dose of 10Gy X-rays (two exposures every 6 days) with an 8-MeV electron beam. The mice were sacrificed and their tumors were dissected, weighed, and fixed in formalin [29].
Immunohistochemistry (IHC)
IHC was performed as previously described. The tissues were probed with primary antibodies targeting APE1(10,203–1-AP, Proteintech, 1:100), Ki67 (27,309–1-AP, Proteintech, 1:100). Patient tissues were obtained from the Department of Pathology of our hospital [30].
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 9.4 software. All data are shown as the mean ± SD of at least three independent experiments, unless otherwise stated. The student's t-test was used to compare any two groups, and multiple groups were compared with one-way analysis. Statistical significance was set at P<0.05 [30].
Results
APE1 is highly expressed in LUAD tissues
We integrated the transcriptomic data of normal lung and LUAD tissues from the TCGA and GEO databases (GSE33532 and GSE75037) and identified several differentially expressed genes (DEGs) in the LUAD samples (Fig. 1A, B). APE1 was significantly upregulated in the tumor tissues relative to the normal lung tissues in the TCGA-LUAD dataset, and correlated with tumor size, lymph node metastasis and TNM stage (Fig. 1C-E), but it was unrelated to smoking (Fig. 1F).
Fig. 1.
APE1 upregulates in the tumor tissues in datasets. (A) APE1 was significantly upregulated in LUAD tissues(n=486) compared to the normal tissues(n=54) through the bioinformatics analysis of the TCGA-LUAD datasets. An independent-sample Student's t-test was performed and significant differences were indicated. (B) The expression distribution of APE1 gene were in lung cancer and adjacent normal tissues in GEO datasets (GSE33532 and GSE75037). There are unpaired samples in GSE33532 including tumor number(n=80), normal lung tissues number(n=20) and paired samples in GSE75037(n=83). The abscissa represents different groups of samples: G1 means tumor tissues and G2 means non-malignant lung tissues and the ordinate represents the expression distribution of the gene, different colors represent different groups, top-left represents the significance P-value. (C) The expression of APE1 was associated with the tumor stage (T stage). (D) The expression of APE1 was associated with the lymphatic metastasis. (E) The expression of APE1 was associated with the pathologic stage. (F) The expression of APE1 was irrelated to smoke. The statistical difference of two groups was compared through the Wilcox test.
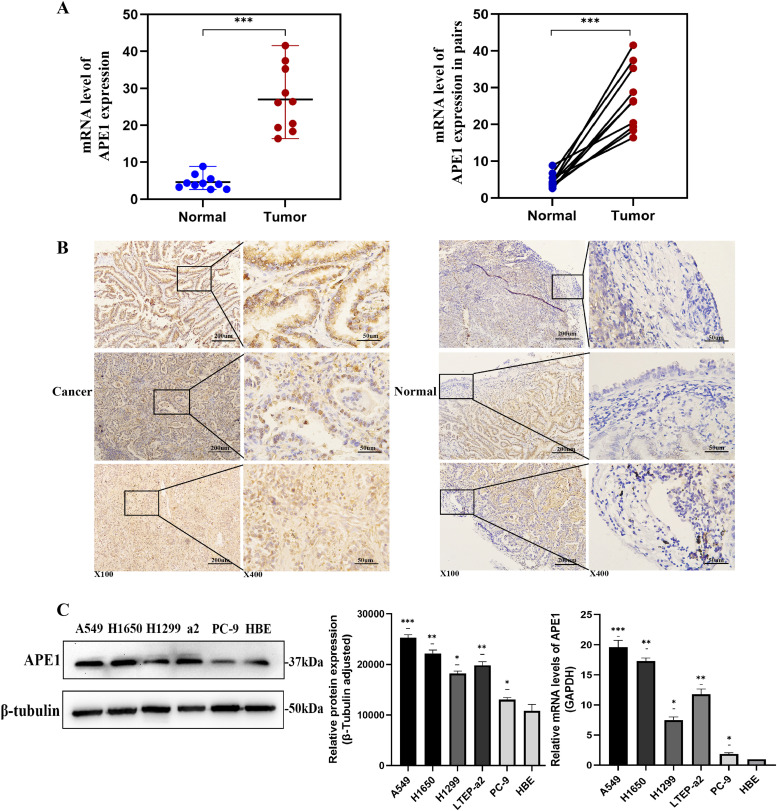
QRT-PCR results showed that APE1 was upregulated in the tumor tissues compared to the corresponding adjacent normal tissues (Fig. 2A). Similar results were obtained by IHC staining of tissues from different patients, which also revealed that APE1 was primarily located in the nucleus (Fig. 2B).
Fig. 2.
The expression of APE1 is higher in LUAD tissues and cells. (A) The expression of APE1 in tumorous and their paired paracarcinoma tissues from 10 LUAD patients were detected in the level of mRNA by qPCR. (B) APE1 was upregulated in the tumor tissues compared with corresponding adjacent normal tissues. APE1 was primarily located in the nucleus. (C) The protein and mRNA expression of APE1 in a normal human lung bronchial epithelial cell line HBE and five LUAD cell lines were tested by western blot and qRT-PCR. *P<0.05, **P<0.01, ***P<0.001.
We also analyzed the expression of APE1 in the normal human lung bronchial epithelial cell line HBE and multiple LUAD cell lines (A549, H1650, H1299, PC9, LTEP-a2). As it was shown that APE1 expression levels were higher in A549, H1650 and H1299 cells compared to those in PC9, LTEP-a2 and HBE cells (Fig. 2C). A549 and H1650 cell lines are known as the most aggressive and malignant. Therefore, A549 and H1650 cells were selected for APE1 knockdown, and the PC9 was used to overexpress APE1 in subsequent experiments.
APE1 promotes the proliferation, migration and invasion of LUAD cells
APE1 was silenced in the A549 and H1650 cell lines by transient transfection with different siRNA sequences. APE1 siRNA-2 (si-APE1–2) and siRNA-3 (si-APE1–3) effectively knocked down APE1 expression, as verified by western blot and qRT-PCR (Fig. S1).
The stably transfected A549 cells were validated by analyzing APE1 mRNA and protein expression by qRT-PCR and western blot to establish A549/shRNA and A549/Vector successfully. In addition, PC9 cells were transfected with APE1 overexpression plasmids and the clones were screened using puromycin (Fig. S2).
The vitro experiments were performed to evaluate the effects of APE1 expression on cell proliferation, apoptosis, migration and invasion of LUAD cells. While APE1 knockdown significantly reduced the proliferation and clonogenic potential of A549 and H1650 cells compared to the respective controls in CCK-8 and EdU assays (Fig. 3A-B), overexpression of APE1 in PC9 cells had opposite effect (Fig. S3A-B). The apoptosis rate increased in the A549 and H1650 cells with APE1 knockdown compared to that in the control cells (Fig. 3C), but it declined in the APE1 overexpressed cells compared to that in PC9/Vector cells (Fig. S3C).
Fig. 3.
APE1 promotes cell proliferation of LUAD cells in vitro. A549 and H1650 cells were transfected with two effective APE1-siRNA (si-APE1–2 and si-APE1–3). (A) Cell proliferation was examined by CCK8. (B) EdU incorporation assay was that in which cells synthesizing DNA stained with EDU (red), nuclei counter stained with Hoechst 33,342 (blue). These three kinds of cells were labelled with EdU about 2h. Scale bar is 100 µm. (C) Flow cytometric was analysis apoptosis in A549 shRNA and H1650 shRNA cells by Annexin-V FITC and PI staining. A representative flow profile is presented (left), and a summary of the percentages for Annexin V-positive cells is shown (right). Error bars correspond to means ± standard deviations from three independent experiments. *P<0.05.
Furthermore, APE1 knockdown in A549 and H1650 cells reduced migration and invasion in vitro (Fig. 4A-B), whereas overexpression of APE1 in PC9 led to a significant increase in migration and invasion (Fig. S3D-E).
Fig. 4.
APE1 promotes cell migration and invasion of LUAD cells in vitro. (A) The wound healing assay was used to show the migration of APE1 in A549 and H1650 cells. The cells migrated into the wounded areas were photographed at 0h and 48h. Error bars correspond to means ± standard deviations from three independent experiments. *P< 0.05. Scale bar is 100µm. (B) The migration and invasion of APE1 in A549 and H1650 cells were also examined by Transwell assay. It showed that cell migration/invasion was significantly inhibited by APE1 knockdown in A549 and H1650 cells compared to that in controls. The results were represented as mean ± SD, *P<0.05. Scale bar is 100 µm.
APE1 affects radiation sensitivity in NSCLC cells
To determine the role of APE1 in the radiosensitivity of LUAD cells, we irradiated A549/Vector and A549/Vector+IR cells with 6Gy X-ray for detection by RNA-seq. The RNA-seq results showed that radiation exposure led to a significant change in APE1 expression compared to non-irradiated controls. Combined with previous reports on the role of APE1 in neuroglioma [31], we hypothesized that APE1 regulates the radiosensitivity of LUAD cells.
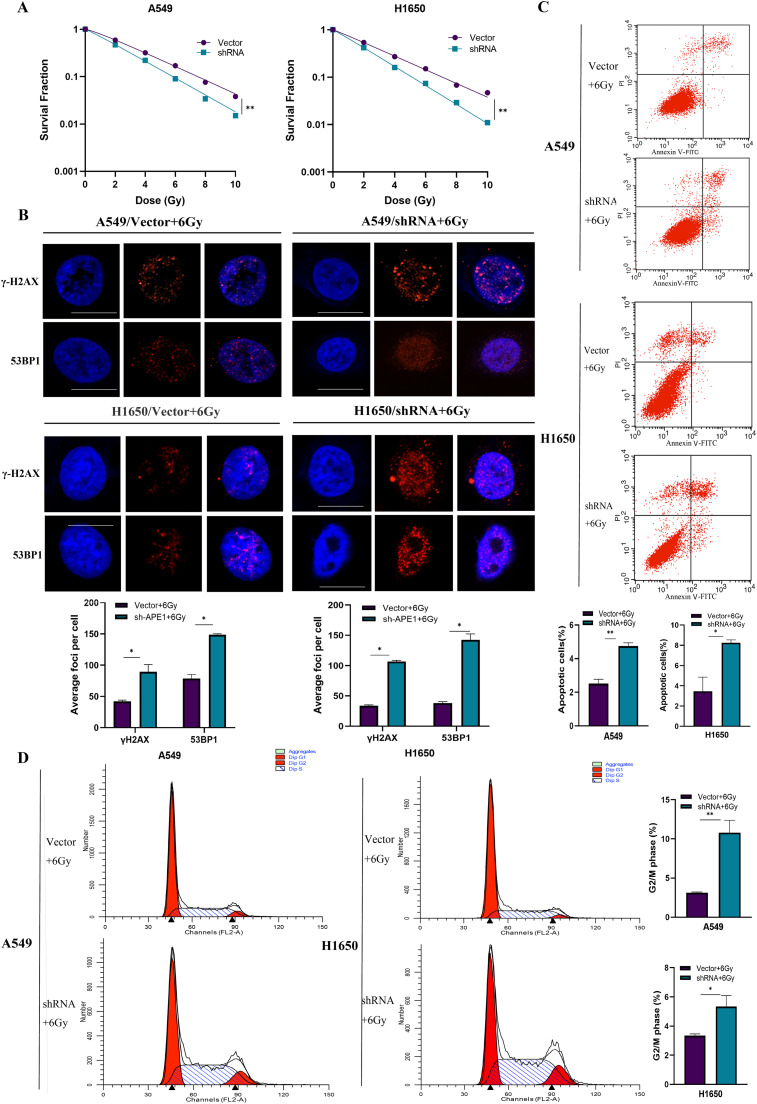
A549 and H1650 cells with APE1 knockdown (A549/shRNA and H1650/shRNA) were more sensitive to X-ray radiation compared to the respective controls (Fig. 5A), while PC9 cells overexpressing APE1 were more radioresistant (Fig. S4A). Because there was a marked decline in survival when irradiated by 6 Gy, this exposure dose was used in subsequent experiments. As expected, the number of γ-H2AX and 53BP1 foci increased significantly in APE1-knockdown A549 and H1650 cells (A549/shRNA and H1650/shRNA) compared to that in control cells 1h post-irradiation with 6Gy X ray (Fig. 5B), which was indicative of radiation-induced DNA damage. In contrast, PC9 cells had fewer γ-H2AX and 53BP1 foci compared to the control cells, indicating that APE1 protects against radiation-induced DNA damage (Fig. S4B). Consistent with this, APE1 knockdown also increased the proportion of apoptotic cells in A549 and H1650 cells (A549/shRNA and H1650/shRNA) following radiation exposure (Fig. 5C), whereas the apoptosis rate in PC9 cells overexpressing APE1 was markedly lower than that in the control group (Fig. S4C).
Fig. 5.
APE1 affects radiation sensitivity in NSCLC cells. The stably transfected A549 cells and H1650 cells were exposed in X-ray irradiation. (A) Clone formation statistical data of A549 and H1650 cells were after APE1 knockdown irradiated by different doses of X-ray. A549/shRNA and H1650/shRNA cells with APE1 knockdown were more sensitive to irradiation than control cells. (B) The number of foci was in γ-H2AX and 53BP1 after irradiation. Scale bars is 10μm. (C) Flow cytometric was analysis of apoptosis in A549/shRNA and H1650/shRNA cells after irradiation by Annexin-V FITC and PI staining. (D) Flow cytometric was analysis of the cell cycle in A549/shRNA and H1650/shRNA cells after irradiation compared to control cells. Error bars correspond to means ± standard deviations from three independent experiments. *P<0.05; **P<0.01.
Furthermore, cell cycle analysis showed that the A549 and H1650 cells with APE1 knockdown (A549/shRNA and H1650/shRNA) were mainly concentrated in the G2/M phase compared to the control cells (Fig. 5D). In contrast, APE1 overexpression decreased the percentage of PC9 cells in the G2/M phase compared to that in control cells (Fig. S3D). These results suggest that APE1 modulates the radiation sensitivity of LUAD cells by inhibiting G2/M arrest and apoptosis.
APE1 negatively regulates p-STING and STING-TBK1-IRF3 pathways by DDX41
To further explore the mechanism underlying the role of APE1 in LUAD, we analyzed the transcriptomic data from TCGA-LUAD datasets. Spearman's correlation analysis revealed that APE1 was involved in pathways related to tumor proliferation, DNA repair and inflammation (Fig. S5).
According to results obtained from the RNA-seq between the Vector and shRNA cell groups (Fig. S6–7), STING was listed as one of the DEGs. In addition, APE1 may negatively correlated with STING in bioinformatics analysis [32]. Furthermore, APE1 is known to exert anti-inflammatory effects by inhibiting the transcriptional activity of NF-κB, and down-regulating TNF-α and iNOS, which are antagonistic to the pro-inflammatory function of STING [33]. In order to further explore the relationship between them, it was carried out that APE1 knockdown in A549 and H1650 cells upregulated p-STING expression (Fig. 6A), whereas overexpression of APE1 in PC9 cells decreased p-STING expression levels (Fig. 6B). Consistent with this, APE1 knockdown also increased the levels of the downstream effectors of STING, including p-TBK1, TBK1, p-IRF3 and IRF3, which indicated that APE1 negatively regulates the STING pathway. As the previous studies show that DDX41 directly binds to STING and DNA via its DEADc domain and triggers the downstream signaling by mitogen-activated protein kinases, TBK1, NF-κB and IRF3 in mDCs [34]. Then we analyzed the transcriptomic data of TCGA-LUAD datasets, Spearman correlation analysis revealed that DDX41 was closely related to APE1 (Fig. 6C). Therefore, we hypothesized that DDX41 is a functional link between APE1 and STING. Indeed, co-IP experiments showed that APE1 physically interacted with DDX41 (Fig. 6D). Taken together, APE1 inhibited the STING-TBK1-IRF3 pathway by directly binding to DDX41 (Fig. S8).
Fig. 6.
APE1 negatively regulates STING-TBK1-IRF3 pathway. (A) P-STING protein expression level was increased after knockdown of APE1 in A549 and H1650 cells, as well as the downstream molecules of STING pathway. (B) P-STING protein expression level and the downstream molecules of STING pathway were decreased in PC9 cells with overexpression of APE1. *P<0.05; **P<0.01 (C) DDX41 was closely related to APE1 in TCGA-LUAD datasets, according to the correlations between two genes. The expression correlation of two genes was analyzed with Spearman. The abscissa represents the expression distribution of the first gene APE1, and the ordinate represents the expression distribution of the second gene DDX41.The density curve on the right represents the trend in distribution of the second gene, the upper density curve represents the trend in distribution of first gene expression. The value on the top represents the correlation p value, correlation coefficient and correlation calculation Amethod. (D) Co-immunoprecipitation (co-IP) was used to validate the interaction of APE1 and DDX41, APE1 was pulled down by anti-DDX41, and western blot was used to detect APE1 and DDX41.
APE1 promotes tumorigenesis and the radiation resistance of LUAD cells in vivo
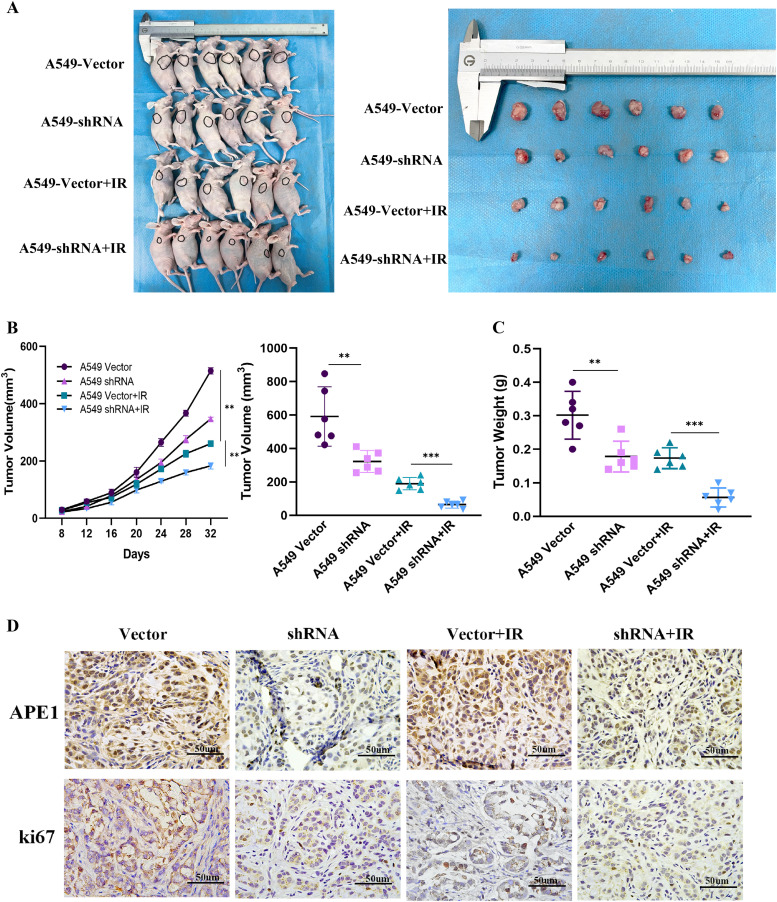
To further assess the role of APE1 in vivo, we established xenograft tumor models in mice using A549 cells stably transfected with shRNA and control vectors. As shown in Fig. 7A, tumors derived from A549/shRNA cells were smaller in terms of volume and weight than those in the control group. The difference in tumor volume was exacerbated following X-ray irradiation (Fig. 7B-C). The number of Ki-67+ proliferating cells also decreased significantly in APE1-knockdown tumors(Fig. 7D, Fig. S9). These data suggest that the absence of APE1 decreases radiation resistance in LUAD cells in vivo.
Fig. 7.
APE1 promotes tumorigenesis and the radiation resistance of LUAD cells in vivo. (A) Representative images of BALB/c nude mice and tumor lumps were at day 32 after inoculation of A549 cells with or without shRNA mediated silencing of APE1. It included Vector control group: mice inoculated with control A549 cells, shRNA group: mice inoculated with A549/shRNA cells, Vector control+IR group: mice inoculated with control A549 cells and treated with irradiation, shRNA+IR group: mice inoculated with A549/shRNA cells and treated with irradiation. The mice in two irradiation groups were irradiated by a total dose of 10Gy X-ray (two exposures every 6 days) with an 8-MeV electron beam. (B) The subcutaneous tumor volume curves were different. (C) Tumor weight was measured. The tumors were weighted immediately after dissection. Tumor weights are represented as means of tumor weights ± SD (n = 12). *P<0.05; **P<0.01; ***P<0.005. (D) Representative images of the immunohistochemical staining of APE1 and Ki-67 expression were in nude mice xenograft tumor sections (magnification, × 400). (*P<0.05. Data were obtained from three independent experiments). Scale bar is 50 µm.
APE1 regulated pyroptosis after irradiation in LUAD cells
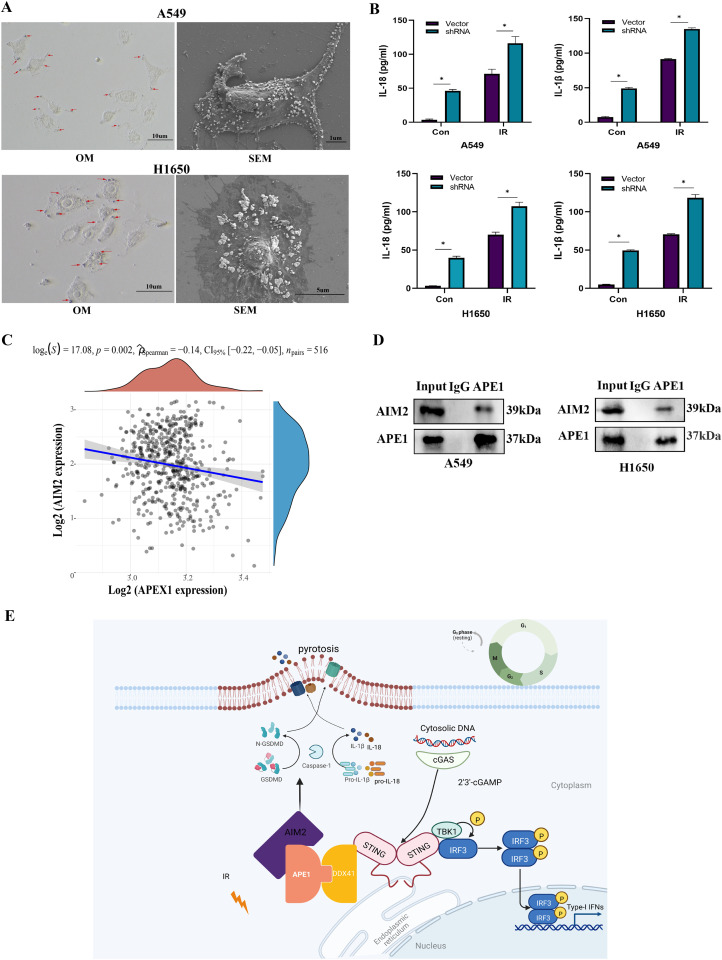
The APE1-knockdown A549 and H1650 cells contracted and displayed typical apoptotic bodies after irradiation, while the cell membrane was structurally intact. Some cells showed cytoplasmic swelling and pyroptosomes, or vesicle-like structures protruding from the plasma membrane, as observed under a light microscope and a scanning electron microscope (Fig. 8A). After pyroptosis, the cells release the proinflammatory cytokines IL-1β and IL-18. The detection release quantity of A549 and H1650 cells after APE1 knockdown was higher than that in control cells as determined by ELISA experiments (Human IL-18 ELISA Kit and Human IL-1β ELISA Kit, JL19261 and JL13662, Shanghai Jianglai Biotech, Shanghai, China). The detection release quantity of A549 and H1650 cells with APE1 knockdown after irradiation was higher than that in control cells These images and ELISA results suggested that APE1 knockdown in A549 cells sensitized them to radiation-induced apoptosis, necrosis and pyroptosis (Fig. 8B).
Fig. 8.
APE1 regulates STING by DDX41 and induce pyroptosis by interacting with AIM2. (A) A549 and H1650 cells were observed with optical and electronic microscope after irradiation. (B) The detection release quantity of A549 and H1650 cells after radiation were higher than control cells by ELISA experiment. *P<0.05. (C) AIM2 was closely related to APE1 in TCGA-LUAD datasets, according to the correlations between two genes. The abscissa represents the expression distribution of the first gene APE1, and the ordinate represents the expression distribution of the second gene AIM2. The density curve on the right represents the trend in distribution of the second gene, the upper density curve represents the trend in distribution of first gene expression. The value on the top represents the correlation p value, correlation coefficient and correlation calculation method. (D) Co-IP also was used to prove the interaction of APE1 and AIM2 in A549 and H1650 cells. APE1 was pulled down by anti-AIM2, and then APE1 and AIM2 were detected by western blot. (E) A schematic diagram of the functional roles of APE1 in LUAD cells. The results were represented as mean ± SD. All experiments were repeated three times.
Then we tried to find some details to explore the relationship between APE1 and pyroptosis. We also analyzed the transcriptomic data of TCGA-LUAD datasets, and Spearman's correlation analysis revealed that AIM2 was closely related to APE1 (Fig. 8C). The co-IP assay revealed that APE1 interacts with the pyroptotic mediator AIM2, as expected (Fig. 8D). The AIM2 inflammasome polymerized is one of the main pathways, which leads to pyroptosis. Based on these findings, we surmised that APE1 knockdown induced DNA damage, activating STING and pyroptosis in LUAD cells after irradiation, which might be the basis of the radiation resistance. The schematic diagram of the roles of APE1 in LUAD is shown in Fig. 8E.
STING reversed radiation resistance in LUAD cells
In this study we found that APE1 knockdown increased the radiosensitivity of LUAD cells by activating the STING pathway, which led us to surmise that activation of the STING pathway may sensitize cells to ionizing radiation. To test this hypothesis, we treated APE1-knockdown A549 cells with a STING inhibitor (C-176, HY-112,906, MedChemExpress) prior to irradiation. Therefore, we suspected that STING could reverse the radiation resistance. As the results mentioned above, A549/shRNA cells showed reduce radiation resistance compared with the control group, while PC9/APE1 cells showed enhanced radiation resistance compared with the control group. C-176 decreased the proportion of apoptotic A549 cells following radiation exposure compared with that in the control group (Fig. S10A). Similarly, cell cycle analysis showed that the C-176 group was less concentrated in the G2/M phase than the control cells (Fig. S10B). Rescue experiments also proved the regulatory relationship between DDX41 and the STING pathway (Fig. S10C). These results showed that STING reversed the radiation resistance of LUAD cells.
Discussion
Although radiation resistance has a strong impact on efficacy, radiotherapy is one of the main treatment methods for NSCLC. We previously found that UBE2T, miRNA, SOD1, mitochondrial dysfunction, cell cycle and the DNA damage response (DDR) are some factors that affect radiosensitivity of lung cancer cells [35,29,36]. Finding these key factors will help us to carry out more effective clinical treatment for patients with NSCLC.
In recent years, large-scale omics studies have identified novel therapeutic targets for multiple cancers [37]. We found the upregulation of APE1 in NSCLC through bioinformatics analysis [38], which is consistent with the fact that APE1 is highly expressed in many cancer types and may function as an oncogene. Likewise, we found that APE1 expressed at significantly higher levels in LUAD cell lines and tissues than in normal lung epithelial cells and para-tumor tissues. Furthermore, analysis of TCGA-LUAD and GEO datasets revealed that APE1 correlated with tumor size (T stage), lymph node metastasis (N stage) and pathological stage, but not with distant metastasis.
Based on GSEA-KEGG pathway analysis of TCGA-LUAD, APE1 was shown to be involved in tumor information signature, tumor proliferation signature, apoptosis, DNA repair, G2M checkpoint, DNA replication, angiogenesis, inflammatory-response and p53 pathway. It is also confirmed that knowing down APE1 in the LUAD cells inhibited their proliferation, invasion and migration, while overexpression of APE1 had the opposite effects. This is consistent with some studies showing that APE1 overexpression promotes epithelial-mesenchymal transition (EMT) of cervical cancer and cutaneous squamous cell carcinoma cells [39,40]. Similar results have been reported for NSCLC [41]. However, there are many factors involved in the regulation of cell proliferation and apoptosis. More significantly, the migration and invasion abilities may be affected by defective cell growth, activated checkpoints and apoptosis signaling. Although the effects of a single gene are limited and incomplete, the role it played cannot be ignored.
APE1 promotes radiation resistance in neuroglioma cells [31]. We speculated that it also plays a similar role in LUAD. RNA-seq was performed on irradiation A549 cells and normal A549 cells. APE1 was listed among the DEGs, as expected. Consistent with this, we found that APE1 knockdown sensitized the A549 cells to X-ray ionizing radiation, whereas cells overexpressing APE1 were more resistant to radiation and showed greater clonogenic survival. Furthermore, APE1 knockdown also increased the number of γ-H2AX and 53BP1 foci in irradiated cells, while APE1 overexpression decreased radiation-induced DNA damage foci. This corresponded to higher apoptosis rates and G2/M phase arrest in the APE1-knockdown cells. These findings suggest that APE1 is a key factor in the induction of radiation resistance in LUAD cells.
Through RNA-seq results and bioinformatics analysis, we found that APE1 expression was negatively correlated with the Type I interferon gene (IFN-I) associated cGAS-STING pathway. The co-IP assay further showed that APE1 interacted directly with DDX41, which is known to bind to STING and DNA through the DEADc domain to form the cytosolic DDX41-STING complex. This indicated that DDX41 likely acts as a functional link between APE1 and STING. We also illustrate that APE1 interacts with the pyroptotic protein AIM2 in the irradiated LUAD cells for the first time. Moreover, our study demonstrated that APE1 interactive with AIM2 induced pyroptosis after irradiation, which is consistent with our RNA-seq results.
Pyroptosis is a type of programmed inflammatory cell death that is mediated through the Caspase‐1 dependent inflammasome pathway, Caspase‐4/5/11 dependent non‐canonical inflammasome pathway and Caspase‐3 dependent pyroptotic pathway [42]. T recent study revealed that there are junction links between inflammasomes and pyroptosis, such as NLRP3 and AIM2 [43,44], which in turn are activated by STING [45]. Taken together, APE1 inhibited radiation-induced pyroptosis in LUAD cells by interacting with AIM2 and inactivating the STING-NLRP3 axis. However, the limitations of our work are that the changes in downstream molecules and the process of recruiting immune cells are not included.
In summary, there are many factors that affect the radiation sensitivity, and our work focus on a certain gene and exploring its specific pathways. From this particular point of view, molecular mechanisms reveal the connection between the radiation sensitivity and pyroptosis. Specifically, APE1 interacts with AIM2 and DDX41 upregulated STING expression, thereby connecting the interaction between radiotherapy sensitivity and pyroptosis.
Conclusions
APE1 promotes the radiation resistance in LUAD cells and protects them against radiation-induced pyroptosis by targeting the STING signaling pathway. Therefore, APE1 inhibitors may be promising prognostic and therapeutic target to enhance radiosensitivity in LUAD. Their potential role in shaping the tumor immune microenvironment and tumor immunotherapy responses deserve further investigation.
Ethics approval
This study was designed in accordance with the Declaration of Helsinki and approved by the ethics committee of Harbin Medical University Cancer Hospital, the ethical document number is 2020-140-IIT.
Consent for publication
All informed consent was obtained from the subject(s) and/or guardian(s).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contributions Statement
Jing Zhou, Dexing Jia, Bo Pan and Yan Yu provided the ideas and designed the research; Jing Zhou, Chuan Yang, Yuan Zheng, Di Sun and Zixin Wei took part in the experiments; Jing Zhou, Dexing Jia and Zixin Wei reviewed the manuscript. All authors read and approved the final manuscript.
Supplementary information
Supplementary files: Supplementary figures were in supplementary material-1
1. The methods of sequencing data analysis
2. Cell Resuscitation and Microscopic Examination & Library Construction
3. Analysis of single-cell transcriptomics data
5. The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2022), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA-Human: HRA004002) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human. Shared URL: https://ngdc.cncb.ac.cn/gsa-human/s/oGN9zj73.6.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
We appreciate the linguistic assistance provided by TopEdit (www.topeditsci.com) during the preparation of this manuscript. The Fig. 8E created with BioRender.com.
Funding
This work was supported by the National Natural Science Foundation of China (81872396).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101749.
Appendix. Supplementary materials
References
- 1.Sun B., Brooks E.D., Komaki R.U., Liao Z., Jeter M.D., McAleer M.F., et al. 7-year follow-up after stereotactic ablative radiotherapy for patients with stage I non-small cell lung cancer: results of a phase 2 clinical trial. Cancer. 2017;123(16):3031–3039. doi: 10.1002/cncr.30693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith R.A., Andrews K.S., Brooks D., Fedewa S.A., Manassaram-Baptiste D., Saslow D., et al. Cancer screening in the United States, 2019: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2019;69(3):184–210. doi: 10.3322/caac.21557. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 4.Lamkanfi M., Dixit V.M. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Man S.M., Karki R., Kanneganti T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017;277(1):61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding J., Wang K., Liu W., She Y., Sun Q., Shi J., et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 7.Gaul S., Leszczynska A., Alegre F., Kaufmann B., Johnson C.D., Adams L.A., et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. 2021;74(1):156–167. doi: 10.1016/j.jhep.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cataldi S., Borrelli A., Ceccarini M.R., Nakashidze I., Codini M., Belov O., et al. Acid and neutral sphingomyelinase behavior in radiation-induced liver pyroptosis and in the protective/preventive role of rMnSOD. Int. J. Mol. Sci. 2020;21(9):3281. doi: 10.3390/ijms21093281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith A.O., Ju W., Adzraku S.Y., Wenyi L., Yuting C., Qiao J., et al. Gamma radiation induce inflammasome signaling and pyroptosis in microvascular endothelial cells. J Inflamm Res. 2021;14:3277–3288. doi: 10.2147/JIR.S318812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M., Wilson D.M., 3rd Human apurinic/apyrimidinic endonuclease 1. Antioxid. Redox. Signal. 2014;20(4):678–707. doi: 10.1089/ars.2013.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Zhao X., Xiao H., Yang B., Liu J., Rao W., et al. APE1 may influence CD4+ naive T cells on recurrence free survival in early stage NSCLC. BMC Cancer. 2021;21(1):233. doi: 10.1186/s12885-021-07950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roychoudhury S., Pramanik S., Harris H.L., Tarpley M., Sarkar A., Spagnol G., et al. Endogenous oxidized DNA bases and APE1 regulate the formation of G-quadruplex structures in the genome. Proc. Natl. Acad. Sci. U.S.A. 2020;117(21):11409–11420. doi: 10.1073/pnas.1912355117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toprani S.M., Das B. Radio-adaptive response, individual radio-sensitivity and correlation of base excision repair gene polymorphism (hOGG1, APE1, XRCC1, and LIGASE1) in human peripheral blood mononuclear cells exposed to gamma radiation. Environ. Mol. Mutagen. 2020;61(5):551–559. doi: 10.1002/em.22383. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding S.M., Benci J.L., Irianto J., Discher D.E., Minn A.J., Greenberg R.A. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548(7668):466–470. doi: 10.1038/nature23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn J., Xia T., Rabasa Capote A., Betancourt D., Barber G.N. Extrinsic phagocyte-dependent STING signaling dictates the immunogenicity of dying cells. Cancer Cell. 2018;33(5):862–873. doi: 10.1016/j.ccell.2018.03.027. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X., Jia M., Sun W., Feng L., Gu C., Wu T. Functional role of RBM10 in lung adenocarcinoma proliferation. Int. J. Oncol. 2019;54:467–478. doi: 10.3892/ijo.2018.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong G., Sun H., Wu S., Mo J. Small interfering RNA against the apurinic or apyrimidinic endonuclease enhances the sensitivity of human pancreatic cancer cells to gemcitabine in vitro. J. Dig. Dis. 2010;11(4):224–230. doi: 10.1111/j.1751-2980.2010.00442.x. [DOI] [PubMed] [Google Scholar]
- 20.Jovic D., Liang X., Zeng H., Lin L., Xu F., Luo Y. Single-cell RNA sequencing technologies and applications: a brief overview. Clin. Transl. Med. 2022;12(3):e694. doi: 10.1002/ctm2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hisada K., Hida Y., Kawabata N., Kawashima Y., Soya Y., Shimada A., Iwano M., Kimura H. Development and evaluation of a novel quenching probe PCR (GENECUBE) assay for rapidly detecting and distinguishing between Chlamydia pneumoniae and Chlamydia psittaci. J. Microbiol. Methods. 2021;184 doi: 10.1016/j.mimet.2021.106212. [DOI] [PubMed] [Google Scholar]
- 22.Habeebunnisa B., Periyasamy M., Anjana D.T. Western blotting: a powerful staple in scientific and biomedical research. BioTechniques. 2022;73:59–69. doi: 10.2144/btn-2022-0003. [DOI] [PubMed] [Google Scholar]
- 23.Tan G., Lin C., Huang C., Chen B., Chen J., Shi Y., Zhi F. Radiosensitivity of colorectal cancer and radiation-induced gut damages are regulated by gasdermin E. Cancer Lett. 2022;529:1–10. doi: 10.1016/j.canlet.2021.12.034. [DOI] [PubMed] [Google Scholar]
- 24.Liu S., Zhan N., Cao C., Xu P., Wang H., Wang S., et al. Long non-coding RNA CBR3-AS1 mediates tumorigenesis and radiosensitivity of non-small cell lung cancer through redox and DNA repair by CBR3-AS1 /miR-409-3p/SOD1 axis. Cancer Lett. 2022;526:1–11. doi: 10.1016/j.canlet.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Sun X., Zhang C., Jin H., Sun G., Tian Y., Shi W., et al. Flow cytometric analysis of T lymphocyte proliferation in vivo by EdU incorporation. Int. Immunopharmacol. 2016;41:56–65. doi: 10.1016/j.intimp.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Zhou F., Geng J., Xu S., Meng Q., Chen K., Liu F. FAM83A signaling induces epithelial-mesenchymal transition by the PI3K/AKT/Snail pathway in NSCLC. Aging (Albany NY) 2019;11(16):6069–6088. doi: 10.18632/aging.102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X., He W., Hu L., Li J., Fang Y., Wang X. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007–1020. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gan J., Zhang Y., Liu S., Mu G., Zhao J., Jiang W., et al. MicroRNA-375 restrains the progression of lung squamous cell carcinoma by modulating the ERK pathway via UBE3A-mediated DUSP1 degradation. Cell Death Discov. 2023 Jun 29;9(1):199. doi: 10.1038/s41420-023-01499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin H., Wang X., Zhang X., Zeng Y., Xu Q., Wang W., et al. UBE2T promotes radiation resistance in non-small cell lung cancer via inducing epithelial-mesenchymal transition and the ubiquitination-mediated FOXO1 degradation. Cancer Lett. 2020;494:121–131. doi: 10.1016/j.canlet.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y., Geng J., Wang X., Meng Q., Xu S., Lang Y., et al. RNA-binding motif protein 10 represses tumor progression through the Wnt/beta- catenin pathway in lung adenocarcinoma. Int. J. Biol. Sci. 2022;18(1):124–139. doi: 10.7150/ijbs.63598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naidu M.D., Mason J.M., Pica R.V., Fung H., Pena L.A. Radiation resistance in glioma cells determined by DNA damage repair activity of Ape1/Ref-1. J. Radiat. Res. 2010;51(4):393–404. doi: 10.1269/jrr.09077. [DOI] [PubMed] [Google Scholar]
- 32.Yang B., Rao W., Luo H., Zhang L., Wang D. Relapse-related molecular signature in early-stage lung adenocarcinomas based on base excision repair, stimulator of interferon genes pathway and tumor-infiltrating lymphocytes. Cancer Sci. 2020;111(10):3493–3502. doi: 10.1111/cas.14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliveira T.T., Coutinho L.G., de Oliveira L.O.A., Timoteo A.R.S., Farias G.C., Agnez-Lima L.F. APE1/Ref-1 role in inflammation and immune response. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.793096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z., Yuan B., Bao M., Lu N., Kim T., Liu Y.-J. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol. 2011;12(10):959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S., Li B., Xu J., Hu S., Zhan N., Wang H., et al. SOD1 promotes cell proliferation and metastasis in non-small cell lung cancer via an miR-409-3p/SOD1/SETDB1 epigenetic regulatory feedforward loop. Front. Cell Dev. Biol. 2020;8:213. doi: 10.3389/fcell.2020.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan N., Li B., Xu X., Xu J., Hu S. Inhibition of FASN expression enhances radiosensitivity in human non-small cell lung cancer. Oncol. Lett. 2018;15(4):4578–4584. doi: 10.3892/ol.2018.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghandi M., Huang F.W., Jane-Valbuena J., Kryukov G.V., Lo C.C., McDonald E.R., 3rd, et al. Next-generation characterization of the cancer cell line encyclopedia. Nature. 2019;569(7757):503–508. doi: 10.1038/s41586-019-1186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia D., Xing Y., Zhan Y., Cao M., Tian F., Fan W., et al. LINC02678 as a novel prognostic marker promotes aggressive non-small-cell lung cancer. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.686975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q., Zhou Z.W., Duan W., Qian C.Y., Wang S.N., Deng M.S., et al. Inhibiting the redox function of APE1 suppresses cervical cancer metastasis via disengagement of ZEB1 from E-cadherin in EMT. J. Exp. Clin. Cancer Res. 2021;40(1):220. doi: 10.1186/s13046-021-02006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng X., Zhen P., Niu X., Dai Y., Wang Y., Zhou M. APE1 promotes proliferation and migration of cutaneous squamous cell carcinoma. J. Dermatol. Sci. 2020;100(1):67–74. doi: 10.1016/j.jdermsci.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Sardar Pasha S.P.B., Sishtla K., Sulaiman R.S., Park B., Shetty T., Shah F., et al. Ref-1/APE1 inhibition with novel small molecules blocks ocular neovascularization. J. Pharmacol. Exp. Ther. 2018;367(1):108–118. doi: 10.1124/jpet.118.248088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herr D.R., Yam T.Y.A., Tan W.S.D., Koh S.S., Wong W.S.F., Ong W.Y., et al. Ultrastructural characteristics of DHA-induced pyroptosis. Neuromol. Med. 2020;22(2):293–303. doi: 10.1007/s12017-019-08586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao J., Peng S., Shan X., Deng G., Shen L., Sun J., et al. Inhibition of AIM2 inflammasome-mediated pyroptosis by Andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis. Cell Death. Dis. 2019;10(12):957. doi: 10.1038/s41419-019-2195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 45.Gaidt M.M., Ebert T.S., Chauhan D., Ramshorn K., Pinci F., Zuber S., et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171(5):1110–1124. doi: 10.1016/j.cell.2017.09.039. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.