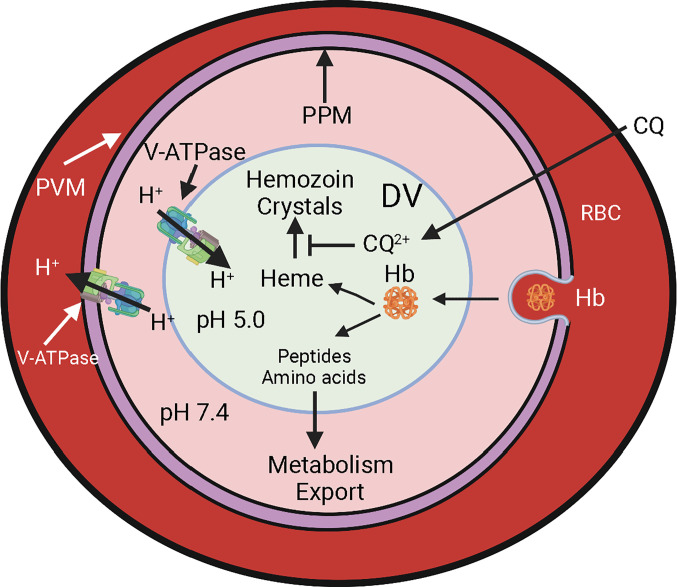
Each year estimated 240 million people in the world suffer from malaria, resulting in over 600,000 deaths. The disease symptoms are due to the parasites’ propensity to replicate in red blood cells (RBC), consuming hemoglobin and destroying the RBC. By a rough estimate, malaria parasites consume about 5,700 kg of human hemoglobin each day, over 2 million kg each year. This staggering sacrifice of human hemoglobin is the work of parasite-encoded proteases that reside within a specialized lysosome-like acidic organelle of the parasite called the digestive vacuole (DV) (1). Each digested hemoglobin tetramer releases four heme molecules, reaching high millimolar concentrations within the DV (2). The released heme would be highly toxic to the parasite, so the parasite manages to detoxify it by biomineralizing it into crystals called hemozoin that remain within the DV (Fig. 1). Antimalarial drugs such as chloroquine interfere with this detoxification process, resulting in heme toxicity to the parasites and their demise (3). Acidification of the DV is critical for both hemoglobin digestion and heme detoxification. Previous studies have implicated proton pumping by a V-type ATPase as the source for DV acidification (4). In PNAS, Alder et al. (5) explore the role of V-ATPase in physiological processes relegated to the DV by using a combination of reverse genetics and imaging approaches that employed about 20 different transgenic parasite lines. These numerous transgenic parasite lines could provide useful tools to investigate an important aspect of malaria parasite physiology involving pH homeostasis In this paper, the authors show the V-ATPase function to be essential for acidification of the DV, hemoglobin digestion, and parasite survival, as well as for correct generation of the DV itself.
Fig. 1.
DV and hemoglobin digestion in a malaria parasite growing inside a RBC. Abbreviations: PVM, parasitophorous vacuole membrane; PPM, parasite plasma membrane; DV, digestive vacuole; Hb, hemoglobin; CQ, chloroquine; CQ2+, diprotonated chloroquine.
The V-ATPase is a widely conserved and extensively investigated rotary pump complex analogous to the mitochondrial F-type adenosine triphosphate (ATP) synthase, with a distinction that it uses ATP hydrolysis to pump protons across membranes rather than synthesizing ATP through protonmotive force (6, 7). In analogy to ATP synthases, V-ATPase complex is divided into two sectors: a membrane-associated VO sector consisting of four subunits designated a, c, d, and e, and a cytosolic V1 sector consisting of eight subunits designated A to H (6, 7). Extensive investigations have revealed that in addition to its role in acidification of intracellular vesicles and organelles, V-ATPase performs multiple activities in animals such as extracellular acidification in kidney, bone, etc., and acting as a signaling hub to control metabolism (7, 8). For instance, a recent study has found lysosomal V-ATPase to be central to the mode of action for the antidiabetes drug metformin (9). Furthermore, the VO and V1 sectors undergo dynamic association/disassociation depending upon the energetic status of the cell (10, 11).
Given the complex role played by V-ATPase in most organisms, it is clearly important to investigate its functions in important pathogens such as malaria parasites. Alder et al. (5) tagged the B subunit of Plasmodium falciparum V-ATPase V1 sector with a fluorescent protein to show the protein to be localized to the DV but also to the parasite membrane. A lack of localization of V-ATPase subunits into the RBC cytosol is counter to a previous report claiming such localization (12). Colocalization of four other V1 sector subunits with the B subunit suggested these proteins to assemble into a complex. Unsurprisingly, conditional knockout of B subunit expression resulted in mislocalization of other V1 sectors subunits. Localization of VO sector subunit a, however, remained unchanged when subunit B was knocked out. This observation is consistent with independent assembly of V1 and VO sector subunits; the former are likely synthesized on cytosolic ribosomes, whereas the latter are likely synthesized on rough endoplasmic reticulum. Authors, however, did not investigate proportions of individual V1 and VO sectors that associate into intact V-ATPases versus those that remain disassociated from each other. In analogy with other organisms (10, 11), this could potentially have a role in regulation of the energetic status of the parasite.
In PNAS, Alder et al. explore the role of V-ATPase in physiological processes relegated to the DV by using a combination of reverse genetics and imaging approaches that employed about 20 different transgenic parasite lines.
Conditional knockout of V-ATPase subunits was lethal to parasites. Under the knockout conditions, Alder et al. (5) show an increase in the pH within the DV as well as a decrease in cytosolic pH. The death of the parasite in the absence of a functional V-ATPase is likely due to a combination of physiological changes. Authors focus on the decreased hemoglobin digestion in the DV as the cause of parasite death. It is also likely that a collapsed or diminished proton gradient across the parasite plasma membrane (PPM) due to the lack of proton pumping by V-ATPase as well as the reduced cytosolic pH contribute to the parasite demise. It is worth pointing out that P. falciparum encodes two membrane-associated H+-pumping pyrophosphatases (PfVP1 and PfVP2), which could also participate in proton homeostasis by using pyrophosphate hydrolysis instead of ATP as the energy source (13, 14). One of these pumps (PfVP1) is localized to the PPM and has been shown to be essential for parasite survival at the ring stage (15). It would be fruitful to examine the possibility of overlapping functions served by these different proton pumps, as a recent study suggested pyrophosphatase-powered H+ pumping into the DV in addition to the V-ATPase (4).
In P. falciparum, the DV is formed very early during intraerythrocytic development cycle and grows as the parasite matures. At this point, not much is known about biogenesis of DV, so the finding by Alder et al. (5) that the DV appears to be fragmented in parasites with genetic ablation of the subunit a and c of the VO sector (but not of the subunit B), is of interest. These fragmented DVs seem to contain hemozoin crystals suggesting at least limited hemoglobin digestion and heme crystal formation. It is interesting to note that while P. falciparum contains a single DV, rodent malaria parasites contain multiple vesicles with hemozoin crystals (16). Reasons for this difference are not clear but being able to induce fragmented DV in P. falciparum could provide hints at mechanisms underlying DV biogenesis.
It has been long recognized that 4-aminoquinoline antimalarials such as chloroquine accumulate in DV at concentrations about 1,000-fold higher than that outside the parasite. This accumulation is believed to be driven by the weak-base nature of chloroquine that gets diprotonated in the acidic DV and thus gets trapped within this compartment. Alder et al. (5) report that conditional knockout of the B subunit increases the DV pH by one unit to about 6, but this does not affect chloroquine accumulation within the DV. Furthermore, they find no change in the efficacy of several 4-aminoquinolines in inhibiting the parasite growth under the knockout condition for subunit B induced 20 h after parasite invasion of the RBC. These are difficult experiments in that inhibition of parasite growth is being assessed in parasites already being inhibited by reduced level of V-ATPase expression. Therefore, caution is required in interpreting the observations to indicate a more sophisticated mechanism underlying chloroquine accumulation in the DV than the currently accepted pH gradient-mediated accumulation. The DV still is acidic, albeit to a reduced level, that may be sufficient to drive drug accumulation.
Acknowledgments
Work in the author’s laboratory is supported by grants from NIH R01AI028398, R01AI132506, and RO1AI154499.
Author contributions
A.B.V. wrote the paper.
Competing interests
The author declares no competing interest.
Footnotes
See companion article, “The role of Plasmodium V-ATPase in vacuolar physiology and antimalarial drug uptake,” 10.1073/pnas.2306420120.
References
- 1.Goldberg D. E., Plasmodial hemoglobin degradation: An ordered pathway in a specialized organelle. Infect. Agents Dis. 1, 207–211 (1992). [PubMed] [Google Scholar]
- 2.Goldberg D. E., Hemoglobin degradation. Curr. Top. Microbiol. Immunol. 295, 275–291 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Ginsburg H., Ward S. A., Bray P. G., An integrated model of chloroquine action. Parasitol. Today 15, 357–360 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Saliba K. J., et al. , Acidification of the malaria parasite’s digestive vacuole by a H+-ATPase and a H+-pyrophosphatase. J. Biol. Chem. 278, 5605–5612 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Alder A., et al. , The role of Plasmodium V-ATPase in vacuolar physiology and antimalarial drug uptake. Proc. Natl. Acad. Sci. U.S.A. 120, e2306420120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forgac M., Vacuolar ATPases: Rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Eaton A. F., Merkulova M., Brown D., The H(+)-ATPase (V-ATPase): From proton pump to signaling complex in health and disease Am. J. Physiol. Cell Physiol. 320, C392–C414 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuli F., Kane P. M., The cytosolic N-terminal domain of V-ATPase a-subunits is a regulatory hub targeted by multiple signals. Front. Mol. Biosci. 10, 1168680 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma T., et al. , Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 603, 159–165 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGuire C., Cotter K., Stransky L., Forgac M., Regulation of V-ATPase assembly and function of V-ATPases in tumor cell invasiveness. Biochim. Biophys. Acta 1857, 1213–1218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kane P. M., Targeting reversible disassembly as a mechanism of controlling V-ATPase activity. Curr. Protein. Pept. Sci. 13, 117–123 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchesini N., Vieira M., Luo S., Moreno S. N., Docampo R., A malaria parasite-encoded vacuolar H(+)-ATPase is targeted to the host erythrocyte J. Biol. Chem. 280, 36841–36847 (2005). [DOI] [PubMed] [Google Scholar]
- 13.McIntosh M. T., Drozdowicz Y. M., Laroiya K., Rea P. A., Vaidya A. B., Two classes of plant-like vacuolar-type H(+)-pyrophosphatases in malaria parasites Mol. Biochem. Parasitol. 114, 183–195 (2001). [DOI] [PubMed] [Google Scholar]
- 14.McIntosh M. T., Vaidya A. B., Vacuolar type H+ pumping pyrophosphatases of parasitic protozoa Int. J. Parasitol. 32, 1–14 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Solebo O., Ling L., Zhou J., Fu T.-M., Ke H., Plasmodium falciparum vacuolar pyrophosphatase 1 for ring stage development and its transition to trophozoite. bioRxiv [Preprint] (2021). 10.1101/2021.10.25.465524 (Accessed 15 July 2023). [DOI]
- 16.Slomianny C., Three-dimensional reconstruction of the feeding process of the malaria parasite. Blood Cells 16, 369–378 (1990). [PubMed] [Google Scholar]