Rheumatology key message.
Myoton can be a practical tool to assess cutaneous SSc.
Dear Editor, SSc, also known as scleroderma, is a chronic autoimmune disease characterized by vasculopathy, fibrosis and immune dysregulation [1]. The skin is the most commonly affected organ and a significant marker of disease progression and mortality. Clinicians currently use the semi-qualitative modified Rodnan skin score (mRSS) to assess skin involvement by palpating skin at 17 body sites [2]. Although high-frequency ultrasound is a reliable tool for quantitatively measuring skin thickness in SSc, it is not widely used in clinical practice due to time limitations for image acquisition and analysis [3]. The Myoton is a handheld device (Supplementary Fig. S1, available at Rheumatology online) that can provide objective and rapid non-invasive assessment of cutaneous involvement in SSc. It measures five biomechanical properties by delivering mechanical impulses to the skin and recording resulting oscillations. The device has been validated for measuring cutaneous sclerosis in chronic graft-vs-host disease [4]. Further details of the operation and reliability of the Myoton compared with other assessment technologies are in Supplementary Data S1, available at Rheumatology online. In this case series, we test the Myoton’s ability to assess cutaneous SSc, comparing the results to mRSS and high-frequency ultrasound thickness measurements.
We used the Myoton to measure the skin stiffness of three SSc patients, each with different cutaneous disease types: limited-early, diffuse-late, diffuse-early/active. Ten sites were measured on each patient’s extremities (bilateral shin, shoulder, bicep, dorsal and volar forearm). At each measurement site, we also calculated skin (epidermis+dermis) and subcutaneous thickness from B-mode images obtained with a VevoMD high-frequency ultrasound device (48 MHz probe). Patients were compared with the Wilcoxon rank sum test. Spearman’s correlation was used to determine the relationship between Myoton measurements, skin and subcutaneous thickness, and mRSS. A glossary of acronyms and abbreviations is available in Supplementary Data S2, available at Rheumatology online.
Patient 1 (limited-early)
This female patient presented at age 46 with positive Scl-70, one-year history of Raynaud’s phenomenon (RP) and progressive bilateral hand pain and skin thickening for 6 months. Her maximum mRSS was 18 with skin thickness of bilateral digits, forearms and hand complaints with arthralgia responsive to 3 months of tocilizumab. At Myoton measurement, the patient was symptomatically stable on hydroxychloroquine monotherapy with a mRSS of 6.
Patient 2 (diffuse-late)
This male patient presented at age 47 with a positive RNApol3, following a 3-month history of rapid onset of severe RP, and skin involvement (mRSS: 28). Given the rapid progression of skin with small and large joint contractures on mycophenolate mofetil (MMF) and IVIg, he underwent autologous hematopoietic stem cell transplant (aHSCT) one year following symptom onset. His maximum mRSS was 40. At Myoton measurement, 5.5 years following diagnosis, he had a mRSS of 33, normal muscle enzymes, and no self-report of disease activity for one year on MMF monotherapy.
Patient 3 (diffuse-early/active)
This male patient presented within 6 months of the onset of RP at age 20, with positive U1RNP, interstitial lung disease, myositis, fixed contractures and rapidly evolving skin (mRSS: 37). At Myoton measurement, 1.5 years after diagnosis, the patient had a mRSS: 24 and normal muscle enzymes on rituximab, MMF, hydroxychloroquine, nintedanib and prednisone 5 mg daily.
The median Myoton stiffness across all 10 sites of the patient with early/active disease (884 N/m; IQR: 566–988 N/m) was significantly higher than both the patient with limited SSc (358 N/m; IQR: 341–411 N/m; P < 0.01) and the patient with late diffuse disease (527 N/m; IQR: 435–630 N/m; P < 0.05] (Supplementary Fig. S2, Supplementary Tables S1 and S2, available at Rheumatology online). Across all 30 measured sites of the three subjects, Myoton stiffness had a high correlation with subcutaneous tissue thickness (Spearman ρ = −0.91, P < 0.001). This correlation was much higher than the correlation of stiffness with skin thickness (ρ = 0.43, P = 0.016). Myoton stiffness also had a highly significant correlation (Spearman ρ = 0.67, P < 0.001) with site mRSS (Supplementary Table S3, available at Rheumatology online).
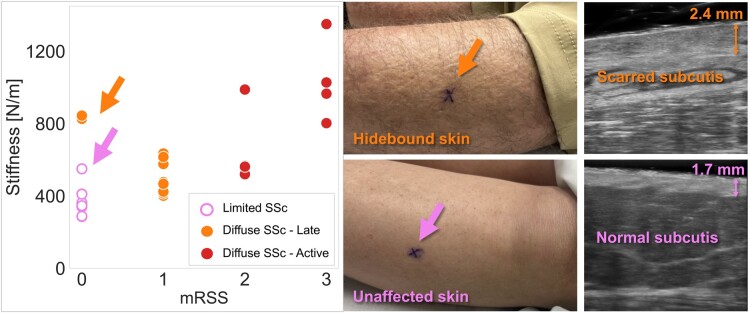
Notably, high Myoton stiffness measurements at the shin of the patient with late disease indicated the presence of sclerosis, despite a mRSS score of 0 (Fig. 1). Ultrasound images at the shin site revealed dermal thickening and scarred soft tissue to the extent of indistinguishable muscle fascia, subcutis and dermis. This highlights Myoton’s ability to capture structural and physiologic alterations missed by mRSS.
Figure 1.
Myoton stiffness parameter plotted against the body site’s local mRSS score (0 unaffected to 3 most severely affected). Outlier with high stiffness for mRSS: 0 (top arrow) is the shin of a patient with late dcSSc. This patient with late diffuse disease had hidebound skin and elevated dermal thickness (2.4 mm) at the shin when compared with the patient with limited disease (1.7 mm)
The patient with early/active diffuse SSc had a severe restrictive pattern on pulmonary function tests (PFT) despite MMF, thus rituximab and nintedanib were initiated, which improved his skin and respiratory symptoms. As the high-resolution chest CT exhibited <10% interstitial lung disease, the severe pulmonary function restriction was likely secondary to hidebound cutaneous involvement of his chest. The chest site mRSS did not change during treatment, but chest stiffness measured by Myoton dropped from 893 N/m to 641 N/m. Thus, Myoton measurements appear to be more sensitive than mRSS to changes in this patient’s chest skin, and in parallel to PFT, objectively quantified his improvement. This quantification helped the patient decide against aHSCT as the next therapeutic step.
In summary, Myoton measurements capture critical information that mechanistically quantifies symptomatic changes to guide medical decision-making. Complex mechanisms contribute to SSc pathogenesis at different disease stages [5, 6]. The Myoton integrates biomechanical data to assess disease phenotype, especially in patients with hidebound skin by mRSS. In conclusion, similar to capillaroscopy [7, 8], Myoton stiffness measurements may be a useful method to distinguish disease activity between early, active and late disease, and quantify response to treatment. A larger research study should validate the results of this case series.
Supplementary Material
Acknowledgements
The authors are grateful for the patients who participated in this research.
Contributor Information
Shramana Ghosh, Medicine Service and Research Service, Department of Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, USA; Department of Dermatology, Vanderbilt University Medical Center, Nashville, TN, USA.
Katie A O’Connell, Department of Dermatology, Vanderbilt University Medical Center, Nashville, TN, USA.
Shinwho Kwun, Medicine Service and Research Service, Department of Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, USA; Department of Dermatology, Vanderbilt University Medical Center, Nashville, TN, USA.
Hayden B Smith, Medicine Service and Research Service, Department of Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, USA; Department of Dermatology, Vanderbilt University Medical Center, Nashville, TN, USA; Vanderbilt University School of Medicine, Nashville, TN, USA.
Catherine H Phillips, Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, USA.
Tyra Silaphet, Medicine Service and Research Service, Department of Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, USA; Department of Dermatology, Vanderbilt University Medical Center, Nashville, TN, USA.
Bohan Jiang, Medicine Service and Research Service, Department of Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, USA; Department of Dermatology, Vanderbilt University Medical Center, Nashville, TN, USA.
Andrew J McNeil, Medicine Service and Research Service, Department of Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, USA; Department of Dermatology, Vanderbilt University Medical Center, Nashville, TN, USA.
Inga Saknite, Department of Dermatology, Vanderbilt University Medical Center, Nashville, TN, USA.
Tracy M Frech, Medicine Service and Research Service, Department of Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, USA; Division of Rheumatology and Immunology, Department of Medicine, Vanderbilt University Medical Center, and Tennessee Valley Healthcare System, Nashville, TN, USA.
Eric R Tkaczyk, Medicine Service and Research Service, Department of Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, USA; Department of Dermatology, Vanderbilt University Medical Center, Nashville, TN, USA; Vanderbilt University School of Medicine, Nashville, TN, USA.
Supplementary material
Supplementary material is available at Rheumatology online.
Data availability
The data underlying this article is available in its online supplementary material, including Supplementary Data S3.
Funding
This work was supported by a Career Development Award (IK2 CX001785) (Tkaczyk) and a VA MERIT award (II01CX0211) (Frech) from the United States Department of Veterans Affairs Clinical Science R&D Service, a CTSA award (UL1 TR002243) from the National Center for Advancing Translational Sciences, and a National Institutes of Health K12 Grant (CA090625) (Tkaczyk).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Herrick AL, Assassi S, Denton CP.. Skin involvement in early diffuse cutaneous systemic sclerosis: an unmet clinical need. Nat Rev Rheumatol 2022;18:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khanna D, Furst DE, Clements PJ. et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord 2017;2:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santiago T, Santos E, Ruaro B. et al. Ultrasound and elastography in the assessment of skin involvement in systemic sclerosis: a systematic literature review focusing on validation and standardization – WSF Skin Ultrasound Group. Semin Arthritis Rheum 2022;52:151954. [DOI] [PubMed] [Google Scholar]
- 4. Baker LX, Chen F, Cronin A. et al. Optimal biomechanical parameters for measuring sclerotic chronic graft-versus-host disease. JID Innov 2021;1:100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allanore Y, Simms R, Distler O. et al. Systemic sclerosis. Nat Rev Dis Primers 2015;1:15002. [DOI] [PubMed] [Google Scholar]
- 6. Cutolo M, Soldano S, Smith V.. Pathophysiology of systemic sclerosis: current understanding and new insights. Expert Rev Clin Immunol 2019;15:753–64. [DOI] [PubMed] [Google Scholar]
- 7. Sulli A, Pizzorni C, Smith V. et al. Timing of transition between capillaroscopic patterns in systemic sclerosis: timing of transition through nailfold microvascular patterns in SSc. Arthritis Rheum 2012;64:821–5. [DOI] [PubMed] [Google Scholar]
- 8. Zumstein Camargo C, Kayser C.. Capillaroscopy changes are associated with disease progression in patients with early systemic sclerosis: a prospective study. Int J Rheum Dis 2019;22:1319–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article is available in its online supplementary material, including Supplementary Data S3.