Abstract
Background:
In October 2018, the allocation policy for adult orthotopic heart transplant (OHTx) in the United States was changed, with the goal of reducing waitlist mortality and providing broader sharing of donor organs within the United States. The aim of this study was to assess the association of this policy change with changes in access to OHTx vs left ventricular assist devices (LVADs), overall and among key sociodemographic subgroups, in the US from 2016 to 2019.
Methods:
We identified all patients receiving OHTx or LVAD between 2016–2019 using the National Inpatient Sample. Controlling for medical comorbidities, pre-policy trends, and within hospital-year effects, we fit a dynamic logistic regression model to evaluate patient and hospital factors associated with receiving OHTx vs LVAD pre- versus post-policy change. We also examined the frequency of temporary mechanical circulatory support (tMCS) in the same fashion.
Results:
We identified 2264 patients who received OHTx and 3157 who received LVADs during the study period. In its first year of implementation, the UNOS policy change of 2018 was associated with no overall change utilization of OHTx vs LVAD. Among OHTx recipients, the frequency of use of tMCS changed from 15.6% in the pre period to 42.6% in the post period (p<0.001). While the policy change was associated with differences in the odds of receiving an OHTx versus LVAD between different regions of the country, there were no significant changes based on age, gender, race/ethnicity, insurance status, or rurality.
Conclusions:
The UNOS policy change on access to OHTx was associated with no overall change in OHTx vs LVAD use in its first year of implementation although we observed small changes in relative odds of transplant based on rurality. Shifts in regional allocation were not significant overall, though certain regions appeared to have a relative increase in their use of OHTx.
Keywords: orthotopic heart transplant (OHTx), left ventricular assist devices (LVADs), health policy, outcomes
Introduction:
The United Network for Organ Sharing (UNOS) heart transplant allocation system has changed considerably since its inception in 1986.1,2 The initial listing strategy focused on illness severity and waitlist time for heart transplant patients using a 2-tiered system. In 1998, with the introduction of left ventricular assist devices (LVADs), a third tier was added. Due to need for broader sharing of organs across geographic areas, the allocation system was changed again in 2005.3 Allocation policy was based on donation service areas (DSA) surrounding donor hospitals with priority placed on local candidates.
From 2006 to 2015 the number of OHTx candidates tripled, and the number of status 1A candidates (patients in an intensive care unit on durable VAD support with significant complications) increased fivefold.1 More than 65% of transplant candidates were listed status 1A.1,2 Within status 1A, significant variability was noted in waitlist mortality; patients receiving extracorporeal membrane oxygenation (ECMO) had waitlist mortality close to 36%, whereas patients receiving other forms of mechanical circulatory support (MCS) had waitlist mortality of roughly 5%.1,2,4 One significant barrier to broader sharing of organs with the prior system was that less critically ill patients in the DSA were favored over sicker patients outside of the designated DSA.3,5 Due to emerging data showing rising waitlist numbers, increased mortality and longer wait list times for patients listed status 1A, UNOS and the Organ Procurement and Transplantation Network (OPTN) initiated the process once again in 2016 to revise the allocation policy.6–9
The most recent policy change took effect in October of 2018.8,9 The goals of the new policy were to address the variability in risk among candidates at higher urgency status, decrease waitlist mortality, reduce the need for listing exceptions, reduce geographic disparities, align listing priority with the evolution in available heart failure therapies, and ensure equitable allocation by medical urgency. The major differences in urgency status included stratification into 6 tiers (Status 1–6) of active listing as opposed to three tiers previously (1A, 1B, 2). These tiers are categorized by the degree of recipient hemodynamic support and require that recipients meet objective hemodynamic criteria. Additionally, geographic prioritization of recipients within a donation service area was changed to allow the highest risk patients within a 500 nautical mile radius greater access to donors.
Further, there are disparities in care and outcomes that many had hoped a new approach to allocation might improve. There are geographic differences in transplant receipt, with some regions of the country having shorter wait times and higher transplant rates than others. Additionally, rural heart failure patients are less likely to receive heart transplants than urban patients,10 likely due to worse access to specialist care and guideline-directed medical therapy for HF.11–13 In addition, due in part to historical and current structural and interpersonal racism, Black and Hispanic individuals in the US have worse access to specialty care, markedly higher heart failure mortality, and lower rates of transplantation compared with White Americans,14 and have a higher rate of allograft failure and death post-transplant.14,15 While the new allocation policies were not designed to mitigate each of these inequities, understanding whether the policy change has improved or worsened them is critically important.
While a number of single-center studies have reported an increase in temporary mechanical support prior to heart transplantation under the new policy,7,16,17 few studies have assessed the national impact of the new policy on allocation patterns or clinical strategies, or its association with changes in use of LVADs as durable support. Understanding whether the new allocation scheme favors or disfavors key sociodemographic groups or different regions across the country is a crucial part of determining whether it has ultimately been successful.
Therefore, the aim of this study was to determine the association of the 2018 UNOS heart transplant allocation policy change with changes in 1) use of OHTx vs LVAD from 2016 to 2019, overall and among key subgroups; 2) clinical strategies in both the OHTx and LVAD cohorts, including utilization of any MCS, ECMO, and IABP, overall and among key subgroups; and 3) clinical outcomes in both the OHTx and LVAD cohorts.
Methods:
Data
This retrospective study was performed using data from 2016 to 2019 from the National Inpatient Sample. The National Inpatient Sample is part of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality. The NIS encompasses a 20% sample from all inpatient hospitalizations within the United States, and includes data from all payor sources.
This study included hospitalizations for adult patients between 18 and 75 years of age with an International Classification of Diseases Tenth Edition (ICD10) procedure code for OHTx (02YA0Z0) or LVAD implantation (02HA0QZ, 02RL0JZ and 02RK0JZ). We excluded patients missing demographic variables of interest, including age, race, payor status, rurality designation, and sex (Supplemental Table 1). Additionally, because HCUP does not track patients across hospital transfers, we excluded hospitalizations that ended with a transfer to another acute care hospital to avoid double-counting patients. We also excluded patients aged <18 or >75, and those with incomplete data with respect to race, insurance status, degree of rurality, sex, or hospital division (see Supplemental Table 1 for details). In addition, we excluded those with medical comorbidities that would preclude transplantation (Lymphoma, Leukemia, Metastatic Cancer, Solid Tumor without Metastasis, HIV/AIDS) as in Supplemental Table 1.
For mechanical circulatory support (MCS), we utilized ICD10 procedure codes to identify implantation of intra-aortic balloon pump (5A02210) and Impella (02HA3RZ, 02HA3RJ, 5A0221D). For ECMO we utilized 5A1522F (central ECMO), 5A1522G (VA ECMO), and 5A1522H (VV ECMO).
Patient-Level Predictors
Patient characteristics included age (18–35, 36–55, 56–75), race (White, Black, Hispanic, Asian or Pacific Islander, Native American, Other, as reported in the HCUP database), sex, insurance status (Medicaid, Medicare, Private, Self-Pay/Charity Care, Other), rurality of patient home residence (>1,000,000, 50k-1mil, <50k), hospital division (New England, Mid-Atlantic, East North Central, West North Central, South Atlantic, East South Central, West South Central, Mountain, Pacific), and medical comorbidities using the Elixhauser approach, which has been validated for risk adjustment in administrative datasets.18
Outcomes
Our primary outcome was receipt of OHTx or an LVAD in the postoperative period. Secondary outcomes included use of any MCS, as well as the individual MCS modalities including ECMO, IABP, Impella, or LVAD, as well as length of stay and in-hospital mortality.
Analysis
First, we assessed baseline patient demographics in the OHTx, LVAD and combined cohorts for age, sex, race, insurance status, degree of rurality, hospital division and Elixhauser comorbidities (Table 1). Next, we compared quarterly count data for both the OHTx and LVAD cohorts from first quarter of 2016 to fourth quarter of 2019 (Figure 1). We then fit a logistic regression model (controlling for medical comorbidities, trends over time and within hospital-year effects) to determine the odds of OHTx within a hospitalization overall and among key subgroups (age, sex, race, insurance, rurality, hospital division). We then assessed hospital outcomes in both the OHTx, LVAD and combined cohorts comparing use of tMCS (any MCS, ECMO, IABP, Impella, VAD, in-hospital mortality, LOS) before and after implementation of the 2018 UNOS allocation policy (Table 3). Lastly, we fit a logistic regression model (controlling for medical comorbidities, trends over time and within hospital-year effects) to determine odds of MCS use in the OHTx cohort within a hospital and among key subgroups (age, sex, race, insurance, rurality, hospital division).
Table 1:
Patient Characteristics
| Total (n=5009) | OHTx (n=2116) | LVAD (n=2893) | P-Value | ||
|---|---|---|---|---|---|
| Age Range | 18–35 | 9.7 | 10.6 | 9.0 | <0.001 |
| 36–55 | 33.4 | 35.8 | 31.7 | ||
| 56–75 | 56.9 | 53.6 | 59.3 | ||
| Race | White | 60.2 | 60.7 | 59.8 | <0.001 |
| Black | 25.2 | 22.4 | 27.3 | ||
| Hispanic | 8.4 | 10.1 | 7.1 | ||
| Asian or Pacific Islander | 2.8 | 3.7 | 2.1 | ||
| Native American | 0.5 | 0.3 | 0.6 | ||
| Other | 2.9 | 2.7 | 3.1 | ||
| Insurance | Medicaid | 12.9 | 12.2 | 13.4 | <0.001 |
| Medicare | 43.1 | 36.5 | 47.9 | ||
| Private | 39.7 | 46.6 | 34.7 | ||
| Self-pay | 0.9 | 0.7 | 1.0 | ||
| Other | 3.4 | 4.0 | 3.0 | ||
| Rurality | Urban | 57.5 | 62.3 | 54.0 | <0.001 |
| Suburban | 27.8 | 25.3 | 29.7 | ||
| Rural | 14.7 | 12.4 | 16.3 | ||
| Sex | Female | 25.2 | 28.5 | 22.8 | <0.001 |
| Male | 74.8 | 71.5 | 77.2 | ||
| Region | New England | 4.8 | 5.1 | 4.5 | <0.001 |
| Mid-Atlantic | 14.9 | 13.0 | 16.3 | ||
| East North Central | 17.3 | 15.5 | 18.6 | ||
| West North Central | 5.8 | 5.1 | 6.3 | ||
| South Atlantic | 20.8 | 18.6 | 22.5 | ||
| East South Central | 7.7 | 9.1 | 6.7 | ||
| West South Central | 12.5 | 11.5 | 13.3 | ||
| Mountain | 3.6 | 4.8 | 2.7 | ||
| Pacific | 12.7 | 17.4 | 9.2 | ||
| Co-morbidities | Cardiac Arrhythmias | 75.8 | 71.3 | 79.1 | <0.001 |
| Valvular disease | 29.8 | 22.5 | 35.1 | <0.001 | |
| Pulmonary circulation disease | 34.1 | 30.3 | 36.9 | <0.001 | |
| Hypertension, complicated | 32.1 | 37.3 | 28.4 | <0.001 | |
| Chronic pulmonary disease | 16.4 | 12.3 | 19.4 | <0.001 | |
| Renal failure | 43.0 | 40.6 | 44.8 | 0.004 | |
| Coagulopathy | 47.1 | 53.8 | 42.3 | <0.001 | |
| Obesity | 20.2 | 14.0 | 24.8 | <0.001 | |
| Diabetes with chronic complications | 30.3 | 29.7 | 30.7 | 0.474 | |
| Liver disease | 11.0 | 10.6 | 11.2 | 0.5313 |
Figure 1:

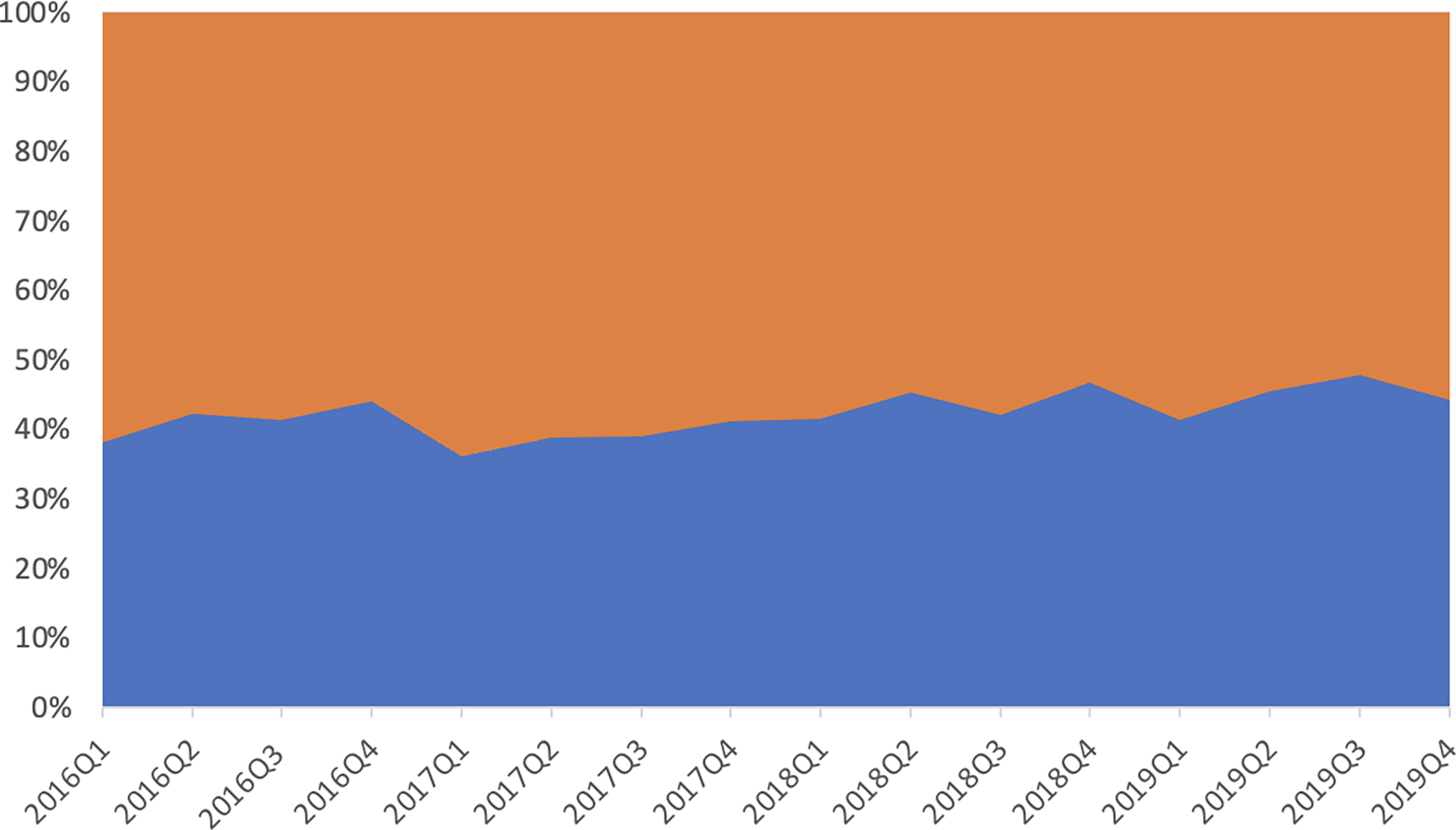
Panel A: Quarterly Counts of Heart Transplants (orange) and LVADs (blue)
Panel B: Quarterly proportions of Heart Transplants (orange) and LVADs (blue)
Tx=transplant; VAD=ventricular assist device
Table 3:
Hospital Outcomes in OHTx/LVAD Cohorts
| Procedure | Pre | Post | P-Value | |||
|---|---|---|---|---|---|---|
| OHTx Cohort (2116) | N (1400) | (%) | N (716) | (%) | ||
| Any MCS | 221 | 15.8 | 304 | 42.5 | <0.001 | |
| ECMO | 6 | 0.4 | 64 | 8.9 | <0.001 | |
| IABP | 190 | 13.6 | 244 | 34.1 | <0.001 | |
| Impella | 43 | 3.1 | 53 | 7.4 | <0.001 | |
| VAD | 26 | 1.9 | 15 | 2.1 | 0.83 | |
| In-Hospital Mortality | 76 | 5.4 | 40 | 5.6 | 0.96 | |
| Mean | St Dev | Mean | St Dev | |||
| LOS | 37.9 | 38.3 | 40.8 | 35.0 | <0.001 | |
| LVAD Cohort (2893) | N (2021) | (%) | N (872) | (%) | ||
| Any MCS | 596 | 29.5 | 320 | 36.7 | 0.00 | |
| ECMO | 6 | 0.3 | 71 | 8.1 | <0.001 | |
| IABP | 464 | 23.0 | 211 | 24.2 | 0.50 | |
| Impella | 174 | 8.6 | 111 | 12.7 | 0.00 | |
| In-Hospital Mortality | 209 | 10.3 | 87 | 10.0 | 0.82 | |
| Mean | St Dev | Mean | St Dev | |||
| LOS | 37.5 | 28.5 | 35.5 | 24.1 | 0.44 | |
| OHTx/LVAD Cohort (5009) | N (3421) | (%) | N (1588) | (%) | ||
| Any MCS | 817 | 23.9 | 624 | 39.3 | <0.001 | |
| ECMO | 12 | 0.4 | 135 | 8.5 | <0.001 | |
| IABP | 654 | 19.1 | 455 | 28.7 | <0.001 | |
| Impella | 217 | 6.3 | 164 | 10.3 | <0.001 | |
| LVAD | 2021 | 59.1 | 872 | 54.9 | 0.01 | |
| OHTx | 1400 | 40.9 | 716 | 45.1 | 0.01 | |
| In-Hospital Mortality | 285 | 8.3 | 127 | 8.0 | 0.73 | |
| Mean | St Dev | Mean | St Dev | |||
| LOS (days) | 37.7 | 32.8 | 37.9 | 29.6 | 0.01 | |
P values less than 0.05 were considered statistically significant. All statistical analyses were performed using R version 4.1.2 and Stan version 2.26.1.19 This study was approved by the Office of Human Research Protection at the Washington University School of Medicine. The requirement for informed consent and HIPAA notification were waived due to the de-identified nature of the data.
Results:
Patient Characteristics
We identified 2116 OHTx patients and 2893 LVAD patients in the study period (2016 to 2019, Table 1). The OHTx cohort had a higher proportion of patients aged 36–55 than the LVAD cohort (35.8 vs 31.7%) and a lower proportion of patients aged 56–75 (53.6 vs 59.3%, p<0.001 for each). From a gender standpoint, female patients made up a greater proportion of the OHTx group (28.5 vs 22.8%, p <0.001). The OHTx cohort had a higher proportion of White (60.7 vs 59.8) and Hispanic (10.1 vs 7.1%) patients than the LVAD cohort, but a lower proportion of Black patients (22.4 vs 27.3%, p<0.001 for each). From an insurance payor perspective, the OHTx group had a lower proportion of Medicaid (12.2 vs 13.4%) and Medicare (36.5 vs 47.9%) but a higher proportion of patients with private insurance (46.6 vs 34.7%, p<0.001 for each). The OHTx cohort had a higher proportion of patients from large population centers, and there was geographic variation in the sample, with the OHTx cohort enriched in the New England, East South Central, Mountain and Pacific regions. Comorbidity burden was high in both cohorts.
Use of OHTx versus LVAD Overall
We next assessed quarterly count data comparing OHTx to LVADs starting in the first quarter of 2016 (114 OHTx vs 185 LVAD patients) through quarter four of 2019 (130 OHTx vs 164 LVAD patients) as shown in Figure 1. Throughout the study period from 2016 to 2019 we saw quarterly variation, but there were consistently more LVADs than OHTx procedures across the study period. Quarter 3 of 2019 saw the closest convergence of the data (156 OHTx vs 171 LVAD patients). On visual inspection, the increase in transplants relative to VADs appeared to pre-date the October 2018 policy change, however.
We assessed the overall trend comparing the rate of difference before and after the 2018 UNOS policy allocation change for OHTx. We calculated the month-to-month expected change in proportion transplanted in both the pre- and post-UNOS allocation change periods. We found the post to pre difference in month-to-month change in proportion transplanted is 0.00, (p = 0.01, 95% CI -0.01, 0.01) which gives no evidence to support the claim that the UNOS allocation change affected the proportion of inpatient hospitalizations with a transplant.
Use of OHTx versus LVAD by Subgroup
Despite the null overall finding, we proceeded to determine whether any key subgroups had a change in the odds of OHTx vs VAD receipt associated with the new allocation policy. Controlling for medical comorbidities, trends over time, and within hospital-year effects, we saw no difference in the change in odds of OHTx versus LVAD based on age (Table 2). Older patients had lower odds of receiving OHTx versus LVAD than patients in the 18–35 age group, but this was similar both pre- and post-policy (for the 56–75 age group, OR 0.70 [0.54, 0.89] pre-policy vs 0.67 [0.47, 0.93] post-policy, p=0.55). Women had higher odds of transplant in both the pre- and post-policy periods, with no significant change associated with the policy. There were also no significant relative changes in the odds of transplant for Black (OR 0.82 [0.67, 1.0] vs 0.89 [0.66, 1.2]), Hispanic (OR 0.97 [0.69, 1.30] vs 1.47 [0.88, 2.35]), or Other race patients (OR 0.84 [0.57, 1.17] vs 1.31 [0.74, 2.18], p=0.088) compared to White patients. Patients with Medicare, Medicaid, or self-pay status had markedly lower odds of OHTx versus LVAD in the pre-policy and post-policy periods compared to privately insured patients, with no significant change over time.
Table 2:
Odds of OHTx versus VAD Among Key Subgroups
| OR (Pre) | OR (Post) | P-Value | ||
|---|---|---|---|---|
| Age | 18–35 | Ref | Ref | - |
| 36–55 | 0.94, (0.72, 1.20) | 0.82, (0.57, 1.13) | 0.49 | |
| 56–75 | 0.70, (0.54, 0.89) | 0.67, (0.47, 0.93) | 0.81 | |
| Sex | Male | ref | Ref | - |
| Female | 1.44, (1.19, 1.72) | 1.80, (1.40, 2.32) | 0.17 | |
| Race | White | Ref | Ref | - |
| Black | 0.82, (0.67, 0.10) | 0.89, (0.66, 1.20) | 0.62 | |
| Hispanic | 0.97, (0.69, 1.30) | 1.47, (0.88, 2.35) | 0.17 | |
| Other | 0.84, (0.57, 1.17) | 1.31, (0.74, 2.18) | 0.16 | |
| Insurance | Private | Ref | Ref | - |
| Medicare | 0.66, (0.56, 0.78) | 0.47, (0.35, 0.60) | 0.03 | |
| Medicaid | 0.66, (0.51, 0.84) | 0.52, (0.36, 0.72) | 0.28 | |
| Self-Pay | 0.88, (0.58, 1.26) | 0.73, (0.38, 1.27) | 0.52 | |
| Rurality | Urban | Ref | Ref | - |
| Suburban | 0.67, (0.56, 0.81) | 0.90, (0.68, 1.18) | 0.09 | |
| Rural | 0.64, (0.50, 0.81) | 0.84, (0.57, 1.21) | 0.26 | |
| Division | New England | Ref | Ref | - |
| Mid Atlantic | 1.30, (0.76, 2.11) | 2.95, (1.35, 5.58) | 0.05 | |
| East North Central | 0.75, (0.51, 1.06) | 0.67, (0.37, 1.04) | 0.60 | |
| West North Central | 1.95, (0.95, 3.42) | 1.56, (0.64, 3.36) | 0.58 | |
| South Atlantic | 1.15, (0.63, 1.87) | 1.58, (0.67, 3.17) | 0.58 | |
| East South Central | 1.82, (1.20, 2.70) | 1.54, (0.86, 2.55) | 0.59 | |
| West South Central | 0.81, (0.56, 1.13) | 0.93, (0.55, 1.46) | 0.67 | |
| Mountain | 0.78, (0.43, 1.32) | 1.61, (0.73, 3.02) | 0.11 | |
| Pacific | 1.00, (0.66, 1.45) | 0.92, (0.50, 1.62) | 0.71 | |
| Overall Trend | Rate of Difference: | 0.00 (−0.01, 0.01) | 0.79 | |
The odds of OHTx for patients in suburban areas were lower than in urban ones at baseline (>50k-1mil population, OR 0.67 [0.56, 0.81]) but similar post-policy (0.90 [0.68, 1.18], p for change in odds=0.111), and a similar pattern was seen in rural areas (<50k population, OR 0.64 [0.50, 0.81] vs 0.84 [0.57, 1.21], p for change in odds=0.111). There were shifts in the relative odds of OHTx versus LVAD by region, which ranged from 0.75 [0.51, 1.06] for the East North Central region to 1.82 [1.20, 2.70] for the East South Central region in the pre-policy period, and 0.67 [0.37, 1.04] for the East North Central region to 2.95 [1.35, 5.58] in the Mid-Atlantic region in the post-policy period, but the overall change was not statistically significant.
MCS Use Overall
To assess the use of temporary mechanical circulatory support as a bridge to OHTx or LVAD pre and post allocation change, we looked at each cohort separately and then as a whole. For the OHTx cohort overall, we saw an increase in any tMCS (15.8 vs 42.5%), ECMO (0.4 vs 8.9%), IABP (13.6 vs 34.1%), and Impella (3.1 vs 7.4%, p<0.001 for each, Table 3). We saw no difference in durable LVAD utilization pre-transplant (1.9 vs 2.1%, p=0.83) in the OHTx cohort. We calculated the month-to-month expected change in proportion use of tMCS as an overall trend comparing pre- to post-policy change. We found a statistically significant increase in proportion tMCS use of 0.02 (p=0.02, 95% CI -0.00, 0.03) comparing pre- to post-policy change.
For the LVAD cohort overall, we saw an increase in any tMCS (29.5 vs 36.7%), ECMO (0.3 vs 8.1%) and Impella (8.6 vs 12.7%, p<0.001 for each), but no difference in IABP utilization (23.0 vs 24.2%, p=0.50). Results for the combined cohort were similar.
Temporary MCS Use Among Key OHTx Subgroups
Among OHTx patients, we saw no significant differences in the pre-post policy change in odds of tMCS use by age group, race, or insurance status, but women had a lower relative odds of tMCS use prior to transplant in the post-period (1.05 [0.71, 1.51] vs 0.59 [0.38, 0.85], p=0.034, Table 4). There was a relative increase in the odds of tMCS utilization in both the suburban (OR 0.61 [0.38, 0.91] vs 1.27 [0.79, 1.91], p=0.027) and rural groups (OR 0.53 [0.26, 0.92] vs 1.02 [0.52, 1.80], p=0.027). Again, while there was regional variation in the odds of tMCS use, there was no significant change from pre- to post-policy.
Table 4:
Odds of MCS Use in OHTx Cohort
| OR (Pre) | OR (Post) | P-Value | ||
|---|---|---|---|---|
| Age | 18–35 | - | ||
| 36–55 | 0.90, (0.50, 1.51) | 1.09, (0.57, 1.90) | 0.61 | |
| 56–75 | 0.83, (0.47, 1.4) | 1.20, (0.64, 2.20) | 0.28 | |
| Sex | Male | - | ||
| Female | 1.05, (0.71, 1.51) | 0.59, (0.38, 0.85) | 0.03 | |
| Race | White | - | ||
| Black | 1.11, (0.70, 1.66) | 1.49, (0.89, 2.31) | 0.30 | |
| Hispanic | 0.56, (0.27, 1.01) | 1.49, (0.71, 2.76) | 0.04 | |
| Other | 1.30, (0.63, 2.33) | 2.33, (1.05, 4.61) | 0.26 | |
| Insurance | Private | - | ||
| Medicare | 1.01, (0.67, 1.50) | 1.41, (0.91, 2.06) | 0.27 | |
| Medicaid | 1.20, (0.65, 1.96) | 1.62, (0.82, 2.91) | 0.45 | |
| Self-Pay | 1.05, (0.39, 2.22) | 0.68, (0.24, 1.56) | 0.49 | |
| Rurality | >=1 million | - | ||
| 50K-1 million | 0.61, (0.38, 0.91) | 1.27, (0.80, 1.91) | 0.02 | |
| <50K | 0.53, (0.26, 0.92) | 1.02, (0.52, 1.80) | 0.12 | |
| Division | New England | - | ||
| Mid Atlantic | 0.61, (0.19, 1.36) | 1.65, (0.52, 3.93) | 0.13 | |
| East North Central | 0.53, (0.23, 1.04) | 1.08, (0.39, 2.27) | 0.23 | |
| West North Central | 0.16, (0.02, 0.56) | 1.38, (0.30, 3.94) | 0.02 | |
| South Atlantic | 0.49, (0.13, 1.28) | 0.85, (0.19, 2.31) | 0.53 | |
| East South Central | 0.85, (0.39, 1.61) | 0.57, (0.21, 1.26) | 0.45 | |
| West South Central | 1.22, (0.62, 2.16) | 1.35, (0.58, 2.88) | 0.88 | |
| Mountain | 2.64, (0.91, 6.29) | 3.86, (1.08, 9.58) | 0.65 | |
| Pacific | 1.20, (0.51, 2.26) | 1.15, (0.38, 2.70) | 0.95 | |
| Overall Trend | Rate of Difference: | 0.02 (0.00, 0.03) | 0.02 | |
In-Hospital Outcomes
Among OHTx patients, length of stay increased post-policy (37.9 vs 40.8 days, p<0.001) but in-hospital mortality was unchanged (5.4 vs 5.6%, p=0.96, Table 2)). Among LVAD patients, there were no changes in length of stay (37.5 vs 38.5 days, p=0.43) or in-hospital mortality (10.3 vs 10.0%, p=0.82) associated with the allocation policy.
Discussion:
Our study demonstrated several significant findings. First, there was no overall change in the odds of OHTx versus LVAD following the change to the heart allocation policy overall, although geographic differences related to rurality declined to some degree. Second, use of tMCS increased dramatically among OHtx patients. Third, outcomes were similar pre- and post-policy change.
While we noted a slight gradual increase in transplant volume over time, it pre-dated the change in allocation policy. Given the durability of dischargeable LVADs and improved survival (close to 80% at 2 years),20 UNOS decreased the priority of durable LVADs from status 1A to status 4 with this policy change. Some had hypothesized that this might disincentivize VAD placement and lead to an increase in OHTx volume overall compared with LVAD volume, and this has been reported by other groups utilizing the UNOS database.21,22 Other concurrent factors also potentially increasing donor supply during the study period that are independent of allocation policy include the continued rise in opiate overdoses23 as well as new hepatitis C viral (HCV) treatments allowing for HCV-positive donors.24–27 However, since there is still a relatively fixed and inadequate overall donor supply, VADs will likely remain a crucial part of heart failure management regardless of donor allocation policies.
We did not find that the new allocation policy was associated with consistent changes in racial inequities in access to transplantation. However, our findings that there were no longer urban-rural disparities in the odds of transplant versus VAD following the policy change are encouraging but exploratory, and warrant further examination as more data become available over time.
We found that the use of temporary MCS increased markedly, particularly among patients who ultimately received heart transplants; in the post-period. These data are consistent with data as reported by others showing specifically an increase in ECMO, IABP and Impella use in the post-transplant era as well as tMCS in total.4,17,28 One hypothesis for this is increased use of tMCS across all centers as technology has become more widely available on the national scale however this increase has been shown to occur disproportionately in transplant centers.28 These data point to a possible change in practice behavior from durable bridge to transplant LVADs to bridge to decision using temporary MCS. The gender difference we observed such that women were less likely to receive tMCS post-policy change has not previously been reported and should be examined in other datasets to confirm, but could be related to technical limitations with smaller body size or differences in physiology prior to transplantation, given that heart failure etiology differs to some degree by gender.
We did not find that the allocation policy change was associated with changes in clinical outcomes. While length of stay increased for OHTx patients, in-hospital mortality was unchanged. Increased length of stay post-policy change may be due to changes in both donor and patient characteristics, or may be related to the additional complexity associated with temporary MCS use. The lack of change in in-hospital mortality is reassuring; future studies should examine mortality on the transplant wait list to determine whether the allocation change improves access for the sickest patients.
This study has limitations, the first of which relates to the use of administrative data. The NIS does not allow researchers to follow individual patients over time to examine long-term outcomes, nor does it provide state-level or hospital-level identifiers with which to determine more granular variability in the response to the allocation policy change. We are also unable to examine waitlist mortality using these data. We only examined patients receiving either OHTx or LVAD; if the policy had led to significant increases in the number of transplants performed in a year, this could raise concerns about a shifting denominator of patients. However, since the overall n of transplants did not increase meaningfully, considering the population of patients receiving one or the other of these two therapies was a reasonable strategy. We only examined the first 5 quarters of the policy’s implementation, and some changes in clinical strategy may have taken longer to emerge. However, due to the COVID-19 pandemic, 2020 data are likely not a pure reflection of the policy’s impact, and longer-term analyses are needed to track the allocation policy’s ultimate impact on access and outcomes.
Conclusions:
The UNOS heart transplant allocation policy change of 2018 was associated with no overall change in the use of OHTx versus LVAD in its first year of implementation, though there were small changes in the relative odds of transplant based on rurality. The use of tMCS prior to transplant increased markedly following the policy, albeit less so among women than men. There were no differences in in-hospital mortality among OHTx or LVAD patients following the allocation change. Further studies are necessary to understand the long-term effect of the policy for access to transplant and for patient outcomes.
Supplementary Material
Acknowledgements:
The authors acknowledge Tierney Lanter for assistance with manuscript preparation. She received no compensation beyond that of regular employment.
Sources of Funding:
Dr. Fox was supported by a National Heart, Lung, and Blood Institute (NHLBI)
Institutional National Research Service Award (5T32HL007081).
Non-standard Abbreviations and Acronyms:
- DSA
Donation Service Areas
- ECMO
extracorporeal membrane oxygenation
- LVADS
left ventricular assist devices
- MCS
mechanical circulatory support
- OHTx
orthotopic heart transplant
- OPTN
Organ Procurement and Transplantation Network
- UNOS
United Network for Organ Sharing
Footnotes
Disclosures: Dr. Joynt Maddox receives research support from the National Heart, Lung, and Blood Institute (R01HL143421 and R01HL164561), National Institute of Nursing Research (U01NR020555) and National Institute on Aging (R01AG060935, R01AG063759, and R21AG065526), and from Humana. She also serves on the Health Policy Advisory Council for the Centene Corporation (St. Louis, MO). The other authors report no conflicts.
Prior presentations: Poster presentation at the 2022 American Heart Association Scientific Sessions, Chicago, IL.
References:
- 1.Callahan LR. OPTN/UNOS Thoracic Organ Transplantation Committee: Proposal to Modify the Adult Heart Allocation System. 2016.
- 2.Department OUP. OPTN Board Briefing. Proposal to Modify the Adult Heart Allocation December 2016. 2016. [Google Scholar]
- 3.Schulze PC, Kitada S, Clerkin K, Jin Z, Mancini DM. Regional differences in recipient waitlist time and pre- and post-transplant mortality after the 2006 United Network for Organ Sharing policy changes in the donor heart allocation algorithm. JACC Heart Fail. 2014;2:166–177. doi: 10.1016/j.jchf.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estep JD, Soltesz E, Cogswell R. The new heart transplant allocation system: Early observations and mechanical circulatory support considerations. J Thorac Cardiovasc Surg. 2020. doi: 10.1016/j.jtcvs.2020.08.113 [DOI] [PubMed] [Google Scholar]
- 5.Parker WF, Anderson AS, Hedeker D, Huang ES, Garrity ER, Jr., Siegler M, Churpek MM. Geographic Variation in the Treatment of U.S. Adult Heart Transplant Candidates. Journal of the American College of Cardiology. 2018;71:1715–1725. doi: 10.1016/j.jacc.2018.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trivedi JR, Slaughter MS. "Unintended" Consequences of Changes in Heart Transplant Allocation Policy: Impact on Practice Patterns. ASAIO J. 2020;66:125–127. doi: 10.1097/MAT.0000000000001128 [DOI] [PubMed] [Google Scholar]
- 7.Hanff TC, Harhay MO, Kimmel SE, Molina M, Mazurek JA, Goldberg LR, Birati EY. Trends in Mechanical Support Use as a Bridge to Adult Heart Transplant Under New Allocation Rules. JAMA Cardiol. 2020;5:728–729. doi: 10.1001/jamacardio.2020.0667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connell G, Wang AS, Kurlansky P, Ning Y, Farr MA, Sayer G, Uriel N, Naka Y, Takeda K. Impact of UNOS allocation policy changes on utilization and outcomes of patients bridged to heart transplant with intra-aortic balloon pump. Clin Transplant. 2022;36:e14533. doi: 10.1111/ctr.14533 [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Yang BQ, Itoh A, Masood MF, Hartupee JC, Schilling JD. Impact of New UNOS Allocation Criteria on Heart Transplant Practices and Outcomes. Transplant Direct. 2021;7:e642. doi: 10.1097/TXD.0000000000001088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axelrod DA, Guidinger MK, Finlayson S, Schaubel DE, Goodman DC, Chobanian M, Merion RM. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA. 2008;299:202–207. doi: 10.1001/jama.2007.50 [DOI] [PubMed] [Google Scholar]
- 11.Gamble JM, Eurich DT, Ezekowitz JA, Kaul P, Quan H, McAlister FA. Patterns of care and outcomes differ for urban versus rural patients with newly diagnosed heart failure, even in a universal healthcare system. Circ Heart Fail. 2011;4:317–323. doi: 10.1161/CIRCHEARTFAILURE.110.959262 [DOI] [PubMed] [Google Scholar]
- 12.Schopfer DW, Whooley MA, Stamos TD. Hospital compliance with performance measures and 30-day outcomes in patients with heart failure. Am Heart J. 2012;164:80–86. doi: 10.1016/j.ahj.2012.04.017 [DOI] [PubMed] [Google Scholar]
- 13.Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA. 2011;306:45–52. doi: 10.1001/jama.2011.902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 15.Lui C, Fraser CD 3rd, Zhou X, Suarez-Pierre A, Kilic A, Zehr KJ, Higgins RSD. Racial Disparities in Patients Bridged to Heart Transplantation With Left Ventricular Assist Devices. Ann Thorac Surg. 2019;108:1122–1126. doi: 10.1016/j.athoracsur.2019.03.073 [DOI] [PubMed] [Google Scholar]
- 16.Goff RR, Uccellini K, Lindblad K, Hall S, Davies R, Farr M, Silvestry S, Rogers JG. A change of heart: Preliminary results of the US 2018 adult heart allocation revision. Am J Transplant. 2020;20:2781–2790. doi: 10.1111/ajt.16010 [DOI] [PubMed] [Google Scholar]
- 17.Cascino TM, Stehlik J, Cherikh WS, Cheng Y, Watt TMF, Brescia AA, Thompson MP, McCullough JS, Zhang M, Shore S, et al. A challenge to equity in transplantation: Increased center-level variation in short-term mechanical circulatory support use in the context of the updated U.S. heart transplant allocation policy. J Heart Lung Transplant. 2022;41:95–103. doi: 10.1016/j.healun.2021.09.004 [DOI] [PubMed] [Google Scholar]
- 18.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 19.(2022) RCT. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. [Google Scholar]
- 20.Mehra MR, Uriel N, Naka Y, Cleveland JC Jr., Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, et al. A Fully Magnetically Levitated Left Ventricular Assist Device - Final Report. The New England journal of medicine. 2019;380:1618–1627. doi: 10.1056/NEJMoa1900486 [DOI] [PubMed] [Google Scholar]
- 21.Doulamis IP, Inampudi C, Kourek C, Mandarada T, Kuno T, Asleh R, Briasoulis A. Characteristics and outcomes of left ventricular assist device recipients transplanted before and after the new donor heart allocation system. Artif Organs. 2022. doi: 10.1111/aor.14363 [DOI] [PubMed] [Google Scholar]
- 22.Mullan CW, Chouairi F, Sen S, Mori M, Clark KAA, Reinhardt SW, Miller PE, Fuery MA, Jacoby D, Maulion C, et al. Changes in Use of Left Ventricular Assist Devices as Bridge to Transplantation With New Heart Allocation Policy. JACC Heart Fail. 2021;9:420–429. doi: 10.1016/j.jchf.2021.01.010 [DOI] [PubMed] [Google Scholar]
- 23.Goldberg DS, Blumberg E, McCauley M, Abt P, Levine M. Improving Organ Utilization to Help Overcome the Tragedies of the Opioid Epidemic. Am J Transplant. 2016;16:2836–2841. doi: 10.1111/ajt.13971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilic A, Hickey G, Mathier MA, Kormos RL, Sultan I, Gleason TG, Keebler ME. Outcomes of the First 1300 Adult Heart Transplants in the United States After the Allocation Policy Change. Circulation. 2020;141:1662–1664. doi: 10.1161/CIRCULATIONAHA.119.045354 [DOI] [PubMed] [Google Scholar]
- 25.Cogswell R, John R, Estep JD, Duval S, Tedford RJ, Pagani FD, Martin CM, Mehra MR. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transplant. 2020;39:1–4. doi: 10.1016/j.healun.2019.11.002 [DOI] [PubMed] [Google Scholar]
- 26.Kilic A, Mathier MA, Hickey GW, Sultan I, Morell VO, Mulukutla SR, Keebler ME. Evolving Trends in Adult Heart Transplant With the 2018 Heart Allocation Policy Change. JAMA Cardiol. 2021;6:159–167. doi: 10.1001/jamacardio.2020.4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlendorf KH, Zalawadiya S, Shah AS, Perri R, Wigger M, Brinkley DM, Danter MR, Menachem JN, Punnoose LR, Balsara K, et al. Expanding Heart Transplant in the Era of Direct-Acting Antiviral Therapy for Hepatitis C. JAMA Cardiol. 2020;5:167–174. doi: 10.1001/jamacardio.2019.4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varshney AS, Berg DD, Katz JN, Baird-Zars VM, Bohula EA, Carnicelli AP, Chaudhry SP, Guo J, Lawler PR, Nativi-Nicolau J, et al. Use of Temporary Mechanical Circulatory Support for Management of Cardiogenic Shock Before and After the United Network for Organ Sharing Donor Heart Allocation System Changes. JAMA Cardiol. 2020;5:703–708. doi: 10.1001/jamacardio.2020.0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


