Abstract
Human erythroleukemic K562 cells represent the prototypical cell culture model of chronic myeloid leukemia (CML). The cells are pseudo-triploid and positive for the Philadelphia chromosome. Therefore, K562 cells have been widely used for investigating the BCR/ABL1 oncogene and the tyrosine kinase inhibitor, imatinib-mesylate. Further, K562 cells overexpress transferrin receptors (TfR) and have been used as a model for targeting cytotoxic therapies, via receptor-mediated endocytosis. Here, we have characterized K562 cells focusing on the karyotype of cells in prolonged culture, regulation of expression of TfR in wildtype (WT) and doxorubicin-resistant cells, and responses to histone deacetylase inhibition (HDACi). Karyotype analysis indicates novel chromosomes and gene expression analysis suggests a shift of cultured K562 cells away from patient-derived leukemic cells. We confirm the high expression of TfR on K562 cells using immunofluorescence and cell-surface receptor binding radioassays. Importantly, high TfR expression is observed in patient-derived cells, and we highlight the persistent expression of TfR following doxorubicin acquired resistance. Epigenetic analysis indicates that permissive histone acetylation and methylation at the promoter region regulates the transcription of TfR in K562 cells. Finally, we show relatively high expression of HDAC enzymes in K562 cells and demonstrate the chemotoxic effects of HDACi, using the FDA-approved hydroxamic acid, vorinostat. Together with a description of morphology, infrared spectral analysis, and examination of metabolic properties, we provide a comprehensive characterization of K562 cells. Overall, K562 cell culture systems remain widely used for the investigation of novel therapeutics for CML, which is particularly important in cases of imatinib-mesylate resistance.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-023-04905-6.
Keywords: K562 cells, Chronic myeloid leukemia, BCR-ABL, Philadelphia chromosome, Transferrin receptors, Doxorubicin, Histone deacetylase inhibitors
Introduction
Following the recognition of the Philadelphia (Ph) chromosome as a significant aberration in chronic myeloid leukemia (CML), the importance of establishing a continuously growing Ph chromosome-positive cell line became evident [1]. Obtained from the pleural effusion of a 53-year-old female with terminal blast crisis disease, the first CML cell line derived by the cytogeneticists Drs Carmen and Bismarck Lozzio and designated K562, demonstrated the capacity to proliferate continuously for 3.5 years (175 serial passages) while retaining their Ph chromosome positivity [2]. Since its derivation, human erythroleukemic K562 cells have become an invaluable cell culture model which may be utilized in a wide variety of experimental designs.
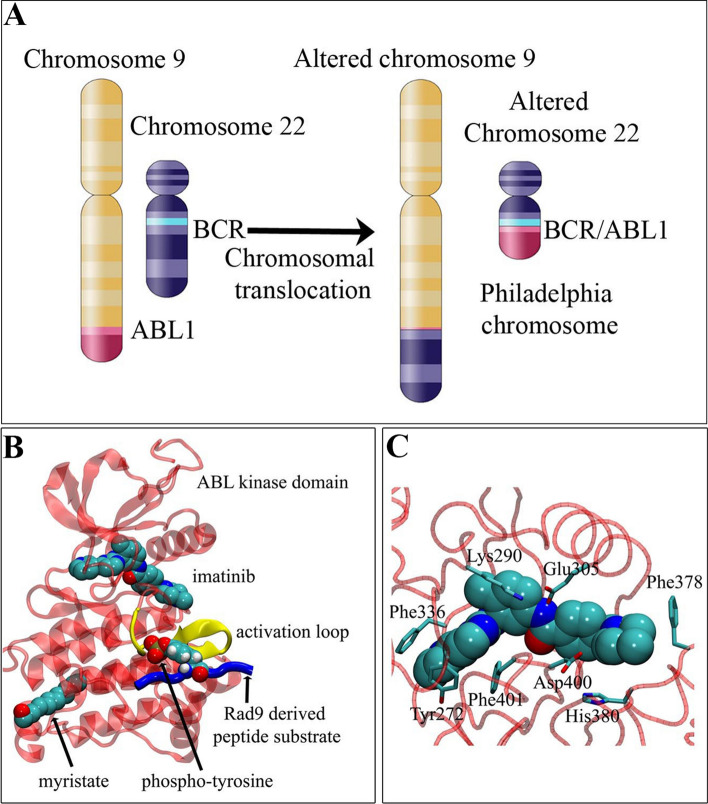
The Ph chromosome (designated der(22)t(9;22)(q34;q11.2), a resultant of a reciprocal translocation between chromosome 9 (ABL1 gene at region q34) and chromosome 22 (BCR gene at region q11.2), gives rise to the breakpoint cluster region-Abelson tyrosine-protein kinase 1 (BCR/ABL1) fusion oncogene (Fig. 1A) [3, 4]. Due to the knowledge that the BCR/ABL1 tyrosine kinase promotes CML development and progression in the majority of cases, the development of its inhibitor, imatinib-mesylate, was part of a revolution in oncology as one of the first molecular targeting cancer therapeutics (Fig. 1B–C) [5]. Despite this, approximately one-third of patients demonstrate various levels of resistance to such therapy by either responding poorly from the outset or relapsing after an initial response [6, 7]. Similarly, resistance to conventional cytotoxic drugs, including the anthracycline doxorubicin, has been observed in advanced phases of CML and remains a major clinical limitation of chemotherapy [8].
Fig. 1.
The importance of the Philadelphia chromosome-positive human erythroleukemic K562 cell line in CML research. A The BCR/ABL1 fusion gene formed by reciprocal translocation of chromosome 9 and chromosome 22. B Molecular model of binding of imatinib (STI-571) and myristate to the kinase domain (PDB 1OPJ, transparent red) of c-ABL. The activation loop (yellow), Rad9-derived peptide substrate (blue; PDB 1K2M) and associated phospho-tyrosine (spheres) are highlighted. The approximate substrate binding position is based on a tyrosine kinase-peptide co-crystal structure (PDB 1IR3). C Close-up of the imatinib binding pocket with nearby amino acids highlighted. Residues numbered according to PDB 1OPJ
The inadequacies in the current clinical management of CML provide rationale for the development of novel therapeutic strategies. Histone deacetylase inhibitors (HDACi) represent a promising class of anticancer compounds that have demonstrated cytotoxic effects in the K562 cell line [9, 10]. Interestingly, vorinostat (FDA-approved for cutaneous T-cell lymphoma) has been shown to be more effective in imatinib-resistant K562 cells compared to their imatinib-sensitive counterpart [3]. In addition, HDACi in combination with imatinib, have demonstrated efficacy in the eradication of CML progenitor cells, which were resistant to imatinib treatment alone [4]. The development of targeted drug delivery systems has also become an important approach to enhance the therapeutic benefit of anticancer compounds and limit resistance. The overexpression of the transferrin receptor on K562 cells, as well as the molecular details of the transferrin-transferrin receptor cycle has led to its use as a routine therapeutic target [11, 12]. Numerous studies, including those from our own laboratory, have utilized transferrin-mediated endocytosis to develop cytotoxic moieties with a significant effect on K562 cells [13]. The results from a phase I clinical trial demonstrated the therapeutic potential of a transferrin-receptor targeting nanoparticle, as siRNA was delivered to malignant cells in melanoma patients [14].
Overall, the use of continuously growing K562 cells has been instrumental in understanding the biology and molecular details of CML. Further, K562 cells have been widely utilized to investigate the mechanisms of action of clinical and experimental therapeutics. In this study, our aim was to investigate properties of K562 cells in prolonged culture in comparison to patient-derived CML cells. Karyotypic analyses and genome-wide expression were performed for comparative purposes. Further, we aimed to investigate the expression and epigenetic regulation of TfR in K562 cells, and the persistence of TfR expression in doxorubicin-resistant cells. Finally, we investigated the expression of HDAC enzymes and responses to the clinically used HDACi, vorinostat.
Methods
Cell culture
Human erythroleukemic K562 cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in complete-Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 20 mmol/L HEPES (pH 7.4; GIBCO-Invitrogen, Carlsbad, CA, USA), 10% (v/v) fetal bovine serum (FBS), 2 mmol/L l-glutamine (GIBCO-Invitrogen), and 1% penicillin/streptomycin (GIBCO-Invitrogen) [2]. Cells were cultured in suspension in a humidified atmosphere of 5% (v/v) CO2 at 37 °C and maintained in exponential growth phase. The K562 growth curve was measured by cell counting daily using a hemocytometer over 9 days [15]. A doxorubicin-resistant cell line was derived from K562 cells by incremental exposure to doxorubicin (1.5–5 µg/mL) over 461 days and is denoted PW-15 cells. Human peripheral blood mononuclear cells (PBMC) were fractionated using the Ficoll Plaque (GE Healthcare, Wauwatosa, Wisconsin, USA) method from buffy coat obtained from the Australian Red Cross Blood Bank (ARCB) under ethics approval (#304/12). Cells were maintained in complete-RPMI-1640 medium supplemented with 10% FBS, 2 mM L-glutamine and 1% penicillin/streptomycin at 37 °C, 5% (v/v) CO2.
3D visualization of K562 cells
K562 cells were stained with β-actin and Hoechst 33,258 to observe cell structure and nuclear volumes. Detailed instructions are outlined in the Supplemental Data.
Flow cytometric analysis
Flow cytometry was utilized for cell cycle analysis. K562 cells were treated with or without 10 µM vorinostat (Sigma–Aldrich, St Louis, MO, USA) for 24 h before collection by centrifugation and prepared as described previously [16].
Synchrotron-based Fourier-Transform InfraRed (FTIR) microspectroscopy
K562 cells were treated with or without 1 µM doxorubicin (Ebewe Pharma, Unterach, Austria) for 1 h at 37 °C and incubated for a further 24 h in fresh media before fixation in 4% paraformaldehyde (Sigma) onto CaF2 windows (Crystran, Dorset, UK). Samples were imaged, and all measurements were recorded on the infrared microspectroscopy beamline (IRM) at the Australian Synchrotron. A more detailed protocol may be found in the Supplemental Data.
Chromosomal G-banding technique
Cells were treated with colcemid (0.2 µg/ml) for 30 min, harvested, incubated in 0.075 M hypotonic KCl at 37 °C for 30 min and fixed in methanol:acetic acid (3:1) to generate a fixed cytogenetic suspension. Fixed cell suspension was dropped onto clean glass slides and G-banded with trypsin and Leishman stain according to standard cytogenetic techniques.
Multiplex fluorescence in situ hybridization (M-FISH)
Fixed cytogenetic suspension was dropped onto clean microscope slides then probed using the 24Xcyte Human Multicolor FISH Probe Kit (MetaSystems, Altlussheim, Germany) according to the manufacturer’s instructions. Metaphases were captured and analyzed using MetaSystems Isis software interfaced with a Zeiss Axioplan Z fluorescence microscope.
Allele-specific FISH for BCR/ABL1
The BCR/ABL Plus Translocation Dual Fusion FISH probe (Cytocell) was used. Probe was added to freshly prepared slides which subsequently underwent co-denaturation at 75 °C for 2 min and were then hybridized overnight at 37 °C. Slides were washed according to the Vysis rapid wash protocol, air dried and mounted with DAPI/antifade.
Microarray data collection, normalization and analysis
Publically accessible data were identified in ArrayExpress, with the previous quality controlled compilation of Affymetrix HG-U133A array-based experiments (accession number: E-MTAB-62) used as reference to manually download raw data from original submissions (Table S1) [17, 18]. CEL files were uploaded into Partek Genomics Suite 6.6 (Partek Inc., St. Louis, Missouri) using RMA default settings, and outliers were removed. Differential gene expression and gene set enrichment analysis (GSEA) was conducted as outlined in the Supplemental Data.
Seahorse metabolic assay
Mitochondrial respiration of the cells was determined using an XF96 extracellular flux analyzer (Seahorse Bioscience, North Billerica, MA) following manufacturer’s protocols (Supplemental Data) [19].
N-SIM microscopy
N-SIM microscopy was utilized to observe the cell-surface expression levels of the transferrin receptor on K562 cells. A more detailed method may be found in the Supplemental Data.
Iron saturation and 125I-labelling of transferrin, and cellular binding assay
To measure the number of transferrin binding sites on K562 cells, optimization of the binding assay involved the complete saturation of human serum apo-transferrin with iron, and the subsequent iodination of the peptide as detailed in the Supplemental Data.
Histone modifications related to the transferrin receptor gene (TFRC) using ChIP sequencing
To assess K562 and PBMC histone modifications, bam files were accessed from the ENCODE project (Table S2; https://www.encodeproject.org/). Reads that align the TFRC gene and 5 kb upstream of the transcription start site (TSS) (chr3:195776155–195814032) were extracted from bam files using SAMtools. The method involved in plotting the data may be referred to in the Supplemental Data.
Cell proliferation and apoptosis
Cell proliferation was determined using the Cell Titer-Blue Assay kit (Promega, Madison, WI, USA) and caspase 3/7 induction was measured using the Apo-ONE Homogeneous Caspase-3/7 Assay kit (Promega) according to manufacturer’s instructions. Data were corrected by subtracting the media control fluorescence reading from the cell florescence measurements.
Immunofluorescence staining for γH2AX and histone deacetylase enzymes
Cells were treated with or without 1 µM doxorubicin for 1 h, washed and incubated for a further 24 h in fresh media before fixing and staining for γH2AX foci as described previously [20]. In separate experiments, untreated cells were stained for the eleven classical metal-dependent histone deacetylase inhibitors (HDAC1-11) following the same protocol using polyclonal rabbit anti-HDAC1-11 antibody panel (Biovision, Milpitas, CA, USA). All analyses were performed using image analysis software, Image J (version 1.48a). Data shown represents the mean ± standard deviation (SD) of a minimum of 5 independent experiments completed in duplicate.
Western blotting
Following 24 h incubation with 10 µM vorinostat, K562 cells were lysed, and cellular and nuclear proteins were harvested, for immunoblotting as previously described [21]. For a list of antibodies used refer to Supplemental Data.
Results
Fundamental properties of the K562 erythroleukemic cell line
Depending on the inducing compound, K562 cells may differentiate along erythroid, macrophage or megakaryocyte lineages (Fig. S1, A) [22–24]. To assess morphology, K562 cells were visualized using 3D fluorescence (Fig. S1, Bi-ii). Cell and nuclear volumes (2975.1 ± 1144.7 µm3 and 949.1 ± 261.5 µm3, respectively), surface area (3057.9 ± 1302.7 µm2 and 884.2 ± 176.8 µm2, respectively) and diameter (15.3 ± 1.7 µm and 12.1 ± 1.6 µm, respectively) were subsequently quantified (Fig. S1, Biii-v). During exponential growth, the doubling time of K562 cells was recorded at 20 h (Fig. S1, C) and cell cycle analysis using flow cytometry showed the amount of cells within G1 (57.7%), S (26.4%), and G2 (15.9%) phase (Fig. S1, D).
FTIR microspectroscopy was utilized to measure vibrational modes of functional groups that characterize major cellular components associated with specific infrared absorbance frequencies [25, 26]. A representative spectrum and its corresponding second derivative were acquired from measurements taken from untreated K562 cell samples (Fig. S1, Ei-ii). Based on the literature, the assignment and interpretation of the major bands resulted in the generation of a characteristic FTIR profile for the cell line (Fig. S1, Eiii) [25, 26]. The protein amide I band at ~ 1654 cm−1 indicated that the secondary structure of cellular protein to be primarily α-helical in nature [27].
To examine the metabolic profile of K562 cells, the XF analyzer was utilized to assess bioenergetic parameters in comparison to normal PBMC, and PBMC stimulated with 25 ng/mL PMA for 4 h to induce proliferation (Fig. S2). K562 cells displayed a higher energetic and metabolic rate in comparison to the low energetic PBMC (Fig. S2, A–B). PBMC stimulated with PMA displayed a higher oxidative phosphorylation (OXPHOS) rate, suggesting that these cells undergo aerobic respiration compared to resting PBMC (Fig. S2, B). K562 cells had a significantly higher glycolytic rate, basal respiration, ATP production, maximal mitochondrial respiration and proton leak in comparison to PMA-stimulated PBMC and lower spare respiratory capacity (Fig. S2, C–H).
K562 cell karyotype is near triploid with multiple structural and numerical abnormalities
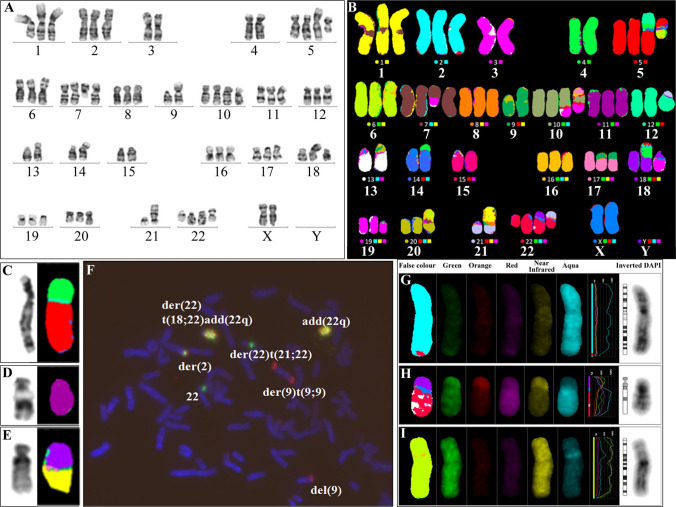
The karyotype of the K562 cell line was analyzed using G-banding, M-FISH and allele specific BCR/ABL1 translocation probe experiments. The formal karyotype according to the International System for Human Cytogenetic Nomenclature (ISCN) may be observed in the Supplemental Data (Table S3). In line with previous findings, the karyotype was shown to be near triploid, including a number of structural and numerical abnormalities (Fig. 2A, B) [28–30]. Most cells (24/34, 71%) contained the new der(5)t(4;5)(p14;p13) (Fig. 2C) but in 10/34 (29%) cells this chromosome had undergone additional rearrangement, incorporating the long arm of chromosome 12, to form the der(5)(12qter- > 12q?::4p?- > 4p14::5p13- > 5qter). This second cell line could also be distinguished by the presence of the der(14)t(4;14) (Fig. 2A, B). A minor subclone, 2/34 (6%) was identified, with deletion of part of the short arm of one chromosome 11 (Fig. 2D). The der(18)t(1;18) was also present only in a minor subclone (2/34 cells, 6%), although it had previously been observed in the dominant clone (Fig. 2E) [28].
Fig. 2.
The karyotype of the erythroleukemic K562 cell line. A G-banded karyotype, and B M-FISH karyotype. Clonal abnormalities present in other subclones but not in this subclone are shown with the G-banded image on the left and M-FISH image on the right: C der(5)t(4;5), D del(11)(p12), and E der(18)t(1;18)(p32;q21). F Allele-specific FISH using the BCR/ABL1 Plus translocation dual fusion probe. Multiple ABL1/BCR fusion signals are present on the add(22q) and the der(22)t(18;22)add(22q) while a single ABL1/BCR fusion is present on the distal long arm of the der(2). One red ABL1 (9q34) signal is present on the del(9p). Two signals are present on the der(9)t(9;9): one on each arm. The intact chromosome 22 and the der(22)t(21;22) each have a single green BCR (22q11.2) signal. The single color gallery created from M-FISH experiments included: G Chromosome 22 (green, red, aqua) but not chromosome 9 (orange, near infrared) material identified on the distal long arm of the derivative chromosome 2, H Alternating regions of diminished green fluorescence intensity present on the long arm of the der(22)t(18;22)add(22q). This is consistent with previously described 13q (red, aqua) amplification on this chromosome, I Material from chromosome 16 (green, near infrared, aqua) inserted into the short arm of chromosome 6
Allele-specific FISH identified a BCR/ABL1 fusion signal on the distal long arm of the derivative chromosome 2, in accordance with previous reports (Fig. 2F) [30]. Tandem duplication of BCR/ABL1 fusion signals on two derivative chromosomes 22 was also consistent with the current literature. These events resulted in BCR/ABL1 amplification with between 15 and 26 fusion signals per cell identified in the 12 interphase nuclei scored. A subsequent translocation event involving one of the BCR/ABL1 amplified derivative chromosomes led to the formation of the der(22)t(18;22)add(22q11.2) (Fig. 2A, B, F–H).
The ABL1 (9q34) component of the der(2), the add(22q) and the der(22)t(18;22)add(22q11.2) could not be detected by M-FISH, suggesting that the amount of chromosome 9 material on these derived chromosomes fell below the lower limit of detection for M-FISH at the chromosome resolution achieved (Fig. 2G, H). Using whole chromosome paint, amplification of chromosome 13 in the tandemly duplicated regions on the derived chromosomes with BCR/ABL1 amplification has also been identified by previous investigators [29]. However, in this study, the M-FISH pattern on the add(22q) and the der(22)t(18;22)add(22q11.2), although not inconsistent with the presence of chromosome 13 material, was inconclusive and this was likely due to limited resolution (Fig. 2H). Furthermore, a der(6) was found to arise as a result of insertion of what is apparently chromosome 16 material into the proximal short arm of one chromosome 6 (Fig. 2I). This is a novel observation, and the study is the first report to characterize this rearrangement as an ins(6;16).
Gene expression profile characteristics of K562 cells
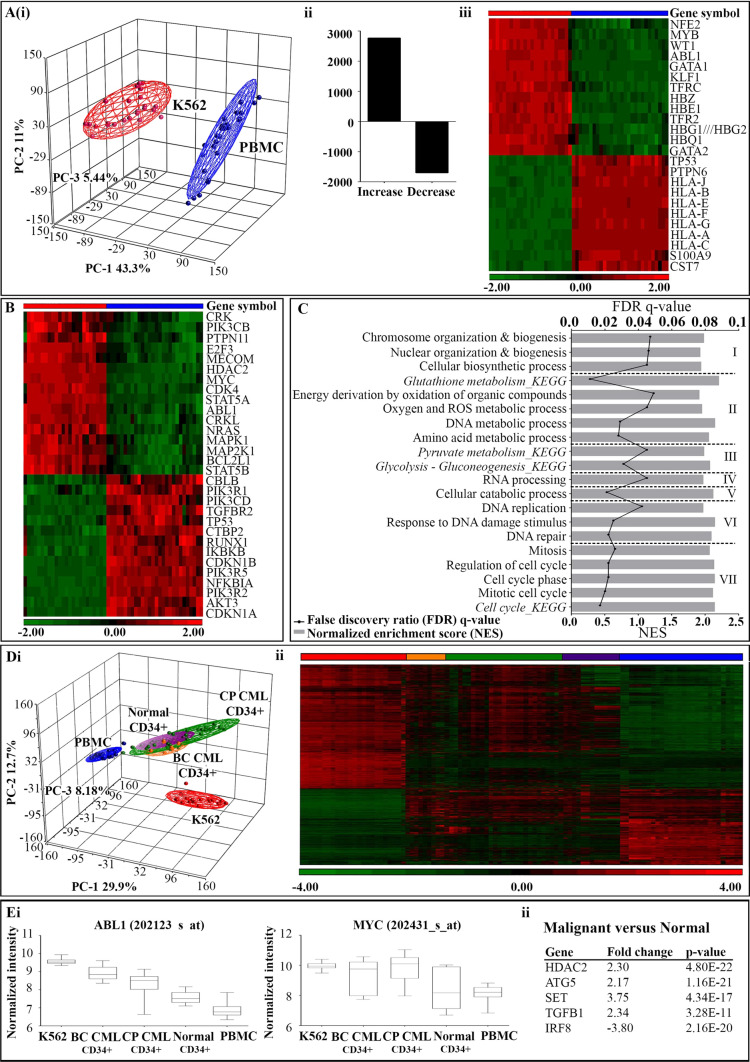
To create a functional gene expression profile for the K562 cell line, microarray data from the malignant cells was compared to that from normal PBMC. A large degree of variance was observed, which was primarily due to cell type (PC-1 43.3%; Fig. 3Ai). In K562 cells, 2772 gene transcripts were significantly upregulated and 1704 gene transcripts were significantly downregulated (Fig. 3Aii). Expression of gene transcripts with products known to be aberrantly expressed in K562 cells, as well as those defined in the KEGG CML gene set (hsa05220) were visualized using hierarchical clustering, with the results in accordance to previous literature (Fig. 3Aiii-B) [22, 31–35].
Fig. 3.
Functional gene expression profile of K562 cells. A Differential gene expression results conducted on microarray gene expression data for K562 cells (red symbols) and normal PBMC (blue symbols). Principal component analysis (PCA) was based on the variance observed in the overall expression profiles between K562 samples and normal PBMC (i). A total of 4476 genes were found to be significantly differentially expressed between the two sample groups: 2772 upregulated, and 1704 downregulated (ii). Hierarchical clustering of select genes which are known to be aberrantly expressed in the K562 cell line validates gene expression profile (iii). B The signature of gene expression in the K562 cell line compared to normal PBMC associated with BCR-ABL mediated leukemogenesis in CML (KEGG pathway; hsa05220). C Gene set enrichment analysis (GSEA) was utilized to characterize the K562 cell line at a functional level using available KEGG and GO biological pathway maps. Categorised based on common cellular function, enriched KEGG and GO gene sets with a FDR q-value of 0.25 were considered significant. D The addition of microarray gene expression data from primary CD34 + hematopoietic stem cell samples was used to further characterize the K562 cell line in the context of clinically relevant disease. PCA results based on the variance observed in the overall profiles between K562 and normal PBMC, as well as CD34 + cell samples from blast crisis (BC) CML (gold symbols), chronic phase (CP) CML (green symbols), and normal individuals (purple symbols) (i). Hierarchical clustering of differentially expressed in the K562 cell line compared to normal PBMC, with the addition of signals from primary samples allows for the visualization of the variance between the immortalized cell line, normal cells and primary samples collected from CML patients (ii). E Select genes which are differentially expressed between malignant and normal cell types, and have the capacity to be therapeutic targeted. Representative box plots of normalized probe intensity signals from each sample group for well known targets, ABL1 and MYC (i). Significant genes differentially expressed in malignant cells compared to normal controls were selected for analysis due to their involvement in the survival and function of the CML progenitor cell population, independent of BCR/ABL1 (ii)
At the functional level, GSEA was performed using available KEGG and GO biological pathway maps to compare cell types [36–38]. In this case, a total of 58 relevant gene sets were shown to be significantly enriched in K562 cells (FDR q-value < 0.05; Table S4). These gene sets were categorized based on common cellular function, including I. Organization and biosynthesis, II. Metabolic processes, III. Carbohydrate metabolism, IV. RNA processing, V. Catabolic processes, VI. DNA replication and repair, and VII. Cell cycle (Fig. 3C).
The overall variance between data sets was found to be primarily due to cell type (PC-1 29.9%; Fig. 3Di). In addition, the PCA plot allowed for the visualization that K562 cells possess a number of genes differentially expressed compared to the primary samples. This was confirmed by hierarchical clustering of the significant transcripts calculated from the direct comparison of K562 cells, primary samples collected from CML patients, and PBMC (Fig. 3Dii).
To assess the potential of a gene as a therapeutic target, the significant transcripts differentially expressed in the CML samples sets in comparison to the normal controls were subject to further analysis. Representative box plots for ABL1 and MYC were displayed due to their well characterized involvement in CML pathogenesis (Fig. 3Ei) [33, 39]. Five genes known to be involved in the survival and function of CML progenitor cells, which have been implicated in the progression of disease independent of BCR/ABL1, were also observed to be differentially expressed in malignant cell groups compared to normal controls (Fig. 3Eii; Table S5) [40].
Cell-surface expression of the transferrin receptor on K562 cells
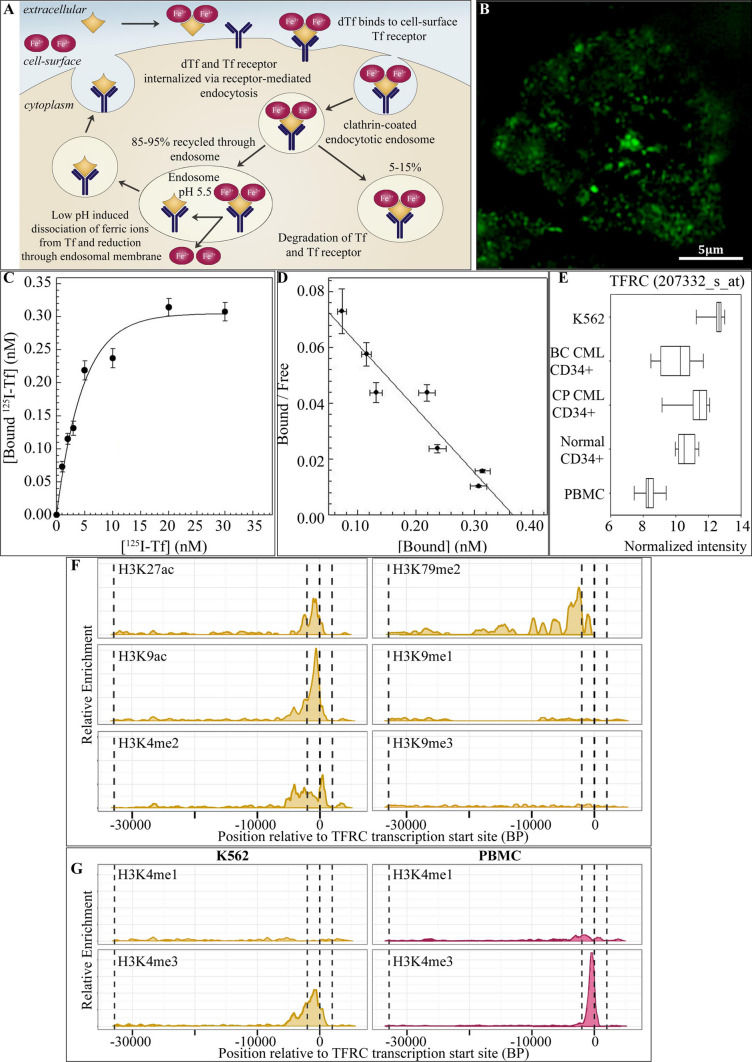
Described previously, transferrin receptor (TfR) expression is significantly upregulated in various malignant tissues, increasing several-fold in a number of cancer types (Fig. 4A) [11, 41]. In accordance with the literature, the TfR is highly expressed in the K562 cell line (Fig. 4B). The binding characteristics of 125I-diferric transferrin were analyzed by estimation of the equilibrium dissociation constant and the number of binding sites per K562 cell from Scatchard analysis of the saturation binding assay (Fig. 4C, D) [42]. Results indicated that the specific binding of 125I-diferric transferrin was saturable as the radioligand concentration exceeded 15 nM (in incubations with 5 × 105 cells), to a population of homogeneous binding sites (Hill coefficient [nH] 0.97 ± 0.11) with an equilibrium dissociation constant of 4.9 ± 0.6 nM (Fig. 4C). Comparable to previous reports, the number of binding sites for unconjugated 125I-diferric transferrin were estimated to be 2.17(± 0.24) × 105 per K562 cell (Fig. 4D) [11].
Fig. 4.
The upregulation of transferrin receptor expression on K562 cells. A Schematic representation of the transferrin receptor-mediated endocytosis pathways. Diferric transferrin binds to transferrin receptors in clathrin coated pits. The pits bud and pinch off from the membrane forming endocytotic vesicles which move into the cytoplasm. In the major transferrin pathway, which represents ~ 85–95% of the internalised transferrin/transferrin receptor complexes, the endocytotic vesicles fuse with early endosomes. At the low pH (~ 5.5) of the endosome the ferric ions dissociate from the transferrin peptide. Within this environment the receptor retains a high affinity for apo-transferrin which is returned to the cell surface bound to the transferrin receptor. At the neutral pH of the extracellular fluid the apo-ligand has a reduced affinity for the receptor and therefore, dissociates from the receptor. The alternative transferrin pathway which represents ~ 5–15% of the internalised transferrin/transferrin receptor complexes, involves trafficking through the trans-Golgi to the lysosomes leading to degradation of transferrin and transferrin receptors. B N-SIM super resolution microphotograph of the TfR1 protein (CD71) in a K562 cell. C Equilibrium binding of 125I-transferrin to cell-surface transferrin receptors on K562 cells. D Scatchard presentation of the binding characteristics of 125I-transferrin to cell-surface transferrin receptors on K562 cells to estimate equilibrium dissociation constants and number of binding sites. E Box plot of normalized probe intensity signals from microarray gene expression data of each sample group for the transferrin receptor (TFRC gene; 207332_s_at). F Chromatin modifications within the TFRC gene in K562 cells using ChIP-Seq data. Dotted lines on plot indicate the transcription start site (0 bp), the promoter region (2000 bp either side of TSS) and the transcription termination site (far left). G Comparison of chromatin modifications, H3K4me1 and H3K4me3, within the TFRC gene in K562 cells and normal PBMC
At the gene expression level, the normalized signal intensity for a TFRC gene probe was elevated in K562 cells and primary CML samples, compared to PBMC (Fig. 4E). A signal increase may also be observed in normal CD34 + cells relative to PBMC. To assess the post-translational histone modifications associated with the increased abundance of the TFRC gene in K562 cells, ChIP-Seq data were accessed from the ENCODE project and subsequently analyzed (Fig. 4F, G). As expected, the transcriptionally permissive modifications including acetylation (H3K27ac and H3K9ac) and methylation (H3K4me2 and H3K4me3) were observed within or in close proximity to the TSS (Fig. 4F). Of interest, the cell cycle-dependent H3K79me2 mark was also observed in the TFRC gene [43, 44]. Only baseline levels of H3K4me1, and the transcriptionally repressive H3K9me1 and H3K9me3 histone modifications were detected. In comparison to PBMC, a broader prevalence of the transcriptionally permissive H3K4me3 histone modification around the TSS was observed in K562 cells (Fig. 4G). Low levels of H3K4me1 were observed in both K562 cells and PBMC.
Sensitivity of K562 cells to doxorubicin and development of doxorubicin-resistant cells
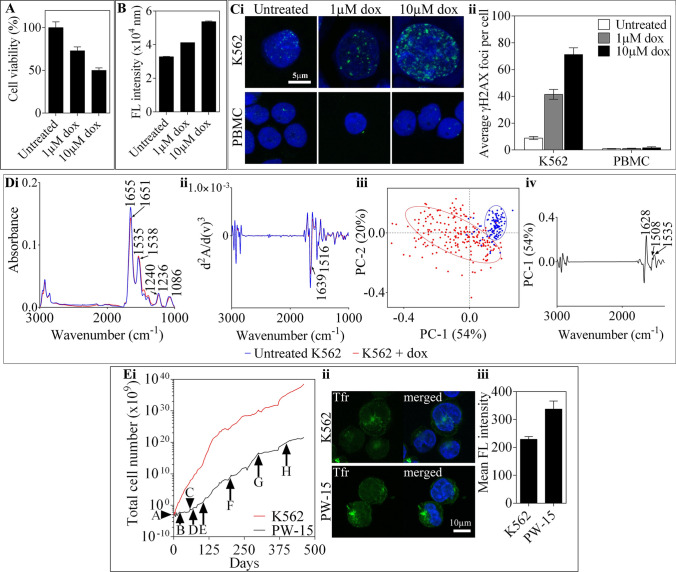
The anthracycline, doxorubicin is routinely used in the treatment of several cancers, with its cytotoxicity related to its capacity to intercalate into DNA and disrupt topoisomerase II, as well as generate free radicals [45, 46]. In this study, K562 cells were treated with 1 or 10 µM doxorubicin for 24 h leading to a dose-dependent reduction in cell viability, and an increase in caspase 3/7 apoptosis (Fig. 5A, B). Immunofluorescence staining for γH2AX was performed to assess the ability of doxorubicin to induce DNA-double strand lesions in K562 cells compared to normal PBMC (Fig. 5C). An increase in the average number of γH2AX foci per cell was observed in K562 cells, from 8.9 ± 1.6 foci per nucleus in the untreated cell to 41.5 ± 5.2 and 71.2 ± 7.1 foci following treatment with 1 µM and 10 µM doxorubicin, respectively. Of note, the accumulation of γH2AX foci in PBMC remained under 2 foci per nucleus for all experimental groups (Fig. 5C).
Fig. 5.
Overview of the biological effects of doxorubicin in K562 cells. A K562 cells treated with 1 and 10 µM doxorubicin for 24 h showed a dose-dependent reduction in cell viability measured using the Cell Titer® (Promega) assay kit (B) and increase in caspase 3/7 apoptosis measured using the Apo-one (Promega) assay kit. C K562 and PBMC cells treated with the indicated concentration of doxorubicin for 1 h, were washed and incubated in fresh media for a further 24 h before fixing and staining for sensitive marker of DNA-double strand breaks – γH2AX (green) and DAPI nuclear stain (blue) (i). The average number of γH2AX foci per cells were quantified in Image J and represented by the mean ± SD of five independent experiments (ii). D Representative FTIR absorbance spectra and PCA analysis from data collected of the K562 cell line following treatment with doxorubicin, in comparison to an untreated control. The averaged vector-normalised spectra calculated from 60 to 150 single K562 cells collected from each experimental group with variance in the major amide and DNA bands labelled (i). The second-order derivatives were also calculated from the corresponding spectra by a Savitzky-Golay algorithm (17 smoothing points) with changes in intensity and frequency labelled, including those in the protein and DNA regions (ii). PC-1/PC-2 scores plot with a largely delineated clustering of the experimental cell sets along PC-1 (iii). The PC-1 loadings plot labelled with major spectral components found to contribute to the variance observed in spectra corresponding to untreated or treated K562 cells (iv). E A doxorubicin-resistant K562 cell line denoted PW-15 was derived from the doxorubicin-sensitive cells by incremental exposure to doxorubicin over a period of 461 days (A—day 0, 2.6 µM, B—day 21, 3.5 µM, C—day 57, 4.3 µM, D—day 68, 5.2 µM, E—day 112, 6 µM, F—day 203, 6.9 µM, G—day 300, 7.8 µM, H—day 404, 8.6 µM). Cells were maintained at a drug concentration until their growth rate approached that of the untreated doxorubicin sensitive K562 cell line (i). The fluorescence intensity of the transferin receptor (Tfr) of PW-15 cells compared to K562 cells was imaged (ii) and quantified in Image J (iii) and showed expression of the Tfr remains a reliable target in the resistant cell line
Calculation of the average FTIR spectra and corresponding second derivative acquired from single K562 cells treated with 1 µM doxorubicin for 1 h, resulted in an observed variance within the protein and DNA regions in comparison to the untreated control (Fig. 5Di-ii). Specifically, a shift in both amide I and amide II bands to lower frequencies, as well changes in the frequency and intensity of the phosphate bands at ~ 1240 cm−1 and 1086 cm−1 were observed [25, 26]. A delineated clustering observed in the PCA scores plot between the experimental sets along PC-1 (54%) confirmed the above results (Fig. 5Diii-iv). Previously, calculation of the integrated ratio of the amide I and amide II bands has been suggested to serve as a marker for a change in DNA concentration [25, 47]. In this case, a significant (p < 0.0001) decrease of this ratio in treated K562 cells (1.9 ± 0.1) in comparison to the untreated control (2.1 ± 0.1) was recorded.
Due to the ability of K562 cells to acquire resistance to conventional chemotherapies such as doxorubicin, the ability to augment their response and regain sensitivity using other modalities has been implicated [48, 49]. Development of doxorubicin-resistant PW-15 cells by incremental exposure to doxorubicin (1.5–5 µg/mL) was conducted over a period of 461 days (Fig. 5E). Despite the acquisition of doxorubicin resistance with increasing concentrations of doxorubicin, a high expression of TfR was observed in PW-15 cells compared to the doxorubicin-sensitive K562 cell line.
Expression of HDAC enzymes and cytotoxicity of the HDACi vorinostat
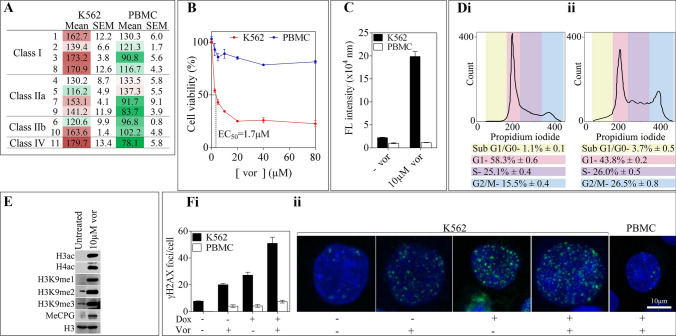
Immunofluorescence staining was used to investigate the expression of the eleven metal-dependent HDAC enzymes in K562 cells compared to normal PBMCs (Fig. 6A). Overall class I and class IV HDACs were shown to be relatively overexpressed in K562 cells. Further, K562 cells were incubated with the broad-spectrum HDACi, vorinostat to investigate its biological effects. It was determined that vorinostat has an EC50 of 1.7 µM in K562 cells (Fig. 6B). K562 cells treated with 10 µM vorinostat for 24 h resulted in significant caspase 3/7 apoptosis, with no effects observed in PBMC (Fig. 6C). Cell cycle was also examined using flow cytometry in K562 cells, with treatment resulting in G2/M cell cycle arrest (Fig. 6D). Immunoblot analysis of treated K562 cells demonstrated increased H3ac and H4ac, as well as increased H3K9me1, H3K9me2, and H3K9me3 (Fig. 6E). An increase in DNA methyl-CpG islands was also observed following treatment.
Fig. 6.
Expression of histone deacetylase enzymes and the cytotoxic effects of vorinostat in K562 and PBMC cells. A Histone deacetylase enzyme expression in K562 vs. PBMC cells was measured by immunofluorescence staining (images not shown) and quantified using Image J. The mean total fluorescence intensity represented as a heat map of 3 independent experiments is shown of all metal-dependent HDAC1-11 enzymes relative to each other and in both cell lines; green = less expression, red = greater expression. B Percentage of relative cell viability and C caspase 3/7 apoptosis in K562 and PBMC cells treated with the indicated concentrations of vorinostat for 24 h. D Vorinostat causes K562 cells to arrest in G2/M phase of the cell cycle. Cells treated with (ii) or without (i) 10 µM vorinostat for 24 h were fixed and stained with propidium iodide and measured using FACs. E 10 µM vorinostat causes hyperacetylation and hypermethylation of histones and methy-CpG islands in K562 cells. F Vorinostat augments doxorubicin -induced γH2AX foci in K562 cells but not PBMC cells
To assess the ability of vorinostat to augment the cellular response to doxorubicin in K562 cells and normal PBMC, immunofluorescence staining for γH2AX was utilized. Both cell types were treated with 10 µM vorinostat for 24 h prior to their 1 h incubation with 1 µM doxorubicin. This was followed by further incubation in fresh media, which resulted in the finding that the combinatorial effect was greatest in K562 cells with 51.0 ± 4.6 foci per nucleus in comparison to PBMC with 7.4 ± 1.0 (Fig. 6F). The accumulation of γH2AX foci per nucleus in PBMC was consistent in each treatment group. Interestingly, the number of γH2AX foci per nucleus was greater in K562 cells treated with the combinatorial treatment compared to the untreated control, or either treatment alone.
Discussion
Overall, this study represents an updated and comprehensive reference of human erythroleukemic K562 cells. The fundamental growth and biological characteristics were confirmed by analyzing the morphology, cell cycle, Synchrotron FTIR spectral profile, and metabolism of K562 cells (Figs. S1 and S2). Moreover, karyotype analysis indicates the presence of novel chromosomes in K562 cells, which are most likely artefacts of long-term culture (Fig. 2).
While K562 cells remain an important cell culture model for investigating various aspects of CML, the role of cancer-initiating stem cells in driving cancer progression requires further investigation. Therefore, exploring the biological and molecular properties of CD34 + CML-initiating cells and their response to conventional and experimental therapeutics has become increasingly common [50]. Detailed analysis of microarray data has highlighted the genetic differences between patient-derived CD34 + CML cells and K562 cells (Fig. 3). With a move towards molecularly targeted therapeutics and personalized medicine, the karyotypic evolution and genetic drift of K562 cells, validates the emerging interest in preclinical studies using patient-derived cells [51].
The dimeric transferrin receptor (TfR) is critical in the uptake of ferric iron (Fe2+)-bound transferrin peptide [52]. The main mechanism involved in the intracellular uptake of iron mediated by the transferrin-transferrin receptor complex, is clathrin-coated endocytosis (Fig. 4) [53–55]. The TfR is ubiquitously expressed on a variety of normal tissue, with the number and distribution varying depending on multiple factors [56]. In line with previous findings, the TFRC gene was found to be highly expressed in K562 cells and primary CML samples compared to PBMC. To gain further insight into the potential epigenetic regulation of TFRC, post-translational histone modifications were evaluated. In K562 cells, transcriptionally permissive modifications (H3K27ac, H3K9ac, H3K4me2, H3K4me3) were detected within or in close proximity to the TSS, while baseline levels of transcriptionally repressive histone modifications were observed (H3K9me1, H3K9me3).
In patients with CML, the development of drug resistance results in disease progression and a poor treatment response [57]. Using a long-term experiment with sequential increasing of the concentration of doxorubicin for a period of over one year, we have highlighted the issue of resistance, which remains a major clinical problem [57]. Due to the doxorubicin-resistant PW-15 cells displaying a high expression of TfR compared to doxorubicin-sensitive K562 cells, targeting via transferrin receptor-mediated endocytosis remains a viable option following resistance to chemotherapy (Fig. 5). Given the progress in transferrin receptor targeting and the promising recent clinical trial results using nanoparticle formulations, there is optimism for this therapeutic approach [58, 59].
Targeting the c-ABL tyrosine kinase with imatinib-mesylate has been transformational in the treatment of CML however resistance, at least in some cases, is an important issue [60]. Several BCR-ABL dependent and independent mechanisms of resistance to imatinib have been proposed including overexpression of the BCR-ABL or its protein product, point mutations, and overexpression of multidrug-resistant P-glycoprotein [61]. Experimental findings indicate that the use of HDACi may be particularly beneficial for the treatment of imatinib-mesylate-resistant CML [62]. We have verified the chromatin modifications and preferential chemotoxicity of the FDA-approved HDACi, vorinostat, in K562 cells compared to normal PBMCs highlighting the potential of this strategy (Fig. 6).
Moreover, the combinatorial effect of vorinostat and doxorubicin was found to be greatest in K562 cells compared to PBMC (Fig. 6). The toxicity, tolerability, and dosing of vorinostat in combination with doxorubicin has previously been investigated in clinical trials [63]. Studies have also investigated the potential of vorinostat to enhance the activity of compounds such as MK-0457, which is an aurora kinase inhibitor, and dasatinib in imatinib-mesylate sensitive and resistant CML cells [64, 65].
Conclusion
In summary, we have characterized the karyotype and gene expression profile of K562 cells in comparison to normal PBMC and patient-derived CML cells. We define novel chromosomes and highlight a genetic drift of K562 cells in prolonged culture compared to patient-derived cells. Nevertheless, cultured K562 cells maintain the classical Philadelphia chromosome, overexpress TfR and HDAC enzymes, which are important targets for potential therapeutics. In this context, high expression of TfR expression persists in doxorubicin-resistant K562 cells, allowing for the possibility of targeting cytotoxic therapies by receptor-mediated endocytosis. Similarly, K562 cells are susceptible to the effects of HDACi, as indicated by the preferential cytotoxicity of vorinostat compared to normal PBMC. Finally, K562 cells remain one of the most used cell lines in CML research, which is critical for cases where patients are resistant to classical therapy with the tyrosine kinase inhibitor, imatinib-mesylate.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge the use of the facilities provided by Monash Micro Imaging at AMREP and particularly, the expert assistance from Drs Stephen Cody and Iśka Carmichael. Also we acknowledge the technical expertise of Dr. Clement Khaw (Singapore Bioimaging Consortium-Nikon Imaging Centre) for N-SIM super resolution imaging and Dr Darren Henstrigde (Cellular and Molecular metabolism, Baker Heart and Diabetes Institute) for assistance with the Seahorse XF96 extracellular flux analyzer. M.W. was supported by the Leukaemia Foundation of Australia. EP is supported by an Australian Government Research Training Program Scholarship. We thank the National Computing Infrastructure (NCI), and the Pawsey Supercomputing Centre in Australia (funded by the Australian Government). Further, we thank the Spartan High Performance Computing service (University of Melbourne), and the Partnership for Advanced Computing in Europe (PRACE) for awarding the access to Piz Daint, hosted at the Swiss National Supercomputing Centre (CSCS), Switzerland. Supported in part by the Victorian Government’s Operational Infrastructure Support Program.
Author contributions
TCK, AH, and AE conceptualized the aims and methodology, were involved in supervision, and production of the first draft of the manuscript. MW, KV, EP, SMT, PAW, HR, IK, SSM, AH, and JV generated data, performed data analysis, curated data, and produced the first draft of the manuscript. All authors contributed to editing and reviewing the manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
Publically-accessible datasets were analyzed in this study and the accession codes have been provided.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Human peripheral blood mononuclear cells (PBMC) were fractionated using the Ficoll Plaque (GE Healthcare, Wauwatosa, Wisconsin, USA) method from buffy coat obtained from the Australian Red Cross Blood Bank (ARCB) under ethics approval (#304/12) and informed consent was obtained from the donors. Cells were maintained in complete-RPMI-1640 medium supplemented with 10% FBS, 2 mM l-glutamine and 1% penicillin/streptomycin at 37 °C, 5% (v/v) CO2.
Consent to participate
Informed consent was obtained from all donors.
Consent for publication
Informed consent was obtained from study participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nowell PC. A minute chromosome in human granulocytic leukemia. Science. 1960;132:1497–1501. [Google Scholar]
- 2.Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45(3):321–334. [PubMed] [Google Scholar]
- 3.Lee SM, Bae JH, Kim MJ, et al. Bcr-Abl-independent imatinib-resistant K562 cells show aberrant protein acetylation and increased sensitivity to histone deacetylase inhibitors. J Pharmacol Exp Ther. 2007;322(3):1084–1092. doi: 10.1124/jpet.107.124461. [DOI] [PubMed] [Google Scholar]
- 4.Zhang B, Strauss AC, Chu S, et al. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell. 2010;17(5):427–442. doi: 10.1016/j.ccr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 6.Bixby D, Talpaz M. Seeking the causes and solutions to imatinib-resistance in chronic myeloid leukemia. Leukemia. 2011;25(1):7–22. doi: 10.1038/leu.2010.238. [DOI] [PubMed] [Google Scholar]
- 7.Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7(5):345–356. doi: 10.1038/nrc2126. [DOI] [PubMed] [Google Scholar]
- 8.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 9.Lozzio CB, Lozzio BB, Machado EA, Fuhr JE, Lair SV, Bamberger EG. Effects of sodium butyrate on human chronic myelogenous leukaemia cell line K562. Nature. 1979;281:709–710. doi: 10.1038/281709b0. [DOI] [PubMed] [Google Scholar]
- 10.Nimmanapalli R, Fuino L, Stobaugh C, Richon V, Bhalla K. Cotreatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) enhances imatinib-induced apoptosis of Bcr-Abl–positive human acute leukemia cells. Blood. 2003;101(8):3236–3239. doi: 10.1182/blood-2002-08-2675. [DOI] [PubMed] [Google Scholar]
- 11.Klausner RD, Ashwell G, Van Renswoude J, Harford JB, Bridges KR. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci. 1983;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Renswoude J, Bridges KR, Harford JB, Klausner RD. Receptor-mediated endocytosis of transferrin and the uptake of Fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci. 1982;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karagiannis TC, Lobachevsky PN, Leung BK, White JM, Martin RF. Receptor-mediated DNA-targeted photoimmunotherapy. Can Res. 2006;66(21):10548–10552. doi: 10.1158/0008-5472.CAN-06-1853. [DOI] [PubMed] [Google Scholar]
- 14.Davis ME, Zuckerman JE, Choi CH, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Absher M. Hemocytometer counting. Tissue culture: methods and applications. New York: Academic Press; 1973. pp. 395–397. [Google Scholar]
- 16.Rafehi H, Smith AJ, Balcerczyk A, et al. Investigation into the biological properties of the olive polyphenol, hydroxytyrosol: mechanistic insights by genome-wide mRNA-Seq analysis. Genes Nutr. 2012;7(2):343–355. doi: 10.1007/s12263-011-0249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang A, Team AP (2014) Functional genomics data and expression look-up tools: array express and expression atlas
- 18.Lukk M, Kapushesky M, Nikkilä J, et al. A global map of human gene expression. Nat Biotechnol. 2010;28(4):322–324. doi: 10.1038/nbt0410-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu M, Neilson A, Swift AL, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292(1):C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- 20.Mah L-J, Vasireddy RS, Tang MM, Georgiadis GT, El-Osta A, Karagiannis TC. Quantification of γH2AX Foci in Response to Ionising Radiation. J Visualiz Exp. 2010 doi: 10.3791/1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ververis K, Rodd AL, Tang MM, El-Osta A, Karagiannis TC. Histone deacetylase inhibitors augment doxorubicin-induced DNA damage in cardiomyocytes. Cell Mol Life Sci. 2011;68(24):4101–4114. doi: 10.1007/s00018-011-0727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson LC, Nilsson K, Gahmberg CG. K562—a human erythroleukemic cell line. Int J Cancer. 1979;23(2):143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- 23.Sutherland JA, Turner AR, Mannoni P, McGann LE, Turc JM. Differentiation of K562 leukemia cells along erythroid, macrophage, and megakaryocyte lineages. J Biol Response Mod. 1986;5(3):250–262. [PubMed] [Google Scholar]
- 24.Baliga BS, Mankad M, Shah AK, Mankad VN. Mechanism of differentiation of human erythroleukaemic cell line K562 by hemin. Cell Prolif. 1993;26(6):519–529. doi: 10.1111/j.1365-2184.1993.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 25.Malek K, Wood BR, Bambery KR. FTIR imaging of tissues: techniques and methods of analysis. Optical spectroscopy and computational methods in biology and medicine. Springer; 2014. pp. 419–473. [Google Scholar]
- 26.Movasaghi Z, Rehman S, ur Rehman DI. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl Spectrosc Rev. 2008;43(2):134–179. [Google Scholar]
- 27.Bellisola G, Della Peruta M, Vezzalini M, et al. Tracking infrared signatures of drugs in cancer cells by Fourier transform microspectroscopy. Analyst. 2010;135(12):3077–3086. doi: 10.1039/c0an00509f. [DOI] [PubMed] [Google Scholar]
- 28.Chen TR. Modal karyotype of human leukemia cell line, K562 (ATCC CCL 243) Cancer Genet Cytogenet. 1985;17(1):55–60. doi: 10.1016/0165-4608(85)90101-3. [DOI] [PubMed] [Google Scholar]
- 29.Gribble SM, Roberts I, Grace C, Andrews KM, Green AR, Nacheva EP. Cytogenetics of the chronic myeloid leukemia-derived cell line K562: karyotype clarification by multicolor fluorescence in situ hybridization, comparative genomic hybridization, and locus-specific fluorescence in situ hybridization. Cancer Genet Cytogenet. 2000;118(1):1–8. doi: 10.1016/s0165-4608(99)00169-7. [DOI] [PubMed] [Google Scholar]
- 30.Naumann S, Reutzel D, Speicher M, Decker HJ. Complete karyotype characterization of the K562 cell line by combined application of G-banding, multiplex-fluorescence in situ hybridization, fluorescence in situ hybridization, and comparative genomic hybridization. Leuk Res. 2001;25(4):313–322. doi: 10.1016/s0145-2126(00)00125-9. [DOI] [PubMed] [Google Scholar]
- 31.Andersson LC, Jokinen M, Gahmberg CG, Klein E, Klein G, Nilsson K. Presence of erythrocytic components in the K562 cell line. Int J Cancer. 1979;24(4):514. doi: 10.1002/ijc.2910240422. [DOI] [PubMed] [Google Scholar]
- 32.Cudkowicz A, Klausner R, Bridges K. Regulation of the transferrin receptor in K562 erythroleukemia cells. Prog Clin Biol Res. 1983;165:509–519. [PubMed] [Google Scholar]
- 33.Leibowitz D, Cubbon R, Bank A. Increased expression of a novel c-abl-related RNA in K562 cells. Blood. 1985;65(3):526–529. [PubMed] [Google Scholar]
- 34.Maziarz RT, Burakoff SJ, Faller DV. The regulation of exogenous and endogenous class I MHC genes in a human tumor cell line, K562. Mol Immunol. 1990;27(2):135–142. doi: 10.1016/0161-5890(90)90108-c. [DOI] [PubMed] [Google Scholar]
- 35.Charnay P, Maniatis T. Transcriptional regulation of globin gene expression in the human erythroid cell line K562. Science. 1983;220(4603):1281–1283. doi: 10.1126/science.6574602. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic acids Res. 2011;40:gkr988. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Consortium GO The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004;32(suppl 1):D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.del Senno L, Barbieri R, Amelotti F, et al. Methylation and expression of c-myc and c-abl oncogenes in human leukemic K562 cells before and after treatment with 5-azacytidine. Cancer Detect Prev. 1985;9(1–2):9–15. [PubMed] [Google Scholar]
- 40.Tortorella SM, Hung A, Karagiannis TC. The implication of cancer progenitor cells and the role of epigenetics in the development of novel therapeutic strategies for chronic myeloid leukemia. Antioxid Redox Signal. 2015;22(16):1425–1462. doi: 10.1089/ars.2014.6096. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton TA, Wada HG, Sussman HH. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci. 1979;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scatchard G. The attractions of proteins for small molecules and ions. Ann N Y Acad Sci. 1949;51(4):660–672. [Google Scholar]
- 43.Fatemiyan N, Davie JR. Broad histone H4 monomethylation marks expressed genes involved in translation. Genome. 2023 doi: 10.1139/gen-2023-0011. [DOI] [PubMed] [Google Scholar]
- 44.Sharma P, Sattarifard H, Fatemiyan N, Lakowski TM, Davie JR. Bioinformatic analyses of Broad H3K79me2 domains in different Leukemia cell line data sets. Cells. 2022 doi: 10.3390/cells11182830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gewirtz D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 46.Praet M, Stryckmans P, Ruysschaert J-M. Cellular uptake, cytotoxicity, and transport kinetics of anthracyclines in human sensitive and multidrug-resistant K562 cells. Biochem Pharmacol. 1996;51(10):1341–1348. doi: 10.1016/0006-2952(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 47.Benedetti E, Bramanti E, Papineschi F, Rossi I, Benedetti E. Determination of the relative amount of nucleic acids and proteins in leukemic and normal lymphocytes by means of Fourier transform infrared microspectroscopy. Appl Spectrosc. 1997;51(6):792–797. [Google Scholar]
- 48.Tsuruo T, Iida H, Nojiri M, Tsukagoshi S, Sakurai Y. Circumvention of vincristine and adriamycin resistance in vitro and in vivo by calcium influx blockers. Can Res. 1983;43(6):2905–2910. [PubMed] [Google Scholar]
- 49.Chen B, Sun Q, Wang X, et al. Reversal in multidrug resistance by magnetic nanoparticle of Fe3O4 loaded with adriamycin and tetrandrine in K562/A02 leukemic cells. Int J Nanomed. 2008;3(2):277. doi: 10.2147/ijn.s2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diaz-Blanco E, Bruns I, Neumann F, et al. Molecular signature of CD34(+) hematopoietic stem and progenitor cells of patients with CML in chronic phase. Leukemia. 2007;21(3):494–504. doi: 10.1038/sj.leu.2404549. [DOI] [PubMed] [Google Scholar]
- 51.Clarke CJ, Holyoake TL. Preclinical approaches in chronic myeloid leukemia: from cells to systems. Exp Hematol. 2017;47:13–23. doi: 10.1016/j.exphem.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West AP, Bennett MJ, Sellers VM, Andrews NC, Enns CA, Bjorkman PJ. Comparison of the interactions of transferrin receptor and transferrin receptor 2 with transferrin and the hereditary hemochromatosis protein HFE. J Biol Chem. 2000;275(49):38135–38138. doi: 10.1074/jbc.C000664200. [DOI] [PubMed] [Google Scholar]
- 53.Mayle KM, Le AM, Kamei DT. The intracellular trafficking pathway of transferrin. Biochimica et Biophysica Acta (BBA)-General Subjects. 2012;1820(3):264–281. doi: 10.1016/j.bbagen.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luck AN, Mason AB. Transferrin-mediated cellular iron delivery. Curr Top Membr. 2011;69:3–35. doi: 10.1016/B978-0-12-394390-3.00001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steere AN, Byrne SL, Chasteen ND, Mason AB. Kinetics of iron release from transferrin bound to the transferrin receptor at endosomal pH. Biochimica et Biophysica Acta (BBA)-General Subjects. 2012;1820(3):326–333. doi: 10.1016/j.bbagen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gatter KC, Brown G, Trowbridge I, Woolston R, Mason D. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983;36(5):539–545. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poudel G, Tolland MG, Hughes TP, Pagani IS. Mechanisms of resistance and implications for treatment strategies in chronic myeloid Leukaemia. Cancers (Basel) 2022 doi: 10.3390/cancers14143300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang HW, Ma KL, Liu H, Zhou JY. Reversal of multidrug resistance in leukemia cells using a transferrin-modified nanomicelle encapsulating both doxorubicin and psoralen. Aging (Albany NY) 2020;12(7):6018–6029. doi: 10.18632/aging.102992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding W, Guo L. Immobilized transferrin Fe3O4@SiO2 nanoparticle with high doxorubicin loading for dual-targeted tumor drug delivery. Int J Nanomed. 2013;8:4631–4639. doi: 10.2147/ijn.S51745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan YL, Zeng SX, Hao RR, Liang MH, Shen ZR, Huang WH. The progress of small-molecules and degraders against BCR-ABL for the treatment of CML. Eur J Med Chem. 2022;238:114442. doi: 10.1016/j.ejmech.2022.114442. [DOI] [PubMed] [Google Scholar]
- 61.Jabbour E, Parikh SA, Kantarjian H, Cortes J. Chronic myeloid leukemia: mechanisms of resistance and treatment. Hematol Oncol Clin North Am. 2011;25(5):981–995. doi: 10.1016/j.hoc.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lernoux M, Schnekenburger M, Dicato M, Diederich M. Epigenetic mechanisms underlying the therapeutic effects of HDAC inhibitors in chronic myeloid leukemia. Biochem Pharmacol. 2020;173:113698. doi: 10.1016/j.bcp.2019.113698. [DOI] [PubMed] [Google Scholar]
- 63.Munster PN, Marchion D, Thomas S, et al. Phase I trial of vorinostat and doxorubicin in solid tumours: histone deacetylase 2 expression as a predictive marker. Br J Cancer. 2009;101(7):1044–1050. doi: 10.1038/sj.bjc.6605293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dai Y, Chen S, Venditti CA, et al. Vorinostat synergistically potentiates MK-0457 lethality in chronic myelogenous leukemia cells sensitive and resistant to imatinib mesylate. Blood. 2008;112(3):793–804. doi: 10.1182/blood-2007-10-116376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiskus W, Pranpat M, Balasis M, et al. Cotreatment with vorinostat (suberoylanilide hydroxamic acid) enhances activity of dasatinib (BMS-354825) against imatinib mesylate-sensitive or imatinib mesylate-resistant chronic myelogenous leukemia cells. Clin Cancer Res. 2006;12(19):5869–5878. doi: 10.1158/1078-0432.Ccr-06-0980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publically-accessible datasets were analyzed in this study and the accession codes have been provided.