ABSTRACT
Integrative cardiovascular responses to heat stress during endurance exercise depend on various variables, such as thermal stress and exercise intensity. This review addresses how increases in skin temperature alter and challenge the integrative cardiovascular system during upright submaximal endurance exercise, especially when skin is hot (i.e. >38°C). Current evidence suggests that exercise intensity plays a significant role in cardiovascular responses to hot skin during exercise. At rest and during mild intensity exercise, hot skin increases skin blood flow and abolishes cutaneous venous tone, which causes blood pooling in the skin while having little impact on stroke volume and thus cardiac output is increased with an increase in heart rate. When the heart rate is at relatively low levels, small increases in heart rate, skin blood flow, and cutaneous venous volume do not compromise stroke volume, so cardiac output can increase to fulfill the demands for maintaining blood pressure, heat dissipation, and the exercising muscle. On the contrary, during more intense exercise, hot skin does not abolish exercise-induced cutaneous venoconstriction possibly due to high sympathetic nerve activities; thus, it does not cause blood pooling in the skin. However, hot skin reduces stroke volume, which is associated with a decrease in ventricular filling time caused by an increase in heart rate. When the heart rate is high during moderate or intense exercise, even a slight reduction in ventricular filling time lowers stroke volume. Cardiac output is therefore not elevated when skin is hot during moderate intensity exercise.
KEYWORDS: Skin temperature, hyperthermia, heat stress, hemodynamic, cardiovascular control, skin blood flow, cutaneous venous volume
Introduction
While cardiovascular responses to heat stress during endurance exercise have been extensively studied, this topic remains inconclusive. The main reason is that the integrative cardiovascular responses to heat stress during endurance exercise depend on various variables, such as thermal stress (i.e. skin and core body temperatures), exercise intensity and duration, type of exercise, and hydration status. Therefore, the specific condition needs to be considered when drawing a conclusion. This review aims to specifically address how increases in skin temperature alter and challenge the integrative cardiovascular system adjustments during upright submaximal endurance exercise (i.e. cycling and running) especially when skin is hot (i.e. >38°C) in euhydrated subjects. Cardiovascular responses to hot skin at rest will also be reviewed to provide a comprehensive understanding of the continuum cardiovascular responses to hot skin from rest to mild exercise and then heavy exercise. High skin temperature was selected because the majority of passive heat stress (i.e. at rest) studies were performed when skin was hot. However, heat stress studies during exercise were often conducted when skin temperature could be quite varied as were the various exercise intensities. Therefore, this review will address the controversy regarding cardiovascular responses to hot skin at rest and during endurance exercise.
Sweating plays a significant role in thermoregulation when exercising in the heat. The loss of body fluid via sweating can result in dehydration, which adversely affects cardiovascular capacity and thermoregulatory [1–3]. Severe hyperthermia can develop during exercise when dehydrated, while skin temperature is stable at lower levels. However, when skin temperature is at very high levels (i.e. >38°C), rapidly developed core hyperthermia is more likely to cause fatigue during endurance exercise before the development of dehydration. Therefore, this review will not discuss studies under dehydrated conditions, which have been covered elsewhere [4].
While several excellent comprehensive reviews on cardiovascular responses to heat stress at rest [5–11] and during exercise [5,6,12–14] have been published, this review provides unique and additional discussions on the effect of increasing skin temperature independent of core temperature on cardiovascular responses at rest and during endurance exercise, since this topic has been relatively less completely addressed yet could be significant when skin is hot. Additionally, the mechanism of how stroke volume is lowered by heat stress has been a contentious topic. Our recent studies [15,16] provided additional and critical evidence to assist in clarifying the controversy between the two mechanisms, but no recent review has comprehensively discussed this topic. Current evidence suggests that exercise intensity (i.e. from resting to intense exercise) plays a significant role in the integrative cardiovascular response to hot skin. Specifically, we will elaborate on the different mechanisms of how hot skin affects cardiovascular responses at different exercise intensities, which are summarized in Figure 1. The exercise referred to in this review is whole-body dynamic submaximal exercise (i.e. running, walking, and cycling) unless specified, and the study populations are young healthy adults. Additionally, the skin referred to in this review is nonglabrous skin (i.e. most of the body surface, including the limbs, head, and trunk) which is regulated differently from glabrous skin (i.e. palms and soles) regarding thermoregulation [17,18].
Figure 1.

Schematic illustration of the cardiovascular responses to hot skin at rest and during submaximal dynamic exercise. (a) At rest and during mild intensity exercise, hot skin increases SBF and abolishes cutaneous venous tone which raises CVV. The extra blood to the skin is provided by an increase in CO which is achieved by an increase in HR and no changes in SV. Hot skin also causes visceral vasoconstriction which redistributes some blood from visceral vascular beds to the skin. At rest and during mild exercise (when core body temperature is relatively low), the extent of the increases in HR and CVV might be not enough to affect SV when TPR (cardiac afterload) is reduced by cutaneous vasodilation. MAP is maintained by an increase in CO while TPR is lowered. (b) During more intense exercise, hot skin increases SBF, but SBF levels off when the core body temperature is above ~38°C. Hot skin does not abolish cutaneous venoconstriction induced by intense exercise, and thus there is no significant blood pooling in the skin. Visceral vasoconstriction is higher during moderate exercise relative to mild exercise as HR is higher. HR can be raised to very high levels by hot skin which decreases ventricular filling time and SV; therefore, CO does not change when skin is hot during moderate intensity exercise. MAP maintains or only slightly decreases. CO, cardiac output; CVV, cutaneous venous volume; HR, heart rate; MAP, mean arterial pressure; SBF, skin blood flow; SV, stroke volume; TPR, total peripheral resistance.
Effect of increasing skin temperature independent of core temperature on cardiovascular responses at rest
It was observed long ago that passive radiant heating of a large area of skin caused a rapid (within 8 seconds) rise in heart rate [19]. The rapidity of the response suggests that the change in heart rate probably depends on nerve impulses from the skin but does not depend on the core temperature in the early stages. Additionally, when the legs were heated and the blood was arrested in the legs by the thigh cuffs to prevent warm blood from going to the other parts of the body, the increase in heart rate was similar compared to without the cuffs [19]. This study also proved that the extent of increase in heart rate is associated with skin temperature. Furthermore, the constancy of the blood pressure ruled out the possibility that the increase in heart rate during this period was dependent on the stimulation of baroreceptors consequent to the cutaneous vasodilation. The heart rate response to an increase in skin temperature is faster than the vasodilation [20], so the increase in heart rate does not originate from the stimulation of the reduction in vasomotor tone. Therefore, there appears to be a neural link between thermal receptors in the skin and the nerves controlling the heart rate. Accordingly, Wyss et al. [21] observed raising whole body skin temperature from 34°C to 40°C during supine rest caused an initial drop in right atrial temperature and it increased thereafter. While the right atrial temperature was depressed, forearm blood flow increased 50–100% and heart rate increased, showing a skin temperature influence on skin blood flow and heart rate. However, when the right atrial temperature increased above the pre-heating value, forearm blood flow and heart rate closely tracked the increase in right atrial temperature, indicating a significant influence of core temperature on forearm blood flow and heart rate. When skin temperature was lowered back to 34°C, heart rate rapidly returned to control levels, whereas forearm blood flow returned more slowly. Therefore, they concluded that decreasing skin temperature has a strong inhibitory influence on heart rate, while only a minor or no influence on skin blood flow. In an animal study [22], the skin and core temperature of baboons were independently controlled by ambient temperature and a heat exchanger in a chronic femoral arteriovenous shunt, respectively. They showed that increasing skin temperature only slightly elevated heart rate and right iliac blood flow when the core temperature was held constant. Whereas elevating core temperature largely increased heart rate and right iliac blood flow either when skin temperature was normal or hot. Therefore, a rapid increase in skin temperature per se elevates heart rate and cutaneous blood flow at rest, but to a much lesser extent compared to the effect of increasing core temperature. Wenger et al. [23] reported that the ratio of the effect of core temperature and skin temperature on the effector responses (i.e. forearm blood flow) was 9:1. Although the influence of increasing skin temperature is small on heart rate and skin blood flow relative to core temperature, the extent to which skin temperature can be elevated is much larger than core temperature. Therefore, the absolute changes in cardiovascular responses via increasing skin temperature per se can be significant.
Increasing skin blood flow increases heat transfer from core to skin. However, heat loss from the skin to the environment is facilitated by moving a large volume of blood close to the body surface as slowly as possible to increase the mean transit time for heat dissipation. Increasing the blood volume in the cutaneous vasculature can be achieved by passive filling of veins following an increase in skin blood flow [6,24] and by increasing cutaneous venous compliance [25]. The amount of blood that a vein can hold is affected by venous tone. If venous tone increases, the vein becomes less compliant and the volume it can hold decreases.
The effect of skin temperature on venous tone has also been investigated. Both local skin and core temperature can affect venous tone [26–28]. Changing the temperature of the blood flowing through the separately perfused dog limb altered the responsiveness of superficial limb veins to sympathetic nerve stimulation, whereas changing core temperature alters the rate of sympathetic stimulation of the veins [26,28]. Wenger et al. [29] performed a study during which subjects rested at ambient temperatures of 25°C and 35°C, while forearm venous volume was measured. Forearm venous volume rose linearly with core temperature, while higher skin temperature shifted the relation between forearm venous volume and core temperature toward lower core temperature without changing the slope. That is, at any given core temperature at rest, higher skin temperature causes higher forearm venous volume.
Effect of increasing skin temperature independent of core temperature on cardiovascular responses during exercise
While cardiovascular responses during exercise under heat stress are extensively investigated, most studies set conditions where both core temperature and whole body skin temperature are elevated. Raising skin temperature during exercise can easily increase core temperature. The inability to separately control core and skin temperature is the main challenge in investigating the independent effect of skin temperature on cardiovascular responses during exercise. Nevertheless, some studies manipulated skin temperature, while core temperature was not affected, at least at some time points, during dynamic exercise. Therefore, the effect of skin temperature independent of core temperature on cardiovascular responses during exercise was revealed.
Cutaneous vasculature
Some studies used exercise intensity as a method to alter core temperature and investigated the effect of skin temperature on skin blood flow at different levels of core temperature. Wenger et al. found that increasing skin temperature decreased the threshold of core temperature at which blood flow begins to increase in the forearm [23] and the finger [30] when subjects cycled at 30%, 50%, and 70% VO2max in ambient temperatures of 15°C, 25°C, and 35°C (Figure 2). Thus, at any given core temperature, skin blood flow was higher when skin temperature was higher before skin blood flow reaches a plateau during exercise. Skin temperature did not affect the slope of the relation between forearm and finger blood flow and core temperature (Figure 2). They also found that the relation between forearm blood flow and core temperature was not affected by exercise intensity. However, different exercise intensities resulted in different core temperatures. The effect of exercise intensity per se, independent of thermal stress, on skin blood flow warrants further investigation. Johnson et al. [31] reported that the reflex increase in skin blood flow to rising skin temperature during exercise is dependent on the level of core temperature, being smaller at low core temperature and larger at higher core temperature. This might be explained by the effect of increasing skin temperature on increasing skin blood flow, which is smaller below the active vasodilation threshold and larger above the threshold. The reflex increase in skin blood flow to rising skin temperature is mediated through vasoconstrictor withdrawal when core temperature is normal at rest, while reflex cutaneous vascular effects with rising skin temperature are largely mediated through the active vasodilation when core temperature is elevated by exercise [32]. Therefore, the reflex effects of whole body skin temperature on the cutaneous vasculature can be mediated through either vasoconstrictor or the active vasodilator arm of the sympathetic nervous system, depending on the background core temperature.
Figure 2.

Influence of skin temperature on forearm (skin) blood flow during dynamic exercise. Increasing skin temperature (Tsk) shifts the threshold of core temperature for cutaneous vasodilation to lower levels during 30–50% VO2max cycling exercise. Therefore, at a given core temperature, increasing Tsk raises skin blood flow during dynamic exercise at least before skin blood flow reaches the plateau level (i.e. when core temperature is above 38°C). Redrawn with permission from Ref [23].
During short-duration exercise, the cutaneous venous tone increased [33–39], caused by increasing sympathetic nervous activity [35,39], in proportion to the intensity of exercise [34–37]. Therefore, forearm venous volume was lower during exercise than at rest at the same skin and core temperature. Additionally, this venoconstrictor effect of exercise was greater with higher baseline skin temperature and forearm venous volume [29]. Locally heating the skin to 40–44°C abolished the normal increase in forearm venous tone which developed in response to mild exercise [34,35,40]. Furthermore, raising whole body skin temperature toward 38°C either abolished or markedly attenuated the venomotor response to 5 minutes of mild cycling exercise (49 and 98 watts). When whole body skin temperature was rapidly cooled toward 34°C during the last 2 minutes of a 98 watts exercise bout, cutaneous veins constricted [37]. This effect of abolishing the venomotor response to exercise was accounted for by a high skin temperature per se, independent of core temperature, since the exercise intensities were low and duration was short. In the follow-up study [40], local forearm skin and whole body skin temperatures were controlled separately during mild (49 watts of cycling exercise) and short duration (10 minutes) exercise. Raising forearm skin temperature to 40°C or whole body skin temperature to 38°C or both abolished cutaneous venoconstriction response to mild exercise. Changing forearm skin temperature alone altered the venomotor tone of the forearm with longer latency and more gradual effect than changing whole body skin temperature. In addition, when the whole body skin temperature was heated to 38°C, forearm veins were not reactive to local cooling. In the dog limbs, cutaneous venomotor responses to sympathetic stimulation depended on the temperature of the limb [26–28,41]. The interpretation is that heating whole body skin reflexly reduces the rate of adrenergic neural constrictor stimulation to cutaneous veins, whereas direct local heating reduces the sensitivity of cutaneous veins to any given level of reflex neural stimulation.
While studies demonstrated that raising local skin temperature to 40–44°C or whole body skin temperature to 38°C, independent of core temperature, abolished the venoconstriction response to exercise during short duration mild-intensity exercise, the independent effect of hot skin on venous tone at higher intensities of exercise has not been established. Nonetheless, studies have shown that forearm venous volume was not higher when skin temperature was above 38°C relative to lower skin temperatures, implying that the exercise-induced increase in cutaneous venous tone was maintained, during 20–30 min of cycling at ~62% VO2peak (Figure 3 and Figure 4(e)) [15,16].
Figure 3.

Weighted mean skin temperature (a), esophageal temperature (b), and core-to-skin temperature gradient (C) responses during exercise with different water temperatures in the suit.
Figure 4.

Cardiovascular responses during exercise with different water temperatures in the suit. (a) Cardiac output; *significant increase in 20°C. (b) Heart rate; all trials were significantly different from each other at every time point (P < 0.05). (c) Stroke volume. (d) Cutaneous blood flow; *15 min and 25 min were significantly higher than 5 min of the same trial in 20°C, 30°C, and 40°C trials (P < 0.05). (e) Forearm venous volume; the resting forearm venous volumes were significantly higher compared to exercising values of the same trial in 30°C, 40°C, and 50°C trials (P < 0.05) indicating exercise caused venoconstriction which was maintained throughout exercise bouts in all trials. Values are means ± SE (n = 8). *Significantly different from 5–10 min of the same trial; †significantly different from 15–20 min of the same trial; ‡significantly different from the 20°C trial at the same time point; §significantly different from the 30°C trial at the same time point, P < 0.05. Used with permission from Chou et al. [15].
Integrative cardiovascular responses
The following studies investigated the effect of increasing skin temperature while controlling for core temperature on cardiovascular responses to exercise. When skin temperature was manipulated by having subjects cycled with 4.3 m/s of airflow or without airflow, the resultant skin temperatures were ~29°C and ~32°C, respectively, while the rectal temperature (~38.2°C) was not different between trials during the first 50 minutes [42]. Shaffrath and Adams [42] observed that higher skin temperature caused higher heart rate and forearm blood flow during prolonged exercise at 60% VO2max. However, the rectal temperature response may be too slow to reflect the difference in core temperature between trials. In another study [43], when endurance-trained subjects cycled at 72% VO2max for 30 minutes in a hot (35°C) or cold (8°C) environment, mean skin temperatures were at 34.5°C and 22.5°C, respectively. Esophageal temperatures were not significantly different at 30 min between the hot (38.5°C) and the cold (38.2°C) trials. Increasing skin temperature elevated cutaneous blood flow by increasing cardiac output. The higher cardiac output in the heat was accomplished by increasing heart rate and maintaining stroke volume. Nadel et al. [44] performed a study whereby subjects exercised on a contour chair cycle ergometer at 40% VO2max for 20–25 minutes in ambient temperatures at 20°C, 26°C, and 36°C, which created skin temperatures at 32.0°C, 33.7°C, and 35.2°C, respectively. Core temperature was unaffected by the ambient temperatures and was at ~37.6°C at the end of the exercise bout for all the trials. Increases in skin temperature raised forearm blood flow, and the extra blood flow to the skin was achieved by an increase in cardiac output. The increase in cardiac output was accomplished by an increase in heart rate and a maintained stroke volume. Lee et al. [45] separately controlled core and skin temperatures during 20 minutes of cycling at 69% VO2peak (Figures 5 and Figure 6). Pre-exercise core temperature was altered by cold (14°C) or hot (40°C) water immersion, and skin temperature during exercise was manipulated by fan cooling or radiant heating. Thus, the effect of skin temperature on cardiovascular responses during exercise when core temperatures were low and high was investigated. When the pre-exercise esophageal temperature was cooled (~35.8°C), warm skin (32.1°C at the end of the exercise) caused greater increases in cutaneous blood flow, heart rate, and cardiac output, while stroke volume was similar compared to cool skin (23.7°C at the end of the exercise) (Figure 5). The effect of skin temperature may be confounded by core temperature as the core temperature was significantly higher after 11 minutes of exercise (0.31°C higher on average from 11 to 20 min of exercise) during warm skin relative to cool skin. However, the magnitude of the increase in heart rate was greater than the effect of increasing core temperature per se on increasing heart rate [46]. In addition, cutaneous blood flow was significantly higher during the first 11 minutes of exercise when the skin was warm compared to when the skin was cool. This suggests that increasing skin temperature per se increases heart rate and cutaneous blood flow but has no effect on stroke volume when the core temperature remained low (<37.7°C). In contrast, during preheating trials (pre-exercise esophageal temperature was initially 37.3°C and reached 38.5°C and 37.7°C at the end of exercise for the warm skin and the cool skin, respectively), heart rate was higher, stroke volume was reduced, and cardiac output was similar throughout the exercise bout when the skin was warm (33.4°C at the end of the exercise) compared to when the skin was cool (25.0°C at the end of the exercise) (Figure 6). Of note, cutaneous blood flow was generally similar, while stroke volume was markedly lower when the skin was warm versus when the skin was cool during preheating trials. Therefore, when cutaneous blood flow reaches its upper limit during exercise caused by a high core temperature, increasing skin temperature does not cause a further elevation in cutaneous blood flow. However, heart rate can be further elevated by increasing skin temperature when the core temperature is high, which might have a secondary effect of lowering stroke volume by reducing ventricular filling time. More recently, Chou et al. [15] progressively pre-heated whole body skin from low to high levels using a water-perfused suit at ~31°C, 34°C, 37°C, and 39°C, respectively, before 20–30 minutes of cycling exercise at 62% VO2peak (Figure 3). Thus, the independent effect of increasing skin temperature was revealed during the early stage (i.e. the first 10 minutes) of exercise when the esophageal was relatively low (37.2°C). Graded increases in skin temperature progressively elevated heart rate, cutaneous blood flow, and cutaneous vascular conductance. There was a trend of increasing skin temperature and heart rate decreasing stroke volume, while cardiac output tended to be lower at the lowest skin temperature (i.e. seeing cardiovascular responses during the first 10 min of Figure 4).
Figure 5.

Cardiovascular responses during exercise with cool skin and warm skin after precooling of core temperature. Warm skin significantly increased heart rate, cutaneous blood flow, and cardiac output while did not impair stroke volume relative to cool skin when core is pre-cooled (~35.8°C) during moderate intensity exercise. Values are means ± SE (n = 8). *Warm skin condition significantly higher than cool skin condition, P < 0.05. Used with permission from Lee et al. [45].
Figure 6.

Cardiovascular responses during exercise with cool skin and warm skin after preheating of core temperature. Warm skin significantly increased heart rate and decreased stroke volume while did not affect cardiac output and cutaneous blood flow relative to cool skin when core is preheated (37.3°C) during moderate intensity exercise. Values are means ± SE (n = 8). *Warm skin condition significantly higher than cool skin condition, P < 0.05. †Main effect of skin temperature condition, P < 0.05. Used with permission from Lee et al. [45].
In summary, when the core temperature is relatively low (<38°C), either achieved by exercising in the cold [43], exercising at low intensities [44], or pre-cooling [45], increasing skin temperature increases heart rate and cutaneous blood flow, while it does not affect stroke volume during low to moderate intensity exercise. As a result, cardiac output is elevated, and the increased cardiac output appears to go to the skin vascular beds. The reason why stroke volume is maintained is not clear because both increasing heart rate and cutaneous blood flow may cause a decline in stroke volume [5,13]. Since the increases in heart rate and cutaneous blood flow are relatively small when the core temperature is low, ventricular filling time might still be sufficient for cardiac filling with only a slightly lower filling pressure. Additionally, the cardiac output under those conditions is also relatively low, which could be further increased by increasing heart rate when skin temperature is warm. Indeed, hot skin (38–39°C) per se seems to further increase heart rate and cutaneous blood flow while tending to lower stroke volume and failing to increase cardiac output during moderate intensity exercise (Figure 4) [15]. On the contrary to lower core temperature, when the core temperature is relatively high (>38°C) during moderate intensity exercise, increasing skin temperature raises heart rate and decreases stroke volume, while cardiac output is maintained [45]. Cutaneous blood flow already reaches its upper limit while in the upright position caused by a high core temperature, therefore, increasing skin temperature has no further effect on cutaneous blood flow when the core temperature is high. Thus, the decrease in stroke volume is probably associated with the increase in heart rate during moderate intensity exercise when the core temperature is high (Figure 6) [45].
Skin temperature was altered during exercise in different environmental conditions in the following studies. While the effect of skin temperature on cardiovascular responses was not the focus of these studies, it is still worth discussing. Subjects exercised at 60% peak aerobic power until exhaustion or for 2 hours with different air velocities (0.2 km/h, 9.9 km/h, 33.3 km/h, and 50.1 km/h for each trial, respectively) in a 33°C ambient temperature and 59% relative humidity environment [47]. Different air velocities resulted in a graded response in skin temperature (~36°C, 35°C, 34°C, and 33°C for each trial, respectively), which caused a graded response in core temperature and heart rate. The core temperature reached ~39°C at the end of exercise for all trials in accordance with the same levels of dehydration [47]. Galloway et al. [48] manipulated skin temperature by having subjects cycle at 70% VO2max at four different ambient temperatures (3.6°C, 10.5°C, 20.6°C, and 30.5°C for each trial, respectively). The skin temperatures were at ~22°C, 26°C, 31°C, and 36°C for each trial, respectively. There was a graded response in core temperatures, which were at ~38.9°C, 39.2°C, 39.4°C, and 39.9°C at the 25 minute of exercise for each trial, respectively. Heart rate was only significantly higher at the highest ambient temperature compared to other ambient temperatures. This suggests that skin temperature might have little effect on heart rate when skin temperature is relatively low (<31°C) during prolonged exercise. When cycling at 70% VO2max until exhaustion in an environment at 30°C in four relatively humidity conditions (24%, 40%, 60%, and 80% for each trial, respectively), skin temperatures were gradedly elevated and stable after 20 minutes of exercise at ~33.6°C, 33.8°C, 34.3°C, and 34.8°C, for each condition, respectively [49]. There were no significant differences in core temperature, cutaneous blood flow, and cutaneous vascular conductance between trails, while there was a tendency (p = 0.097) for higher heart rate with increasing relative humidity or skin temperature. Another similar study performed by Muhamed et al. [50] had trained runners run for 60 minutes at 70% VO2max followed by an incremental test to exhaustion in an environment at 31°C in relative humidities of 23%, 43%, 52%, 61%, and 71%. Skin temperatures were gradedly elevated and stable after 30 minutes of exercise at ~31.7°C, 32.3°C, 32.5°C, 32.9°C, and 33.3°C, for each condition, respectively. Heart rate during steady-state exercise was significantly higher in the 61% and 71% relative humidity trials compared to the 23% relative humidity trial. Stroke volume was systematically decreased as heart rate rose and thus cardiac output remained unchanged between trials. Otani et al. [51] used another approach to alter skin temperature by applying different intensities of solar radiation (0 W/m2, 250 W/m2, 500 W/m2, and 800 W/m2 for each condition, respectively) on the subjects during 70% VO2max exercise to exhaustion. Different intensities of solar radiation caused a graded response in skin temperature at ~33°C, 33.5°C, 33.9°C, and 34.5°C for each condition, respectively. However, for this small range of skin temperatures, there were no significant differences in core temperature, heart rate, cutaneous blood flow, cutaneous vascular conductance, sweat rate, and changes in plasma volume between trials. Of note, with only slight increases in skin temperature and without any differences in other physiological variables between trials, the rate of perceived exertion was significantly increased and time to exhaustion during 70% VO2max was significantly reduced by increasing the relative humidity [49] and solar radiation [51] of the environment. This suggests that skin temperature per se can affect perceived exertion and endurance exercise capacity, which indeed has been previously shown [52]. In summary, when skin temperature is elevated in a graded manner during exercise, it causes graded increases in core temperature and heart rate and a graded decrease in stroke volume while cardiac output is maintained if the differences in skin temperature between each trial are large enough. In addition, when skin temperature is below 31°C, it may not affect the heart rate during moderate intensity exercise. While differences in skin temperature between trials are only slight, skin temperature does not affect heart rate, cutaneous blood flow, and core temperature during exercise. However, even without any physiological differences, a slight increase in skin temperature appears to be enough to increase perceived exertion and cause earlier fatigue.
Overall, increasing skin temperature decreases the threshold of core temperature at which skin blood flow begins to increase, but the slope of the relation between skin blood flow and core temperature is not affected by skin temperature [23,30]. Therefore, at any given core temperature, skin blood flow is higher when skin temperature is higher. However, skin blood flow reaches an upper limit when the core temperature is at ~38°C during exercise [53,54]. Increasing skin temperature does not further elevate skin blood flow once it reaches its upper limit (when the core temperature is above 38°C) during exercise [45]. Similar to skin blood flow, higher skin temperature causes a higher cutaneous venous volume at any given core temperature [29]. However, exercise causes cutaneous venoconstriction, and this venoconstrictor effect of exercise is greater when cutaneous venous blood volume is larger [29]. Raising local or whole body skin temperature to a high level (>38°C) abolishes the venoconstrictor effect of exercise at least during short duration mild intensity exercise [34,35,37,40] but not during moderate intensity exercise [15,16]. Additionally, it appears that even when skin temperature is only slightly increased without affecting other physiological variables during exercise, raising skin temperature causes higher perceived exertion and diminishes exercise tolerance [49,51,52]. A larger increase in skin temperature increases the heart rate [15,19,21,22,42–45,47] which is in proportion to the increase in skin temperature [15,19]. The increases in heart rate are rapid and prior to the changes in core temperature and cutaneous vasodilation, suggesting that there is a direct neural link between thermal receptors in the skin and the nerves controlling the heart [20]. However, when skin temperature is below 31°C, increasing skin temperature may not affect heart rate during moderate intensity exercise. Raising skin temperature does not affect stroke volume when the core temperature is low (heart rate and skin blood flow are relatively low) [15,43–45]. Raising skin temperature decreases stroke volume when the core temperature is high (heart rate and skin blood flow are relatively high) [45]. As a result, cardiac output either increases or is maintained when increasing skin temperature during exercise [15,43–45].
Cardiovascular responses to hot skin at rest
A series of studies performed by Rowell et al. established the fundamentals of the current understanding of the cardiovascular responses to hot skin at rest [21,55–61]. Several excellent reviews on cardiovascular responses to passive heat stress have also been published [5–11]. In general, skin blood flow increases as the internal temperature increases to dissipate heat by transporting heat from the core to the skin [21,60,62]. The increase in skin blood flow is achieved by cutaneous vasodilation, which causes a decrease in total peripheral resistance [57]. Cutaneous venous volume can be elevated passively by increasing skin blood flow or actively by venodilation [63]. Increasing venous volume during heat stress at rest [64] can cause pooling of the blood to the skin and thus reduce ventricular filling pressure and central blood volume. The right atrium mean pressure falls relatively rapidly during the first 10 min of passive heating and declines steadily throughout the heat stress, approaching 0 mm Hg [57].
Despite the fall in right ventricular and presumably left ventricular filling pressure, stroke volume generally stays the same or slightly increases during passive heat stress at rest [5,9,57,59,65–69]. Stroke volume is a combination of the net effects of cardiac preload, cardiac afterload, and contractility. The diastolic function of the heart may also influence stroke volume during passive heat stress. Numerous studies that measured the indexes of cardiac preload, such as right atrial pressure, central venous pressure, pulmonary capillary wedge pressure, left-ventricular end-diastolic volume, and central blood volume, showed that passive heat stress decreases cardiac preload at rest [11,66,69–73]. Additionally, heat stress-induced increases in heart rate would theoretically decrease cardiac preload by reducing ventricular filling time. Cardiac afterload also decreases during passive heat stress as indicated by the decrease in total peripheral resistance [11,57], which may be due to cutaneous vasodilation. Nelson et al. [74] observed that a decrease in end-systolic wall stress also denoted a reduction in cardiac afterload. Heat stress causes an increase in contractility of the heart as heat stress elevates sympathetic nervous activities [61,75,76]. Evidence supports an improvement of contractility during passive heat stress including increases in ejection fraction [71,74], isovolumic acceleration of the septal and lateral mitral annulus [77], and left-ventricular twist rates [69,74]. Left-ventricular diastolic function is preload dependent. That is, when preload decreases, so does diastolic function. However, some studies observed that diastolic function is preserved when preload is decreased during passive heat stress [74,77] suggesting that heat stress improves diastolic function with a background of lowered preload. The combined effect of passive heat stress on preload, afterload, contractility, and diastolic function can be revealed from Frank-Starling curves (Figure 7) [70,78]. Passive heat stress induced decreases in afterload and increases cardiac contractility and a shift of the Frank-Starling curve upward and to the left, which allows greater stroke volume at any given ventricular filling pressure. However, the slope of the curve is steeper during heat stress. Thus, when cardiac preload is reduced by heat stress, it causes greater decreases in stroke volume compared to when normothermic (Figure 7).
Figure 7.

Schematic of the effect of thermal stress on the Frank. Starling relations.
Heat stress shifts the Frank-Starling curve upward and to the left and alters the operating points. The steep slope indicates that a similar decrease in pulmonary capillary wedge pressure would cause a relatively large decrease in stroke volume during heat stress compared to normothermia. Used with permission from Ref [11].
The heart rate invariably increases with core hyperthermia [21,65,75,79,80]. Heart rate is affected by temperature in two ways: 1) the direct effects on intrinsic heart rate through cardiac nodal cells (sinoatrial and atrioventricular) and 2) the effects on autonomic nervous system activity. The direct effect of temperature on intrinsic heart rate, independent of autonomic nervous system activity, has been demonstrated in animals [81,82], isolated heart [83,84], embryo [85–87], and human [46]. Heat stress is a hyperadrenergic state that increases sympathetic noradrenergic signaling and circulating catecholamines [61,75,76]. Heat stress also decreases cardiac parasympathetic effects [88–91]. During passive heat stress in baboons, Gorman and Proppe [92] reported that ~40% of the increase in heart rate was accounted for by cardiac temperature and ~60% was due to autonomic influences. In addition, the autonomic control of the increases in heart rate with heat stress was attributed to ~25% sympathetic activation and ~75% parasympathetic vagal withdrawal.
Maintained stroke volume and increased heart rate allow a large increase in cardiac output [5,65,66,93] when the skin vasculature is dilated and no muscle pump is active at rest. Mean arterial blood pressure is usually maintained or only slightly decreased [57,59,94]. Maintained blood pressure is partially achieved by an increase in cardiac output. In addition to an increase in cardiac output, other vascular beds also make adjustments to passive heat stress. For example, splanchnic blood flow reduces by about 40% [55–59,61,66,95,96], and renal blood flow decreases by 15–40% during passive heating [58,66,97,98]. The decrease in splanchnic and renal blood flow can be caused by reducing perfusion pressure as arterial blood pressure sometimes slightly decreases during passive heat stress [57,99], and by sympathetically mediated vasoconstriction as splanchnic vascular resistance increases during passive heat stress (Figure 8) [58,59]. Vasoconstriction of splanchnic and renal vascular beds helps maintain mean arterial blood pressure and reduces the distending pressure of the highly compliant vasculature, which results in a reduction in blood volume in those regions. Reductions in splanchnic blood volume during heat stress have been qualitatively assessed from x-ray dimensions of the liver, showing reduced volume [100]. Crandall et al. [71,101] also observed that liver and spleen blood volumes are reduced during passive heat stress. Therefore, blood redistributes from visceral organs to the skin during passive heat stress.
Figure 8.

The relationships between splanchnic and renal blood flow, heart rate, and sympathetic nervous activity. The relationships between splanchnic blood flow (SBF) or renal blood flow (RBF) presented as a percent of resting values vs. heart rate, plasma norepinephrine (NE) concentration vs. heart rate, and plasma renin activity (PRA) vs. heart rate are illustrated. Note that NE and PRA begin to rise and SBF (RBF) begins to fall at the same heart rates (i.e. at 50–60 bpm when stresses are applied at rest and at 90–100 bpm when the stress is exercising under a variety of conditions including heat stress) indicating an overall increase in sympathetic nervous activity under stress which increases heart rate and decreases SBF and RBF. Redrawn with permission from Ref [144].
Studies that measured forearm muscle blood flow showed that passive heat stress did not affect resting skeletal muscle blood flow [60,62,102,103], while other studies observed calf and leg muscle blood flow slightly increased (i.e. ~1 ml/min/100 g tissue) during passive heat stress [104–108]. The increase in skeletal muscle blood flow is the net result of the increased vasodilator activity, overriding the parallel elevated vasoconstrictor activity caused by direct local heating of the skeletal muscle while not eliciting whole body heating of the other part of the body [104,107,109,110]. Nonetheless, a 1 ml/min/100 g tissue increase in skeletal muscle blood flow remains small, probably ~350 ml/min for the entire body in a person with an average muscle mass of 35 kg. Cerebral blood flow reduces by ~0.1 L/min or 15% during passive heat stress [111,112], and this reduction in cerebral blood flow is caused by a hyperventilation-induced reduction in the potent cerebral vasodilator CO2 [113].
Therefore, cutaneous vascular beds seem to be the only vascular beds that largely increase blood flow during heat stress at rest. Therefore, the majority of the increased cardiac output during passive heat stress is directed to the skin. More specifically, when both skin and core temperatures are high (i.e. above 39°C), cardiac output can increase by an average of 6.6 L/min [5]. While others observed smaller increases in cardiac output, Koroxenidis et al. [65] reported increases averaging 3.3 L/min in cardiac output when the oral temperature reached ~37.8°C (~1.1°C elevation while skin temperature was not measured). Minson et al. [66] measured an average of 4.5 L/min increase in cardiac output when the esophageal temperature was at ~38.8°C (~2.5°C elevation), and skin temperature was at ~39°C. Ganio et al. [93] showed an average of ~5 L/min elevation in cardiac output when pulmonary artery blood temperature was at ~38.7°C (~1.2° elevation) and skin temperature was at ~39°C. This variation of the increase in cardiac output with heat stress at rest may reflect the degree of increases in skin and internal temperature and the fraction of the body surface directly heated. Splanchnic blood flow was reduced by ~0.6 L/min, and renal blood flow was reduced by ~0.4 L/min during passive heat stress [5,66]. The effect of increased cardiac output and the redistribution of blood flow has the potential for a 7–8 L/min increase in blood flow to the skin. This high level of skin blood flow is 50–60% of cardiac output during supine rest, and the cutaneous vascular beds are receiving the largest blood flow in the context of passive heating. Therefore, skin blood flow is not only important for temperature regulation but also for systemic hemodynamics, including blood pressure regulation and the delivery of blood flow to other regions at rest [6].
In summary, passive heat stress at rest elevates skin temperature and eventually core temperature. Increasing skin and core temperature causes skin vasodilation and an increase in cutaneous venous volume, which causes decreases in ventricular filling pressure and central blood volume. However, stroke volume is maintained when cardiac preload is decreased because cardiac afterload is reduced and contractility and diastolic function are increased by passive heat stress. The rise in skin blood flow is achieved by an increase in cardiac output and blood redistribution from other vascular beds. Cardiac output rises due to an increased heart rate and a maintained stroke volume; therefore, blood pressure is maintained when total peripheral resistance is decreased by skin vasodilation. Visceral organs vasoconstrict and passively release their volume, compensating in part for the displacement of volume to the cutaneous veins. Skeletal muscle blood flow is maintained or slightly increased due to the effect of local heating, and cerebral blood flow decreases due to hyperventilation-induced reduction in PaCO2 (Figure 1(a)).
Cardiovascular responses to hot skin during endurance exercise
Cutaneous vasculature
Dynamic exercise affects skin blood flow via several modifications of the vasoconstrictor and active vasodilator systems (Figure 9) [114–116]. In general, exercise affects the relationship between core body temperature and skin blood flow in three ways (Figure 9). First, at the beginning of exercise, there is a prompt cutaneous vasoconstrictor response both in normothermic and hyperthermic conditions, and this vasoconstriction is a function of the baseline level of skin blood flow. That is, the largest vasoconstriction occurs when the initial skin blood flow is the highest [114,117–119]. This vasoconstriction is caused by an increase in sympathetic adrenergic vasoconstrictor system activity [118]. The initiation of exercise does not appear to have an immediate inhibitory effect on the active vasodilator system since a local iontophoresis of bretylium treatment, which selectively blocks noradrenergic vasoconstrictor nerves, completely prevents the vasoconstriction response to the initiation of exercise [118]. As exercise continues in a hot environment outside the prescriptive zone, core temperature rises. There is a threshold core temperature at which vasodilation begins, after which skin blood flow rises with core body temperature [23,120,121]. Second, exercise is associated with an increase in the threshold core temperature for the onset of skin vasodilation [115,121–125]. This is achieved by a delay in initiation of active cutaneous vasodilation to a higher core temperature because the increase in the threshold core temperature with exercise is unaffected by the vasoconstrictor nerve inhibition [122]. In addition, the threshold core temperature for the initial cutaneous vasodilation is dependent on the intensity of exercise [126,127]. Studies also showed a strong correlation between changes in plasma osmolality and the delay in the onset of active cutaneous vasodilation [124,128,129]. Increases in plasma osmolality are proportional to the intensity of exercise [130], which coincides with the graded increases of the threshold core temperature for the onset of vasodilation as exercise intensity is increased [126]. Therefore, the hyperosmotic effect of exercise is a possible mechanism for the delayed onset of active cutaneous vasodilation. Lastly, exercise lowers both the upper limit of skin blood flow and the core temperature when the plateau is reached compared to the resting condition (Figure 9). Skin blood flow reaches an apparent upper limit at a core temperature of approximately 38°C during exercise [53,54]. Above this temperature, the rate of the rise in skin blood flow sharply decreases or no further increase in skin blood flow occurs despite further increases in core temperature, whereas skin blood flow at rest would continue to rise with core temperature until the true maximal level is achieved. The upper limit of skin blood flow during exercise is about 50–60% of maximal skin blood flow and well below the level that can be seen during rest at the same core and whole body skin temperature [53,131,132]. This upper limit of skin blood flow during exercise is achieved by limiting the active vasodilator system because pharmacological blockage of the vasoconstrictor function does not eliminate the plateau phase when the core temperature is above 38°C. Instead, vasoconstrictor blockage tends to make the plateau more apparent [133], inferring that during the plateau phase, there might be a slow withdrawal of vasoconstrictor activity, while further increases in vasodilator activity are inhibited.
Figure 9.

Schematic description of the effect of dynamic exercise on skin blood flow response to hyperthermia. Exercise lowers skin blood flow relative to resting conditions at a given core body temperature by three ways: (a) an initial adrenergic cutaneous vasoconstriction at the onset of exercise, (b) an increase in core body temperature threshold at which cutaneous vasodilation begins, and (c) a plateau of skin blood flow at 50–60% maximal resting level above a core body temperature of 38°C. Redrawn with permission from Ref [14].
One may notice that there is an inconsistency regarding the control of cutaneous vasoconstrictor and vasodilator pathways by exercise. The onset of exercise only increases vasoconstrictor activity, whereas the delayed initiation of active cutaneous vasodilation and the lower upper limit of skin blood flow are only controlled by inhibition of the vasodilation system. The explanation is that the classical “exercise reflexes” affect the vasoconstrictor system as in other vascular beds at the onset of exercise [5], whereas changes in plasma osmolality affect the vasodilator system and delay the initiation of cutaneous vasodilation. The lower upper limit of skin blood flow during exercise is achieved by limiting the active vasodilator system, but the specific mechanism is unknown. In addition, the effect of vasoconstrictor activity on cutaneous blood flow may be too small once the active cutaneous vasodilation is triggered to lower skin blood flow. Therefore, no effect is seen with vasoconstrictor blockage on skin blood flow during exercise except at the onset of exercise.
In summary, dynamic exercise affects skin blood flow in several ways. First, at the onset of exercise, there is a cutaneous vasoconstriction mediated by the increase in vasoconstrictor activity. As exercise continues and core temperature rises, there is a thermoregulatory component responding to the increases in core and skin temperatures largely mediated by the sympathetic activation of the cutaneous vasodilator system. Second, the threshold core temperature at which cutaneous vasodilation begins moves to a higher temperature. This effect is probably mediated by a hyperosmotic effect inhibiting the vasodilator system and delaying its onset, and the extent of the delay is dependent on the intensity of the exercise. Third, there is an apparent upper limit to cutaneous vasodilation as exercise continues. This is due to an upper limit of the vasodilator system function. Generally, the effect of dynamic exercise is to keep skin blood flow below the levels that can be seen at rest with the same thermal condition.
Of note, while exercise reduces skin blood flow at a given core temperature, this exercise-induced decrease in skin blood flow may not compromise thermoregulation despite increases in core temperature because the core-to-skin temperature gradient, mean transit time for heat exchange between skin and the environment, and heat transportation from core to peripheral through a high blood flow of exercising limbs are increased during exercise [134]. This was shown by the fact that the rate of increase in core temperature was not altered after skin blood flow leveled off during exercise (Figures 3(b) and Figure 4(d)) [15]. The core-to-skin temperature gradient affects the amount of heat being transferred [24,135]. Based on the equation: Qs = 1/C × H/(Tc–Tsk), in which Qs is the minimal requirement for skin blood flow to maintain thermal balance, C is the specific heat of blood (1 kcal°C−1kg−1), H is the heat production during a steady state, and Tc-Tsk is the difference between core and skin temperature. Widening the core-to-skin gradient increases the amount of heat transferred at the same level of skin blood flow. Thus, the elevation of core temperature can be a strategy to increase heat dissipation or decrease the demand for skin blood flow. Furthermore, when skin temperature is higher than core temperature, the core-to-skin temperature gradient is reversed. Therefore, an increase in skin blood flow would actually have no benefit on heat dissipation.
Integrative cardiovascular responses
In contrast to passive heat stress, during which the core temperature is elevated by exogenous heat, exercise increases endogenous heat production. Exercise in the heat narrows the core-to-skin temperature gradient, and thus increases the demand for skin blood flow to maintain thermal balance. However, the pumping capacity of the heart is not without a limit. The demands for maintaining blood pressure, oxygen transport to exercising muscles, and heat transport to the skin can easily exceed the pumping capacity of the heart. While blood pressure may slightly decrease, it is usually maintained at reasonable levels during endurance exercise in the heat [5,24,136–139]. Heat stress increases exercising limb blood flow only during small muscle-mass exercise (i.e. single-leg knee-extensor exercise) at low intensities [106,107]. Blood flow to exercising muscle is generally not affected by heat stress during high intensity (85% of maximal capacity) one-legged knee extension exercise [140] and more dynamic exercise (i.e. cycling and uphill walking) [137,138,141,142]. Exercise affects skin blood flow in several ways and generally reduces it to levels below that can be seen during rest at the same core temperature as previously discussed (Figure 9) [14,143]. Nevertheless, skin blood flow is still higher during exercise in the heat compared to during exercise in a thermal neutral environment. Where does the extra blood flow to the skin come from? As during passive heat stress, vasoconstriction of splanchnic and renal vascular beds may also redistribute blood to the skin during exercise in the heat. Splanchnic and renal blood flow falls in inverse proportion as heart rate increases, and this relationship between heart rate and both splanchnic and renal blood flow during exercise is not affected by environment, body temperature, and the duration and intensity of exercise (Figure 8) [5,144–147]. Therefore, exercise in the heat elevates heart rate at a given intensity when compared to in a thermal neutral environment indicating higher vasoconstriction of the splanchnic and renal vascular beds during exercise in the heat. Interestingly, plasma norepinephrine concentration and plasma renin activity are also closely correlated with heart rate during heat stress, exercise, or both. In addition, the onset of the increases in plasma norepinephrine concentration and plasma renin activity and the decrease in splanchnic blood flow occur at the same heart rate. Exercise shifts the heart rate where the plasma norepinephrine concentration and plasma renin activity begin to rise and the splanchnic blood flow begins to fall to a higher value, but exercise does not alter the slope of heart rate versus plasma norepinephrine concentration, plasma renin activity, and splanchnic blood flow (Figure 8) [144]. Thus, this suggests that heart rate, splanchnic vascular resistance, and renal vascular resistance increase in proportion to the increase in sympathetic nervous activity, and this close relationship is maintained during heat stress, exercise, or both.
The effect of heat stress on cardiac output responses during exercise may depend on the exercise intensity. Mild-intensity exercise is more similar to passive heat stress, that is, cardiac output is well below its maximal value. Thus, while not all the studies reached statistical significance, many studies observed that cardiac output increased during mild-to-moderate exercise in the heat compared to the thermoneutral control trial [42,44,137,140,148,149]. In addition, Rowell et al. [150] observed cardiac output gradually increased during prolonged mild exercise in the heat. This increase in cardiac output is achieved by an increase in heart rate and maintenance of stroke volume [42,44,137,140]. As exercise intensity increases, the cardiac output reserved to provide blood flow to the cutaneous circulation becomes less. In fact, cardiac pumping capacity may be reduced at a time when the demands for flow are very high during more intense exercise in the heat. While heart rate increases, stroke volume declines during moderate to heavy exercise in the heat compared to the thermoneutral control [3,44,45,54,136,148]. Therefore, during moderate to heavy exercise in the heat, cardiac output is usually similar to or even lower compared to the normothermic control [3,44,45,54,136]. Two mechanisms have been proposed to explain how stroke volume is affected by heat stress. We will elaborate on the evidence that supports each mechanism to address this long-standing debate in the next section.
Different mechanisms of how hot skin affects stroke volume during exercise
Traditional mechanism
The traditional mechanism is that an increase in skin blood flow, as body temperature rises (skin temperature, core temperature, or both), causes an increase in cutaneous venous volume, which results in a reduction in ventricular filling pressure, end-diastolic volume (venous return), and stroke volume during dynamic exercise. Heart rate increases only to maintain cardiac output [24]. However, direct evidence showing that the increases in cutaneous blood flow and cutaneous blood volume closely correlate with the decrease in stroke volume during dynamic exercise is lacking. The indirect evidence used to support this hypothesis is discussed next.
Johnson and Rowell [151] reported that skin blood flow increased progressively during prolonged exercise, suggesting that the progressive decline in stroke volume that is usually observed (not measured in this study) during prolonged exercise is due in part to progressive increases in skin blood flow. Shaffrath et al. [42] also observed a progressive increase in skin blood flow and a progressive decrease in stroke volume during prolonged exercise at 60% VO2max. Skin blood flow significantly correlated with the heart rate, but the correlation between skin blood flow and stroke volume was not reported. However, many studies have shown that skin blood flow does not rise continuously throughout the prolonged exercise. When exercise in thermal neutral environments or during compensable heat stress, core temperature and skin blood flow increase during the first 20 minutes of exercise. Once the new thermal balance is reached, skin blood flow remains fairly stable thereafter, while core temperature may gradually and slightly rise as exercise duration increases [23,152,153]. During uncompensable heat stress, core temperature rises continuously throughout the exercise duration, while skin blood flow reaches a plateau when core temperature is above ~38°C [15,16,49,53,54,154–158]. However, stroke volume progressively decreases as the duration of exercise and core temperature increase during both compensable and uncompensable heat stress when skin temperature increased up to 39°C (Figure 4) [15,152,154,157,158]. Therefore, the increase in skin blood flow is not temporally related to the progressive reduction in stroke volume. In addition, if the increase in skin blood flow is the driver for raising heart rate, they should be closely associated with each other. However, in the studies mentioned above, the heart rate continuously rose, while skin blood flow reached a plateau as exercise duration or core temperature increased (Figure 4) [15,16,54,152,154,155]. In addition, when skin blood flow was lowered by adrenaline infusion during prolonged exercise, heart rate increased compared to the control trial [159]. The increase in heart rate, however, might be caused by the adrenaline infusion itself, or by a higher core temperature due to impaired heat dissipation caused by cutaneous vasoconstriction, or both. Although adrenaline infusion may raise heart rate independent of the reduction of cutaneous blood flow, this study also argues against the traditional mechanism that an increase in skin blood flow drives the increase in heart rate.
We have discussed that skin blood flow usually does not rise continuously during prolonged exercise or during exercise in the heat, and skin blood flow does not temporally relate to stroke volume during exercise. The next question is whether increases in cutaneous venous volume are associated with reduced stroke volume during exercise? Some data suggest that venous volume can affect stroke volume during exercise. Patients with congenital absence of venous valves showed considerably lower stroke volume during exercise in the sitting position than in the supine position, and the difference was significantly larger than in normal subjects [160]. Patients with large varicose veins showed higher stroke volume and cardiac output during submaximal exercise in a sitting position when the legs were bandaged compared to those not bandaged [161]. Bandaging the legs also raised stroke volume in normal subjects during prolonged exercise in a neutral environment, however, it did not prevent the gradual decreases in stroke volume as exercise duration increased [162]. These studies demonstrated that reducing venous volume in the lower limbs elevates stroke volume during upright exercise, suggesting that an increase in cutaneous blood volume can cause a decrease in stroke volume. The following question is, does an increase in cutaneous venous volume actually occur during exercise in the heat?
Forearm venous volume increased linearly with esophageal temperature during 25–40 min of cycling exercise at 40–45% VO2max [29]. Additionally, raising local or whole body skin temperature to a high level (>38°C) abolishes the venoconstrictor effect of exercise during short duration (5–10 min) mild-intensity exercise (~100 watts of cycling exercise) [34,35,37,40]. However, this venoconstrictor effect of exercise seems to be maintained at higher intensities of exercise as exercise causes cutaneous venoconstriction in proportion to the intensity of exercise [34–37]. Indeed, Tripathi et al. [156] found that forearm venous compliance (forearm venous volume divided by the changes in forearm venous pressure) decreased progressively during 30 minutes of cycling exercise at 50% VO2max in the heat (35°C ambient temperature, <40% relative humidity). Fortney et al. [163] also observed that forearm venous volume decreased progressively during the 30 minutes of cycling at 65–70% VO2max in an ambient temperature of 30°C. Chou et al. [15,16] further showed progressive decreases in forearm venous volume during 20–30 min of cycling at ~62% VO2peak even when skin temperature was at 39°C (Figure 4(e)). They also revealed that forearm venous volume was not different when skin temperature increased from 31°C to 39°C during cycling exercise (Figure 4(e)) [15,16]. These studies suggest that cutaneous venous tone diminishes when core temperature increases during low-intensity exercise, while marked cutaneous venoconstriction occurred and persisted during moderate intensity exercise despite large increases in skin and core temperatures. Therefore, these results imply that the decline in stroke volume during prolonged moderate-to-high intensity exercise in the heat is not caused by an increase in cutaneous venous volume. However, low-intensity exercise in the heat may be different because stroke volume is usually maintained [42,44,137,140], while cutaneous venous volume increases [29]. On the other hand, stroke volume largely decreases [3,15,16,44,45,54,136,148], while cutaneous venous volume also declines [15,16,163] during moderate intensity exercise.
We have concluded that skin blood flow and volume are not temporally correlated with stroke volume and that cutaneous venous volume either increases or decreases depending on the exercise intensity and thermal stress during exercise in the heat. The next question is: does the ventricular filling pressure decrease as skin and/or core temperature increases during exercise? Rowell et al. [148] rapidly altered whole body skin temperature in subjects wearing a water-perfused suit during mild and moderate intensities walking exercise (Figures 10 and Figure11). Skin temperatures were altered at 30-min intervals and were at 32°C, 38°C, 27°C, and 40°C for each 30-min interval, respectively. While the left ventricular filling pressure was not directly measured, its changes were thought to closely parallel the changes in right atrial mean pressure [164]. During mild exercise, subjects walked on a treadmill at 0% grade and at either 2.7 or 3.5 mph, which elicited 26.3–36.6% VO2max. When skin temperature was raised to 38°C during mild exercise, cardiac output and heart rate progressively increased, while right atrial mean pressure, aortic mean pressure, total peripheral resistance, and stroke volume progressively fell during the exercise and heating periods (Figure 10). Central blood volume fell relatively rapidly during the first 10 minutes of heating and stayed low until the end of heating periods, which suggests that there was a displacement of blood from the central to peripheral circulation. Since the exercise intensities were low, the highest average rectal temperature only reached 38.5°C. When rapidly cooling the skin toward 27°C, heart rate, stroke volume, central blood volume, mean aortic pressure, right atrial mean pressure, and total peripheral resistance rapidly returned to control levels. The abrupt increase in central blood volume indicates that blood volume shifted from the peripheral to the central circulation. Raising skin temperature again to 40°C reproduced the same changes as during the first heating period. However, skin blood flow and cutaneous venous blood volume were not measured in this study, and thus the traditional mechanism was not proven directly. Nevertheless, the right atrial mean pressure and central blood volume responses agreed with the venous tone studies discussed above. That is, raising local [34,35,40] or whole body skin temperature to a very high level (≥38°C) [37,40] abolished the cutaneous venoconstriction response to exercise during short duration mild intensity exercise, and forearm venous volume increased during prolonged 40–45% VO2max exercise in the heat [29] as exercise duration and core temperature increase. Therefore, the decreases in atrial mean pressure and central blood volume might be caused, at least in part, by the redistribution of blood from central to the cutaneous vasculatures during mild exercise when skin temperature is above 38°C.
Figure 10.
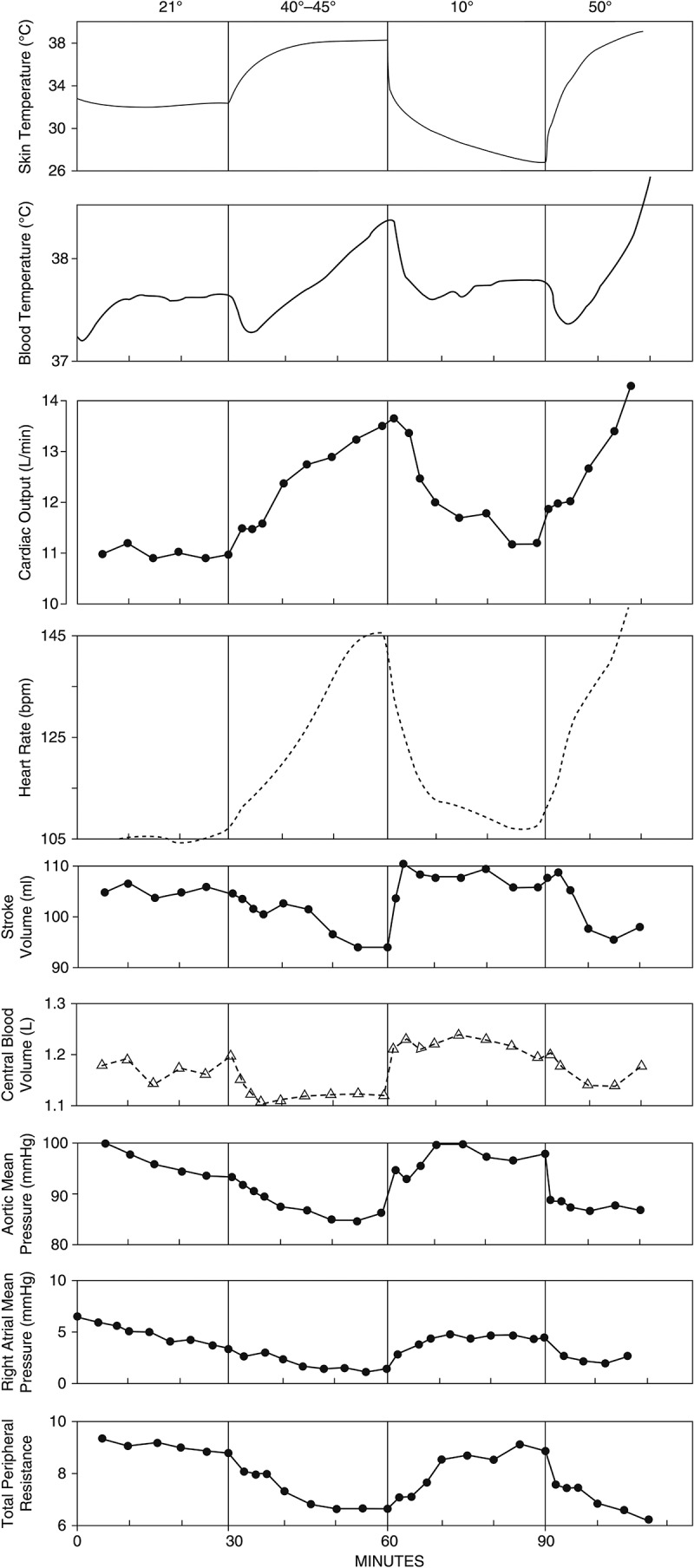
Average cardiovascular and temperature data from six subjects during mild exercise (0% grade, 2.7 or 3.5 mph) when heating and cooling the skin. During heating periods, right atrial mean pressure, central blood volume, aortic mean pressure, total peripheral resistance, and stroke volume fell suggesting that there was a displacement of blood from the central to the peripheral circulations. Blood volume was most likely redistributed from the central to the skin during heating, but skin blood flow and volume were not measured. Redrawn with permission from Ref [148].
Figure 11.

Average cardiovascular and temperature data from four subjects during moderate intensity exercise (7.5% grade, 3.5 mph) when heating and cooling the skin. In contrast to mild exercise, aortic mean pressure and central blood volume increased while total peripheral resistance was only slightly reduced during heating. The right atrial mean pressure was not measured. Therefore, it seems that blood was not redistributed from the central to the skin while stroke volume still declined when heating the skin during moderate intensity exercise. Redrawn with permission from Ref [148].
In the same study [148], another four subjects walked on the treadmill at 7.5% gradient and 3.5 mph, eliciting 44.5–64.0% VO2max (Figure 11). During this moderate intensity exercise, skin temperatures were the same as during mild exercise, but the average rectal temperature reached 39.3°C at the end of the heating exercise. In agreement with the mild exercise data, cardiac output and heart rate increased, and stroke volume decreased during heating. However, the aortic mean pressure and central blood volume increased, while total peripheral resistance was maintained during heating. The right atrial mean pressure measurements could not be made at higher workloads due to moving artifacts. When suddenly cooled during moderate intensity exercise, there was a drop in mean aortic pressure, in contrast to the increase seen during mild exercise. Therefore, the cardiovascular responses during moderate intensity exercise did not suggest that blood was redistributed from the central to the peripheral circulation during heating. The decreases in right atrial mean pressure, aortic mean pressure, central blood volume, and total peripheral resistance occurred when heating the skin during mild but not during moderate exercise.
Summarizing the studies discussed above, after the initial increase, cutaneous venous tone returned to its low baseline level during mild exercise [36], and cutaneous venous volume increased [29] as skin and core temperatures increased during mild exercise (<40–45% VO2max). The increase in cutaneous venous volume may be the cause of the decreases in central blood volume, right atrial mean pressure, and thus stroke volume during mild exercise (26.3–36.6% VO2max) observed in Rowell’s study [148]. However, the stroke volume does not always decrease during mild exercise in the heat [44,155]. On the other hand, cutaneous venous tone was maintained to the end of the exercise during moderate exercise (160 watts) [36], and cutaneous venous volume decreased as exercise duration increased when skin is hot during moderate intensity exercise (60–70% VO2max) [15,16,163]. This might explain the lack of reduction in central blood volume during moderate exercise (44.5–64.0% VO2max) when heating, as observed by Rowell et al. [148]. However, not all studies fit into this scenario. One study reported that the central blood volume was constantly lower at different intensities of exercise (VO2 range from 1.8 to 2.8 l/min) when exercising in a hot dry environment (43.3°C) compared to in a neutral environment (25.6°C) [136]. In Rowell’s studies [136,148], skin blood flow and right atrial pressure were not measured during moderate exercise. Nose et al. [155] performed a study that specifically tested the relation between forearm blood flow and right atrial pressure during 50 minutes of moderate exercise at 60% VO2peak. Right atrial pressure increased rapidly at the onset of exercise due to the pumping of contracting muscles and progressively decreased thereafter. However, the right atrial pressure was not temporally correlated with forearm blood flow and stroke volume responses during exercise. Forearm blood flow reached a plateau when core temperature was above 38°C. Stroke volume stayed at the same level after 20 minutes of exercise. Therefore, the current evidence supports the mechanism that cutaneous venous pooling causes the decreases in stroke volume only during mild but not moderate exercise even when whole body skin temperature is elevated to high levels (above 38°C) [15,16]. The potential explanation is that higher sympathetic nervous activities during heavier exercise cause increase venous tone and cutaneous venoconstriction that prevents cutaneous venous pooling of blood. If this interpretation is correct, we are left with the question “what causes the decreases in stroke volume as skin and/or core temperature increase during moderate intensity exercise”? The alternative mechanism may provide a more plausible explanation.
Alternative mechanism
The alternative mechanism proposes that the decline in stroke volume during prolonged moderate intensity exercise or during exercise with elevated skin and/or core temperature is simply due to an increase in heart rate [13]. An increase in heart rate would decrease ventricular filling time, end-diastolic volume, and thus stroke volume [165–170]. The heart rate that is increased by a cardiac pacemaker decreases stroke volume at rest and during exercise [165–167,171]. Ross et al. [167] also observed that ejection periods and mean rates of ejection decreased as the heart rate was increased by a pacemaker. A decrease in stroke volume at rest and during exercise has also been observed in patients developing supraventricular tachycardia [172]. The consistent cardiovascular response to increasing skin or core temperature during constant intensity exercise is an increase in heart rate compared to when the skin and/or core temperature are lower (Figures 3 and 4) [3,15,42,46,148,173,174]. Heart rate was strongly correlated with core temperature during moderate intensity exercise when skin temperature increased from 32°C to 39°C (r = 0.92) [15]. Heart rate was also strongly correlated with body temperature, and the combination of skin and core temperatures, during prolonged exercise was highly correlated using different thermal stresses (r = 0.96) [45]. Heat-induced increases in heart rate can be caused by the cardiac temperature per se [46,81–85] or by autonomic nervous activities [61,75,76].
Since increasing heart rate has the potential to decrease stroke volume by reducing the ventricular filling time, Coyle and Gonzalez-Alonso hypothesized that the hyperthermia-induced increases in heart rate cause the decreases in stroke volume during prolonged exercise or exercise in the heat [13]. When an increase in core temperature is absent during prolonged exercise, an increase in heart rate and a decrease in stroke volume are also absent [2,175]. In order to test the hypothesis, a small dose of β1-adrenoceptorblocker (i.e. Atenolol) was ingested by subjects immediately before prolonged exercise to prevent the heart rate from increasing during the 15–55 min of exercise at 57% VO2peak in a thermoneutral environment [152]. When the increase in heart rate was prevented, the decline in stroke volume was also prevented, despite a normal cutaneous blood flow response. Therefore, this study demonstrates that the increase in heart rate is largely responsible for the decline in stroke volume during exercise in a thermoneutral environment (skin temperature was ~31°C and esophageal temperature was up to ~37.7°C). Two follow-up β-blocker studies have also been performed in the same laboratory when subjects exercise at ~60% VO2peak with skin temperature at ~36°C [154] and ~38°C (Figure 12) [16]. In particular, the high skin temperature was purposely matched to the skin temperature of Rowell’s study discussed previously [148]. In addition, skin was rapidly cooled after 20 min of exercise to mimic the rapid skin cooling in Rowell’s study. The novelty and the significance of this study relative to Rowell’s study [148] are as follows: (1) cutaneous blood flow, forearm venous volume, and stroke volume were measured simultaneously; (2) β-blocker was used to reveal the independent effect of increasing heart rate on stroke volume when skin is hot during exercise. When the heart rate responses during exercise in the elevated skin temperature conditions were lowered by a β-blocker and matched to the heart rate of the normothermic control conditions, stroke volume in the elevated skin temperature conditions was either higher [154] or restored [16] compared to the normothermic conditions (Figure 12). That is, while increasing skin temperature elevated cutaneous blood flow, it did not reduce stroke volume when the heart rate was the same as normothermia during moderate intensity exercise. Additionally, forearm venous volume during exercise was not different when skin was hot relative to normothermic conditions indicating no further blood pooling in the skin when skin was hot (Figure 12). Furthermore, when core and skin temperatures were elevated, rapid cooling of the skin decreased cardiac output, heart rate, and cutaneous blood flow, yet had no effects on mean arterial pressure and forearm venous volume. Stroke volume only increased during cooling when the decrease in heart rate was amplified by β-blocker (Figure 12) [16]. These results suggest that the increases in heart rate seem largely responsible for the decline in stroke volume during moderate intensity exercise when skin is warm (~36°C) and hot (~38°C) and core temperature is up to ~39°C.
Figure 12.

Temperature and cardiovascular responses during moderate intensity exercise (62% VO2peak) when heating and cooling the skin with or without β-blocker ingestion. Skin temperature (Tsk) was preheated to 38°C or 33°C before exercise bouts and was rapidly cooled toward 28°C at 20 min of exercise by a water-perfused suit. Subjects ingested either β-blocker (βB) or placebo (PL) before exercise in two hot skin trials. (a) Skin temperature, (b) esophageal temperature (c) cardiac output, (d) heart rate, (e) stroke volume, (f) cutaneous blood flow, and (g) forearm venous volume responses during exercise. *Tsk 38°C-PL significantly different from Tsk 33°C-PL and Tsk 38°C-βB at the same time point, P < 0.05; †Tsk 38°C-βB significantly different from Tsk 33°C-PL at the same time point, P < 0.05; ‡significantly different from 5–10 min within trials, P < 0.05; §significantly different from 15–20 min within trials, P < 0.05; #Tsk 33°C-PL significantly different from Tsk 38°C-PL and Tsk 38°C-βB at the same time point. The shaded areas represent skin cooling from min 20 to min 40. Values are means ± SE (n = 9). Used with permission from Chou et al. [16].
While there is no direct evidence showing that cutaneous venous pooling occurs during moderate intensity exercise in the heat, some studies have shown declines in the index of ventricular filling pressure during prolonged moderate intensity exercise [155,176]. If there is little blood pooling in the skin, what causes the decline in ventricular filling pressure during prolonged moderate intensity exercise in the heat? Is the decrease in ventricular filling pressure also caused by an increase in heart rate? Some studies observed that an increase in heart rate was associated with a decrease in ventricular filling pressure. Studies of patients with pacemaker showed that an increase in heart rate by pacemaker was accompanied by decreases in the right ventricular end-diastolic pressure, right atrial mean pressure, and stroke volume during supine exercise at 50 watts [165,171], while the marked constancy of the central blood volume indicated no shift of the blood volume when heart rate was increased by pacemaker [165]. In addition, patients wore anti-gravity suits to produce a shift of blood from the lower body to the thorax, which did not increase stroke volume at high ventricular rates [165]. Heart rate increased by the anticholinergic substance (methyl-scopolamine nitrate) also showed lower ventricular filling pressure and stroke volume at rest and during exercise [170]. Intravenous injection of atropine increased heart rate and cardiac output while decreasing stroke volume and central venous pressure during supine rest [177,178]. The decreases in ventricular filling pressure and central venous pressure might be explained by vasodilation of peripheral blood vessels as peripheral resistance was also reduced by atropine injection [178]. Atropine flush is also a well-known phenomenon that indicates vasodilation of superficial blood vessels. However, certain maneuvers intended to increase venous return, such as hyperventilation, passive leg-raising, and head-down tilting failed to increase the cardiac output and stroke volume after atropine injection [177]. Therefore, studies have shown that increasing heart rate by various methods was accompanied by a decline in ventricular filling pressure. Thus, the mechanism for the decline in stroke volume is not caused by peripheral blood pooling.
In summary, direct evidence showed that using β-blocker to lower the heart rate fully restored or further elevated stroke volume during moderate intensity endurance exercise, even when increasing whole body skin temperature up to 39°C [16,154]. Additionally, neither cutaneous blood flow nor cutaneous venous volume was higher when skin was hot compared to lower skin temperatures, implying no pooling of blood in the skin. The potential mechanism for the stroke volume reduction during exercise when skin is hot seems to be that an increase in heart rate shortens the ventricular filling time and possibly decreases ventricular filling pressure and thus stroke volume. While it seems an increase in heart rate is mainly responsible for the decline in stroke volume, one should remember that stroke volume is the result of various factors that influence cardiac preload, afterload, contractility, and diastolic function. However, it is reasonable to state that eliminating the increase in heart rate can prevent the decline in stroke volume during exercise. It is also apparent that the elevation of heart rate from skin and core hyperthermia during moderate intensity endurance exercise will markedly reduce stroke volume.
Conclusions
In conclusion, integrative cardiovascular responses to increasing skin temperature to high levels (i.e. >38°C) at rest and during submaximal endurance exercise were discussed. Generally, heat stress, including when skin temperature is hot, does not compromise mean arterial pressure and blood flow to exercising muscle during submaximal endurance exercise. Also, both heat stress and exercise cause visceral vasoconstriction, which is highly associated with increased heart rate and sympathetic nerve activity. An increase in skin temperature when core temperature remains low (i.e. at rest, during mild exercise, during pre-cooling, or early stages of moderate intensity exercise), increases heart rate while maintaining stroke volume and thus raising cardiac output. The increase in cardiac output provides extra blood flow to the skin for heat dissipation. At rest and during mild exercise (Figure 1(a)), hot skin also raises cutaneous venous volume, which causes blood pooling in the skin and reduces ventricular filling pressure. However, stroke volume is maintained despite reductions in ventricular filling pressure, mainly caused by skin blood pooling, and reduced ventricular filling time caused by increase in heart rate. Therefore, there is still sufficient time for cardiac filling with slightly lower filling pressure and filling time. When cardiac output is well below its maximal capacity (i.e. at rest and during mild exercise when core temperature is at low levels), small increases in heart rate, skin blood flow, and cutaneous venous volume do not compromise stroke volume, so cardiac output can increase to fulfill the demands for maintaining blood pressure, heat dissipation, and the exercising muscle (Figure 1(a)).
In contrast, during more intense moderate exercise (Figure 1(b)), hot skin does not further increase skin blood flow once core temperature reaches ~>38°C and does not abolish the venoconstriction induced by high sympathetic nerve activity during intense exercise, causing little blood pooling in the skin. However, stroke volume decreases, which is largely associated with an increase in heart rate; therefore, cardiac output is not elevated when skin is hot during moderate intensity exercise. Thus, an increase in heart rate plays a significant role in reducing stroke volume when skin is hot during moderate exercise. The interpretation is that when the heart rate is so high (i.e. approaching maximal values) and skin blood flow is at the plateau level, even a slight reduction in ventricular filling time from accelerated heart rate lowers the stroke volume.
Biographies
Dr. Chou completed his Ph.D. at the Department of Kinesiology and Health Education at The University of Texas at Austin. While under the supervision of Dr. Coyle, his dissertation focused on the integrative cardiovascular responses to increasing whole body skin temperature during endurance exercise. Currently, he is a Postdoctoral Scientist at the Nationwide Children’s Hospital where he applies state-of-the-art non-invasive molecular imaging techniques to assess physiological processes such as skeletal muscle perfusion and metabolism and arterial inflammation in patients with cardiovascular diseases.
Dr. Coyle is a long-time professor at The University of Texas at Austin, where he directs the Human Performance Laboratory. In addition to his research in cardiovascular responses to exercise in the heat, he has studied dehydration, the physiological determinants of endurance performance and the metabolic responses to exercise. Recently, he has found that inactivity blunts the metabolic responses to acute exercise.
Disclosure statement
No potential conflict of interest was reported by the author(s).
List of Abbreviations
CO cardiac output
CVV cutaneous venous volume
HR heart rate
MAP mean arterial pressure
SBF skin blood flow
SV stroke volume
TPR total peripheral resistance.
References
- [1].Montain SJ, Coyle EF.. Influence of graded dehydration on hyperthermia and cardiovascular drift during exercise. J Appl Physiol (1985). 1992;73(4):1340–1350. [DOI] [PubMed] [Google Scholar]
- [2].Gonzalez-Alonso J, Mora-Rodriguez R, Below PR, et al. Dehydration reduces cardiac output and increases systemic and cutaneous vascular resistance during exercise. J Appl Physiol (1985). 1995;79(5):1487–1496. [DOI] [PubMed] [Google Scholar]
- [3].Gonzalez-Alonso J, Mora-Rodriguez R, Below PR, et al. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. J Appl Physiol (1985). 1997;82(4):1229–1236. [DOI] [PubMed] [Google Scholar]
- [4].Trangmar SJ, Gonzalez-Alonso J. New insights into the impact of dehydration on blood flow and metabolism during exercise. Exerc Sport Sci Rev. 2017;45(3):146–153. [DOI] [PubMed] [Google Scholar]
- [5].Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54(1):75–159. [DOI] [PubMed] [Google Scholar]
- [6].Rowell LB. Cardiovascular Adjustments to Thermal Stress. In R. Terjung, editor. Comprehensive Physiology. 2011. doi: 10.1002/cphy.cp020327. [DOI] [Google Scholar]
- [7].Rowell LB. Cardiovascular aspects of human thermoregulation. Circ Res. 1983;52(4):367–379. [DOI] [PubMed] [Google Scholar]
- [8].Rowell LB. Thermal stress, in human circulation regulation during physical stress. New York: Oxford University Press; 1986. p. 174–212. [Google Scholar]
- [9].Johnson JM, Proppe, DW. Compr Physiol. In R. Terjung, editor. 2011. 10.1002/cphy.cp020327 [DOI] [Google Scholar]
- [10].Rowell LB. Cutaneous and skeletal muscle circulations, in human circulation regulation during physical stress. New York: Oxford University Press; 1986. p. 96–116. [Google Scholar]
- [11].Crandall CG, Wilson TE. Human cardiovascular responses to passive heat stress. Compr Physiol. 2015;5(1):17–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Periard JD, Eijsvogels TMH, Daanen HAM. Exercise under heat stress: thermoregulation, hydration, performance implications, and mitigation strategies. Physiol Rev. 2021;101(4):1873–1979. [DOI] [PubMed] [Google Scholar]
- [13].Coyle EF, Gonzalez-Alonso J. Cardiovascular drift during prolonged exercise: new perspectives. Exerc Sport Sci Rev. 2001;29(2):88–92. [DOI] [PubMed] [Google Scholar]
- [14].Gonzalez-Alonso J, Crandall CG, Johnson JM. The cardiovascular challenge of exercising in the heat. J Physiol. 2008;586(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chou TH, Allen JR, Hahn D, et al. Cardiovascular responses to exercise when increasing skin temperature with narrowing of the core-to-skin temperature gradient. J Appl Physiol (1985). 2018;125(3):697–705. [DOI] [PubMed] [Google Scholar]
- [16].Chou TH, Akins JD, Crawford CK, et al. Low stroke volume during exercise with hot skin is due to elevated heart rate. Med Sci Sports Exerc. 2019;51(10):2025–2032. [DOI] [PubMed] [Google Scholar]
- [17].Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol (Oxf). 2014;210(3):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Romanovsky AA. The thermoregulation system and how it works. Handb Clin Neurol. 2018;156:3–43. [DOI] [PubMed] [Google Scholar]
- [19].Cooper KE, Kerslake DM. Changes in heart rate during exposure of the skin to radiant heat. Clin Sci (Lond). 1955;14(1):125–135. [PubMed] [Google Scholar]
- [20].Kerslake DM, Cooper KE. Vasodilation in the hand in response to heating the skin elsewhere. Clin Sci (Lond). 1950;9:31–47. [Google Scholar]
- [21].Wyss CR, Brengelmann GL, Johnson JM, et al. Control of skin blood flow, sweating, and heart rate: role of skin vs. core temperature. J Appl Physiol. 1974;36(6):726–733. [DOI] [PubMed] [Google Scholar]
- [22].Proppe DW, Brengelmann GL, Rowell LB. Control of baboon limb blood flow and heart rate-role of skin vs. core temperature. Am J Physiol. 1976;231(5 Pt. 1):1457–1465. [DOI] [PubMed] [Google Scholar]
- [23].Wenger CB, Roberts MF, Stolwijk JA, et al. Forearm blood flow during body temperature transients produced by leg exercise. J Appl Physiol. 1975;38(1):58–63. [DOI] [PubMed] [Google Scholar]
- [24].Rowell LB. Circulatory adjustments to dynamic exercise and heat stress: competing controls. In Rowell LB.editor. Human circulation regulation during physical stress. New York: Oxford University Press; 1986. p. 363–406. [Google Scholar]
- [25].Gauer OH, Thron HL. Postural changes in the circulation. Handbook of physiology. Circulation. 1965;2(3):2409–2439. [Google Scholar]
- [26].Webb-Peploe MM, Shepherd JT. Response of dogs’ cutaneous veins to local and central temperature changes. Circ Res. 1968;23(6):693–699. [DOI] [PubMed] [Google Scholar]
- [27].Vanhoutte PM, Shepherd JT. Effect of temperature on reactivity of isolated cutaneous veins of the dog. Am J Physiol. 1970;218(1):187–190. [DOI] [PubMed] [Google Scholar]
- [28].Webb-Peploe MM, Shepherd JT. Peripheral mechanism involved in response of dog’s cutaneous veins to local temperature change. Circ Res. 1968;23(6):701–708. [DOI] [PubMed] [Google Scholar]
- [29].Wenger CB, Roberts MF. Control of forearm venous volume during exercise and body heating. J Appl Physiol Respir Environ Exerc Physiol. 1980;48(1):114–119. [DOI] [PubMed] [Google Scholar]
- [30].Wenger CB, Roberts MF, Nadel ER, et al. Thermoregulatory control of finger blood flow. J Appl Physiol. 1975;38(6):1078–1082. [DOI] [PubMed] [Google Scholar]
- [31].Johnson JM, Park MK. Reflex control of skin blood flow by skin temperature: role of core temperature. J Appl Physiol Respir Environ Exerc Physiol. 1979;47(6):1188–1193. [DOI] [PubMed] [Google Scholar]
- [32].Pergola PE, Kellogg DL, Johnson JM, et al. Reflex control of active cutaneous vasodilation by skin temperature in humans. Am J Physiol. 1994;266(5 Pt 2):H1979–84. [DOI] [PubMed] [Google Scholar]
- [33].Page EB, Hickam JB, Sieker HO, et al. Reflex venomotor activity in normal persons and in patients with postural hypotension. Circulation. 1955;11(2):262–270. [DOI] [PubMed] [Google Scholar]
- [34].Bevegard BS, Shepherd JT. Reaction in man of resistance and capacity vessels in forearm and hand to leg exercise. J Appl Physiol. 1966;21(1):123–132. [DOI] [PubMed] [Google Scholar]
- [35].Bevegard BS, Shepherd JT. Changes in tone of limb veins during supine exercise. J Appl Physiol. 1965;20:1–8. [DOI] [PubMed] [Google Scholar]
- [36].Hanke D, Schlepper M, Westermann K, et al. [Venous tone, skin- and muscle blood flow in forearm and hand during exercise]. Pflugers Arch. 1969;309(2):115–127. [DOI] [PubMed] [Google Scholar]
- [37].Rowell LB, Brengelmann GL, Detry JM, et al. Venomotor responses to rapid changes in skin temperature in exercising man. J Appl Physiol. 1971;30(1):64–71. [DOI] [PubMed] [Google Scholar]
- [38].Samueloff SL, Bevegard BS, Shepherd JT. Temporary arrest of circulation to a limb for the study of venomotor reactions in man. J Appl Physiol. 1966;21(1):341–346. [DOI] [PubMed] [Google Scholar]
- [39].Merritt FL, Weissler AM. Reflex venomotor alterations during exercise and hyperventilation. Am Heart J. 1959;58(3):382–387. [Google Scholar]
- [40].Rowell LB, Brengelmann GL, Detry JM, et al. Venomotor responses to local and remote thermal stimuli to skin in exercising man. J Appl Physiol. 1971;30(1):72–77. [DOI] [PubMed] [Google Scholar]
- [41].Webb-Peploe MM, Shepherd JT. Responses of the superficial limb veins of the dog to changes in temperature. Circ Res. 1968;22(6):737–746. [DOI] [PubMed] [Google Scholar]
- [42].Shaffrath JD, Adams WC. Effects of airflow and work load on cardiovascular drift and skin blood flow. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(5):1411–1417. [DOI] [PubMed] [Google Scholar]
- [43].Gonzalez-Alonso J, Mora-Rodriguez R, Coyle EF. Stroke volume during exercise: interaction of environment and hydration. Am J Physiol Heart Circ Physiol. 2000;278(2):H321–30. [DOI] [PubMed] [Google Scholar]
- [44].Nadel ER, Cafarelli E, Roberts MF, et al. Circulatory regulation during exercise in different ambient temperatures. J Appl Physiol Respir Environ Exerc Physiol. 1979;46(3):430–437. [DOI] [PubMed] [Google Scholar]
- [45].Lee JF, Christmas KM, Machin DR, et al. Warm skin alters cardiovascular responses to cycling after preheating and precooling. Med Sci Sports Exerc. 2015;47(6):1168–1176. [DOI] [PubMed] [Google Scholar]
- [46].Jose AD, Stitt F, Collison D. The effects of exercise and changes in body temperature on the intrinsic heart rate in man. Am Heart J. 1970;79(4):488–498. [DOI] [PubMed] [Google Scholar]
- [47].Saunders AG, Dugas JP, Tucker R, et al. The effects of different air velocities on heat storage and body temperature in humans cycling in a hot, humid environment. Acta Physiol Scand. 2005;183(3):241–255. [DOI] [PubMed] [Google Scholar]
- [48].Galloway SD, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc. 1997;29(9):1240–1249. [DOI] [PubMed] [Google Scholar]
- [49].Maughan RJ, Otani H, Watson P. Influence of relative humidity on prolonged exercise capacity in a warm environment. Eur J Appl Physiol. 2012;112(6):2313–2321. [DOI] [PubMed] [Google Scholar]
- [50].Che Muhamed AM, Atkins K, Stannard SR, et al. The effects of a systematic increase in relative humidity on thermoregulatory and circulatory responses during prolonged running exercise in the heat. Temperature. 2016;3(3):455–464. doi: 10.1080/23328940.2016.1182669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Otani H, Kaya M, Tamaki A, et al. Effects of solar radiation on endurance exercise capacity in a hot environment. Eur J Appl Physiol. 2016;116(4):769–779. [DOI] [PubMed] [Google Scholar]
- [52].Schlader ZJ, Simmons SE, Stannard SR, et al. Skin temperature as a thermal controller of exercise intensity. Eur J Appl Physiol. 2011;111(8):1631–1639. [DOI] [PubMed] [Google Scholar]
- [53].Brengelmann GL, Johnson JM, Hermansen L, et al. Altered control of skin blood flow during exercise at high internal temperatures. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(5):790–794. [DOI] [PubMed] [Google Scholar]
- [54].Gonzalez-Alonso J, Teller C, Andersen SL, et al. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol (1985). 1999;86(3):1032–1039. [DOI] [PubMed] [Google Scholar]
- [55].Rowell LB, Blackmon JR, Martin RH, et al. Hepatic clearance of indocyanine green in man under thermal and exercise stresses. J Appl Physiol. 1965;20(3):384–394. [DOI] [PubMed] [Google Scholar]
- [56].Rowell LB, Brengelmann GL, Blackmon JR, et al. Splanchnic blood flow and metabolism in heat-stressed man. J Appl Physiol. 1968;24(4):475–484. [DOI] [PubMed] [Google Scholar]
- [57].Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol. 1969;27(5):673–680. [DOI] [PubMed] [Google Scholar]
- [58].Rowell LB, Brengelmann GL, Blackmon JR, et al. Redistribution of blood flow during sustained high skin temperature in resting man. J Appl Physiol. 1970;28(4):415–420. [DOI] [PubMed] [Google Scholar]
- [59].Rowell LB, Detry JR, Profant GR, et al. Splanchnic vasoconstriction in hyperthermic man–role of falling blood pressure. J Appl Physiol. 1971;31(6):864–869. [DOI] [PubMed] [Google Scholar]
- [60].Detry JM, Brengelmann GL, Rowell LB, et al. Skin and muscle components of forearm blood flow in directly heated resting man. J Appl Physiol. 1972;32(4):506–511. [DOI] [PubMed] [Google Scholar]
- [61].Escourrou P, Freund PR, Rowell LB, et al. Splanchnic vasoconstriction in heat-stressed men: role of renin-angiotensin system. J Appl Physiol Respir Environ Exerc Physiol. 1982;52(6):1438–1443. [DOI] [PubMed] [Google Scholar]
- [62].Edholm OG, Fox RH, Macpherson RK. The effect of body heating on the circulation in skin and muscle. J Physiol. 1956;134(3):612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Shepherd JT, Vanhoutte PM. Role of the venous system in circulatory control. Mayo Clin Proc. 1978;53(4):247–255. [PubMed] [Google Scholar]
- [64].Deschamps A, Magder S. Skin vascular bed is a potential blood reservoir during heat stress. Am J Physiol. 1990;259(6 Pt 2):H1796–802. [DOI] [PubMed] [Google Scholar]
- [65].Koroxenidis GT, Shepherd JT, Marshall RJ. Cardiovascular response to acute heat stress. J Appl Physiol. 1961;16:869–872. [DOI] [PubMed] [Google Scholar]
- [66].Minson CT, Wladkowski SL, Cardell AF, et al. Age alters the cardiovascular response to direct passive heating. J Appl Physiol (1985). 1998;84(4):1323–1332. [DOI] [PubMed] [Google Scholar]
- [67].Peters JK, Nishiyasu T, Mack GW. Reflex control of the cutaneous circulation during passive body core heating in humans. J Appl Physiol (1985). 2000;88(5):1756–1764. [DOI] [PubMed] [Google Scholar]
- [68].Murray RH. Cardiopulmonary effects of brief, intense thermal exposures. J Appl Physiol. 1966;21(6):1717–1724. [DOI] [PubMed] [Google Scholar]
- [69].Stohr EJ, González-Alonso J, Pearson J, et al. Effects of graded heat stress on global left ventricular function and twist mechanics at rest and during exercise in healthy humans. Exp Physiol. 2011;96(2):114–124. [DOI] [PubMed] [Google Scholar]
- [70].Wilson TE, Crandall CG. Effect of thermal stress on cardiac function. Exerc Sport Sci Rev. 2011;39(1):12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Crandall CG, Wilson TE, Marving J, et al. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol. 2008;586(1):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Keller DM, Low DA, Wingo JE, et al. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. J Physiol. 2009;587(Pt 5):1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wilson TE, Brothers RM, Tollund C, et al. Effect of thermal stress on Frank-Starling relations in humans. J Physiol. 2009;587(Pt 13):3383–3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nelson MD, Haykowsky MJ, Petersen SR, et al. Increased left ventricular twist, untwisting rates, and suction maintain global diastolic function during passive heat stress in humans. Am J Physiol Heart Circ Physiol. 2010;298(3):H930–7. [DOI] [PubMed] [Google Scholar]
- [75].Kim YD, Lake CR, Lees DE, et al. Hemodynamic and plasma catecholamine responses to hyperthermic cancer therapy in humans. Am J Physiol. 1979;237(5):H570–4. [DOI] [PubMed] [Google Scholar]
- [76].Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension. 1990;15(5):505–507. [DOI] [PubMed] [Google Scholar]
- [77].Brothers RM, Bhella PS, Shibata S, et al. Cardiac systolic and diastolic function during whole body heat stress. Am J Physiol Heart Circ Physiol. 2009;296(4):H1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bundgaard-Nielsen M, Wilson TE, Seifert T, et al. Effect of volume loading on the Frank-Starling relation during reductions in central blood volume in heat-stressed humans. J Physiol. 2010;588(Pt 17):3333–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].el-Sherif N, Shahwan L, Sorour AH. The effect of acute thermal stress on general and pulmonary hemodynamics in the cardiac patient. Am Heart J. 1970;79(3):305–317. [DOI] [PubMed] [Google Scholar]
- [80].Faithfull NS, Reinhold HS, van den Berg AP, et al. Cardiovascular changes during whole body hyperthermia treatment of advanced malignancy. Eur J Appl Physiol Occup Physiol. 1984;53(3):274–281. [DOI] [PubMed] [Google Scholar]
- [81].Badeer H. Influence of temperature on S-A rate of dog’s heart in denervated heart-lung preparation. Am J Physiol. 1951;167(1):76–80. [DOI] [PubMed] [Google Scholar]
- [82].Bolter CP, Atkinson KJ. Influence of temperature and adrenergic stimulation on rat sinoatrial frequency. Am J Physiol. 1988;254(5 Pt 2):R840–4. [DOI] [PubMed] [Google Scholar]
- [83].Clark AJ. The effect of alterations of temperature upon the functions of the isolated heart. J Physiol. 1920;54(4):275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Knowlton FP, Starling EH. The influence of variations in temperature and blood-pressure on the performance of the isolated mammalian heart. J Physiol. 1912;44(3):206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Garrey WE, Townsend SE. Neural responses and reactions of the heart of a human embryo. Am J Physiol. 1948;152(2):219–224. [DOI] [PubMed] [Google Scholar]
- [86].Nakazawa M, Miyagawa ST, Morishima M, et al. Effects of environmental hyperthermia on cardiovascular function in the rat embryo. Pediatr Res. 1991;30(6):505–508. [DOI] [PubMed] [Google Scholar]
- [87].Nakazawa M, Miyagawa S, Takao A, et al. Hemodynamic effects of environmental hyperthermia in stage 18, 21, and 24 chick embryos. Pediatr Res. 1986;20(12):1213–1215. [DOI] [PubMed] [Google Scholar]
- [88].Bruce-Low SS, Cotterrell D, Jones GE. Heart rate variability during high ambient heat exposure. Aviat Space Environ Med. 2006;77(9):915–920. [PubMed] [Google Scholar]
- [89].Crandall CG, Zhang R, Levine BD. Effects of whole body heating on dynamic baroreflex regulation of heart rate in humans. Am J Physiol Heart Circ Physiol. 2000;279(5):H2486–92. [DOI] [PubMed] [Google Scholar]
- [90].Cui J, Zhang R, Wilson TE, et al. Spectral analysis of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2004;286(3):H1101–6. [DOI] [PubMed] [Google Scholar]
- [91].Yamamoto S, Iwamoto M, Inoue M, et al. Evaluation of the effect of heat exposure on the autonomic nervous system by heart rate variability and urinary catecholamines. J Occup Health. 2007;49(3):199–204. [DOI] [PubMed] [Google Scholar]
- [92].Gorman AJ, Proppe DW. Mechanisms producing tachycardia in conscious baboons during environmental heat stress. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(2):441–446. [DOI] [PubMed] [Google Scholar]
- [93].Ganio MS, Overgaard M, Seifert T, et al. Effect of heat stress on cardiac output and systemic vascular conductance during simulated hemorrhage to presyncope in young men. Am J Physiol Heart Circ Physiol. 2012;302(8):H1756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Johnson JM, Niederberger M, Rowell LB, et al. Competition between cutaneous vasodilator and vasoconstrictor reflexes in man. J Appl Physiol. 1973;35(6):798–803. [DOI] [PubMed] [Google Scholar]
- [95].Minson CT, Wladkowski SL, Pawelczyk JA, et al. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol. 1999;276(1 Pt 2):R203–12. [DOI] [PubMed] [Google Scholar]
- [96].Rowell LB. Regulation of splanchnic blood flow in man. Physiologist. 1973;16(2):127–142. [PubMed] [Google Scholar]
- [97].Radigan LR, Robinson S. Effects of environmental heat stress and exercise on renal blood flow and filtration rate. J Appl Physiol. 1949;2(4):185–191. [DOI] [PubMed] [Google Scholar]
- [98].Kenney RA. The effect of hot, humid environments on the renal function of West Africans. J Physiol. 1952;118(2):25–26. [PubMed] [Google Scholar]
- [99].Ganio MS, Brothers RM, Lucas RAI, et al. Validity of auscultatory and Penaz blood pressure measurements during profound heat stress alone and with an orthostatic challenge. Am J Physiol Regul Integr Comp Physiol. 2011;301(5):R1510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Glaser EM, Berridge FR, PRIOR KM. Effects of heat and cold on the distribution of blood within the human body; radiological investigations of the liver, lungs and heart. Clin Sci. 1950;9(2):181–187. [PubMed] [Google Scholar]
- [101].Crandall CG, Wilson TE, Marving J, et al. Colloid volume loading does not mitigate decreases in central blood volume during simulated haemorrhage while heat stressed. J Physiol. 2012;590(5):1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Johnson JM, Brengelmann GL, Rowell LB. Interactions between local and reflex influences on human forearm skin blood flow. J Appl Physiol. 1976;41(6):826–831. [DOI] [PubMed] [Google Scholar]
- [103].Roddie IC, Shepherd JT, Whelan RF. Evidence from venous oxygen saturation measurements that the increase in forearm blood flow during body heating is confined to the skin. J Physiol. 1956;134(2):444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Keller DM, Sander M, Stallknecht B, et al. alpha-Adrenergic vasoconstrictor responsiveness is preserved in the heated human leg. J Physiol. 2010;588(Pt 19):3799–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Barcroft H, Bonnar WM, Edholm OG. Reflex vasodilatation in human skeletal muscle in response to heating the body. J Physiol. 1947;106(3):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pearson J, Low DA, Stöhr E, et al. Hemodynamic responses to heat stress in the resting and exercising human leg: insight into the effect of temperature on skeletal muscle blood flow. Am J Physiol Regul Integr Comp Physiol. 2011;300(3):R663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Chiesa ST, Trangmar SJ, Kalsi KK, et al. Local temperature-sensitive mechanisms are important mediators of limb tissue hyperemia in the heat-stressed human at rest and during small muscle mass exercise. Am J Physiol Heart Circ Physiol. 2015;309(2):H369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chiesa ST, Trangmar SJ, Gonzalez-Alonso J. Temperature and blood flow distribution in the human leg during passive heat stress. J Appl Physiol (1985). 2016;120(9):1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Heinonen I, Brothers RM, Kemppainen J, et al. Local heating, but not indirect whole body heating, increases human skeletal muscle blood flow. J Appl Physiol (1985). 2011;111(3):818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kalsi KK, Chiesa ST, Trangmar SJ, et al. Mechanisms for the control of local tissue blood flow during thermal interventions: influence of temperature-dependent ATP release from human blood and endothelial cells. Exp Physiol. 2017;102(2):228–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ogoh S, Sato K, Okazaki K, et al. Blood flow distribution during heat stress: cerebral and systemic blood flow. J Cereb Blood Flow Metab. 2013;33(12):1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Bain AR, Smith KJ, Lewis NC, et al. Regional changes in brain blood flow during severe passive hyperthermia: effects of Pa CO 2 and extracranial blood flow. J Appl Physiol (1985). 2013;115(5):653–659. [DOI] [PubMed] [Google Scholar]
- [113].Willie CK, Tzeng Y-C, Fisher JA, et al. Integrative regulation of human brain blood flow. J Physiol. 2014;592(5):841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Johnson JM. Exercise and the cutaneous circulation. Exerc Sport Sci Rev. 1992;20:59–97. [PubMed] [Google Scholar]
- [115].Kenney WL, Johnson JM. Control of skin blood flow during exercise. Med Sci Sports Exerc. 1992;24(3):303–312. [PubMed] [Google Scholar]
- [116].Johnson JM, Minson CT, Kellogg DL. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Comprehensive Physiology. 2014;4:33–89. [DOI] [PubMed] [Google Scholar]
- [117].Johnson JM, Park MK. Effect of heat stress on cutaneous vascular responses to the initiation of exercise. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(3):744–749. [DOI] [PubMed] [Google Scholar]
- [118].Kellogg DL Jr., Johnson JM, Kosiba WA. Competition between cutaneous active vasoconstriction and active vasodilation during exercise in humans. Am J Physiol. 1991;261(4 Pt 2):H1184–9. [DOI] [PubMed] [Google Scholar]
- [119].Taylor WF, Johnson JM, O’Leary DS, et al. Modification of the cutaneous vascular response to exercise by local skin temperature. J Appl Physiol Respir Environ Exerc Physiol. 1984;57(6):1878–1884. [DOI] [PubMed] [Google Scholar]
- [120].Benzinger TH. Heat regulation: homeostasis of central temperature in man. Physiol Rev. 1969;49(4):671–759. [DOI] [PubMed] [Google Scholar]
- [121].Johnson JM, Park MK. Effect of upright exercise on threshold for cutaneous vasodilation and sweating. J Appl Physiol Respir Environ Exerc Physiol. 1981;50(4):814–818. [DOI] [PubMed] [Google Scholar]
- [122].Kellogg DL Jr., Johnson JM, Kosiba WA. Control of internal temperature threshold for active cutaneous vasodilation by dynamic exercise. J Appl Physiol (1985). 1991;71(6):2476–2482. [DOI] [PubMed] [Google Scholar]
- [123].Sugenoya J, Ogawa T, Jmai K, et al. Cutaneous vasodilatation responses synchronize with sweat expulsions. Eur J Appl Physiol Occup Physiol. 1995;71(1):33–40. [DOI] [PubMed] [Google Scholar]
- [124].Takamata A, Nagashima K, Nose H, et al. Role of plasma osmolality in the delayed onset of thermal cutaneous vasodilation during exercise in humans. Am J Physiol. 1998;275(1 Pt 2):R286–90. [DOI] [PubMed] [Google Scholar]
- [125].Smolander J, Saalo J, Korhonen O. Effect of work load on cutaneous vascular response to exercise. J Appl Physiol (1985). 1991;71(4):1614–1619. [DOI] [PubMed] [Google Scholar]
- [126].Taylor WF, Johnson JM, Kosiba WA, et al. Graded cutaneous vascular responses to dynamic leg exercise. J Appl Physiol (1985). 1988;64(5):1803–1809. [DOI] [PubMed] [Google Scholar]
- [127].Mack GW, Nose H, Takamata A, et al. Influence of exercise intensity and plasma volume on active cutaneous vasodilation in humans. Med Sci Sports Exerc. 1994;26(2):209–216. [DOI] [PubMed] [Google Scholar]
- [128].Mitono H, Endoh H, Okazaki K, et al. Acute hypoosmolality attenuates the suppression of cutaneous vasodilation with increased exercise intensity. J Appl Physiol (1985). 2005;99(3):902–908. [DOI] [PubMed] [Google Scholar]
- [129].Leslie SJ, Rahman MQ, Denvir MA, et al. Endothelins and their inhibition in the human skin microcirculation: ET[1-31], a new vasoconstrictor peptide. Br J Clin Pharmacol. 2004;57(6):720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Convertino VA, Keil LC, Bernauer EM, et al. Plasma volume, osmolality, vasopressin, and renin activity during graded exercise in man. J Appl Physiol Respir Environ Exerc Physiol. 1981;50(1):123–128. [DOI] [PubMed] [Google Scholar]
- [131].Kenney WL, Tankersley CG, Newswanger DL, et al. Alpha 1-adrenergic blockade does not alter control of skin blood flow during exercise. Am J Physiol. 1991;260(3 Pt 2):H855–61. [DOI] [PubMed] [Google Scholar]
- [132].Kenney WL, Zappe DH, Tankersley CG, et al. Effect of systemic yohimbine on the control of skin blood flow during local heating and dynamic exercise. Am J Physiol. 1994;266(2 Pt 2):H371–6. [DOI] [PubMed] [Google Scholar]
- [133].Kellogg DL Jr., Johnson JM, Kenney WL, et al. Mechanisms of control of skin blood flow during prolonged exercise in humans. Am J Physiol. 1993;265(2 Pt 2):H562–8. [DOI] [PubMed] [Google Scholar]
- [134].Kenney WL, Stanhewicz AE, Bruning RS, et al. Blood pressure regulation III: what happens when one system must serve two masters: temperature and pressure regulation? Eur J Appl Physiol. 2014;114(3):467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kenefick RW, Cheuvront SN, Sawka MN. Thermoregulatory function during the marathon. Sports Med. 2007;37(4–5):312–315. [DOI] [PubMed] [Google Scholar]
- [136].Rowell LB, Marx HJ, Bruce RA, et al. Reductions in cardiac output, central blood volume, and stroke volume with thermal stress in normal men during exercise. J Clin Invest. 1966;45(11):1801–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Nielsen B, Savard G, Richter EA, et al. Muscle blood flow and muscle metabolism during exercise and heat stress. J Appl Physiol (1985). 1990;69(3):1040–1046. [DOI] [PubMed] [Google Scholar]
- [138].Nielsen B, Hales JR, Strange S, et al. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 1993;460:467–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Nielsen B, Strange S, Christensen NJ, et al. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflugers Arch. 1997;434(1):49–56. [DOI] [PubMed] [Google Scholar]
- [140].Savard GK, Nielsen B, Laszczynska J, et al. Muscle blood flow is not reduced in humans during moderate exercise and heat stress. J Appl Physiol (1985). 1988;64(2):649–657. [DOI] [PubMed] [Google Scholar]
- [141].Gonzalez-Alonso J, Calbet JA, Nielsen B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. J Physiol. 1998;513(3):895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Trangmar SJ, Chiesa ST, Kalsi KK, et al. Whole body hyperthermia, but not skin hyperthermia, accelerates brain and locomotor limb circulatory strain and impairs exercise capacity in humans. Physiol Rep. 2017;5(2):e13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Johnson JM, Minson CT, Kellogg DL. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol. 2014;4:33–89. [DOI] [PubMed] [Google Scholar]
- [144].Rowell LB. Reflex control of regional circulations in humans. J Auton Nerv Syst. 1984;11(2):101–114. [DOI] [PubMed] [Google Scholar]
- [145].Rowell LB. circulatory adjustments to dynamic exercise, in human circulation: regulation during physical stress. New York: Oxford University Press; 1986. p. 213–256. [Google Scholar]
- [146].Grimby G. Renal clearances during prolonged supine exercise at different loads. J Appl Physiol. 1965;20(6):1294–1298. [Google Scholar]
- [147].Castenfors J. Renal function during prolonged exercise. Ann N Y Acad Sci. 1977;301:151–159. [DOI] [PubMed] [Google Scholar]
- [148].Rowell LB, MURRAY JA, BRENGELMANN GL, et al. Human cardiovascular adjustments to rapid changes in skin temperature during exercise. Circ Res. 1969;24(5):711–724. [DOI] [PubMed] [Google Scholar]
- [149].MacDougall JD, Reddan WG, Layton CR, et al. Effects of metabolic hyperthermia on performance during heavy prolonged exercise. J Appl Physiol. 1974;36(5):538–544. [DOI] [PubMed] [Google Scholar]
- [150].Rowell LB, Kraning KK, Kennedy JW, et al. Central circulatory responses to work in dry heat before and after acclimatization. J Appl Physiol. 1967;22(3):509–518. [DOI] [PubMed] [Google Scholar]
- [151].Johnson JM, Rowell LB. Forearm skin and muscle vascular responses to prolonged leg exercise in man. J Appl Physiol. 1975;39(6):920–924. [DOI] [PubMed] [Google Scholar]
- [152].Fritzsche RG, Switzer TW, Hodgkinson BJ, et al. Stroke volume decline during prolonged exercise is influenced by the increase in heart rate. J Appl Physiol (1985). 1999;86(3):799–805. [DOI] [PubMed] [Google Scholar]
- [153].Nose H, Mack GW, Shi XR, et al. Effect of saline infusion during exercise on thermal and circulatory regulations. J Appl Physiol (1985). 1990;69(2):609–616. [DOI] [PubMed] [Google Scholar]
- [154].Trinity JD, Pahnke MD, Lee JF, et al. Interaction of hyperthermia and heart rate on stroke volume during prolonged exercise. J Appl Physiol (1985). 2010;109(3):745–751. [DOI] [PubMed] [Google Scholar]
- [155].Nose H, Takamata A, Mack GW, et al. Right atrial pressure and forearm blood flow during prolonged exercise in a hot environment. Pflugers Arch. 1994;426(3–4):177–182. [DOI] [PubMed] [Google Scholar]
- [156].Tripathi A, Mack GW, Nadel ER. Cutaneous vascular reflexes during exercise in the heat. Med Sci Sports Exerc. 1990;22(6):796–803. [DOI] [PubMed] [Google Scholar]
- [157].Fortney SM, Nadel ER, Wenger CB, et al. Effect of acute alterations of blood volume on circulatory performance in humans. J Appl Physiol Respir Environ Exerc Physiol. 1981;50(2):292–298. [DOI] [PubMed] [Google Scholar]
- [158].Nadel ER, Fortney SM, Wenger CB. Effect of hydration state of circulatory and thermal regulations. J Appl Physiol Respir Environ Exerc Physiol. 1980;49(4):715–721. [DOI] [PubMed] [Google Scholar]
- [159].Mora-Rodriguez R, Gonzalez-Alonso J, Below PR, et al. Plasma catecholamines and hyperglycaemia influence thermoregulation in man during prolonged exercise in the heat. J Physiol. 1996;491(2):529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Bevegard S, Lodin A. Postural circulatory changes at rest and during exercise in five patients with congenital absence of valves in the deep veins of the legs. Acta Med Scand. 1962;172:21–29. [DOI] [PubMed] [Google Scholar]
- [161].Grimby G, Nilsson NJ, Sanne H. Cardiac output during exercise in patients with varicose veins. Scand J Clin Lab Invest. 1964;16:21–30. [DOI] [PubMed] [Google Scholar]
- [162].Grimby G, Nilsson NJ, Sanne H. Repeated serial determination of cardiac output during 30 min exercise. J Appl Physiol. 1966;21(6):1750–1756. [DOI] [PubMed] [Google Scholar]
- [163].Fortney SM, Wenger CB, Bove JR, et al. Effect of blood volume on forearm venous and cardiac stroke volume during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(3):884–890. [DOI] [PubMed] [Google Scholar]
- [164].Reeves JT, Groves BM, Cymerman A, et al. Operation Everest II: cardiac filling pressures during cycle exercise at sea level. Respir Physiol. 1990;80(2–3):147–154. [DOI] [PubMed] [Google Scholar]
- [165].Bevegard S, Jonsson B, Karlof I, et al. Effect of changes in ventricular rate on cardiac output and central pressures at rest and during exercise in patients with artificial pacemakers. Cardiovasc Res. 1967;1(1):21–33. [DOI] [PubMed] [Google Scholar]
- [166].Sheriff DD, Zhou XP, Scher AM, et al. Dependence of cardiac filling pressure on cardiac output during rest and dynamic exercise in dogs. Am J Physiol. 1993;265(1 Pt 2):H316–22. [DOI] [PubMed] [Google Scholar]
- [167].Ross J Jr., Linhart JW, Brauwald E. Effects of changing heart rate in man by electrical stimulation of the right atrium. studies at rest, during exercise, and with isoproterenol. Circulation. 1965;32(4):549–558. [DOI] [PubMed] [Google Scholar]
- [168].Turkevich D, Micco A, Reeves JT. Noninvasive measurement of the decrease in left ventricular filling time during maximal exercise in normal subjects. Am J Cardiol. 1988;62(9):650–652. [DOI] [PubMed] [Google Scholar]
- [169].Strandell T. Mechanical systole at rest, during and after exercise in supine and sitting position in young and old men. Acta Physiol Scand. 1964;61:279–298. [DOI] [PubMed] [Google Scholar]
- [170].Bevegard S. The effect of cardioacceleration by methyl-scopolamine nitrate on the circulation at rest and during exercise in supine position, with special reference to the stroke volume. Acta Physiol Scand. 1963;57:61–80. [DOI] [PubMed] [Google Scholar]
- [171].Bevegard S. Observations on the effect of varying ventricular rate on the circulation at rest and during exercise in two patients with an artificial pacemaker. Acta Med Scand. 1962;172:615–622. [PubMed] [Google Scholar]
- [172].Saunders DE Jr., Ord JW. The hemodynamic effects of paroxysmal supraventricular tachycardia in patients with the Wolff-Parkinson-white syndrome. Am J Cardiol. 1962;9:223–236. [DOI] [PubMed] [Google Scholar]
- [173].Gonzalez-Alonso J, Mora-Rodriguez R, Coyle EF. Supine exercise restores arterial blood pressure and skin blood flow despite dehydration and hyperthermia. Am J Physiol. 1999;277(2 Pt 2):H576–83. [DOI] [PubMed] [Google Scholar]
- [174].Eiken T, Harrison AJ, Burdon CA, et al. Elevated body temperature contributes to the increased heart rate response during eccentric compared to concentric cycling when matched for oxygen consumption. Temperature. 2021;8(1):30–38. doi: 10.1080/23328940.2020.1810199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Hamilton MT, Gonzalez-Alonso J, Montain SJ, et al. Fluid replacement and glucose infusion during exercise prevent cardiovascular drift. J Appl Physiol (1985). 1991;71(3):871–877. [DOI] [PubMed] [Google Scholar]
- [176].Ekelung LG, Holmgren A. Circulatory and respiratory adaptation, during long-term, non-steady state exercise, in the sitting position. Acta Physiol Scand. 1964;62:240–255. [DOI] [PubMed] [Google Scholar]
- [177].Berry JN, Thompson, HK, Jr., Miller, DE, et al. Changes in cardiac output, stroke volume, and central venous pressure induced by atropine in man. Am Heart J. 1959;58(2):204–213. [DOI] [PubMed] [Google Scholar]
- [178].Gorten R, Gunxells JC, WEISSLER AM, et al. Effects of atropine and isoproterenol on cardiac output, central venous pressure, and mean transit time of indicators placed at three different sites in the venous system. Circ Res. 1961;9:979–983. [DOI] [PubMed] [Google Scholar]


