Abstract
Background
Birth asphyxia is of significant concern because it impacts newborn health from low to severe levels. In Thailand, birth asphyxia remains a leading cause of delayed developmental health in children under 5 years old. The study aimed to determine the maternal, fetal and health service factors contributing to birth asphyxia.
Methods
A case–control design was conducted on a sample of 4256 intrapartum chart records. The samples were selected based on their Apgar scores in the first minute of life. A low Apgar score (≤ 7) was chosen for the case group (852) and a high Apgar score (> 7) for the control group (3408). In addition, a systematic random technique was performed to select 23 hospitals, including university, advanced and secondary, in eight health administration areas in Thailand for evaluating the intrapartum care service. Data analysis was conducted using SPSS statistical software.
Results
The odds of birth asphyxia increases in the university and advanced hospitals but the university hospitals had the highest quality of care. The advanced and secondary hospitals had average nurse work-hours per week of more than 40 h. Multivariable logistic regression analysis found that intrapartum care services and maternal–fetal factors contributed to birth asphyxia. The odd of birth asphyxia increases significantly in late–preterm, late–term pregnancies, low-birth weight, and macrosomia. Furthermore, maternal comorbidity, non-reassuring, and obstetric emergency conditions significantly increase the odd of birth asphyxia. In addition, an excellent quality of intrapartum care, a combined nursing model, low nurse work-hours, and obstetrician-conducted delivery significantly reduced birth asphyxia.
Conclusion
Birth asphyxia problems may be resolved in the health service management offered by reducing the nurse work-hours. Excellent quality of care required the primary nursing care model combined with a team nursing care model. However, careful evaluation and monitoring are needed in cases of comorbidity, late–preterm, late–term pregnancies, low-birth weight, and macrosomia. Furthermore, increasing the obstetrician availability in obstetric emergencies and non-reassuring fetal status is important.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-023-05885-y.
Keywords: Birth asphyxia, Intrapartum care service, Quality of care
Introduction
Birth asphyxia is the failure to initiate and sustain spontaneous breathing at birth, causing permanent brain cell damage and threatening the newborn's life. The recovery process requires lengthy hospital observation and intensive care, and may take a lifetime [1–4]. Birth asphyxia is still the leading cause of neonatal morbidity and mortality and remains a significant cause of delayed developmental health in children under 5 years old worldwide. Previous studies reported that maternal–fetal factors affected birth asphyxia [5, 6]. In addition, the appropriate health care service components, such as human resource allocation, specialist availability, competency improvement and an experienced provider contributing to care quality, also impact neonatal outcomes [7]. Therefore, identifying the intrapartum health service factors and maternal–fetal factors contributing to birth asphyxia in Thailand will help to improve the quality of intrapartum care and save neonatal lives.
Birth asphyxia is a significant concern in Thailand due to its higher birth rate (16.0 per 1000 live births in 2021) compared to developed countries (1.5 per 1000 live births) [8, 9]. The range of birth asphyxia rates varies across hospitals in Thailand according to the different levels of hospital. According to health statistics in 2017, the average number of birth asphyxia cases was 40.64 per 1000 live births in advanced hospitals, 41.68 per 1000 live births in university hospitals and 13.42 per 1000 live births in secondary hospitals [10]. These figures show that different hospital types might have different associated factors that affect birth outcomes.
Previous studies reported that maternal–fetal factors affect birth asphyxia such as maternal age, BMI, gestational age, ANC visit, maternal health and obstetric complications [8, 11–14]. Furthermore, the literature reviews reported that healthcare service factors are associated with maternal and neonatal health outcomes such as unequally healthcare providers allocation, nurse staffing, nurse workload, nurse work-hours, nurse-to-patient ratio, and provider performance improvement [15–22]. The appropriate health service resources provide quality intrapartum care. The quality of care during the intrapartum period is crucial to reducing neonatal morbidity and mortality. The two main processes of intrapartum care are the initial evaluation and risk screening and also intrapartum monitoring and care. The initial evaluation and risk screening includes the main strategies recommended for intrapartum practice guidelines during admission. Intrapartum monitoring and care includes labour progress monitoring by using the partograph and close monitoring of the fetal heart rate. All intrapartum women should receive immediate care to reduce the chance of complications during delivery, such as encouraging an upright position, lying on the left side and relaxation. Furthermore, the appropriate duration of expulsion management and an upright position during the second stage provided a positive outcome. The team activated immediately after detecting a severe non-reassuring fetus would resolve the problem of severe hypoxic-ischaemic encephalopathy or perinatal death [23–25]. Timely and appropriate intervention by the in-utero resuscitation technique during non-reassuring fetal status includes changing maternal position, oxygen administration, uterine relaxation and an intravenous fluid bolus to reduce severe hypoxia [23, 26–28].
Moreover, regarding the model of intrapartum care, literature reviews indicated that continuing care or the primary nursing care model was beneficial to perinatal outcomes and reduced neonatal death rates [29–33]. Although the primary nursing care model has better outcomes than the team nursing care model, most labour and delivery care units in Thailand employ the team nursing model or a combined primary nursing care and team model due to provider shortage. However, some reports showed that the team nursing model left tasks undone and decreased patient safety and care quality. In addition to the care model, a professional healthcare team of labour and delivery is also important.
Despite a growing research interest in human resources and health outcomes, there is still a lack of evidence on the effects of healthcare service factors on birth asphyxia. Most research has focused on maternal and fetal factors, with less attention given to hospital factors and birth asphyxia. Therefore, this case–control study was aimed at determining intrapartum health services and maternal–fetal factors that contribute to birth asphyxia at three levels of hospital: university, advanced and secondary. In addition, it is hoped that the research findings will serve as an evidence base for developing national strategic proposals for improving maternal and fetal health outcomes and solving the disparity among intrapartum health services in Thailand.
Methods
The case–control study was designed to collect data from 4256 intrapartum care charts recorded in 23 hospitals in Thailand from 2016 to 2017. Pregnant women of gestational age 34+0–41+6 weeks, admitted with signs of labour onset and delivered with a low Apgar score (≤ 7) at the first minute of life, were selected as the case group (852) and those with an Apgar score of > 7 as the control group (3408). Month and type of delivery were matched in the case and control groups. Pregnant women with twin or congenitally abnormal fetuses were excluded.
The sample size was determined from the study by Berazategui et al. [34]. The number of antenatal care visits was selected as a variable and calculated in the SMART program. For a two-sided test with a 5% type I error, the study required a sample size of at least 852 to make this comparison with 80% power. Therefore, the research proportion of case and control samples was 1 to 4 [35]. The overall research sample size was 4260 when the number of control samples was 3408.
The number of estimated hospitals required for collecting data was based on a multilevel research design. The standardized proportion difference as an effect measure was applied to calculate the number of research settings needed to conduct the research [36].
where.
d = proportion difference as an effect size
p 0 = proportion of birth asphyxia in secondary hospitals (0.011)
p 1 = proportion of birth asphyxia in primary hospitals (0.0068)
σpooled = pooled standard error of birth asphyxia
n 0 = number of cases of birth asphyxia in secondary hospitals (7231)
n 1 = number of cases of birth asphyxia in primary hospitals (6433)
Z α/2 = percentile at (1 – α/2)100% of standard normal for two-sided t-test with α level of significance (i.e. Z0.025 = 1.96)
Zβ = percentile at (1 – β) 100% of standard normal for power of test with 1 – β (i.e. Z0.2 = 0.84)
ρ = intraclass correlation of birth asphyxia with hospitals (pre-setting value)
ni = sample size average per hospital of birth asphyxia (pre-setting value)
nj = hospital size estimates
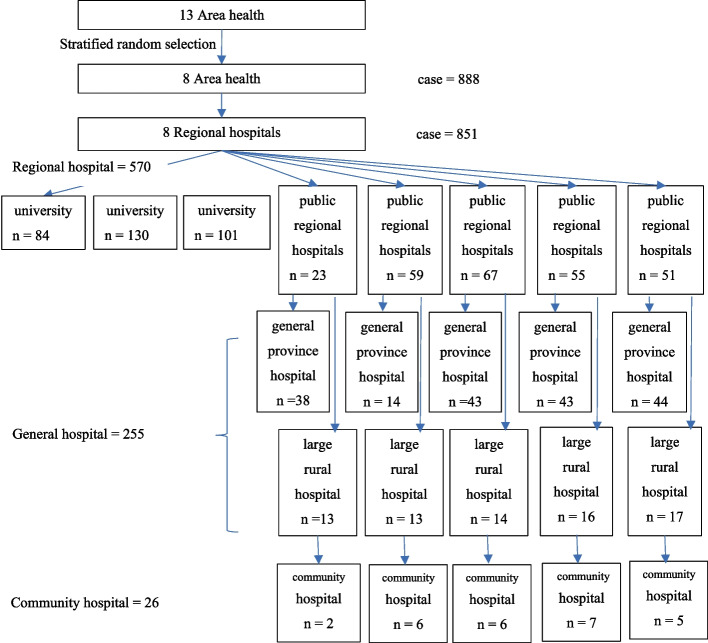
The multilevel study by Ensing et al. [13] was applied to estimate an adequate number of hospitals. According to the formula, the number of research settings (nj) equals 23 hospitals. A Systematic random sampling technique was used to select the research setting (Fig. 1).
Fig. 1.
The number and type of hospital settings for data collection
Instruments
The Intrapartum Care Record Form (Supplementary I) collected the intrapartum care quality data via medical chart review. The record form was modified from the Fistula Care Monitoring Tool for Partograph Review of the US Agency for International Development and from the Assessment Tool for the Quality of Hospital Care for Mothers and Newborn Babies of the World Health Organization (2009) [37, 38]. The modified instrument had 12 indicators with 28 items. The score of intrapartum care for pregnant women with reassuring fetuses was 0–30 points and for pregnant women with non-reassuring fetuses was 1–46 points. Therefore, each condition was summed and weighted to 100%. The data were interpreted as four levels: 100%, excellent care quality; 80–99%, good care quality; 50–79%, fair care quality; and 2–49%, poor care quality.
The Asphyxia Risk Factors Record Form (Supplementary II) was used to extract the maternal and fetal risk factor data. The form was modified from the Risk Factors Questionnaire by Aslam et al. (2014) [14]. The modified form comprised 22 Yes/No questions that were separated into three parts: antepartum risk factors (Items 1–9), intrapartum risk factors (Items 10–16) and fetal risk factors (Items 17–22).
The Intrapartum Health Care System Questionnaire (Supplementary III) was modified from the World Health Organization’s Safe Motherhood Needs Assessment Instrument, version 1.1 [39]. The modified questionnaire contained 12 items to collect health services data, the number of pregnant women served by this facility, deliveries per year, high-risk pregnancy, maternity beds, intrapartum healthcare providers and nurse-midwife training. The four additional items include nurse allocation, nurse experience, nurse work-hours and number of cases of birth asphyxia. In addition, all instruments were tested for content validity regarding linguistics, objectivity and comprehensiveness.
Statistical analysis
Data analysis was conducted using the SPSS statistical software package (version 23). Frequencies and percentages were used to describe the maternal–fetal factors and health service structure. The intrapartum care quality data were calculated for frequency and rate. The intra-class correlation coefficient (ICC) tested for intrapartum health service level variance had a value of > 0.1 [35].
Based on the results of the ICC tests for this study, the ICC value was < 0.1, which indicates that there were no variations of hospital level. Nevertheless, the variations of hospital levels were already explained as the independent variables of research. Therefore, multivariable logistic regression analysis was used to estimate the odds ratio (OR) and 95% confidence interval (95% CI) for predictive factors associated with birth asphyxia. All independent variables were analysed using a univariable model to select the independent variables (presented as p < 0.05) with the entering method. Furthermore, model testing for predictive factors was performed by considering the assumption for statistics using multilevel logistic regression analysis. The two required assumptions were that there was no multicollinearity for each independent variable and that the variance–covariance matrices were equal [35].
Because all variables were coded on a dichotomous scale, there was no necessity to test with the two required assumptions. Thus, it was appropriate to analyse the multivariable logistic regression analysis.
Results
Overall, 23 hospitals delivered health services to 72,005 pregnant women (range 500–9117) during October 2017 to September 2018. The total rate of birth asphyxia was 38.19 per 1000 live births. The total rates of birth asphyxia in advanced hospitals (median = 52.91, range = 17.31–75.24/1000 live births) and university hospitals (median = 30.72, range = 19.40–99.14/1000 live births) were higher than in secondary hospitals (median = 18.87, range = 5.13–51.93/1000 live births). Furthermore, university hospitals had the highest rate of severe birth asphyxia (median = 3.41, range = 0.39–22.44/1000 live births), followed by advanced hospitals (median = 2.29, range = 0–3.39/1000 live birth). The highest number of non-delivered high-risk pregnancies was found in university hospitals (median = 21.93%, range = 1.23–55.70%). Furthermore, the caesarean section rate in university hospitals was the highest (median = 47.28%, range = 7.26–72.70%), followed by advanced hospitals (median = 44.02%, range = 6.03–41.56%; Table 1).
Table 1.
Number of intrapartum women, type of delivery and newborn health in the three levels of hospital
| Variable | Hospital level | |||
|---|---|---|---|---|
| University ( n = 3) | Advanced ( n = 5) | Secondary ( n = 15) | Total ( n = 23) | |
| Total number of intrapartum women | ||||
| - Total | 16,348 (22.77%) | 24,447 (33.95%) | 31,210 (43.34%) | 72,005 |
| - Range | 1468 (8.98%)–9117 (55.77%) | 2979 (12.19%)–6969 (28.51%) | 500 (1.60%)–4010 (12.85%) | 500 (0.69%)–9117 (12.66%) |
| Non-deliveries with high-risk conditions | ||||
| - Total | 5228 (31.98%) | 3,534 (14.46%) | 5888 (18.87%) | 14,650 (20.35%) |
| - Median | 21.94% | 12.44% | 15.27% | 14.34% |
| - Range | 18 (1.23%)–3210 (55.70%) | 50 (1.22%)–1678 (56.33%) | 0–1354 (59.94%) | 0–3210 (54.52%) |
| Deliveries | ||||
| - Total | 11,120 (19.39%) | 20,913 (36.46%) | 25,322 (44.15%) | 57,355 (79.65%) |
| - Median | 84.73% | 87.56% | 78.06% | 85.65% |
| - Range | 1013 (13.04%)–2728 (64.00%) | 1301 (6.22%)–6102 (29.18%) | 494 (1.95%)–3296 (13.26%) | 375 (1.20%) 4388 (14.05%) |
| Type of Delivery | ||||
| - Vaginal birth | ||||
| - Total | 5097 (16.32%) | 12,295 (39.37%) | 13,840 (44.34%) | 31,232 (54.45%) |
| - Median | 52.72% | 55.99% | 60.78% | 58.81% |
| - Range | 1013 (19.87%)–2738 (53.72%) | 781 (6.35%)–4388 (35.69%) | 375 (0%)–1733 (12.52%) | 375 (0%)–4388 (59.94%) |
| - Caesarean birth | ||||
| - Total | 6023 (23.06%) | 8618 (32.09%) | 11,482 (43.95%) | 26,123 (45.55%) |
| - Median | 47.28% | 44.01% | 39.22% | 41.19% |
| - Range | 437 (7.26%)–4379 (72.70%) | 520 (6.03%)–3582 (41.56%) | 102 (0.83%)–1988 (17.31%) | 102 (0.39%)–4379 (16.76%) |
| Newborn Health Outcomes | ||||
| - Live birth | ||||
| - Total | 12,404 (19.81%) | 22,742 (36.33%) | 27,459 (43.86%) | 62,605 |
| - Range | 1465 (11.81%)–7681 (61.92%) | 3004 (13.21%)–6067 (26.68%) | 390 (1.42%)–3445 (12.55%) | 390 (0.62%)–7681 (12.27%) |
| - Asphyxia /1000 live births (1st min) | ||||
| - Apgar ≤ 7 | ||||
| - Total | 517 (41.6/1000) | 1188 (52.24/1000) | 686 (24.98/1000) | 2391 (38.19/1000) |
| - Median | 30.72/1000 | 52.91/1000 | 18.87/1000 | 22.13 1000 |
| - Range | (19.40–99.14/1000) | (17.31–75.24/1000) | (5.13–51.93/1000) | (0.84–16.69/1000) |
| - Apgar ≤ 3 | ||||
| - Total | 81 (6.53/1000) | 42 (1.85/1000) | 29 (1.06/1000) | 152 (2.43/1000) |
| - Median | 3.41/1000 | 2.29/1000 | 1.15/1000 | 1.23/1000 |
| - Range | (0.39–22.44/1000) | (0–3.39/1000) | (0–2.56/1000) | (0–1.17/1000) |
Thailand's tertiary level comprised advanced and university hospitals, which can care for more than 500 beds. The secondary level included general and large/middle community hospitals, which can care for more than 90 beds
Among the 23 hospitals, 108 obstetricians, 100 obstetric residents/general physicians and 325 nurse-midwives worked in the intrapartum units. Obstetricians were allocated to all hospitals. The university and advanced hospitals had one or two residents/general physicians assigned to the intrapartum care unit. Of the 325 nurse-midwives, 285 were registered nurse-midwives, 30 received four-month midwifery specialist programme training and 10 were advanced practice nurses. The highest ratio of obstetricians to intrapartum women was found in the university hospitals (1:1440.75; range 209.71–1519.50) and the lowest in the secondary hospitals (1:537.00; range 277.86–1468.00). The nurse-to-client ratios in the university hospitals (median = 260.49, range = 97.87–274.43) and the advanced hospitals (median = 290.38, range = 257.29–383.75) were also higher than in the secondary hospitals (median = 184.46, range = 71.43–300.00). Of the 325 nurse-midwives, 44.62% had worked for ≥ 10 years. The mean nurse-midwife work-hours per week were 46.38 ± 6.78 h. Furthermore, the study found that nurse-midwives in advanced and secondary hospitals work for > 40 h per week (mean = 47.90 ± 4.48 and 47.58 ± 6.90, respectively). The study found that the team nursing care model was the most common (Table 3). Advanced hospitals had the least adequately allocated nurse-midwives for all work shifts (20% in day, 20% in afternoon, 60% at night). Nurse-midwives in all university and advanced hospitals received training on partograph and cardiotocograph monitoring. Furthermore, all nurse-midwives in university hospitals received training in neonatal resuscitation (Table 2).
Table 3.
Quality of intrapartum care classified by hospital level
| Intrapartum care | University | Advanced | Secondary | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Quality of intrapartum care | (n = 1581) | (n = 1254) | (n = 1421) | (n = 4256) | ||||
| Mean | 93.94 ± 7.24 | 83.04 ± 14.56 | 86.07 ± 9.40 | 88.10±11.53 | ||||
| Care quality level | (n = 1581) | (n =1254) | (n = 1421) | (n = 4256) | ||||
| Fair (< 80%) | 95 | 6.00 | 394 | 31.40 | 333 | 23.40 | 822 | 19.30 |
| Good (> 80%) | 1486 | 94.00 | 860 | 68.60 | 1088 | 76.60 | 3434 | 80.70 |
| - Careful monitoring | (n = 1581) | (n = 1254) | (n = 1421) | (n = 4256) | ||||
| No | 377 | 23.80 | 286 | 22.80 | 223 | 15.70 | 886 | 20.80 |
| Yes | 1204 | 76.20 | 968 | 77.20 | 1198 | 84.30 | 3370 | 79.20 |
| Partograph use (n = 3563) | ||||||||
| No | 15 | 2.55 | 287 | 48.81 | 286 | 48.64 | 588 | 16.50 |
| Yes | 1011 | 33.98 | 925 | 31.09 | 1039 | 34.92 | 2975 | 83.50 |
| Cervical dilatation screening (n = 4250) | ||||||||
| No | 62 | 25.41 | 152 | 62.3 | 30 | 12.30 | 244 | 5.74 |
| Yes | 1453 | 36.27 | 1175 | 29.33 | 1378 | 34.40 | 4006 | 94.26 |
| Partograph plot during active labour (n = 3397) | ||||||||
| No | 8 | 2.01 | 244 | 61.15 | 147 | 36.84 | 399 | 11.75 |
| Yes | 1010 | 33.69 | 912 | 30.42 | 1076 | 35.89 | 2998 | 88.25 |
| Cervix dilation observed every 4 h during active labour (n = 3507) | ||||||||
| No | 218 | 41.76 | 143 | 27.39 | 161 | 30.84 | 522 | 14.88 |
| Yes | 1049 | 35.14 | 812 | 27.20 | 1,124 | 37.65 | 2985 | 85.12 |
| Descending fetal head observed during active labour (n = 4072) | ||||||||
| No | 199 | 52.93 | 162 | 43.09 | 15 | 3.99 | 376 | 9.23 |
| Yes | 1270 | 34.36 | 1128 | 30.52 | 1298 | 35.12 | 3696 | 90.77 |
| Contractions observed every 30 min during active labour (n = 3697) | ||||||||
| No | 101 | 10.43 | 427 | 44.11 | 440 | 45.45 | 968 | 26.18 |
| Yes | 1260 | 46.17 | 672 | 24.62 | 797 | 29.20 | 2729 | 73.82 |
| Amniotic fluid membrane monitoring (n = 3998) | ||||||||
| No | 8 | 4.23 | 144 | 76.19 | 37 | 19.58 | 189 | 4.73 |
| Yes | 1360 | 35.70 | 1131 | 29.69 | 1318 | 34.60 | 3809 | 95.27 |
| Amniotic fluid characteristic record (n = 3838) | ||||||||
| No | 9 | 6.21 | 129 | 88.97 | 7 | 4.83 | 145 | 3.78 |
| Yes | 1356 | 36.72 | 1059 | 28.68 | 1278 | 34.61 | 3693 | 96.22 |
| FHR monitoring every 30 min during active labour (n = 3628) | ||||||||
| No | 67 | 7.25 | 405 | 43.83 | 452 | 48.92 | 924 | 25.47 |
| Yes | 1199 | 44.34 | 708 | 26.18 | 797 | 29.47 | 2704 | 74.53 |
| Blood pressure screening on admission (n = 4183) | ||||||||
| No | 0 | 0.00 | 110 | 98.21 | 2 | 1.79 | 112 | 2.68 |
| Yes | 1453 | 35.69 | 1212 | 29.77 | 1406 | 34.54 | 4071 | 97.32 |
| Blood pressure monitoring every 4 h (n = 3804) | ||||||||
| No | 3 | 2.38 | 86 | 68.25 | 37 | 29.37 | 126 | 3.31 |
| Yes | 1381 | 37.55 | 992 | 26.97 | 1305 | 35.48 | 3678 | 96.69 |
| Pulse screening on admission (n = 4121) | ||||||||
| No | 1 | 0.78 | 125 | 96.9 | 3 | 2.33 | 129 | 3.06 |
| Yes | 1420 | 34.78 | 1248 | 30.57 | 1415 | 34.66 | 4083 | 96.94 |
| Pulse monitoring every 4 h (n = 3804) | ||||||||
| No | 3 | 2.38 | 86 | 68.25 | 37 | 29.37 | 126 | 3.31 |
| Yes | 1381 | 37.55 | 992 | 26. 97 | 1305 | 35.48 | 3678 | 96.69 |
| Temperature monitoring every 4 h (n = 4210) | ||||||||
| No | 7 | 4.35 | 124 | 77.02 | 30 | 18.63 | 161 | 3.82 |
| Yes | 1416 | 34.97 | 1248 | 30.82 | 1385 | 34.21 | 4049 | 96.18 |
| FHR monitoring in 2nd stage (n = 2248) | ||||||||
| No | 4 | 0.47 | 360 | 42.2 | 489 | 57.33 | 853 | 37.94 |
| Yes | 457 | 32.76 | 490 | 35.13 | 448 | 32.11 | 1395 | 62.06 |
| Severe NRFS monitoring every 5 min during 2nd stage (n = 771) | ||||||||
| No | 117 | 28.61 | 216 | 52.81 | 76 | 18.58 | 409 | 53.05 |
| Yes | 163 | 45.03 | 140 | 38.67 | 59 | 16.30 | 362 | 46.95 |
| - Appropriate intervention | (n = 1521) | (n = 1254) | (n = 1421) | (n = 4256) | ||||
| No | 1006 | 67.40 | 1065 | 84.90 | 1199 | 84.40 | 3330 | 78.20 |
| Yes | 515 | 32.60 | 189 | 15.10 | 222 | 15.60 | 926 | 21.80 |
| Encourage position change during active labour (n = 3645) | ||||||||
| No | 551 | 30.26 | 545 | 29.93 | 725 | 39.81 | 1821 | 49.96 |
| Yes | 721 | 39.53 | 511 | 28.02 | 592 | 32.46 | 1824 | 50.04 |
| Clear bladder during active labour (n = 3607) | ||||||||
| No | 543 | 34.90 | 535 | 34.38 | 478 | 30.72 | 1556 | 43.14 |
| Yes | 733 | 35.74 | 496 | 24.18 | 822 | 40.08 | 2051 | 56.86 |
| Uterus stimulated during active labour (n = 4021) | ||||||||
| No | 739 | 30.93 | 727 | 30.43 | 923 | 38.64 | 2389 | 59.41 |
| Yes | 793 | 48.59 | 482 | 29.53 | 357 | 21.88 | 1632 | 40.59 |
| Encourage position change during NRFS (n = 829) | ||||||||
| No | 81 | 39.71 | 63 | 30.88 | 60 | 29.41 | 204 | 24.61 |
| Yes | 401 | 64.16 | 104 | 16.64 | 120 | 19.20 | 625 | 75.39 |
| Oxygen management during NRFS (n = 839) | ||||||||
| No | 30 | 28.85 | 50 | 48.08 | 24 | 23.08 | 104 | 12.40 |
| Yes | 452 | 61.50 | 127 | 17.28 | 156 | 21.22 | 735 | 87.60 |
| Reduced oxytocin during NRFS (n = 823) | ||||||||
| No | 304 | 59.49 | 94 | 18.4 | 113 | 22.11 | 511 | 62.09 |
| Yes | 178 | 57.05 | 67 | 21.47 | 67 | 21.47 | 312 | 37.91 |
| Intravenous fluid loading during NRFS (n = 845) | ||||||||
| No | 220 | 52.63 | 111 | 26.56 | 87 | 20.81 | 418 | 49.47 |
| Yes | 262 | 61.36 | 72 | 16.86 | 93 | 21.78 | 427 | 50.53 |
| - Team activated | (n = 332) | (n = 1001) | (n = 248) | (n = 1581) | ||||
| No | 88 | 26.5 | 553 | 55.2 | 69 | 27.8 | 710 | 44.9 |
| Yes | 244 | 73.5 | 448 | 44.8 | 179 | 72.2 | 871 | 55.1 |
| For prolonged active labour (n = 1338) | ||||||||
| No | 48 | 10.28 | 345 | 73.88 | 74 | 15.85 | 467 | 34.90 |
| Yes | 210 | 24.11 | 360 | 41.33 | 301 | 34.56 | 871 | 65.10 |
| For NRFS (n = 831) | ||||||||
| No | 19 | 32.20 | 34 | 57.63 | 6 | 10.17 | 59 | 7.10 |
| Yes | 463 | 59.97 | 135 | 17.49 | 174 | 22.54 | 772 | 92.90 |
| For prolonged 2nd stage (n = 181) | ||||||||
| No | 53 | 54.64 | 28 | 28.87 | 16 | 16.49 | 97 | 53.59 |
| Yes | 9 | 10.71 | 39 | 46.43 | 36 | 42.86 | 84 | 46.41 |
| Severe NRFS terminated in 30 min (n = 86) | ||||||||
| No | 38 | 56.72 | 3 | 4.48 | 26 | 38.81 | 67 | 77.91 |
| Yes | 6 | 31.58 | 1 | 5.26 | 12 | 63.16 | 19 | 22.09 |
Totals do not necessarily add up across all variables because of missing data in the medical chart records
FHR Fetal heart rate, NRFS Non-reassuring fetal status
Table 2.
The intrapartum health service variables classified by hospital level
| Provider variables | Hospital level | |||
|---|---|---|---|---|
| University ( n = 3) | Advanced ( n = 5) | Secondary ( n = 15) | Total ( n = 23) | |
| - Obstetricians | 17 | 32 | 59 | 108 |
| - Resident/general physicians | 72 | 28 | - | 100 |
| - Nurse-widwives | 71 | 81 | 173 | 325 |
| Registered nurse | 67 | 72 | 146 | 285 |
| Specialized nurse | 3 | 7 | 20 | 30 |
| Advanced practice nurse | 1 | 2 | 7 | 10 |
| Nurse-midwife experience | ||||
| ≤ 3 years | 24 (33.80%) | 17 (20.90%) | 41 (23.70%) | 82 (25.23%) |
| 4–9 years | 15 (21.13%) | 25 (30.86%) | 58 (33.53%) | 98 (30.15%) |
| ≥ 10 years | 32 (45.07%) | 39 (48.15%) | 74 (42.77%) | 145 (44.62%) |
| Obstetrician-to-client ratio | ||||
| - Median | 1440.75 | 1023.33 | 537.00 | 551.60 |
| - Range | 209.71–1519.50 | 400.22–1189.25 | 277.86–1468.00 | 209.71–1519.50 |
| Nurse-to-client ratio | ||||
| - Median | 260.49 | 290.38 | 184.46 | 209.75 |
| - Range | 97.87–274.43 | 257.29–383.75 | 71.43–300.00 | 71.43–383.75 |
| Average nurse work-hours (hours/week) | ||||
| - Mean ± SD | 37.8 ± 2.57 | 47.90 ± 4.48 | 47.58 ± 6.90 | 46.38 ± 6.78 |
| - Range | 35.00–40.00 | 42.00–54.00 | 36.75–56.00 | 35.00–56.00 |
| Nursing care model | ||||
| Team | 2 (66.67%) | 3 (60.00%) | 9 (60.00%) | 14 (60.87%) |
| Primary | 1 (33.33%) | 1 (20.00%) | 4 (26.67%) | 6 (26.09%) |
| Combined team and primary | – | 1 (20.00%) | 2 (13.33%) | 3 (13.04%) |
| Appropriate nurse staff allocation/shift | ||||
| Day (08:00 am–04:00 pm) | 2 (66.67%) | 1 (20.00%) | 14 (93.33%) | 17 (73.91%) |
| Afternoon (04:00 pm–12:00 am) | 2 (66.67%) | 1 (20.00%) | 12 (80.00%) | 15 (65.22%) |
| Night (12:00 am–08:00 am) | 2 (66.67%) | 3 (60.00%) | 15 (100%) | 20 (86.96%) |
| In-house training programme | ||||
| Partograph use | 3 (100%) | 5 (100%) | 10 (6.67%) | 18 (78.26%) |
| Cardiotocograph monitoring | 3 (100%) | 5 (100%) | 11 (73.33%) | 19 (82.61%) |
| Obstetric emergency management | 2 (66.67%) | 4 (80.00%) | 10 (66.67%) | 16 (69.57%) |
| Neonatal resuscitation | 3 (100%) | 4 (80.00%) | 14 (93.33%) | 21 (91.30%) |
Appropriate nurse staff allocation based on recommendations of the Thai Nursing Council (nurse-to-intrapartum women ratio of 1:2) (Nursing and Midwifery Council, 2008) and the Association of Women’s Health Obstetric and Neonatal Nurses (women with medical or obstetric complications during labour ratio of 1:1) (Association of Women’s Health Obstetric and Neonatal Nurses, 2010)
Table 3 The quality of intrapartum care provided to 4256 intrapartum women. The study found that 3434 (83.08%) received good quality care. The highest quality was found in university hospitals (93.94%) and the lowest in advanced hospitals (83.04%). Regarding the three main intrapartum care procedures (i.e. careful monitoring, appropriate intervention and activated team alert), secondary hospitals had the most careful monitoring (84.30%), such as partograph plot during active labour (35.89%), cervix dilation observed every 4 h during active labour (37.65%) and descending fetal head observation during active labour (35.12%). University hospitals provided the highest appropriate intervention (32.60%) compared to advanced and secondary hospitals, such as encouraging pregnant women to change their position (64.16%), oxygen management (61.50%), reduced oxytocin (57.05%) and IV fluid loading (61.36%) during non-reassuring fetal status. Regarding team activation, the study found that advanced hospitals were the lowest (44.8%), with a non-reassuring fetal response of 17.49% and a severe non-reassuring fetal termination in 30 min of 5.26%.
Table 4 presents the binary logistic regression results for factors contributing to birth asphyxia. The univariablete analysis found factors predicted birth asphyxia such as the quality of intrapartum care, delivery conducted provider, gestational age, parity, birth weight, maternal comorbidity, fetal complication, obstetric emergency, and non-reassuring fetal status. The odd of birth asphyxia was significantly increased 1.45-fold in pregnant women who received good quality care compared to excellent care (95% CI = 1.19–1.77, p < 0.001). Furthermore, deliveries conducted by residents or general physicians significantly increased the odds of birth asphyxia to 2.06 times for deliveries by obstetricians (95% CI = 1.68–2.52, p < 0.001). As for gestational age, the odd of birth asphyxia increased significantly by 1.69- and 1.53-fold at late-preterm (34+0–36+6 weeks) and late-term (41+0–41+6 weeks), respectively, compared to full-term deliveries (37+0–40+6 weeks): 95% CI = 1.43–2.01 (p = 0.000) and 95% CI = 1.08–2.16 (p = 0.018), respectively. The odd of birth asphyxia was also significantly higher in the nulliparous group than the multiparous group (OR = 1.25, 95% CI = 1.073–1.467, p = 0.004). The odd of birth asphyxia increased significantly by 2.31- and 1.99-fold in low-birthweight newborns (≤ 2500 g) and newborns with macrosomia (≥ 4000 g), respectively, compared to the normal-weight newborns (2501–3999 g): 95% CI = 1.81–2.95 (p = 0.000) and 95% CI = 1.32–3.00 (p = 0.001), respectively. Furthermore, the odd of birth asphyxia was significantly increased in the maternal–fetal high-risk groups: maternal comorbidity, 1.73-fold (95% CI = 1.47–2.03, p < 0.001); fetal complications, 2.21 -fold (95% CI = 1.69–2.88, p < 0.001); and obstetric emergency, 1.87-fold (95% CI = 1.50–2.33, p < 0.001). In particular, non-reassuring fetal status significantly increased the odd of birth asphyxia by 3.07-fold (95% CI = 2.59–3.63, p < 0.001).
Table 4.
The Binary Logistic Regression Analysed Factors Contributing to Birth Asphyxia in Thailand, 2016 – 2017
| Variable | Asphyxia | No Asphyxia | OR | 95% CI | p | aOR | 95% CI | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||||||
| Quality of intrapartum care | 851 | 20.00 | 3405 | 80.00 | ||||||||
| - Excellent (100%) | 153 | 17.97 | 773 | 22.70 | ref | ref | ||||||
| - Good (80–99%) | 139 | 16.33 | 1949 | 57.24 | 1.45 | 1.19 | 1.77 | < 0.001 | 1.47 | 1.17 | 1.84 | 0.001 |
| - Fair–Low (< 50%) | 559 | 65.69 | 683 | 20.06 | 1.03 | 0.80 | 1.32 | 0.828 | 1.01 | 0.77 | 1.34 | 0.924 |
| Hospital level | ||||||||||||
| - University | 315 | 37.02 | 1266 | 37.18 | ref | |||||||
| - Advanced | 298 | 35.02 | 1180 | 34.65 | 1.015 | 0.850 | 1.23 | 0.869 | ||||
| - Secondary | 238 | 27.97 | 959 | 28.16 | 0.997 | 0.826 | 1.19 | 0.979 | ||||
| Nursing care model | ||||||||||||
| - Team | 476 | 55.90 | 1963 | 57.70 | ref | ref | ||||||
| - Primary | 234 | 27.50 | 917 | 26.90 | 1.052 | 0.883 | 1.254 | 0.568 | 1.58 | 1.24 | 2.02 | < 0.001 |
| - Combined | 141 | 16.60 | 525 | 15.40 | 1.108 | 0.897 | 1.368 | 0.343 | 0.74 | 0.57 | 0.96 | 0.021 |
| Specialized nurse | ||||||||||||
| - Unavailable | 298 | 35.00 | 1207 | 35.40 | ref | ref | ||||||
| - Available | 553 | 65.00 | 2198 | 64.60 | 1.02 | 0.87 | 1.19 | 0.814 | 1.21 | 0.96 | 1.53 | 0.106 |
| Advanced practice nurse | ||||||||||||
| - Unavailable | 660 | 20.00 | 2645 | 80.00 | ref | |||||||
| - Available | 191 | 20.10 | 760 | 79.90 | 1.01 | 0.84 | 1.21 | 0.938 | ||||
| Nurse work-hours (hours/week) | ||||||||||||
| - ≥ 40 h | 667 | 78.37 | 2698 | 79.27 | ref | ref | ||||||
| - < 40 h | 184 | 21.62 | 707 | 20.76 | .095 | 0.79 | 1.14 | 0.582 | 0.62 | 0.50 | 0.77 | < 0.001 |
| Delivery conducted by: | ||||||||||||
| - Obstetrician | 481 | 58.40 | 2070 | 65.40 | ref | ref | ||||||
| - Nurse-midwives | 155 | 18.80 | 703 | 22.20 | 0.95 | 0.78 | 1.16 | 0.607 | 1.02 | 0.82 | 1.27 | 0.880 |
| - Resident or general physician | 187 | 22.70 | 391 | 12.40 | 2.06 | 1.68 | 2.52 | < 0.001 | 1.99 | 1.58 | 2.49 | < 0.001 |
| Gestational group | ||||||||||||
| - Term | 556 | 65.50 | 2584 | 76.00 | ref | ref | ||||||
| - Late-preterm(34+0–36+6 weeks) | 248 | 29.20 | 680 | 20.00 | 1.69 | 1.43 | 2.01 | < 0.001 | 1.63 | 1.34 | 1.98 | < 0.001 |
| - Late-term (40+1–41+6 weeks) | 45 | 5.30 | 137 | 4.00 | 1.53 | 1.08 | 2.16 | 0.018 | 1.61 | 1.11 | 2.34 | 0.012 |
| Parity | ||||||||||||
| - Multiparous | 550 | 35.40 | 2019 | 40.70 | ref | |||||||
| - Nulliparous | 301 | 64.60 | 1386 | 59.30 | 1.25 | 1.073 | 1.467 | 0.004 | ||||
| Pre-pregnancy BMI | ||||||||||||
| - Normal weight | 304 | 42.70 | 1235 | 42.00 | ref | |||||||
| - Underweight | 277 | 38.90 | 1289 | 43.80 | 0.87 | 0.73 | 1.05 | 0.140 | ||||
| - Overweight | 91 | 12.80 | 288 | 9.80 | 1.28 | 0.98 | 1.68 | 0.067 | ||||
| - Obese | 40 | 5.60 | 128 | 4.40 | 1.27 | 0.87 | 1.85 | 0.214 | ||||
| Completion of ANC visits | ||||||||||||
| - Incomplete | 322 | 40.20 | 1292 | 40.80 | ref | |||||||
| - Complete | 479 | 59.80 | 1877 | 59.20 | 1.02 | 0.87 | 1.20 | 0.769 | ||||
| Birth weight | ||||||||||||
| - Normal | 128 | 15.00 | 231 | 6.80 | ref | ref | ||||||
| - Low | 689 | 81.00 | 3096 | 90.90 | 2.31 | 1.81 | 2.95 | < 0.001 | 2.29 | 1.77 | 2.96 | < 0.001 |
| - Macrosomia | 34 | 4.00 | 78 | 2.30 | 1.99 | 1.32 | 3.00 | 0.001 | 2.14 | 1.37 | 3.35 | 0.001 |
| Maternal and fetal high-risk conditions | ||||||||||||
| Maternal comorbidity | ||||||||||||
| - No | 562 | 66.04 | 2624 | 77.06 | ref | ref | ||||||
| - Yes | 289 | 33.96 | 781 | 22.94 | 1.73 | 1.47 | 2.03 | < 0.001 | 1.65 | 1.35 | 1.94 | < 0.001 |
| Intrapartum complications | ||||||||||||
| - No | 737 | 86.60 | 2904 | 85.30 | ref | |||||||
| - Yes | 114 | 13.40 | 501 | 14.70 | 0.90 | 0.72 | 1.12 | 0.328 | ||||
| Fetal complications | ||||||||||||
| - No | 760 | 89.30 | 3230 | 94.90 | ref | |||||||
| - Yes | 91 | 10.70 | 175 | 5.10 | 2.21 | 1.69 | 2.88 | < 0.001 | ||||
| Obstetric emergency | ||||||||||||
| - No | 722 | 84.80 | 3108 | 91.30 | ref | ref | ||||||
| - Yes | 129 | 15.20 | 297 | 8.70 | 1.87 | 1.50 | 2.33 | < 0.001 | 1.64 | 1.283 | 2.11 | < 0.001 |
| Non-reassuring fetal status | ||||||||||||
| - Reassuring | 552 | 64.86 | 2894 | 83.98 | ref | ref | ||||||
| - Non-reassuring | 299 | 35.14 | 511 | 14.83 | 3.07 | 2.59 | 3.633 | < 0.001 | 3.04 | 2.51 | 3.69 | < 0.001 |
| Nagelkerke R2 = 0.140 | ||||||||||||
CTG Cardiotocography, ref reference, ANC Antenatal care, BMI Body mass index, aOR adjusted odds ratio
Table 4 presents the binary logistic regression results for factors contributing to birth asphyxia. The univariable analysis found factors predicted birth asphyxia such as the quality of intrapartum care, delivery conducted provider, gestational age, parity, birth weight, maternal comorbidity, fetal complication, obstetric emergency, and non-reassuring fetal status. The odd of birth asphyxia was significantly increased 1.45-fold in pregnant women who received good quality care compared to excellent care (95% CI = 1.19–1.77, p < 0.001). Furthermore, deliveries conducted by residents or general physicians significantly increased the odds of birth asphyxia to 2.06 times for deliveries by obstetricians (95% CI = 1.68–2.52, p < 0.001). As for gestational age, the odd of birth asphyxia increased significantly by 1.69- and 1.53-fold at late-preterm (34+0–36+6 weeks) and late-term (41+0–41+6 weeks), respectively, compared to full-term deliveries (37+0–40+6 weeks): 95% CI = 1.43–2.01 (p = 0.000) and 95% CI = 1.08–2.16 (p = 0.018), respectively. Birth asphyxia was also significantly higher in the nulliparous group than the multiparous group (OR = 1.25, 95% CI = 1.073–1.467, p = 0.004). The odd of birth asphyxia increased significantly by 2.31- and 1.99-fold in low-birthweight newborns (≤ 2500 g) and newborns with macrosomia (≥ 4000 g), respectively, compared to normal-weight newborns (2501–3999 g): 95% CI = 1.81–2.95 (p = 0.000) and 95% CI = 1.32–3.00 (p = 0.001), respectively. Furthermore, odd of birth asphyxia was significantly increased in the maternal–fetal high-risk groups: maternal comorbidity, 1.73-fold (95% CI = 1.47–2.03, p < 0.001); fetal complications, 2.21 -fold (95% CI = 1.69–2.88, p < 0.001); and obstetric emergency, 1.87-fold (95% CI = 1.50–2.33, p < 0.001). In particular, non-reassuring fetal status significantly increased the odd of birth asphyxia by 3.04-fold (95% CI = 2.51–3.69, p < 0.001).
However, the multivariable logistic regression results for the factors contributing to birth asphyxia. According to the chi-square statistic model, the overall model is significant at p < 0.001. The Nagelkerke R2 value of 0.140 suggests that approximately 14% of the variation in the response variable is explained by the predictors included in the logistic regression model. The analysis indicated that the odd of birth asphyxia was significantly higher with good care quality compared to excellent care quality (aOR = 1.47, 95% CI = 1.17–1.84, p = 0.001). Birth asphyxia was significantly reduced by 38% for nurse work-hours of < 40 h per week (aOR = 0.62, 95% CI = 0.50–0.77, p < 0.001). Furthermore, the results show that birth asphyxia was significantly reduced by 26% when the combined nursing care model (primary and team) was applied in hospitals compared to the team nursing care model (aOR = 0.74, 95% CI = 0.57–0.96, p = 0.021). In contrast, the primary care model had significantly higher the odd of birth asphyxia than the team nursing care model (aOR = 1.58, 95% CI = 1.24–2.02, p ≤ 0.001). The odd of birth asphyxia was significantly increased when the newborn was delivered by a resident or general physician rather than by an obstetrician (aOR = 1.99, 95% CI = 1.58–2.49, p < 0.001). Moreover, delivery in the late-preterm and late-term groups significantly increased odd of birth asphyxia compared to the full-term pregnancy group (aOR = 1.63, 95% CI = 1.34–1.98, p < 0.001; aOR = 1.61, 95% CI = 1.11–2.34, p = 0.012). Pregnancies with comorbidity, obstetric emergency, non-reassuring fetal status, low birthweight or macrosomia significantly increased odd of birth asphyxia (aOR = 1.62, 95% CI = 1.35–1.94, p < 0.001; aOR = 1.64, 95% CI = 1.29–2.11, p < 0.001; aOR = 3.04, 95% CI = 2.50–3.69, p < 0.001; aOR = 2.29, 95% CI = 1.77–2.96, p < 0.001; aOR = 2.14, 95% CI = 1.37–3.35, p = 0.001).
Discussion
This case–control study aimed to determine the maternal–fetal and health service factors contributing to birth asphyxia. Among the 4256 samples, the average rate of birth asphyxia was 38.19 per 1000 live births. Although advanced hospitals had the highest rate of birth asphyxia (52.24 per 1000 live births), university hospitals had the highest rate of severe birth asphyxia (6.53 per 1000 live births), possibly due to the number of high-risk deliveries referred from other facilities.
Multivariable logistic regression analysis found significant increases the odds of birth asphyxia in late-preterm, late-term, low-birth weight, and macrosomia fetuses. This result was supported by previous studies finding that neonates born at 34+0–36+6 weeks and of low birthweight are associated with birth asphyxia. The immature pulmonary function may make it difficult for the fetus to maintain breathing after birth. Likewise, the placental deterioration in post-term neonates increases the likelihood of birth asphyxia more than in pregnancies delivered at term. Therefore, non-spontaneous late-term deliveries should be avoided in order to reduce birth asphyxia. Antenatal surveillance is recommended at 40+0–41+6 weeks, beginning twice weekly with the biophysical profile or non-stress testing plus amniotic fluid index measure, along with induction at 41+0 weeks’ gestation [40–43].
Furthermore, the study found that birth asphyxia increases significantly in pregnant women with comorbidities and obstetric emergency conditions. Maternal complications such as gestational hypertension and diabetes may cause utero-placental insufficiency, resulting in reduced blood flow and loss of placental integrity that predisposes the fetus to intrauterine hypoxia [44–46]. An obstetric emergency during the intrapartum period significantly increases the likelihood of birth asphyxia. Maternal–fetal circulatory problems could also occur in placenta abruption, cord prolapse, shoulder dystocia and non-reassuring fetus [47–50]. Therefore, intrapartum care by carefully screening, close monitoring, timely detection and appropriate response from a multidisciplinary team are needed to resolve the problem.
The research findings revealed that an excellent level of intrapartum care quality reduces birth asphyxia. Careful monitoring, such as partograph use for monitoring the descent of the fetal head to identify the cephalo-pelvic disproportion promptly, can effectively reduce birth asphyxia. Continuous fetal heart rate monitoring in non-reassuring fetal conditions can shorten the decision-to-delivery time and reduce the severity of hypoxic-ischaemic encephalopathy in newborns [51, 52]. Appropriate interventions, such as placing intrapartum women in a side-lying position with the head elevated, can significantly reduce the risk of birth asphyxia. In a severe non-reassuring fetus, the appropriate intrauterine resuscitation reduced fetal hypoxia. Activating an alert team to respond, such as obstetricians, paediatricians, anaesthetists and specialized or experienced nurses, could reduce birth asphyxia [23–28, 34]. Furthermore, the study found that caesarean delivery reduced birth asphyxia significantly. Under high-risk pregnancy conditions or obstetric emergencies, caesarean delivery may be required to save the mother’s and the baby's lives and prevent unexpected adverse outcomes.
Furthermore, the research found that the combined primary and team nursing care model significantly lowered birth asphyxia compared to the team nursing care model. The result is different from the previous study, which found that the one-on-one care or primary/total patient care model can ensure that pregnant women received closer and more continuous care and had more desirable outcomes than other nursing models [53]. However, the primary care model had a limitation for nurses with less experience because it requires that they work and make decisions alone [54]. Therefore, in a nurse staff shortage situation such as that in Thailand, primary nursing care combined with the team nursing care model may be reasonable for intrapartum care. Furthermore, training to improve competency and confidence in providing intrapartum care, including electronic fetal monitoring, emergency obstetric management and a neonatal resuscitation training programme to improve neonatal outcomes, should be provided to fewer experienced nurse-midwives in labour and delivery units [23, 55].
Lastly, the study found that newborns delivered by a resident or general physician had a higher risk of birth asphyxia. This may be because the general physicians or residents may not be trained to deal with complicated patients referred to the advanced and university-level hospitals. The high workload condition made it difficult to improve the quality of nursing care, therefore medical work experience requires the obstetrician to be closely supervised in order to improve client health outcomes [56].
Implications for practice
Pregnant women with comorbidity and late-preterm, late-term pregnancies, low-birth weigh, and macrosomia need close monitoring to prevent birth asphyxia. In addition, an appropriate number of nurse-midwives allocated in the advanced hospitals is needed to resolve the high workload, transferring non-intrapartum pregnant women with high-risk conditions to the appropriate unit to be managed by a specialized multidisciplinary team. Furthermore, the intrapartum nursing care model driven by applying the primary care model combined with the team model is feasible for providing good quality care.
Limitations
The retrospective case–control study was designed for data extraction from medical chart reviews, therefore unreported healthcare activity was not available to clarify the quality of intrapartum care.
To ensure that birth asphyxia did not develop from immature fetal lungs or placental insufficiency, the research samples did not include very preterm and or post-term fetuses in the selection criteria and therefore such fetuses were not considered as factors affecting birth asphyxia in this study.
Conclusion
Birth asphyxia in Thailand is a serious problem that requires more attention. An excellent level of intrapartum care quality is required to reduce the birth asphyxia rate, carefully evaluating and monitoring pregnant women with comorbidity, late-preterm and late-term pregnancies and newborns with low birthweight or macrosomia. The primary care model combined with the team nursing care model is the alternative strategy to improve the quality of care in the intrapartum unit. In addition, obstetric emergencies need early detection and appropriate intervention by a specialized care team to enhance staff allocation and reduce the nurse work-hours per week .
Supplementary Information
Additional file 1. Intrapartum Care Record Form.
Additional file 2. Asphyxia Risk Factors Record Form.
Additional file 3. Intrapartum Health Care System Questionnaire.
Acknowledgements
The authors would like to thank the Thai Society of Maternal and Fetal Medicine for funding this research study. In addition, we would like to thank Clinical Professor Prasert Sunsaneevithayakul (Department of Obstetrics & Gynaecology, Faculty of Medicine Siriraj Hospital, Mahidol University), Associate Professor Thitima Suntharasaj (Department of Obstetrics and Gynecology, Faculty of Medicine, Prince of Songkla University) and Clinical Professor Supatra Sirichotiyakul (Department of Obstetrics and Gynecology, Faculty of Medicine, Chiang Mai University) for valuable suggestions in relation to research data collection.
Authors’ contributions
P.R. and A.R. wrote the main manuscript text and prepared tables 1, 2, 3 and 4.
All authors reviewed the manuscript.
Authors’ information
Panida Rattanaprom obtained a Doctoral degree in Nursing from the Faculty of Nursing, Mahidol University, Bangkok, Thailand. Currently, she is working as a lecturer at the Borommarajchonnani Nursing College Suratthani, Faculty of Nursing, Praboromarajchanok Institute, Thailand.
Assoc. Prof. Dr Ameporn Ratinthorn* obtained a PhD in Nursing from the University of California, San Francisco, USA. Currently, she is working as a lecturer in the Department of Obstetrics and Gynaecological Nursing, Faculty of Nursing, Mahidol University, Thailand.
Prof. Dr Siriorn Sindhu obtained a PhD in Nursing from the University of California, San Francisco, USA. Currently, she is working as a lecturer in the Doctoral of Nursing Science Program, Faculty of Nursing, Mahidol University, Thailand.
Prof. Dr Chukiat Viwatwongkasem obtained a PhD in Statistics from the National Institute of Development Administration, Thailand. Currently, he is working as a lecturer in the Department of Biostatistics (Faculty of Public Health, Mahidol University, Bangkok, Thailand), with expertise in Probability Theory, Statistics and Addiction Medicine.
Funding
The study was partly supported by the Thai Society of Maternal and Fetal Medicine. However, the funder played no role in the study design, data collection, analysis/interpretation of data or writing of the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Siriraj Institutional Review Board of the Faculty of Medicine, Mahidol University (No. Si 705/2018); the Human Research Ethics Committee, Faculty of Medicine, Prince of Songkla University (REC. 61–317-19–6); and the Research Ethics Committee, Faculty of Medicine, Chiang Mai University (ID: 5649). The ethics committee complies fully with international guidelines for human research protection, such as the Declaration of Helsinki, the Belmont Report, CIOMS Guidelines and the International Conference on Harmonization in Good Clinical Practice (ICH-GCP). The research proposal, participant information sheet, informed consent form, case record form and questionnaire were approved. Before data collection, human research protection certificates were presented to each hospital director of the research setting for permission. All agreed to permit data collection before the data collection began. All the enrolled head nurses of the intrapartum unit were accurately informed about the research purpose, and the researcher randomly selected the samples based on the inclusion criteria.
The data collection progress must be reported at least once a year except where required more frequent by the Research Ethics Committee. Prior Research Ethics Committee approval is required before implementing any changes in the consent documents or protocol unless those changes are needed urgently for the safety of subjects. In addition, any event or new information that may affect the benefit/risk ratio of the study must be reported to the research ethic committee promptly.
The informed consent was performed in a voluntary approached before data collection from the participant. Each participant was adequately informed of the aims, methods, possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits and potential risks of the study and the discomfort it may entail, post-study provisions and any other relevant aspects of the study. In addition, the participant was informed about the right to withdraw consent to participate at any time without reprisal.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahearne CE, Boylan GB, Murray DM. Short and long term prognosis in perinatal asphyxia: An update. World J. Clin. Pediatr. 2016;5(1):67–74. doi: 10.5409/wjcp.v5.i1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Harrison MS, McClure EM. Stillbirths: The hidden birth asphyxia - US and global perspectives. Clin. Perinatol. 2016;43(3):439–53. doi: 10.1016/j.clp.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Pappas A, Korzeniewski SJ. Long-term cognitive outcomes of birth asphyxia and the contribution of identified perinatal asphyxia to cerebral palsy. Clin. Perinatol. 2016;43(3):559–72. doi: 10.1016/j.clp.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Garfinkle J, Wintermark P, Shevell MI, Oskoui M. Cerebral palsy after neonatal encephalopathy: Do neonates with suspected asphyxia have worse outcomes? Dev. Med. Child Neurol. 2016;58(2):189–94. doi: 10.1111/dmcn.12953. [DOI] [PubMed] [Google Scholar]
- 5.Sendeku FW, Azeze GG, Fenta SL. Perinatal asphyxia and its associated factors in Ethiopia: A systematic review and meta-analysis. BMC Pediatr. 2020;20:135. doi: 10.1186/s12887-020-02039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdo RA, Halil HM, Kebede BA, Anshebo AA, Gejo NG. Prevalence and contributing factors of birth asphyxia among the neonates delivered at Nigist Eleni Mohammed memorial teaching hospital, Southern Ethiopia: A cross-sectional study. BMC Pregnancy Childbirth. 2019;19:536. doi: 10.1186/s12884-019-2696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Workineh Y, Semachew A, Ayalew E, Animaw W, Tirfie M, Birhanu M. Prevalence of perinatal asphyxia in East and Central Africa: Systematic review and meta-analysis. Heliyon. 2020;6(4):e03793. doi: 10.1016/j.heliyon.2020.e03793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Public Health (Thailand), Department of Health. Birth Asphyxia Rate 2021. Department of Health Dashboard. 2021. Available from: https://dashboard.anamai.moph.go.th/dashboard/birthasphyxia/index?year=2021.
- 9.Greco P, Nencini G, Piva I, Scioscia M, Volta CA, Spadaro S, Nappi L. Pathophysiology of hypoxic-ischemic encephalopathy: A review of the past and a view on the future. Acta Neurol. Belg. 2020;120(2):277–88. doi: 10.1007/s13760-020-01308-3. [DOI] [PubMed] [Google Scholar]
- 10.Strategy and Planning Division. Health Statistic 2017. Strategy and Planning Division, Ministry of Public Health, Thailand. 2018. Available from: https://bps.moph.go.th/new_bps/sites/default/files/stratistics60.pdf.
- 11.Gillam-Krakauer M, Gowen CW., Jr . Birth Asphyxia. Treasure Island, FL: StatPearls Publishing; 2020. [Google Scholar]
- 12.Ayuk Widiani N, Yuli Kurniati D, Windiani IT. Maternal and infant risk factors on the incidence of neonatal asphyxia in Bali: A case-control study. Publ. Health Prev. Med. Arch. 2016;4(2):120–26. doi: 10.24843/PHPMA.2016.v04.i02.p01. [DOI] [Google Scholar]
- 13.Ensing S, Abu-Hanna A, Schaaf JM, Mol BWJ, Ravelli ACJ. Trends in birth asphyxia, obstetric interventions and perinatal mortality among term singletons: A nationwide cohort study. J. Matern. Fetal Neonatal Med. 2015;28(6):632–7. doi: 10.3109/14767058.2014.929111. [DOI] [PubMed] [Google Scholar]
- 14.Aslam HM, Saleem S, Afzal R, Iqbal U, Saleem S, Shaikh MWA, Shahid N. Risk factors of birth asphyxia. Ital. J. Pediatr. 2014;40:94. doi: 10.1186/s13052-014-0094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu JH, Profit J, Melsop K, Brown T, Davis A, Main E, Lee HC. Relationship of hospital staff coverage and delivery room resuscitation practices to birth asphyxia. Am. J. Perinatol. 2017;07(03):259–63. doi: 10.1055/s-0036-1586505. [DOI] [PubMed] [Google Scholar]
- 16.Morioka N, Tomio J, Seto T, Kobayashi Y. The association between higher nurse staffing standards in the fee schedules and the geographic distribution of hospital nurses: A cross-sectional study using nationwide administrative data. BMC Nursing. 2017;16:25. doi: 10.1186/s12912-017-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Zheng J, Liu K, Baggs JG, Liu J, Wu Y, You L. Hospital nursing organisational factors, nursing care left undone, and nurse burnout as predictors of patient safety: A structural equation modeling analysis. Int. J. Nurs. Stud. 2018;86:82–9. doi: 10.1016/j.ijnurstu.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Amiri A, Vehviläinen-Julkunen K, Solankallio-Vahteri T, Tuomi S. Impact of nurse staffing on reducing infant, neonatal and perinatal mortality rates: Evidence from panel data analysis in 35 OECD countries. Int. J. Nurs. Sci. 2020;7(2):161–9. doi: 10.1016/j.ijnss.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stimpfel AW, Aiken LH. Hospital staff nurses' shift length associated with safety and quality of care. J. Nurs. Care Qual. 2013;28(2):122–9. doi: 10.1097/NCQ.0b013e3182725f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma S, Rani R. Nurse-to-patient ratio and nurse staffing norms for hospitals in India: A critical analysis of national benchmarks. J. Family Med. Prim. Care. 2020;9(6):2631–7. doi: 10.4103/jfmpc.jfmpc_248_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mildenberger C, Ellis C, Lee K. Neonatal resuscitation training for midwives in Uganda: Strengthening skill and knowledge retention. Midwifery. 2017;50:36–41. doi: 10.1016/j.midw.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Ljungblad LW, Sandvik SO, Lyberg A. The impact of skilled birth attendants trained on newborn resuscitation in Tanzania: A literature review. Int. J. Afr. Nurs. Sci. 2019;11:100168. doi: 10.1016/j.ijans.2019.100168. [DOI] [Google Scholar]
- 23.Johnson MJ. Intrauterine fetal resuscitation: A midwife’s role. Glob. J. Res. Anal. 2020;9(8):115–19. [Google Scholar]
- 24.Housseine N, Punt MC, Mohamed AG, Said SM, Maaløe N, Zuithoff NPA, Rijken MJ. Quality of intrapartum care: Direct observations in a low-resource tertiary hospital. Reprod. Health. 2020;17(1):36. doi: 10.1186/s12978-020-0849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kc A, Wrammert J, Clark RB, Ewald U, Målqvist M. Inadequate fetal heart rate monitoring and poor use of partogram associated with intrapartum stillbirth: A case-referent study in Nepal. BMC Pregnancy Childbirth. 2016;16:233. doi: 10.1186/s12884-016-1034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masereka EM, Naturinda A, Tumusiime A, Munguiko C. Implementation of the Perinatal Death Surveillance and Response guidelines: Lessons from annual health system strengthening interventions in the Rwenzori Sub-Region Western Uganda . Nurs. Open. 2020;7(5):1497–505. doi: 10.1002/nop2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim Gouda AM, Hassan Khedr NF. Effect of left lateral position on intrauterine fetal resuscitation among pregnant women with reduced fetal movements. Int. J. Nurs. Didact. 2018;8(07):40–6. doi: 10.15520/ijnd.v8i07.2225. [DOI] [Google Scholar]
- 28.Prescott KM, Semroc B. Creating a culture of excellence around fetal heart rate tracings through listening and collaboration. J. Obstet. Gynecol. Neonatal Nurs. 2019;48(3):S42. ISSN 0884–2175. doi: 10.1016/j.jogn.2019.04.072. [DOI] [Google Scholar]
- 29.Havaei F, MacPhee M, Dahinten VS. The effect of nursing care delivery models on quality and safety outcomes of care: A cross-sectional survey study of medical-surgical nurses. J. Adv. Nurs. 2019;75(10):2144–55. doi: 10.1111/jan.13997. [DOI] [PubMed] [Google Scholar]
- 30.Sosa GA, Crozier KE, Stockl A. Midwifery one-to-one support in labour: More than a ratio. Midwifery. 2018;62:230–39. doi: 10.1016/j.midw.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 31.Gidaszewski B, Khajehei M, Gibbs E, Chua SC. Comparison of the effect of caseload midwifery program and standard midwifery-led care on primiparous birth outcomes: A retrospective cohort matching study. Midwifery. 2019;69:10–16. doi: 10.1016/j.midw.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Dal Molin A, Gatta C, Boggio Gilot C, Ferrua R, Cena T, Manthey M, Croso A. The impact of primary nursing care pattern: Results from a before–after study. J. Clin. Nurs. 2018;27(5–6):1094–102. doi: 10.1111/jocn.14135. [DOI] [PubMed] [Google Scholar]
- 33.Wan H, Hu S, Thobaben M, Hou Y, Yin T. Continuous primary nursing care increases satisfaction with nursing care and reduces postpartum problems for hospitalised pregnant women. Contemp. Nurse. 2011;37(2):149–59. doi: 10.5172/conu.2011.37.2.149. [DOI] [PubMed] [Google Scholar]
- 34.Berazategui JP, Aguilar A, Escobedo M, Dannaway D, Guinsburg R, de Almeida MFB, Szyld E. Risk factors for advanced resuscitation in term and near-term infants: A case–control study. Arch. Dis. Child. Fetal Neonatal Ed. 2017;102(1):F44. doi: 10.1136/archdischild-2015-309525. [DOI] [PubMed] [Google Scholar]
- 35.Plichta SB, Kelvin EA. Munro's Statistical Methods for Health Care Research. 6. China: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 36.Cohen J. Statistical Power Analysis for the Behavioural Sciences. Rev. Hillsdale, NJ: Lawrence Erlbaum Associates Inc.; 1977. [Google Scholar]
- 37.USAID (2014). Fistula Care and Engender Health. https://www.fistulacare.org. Accessed 12 May 2021.
- 38.World Health Organization. Making Pregnancy Safer Assessment Tool for the Quality of Hospital Care for Mothers and Newborn Babies. 2009. https://apps.who.int/iris/handle/10665/107968. Accessed 12 May 2021.
- 39.World Health Organization. Safe Motherhood Needs Assessment Version 1.1 – 2001 (Revised edition). Sexual and Reproductive Health. 2021. Retrieved from: https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/rht_msm_96_18/en/. Accessed 12 May 2021.
- 40.Ranjbar A, Mehrnoush V, Darsareh F, Pariafsay F, Shirzadfardjahromi M, Shekari M, Darsareh Sr F. The Incidence and Outcomes of Late-Term Pregnancy. Cureus. 2023;15(1). [DOI] [PMC free article] [PubMed]
- 41.Murzakanova G, Räisänen S, Jacobsen AF, Sole KB, Bjarkø L, Laine K. Adverse perinatal outcomes in 665,244 term and post-term deliveries—a Norwegian population-based study. Euro J Obstet Gynecol Reprod Biol. 2020;247:212–8. [DOI] [PubMed]
- 42.Kortekaas JC, Bruinsma A, Keulen J, Vandenbussche F, van Dillen J, de Miranda E. Management of late-term pregnancy in midwifery- and obstetrician-led care. BMC Pregnancy Childbirth. 2019;19(1):181. https://doi-org.ejournal.mahidol.ac.th/10.1186/s12884-019-2294-7. [DOI] [PMC free article] [PubMed]
- 43.Maoz O, Wainstock T, Sheiner E, Walfisch A. Immediate perinatal outcomes of postterm deliveries. J Maternal Fetal Neonatal Med. 2018;32(11):1847–52. [DOI] [PubMed]
- 44.Newman C, Egan AM, Ahern T, Al-Kiyumi M, Balan G, Brassill MJ, Brosnan E, Carmody L, Clarke H, Coogan Kell, C, Culliney L, Davern R, Durkan M, Fenlon M, Ferry P, Hanlon G, Higgins T, Hoashi S, Khamis A, Kinsley B, Dunne FP. Diabetes care and pregnancy outcomes for women with pregestational diabetes in Ireland. Diabetes Res Clin Pract. 2021;173:108685. 10.1016/j.diabres.2021.108685. [DOI] [PubMed]
- 45.Agrawal A, nger NK. Hypertension during pregnancy. Curr Hypertens Rep. 2020;22(9):64. 10.1007/s11906-020-01070-0. [DOI] [PubMed]
- 46.Smith C, Teng F, Branch E, Chu S, Joseph KS. Maternal and perinatal morbidity and mortality associated with anemia in pregnancy. Obstet. Gynecol. 2019;134(6):1234–44. 10.1097/AOG.0000000000003557. [DOI] [PMC free article] [PubMed]
- 47.Downes KL, Grantz KL, Shenassa ED. Maternal, labor, delivery, and perinatal outcomes associated with placental abruption: A systematic review. Am J Perinatol. 2017;34(10): 935–57. 10.1055/s-0037-1599149. [DOI] [PMC free article] [PubMed]
- 48.Li Y, Tian Y, Liu N, Chen Y, Wu F. Analysis of 62 placental abruption cases: Risk factors and clinical outcomes. Taiwan J Obstet Gynecol. 2019;58(2):223–6. 10.1016/j.tjog.2019.01.010. [DOI] [PubMed]
- 49.Adeniran AS, Imhoagene A, Ezeoke GG. Presentation and perinatal outcome following umbilical cord prolapse in Ilorin. J Trop Med. 2017;19(1):31–5. 10.4103/jomt.jomt_39_16.
- 50.Cesari E, Ghirardello S, Brembilla G, Svelato A, Ragusa A. Clinical features of a fatal shoulder dystocia: The hypovolemic shock hypothesis, Med Hypotheses. 2018;118:139–41. https://doi.org/30037602. [DOI] [PubMed]
- 51.Salma U, Jabeen M, Shimul S, Akhter D. Analysis of cardiotocography findings in pregnancy with less fetal movement and its association with perinatal outcome. Med Today. 2018;30(1):19-22. https://doi.org/58484206.
- 52.Kitaw TM, Limenh SK, Chekole FA, Getie SA, Gemeda BN, Engda AS. Decision to delivery interval and associated factors for emergency cesarean section: A cross-sectional study. BMC Pregnancy Childbirth. 2021;21:224. https://doi.org/https://doi-org.ejournal.mahidol.ac.th/10.1186/s12884-021-03706-8. [DOI] [PMC free article] [PubMed]
- 53.Fernandez R, Johnson M, Tran DT, Miranda C. Models of care in nursing: A systematic review. Int J Evid‐Based Healthc. 2012;10(4):324–37. https://doi.org/https://scholar-google-com.ejournal.mahidol.ac.th/scholar?hl=th&as_sdt=0%2C5&q=Models+of+care+in+nursing%3A+A+systematic+review.&btnG=. [DOI] [PubMed]
- 54.Fairbrother G, Chiarella M, Braithwaite J. Models of care choices in today's nursing workplace: Where does team nursing sit? Aust Health Rev. 2015;39(5):489–93. https://doi.org/https://scholar-google-com.ejournal.mahidol.ac.th/scholar?hl=th&as_sdt=0%2C5&q=Models+of+care+choices+in+today%27s+nursing+workplace%3A+Where+does+team+nursing+sit%3F+&btnG=. [DOI] [PubMed]
- 55.Desta M, Akalu TY, Alamneh YM, Talie A, Alemu AA, Tessema Z, Getaneh T. Perinatal mortality and its association with antenatal care visit, maternal tetanus toxoid immunization and partograph utilization in Ethiopia: a meta-analysis. Sci Rep. 2021;11(1):19641. https://doi.org/https://scholar-google-com.ejournal.mahidol.ac.th/scholar?hl=th&as_sdt=0%2C5&q=Perinatal+mortality+and+its+association+with+antenatal+care+visit%2C+maternal+tetanus+toxoid+immunization+and+partograph+utilization+in+Ethiopia%3A+a+meta-analysis&btnG=. [DOI] [PMC free article] [PubMed]
- 56.Bardos J, Loudon H, Rekawek P, Friedman F, Brodman M, Fox NS. Association between senior obstetrician supervision of resident deliveries and mode of delivery. Obstet. Gynecol. 2017;129(3):486–90. https://doi.org/https://journals-lww-com.ejournal.mahidol.ac.th/greenjournal/fulltext/2017/03000/Association_Between_Senior_Obstetrician.12.aspx. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Intrapartum Care Record Form.
Additional file 2. Asphyxia Risk Factors Record Form.
Additional file 3. Intrapartum Health Care System Questionnaire.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.