Abstract
Purpose of Review:
To summarize the recent pre-clinical findings investigating dopaminergic circuits for their involvement in reversing anesthetic-induced unconsciousness.
Recent Findings:
The release of dopamine from the ventral tegmental area onto dopamine D1 receptor-expressing neurons in the nucleus accumbens promotes emergence following general anesthesia. Two relevant targets of dopamine D1 receptor-expressing neurons in the nucleus accumbens include the lateral hypothalamus and ventral pallidum. Activating mesocortical dopaminergic projections from the ventral tegmental area to the prelimbic cortex has also been shown to hasten emergence from general anesthesia. In contrast, the nigrostriatal dopamine pathway is not involved in regulating anesthetic emergence. The role of the tuberoinfundibular endocrine dopamine pathway remains to be tested; however, recent studies have identified an important function of neuroendocrine signaling on modulating general anesthesia.
Summary:
Potential avenues for accelerating anesthetic emergence may be found through targeting specific arousal-promoting pathways in the brain. Accumulating evidence from rodent studies manipulating cell type- and circuit-specific signaling pathways have identified dopamine as a potent modulator of general anesthesia. Specifically, dopamine signaling along the mesolimbic and mesocortical pathways plays a fundamental role in regulating consciousness.
Keywords: Dopamine, mesolimbic, mesocortical, emergence, neural circuits
Introduction
Despite the number of pharmaceuticals available to tightly regulate and maintain physiological stability during general anesthesia, anesthetic emergence remains a process largely driven by the pharmacokinetics of anesthetic drug elimination. Consequently, delayed emergence remains a common postoperative complication, estimated to occur in 9.2% of surgical patients (1). Inappropriate dosing, impaired metabolism or elimination, prolonged surgery, and drug interactions can all adversely affect the pharmacokinetics of anesthetic drug elimination, contributing to delays in recovery (2). There are also several patient risk factors associated with delayed emergence; geriatric patients, pediatric patients, patients who are obese, and those with preexisting cardiac and pulmonary diseases are all more likely to experience delayed emergence following general anesthesia (3). In addition to decreasing operating room efficiency, delays in emergence impose greater health care costs, poorer health outcomes (ex. respiratory complications, increased risk of aspiration, and hypoventilation leading to hypoxia), and are associated with a greater incidence of emergence agitation and delirium (2). Developing strategies to accelerate the process of emergence or reverse anesthesia may provide significant clinical benefits.
Growing evidence from preclinical research suggests that arousal pathways in the brain can be targeted to promote the return of consciousness following general anesthesia (6-8). There are several arousal-promoting pathways under active investigation, including orexinergic (9-16), cholinergic (17-19), and noradrenergic (20-24) circuits; however, in the past few years there has been a surge of new findings investigating dopaminergic circuits involved in modulating general anesthesia and conscious recovery (25, 26).
The role of dopamine as an arousal-promoting neuromodulator has been well established. In 1973, Jones et al. lesioned catecholamine-containing neurons in cats to demonstrate that more extensive lesions in the ventral tegmentum, a posterior region of the midbrain, produced larger decreases in dopamine release concomitant with a more profound loss of behavioral arousal (27). In contrast, cats with dopamine-sparing lesions showed either normal or, in the case of lesions to noradrenergic nuclei, ataxic behavioral states without changes in arousal (27). The eventual development of a transgenic mouse strain incapable of endogenously synthesizing dopamine, i.e. dopamine-deficient mice, provided an ideal testing ground to investigate behavioral consequences of brain-wide dopamine signaling (28). Dopamine-deficient mice are inactive, unmotivated, lack the capacity to learn simple tasks, and do not eat enough to avoid starvation and dehydration; however, restoring dopamine signaling to the striatum through levodopa (L-Dopa, a precursor to dopamine) treatment is sufficient to rescue most basic behaviors (29). The behavioral and neurocognitive deficits displayed by dopamine-deficient mice are so severe that the mice have been described as semi-conscious at most, highlighting the fundamental role dopamine plays in maintaining consciousness (29).
The advent of techniques capable of interrogating the involvement of cell type- and projection-specific circuits has given rise to a boon of neural circuit-focused research over the past decade. Widespread access to these tools has borne new findings mapping out the neuroanatomical loci and pathways capable of regulating emergence from general anesthesia. Here we review recent developments manipulating dopamine signaling pathways and their impact on conscious recovery following general anesthesia in rodent models, as well as the emerging evidence generated from clinical investigations of anesthetized and minimally conscious patients.
Dopamine pathways in the brain
Dopaminergic signaling directs a diverse array of neurocognitive and behavioral outcomes through its actions across distinct neural circuits. These circuits are categorized into four major pathways: the mesolimbic, mesocortical, nigrostriatal, and tuberoinfundibular pathways (Figure 1). Two of the four pathways, mesolimbic and mesocortical, originate in the ventral tegmental area (VTA), a dopamine rich hub in the midbrain. Early pharmacologic studies identified dopamine as a potent modulator of behavioral arousal in animals under general anesthesia (30-33). However, the direct involvement of the VTA in anesthetic emergence was first described using a stimulating electrode to activate VTA neurons in rats under continuous isoflurane anesthesia, resulting in the profound recovery of behavioral arousal (34). Soon thereafter, it was found that selectively lesioning VTA neurons expressing tyrosine hydroxylase, an enzyme required for dopamine synthesis, significantly delayed emergence latency following propofol (35). Since then, more refined tools – such as optogenetic and chemogenetic techniques - have been applied to excite and inhibit specific neural populations and their projections to elucidate their roles in promoting arousal.
Figure 1.
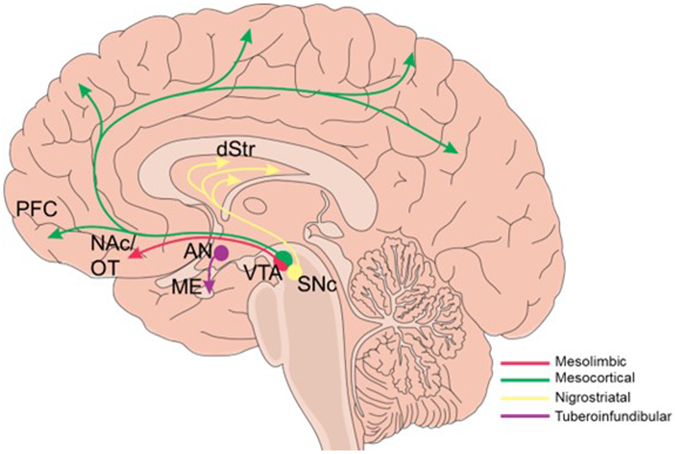
The four major dopaminergic pathways of the brain. A mid-sagittal view of the human brain with the four main dopaminergic signaling pathways outlined. Mesolimbic (pink): ventral tegmental area (VTA) dopaminergic projections to the nucleus accumbens (NAc) and olfactory tubercle (OT). Mesocortical (green): VTA projections to the cortex, and predominantly the prefrontal cortex (PFC). Nigrostriatal (yellow): substantia nigra pars compacta (SNc) to the dorsal striatum (dStr). Tuberoinfundibular (purple): arcuate nucleus (AN) of the hypothalamus to the median eminence (ME).
Taylor et al. employed the now common Cre-lox technique to selectively express the light-sensitive cation channel, channelrhodopsin-2 (ChR2), in VTA dopaminergic neurons (36). The technique uses a transgenic mouse line expressing the enzyme Cre recombinase under a cell type-selective promoter; in this case, the dopamine transporter (DAT) promoter was selected to target dopamine neurons. When combined with the local injection of an adeno-associated virus that induces Cre-dependent expression of ChR2 into the VTA, these neurons can be selectively activated with blue light pulses, i.e., photostimulation. The study demonstrated that photostimulating dopaminergic VTA neurons using a fiber optic probe implanted in the VTA induces behavioral and neurophysiological signs of arousal in mice under steady-state isoflurane anesthesia (36). The effect was lost by pretreating mice systemically with the D1 dopamine receptor (D1R) antagonist, SCH-23390 (36). This investigation demonstrated that VTA dopamine release, and specifically dopamine acting on D1R at downstream sites, was sufficient to induce behavioral arousal in anesthetized animals. More recently, it has been shown that selective knockdown of DAT in the VTA decreases anesthetic depth score and hastens emergence following propofol (37).
The mesolimbic pathway
D1Rs are excitatory G protein-coupled receptors expressed throughout the striatum. To determine which downstream targets of the VTA containing D1R may be involved in promoting emergence, studies began investigating the nucleus accumbens (NAc), a structure in the basal ganglia primary composed of D1R- and D2R-expressing GABAergic neurons heavily innervated by dopaminergic VTA projections. VTA dopaminergic projections to the NAc (VTADA→NAc) are the primary component of the mesolimbic dopamine pathway. While most well-known for its involvement in reward, addiction, and motivation, the mesolimbic pathway also plays a role in maintaining arousal and delaying sleep (38, 39). Investigations revealed that microinjections of the D1R agonist, chloro-APB, into the NAc accelerated emergence latency following isoflurane anesthesia, whereas microinjections of the D1R antagonist, SCH-23390, delayed emergence following propofol and isoflurane anesthesia (40, 41).
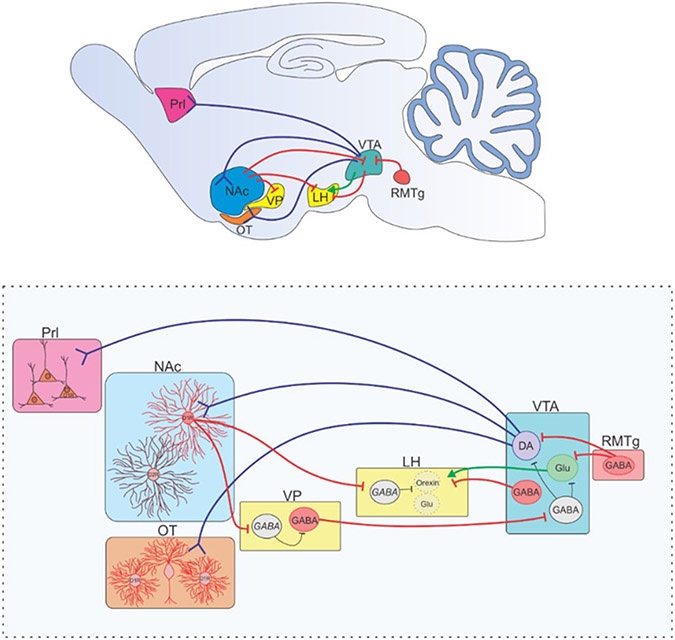
To directly investigate the VTADA→NAc circuit, Gui et al. optogenetically stimulated dopaminergic projections from the VTA to the NAc by placing the fiber optic probe in the NAc of DAT-Cre mice expressing ChR2 in the VTA. Because ChR2 is anterogradely expressed along axon processes, photostimulation can be directed at the subset of neurons projecting to, or through, a specific region. Selectively activating VTADA→NAc projections delayed induction and accelerated emergence from sevoflurane anesthesia (42). Additionally, fiber photometry recordings of dopaminergic VTA neurons revealed that their activity, as measured by population-level calcium activity, declines sharply during induction with sevoflurane and markedly increases immediately prior to return of the righting reflex, a standard endpoint used to indicate return of consciousness (42). Extracellular dopamine concentration in the NAc displays a parallel drop during induction and a resurgence during emergence (42). Thus, activity along the VTADA→NAc pathway correlates with anesthetic-induced loss and return of consciousness, and exogenous activation of this pathway promotes emergence.
To confirm the target of NAc dopamine, Bao et al. measured the activity of D1R-expressing NAc neurons in mice during sevoflurane anesthesia (43). NAc D1R neuronal activity follows the same pattern of activity as VTA dopaminergic neurons (43). Moreover, optogenetically stimulating D1R NAc neurons restores the righting reflex in the majority (66%) of mice under steady-state sevoflurane anesthesia (43). Taken together, these results suggest that general anesthetics reduce VTADA→NAcD1R signaling, and conscious recovery can be accelerated by restoring activity along this pathway.
In a follow-up investigation by Bao et al., selectively activating D1R NAc projections to either the ventral pallidum (VP) or the lateral hypothalamus (LH) was found to likewise induce emergence under steady stage sevoflurane anesthesia (44). Currently, it is unclear which neurons are targeted in these regions as both the VP and LH contain diverse neural cell types with broad and varied projections. However, D1R NAc neurons are GABAergic, and activating these GABAergic projections innervating the VP and LH increased neural firing of the resident neural populations, suggesting NAcD1R projections primarily act on inhibitory interneurons to cause disinhibition of both regions. Evidence from sleep studies suggest GABAergic neurons in the VP project to the VTA and predominantly inhibit GABAergic VTA interneurons to promote wakefulness (45). Thus, the VP may be part of a feedforward mechanism of mesolimbic activity. LH neurons also consist of a diverse array of neuronal subtypes, one of which are arousal-promoting orexinergic neurons (9). Importantly, both GABAergic and glutamatergic neurons in the VTA project directly to the LH (46). Optogenetic and chemogenetic activation of VTAGABA→LH neurons accelerates loss of righting and delays return of righting under isoflurane anesthesia whereas activation of VTAGlu→LH neurons has been shown to promote wakefulness (46). Hence, the VTA may promote emergence by targeting the LH via both direct (VTAGlu→LH) and indirect (VTADA→NAcD1R→LH) pathways.
Another node intersecting the mesolimbic pathway includes dopaminergic projections from the VTA to the olfactory tubercle. This node regulates odor-guided motivated behaviors via D1R- and D2R-expressing neurons (47). While pharmacologically activating or inhibiting olfactory tubercle D1Rs via direct microinjection has no impact on isoflurane induction times in rodents, activating D1R accelerates emergence while inhibiting D1Rs delays emergence from isoflurane (48). Furthermore, selective optogenetic stimulation of VTA dopaminergic projections to the olfactory tubercle accelerates emergence latency following isoflurane anesthesia (48).
Mesocortical pathway
Like the mesolimbic pathway, the mesocortical pathway originates in the VTA. However, the mesocortical pathway, as the name suggests, projects to cortical regions, primarily the medial prefrontal cortex in humans. In rodents the analogous structure is the prelimbic cortex (Prl). Microinjections of the D1R antagonist, SCH-23390, into the prelimbic cortex has been shown to decrease induction time and delay emergence time in rats under sevoflurane anesthesia (49). Conversely, microinjecting the D1R agonist, chloro-APB, into the rat prelimbic cortex delays induction and accelerates emergence. Chemogenetic activation of dopaminergic projections from the VTA to the prelimbic cortex likewise delays induction time and accelerates time to emergence in rats under sevoflurane anesthesia (49). Thus, the VTADA→PrlD1R pathway may likewise be involved in regulating conscious recovery following general anesthesia.
Nigrostriatal pathway
The nigrostriatal dopamine pathway originates in the substantia nigra pars compacta (SNc), located in the midbrain lateral to the VTA, and projects to the dorsal striatum. Unlike the mesocortical and mesolimbic pathways which are both largely implicated in reward processing, the nigrostriatal pathway is more closely associated with coordinating movement. Early data from electrical stimulation studies found that electrically stimulating SNc neurons, unlike VTA neurons, produced neither behavioral nor neurophysiological signs of arousal in rats under isoflurane anesthesia (34). Later studies selectively lesioned dopaminergic SNc neurons and found a modest attenuation in modafinil-induced wakefulness; however, SNc lesions did not alter physiological sleep levels (50). Dopamine concentration in the dorsal striatum of rats has also been shown to not change under surgical doses of sevoflurane, suggesting the nigrostriatal dopamine pathway does not play a significant role in regulating general anesthesia (51).
Tuberoinfundibular pathway
The tuberoinfundibular pathway is involved in endocrine signaling, and consists of dopamine neurons within the arcuate nucleus, a region in the hypothalamus, projecting to the median eminence, found at the base of the hypothalamus. While the specific involvement of dopamine signaling along this path has yet to be investigated, an ensemble of hypothalamic neuroendocrine neurons activated by a diverse array of anesthetics has been identified (52). These neurons have been dubbed anesthesia-activated neurons (AANs) of the supraoptic nucleus. The study mapped AAN projections in the mouse brain and found these neurons target several areas involved in regulating arousal, including the VTA, lateral habenula, and periaqueductal gray. AANs were also found to send a large axonal track to the arcuate nucleus and median eminence.
AANs in the supraoptic nucleus appear to be predominantly glutamatergic neurons (95% expressing vGlut2, the vesicular glutamate transporter 2) and express the neuropeptides vasopressin (85%) and prodynorphin (81%), but a subset also expresses to varying degrees oxytocin and galanin (52). Chemogenetically activating vasopressin-expressing supraoptic nucleus neurons was sufficient to potentiate slow-wave sleep in mice, and optogenetic activation delayed emergence from isoflurane general anesthesia (52). The work also identified a population of neurons in the supraoptic nucleus that showed depressed activity following loss of consciousness and a return of activity prior to return of consciousness (52). These neurons and the relevant circuits have yet to be characterized, but the involvement of neuroendocrine neurons in regulating general anesthesia suggests the tuberoinfundibular pathway may warrant explicit investigation.
Clinical extrapolations
With 100 million years of evolutionary divergence between us, how well rodent neural circuitry maps onto the human brain remains a topic of active investigation (53). However, susceptibility to anesthesia is not entirely constrained by organism complexity – invertebrates, vertebrates, and even plants (54), respond to anesthetics – thus some mechanisms of anesthesia appear to be organism-invariant. However, how the brain orchestrates its return to a conscious state will entail some organism-specific mechanisms. Currently, human studies are limited by the available techniques, but there is some human data supporting the role of dopaminergic signaling in conscious recovery. Recent functional magnetic resonance imaging (fMRI) studies on healthy participants treated with propofol and patients with disturbed consciousness have demonstrated that both groups display reduced connectivity between the VTA and the precuneus and posterior cingulate cortex – two regions implicated in consciousness and the default mode network (55). Patients with disorders of consciousness that had greater connectivity among these regions were more likely to improve at follow-up. In addition, patients treated with methylphenidate, a drug shown to reverse general anesthesia pre-clinically via a dopamine-dependent mechanism (30, 31, 56), developed increased connectivity among these regions (55).
Case reports from patients in persistent vegetative states or minimally conscious states also lend support to the notion that dopamine-promoting treatments may increase the level of consciousness. In a report of five patients in minimally conscious states or persistent vegetative states, treatment with levodopa led to a recovery from the vegetative/minimally conscious state within 1.5 months of treatment (57). Similarly, in five patients in a vegetative state, bromocriptine (a dopamine receptor agonist) promoted improvement to a minimally conscious state in all patients (58). While rigorous clinical evidence has yet to be disseminated, the ability of dopaminergic drugs to promote conscious recovery in patients with disrupted consciousness is a topic under active investigation (59).
Conclusions
Compelling evidence from in vivo circuit manipulation studies identifies mesolimbic and mesocortical dopamine signaling pathways as potential targets to facilitate anesthetic emergence (summarized in Figure 2). Both circuits modulate arousal largely through VTA dopamine release onto postsynaptic D1R-expressing neurons. Thus far, D1R-expressing neuronal populations involved in regulating emergence have been identified in the nucleus accumbens, prelimbic cortex, and olfactory tubercle in rodent models. Elucidating these pathways will not only further our understanding of how anesthetics exert their effects at the circuit level but will also be fundamental for developing strategies to reverse the anesthetized state.
Figure 2.
Dopaminergic circuits involved in anesthetic emergence. (Top) A mid-sagittal view of a rodent brain is depicted with dopaminergic (blue), GABAergic (red), and glutamatergic (green) projections depicted summarizing the dopamine signaling pathways involved in regulating anesthetic emergence and detailed in the bottom panel. Activation of dopaminergic neurons in the VTA induce arousal under general anesthesia (33). Activation of the RMTg, a structure with dense inhibitory projections to VTA dopamine and glutamate neurons, enhances general anesthesia (60). Dopamine release from the VTA onto D1R-expressing neurons in the NAc (40, 42, 43) and OT (48) (Mesolimbic pathway) or the Prl (mesocortical pathway)(49) has been shown to induce emergence from general anesthesia in rodent studies. Recently, D1R NAc neurons projecting to the VP and LH have been shown to both induce emergence in rodents under general anesthesia(44). The target in the VP and LH are thought to be inhibitory interneurons. GABAergic VP projections have been shown to disinhibit VTA neurons, increasing arousal(45). VTA GABAergic neurons projection to the LH has been shown to induce emergence following anesthesia(61), and glutamatergic VTA projections to the LH enhance arousal(46). VTA = ventral tegmental nucleus, RMTg = rostromedial tegmental nucleus, NAc = nucleus accumbens, Prl = prelimbic cortex, VP = ventral pallidum, LH = lateral hypothalamus, OT = olfactory tubercle. Blue projections are dopaminergic, red are GABAergic, green are glutamatergic. Inhibitory interneurons are in black.
Key Points.
Arousal-promoting pathways in the brain represent potential targets for accelerating anesthetic emergence.
Evidence from rodent studies indicates that dopamine signaling along the mesolimbic and mesocortical pathway actively promotes emergence from general anesthesia.
Dopamine D1 receptor activation mediates the arousal-promoting effects of dopamine signaling, particularly in neurons in the nucleus accumbens, olfactory tubercle, and prelimbic cortex.
While evidence for the role of dopaminergic circuits in anesthetic emergence in humans is limited, clinical research investigating these circuits in patients with disrupted consciousness is ongoing.
Funding:
This work was supported by grants from the National Institutes of Health (NIH grants R01-GM126155 to K.S., and K99-AG076877 to K.F.V.); Departmental support (Scholar Award to K.S.); and the Canadian Institutes of Health Research (BPF180179 to K.F.V.)
Footnotes
Conflicts of interest: None
References
- 1.Zelcer J, Wells DG. Anaesthetic-related recovery room complications. Anaesth Intensive Care. 1987;15(2):168–74. Epub 1987/05/01. doi: 10.1177/0310057X8701500209. [DOI] [PubMed] [Google Scholar]
- 2.Cascella M, Bimonte S, Di Napoli R. Delayed Emergence from Anesthesia: What We Know and How We Act. Local Reg Anesth. 2020;13:195–206. Epub 2020/11/13. doi: 10.2147/LRA.S230728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misal US, Joshi SA, Shaikh MM. Delayed recovery from anesthesia: A postgraduate educational review. Anesth Essays Res. 2016;10(2):164–72. Epub 2016/05/24. doi: 10.4103/0259-1162.165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi GJ, Baek CW, Kang H, et al. Emergence agitation after orthognathic surgery: a randomised controlled comparison between sevoflurane and desflurane. Acta Anaesthesiol Scand. 2015;59(2):224–31. Epub 2014/11/15. doi: 10.1111/aas.12435. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Sung TY. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol. 2020;73(6):471–85. Epub 2020/03/27. doi: 10.4097/kja.20097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelz MB, Garcia PS, Mashour GA, Solt K. Escape From Oblivion: Neural Mechanisms of Emergence From General Anesthesia. Anesth Analg. 2019;128(4):726–36. Epub 2019/03/19. doi: 10.1213/ANE.0000000000004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moody OA, Zhang ER, Vincent KF, et al. The Neural Circuits Underlying General Anesthesia and Sleep. Anesth Analg. 2021;132(5):1254–64. Epub 2021/04/16. doi: 10.1213/ANE.0000000000005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reimann HM, Niendorf T. The (Un)Conscious Mouse as a Model for Human Brain Functions: Key Principles of Anesthesia and Their Impact on Translational Neuroimaging. Frontiers in Systems Neuroscience. 2020;14(8):42. Epub May 19, 2020. doi: 10.3389/fnsys.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelz MB, Sun Y, Chen J, et al. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105(4):1309–14. Epub 2008/01/16. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong H, Niu J, Su B, et al. Activation of orexin signal in basal forebrain facilitates the emergence from sevoflurane anesthesia in rat. Neuropeptides. 2009;43(3):179–85. Epub 2009/05/26. doi: 10.1016/j.npep.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Zhang LN, Li ZJ, Tong L, et al. Orexin-A facilitates emergence from propofol anesthesia in the rat. Anesth Analg. 2012;115(4):789–96. Epub 2012/07/17. doi: 10.1213/ANE.0b013e3182645ea3. [DOI] [PubMed] [Google Scholar]
- 12.Ran MZ, Wu W, Li JN, et al. Reduction of orexin-A is responsible for prolonged emergence of the rat subjected to sleep deprivation from isoflurane anesthesia. CNS Neurosci Ther. 2015;21(3):298–300. Epub 2015/02/14. doi: 10.1111/cns.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang LN, Yang C, Ouyang PR, et al. Orexin-A facilitates emergence of the rat from isoflurane anesthesia via mediation of the basal forebrain. Neuropeptides. 2016;58:7–14. Epub 2016/02/28. doi: 10.1016/j.npep.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Ran M, Wang Z, Yang H, et al. Orexin-1 receptor is involved in ageing-related delayed emergence from general anaesthesia in rats. Br J Anaesth. 2018;121(5):1097–104. Epub 2018/10/20. doi: 10.1016/j.bja.2018.05.073. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Cheung K, Kyu S, et al. Activation of orexin system facilitates anesthesia emergence and pain control. Proc Natl Acad Sci U S A. 2018;115(45):E10740–E7. Epub 2018/10/24. doi: 10.1073/pnas.1808622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Li H, Wang D, et al. Orexin activated emergence from isoflurane anaesthesia involves excitation of ventral tegmental area dopaminergic neurones in rats. Br J Anaesth. 2019;123(4):497–505. Epub 2019/08/11. doi: 10.1016/j.bja.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Pal D, Silverstein BH, Lee H, Mashour GA. Neural Correlates of Wakefulness, Sleep, and General Anesthesia: An Experimental Study in Rat. Anesthesiology. 2016;125(5):929–42. Epub 2016/10/19. doi: 10.1097/ALN.0000000000001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo TY, Cai S, Qin ZX, et al. Basal Forebrain Cholinergic Activity Modulates Isoflurane and Propofol Anesthesia. Front Neurosci. 2020;14:559077. Epub 2020/11/17. doi: 10.3389/fnins.2020.559077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung LS, Chu L, Prado MAM, Prado VF. Forebrain Acetylcholine Modulates Isoflurane and Ketamine Anesthesia in Adult Mice. Anesthesiology. 2021;134(4):588–606. Epub 2021/02/27. doi: 10.1097/ALN.0000000000003713. [DOI] [PubMed] [Google Scholar]
- 20.Mason ST, Angel A. Anaesthesia: the role of adrenergic mechanisms. Eur J Pharmacol. 1983;91(1):29–39. Epub 1983/07/15. doi: 10.1016/0014-2999(83)90358-8. [DOI] [PubMed] [Google Scholar]
- 21.Kushikata T, Yoshida H, Kudo M, et al. Role of coerulean noradrenergic neurones in general anaesthesia in rats. Br J Anaesth. 2011;107(6):924–9. Epub 2011/10/04. doi: 10.1093/bja/aer303. [DOI] [PubMed] [Google Scholar]
- 22.McCarren HS, Chalifoux MR, Han B, et al. alpha2-Adrenergic stimulation of the ventrolateral preoptic nucleus destabilizes the anesthetic state. J Neurosci. 2014;34(49):16385–96. Epub 2014/12/05. doi: 10.1523/JNEUROSCI.1135-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazey EM, Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci U S A. 2014;111(10):3859–64. Epub 2014/02/26. doi: 10.1073/pnas.1310025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu B, Yu T, Yuan J, et al. Noradrenergic transmission in the central medial thalamic nucleus modulates the electroencephalographic activity and emergence from propofol anesthesia in rats. J Neurochem. 2017;140(6):862–73. Epub 2017/01/17. doi: 10.1111/jnc.13939. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Miao X, Sun Y, et al. Dopaminergic System in Promoting Recovery from General Anesthesia. Brain Sciences. 2023;13(4). doi: 10.3390/brainsci13040538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heshmati M, Bruchas MR. Historical and Modern Evidence for the Role of Reward Circuitry in Emergence. Anesthesiology. 2022;136(6):997–1014. Epub 2022/04/02. doi: 10.1097/ALN.0000000000004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones BE, Bobillier P, Pin C, Jouvet M. The effect of lesions of catecholamine-containing neurons upon monoamine content of the brain and EEG and behavioral waking in the cat. Brain Res. 1973;58(1):157–77. Epub 1973/08/17. doi: 10.1016/0006-8993(73)90830-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83(7):1197–209. Epub 1995/12/29. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 29.Palmiter RD. Dopamine signaling as a neural correlate of consciousness. Neuroscience. 2011;198:213–20. Epub 2011/08/16. doi: 10.1016/j.neuroscience.2011.06.089. [DOI] [PubMed] [Google Scholar]
- 30.Solt K, Cotten JF, Cimenser A, et al. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011;115(4):791–803. Epub 2011/09/22. doi: 10.1097/ALN.0b013e31822e92e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology. 2012;116(5):998–1005. Epub 2012/03/27. doi: 10.1097/ALN.0b013e3182518bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenny JD, Taylor NE, Brown EN, Solt K. Dextroamphetamine (but Not Atomoxetine) Induces Reanimation from General Anesthesia: Implications for the Roles of Dopamine and Norepinephrine in Active Emergence. PLoS One. 2015;10(7):e0131914. Epub 2015/07/07. doi: 10.1371/journal.pone.0131914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology. 2013;118(1):30–9. Epub 2012/12/12. doi: 10.1097/ALN.0b013e318278c896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solt K, Van Dort CJ, Chemali JJ, et al. Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology. 2014;121(2):311–9. Epub 2014/01/09. doi: 10.1097/ALN.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X, Wang Y, Zhang C, et al. The Role of Dopaminergic VTA Neurons in General Anesthesia. PLoS One. 2015;10(9):e0138187. Epub 2015/09/24. doi: 10.1371/journal.pone.0138187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor NE, Van Dort CJ, Kenny JD, et al. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A. 2016;113(45):12826–31. Epub 2016/10/30. doi: 10.1073/pnas.1614340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * 37.Guo J, Xu K, Yin JW, et al. Dopamine transporter in the ventral tegmental area modulates recovery from propofol anesthesia in rats. J Chem Neuroanat. 2022;121:102083. Epub 2022/02/20. doi: 10.1016/j.jchemneu.2022.102083. [DOI] [PubMed] [Google Scholar]; This work identified that knockdown of the dopamine transporter, a transmembrane transporter expressed in dopamine neurons involved in regulating the concentration of extracellular dopamine, in VTA neurons accelerates conscious recovery following propofol. Thus confirming that the release of dopamine from VTA neurons can be exploited to drive anesthetic emergence. [Google Scholar]
- 38.Luo YJ, Li YD, Wang L, et al. Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D(1) receptors. Nat Commun. 2018;9(1):1576. Epub 2018/04/22. doi: 10.1038/s41467-018-03889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eban-Rothschild A, Rothschild G, Giardino WJ, et al. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci. 2016;19(10):1356–66. Epub 2016/09/07. doi: 10.1038/nn.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Gui H, Duan Z, et al. Dopamine D1 Receptor in the Nucleus Accumbens Modulates the Emergence from Propofol Anesthesia in Rat. Neurochem Res. 2021;46(6):1435–46. Epub 2021/03/09. doi: 10.1007/s11064-021-03284-3. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Gui H, Hu L, et al. Dopamine D1 receptor in the NAc shell is involved in delayed emergence from isoflurane anesthesia in aged mice. Brain Behav. 2021;11(1):e01913. Epub 2020/10/24. doi: 10.1002/brb3.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ** 42.Gui H, Liu C, He H, et al. Dopaminergic Projections From the Ventral Tegmental Area to the Nucleus Accumbens Modulate Sevoflurane Anesthesia in Mice. Front Cell Neurosci. 2021;15:671473. Epub 2021/05/18. doi: 10.3389/fncel.2021.671473. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fiber photometry recordings of extracellular dopamine in the NAc of mice during sevoflurane anesthesia demonstrate that dopamine levels drop in the NAc prior to loss of righting. These data suggest the drop in NAc dopamine levels promote loss of consciousness rather than being a consequence of loss of consciousness. The drop in extracellular dopamine coincides with a decrease in NAc neuronal activity. This work was also the first to show that VTA projections which terminate in the NAc are involved in regulating sevoflurane anesthesia. The authors use retrograde viruses to target only the VTA projections terminating in the NAc with excitatory/inhibitory opsins or DREADDs to modulate loss and return of righting in mice. [Google Scholar]
- ** 43.Bao WW, Xu W, Pan GJ, et al. Nucleus accumbens neurons expressing dopamine D1 receptors modulate states of consciousness in sevoflurane anesthesia. Curr Biol. 2021;31(9):1893–902 e5. Epub 2021/03/12. doi: 10.1016/j.cub.2021.02.011. [DOI] [PubMed] [Google Scholar]; The first demonstration that endogenous activity in D1 dopamine receptor (D1R) expressing NAc neurons decreases prior to sevoflurane-induced loss of consciousness and returns following return of consciousness in mice. Optogenetic activation of D1R NAc neurons induces righting in two thirds of mice under light sevoflurane anesthesia. This work conclusively showed that D1R neurons of the NAc are a target of VTA dopamine projections involved in mediating arousal. [Google Scholar]
- * 44.Bao W, Ding J, Jiang S, et al. Selective activation of NAc D1R-VP/LH circuits promotes reanimation from sevoflurane anesthesia. Anesthesia & Analgesia. 2023. [DOI] [PubMed] [Google Scholar]; The authors used transgenic mouse models and optogenetic techniques to identify two targets of NAc D1R neuronal projections involved in regulating emergence from sevoflurane anesthesia: the ventral pallidum and the lateral hypothalamus. This represents the first identified direct downstream targets of NAc D1R projections involved in regulating emergence. [Google Scholar]
- 45.Li YD, Luo YJ, Xu W, et al. Ventral pallidal GABAergic neurons control wakefulness associated with motivation through the ventral tegmental pathway. Mol Psychiatry. 2021;26(7):2912–28. Epub 2020/10/16. doi: 10.1038/s41380-020-00906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu X, Li W, Ma Y, et al. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat Neurosci. 2019;22(1):106–19. Epub 2018/12/19. doi: 10.1038/s41593-018-0288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murata K. Hypothetical Roles of the Olfactory Tubercle in Odor-Guided Eating Behavior. Front Neural Circuits. 2020;14:577880. Epub 2020/12/03. doi: 10.3389/fncir.2020.577880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang B, Ao Y, Liu Y, et al. Activation of Dopamine Signals in the Olfactory Tubercle Facilitates Emergence from Isoflurane Anesthesia in Mice. Neurochem Res. 2021;46(6):1487–501. Epub 2021/03/13. doi: 10.1007/s11064-021-03291-4. [DOI] [PubMed] [Google Scholar]
- * 49.Song Y, Chu R, Cao F, et al. Dopaminergic Neurons in the Ventral Tegmental-Prelimbic Pathway Promote the Emergence of Rats from Sevoflurane Anesthesia. Neurosci Bull. 2022;38(4):417–28. Epub 2021/12/27. doi: 10.1007/s12264-021-00809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; First evidence for the involvement of the mesocortical dopaminergic pathway in regulating arousal from general anesthesia. The work uses selective chemogenetic and optogenetic activation of VTA dopamine projections to the prelimbic cortex in rats to delay loss of righting and accelerate return of righting in rats following sevoflurane anesthesia. [Google Scholar]
- 50.Yang YF, Dong H, Shen Y, et al. Mesencephalic dopamine neurons are essential for modafinil-induced arousal. Br J Pharmacol. 2021;178(24):4808–25. Epub 2021/08/17. doi: 10.1111/bph.15660. [DOI] [PubMed] [Google Scholar]
- 51.He E, Xu S, Dai Y, et al. SWCNTs/PEDOT:PSS-Modified Microelectrode Arrays for Dual-Mode Detection of Electrophysiological Signals and Dopamine Concentration in the Striatum under Isoflurane Anesthesia. ACS Sens. 2021;6(9):3377–86. Epub 2021/08/20. doi: 10.1021/acssensors.1c01241. [DOI] [PubMed] [Google Scholar]
- 52.Jiang-Xie LF, Yin L, Zhao S, et al. A Common Neuroendocrine Substrate for Diverse General Anesthetics and Sleep. Neuron. 2019;102(5):1053–65 e4. Epub 2019/04/23. doi: 10.1016/j.neuron.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loomba S, Straehle J, Gangadharan V, et al. Connectomic comparison of mouse and human cortex. Science. 2022;377(6602):eabo0924. Epub 2022/06/24. doi: 10.1126/science.abo0924. [DOI] [PubMed] [Google Scholar]
- 54.Yokawa K, Kagenishi T, Baluska F. Anesthetics, Anesthesia, and Plants. Trends Plant Sci. 2019;24(1):12–4. Epub 2018/11/18. doi: 10.1016/j.tplants.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Spindler LRB, Luppi AI, Adapa RM, et al. Dopaminergic brainstem disconnection is common to pharmacological and pathological consciousness perturbation. Proc Natl Acad Sci U S A. 2021;118(30). Epub 2021/07/25. doi: 10.1073/pnas.2026289118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kenny JD, Chemali JJ, Cotten JF, et al. Physostigmine and Methylphenidate Induce Distinct Arousal States During Isoflurane General Anesthesia in Rats. Anesth Analg. 2016;123(5):1210–9. Epub 2016/10/21. doi: 10.1213/ANE.0000000000001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuda W, Komatsu Y, Yanaka K, Matsumura A. Levodopa treatment for patients in persistent vegetative or minimally conscious states. Neuropsychol Rehabil. 2005;15(3-4):414–27. Epub 2005/12/15. doi: 10.1080/09602010443000588. [DOI] [PubMed] [Google Scholar]
- 58.Passler MA, Riggs RV. Positive outcomes in traumatic brain injury-vegetative state: patients treated with bromocriptine. Arch Phys Med Rehabil. 2001;82(3):311–5. Epub 2001/03/14. doi: 10.1053/apmr.2001.20831. [DOI] [PubMed] [Google Scholar]
- 59.Edlow BL, Barra ME, Zhou DW, et al. Personalized Connectome Mapping to Guide Targeted Therapy and Promote Recovery of Consciousness in the Intensive Care Unit. Neurocrit Care. 2020;33(2):364–75. Epub 2020/08/15. doi: 10.1007/s12028-020-01062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vlasov K, Pei J, Nehs CJ, et al. Activation of GABAergic Neurons in the Rostromedial Tegmental Nucleus and Other Brainstem Regions Promotes Sedation and Facilitates Sevoflurane Anesthesia in Mice. Anesth Analg. 2021;132(4):e50–e5. Epub 2021/02/10. doi: 10.1213/ANE.0000000000005387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin L, Li L, Deng J, et al. Optogenetic/Chemogenetic Activation of GABAergic Neurons in the Ventral Tegmental Area Facilitates General Anesthesia via Projections to the Lateral Hypothalamus in Mice. Front Neural Circuits. 2019;13:73. Epub 2019/12/05. doi: 10.3389/fncir.2019.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]